Modulation of Gut Microbiota and Short-Chain Fatty Acid Production by Simulated Gastrointestinal Digests from Microalga Chlorella vulgaris
Abstract
1. Introduction
2. Results and Discussion
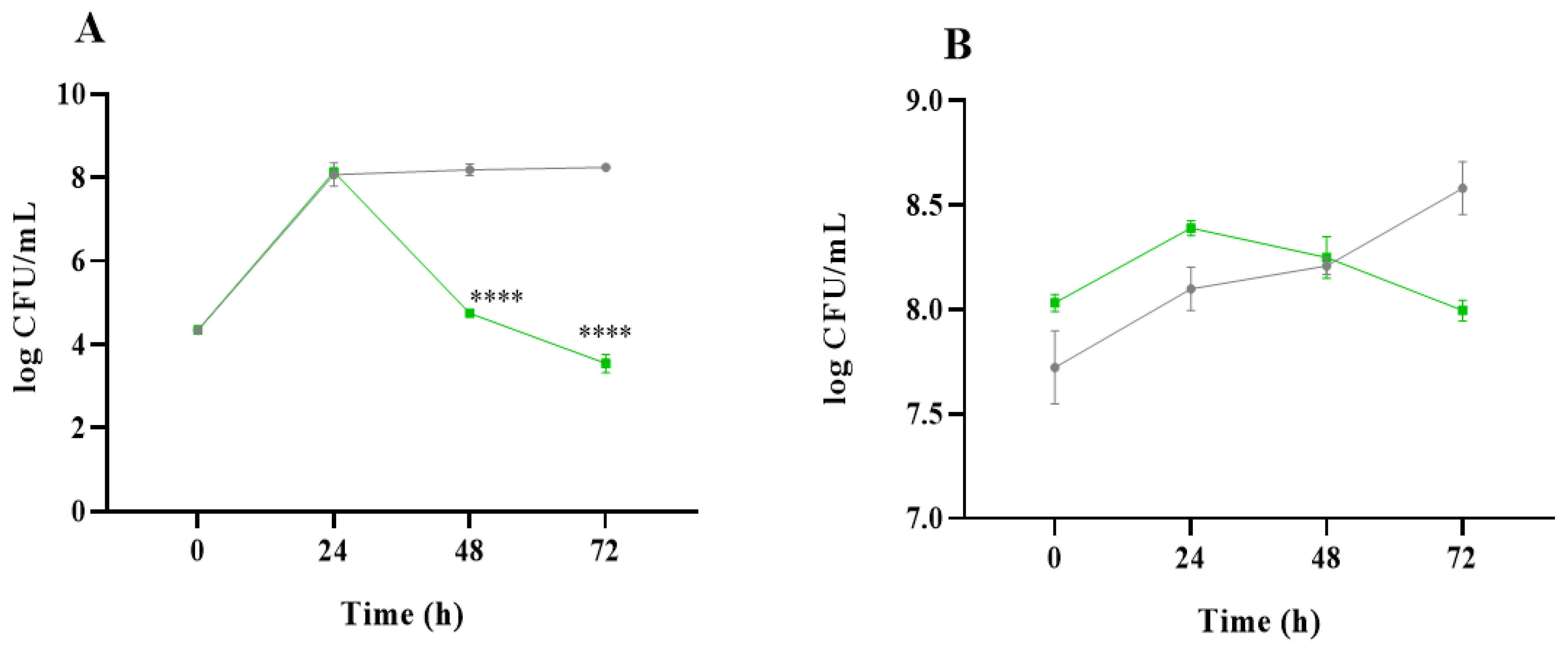
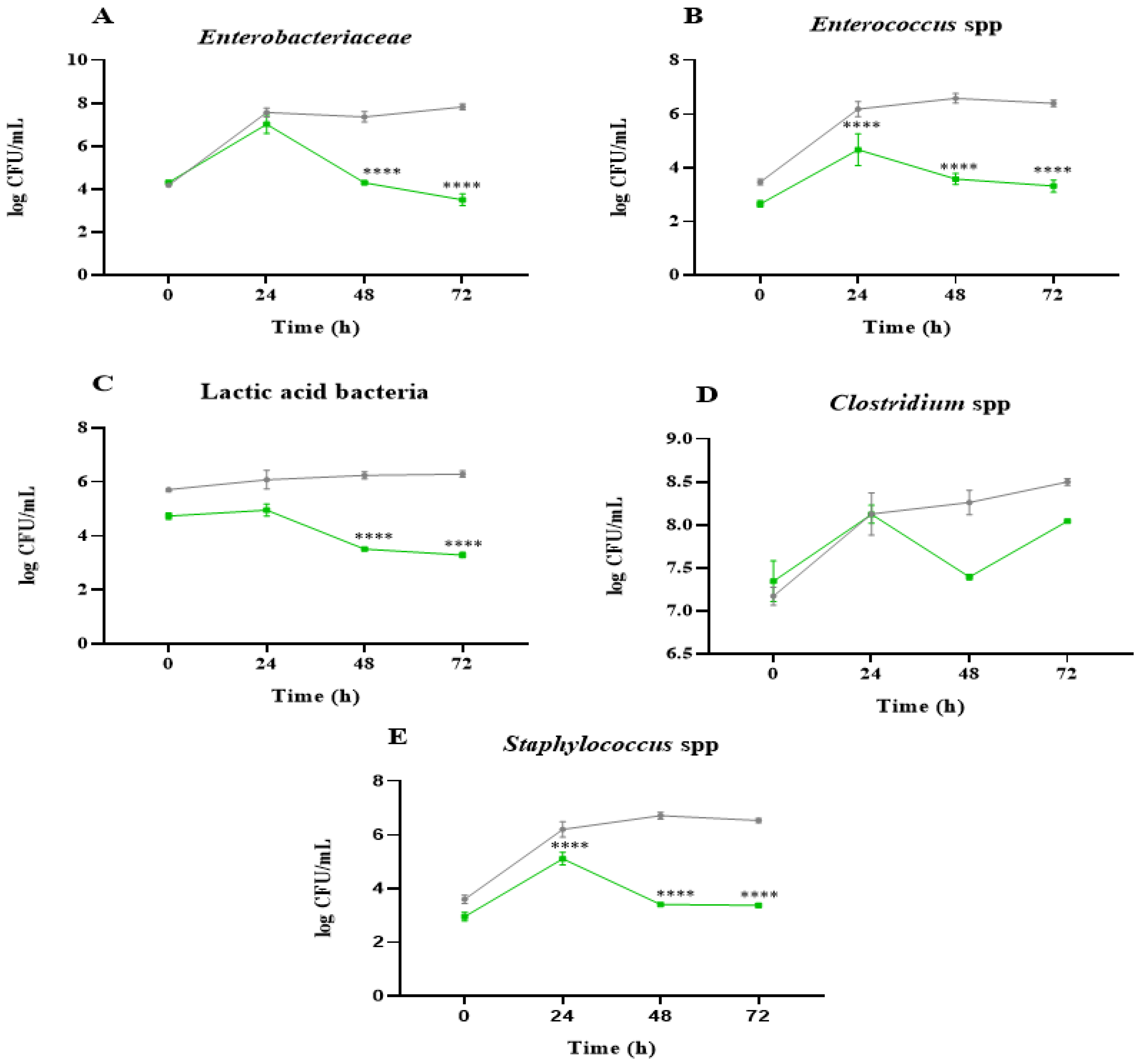
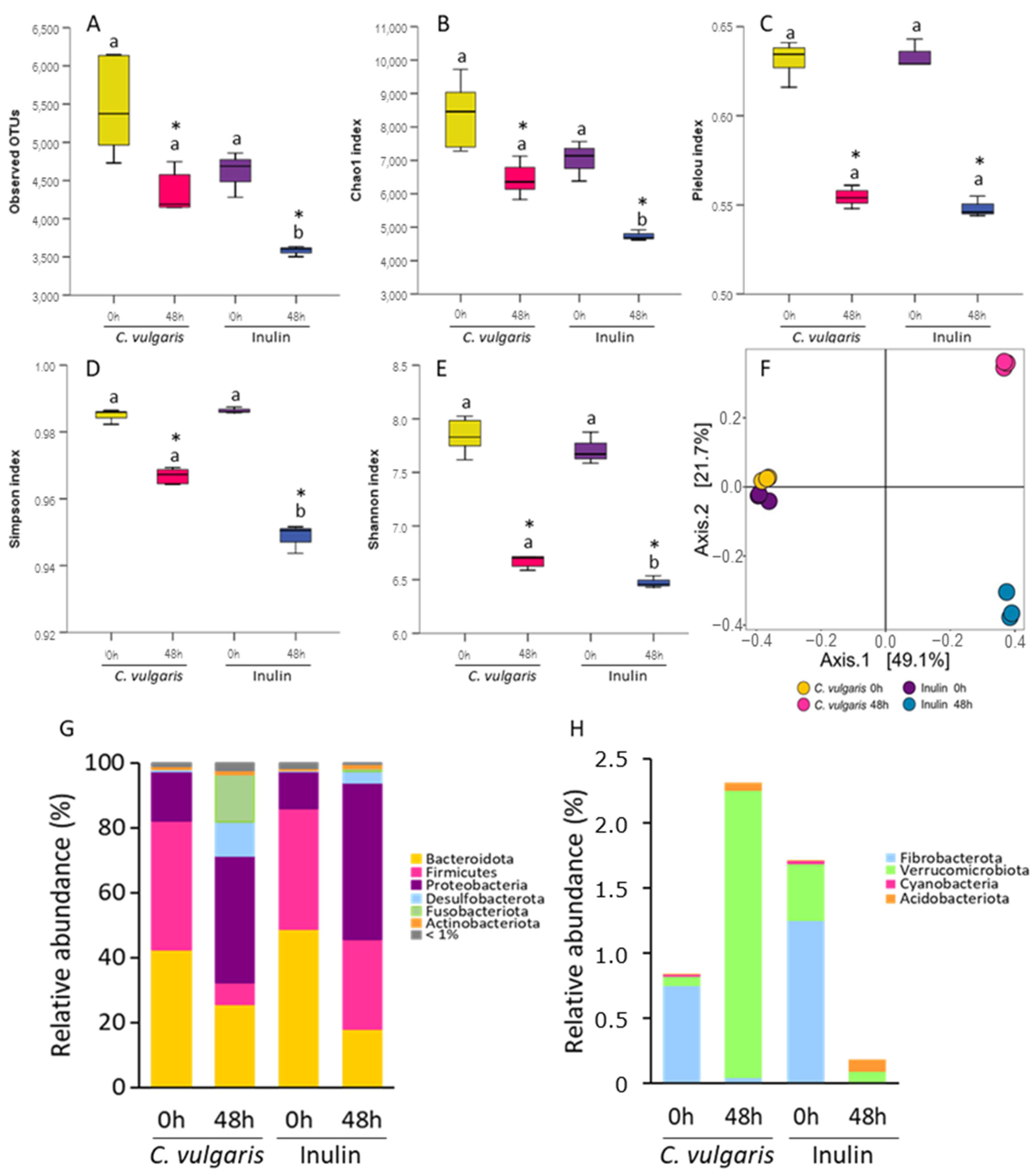
2.1. Analysis of Microbial Communities
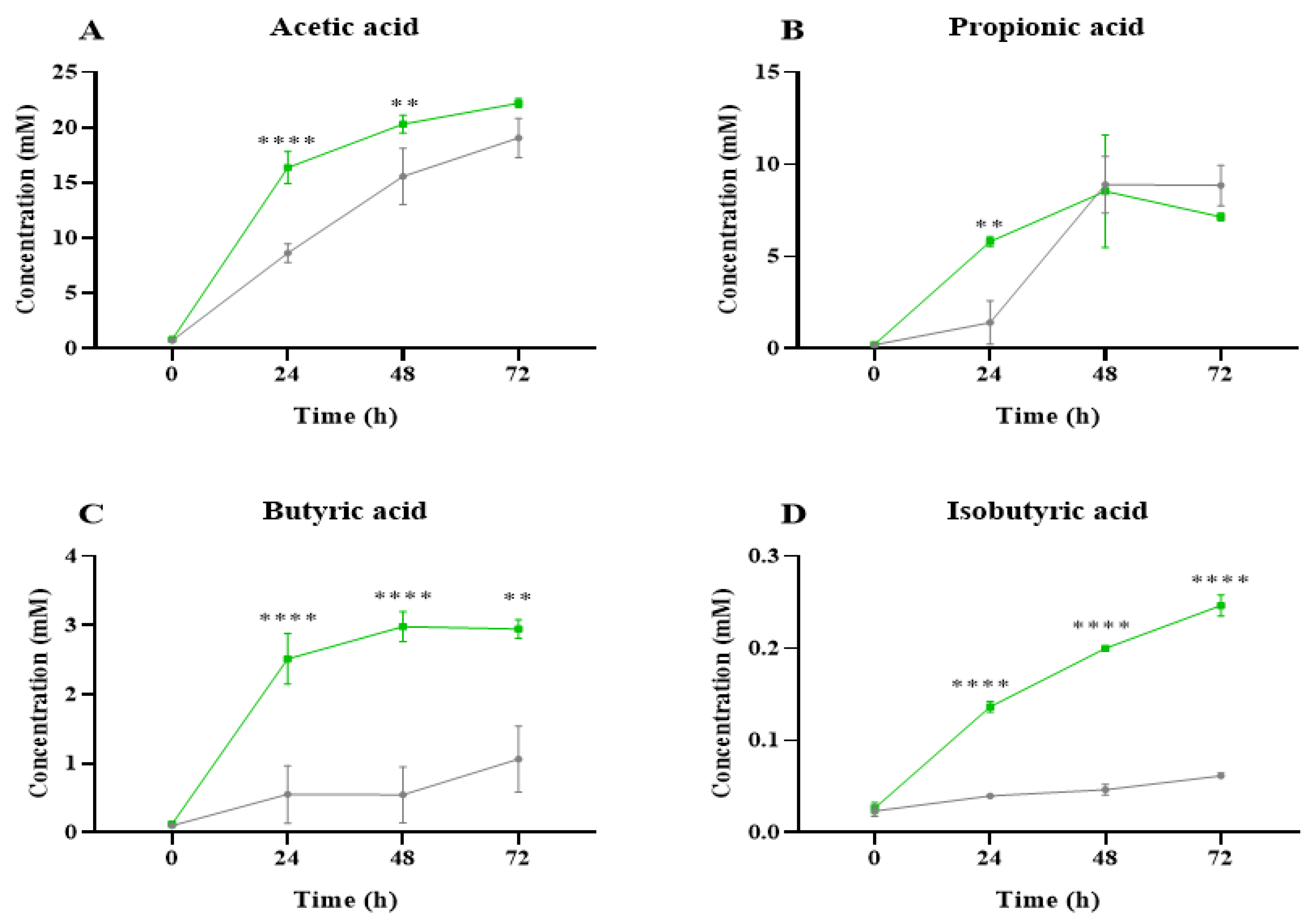
2.2. Short-Chain Fatty Acid Analysis
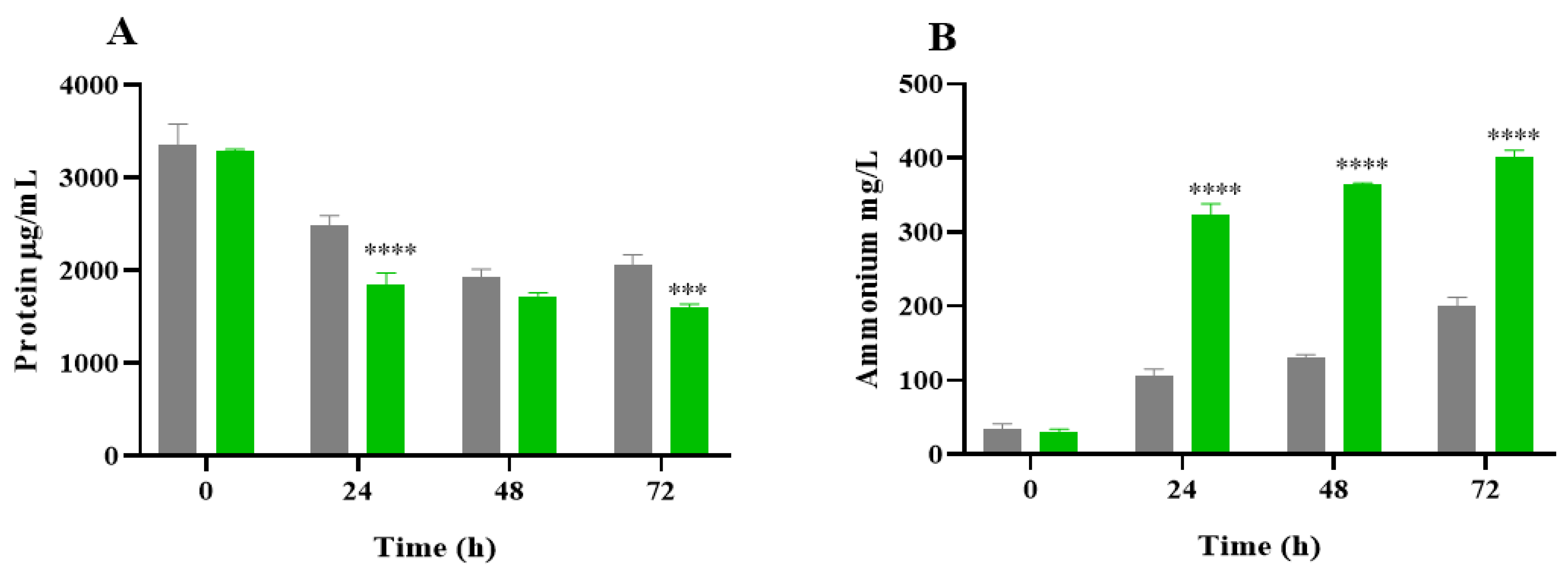
2.3. Protein Degradation and Ammonium Production
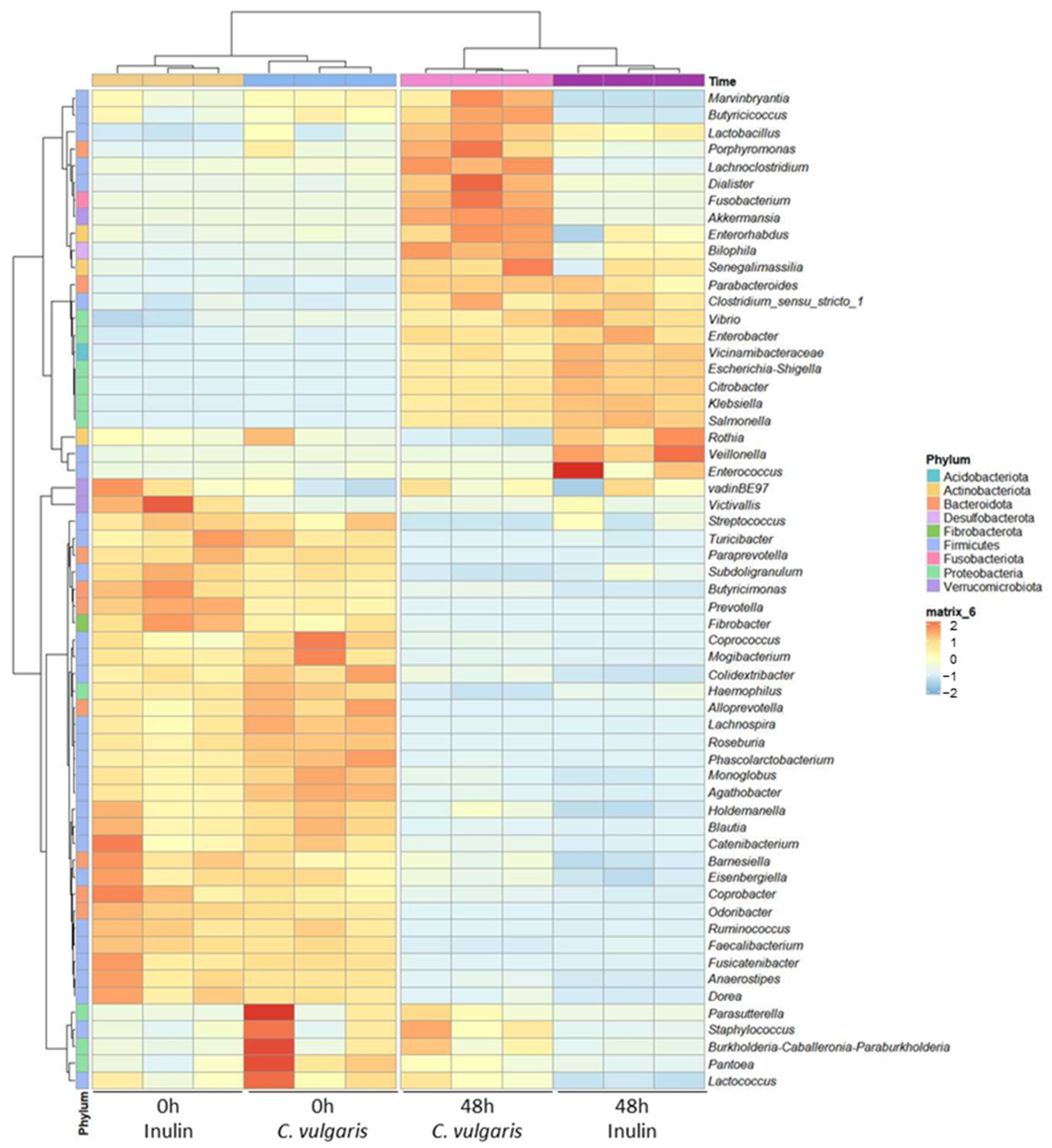
2.4. 16S Ribosomal RNA Gene Analysis
3. Materials and Methods
3.1. Samples and Reagents
3.2. Simulated Gastrointestinal Digestion
3.3. Static Colonic Fermentation
3.3.1. Microbial Plate Counting
3.3.2. Short-Chain Fatty Acid (SCFA) Analysis
3.3.3. Protein and Ammonium Content
3.4. 16S Ribosomal RNA Gene Analysis
3.4.1. Extraction and Quantification of Microbial DNA
3.4.2. 16S Ribosomal RNA Gene Sequencing and Bioinformatics Analysis
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barros de Medeiros, V.P.; da Costa, W.K.A.; da Silva, R.T.; Pimentel, T.C.; Magnani, M. Microalgae as source of functional ingredients in new-generation foods: Challenges, technological effects, biological activity, and regulatory issues. Crit. Rev. Food. Sci. Nutr. 2022, 62, 4929–4950. [Google Scholar] [CrossRef] [PubMed]
- Canelli, G.; Tarnutzer, C.; Carpine, R.; Neutsch, L.; Bolten, C.J.; Dionisi, F.; Mathys, A. Biochemical and nutritional evaluation of Chlorella and Auxenochlorella biomasses relevant for food application. Front. Nutr. 2020, 7, 565996. [Google Scholar] [CrossRef]
- Dolganyuk, V.; Belova, D.; Babich, O.; Prosekov, A.; Ivanova, S.; Katserov, D.; Patyukov, N.; Sukhikh, S. Microalgae: A promising source of valuable bioproducts. Biomolecules 2020, 10, 1153. [Google Scholar] [CrossRef] [PubMed]
- Ennaji, H.; Bourhia, M.; Taouam, I.; Falaq, A.; Bellahcen, T.O.; Salamatullah, A.M.; Alzahrani, A.; Alyahya, H.K.; Ullah, R.; Ibenmoussa, S. Physicochemical evaluation of edible Cyanobacterium Arthrospira platensis collected from the south atlantic coast of Morocco: A promising source of dietary supplements. eCAM 2021, 2021, 3337231. [Google Scholar] [CrossRef]
- Prüser, T.; Braun, P.; Wiacek, C. Microalgae as a novel food. Potential and legal framework. Ernahr. Umsch. 2021, 68, 78–85. [Google Scholar]
