Special Issue “New Challenges and Perspectives in Polycystic Ovary Syndrome”
Author Contributions
Conflicts of Interest
List of Contributions
- Varhegyi, V.; Modos, A.; Trager, D.; Gerszi, D.; Horvath, E.M.; Sipos, M.; Acs, N.; Molnar, M.J.; Varbiro, S.; Gal, A. GDF-15 and mtDNA Deletions Are Useful Biomarkers of Mitochondrial Dysfunction in Insulin Resistance and PCOS. Int. J. Mol. Sci. 2024, 25, 916. https://doi.org/10.3390/ijms252010916.
- Kukaev, E.; Kirillova, E.; Tokareva, A.; Rimskaya, E.; Starodubtseva, N.; Chernukha, G.; Priputnevich, T.; Frankevich, V.; Sukhikh, G. Impact of Gut Microbiota and SCFAs in the Pathogenesis of PCOS and the Effect of Metformin Therapy. Int. J. Mol. Sci. 2024, 25, 10636. https://doi.org/10.3390/ijms251910636.
- Alarcón-Granados, M.C.; Camargo-Villalba, G.E.; Forero-Castro, M. Exploring Genetic Interactions in Colombian Women with Polycystic Ovarian Syndrome: A Study on SNP-SNP Associations. Int. J. Mol. Sci. 2024, 25, 9212. https://doi.org/10.3390/ijms25179212
- Marie, C.; Pierre, A.; Mayeur, A.; Giton, F.; Corre, R.; Grynberg, M.; Cohen-Tannoudji, J.; Guigon, C.J.; Chauvin, S. Dysfunction of Human Estrogen Signaling as a Novel Molecular Signature of Polycystic Ovary Syndrome. Int. J. Mol. Sci. 2023, 24, 16689. https://doi.org/10.3390/ijms242316689.
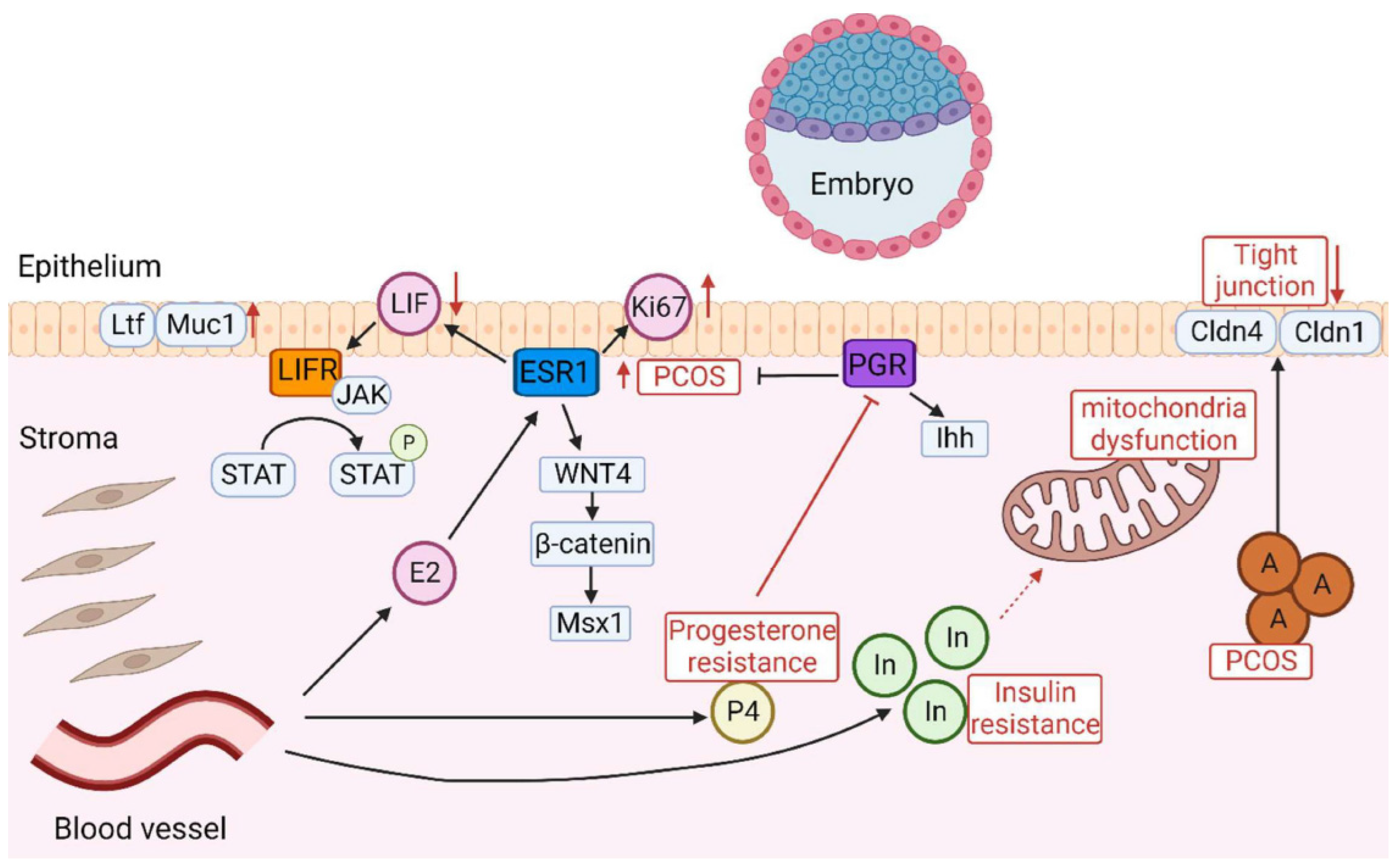
- Matsuyama, S.; Whiteside, S.; Li, S.-Y. Implantation and Decidualization in PCOS: Unraveling the Complexities of Pregnancy. Int. J. Mol. Sci. 2024, 25, 1203. https://doi.org/10.3390/ijms25021203.
References
- Teede, H.J.; Tay, C.T.; Laven, J.; Dokras, A.; Moran, L.; Piltonen, T.; Costello, M.; Boivin, J.; Redman, L.; Boyle, J.; et al. International evidence-based guideline for the assessment and management of polycystic ovary syndrome 2023. Natl. Health Med. Res. Counc. 2023, 1–258. [Google Scholar]
- Parker, J.; O’Brien, C.; Hawrelak, J.; Gersh, F.L. Polycystic Ovary Syndrome: An Evolutionary Adaptation to Lifestyle and the Environment. Int. J. Environ. Res. Public Health 2022, 19, 1336. [Google Scholar] [CrossRef] [PubMed]
- Dumesic, D.A.; Abbott, D.H.; Chazenbalk, G.D.; Scholar, G. An Evolutionary Model for the Ancient Origins of Polycystic Ovary Syndrome. J. Clin. Med. 2023, 12, 6120. [Google Scholar] [CrossRef] [PubMed]
- Stener-Victorin, E.; Deng, Q. Epigenetic inheritance of PCOS by developmental programming and germline transmission. Trends Endocrinol. Metab. 2025, 1–10. [Google Scholar] [CrossRef]
