Optimization and Standardization of Plant-Derived Vascular Scaffolds
Abstract
:1. Introduction
2. Results
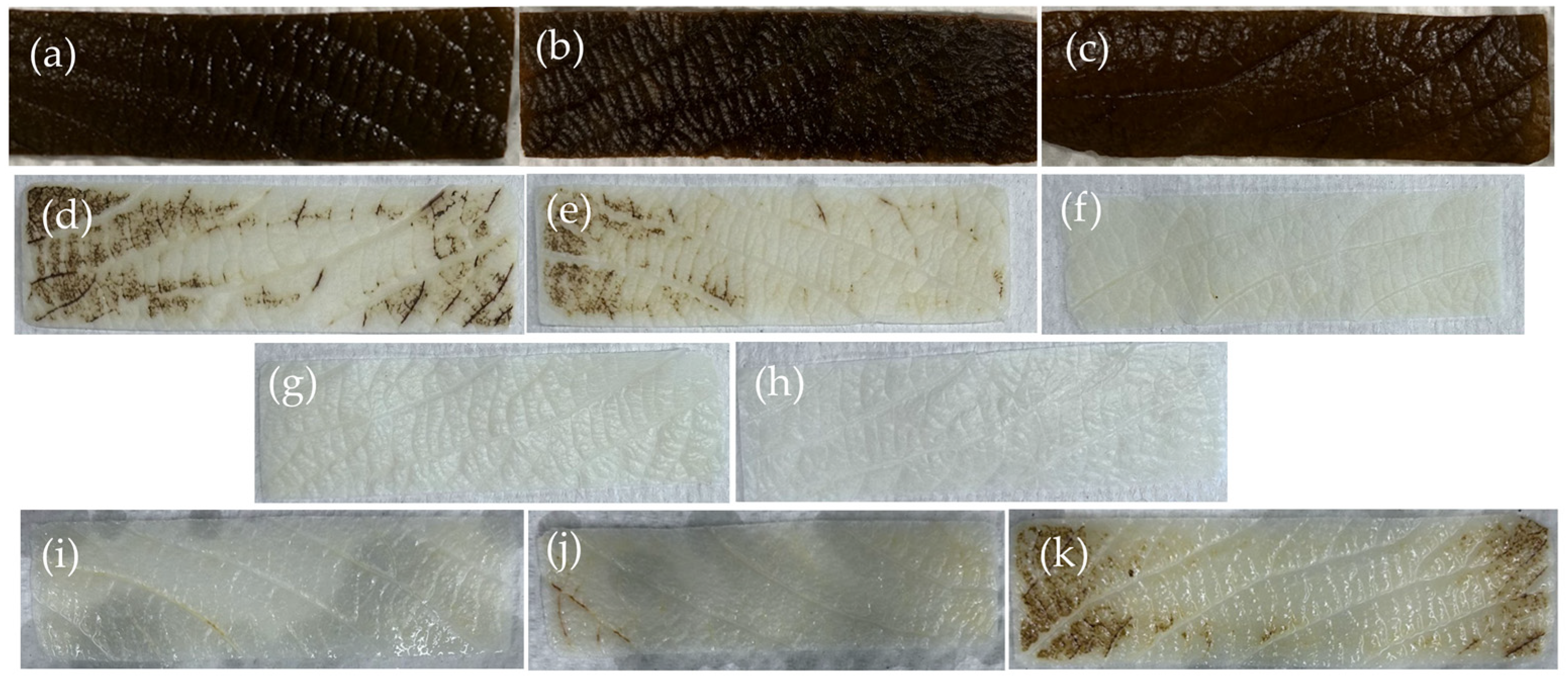
2.1. Decellularization Efficiency
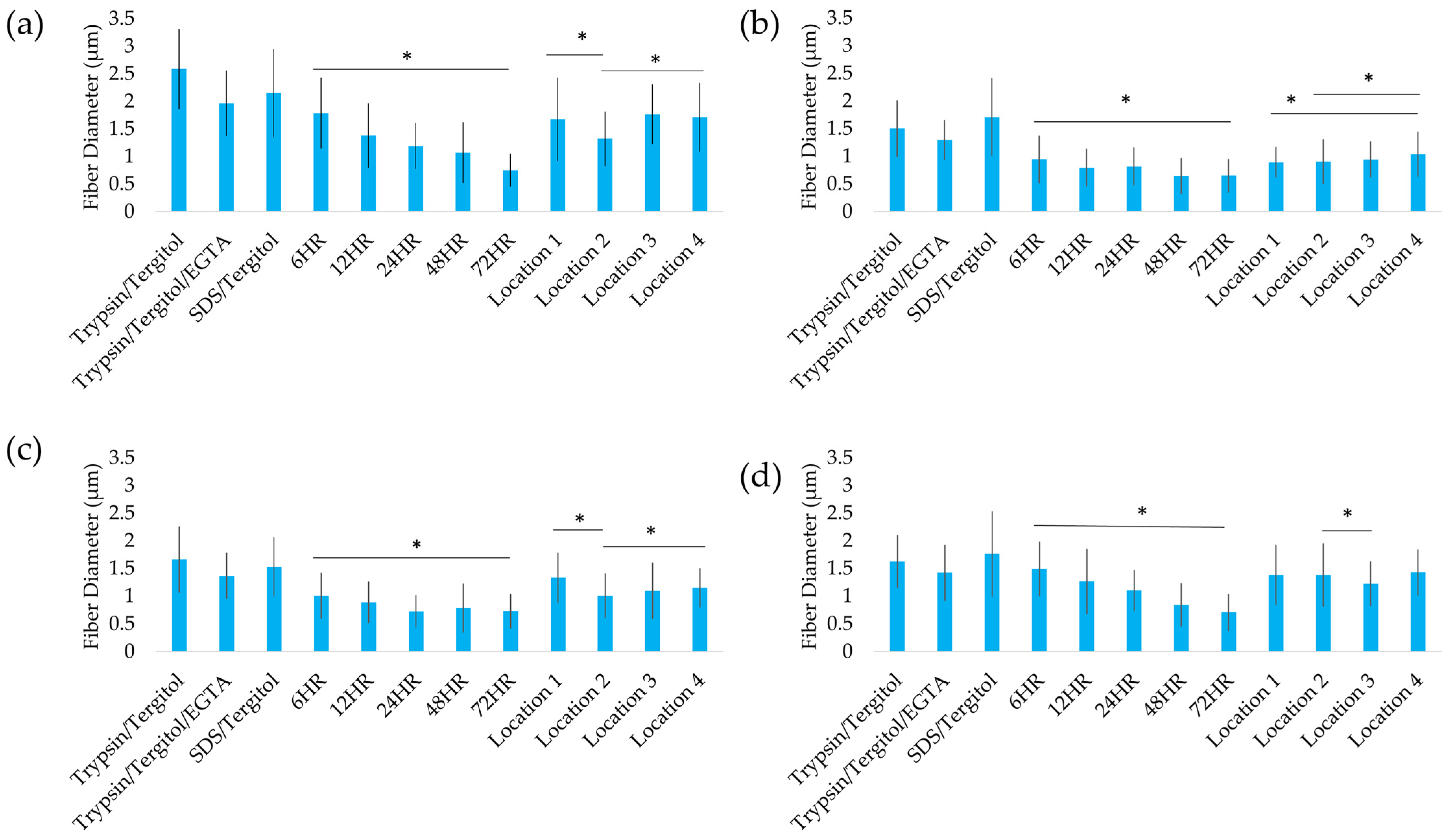