- Camacho, F.; Macedo, A.; Malcata, F. Potential industrial applications and commercialization of microalgae in the functional food and feed industries: A short review. Mar. Drugs 2019, 17, 312. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, R.A.; Abd El Latif, A.; Elkaw, E.M.; Alotaibi, A.S.; Alenzi, A.M.; Hamza, H.A. Assessment of antioxidant and anticancer activities of microgreen alga Chlorella vulgaris and its blend with different vitamins. Molecules 2022, 27, 1602. [Google Scholar] [CrossRef]
- Gentscheva, G.; Nikolova, K.; Panayotova, V.; Peycheva, K.; Makedonski, L.; Slavov, P.; Radusheva, P.; Petrova, P.; Yotkovska, I. Application of Arthrospira platensis for medicinal purposes and the food industry: A review of the literature. Life 2023, 13, 845. [Google Scholar] [CrossRef]
- Maurício, T.; Couto, D.; Lopes, D.; Conde, T.; Pais, R.; Batista, J.; Melo, T.; Pinho, M.; Moreira, A.S.; Trovão, M. Differences and similarities in lipid composition, nutritional value, and bioactive potential of four edible Chlorella vulgaris strains. Foods 2023, 12, 1625. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, M.; Grimi, N.; Dar, B.N.; Calvo-Lerma, J.; Barba, F.J. Research progress in microalgae nutrients: Emerging extraction and purification technologies, digestive behavior, and potential effects on human gut. Crit. Rev. Food Sci. Nutr. 2023, 64, 11375–11395. [Google Scholar] [CrossRef]
- Chabni, A.; Bañares, C.; Reglero, G.; Torres, C.F. A comparative study of in vitro gastrointestinal digestion of three strategic edible oils. J. Food Sci. 2022, 87, 3268–3278. [Google Scholar] [CrossRef]
- McClements, D.J.; Peng, S.-F. Current status in our understanding of physicochemical basis of bioaccessibility. Curr. Opin. Food Sci. 2020, 31, 57–62. [Google Scholar] [CrossRef]
- Duijsens, D.; Pälchen, K.; Guevara-Zambrano, J.; Verkempinck, S.; Infantes-Garcia, M.; Hendrickx, M.; Van Loey, A.; Grauwet, T. Strategic choices for in vitro food digestion methodologies enabling food digestion design. Trends Food Sci. 2022, 126, 61–72. [Google Scholar] [CrossRef]
- McClements, D.J.; Öztürk, B. Utilization of nanotechnology to improve the handling, storage and biocompatibility of bioactive lipids in food applications. Foods 2021, 10, 365. [Google Scholar] [CrossRef]