- Shaw, L.M.A.; Elton, S. Polycystic ovary syndrome: A transgenerational evolutionary adaptation. BJOG Int. J. Obstet. Gynaecol. 2008, 115, 144–148. [Google Scholar] [CrossRef]
- Charifson, M.A.; Trumble, B.C. Evolutionary origins of polycystic ovary syndrome: An environmental mismatch disorder. Evol. Med. Public Health 2019, 2019, 50–63. [Google Scholar] [CrossRef]
- Dumesic, D.A.; Padmanabhan, V.; Chazenbalk, G.D.; Abbott, D.H. Polycystic ovary syndrome as a plausible evolutionary outcome of metabolic adaptation. Reprod. Biol. Endocrinol. 2022, 20, 12. [Google Scholar] [CrossRef]
- Benton, M.L. The influence of evolutionary history on human health and disease. Nat. Rev. Genet. 2021, 22, 269–283. [Google Scholar] [CrossRef]
- Fay, J.C. Disease consequences of human adaptation. Appl. Transl. Genom. 2013, 2, 42–47. [Google Scholar] [CrossRef]
- Tsatsoulis, A.; Mantzaris, M.D.; Bellou, S.; Andrikoula, M. Insulin resistance: An adaptive mechanism becomes maladaptive in the current environment—An evolutionary perspective. Metabolism 2013, 62, 622–633. [Google Scholar] [CrossRef]
- Joham, A.E.; Tay, C.T.; Laven, J.; Louwers, Y.V.; Azziz, R. Approach to the Patient: Diagnostic Challenges in the Workup for Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 2025, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hoeger, K.M.; Dokras, A.; Piltonen, T. Update on PCOS: Consequences, Challenges, and Guiding Treatment. J. Clin. Endocrinol. Metab. 2021, 106, E1071–E1083. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.; Hofstee, P.; Brennecke, S. Prevention of Pregnancy Complications Using a Multimodal Lifestyle, Screening, and Medical Model. J. Clin. Med. 2024, 13, 4344. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Gibson, M.; Laven, J.; Dokras, A.; Moran, L.J.; Piltonin, T.; Costello, M.; Mousa, A.; Joham, A.E.; Tay, C.T.; et al. International PCOS guideline clinical research priorities roadmap: A co-designed approach aligned with end-user priorities in a neglected women’s health condition. Eclinicalmedicine 2024, 78, 102927. [Google Scholar] [CrossRef]
- Parker, J. Pathophysiological Effects of Contemporary Lifestyle on Evolutionary-Conserved Survival Mechanisms in Polycystic Ovary Syndrome. Life 2023, 13, 1056. [Google Scholar] [CrossRef]
- Siemers, K.M.; Klein, A.K.; Baack, M.L. Mitochondrial Dysfunction in PCOS: Insights into Reproductive Organ Pathophysiology. Int. J. Mol. Sci. 2023, 24, 13123. [Google Scholar] [CrossRef]
- Boenzi, S.; Diodato, D. Biomarkers for mitochondrial energy metabolism diseases. Essays Biochem. 2018, 62, 443–454. [Google Scholar]
