Study on Volatile Organic Compounds and Antioxidant Polyphenols in Cumin Produced in Xinjiang
Abstract
:1. Introduction
2. Results and Discussion
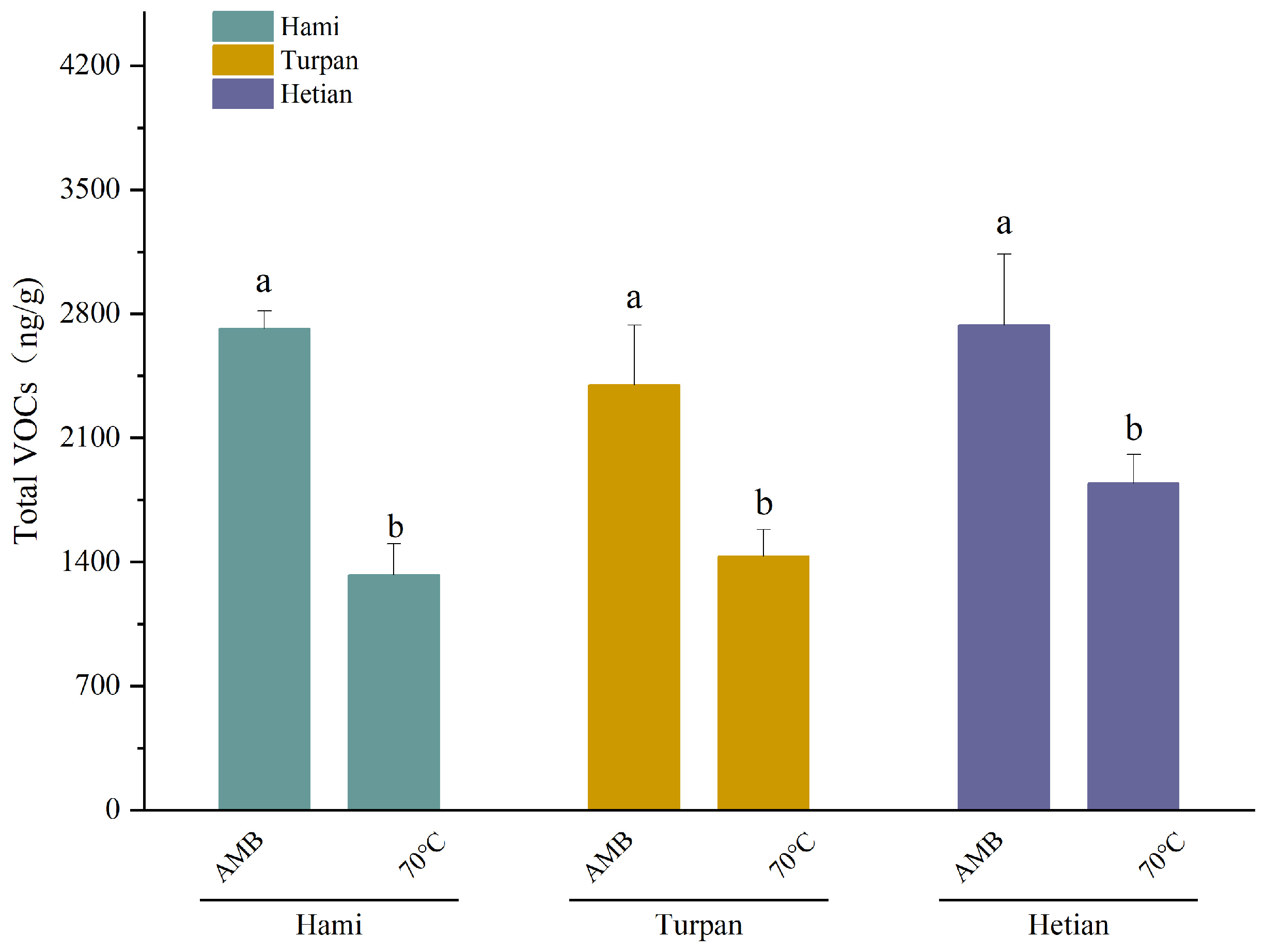
2.1. Changes in VOC Content of Cumin from Different Origins Under Different Processing Temperatures
2.2. Changes in VOC Types and Contents of Cumin from Different Origins Under Different Processing Temperatures
2.3. Differences in VOCs Under Different Treatment Conditions
2.4. Identification of Polyphenolic Compounds in Hetian Cumin Extract
3. Materials and Methods
3.1. Experimental Materials
3.2. Instruments
3.3. Preparation of Cumin Ethanol Extract
3.4. Headspace Solid-Phase Microextraction
3.5. GC-MS Analysis Conditions
3.6. Establishment of β-Carboline HAAs Simulation System
3.7. Identification of Polyphenolic Compounds in the Simulation System
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ravi, R.; Prakash, M.; Bhat, K.K. Characterization of Aroma Active Compounds of Cumin (Cuminum cyminum L.) by GC-MS, E-Nose, and Sensory Techniques. Int. J. Food Prop. 2013, 16, 1048–1058. [Google Scholar] [CrossRef]
- Mnif, S.; Aifa, S. Cumin (Cuminum cyminum L.) from Traditional Uses to Potential Biomedical Applications. Chem. Biodivers. 2015, 12, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Yadav, S.S.; Kumar, S.; Narashiman, B. A review on traditional uses, phytochemistry, pharmacology, and clinical research of dietary spice Cuminum cyminum L. Phytother. Res. 2021, 35, 5007–5030. [Google Scholar] [CrossRef]
- Usman, M.; Sahar, A.; Ahmad, M.H.; Khan, M.I.; Afzal, M.F.; Rahman, H.U.U.; Ali, M.A.; Khalid, W.; Khalid, M.Z.; Madilo, F.K. Augmenting the Oxidative Stability of Chicken Nuggets by incorporating cumin and Black cumin as natural preservatives. Int. J. Food Prop. 2024, 27, 352–366. [Google Scholar] [CrossRef]
- Satyanarayana, S.; Sushruta, K.; Sarma, G.S.; Srinivas, N.; Subba Raju, G.V. Antioxidant activity of the aqueous extracts of spicy food additives—Evaluation and comparison with ascorbic acid in in-vitro systems. J. Herb. Pharmacother. 2004, 4, 1–10. [Google Scholar] [CrossRef]
- Gholamnezhad, Z.; Havakhah, S.; Boskabady, M.H. Preclinical and clinical effects of Nigella sativa and its constituent, thymoquinone: A review. J. Ethnopharmacol. 2016, 190, 372–386. [Google Scholar] [CrossRef] [PubMed]