- Zhou, H.; Tan, Y.; McClements, D.J. Applications of the INFOGEST in vitro digestion model to foods: A review. Ann. Rev. Food Sci. 2023, 14, 135–156. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Niccolai, A.; Zittelli, G.C.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of interest as food source: Biochemical composition and digestibility. Algal Res. 2019, 42, 101617. [Google Scholar] [CrossRef]
- Chen, L.; Xu, W.; Chen, D.; Chen, G.; Liu, X.; Zeng, X.; Shao, R.; Zhu, H. Digestibility of sulfated polysaccharide from the brown seaweed Ascophyllum nodosum and its effect on the human gut microbiota in vitro. Int. J. Biol. Macromol. 2018, 112, 1055–1061. [Google Scholar] [CrossRef]
- Qazi, W.M.; Ballance, S.; Kousoulaki, K.; Uhlen, A.K.; Kleinegris, D.M.M.; Skjånes, K.; Rieder, A. Protein Enrichment of Wheat Bread with Microalgae: Microchloropsis Gaditana, Tetraselmis Chui and Chlorella vulgaris. Foods 2021, 10, 3078. [Google Scholar] [CrossRef]
- Zhang, Y.-J.; Li, S.; Gan, R.-Y.; Zhou, T.; Xu, D.-P.; Li, H.-B. Impacts of gut bacteria on human health and diseases. Int. J. Mol. Sci. 2015, 16, 7493–7519. [Google Scholar] [CrossRef]
- Blaak, E.; Canfora, E.; Theis, S.; Frost, G.; Groen, A.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; Van Harsselaar, J. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Ashraf, N.; Ahmad, F.; Lu, Y. Synergy between microalgae and microbiome in polluted waters. Trends Microbiol. 2022, 31, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Gao, J.; Peng, C.; Song, J.; Xie, Z.; Jia, J.; Li, H.; Zhao, S.; Liang, Y.; Gong, B. The effect of the microalgae Chlorella vulgaris on the gut microbiota of juvenile Nile tilapia (Oreochromis niloticus) is feeding-time dependent. Microorganisms 2023, 11, 1002. [Google Scholar] [CrossRef]
- Barros de Medeiros, V.P.; de Souza, E.L.; de Albuquerque, T.M.R.; da Costa Sassi, C.F.; dos Santos Lima, M.; Sivieri, K.; Pimentel, T.C.; Magnani, M. Freshwater microalgae biomasses exert a prebiotic effect on human colonic microbiota. Algal Res. 2021, 60, 102547. [Google Scholar] [CrossRef]
- Baldelli, V.; Scaldaferri, F.; Putignani, L.; Del Chierico, F. The role of Enterobacteriaceae in gut microbiota dysbiosis in inflammatory bowel diseases. Microorganisms 2021, 9, 697. [Google Scholar] [CrossRef]