- Pence, B.D. Growth Differentiation Factor-15 in Immunity and Aging. Front. Aging 2022, 3, 837575. [Google Scholar] [CrossRef]
- Tremellen, K.; Pearce, K. Dysbiosis of Gut Microbiota (DOGMA)-A novel theory for the development of Polycystic Ovarian Syndrome. Med. Hypotheses 2012, 79, 104–112. [Google Scholar] [CrossRef]
- Parker, J.; O’Brien, C.; Hawrelak, J. A review of the role of gastrointestinal dysbiosis in thepathogenesis of polycystic ovary syndrome. J. Obstet. Gynecol. Res. 2021, 65, 14–28. [Google Scholar]
- Guo, J.; Shao, J.; Yang, Y.; Niu, X.; Liao, J.; Zhao, Q.; Wang, D.; Li, S.; Hu, J. Gut Microbiota in Patients with Polycystic Ovary Syndrome: A Systematic Review. Reprod. Sci. 2022, 29, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Naessen, T.; Kushnir, M.M.; Chaika, A.; Nosenko, J.; Mogilevkina, I.; Rockwood, A.L.; Carlstrom, K.; Bergquist, J.; Kirilovas, D. Steroid profiles in ovarian follicular fluid in women with and without polycystic ovary syndrome, analyzed by liquid chromatography-tandem mass spectrometry. Fertil. Steril. 2010, 94, 2228–2233. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, W.; Zhou, C.; Zhou, Y.; Liu, X.; Ding, G.; Hu, Y.; Pan, J.; Sheng, J.; Jin, L.; et al. Steroid metabolome profiling of follicular fluid in normo- and hyperandrogenic women with polycystic ovary syndrome. J. Steroid Biochem. Mol. Biol. 2021, 206, 105806. [Google Scholar] [CrossRef]
- Jakimiuk, A.J.; Weitsman, S.R.; Brzechffa, P.R.; Magoffin, D.A. Aromatase mRNA expression in individual follicles from polycystic ovaries. Mol. Hum. Reprod. 1998, 4, 1–8. [Google Scholar] [CrossRef]
- Panghiyangani, R.; Soeharso, P.; Pujianto, A.; Suryandari, D.; Wiweko, B.; Kurniati, M.; Pujianto, D.A. CYP19A1 gene expression in patients with polycystic ovarian syndrome. J. Hum. Reprod. Sci. 2020, 13, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Narayana, S.; Ananad, C.; Kumari, N.S.; Sonkusere, S.; Babu, S.V.S. Impact of Aromatase Enzyme and its Altered Regulation on Polycystic Ovary Syndrome (PCOS): A Key Factor in Pathogenesis of PCOS. Indian J. Med. Spec. 2023, 14, 206–211. [Google Scholar] [CrossRef]
- Xue, Z.; Li, J.; Feng, J.; Han, H.; Zhao, J.; Zhang, J.; Han, Y.; Wu, X.; Zhang, Y. Research Progress on the Mechanism Between Polycystic Ovary Syndrome and Abnormal Endometrium. Front. Physiol. 2021, 12, 788772. [Google Scholar] [CrossRef]