- Hajhashemi, V.; Ghannadi, A.; Jafarabadi, H. Black cumin seed essential oil, as a potent analgesic and antiinflammatory drug. Phytother. Res. PTR 2004, 18, 195–199. [Google Scholar] [CrossRef]
- Padilla-Camberos, E.; Sanchez-Hernandez, I.M.; Torres-Gonzalez, O.R.; Gallegos-Ortiz, M.D.; Mendez-Mona, A.L.; Baez-Moratilla, P.; Flores-Fernandez, J.M. Natural essential oil mix of sweet orange peel, cumin, and allspice elicits anti-inflammatory activity and pharmacological safety similar to non-steroidal anti-inflammatory drugs. Saudi J. Biol. Sci. 2022, 29, 3830–3837. [Google Scholar] [CrossRef]
- Jun, Y.W.; Kant, M.; Coskun, E.; Kato, T.A.; Jaruga, P.; Palafox, E.; Dizdaroglu, M.; Kool, E.T. Possible Genetic Risks from Heat-Damaged DNA in Food. ACS Cent. Sci. 2023, 9, 1170–1179. [Google Scholar] [CrossRef]
- Bai, X.; Li, Y.; Liang, W.W.; Xia, X.F.; Bian, C. Formation of advanced glycation end products of chicken breast meat induced by freeze-thaw cycles and subsequent cooking. Int. J. Biol. Macromol. 2023, 244, 125387. [Google Scholar] [CrossRef]
- Xiong, K.; Li, M.M.; Chen, Y.Q.; Hu, Y.M.; Jin, W. Formation and Reduction of Toxic Compounds Derived from the Maillard Reaction During the Thermal Processing of Different Food Matrices. J. Food Prot. 2024, 87, 100338. [Google Scholar] [CrossRef]
- Zeng, Y.; Jia, X.; Wei, L.; Wan, N. The effect of rosemary essential oil on acrylamide content and response surface optimization on heating treatment for fried dried beef. Food Ferment. Ind. 2015, 41, 208–214. [Google Scholar]
- Upadhyay, R.; Mishra, H.N. Antioxidant activity measurement of oleoresin from rosemary and sage. Ind. Crops Prod. 2014, 61, 453–459. [Google Scholar] [CrossRef]
- Puangsombat, K.; Jirapakkul, W.; Smith, J.S. Inhibitory Activity of Asian Spices on Heterocyclic Amines Formation in Cooked Beef Patties. J. Food Sci. 2011, 76, T174–T180. [Google Scholar] [CrossRef]
- Kesen, S.; Amanpour, A.; Sarhir, S.T.; Sevindik, O.; Guclu, G.; Kelebek, H.; Selli, S. Characterization of Aroma-Active Compounds in Seed Extract of Black Cumin (Nigella sativa L.) by Aroma Extract Dilution Analysis. Foods 2018, 7, 98. [Google Scholar] [CrossRef]
- Khan, I.U.; Rathore, B.S.; Mehriya, M.L.; Singh, B. Evaluation, Estimation and Identification of Essential Oil Constituents in Cumin (Cuminum cyminum) Genotypes Grown in Western Rajasthan. J. Essent. Oil Bear. Plants 2017, 20, 769–778. [Google Scholar] [CrossRef]