- Sannasiddappa, T.H.; Costabile, A.; Gibson, G.R.; Clarke, S.R. The influence of Staphylococcus aureus on gut microbial ecology in an in vitro continuous culture human colonic model system. PLoS ONE 2011, 6, e23227. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.; Santoyo, S.; Jaime, L.; Avalo, B.; Cifuentes, A.; Reglero, G.; Reina, G.G.-B.; Señoráns, F.J.; Ibáñez, E. Comprehensive characterization of the functional activities of pressurized liquid and ultrasound-assisted extracts from Chlorella vulgaris. LWT-Food. Sci. Technol. 2012, 46, 245–253. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, M.; Bäuerl, C.; Cortés-Macías, E.; Calvo-Lerma, J.; Collado, M.C.; Barba, F.J. The impact of liquid-pressurized extracts of Spirulina, Chlorella and Phaedactylum tricornutum on in vitro antioxidant, antiinflammatory and bacterial growth effects and gut microbiota modulation. Food Chem. 2023, 401, 134083. [Google Scholar] [CrossRef]
- Wu, T.; Nie, X.-R.; Gan, R.-Y.; Guo, H.; Fu, Y.; Yuan, Q.; Zhang, Q.; Qin, W. In vitro digestion and fecal fermentation behaviors of a pectic polysaccharide from okra (Abelmoschus esculentus) and its impacts on human gut microbiota. Food Hydrocoll. 2021, 114, 106577. [Google Scholar] [CrossRef]
- Guo, C.; Wang, Y.; Zhang, S.; Zhang, X.; Du, Z.; Li, M.; Ding, K. Crataegus pinnatifida polysaccharide alleviates colitis via modulation of gut microbiota and SCFAs metabolism. Inter. J. Biol. Macromol. 2021, 181, 357–368. [Google Scholar] [CrossRef]
- Tiwari, D.P.; Shah, P.; Van den Abbeele, P.; Marzorati, M.; Calatayud, M.; Ghyselinck, J.; Dubey, A.K.; Narayanan, S.; Jain, M. Microbial fermentation of Fossence™, a short-chain fructo-oligosaccharide, under simulated human proximal colonic condition and assessment of its prebiotic effects—A pilot study. FEMS Microbiol. Lett. 2021, 368, fnab147. [Google Scholar] [CrossRef]
- Yousi, F.; Kainan, C.; Junnan, Z.; Chuanxing, X.; Lina, F.; Bangzhou, Z.; Jianlin, R.; Baishan, F. Evaluation of the effects of four media on human intestinal microbiota culture in vitro. AMB Express 2019, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cortés, P.; Juárez, M.; de la Fuente, M.A. Milk fatty acids and potential health benefits: An updated vision. Trends Food Sci. 2018, 81, 1–9. [Google Scholar] [CrossRef]
- Lin, H.V.; Frassetto, A.; Kowalik, E.J., Jr.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE 2012, 7, e35240. [Google Scholar] [CrossRef]
- Salazar, N.; González, S.; de los Reyes Gavilan, C.G.; Rios-Covian, D. Branched short-chain fatty acids as biological indicators of microbiota health and links with anthropometry. In Biomarkers in Nutrition, Biomarkers in Disease: Methods, Discoveries and Applications; Patel, V.B., Preedy, V.R., Eds.; Springer Nature: Cham, Switzerland, 2022; pp. 1–17. [Google Scholar]