2.2. Mechanical Properties
2.3. Permeability Testing
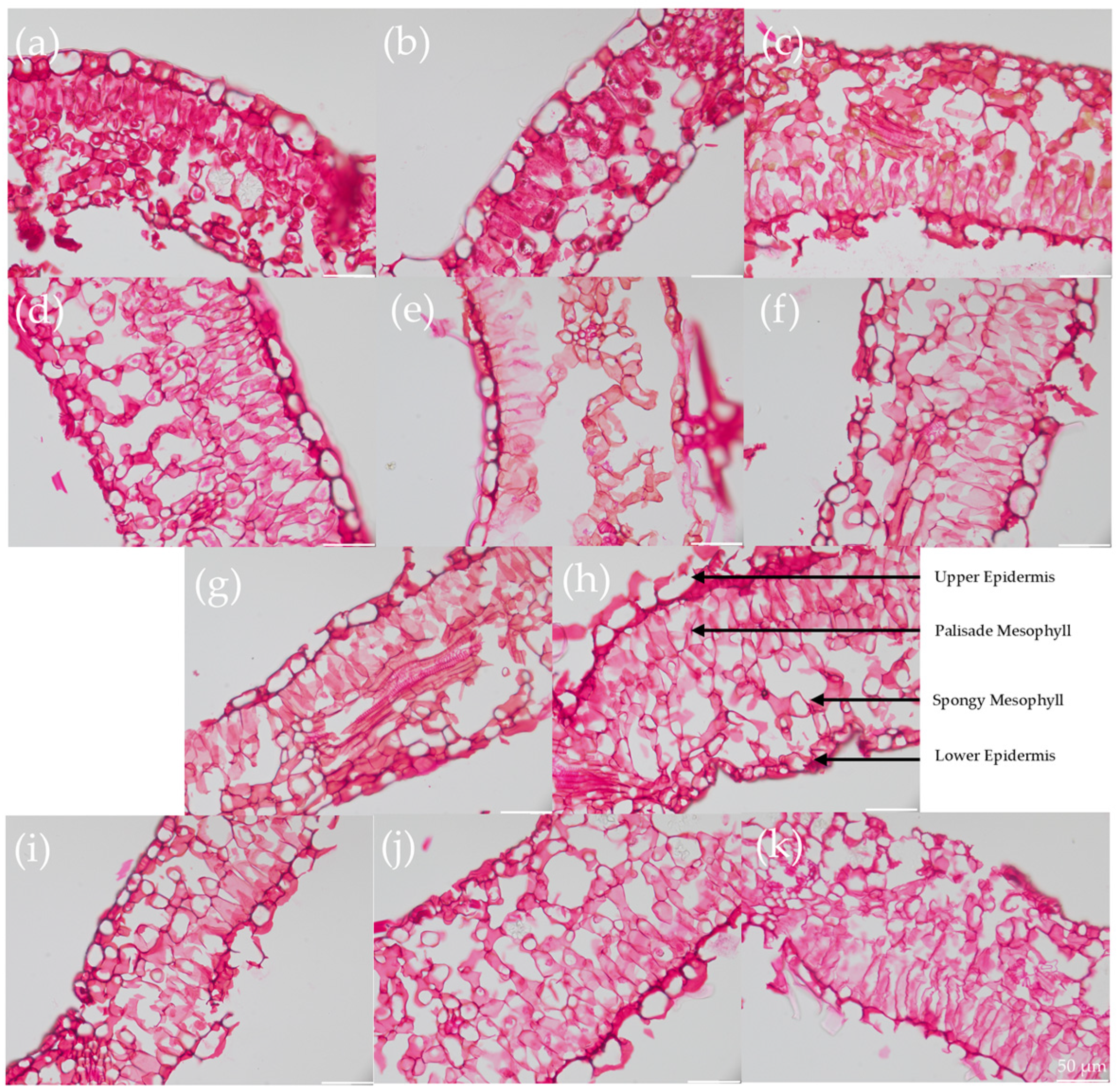
2.4. Structural Analysis
2.5. Recellularization of Decellularized Leatherleaf with ECs
3. Discussion
4. Materials and Methods