- Ali, R.; Ahmed, T.; Gul, H.; Rehman, R. An interplay of Progesterone, Leukemia Inhibitor Factor and Interleukin-6 in the window of implantation; Impact on fertility. Cytokine 2023, 170, 156332. [Google Scholar] [CrossRef]
- Yusuf, M.; Amri, M.F.; Ugusman, A.; Hamid, A.A.; Wahab, N.A.; Mokhtar, M.H. Hyperandrogenism and Its Possible Effects on Endometrial Receptivity: A Review. Int. J. Mol. Sci. 2023, 24, 12026. [Google Scholar] [CrossRef]
- Mokhtar, M.H.; Giriabu, N.; Salleh, N. Testosterone Reduces Tight Junction Complexity and Down-regulates Expression of Claudin-4 and Occludin in the Endometrium in Ovariectomized, Sex-steroid Replacement Rats. Vivo 2020, 34, 225–231. [Google Scholar] [CrossRef]
- Li, S.; Song, Z.; Song, M.; Qin, J.; Zhao, M.; Yang, Z. Impaired receptivity and decidualization in DHEA-induced PCOS mice. Sci. Rep. 2016, 6, 38134. [Google Scholar] [CrossRef] [PubMed]
- Lindheim, L.; Bashir, M.; Munzker, J.; Trummer, C.; Zachhuber, V.; Leber, B.; Horvath, A.; Pieber, T.R.; Gorkiewicz, G.; Stadlbauer, V.; et al. Alterations in Gut Microbiome Composition and Barrier Function Are Associated with Reproductive and Metabolic Defects in Women with Polycystic Ovary Syndrome (PCOS): A Pilot Study. PLoS ONE 2017, 12, e0168390. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Bartos, A.; Whitsett, J.A.; Dey, S.K. Uterine Deletion of Gp130 or Stat3 Shows Implantation Failure with Increased Estrogenic Responses. Mol. Endocrinol. 2013, 27, 1492–1501. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Disruption of aromatase homeostasis as the cause of a multiplicity of ailments: A comprehensive review. J. Steroid Biochem. Mol. Biol. 2017, 168, 19–25. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parker, J.; Hofstee, P. Special Issue “New Challenges and Perspectives in Polycystic Ovary Syndrome”. Int. J. Mol. Sci. 2025, 26, 2665. https://doi.org/10.3390/ijms26062665
Parker J, Hofstee P. Special Issue “New Challenges and Perspectives in Polycystic Ovary Syndrome”. International Journal of Molecular Sciences. 2025; 26(6):2665. https://doi.org/10.3390/ijms26062665
Chicago/Turabian StyleParker, Jim, and Pierre Hofstee. 2025. "Special Issue “New Challenges and Perspectives in Polycystic Ovary Syndrome”" International Journal of Molecular Sciences 26, no. 6: 2665. https://doi.org/10.3390/ijms26062665
APA StyleParker, J., & Hofstee, P. (2025). Special Issue “New Challenges and Perspectives in Polycystic Ovary Syndrome”. International Journal of Molecular Sciences, 26(6), 2665. https://doi.org/10.3390/ijms26062665