- Wu, S.; Bhat, Z.F.; Gounder, R.S.; Mohamed Ahmed, I.A.; Al-Juhaimi, F.Y.; Ding, Y.; Bekhit, A.E.-D. Effect of dietary protein and processing on gut microbiota—A systematic review. Nutrients 2022, 14, 453. [Google Scholar] [CrossRef] [PubMed]
- Gil-Sánchez, I.; Ayuda-Durán, B.; González-Manzano, S.; Santos-Buelga, C.; Cueva, C.; Martín-Cabrejas, M.A.; Sanz-Buenhombre, M.; Guadarrama, A.; Moreno-Arribas, M.V.; Bartolomé, B. Chemical characterization and in vitro colonic fermentation of grape pomace extracts. J. Sci. Food Agric. 2017, 97, 3433–3444. [Google Scholar] [CrossRef]
- Casarotti, S.N.; Borgonovi, T.F.; de Mello Tieghi, T.; Sivieri, K.; Penna, A.L.B. Probiotic low-fat fermented goat milk with passion fruit by-product: In vitro effect on obese individuals’ microbiota and on metabolites production. Int. Food Res. J. 2020, 136, 109453. [Google Scholar] [CrossRef]
- Käferböck, A.; Smetana, S.; de Vos, R.; Schwarz, C.; Toepfl, S.; Parniakov, O. Sustainable extraction of valuable components from Spirulina assisted by pulsed electric fields technology. Algal Res. 2020, 48, 101914. [Google Scholar] [CrossRef]
- Qin, R.; Wang, J.; Chao, C.; Yu, J.; Copeland, L.; Wang, S.; Wang, S. RS5 produced more butyric acid through regulating the microbial community of human gut microbiota. J. Agric. Food Chem. 2021, 69, 3209–3218. [Google Scholar] [CrossRef]
- Xu, M.; Pan, L.; Wang, B.; Zou, X.; Zhang, A.; Zhou, Z.; Han, Y. Simulated digestion and fecal fermentation behaviors of levan and its impacts on the gut microbiota. J. Agric. Food Chem. 2023, 71, 1531–1546. [Google Scholar] [CrossRef] [PubMed]
- Firrman, J.; Liu, L.; Mahalak, K.; Tanes, C.; Bittinger, K.; Tu, V.; Bobokalonov, J.; Mattei, L.; Zhang, H.; Van den Abbeele, P. The impact of environmental pH on the gut microbiota community structure and short chain fatty acid production. FEMS Microbiol. Ecol. 2022, 98, fiac038. [Google Scholar] [CrossRef] [PubMed]
- Segers, A.; de Vos, W.M. Mode of action of Akkermansia muciniphila in the intestinal dialogue: Role of extracellular proteins, metabolites and cell envelope components. Microbiome Res. Rep. 2023, 2, 6. [Google Scholar] [CrossRef]
- Li, H.-Y.; Zhou, D.-D.; Gan, R.-Y.; Huang, S.-Y.; Zhao, C.-N.; Shang, A.; Xu, X.-Y.; Li, H.-B. Effects and mechanisms of probiotics, prebiotics, synbiotics, and postbiotics on metabolic diseases targeting gut microbiota: A narrative review. Nutrients 2021, 13, 3211. [Google Scholar] [CrossRef]