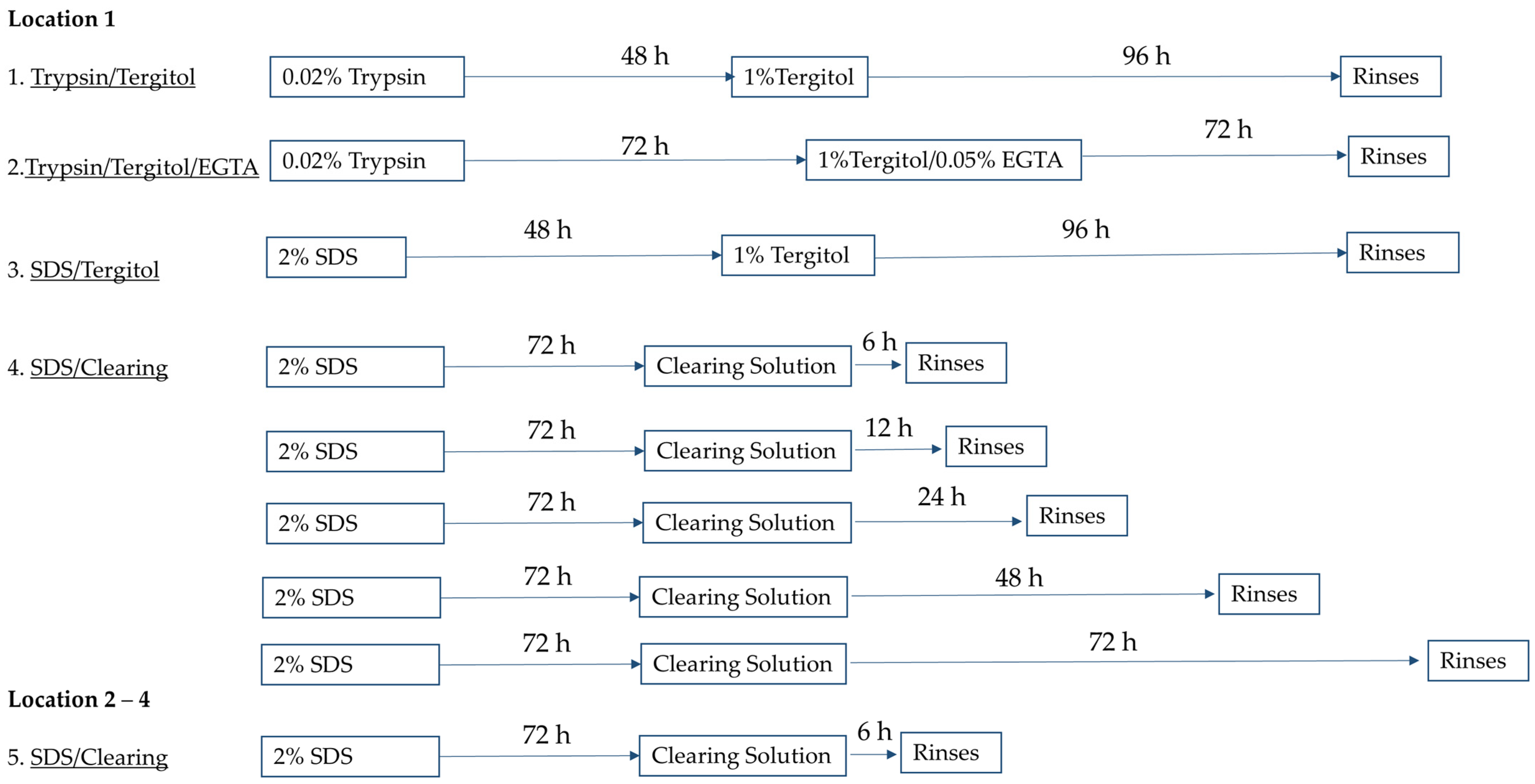
4.1. Plant Leaf Decellularization
- Trypsin/Tergitol: Samples were treated for 48 h in 0.02% Trypsin (Sigma-Aldrich, St. Louis, MO, USA) and 96 h in 1% Tergitol (Sigma-Aldrich) in deionized water, with daily solution replacement.
- Trypsin/Tergitol/EGTA: Samples were treated for 72 h in 0.02% Trypsin, followed by 72 h 1% Tergitol and 0.05% EGTA (Sigma-Aldrich) in deionized water, with solutions refreshed every 24 h.