- Kiralan, M. Volatile Compounds of Black Cumin Seeds (Nigella sativa L.) from Microwave-Heating and Conventional Roasting. J. Food Sci. 2012, 77, C481–C484. [Google Scholar] [CrossRef]
- Ma, Y.C.; Zhang, K.; Xu, C.W.; Lai, C.R.; Liu, Y.; Cao, Y.; Zhao, L.C. Contribution of lipid to the formation of characteristic volatile flavor of peanut oil. Food Chem. 2024, 442, 138496. [Google Scholar] [CrossRef]
- Ashokkumar, K.; Vellaikumar, S.; Murugan, M.; Dhanya, M.K.; Karthikeyan, A.; Ariharasutharsan, G.; Arjun, P.; Sivakumar, P.; Aiswarya, S. GC/MS Analysis of Essential Oil Composition from Selected Seed Spices. Natl. Acad. Sci. Lett. 2021, 44, 503–506. [Google Scholar] [CrossRef]
- Elyasi, R.; Majdi, M.; Krause, S.T.; Kücükay, N.; Azizi, A.; Degenhardt, J. Identification and functional characterization of a γ-terpinene synthase in Nigella sativa L (black cumin). Phytochemistry 2022, 202, 113290. [Google Scholar] [CrossRef]
- Antonella, D.; Federico, D.; Grazia, S.M.; Gabriela, M. Antimutagenic and antioxidant activities of some bioflavours from wine. Food Chem. Toxicol. 2013, 60, 141–146. [Google Scholar] [CrossRef]
- Yeddes, W.; Mejri, I.; Grati-Affes, T.; Khammassi, S.; Hammami, M.; Aidi-Wannes, W.; Saidani-Tounsi, M. Synergistic Effect of Cloves (Syzygium aromaticum), Thyme (Thymus vulgaris) and Lemon (Citrus limon) Blended Essential Oils Optimized by Mixture Design for Improving the Antioxidant Activity. Avicenna J. Med. Biochem. 2022, 10, 20–29. [Google Scholar] [CrossRef]
- Shen, X.; Chen, Y.; Omedi, J.O.; Oz, E.; Oz, F.; Xiao, C.; Zhou, Y.; Chen, J.; Zeng, M. The Effects of Volatile Organic Compounds (VOCs) on the Formation of Heterocyclic Amines (HAs) in Meat Patties, under Different Smoking Temperatures and Durations. Foods 2022, 11, 3687. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, H.Y.; Kang, K.S.; Lee, J.G.; Yokozawa, T.; Park, J.H. The chemical and hydroxyl radical scavenging activity changes of ginsenoside-Rb1 by heat processing. Bioorg. Med. Chem. Lett. 2008, 18, 4515–4520. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.X.; Luo, X.L.; Huang, Y.; Zhao, M.J.; Liu, T.; Wang, J.; Feng, F.Q. Influence of cooking techniques on food quality, digestibility, and health risks regarding lipid oxidation. Food Res. Int. 2023, 167, 112685. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.M.; Wang, S.; Zhao, Z.Q.; Dou, S.H.; Zhang, S.F.; Wang, Y.; Gao, P.X.; Wang, B.C.; Xu, X.B.; Dong, L. Analysis of volatiles from the thermal decomposition of Amadori rearrangement products in the cysteine-glucose Maillard reaction and density functional theory study. Food Res. Int. 2024, 188, 114454. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, J.; Stojkovic, D.; Sokovic, M. Terpene core in selected aromatic and edible plants: Natural health improving agents. Adv. Food Nutr. Res. 2019, 90, 423–451. [Google Scholar] [CrossRef]
- Çakmakçi, R.; Mosber, G.; Milton, A.H.; Alatürk, F.; Ali, B. The Effect of Auxin and Auxin-Producing Bacteria on the Growth, Essential Oil Yield, and Composition in Medicinal and Aromatic Plants. Curr. Microbiol. 2020, 77, 564–577. [Google Scholar] [CrossRef]
- Wang, L.; Hu, G.Y.; Lei, L.B.; Lin, L.; Wang, D.Q.; Wu, J.X. Identification and Aroma Impact of Volatile Terpenes in Moutai Liquor. Int. J. Food Prop. 2016, 19, 1335–1352. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; Ma, Y.; Ma, H.; Deng, N.; Yuan, X.; Ye, Q. Analysis of Aromatic Compounds in Blackberry Juice Subjected to Thermal Treatment by SPME-GC-MS. Food Sci. 2013, 34, 212–217. [Google Scholar]
- de Oliveira, T.M.; de Carvalho, R.B.F.; da Costa, I.H.F.; de Oliveira, G.A.L.; de Souza, A.A.; de Lima, S.G.; de Freitas, R.M. Evaluation of p-cymene, a natural antioxidant. Pharm. Biol. 2015, 53, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.F.; Baschieri, A.; Amorati, R.; Valgimigli, L. Synergic antioxidant activity of γ-terpinene with phenols and polyphenols enabled by hydroperoxyl radicals. Food Chem. 2021, 345, 128468. [Google Scholar] [CrossRef]
- Aristatile, B.; Al-Numair, K.S.; Veeramani, C.; Pugalendi, K.V. Effect of carvacrol on hepatic marker enzymes and antioxidant status in d-galactosamine-induced hepatotoxicity in rats. Fundam. Clin. Pharmacol. 2009, 23, 757–765. [Google Scholar] [CrossRef]
- Xu, S.-f.; Zou, B.; Yang, J.; Yao, P.; Li, C.-m. Characterization of a highly polymeric proanthocyanidin fraction from persimmon pulp with strong Chinese cobra PLA2 inhibition effects. Fitoterapia 2012, 83, 153–160. [Google Scholar] [CrossRef]