- Geerlings, S.Y.; Kostopoulos, I.; De Vos, W.M.; Belzer, C. Akkermansia muciniphila in the human gastrointestinal tract: When, where, and how? Microorganisms 2018, 6, 75. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, J.; Wu, C. Modulation of gut microbiota and immune system by probiotics, pre-biotics, and post-biotics. Front. Nutr. 2022, 8, 634897. [Google Scholar] [CrossRef] [PubMed]
- Paterson, S.; Majchrzak, M.; Alexandru, D.; Di Bella, S.; Fernández-Tomé, S.; Arranz, E.; de la Fuente, M.A.; Gómez-Cortés, P.; Hernández-Ledesma, B. Impact of the biomass pretreatment and simulated gastrointestinal digestion on the digestibility and antioxidant activity of microalgae Chlorella vulgaris and Tetraselmis chuii. Food Chem. 2024, 453, 139686. [Google Scholar] [CrossRef]
- Wang, C.; Lan, C.Q. Effects of Shear Stress on Microalgae—A Review. Biotechnol. Adv. 2018, 36, 986–1002. [Google Scholar] [CrossRef]
- Velankanni, P.; Go, S.H.; Jin, J.B.; Park, J.S.; Park, S.; Lee, S.B.; Kwon, H.K.; Pan, C.H.; Cha, K.H.; Lee, C.G. Chlorella Vulgaris Modulates Gut Microbiota and Induces Regulatory T Cells to Alleviate Colitis in Mice. Nutrients 2023, 15, 3293. [Google Scholar] [CrossRef]
- Nishimoto, Y.; Nomaguchi, T.; Mori, Y.; Ito, M.; Nakamura, Y.; Fujishima, M.; Murakami, S.; Yamada, T.; Fukuda, S. The Nutritional Efficacy of Chlorella Supplementation Depends on the Individual Gut Environment: A Randomised Control Study. Front. Nutr. 2021, 8, 648073. [Google Scholar] [CrossRef]
- Hosseini, A.M.; Keshavarz, S.A.; Nasli-Esfahani, E.; Amiri, F.; Janani, L. The Effects of Chlorella Supplementation on Glycemic Control, Lipid Profile and Anthropometric Measures on Patients with Type 2 Diabetes Mellitus. Eur. J. Nutr. 2021, 60, 3131–3141. [Google Scholar] [CrossRef] [PubMed]
- Fields, F.J.; Lejzerowicz, F.; Schroeder, D.; Ngoi, S.M.; Tran, M.; McDonald, D.; Jiang, L.; Chang, J.T.; Knight, R.; Mayfield, S. Effects of the Microalgae Chlamydomonas on Gastrointestinal Health. J. Funct. Foods 2020, 65, 103738. [Google Scholar] [CrossRef]
- Nadia, J.; Roy, D.; Montoya, C.A.; Singh, H.; Acevedo-Fani, A.; Bornhorst, G.M. A Proposed Framework to Establish in Vitro-in Vivo Relationships Using Gastric Digestion Models for Food Research. Food Funct. 2024, 15, 10233–10261. [Google Scholar] [CrossRef] [PubMed]