- SDS/Tergitol: Samples were treated for 48 h in 2% SDS and 96 h in 1% Tergitol.
- SDS/Clearing Solution: Samples were treated in 2% SDS in deionized water for 72 h, followed by treatment in a clearing solution (10% bleach and 0.1% Triton X-100 in water) for 6, 12, 24, 48, or 72 h.
4.2. Tensile Testing
4.3. Permeability Testing Protocol
4.4. DNA Quantification
4.5. Histological Staining
4.6. Cell Culture
4.7. 2D Seeding
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ePTFE | Expanded polytetrafluoroethylene |
| ECM | Extracellular matrix |
| SDS | Sodium dodecyl sulfate |
| EC | Endothelial cell |
| PBS | Phosphate-buffered saline |
References
- Weintraub, W.S. The economic burden of illness. JAMA Netw. Open 2023, 6, e232663. [Google Scholar] [CrossRef]
- Gaudino, M.; Hameed, I.; Farkouh, M.E.; Rahouma, M.; Naik, A.; Robinson, N.B.; Ruan, Y.; Demetres, M.; Biondi-Zoccai, G.; Angiolillo, D.J.; et al. Overall and cause-specific mortality in randomized clinical trials comparing percutaneous interventions with coronary bypass surgery: A meta-analysis. JAMA Intern. Med. 2020, 180, 1638–1646. [Google Scholar] [CrossRef]
- Latif, F.; Uyeda, L.; Edson, R.; Bhatt, D.L.; Goldman, S.; Holmes, D.R.; Rao, S.V.; Shunk, K.; Aggarwal, K.; Uretsky, B.; et al. Stent-only versus adjunctive balloon angioplasty approach for saphenous vein graft percutaneous coronary intervention: Insights from DIVA trial. Circ. Cardiovasc. Interv. 2020, 13, e008494. [Google Scholar] [CrossRef] [PubMed]
- Fayon, A.; Menu, P.; El Omar, R. Cellularized small-caliber tissue-engineered vascular grafts: Looking for the ultimate gold standard. NPJ Regen. Med. 2021, 6, 46. [Google Scholar] [CrossRef] [PubMed]
- Obiweluozor, F.O.; Emechebe, G.A.; Kim, D.-W.; Cho, H.-J.; Park, C.H.; Kim, C.S.; Jeong, I.S. Considerations in the development of small-diameter vascular graft as an alternative for bypass and reconstructive surgeries: A review. Cardiovasc. Eng. Technol. 2020, 11, 495–521. [Google Scholar] [CrossRef] [PubMed]
- Pashneh-Tala, S.; MacNeil, S.; Claeyssens, F. The Tissue-Engineered Vascular Graft-Past, Present, and Future. Tissue Eng. Part B Rev. 2016, 22, 68–100. [Google Scholar] [CrossRef]
- Mallis, P.; Kostakis, A.; Stavropoulos-Giokas, C.; Michalopoulos, E. Future perspectives in small-diameter vascular graft engineering. Bioengineering 2020, 7, 160. [Google Scholar] [CrossRef]
- Li, M.-X.; Wei, Q.-Q.; Mo, H.-L.; Ren, Y.; Zhang, W.; Lu, H.-J.; Joung, Y.K. Challenges and advances in materials and fabrication technologies of small-diameter vascular grafts. Biomater. Res. 2023, 27, 58. [Google Scholar]
- Das, K.K.; Tiwari, R.M.; Shankar, O.; Maiti, P.; Dubey, A.K. Tissue-engineered vascular grafts for cardiovascular disease management: Current strategies, challenges, and future perspectives. MedComm Biomater. Appl. 2024, 3, e88. [Google Scholar] [CrossRef]
- Gershlak, J.R.; Hernandez, S.; Fontana, G.; Perreault, L.R.; Hansen, K.J.; Larson, S.A.; Binder, B.Y.; Dolivo, D.M.; Yang, T.; Dominko, T.; et al. Crossing kingdoms: Using decellularized plants as perfusable tissue engineering scaffolds. Biomaterials 2017, 125, 13–22. [Google Scholar] [CrossRef]