- Voxeur, A.; Wang, Y.; Sibout, R. Lignification: Different mechanisms for a versatile polymer. Curr. Opin. Plant Biol. 2015, 23, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Bueno, M.; Franco-Luesma, E.; Culleré, L.; Fernández-Zurbano, P. Key Changes in Wine Aroma Active Compounds during Bottle Storage of Spanish Red Wines under Different Oxygen Levels. J. Agric. Food Chem. 2014, 62, 10015–10027. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gao, M.; Wang, W.; Ren, X.; Xu, Q.; Peng, Z. Research Progress on the Inhibitory Mechanism of Plant Polyphenols on the Formation of Heterocyclic Amines in Thermally Processed Meat Products. Food Sci. 2023, 44, 211–220. [Google Scholar]
- Valois, F.P.; Bezerra, K.C.A.; Assunçao, F.P.D.; Bernar, L.P.; da Paz, S.P.A.; Santos, M.C.; Feio, W.P.; Silva, R.M.P.; Mendonça, N.M.; de Castro, D.A.R.; et al. Improving the Antioxidant Activity, Yield, and Hydrocarbon Content of Bio-Oil from the Pyrolysis of Açaí Seeds by Chemical Activation: Effect of Temperature and Molarity. Catalysts 2024, 14, 44. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Zhou, J.; Sun, M.; Wang, H.; Yan, X.; Yao, M.; Zhu, C.; Wang, L.; Zhou, J.; Liu, B. Studies on Antibacteria and Antioxidant Properies of Sesamin. Food Sci. 2004, 25, 102–105. [Google Scholar]
- Plumb, G.W.; Price, K.R.; Williamson, G. Antioxidant properties of flavonol glycosides from green beans. Redox Rep. Commun. Free. Radic. Res. 1999, 4, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.R.; Park, J.; Jung, S.K.; Chang, Y.H. Synthesis, characterization, and functional properties of chlorophylls, pheophytins, and Zn-pheophytins. Food Chem. 2018, 245, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Ramanan, M.; Sinha, S.; Sudarshan, K.; Aidhen, I.S.; Doble, M. Inhibition of the enzymes in the leukotriene and prostaglandin pathways in inflammation by 3-aryl isocoumarins. Eur. J. Med. Chem. 2016, 124, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Sudarshan, K.; Aidhen, I.S. Convenient Synthesis of 3-Glycosylated Isocoumarins. Eur. J. Org. Chem. 2017, 2017, 34–38. [Google Scholar] [CrossRef]
- Yeoh, W.K.; Ali, A. Ultrasound treatment on phenolic metabolism and antioxidant capacity of fresh-cut pineapple during cold storage. Food Chem. 2017, 216, 247–253. [Google Scholar] [CrossRef]
- Rizzo, P.V.; Del Toro-Gipson, R.S.; Cadwallader, D.C.; Drake, M.A. Identification of aroma-active compounds in Cheddar cheese imparted by wood smoke. J. Dairy Sci. 2022, 105, 5622–5640. [Google Scholar] [CrossRef]