- Medvedeva, S.; Achasova, K.; Boldyreva, L.; Ogienko, A.; Kozhevnikova, E. The Application of Explants, Crypts, and Organoids as Models in Intestinal Barrier Research. Tissue Barriers 2024, 5, 2423137. [Google Scholar] [CrossRef]
- Salman, C.K.M.; Beura, M.; Singh, A.; Dahuja, A.; Kamble, V.B.; Shukla, R.P.; Thandapilly, S.J.; Krishnan, V. Biomimic Models for in Vitro Glycemic Index: Scope of Sensor Integration and Artificial Intelligence. Food Chem. X 2024, 25, 102132. [Google Scholar] [CrossRef]
- Tamargo, A.; de Llano, D.G.; Cueva, C.; Del Hierro, J.N.; Martin, D.; Molinero, N.; Bartolomé, B.; Moreno-Arribas, M.V. Deciphering the interactions between lipids and red wine polyphenols through the gastrointestinal tract. Food Res. Int. 2023, 165, 112524. [Google Scholar] [CrossRef]
- Cebrian-Lloret, V.; Martínez-Abad, A.; Recio, I.; Lopez-Rubio, A.; Martínez-Sanz, M. In vitro digestibility of proteins from red seaweeds: Impact of cell wall structure and processing methods. Food Res. Int. 2024, 178, 113990. [Google Scholar] [CrossRef]
- Hiel, S.; Bindels, L.B.; Pachikian, B.D.; Kalala, G.; Broers, V.; Zamariola, G.; Chang, B.P.; Kambashi, B.; Rodriguez, J.; Cani, P.D. Effects of a diet based on inulin-rich vegetables on gut health and nutritional behavior in healthy humans. Am. J. Clin. Nutr. 2019, 109, 1683–1695. [Google Scholar] [CrossRef]
- Jin, J.B.; Cha, J.W.; Shin, I.S.; Jeon, J.Y.; Cha, K.H.; Pan, C.H. Supplementation with Chlorella vulgaris, Chlorella protothecoides, and Schizochytrium sp. increases propionate-producing bacteria in in vitro human gut fermentation. J. Sci. Food Agric. 2020, 100, 2938–2945. [Google Scholar] [CrossRef]
- Tamargo, A.; Cueva, C.; Alvarez, M.D.; Herranz, B.; Bartolome, B.; Moreno-Arribas, M.V.; Laguna, L. Influence of viscosity on the growth of human gut microbiota. Food Hydrocoll. 2018, 77, 163–167. [Google Scholar] [CrossRef]
- Gil-Sánchez, I.; Cueva, C.; Sanz-Buenhombre, M.; Guadarrama, A.; Moreno-Arribas, M.V.; Bartolomé, B. Dynamic gastrointestinal digestion of grape pomace extracts: Bioaccessible phenolic metabolites and impact on human gut microbiota. J. Food Compos. Anal. 2021, 68, 41–52. [Google Scholar] [CrossRef]
- Jiménez-Arroyo, C.; Tamargo, A.; Molinero, N.; Moreno-Arribas, M.V. The gut microbiota, a key to understanding the health implications of micro(nano)plastics and their biodegradation. Microb. Biotechnol. 2023, 16, 34–53. [Google Scholar] [CrossRef] [PubMed]