- Gorbenko, N.; Vaccaro, J.C.; Fagan, R.; Cerro, R.A.; Khorrami, J.M.; Galindo, L.; Merna, N. Perfusion Bioreactor Conditioning of Small-diameter Plant-based Vascular Grafts. Tissue Eng. Regen. Med. 2024, 21, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, Q.; Wang, S.; Zhang, J.; Fan, S.; Lin, X. Current advances in the development of decellularized plant extracellular matrix. Front. Bioeng. Biotechnol. 2021, 9, 712262. [Google Scholar] [CrossRef]
- Hasan, M.M.; Swapon, A.R.; Dipti, T.I.; Choi, Y.-J.; Yi, H.-G. Plant-Based Decellularization: A Novel Approach for Perfusion-Compatible Tissue Engineering Structures. J. Microbiol. Biotechnol. 2024, 34, 1003. [Google Scholar] [CrossRef] [PubMed]
- Modulevsky, D.J.; Cuerrier, C.M.; Pelling, A.E. Biocompatibility of subcutaneously implanted plant-derived cellulose biomaterials. PLoS ONE 2016, 11, e0157894. [Google Scholar] [CrossRef] [PubMed]
- Latour, M.L.; Tarar, M.; Hickey, R.J.; Cuerrier, C.M.; Catelas, I.; Pelling, A.E. Plant-derived cellulose scaffolds for bone tissue engineering. BioRxiv 2020. [Google Scholar] [CrossRef]
- Cheng, Y.-W.; Shiwarski, D.J.; Ball, R.L.; Whitehead, K.A.; Feinberg, A.W.W. Engineering aligned skeletal muscle tissue using decellularized plant-derived scaffolds. ACS Biomater. Sci. Eng. 2020, 6, 3046–3054. [Google Scholar] [CrossRef]
- Cevik, M.; Dikici, S. Development of tissue-engineered vascular grafts from decellularized parsley stems. Soft Matter 2023, 20, 338–350. [Google Scholar] [CrossRef]
- Shang, L.; Wang, S.; Mao, Y. Recent advances in plant-derived polysaccharide scaffolds in tissue engineering: A review. International. J. Biol. Macromol. 2024, 277, 133830. [Google Scholar] [CrossRef]
- Asadian, E.; Abbaszadeh, S.; Ghorbani-Bidkorpeh, F.; Rezaei, S.; Xiao, B.; Santos, H.A.; Shahbazi, M.-A. Hijacking plant skeletons for biomedical applications: From regenerative medicine and drug delivery to biosensing. Biomater. Sci. 2025, 13, 9–92. [Google Scholar] [CrossRef]
- Merna, N.; Robertson, C.; La, A.; George, S.C. Optical imaging predicts mechanical properties during decellularization of cardiac tissue. Tissue Eng. Part C Methods 2013, 19, 802–809. [Google Scholar] [CrossRef]
- Wong, V.; Gada, S.; Singh, M.; Merna, N.J. Development of Small-Caliber Vascular Grafts using Human Umbilical Artery: An Evaluation of Methods. Tissue Eng. Part C Methods 2022, 29, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sehic, E.; de Miguel-Gómez, L. Standardizing decellularization protocols for optimized uterine tissue bioengineering. Regen. Ther. 2025, 28, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Naegeli, K.M.; Kural, M.H.; Li, Y.; Wang, J.; Hugentobler, E.A.; Niklason, L.E. Bioengineering human tissues and the future of vascular replacement. Circ. Res. 2022, 131, 109–126. [Google Scholar] [CrossRef]
- Gorbenko, N.; Rinaldi, G.; Sanchez, A.; Merna, N.J. Small-caliber vascular grafts engineered from decellularized leaves and cross-linked gelatin. Tissue Eng. Part A 2023, 29, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Yavari, N.; Tripathi, R.; Wu, B.-S.; MacPherson, S.; Singh, J.; Lefsrud, M. The effect of light quality on plant physiology, photosynthetic, and stress response in Arabidopsis thaliana leaves. PLoS ONE 2021, 16, e0247380. [Google Scholar] [CrossRef]
- Hanson, H.C. Leaf-structure as related to environment. Am. J. Bot. 1917, 4, 533–560. [Google Scholar]
- de Valence, S.; Tille, J.C.; Mugnai, D.; Mrowczynski, W.; Gurny, R.; Möller, M.; Walpoth, B.H. Long term performance of polycaprolactone vascular grafts in a rat abdominal aorta replacement model. Biomaterials 2012, 33, 38–47. [Google Scholar] [CrossRef]
- Lee, M.K.; Song, J.Y.; Kim, T.Y.; Kim, J.H.; Choi, J.B.; Kuh, J.H. Simple anastomotic techniques for coronary artery bypass surgery in patients with small coronary arteries or a marked size discrepancy between the coronary artery and graft. Korean J. Thorac. Cardiovasc. Surg. 2016, 49, 485. [Google Scholar] [CrossRef]
- Meng, X.; Wang, X.; Jiang, Y.; Zhang, B.; Li, K.; Li, Q. Suture retention strength of P (LLA-CL) tissue-engineered vascular grafts. RSC Adv. 2019, 9, 21258–21264. [Google Scholar] [CrossRef]
- Toker, M.; Rostami, S.; Kesici, M.; Gul, O.; Kocaturk, O.; Odabas, S.; Garipcan, B. Decellularization and characterization of leek: A potential cellulose-based biomaterial. Cellulose 2020, 27, 7331–7348. [Google Scholar] [CrossRef]
- Mohan, C.C.; Unnikrishnan, P.S.; Krishnan, A.G.; Nair, M.B. Decellularization and oxidation process of bamboo stem enhance biodegradation and osteogenic differentiation. Mater. Sci. Eng. C 2021, 119, 111500. [Google Scholar]
- Wang, J.; Qin, X.; Kong, B.; Ren, H. Celery-derived scaffolds with liver lobule-mimicking structures for tissue engineering transplantation. Smart Med. 2022, 1, e20220002. [Google Scholar] [CrossRef] [PubMed]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef]
- Khanafer, K.; Duprey, A.; Zainal, M.; Schlicht, M.; Williams, D.; Berguer, R. Determination of the elastic modulus of ascending thoracic aortic aneurysm at different ranges of pressure using uniaxial tensile testing. J. Thorac. Cardiovasc. Surg. 2011, 142, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Adamski, M.; Fontana, G.; Gershlak, J.R.; Gaudette, G.R.; Le, H.D.; Murphy, W.L. Two Methods for Decellularization of Plant Tissues for Tissue Engineering Applications. JoVE 2018, 135, e57586. [Google Scholar]
- Wang, Y.; Dominko, T.; Weathers, P.J. Using decellularized grafted leaves as tissue engineering scaffolds for mammalian cells. In Vitro Cell. Dev. Biol. Plant 2020, 56, 765–774. [Google Scholar] [CrossRef]
- Radzali, N.A.M.; Hidzir, N.M.; Rahman, I.A.; Mokhtar, A.K. Mechanical properties of polymeric biomaterials: Modified ePTFE using gamma irradiation. Open Chem. 2021, 19, 1207–1215. [Google Scholar] [CrossRef]
- Coirbay, A.; Kerckhofs, G.; Lacroix, V. Biomechanics: Mechanical Characterization of Artery Tissue and Synthetic Arterial Grafts; The Université catholique de Louvain (UCL): Ottignies-Louvain-la-Neuve, Belgium, 2020. [Google Scholar]
- Hiob, M.A.; She, S.; Muiznieks, L.D.; Weiss, A.S. Biomaterials and modifications in the development of small-diameter vascular grafts. ACS Biomater. Sci. Eng. 2017, 3, 712–723. [Google Scholar] [CrossRef]