- García-Villalba, R.; Giménez-Bastida, J.A.; García-Conesa, M.T.; Tomás-Barberán, F.A.; Espín, J.C.; Larrosa, M. Alternative method for gas chromatography-mass spectrometry analysis of short-chain fatty acids in faecal samples. J. Sep. Sci. 2012, 35, 1906–1913. [Google Scholar] [CrossRef]
- Paterson, S.; Fernández-Tomé, S.; Galvez, A.; Hernández-Ledesma, B. Evaluation of the multifunctionality of soybean proteins and peptides in immune cell models. Nutrients 2023, 15, 1220. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, 1–18. [Google Scholar] [CrossRef]
- Lin, H.; Peddada, S.D. Analysis of microbial compositions: A review of normalization and differential abundance analysis. NPJ Biofilms Microbiomes 2020, 6, 60. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bañares, C.; Paterson, S.; Gómez-Garre, D.; Ortega-Hernández, A.; Sánchez-González, S.; Cueva, C.; de la Fuente, M.Á.; Hernández-Ledesma, B.; Gómez-Cortés, P. Modulation of Gut Microbiota and Short-Chain Fatty Acid Production by Simulated Gastrointestinal Digests from Microalga Chlorella vulgaris. Int. J. Mol. Sci. 2025, 26, 2754. https://doi.org/10.3390/ijms26062754
Bañares C, Paterson S, Gómez-Garre D, Ortega-Hernández A, Sánchez-González S, Cueva C, de la Fuente MÁ, Hernández-Ledesma B, Gómez-Cortés P. Modulation of Gut Microbiota and Short-Chain Fatty Acid Production by Simulated Gastrointestinal Digests from Microalga Chlorella vulgaris. International Journal of Molecular Sciences. 2025; 26(6):2754. https://doi.org/10.3390/ijms26062754
Chicago/Turabian StyleBañares, Celia, Samuel Paterson, Dulcenombre Gómez-Garre, Adriana Ortega-Hernández, Silvia Sánchez-González, Carolina Cueva, Miguel Á. de la Fuente, Blanca Hernández-Ledesma, and Pilar Gómez-Cortés. 2025. "Modulation of Gut Microbiota and Short-Chain Fatty Acid Production by Simulated Gastrointestinal Digests from Microalga Chlorella vulgaris" International Journal of Molecular Sciences 26, no. 6: 2754. https://doi.org/10.3390/ijms26062754
APA StyleBañares, C., Paterson, S., Gómez-Garre, D., Ortega-Hernández, A., Sánchez-González, S., Cueva, C., de la Fuente, M. Á., Hernández-Ledesma, B., & Gómez-Cortés, P. (2025). Modulation of Gut Microbiota and Short-Chain Fatty Acid Production by Simulated Gastrointestinal Digests from Microalga Chlorella vulgaris. International Journal of Molecular Sciences, 26(6), 2754. https://doi.org/10.3390/ijms26062754