- Azevedo, E.P.; Retarekar, R.; Raghavan, M.L.; Kumar, V. Mechanical properties of cellulose: Chitosan blends for potential use as a coronary artery bypass graft. J. Biomater. Sci. Polym. Ed. 2013, 24, 239–252. [Google Scholar] [CrossRef]
- Haga, M.; Yamamoto, S.; Okamoto, H.; Hoshina, K.; Asakura, T.; Watanabe, T. Histological reactions and the in vivo patency rates of small silk vascular grafts in a canine model. Ann. Vasc. Dis. 2017, 10, 132–138. [Google Scholar] [CrossRef]
- Edgar, L.; McNamara, K.; Wong, T.; Tamburrini, R.; Katari, R.; Orlando, G. Heterogeneity of Scaffold Biomaterials in Tissue Engineering. Materials 2016, 9, 332. [Google Scholar] [CrossRef] [PubMed]
- Tenreiro, M.F.; Almeida, H.V.; Calmeiro, T.; Fortunato, E.; Ferreira, L.; Alves, P.M.; Serra, M. Interindividual heterogeneity affects the outcome of human cardiac tissue decellularization. Sci. Rep. 2021, 11, 20834. [Google Scholar] [CrossRef]
- Di Nubila, A.; Dilella, G.; Simone, R.; Barbieri, S.S. Vascular Extracellular Matrix in Atherosclerosis. Int. J. Mol. Sci. 2024, 25, 12017. [Google Scholar] [CrossRef] [PubMed]
- Valaris, S.; Kostourou, V. Cell–Extracellular Matrix Adhesions in Vascular Endothelium. In Matrix Pathobiology and Angiogenesis; Papadimitriou, E., Mikelis, C.M., Eds.; Springer International Publishing: Cham, Switzerland, 2023; pp. 175–204. [Google Scholar]
- Schutte, R.J.; Parisi-Amon, A.; Reichert, W.M. Cytokine profiling using monocytes/macrophages cultured on common biomaterials with a range of surface chemistries. J. Biomed. Mater. Res. Part A Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2009, 88, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.D.; Sawh-Martinez, R.; Brennan, M.P.; Jay, S.M.; Devine, L.; Rao, D.A.; Yi, T.; Mirensky, T.L.; Nalbandian, A.; Udelsman, B.; et al. Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc. Natl. Acad. Sci. USA 2010, 107, 4669–4674. [Google Scholar] [CrossRef]
- Bilirgen, A.C.; Toker, M.; Odabas, S.; Yetisen, A.K.; Garipcan, B.; Tasoglu, S. Plant-based scaffolds in tissue engineering. ACS Biomater. Sci. Eng. 2021, 7, 926–938. [Google Scholar] [CrossRef]
- Fernández-Pérez, J.; van Kampen, K.A.; Mota, C.; Baker, M.; Moroni, L. Flexible, Suturable, and Leak-free Scaffolds for Vascular Tissue Engineering Using Melt Spinning. ACS Biomater. Sci. Eng. 2023, 9, 5006–5014. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imeidopf, G.; Khaimov, D.; John, S.; Merna, N. Optimization and Standardization of Plant-Derived Vascular Scaffolds. Int. J. Mol. Sci. 2025, 26, 2752. https://doi.org/10.3390/ijms26062752
Imeidopf G, Khaimov D, John S, Merna N. Optimization and Standardization of Plant-Derived Vascular Scaffolds. International Journal of Molecular Sciences. 2025; 26(6):2752. https://doi.org/10.3390/ijms26062752
Chicago/Turabian StyleImeidopf, Gianna, Dara Khaimov, Sashane John, and Nick Merna. 2025. "Optimization and Standardization of Plant-Derived Vascular Scaffolds" International Journal of Molecular Sciences 26, no. 6: 2752. https://doi.org/10.3390/ijms26062752
APA StyleImeidopf, G., Khaimov, D., John, S., & Merna, N. (2025). Optimization and Standardization of Plant-Derived Vascular Scaffolds. International Journal of Molecular Sciences, 26(6), 2752. https://doi.org/10.3390/ijms26062752