- Xu, L.; He, W.J.; Lu, M.; Yuan, B.; Zeng, M.M.; Tao, G.J.; Qin, F.; Chen, J.; Guan, Y.M.; He, Z.Y. Enzyme-assisted ultrasonic-microwave synergistic extraction and UPLC-QTOF-MS analysis of flavonoids from Chinese water chestnut peels. Ind. Crops Prod. 2018, 117, 179–186. [Google Scholar] [CrossRef]
- Pavlović, A.V.; Papetti, A.; Zagorac, D.Č.D.; Gašić, U.M.; Mišić, D.M.; Tešić, Ž.L.; Natić, M.M. Phenolics composition of leaf extracts of raspberry and blackberry cultivars grown in Serbia. Ind. Crops Prod. 2016, 87, 304–314. [Google Scholar] [CrossRef]
- Liu, X.X.; Chen, W.Y.; Sun, M.H.; Lv, X.F.; Shen, X.; Chai, Z.P.; Zeng, M.M. The Effect of Cumin on the Formation of β-Carboline Heterocyclic Amines in Smoked Meat and Simulated Systems. Foods 2025, 14, 299. [Google Scholar] [CrossRef]

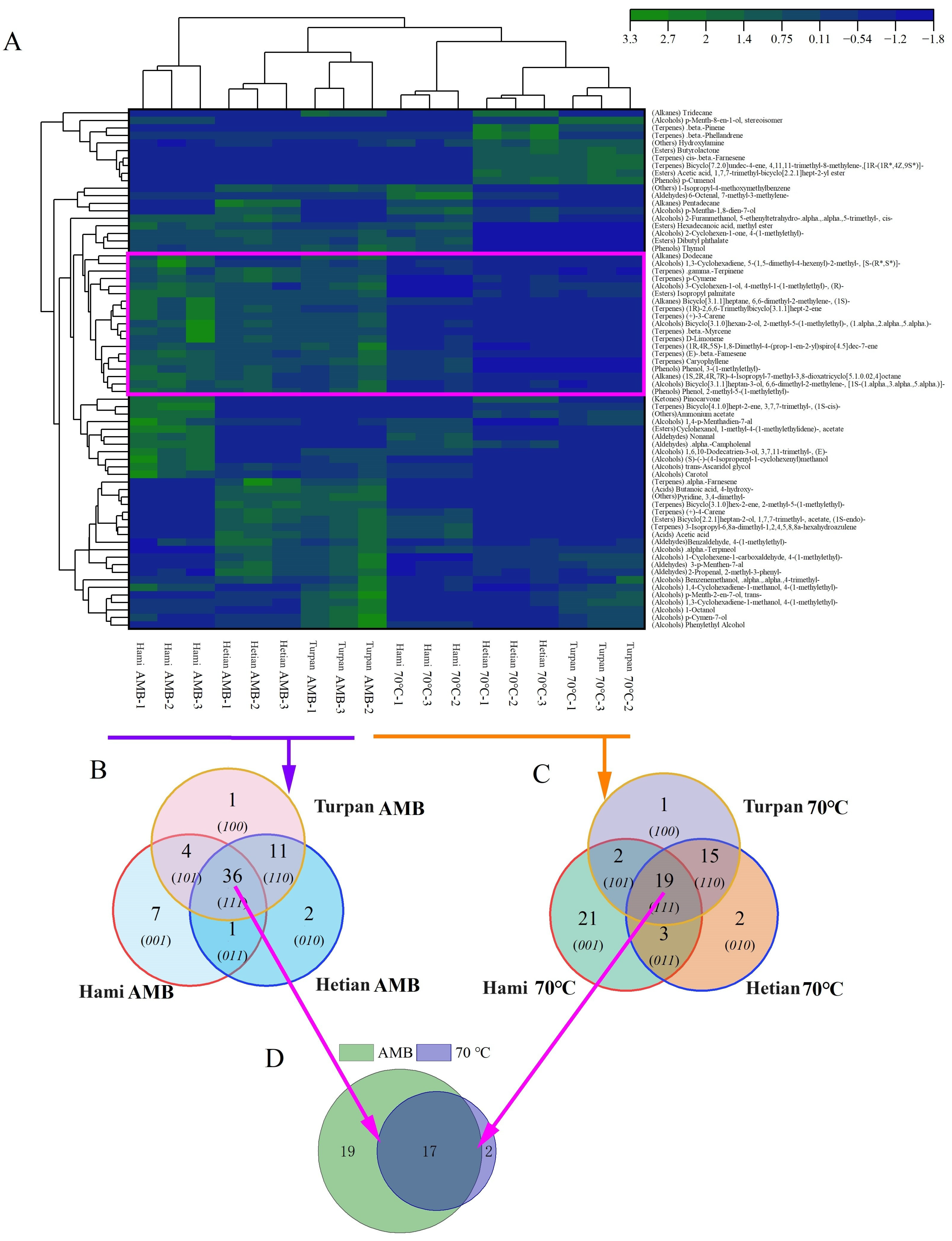

| Number | Name | Hami AMB µg/g | Hami 70 °C µg/g | Hetian AMB µg/g | Hetian 70 °C µg/g | Turpan AMB µg/g | Turpan 70 °C µg/g | |
|---|---|---|---|---|---|---|---|---|
| 1 | Butanoic acid, 4-hydroxy- | Acids | n.d. | n.d. | 1.17 ± 0 | n.d. | 1.11 ± 0.24 | n.d. |
| 2 | Acetic acid | Acids | n.d. | 0.69 ± 0.14 | 0.55 ± 0.48 | n.d. | 0.88 ± 0.22 | n.d. |
| Total | n.d. | 0.69 ± 0.14 | 1.72 ± 0.48 | n.d. | 1.99 ± 0.46 | n.d. | ||
| 3 | 1,4-p-Menthadien-7-al | Alcohols | 428.86 ± 186.31 | 243.83 ± 37.73 | 178.46 ± 21.96 | n.d. | 109.83 ± 20.79a | 239.95 ± 25.14b |
| 4 | Bicyclo[3.1.0]hexan-2-ol, 2-methyl-5-(1-methylethyl)-, (1.α.,2.α.,5.α.)- | Alcohols | 143.9 ± 57.76a | 41.53 ± 6.07b | 97.9 ± 13.46a | 23.26 ± 2.31b | 90.85 ± 16.94a | 14.67 ± 2.11b |
| 5 | p-Cymen-7-ol | Alcohols | 12.1 ± 5.46 | 8.8 ± 1.2 | 14.04 ± 2.53a | 7.83 ± 1.1b | 34.4 ± 10.27 | 18.74 ± 2.02 |
| 6 | p-Menth-2-en-7-ol, trans- | Alcohols | 3.45 ± 0.33 | 3.28 ± 0.47 | 8.61 ± 1.55a | 5.37 ± 0.76b | 21.35 ± 7.21 | 13.62 ± 1.67 |
| 7 | Bicyclo[3.1.1]heptan-3-ol, 6,6-dimethyl-2-methylene-, [1S-(1.α.,3.α.,5.α.)]- | Alcohols | 4.74 ± 0.47a | 2.62 ± 0.32b | 4.33 ± 0.75a | 2.61 ± 0.32b | 4.26 ± 1.21 | 2.51 ± 0.32 |
| 8 | 3-Cyclohexen-1-ol, 4-methyl-1-(1-methylethyl)-, (R)- | Alcohols | 8.98 ± 0.89 | n.d. | 5.46 ± 2.32 | 2.58 ± 0.94 | 8.04 ± 2.47a | 3.93 ± 0.66b |
| 9 | 1,4-Cyclohexadiene-1-methanol, 4-(1-methylethyl)- | Alcohols | 9.5 ± 3.17 | 4.44 ± 0.46 | 3.5 ± 0.68a | 1.91 ± 0.33b | 11.36 ± 3.64a | 7.13 ± 0.83b |
| 10 | .α.-Farnesene | Alcohols | n.d. | n.d. | 0.54 ± 0.26 | n.d. | 0.31 ± 0 | n.d. |
| 11 | 2-Furanmethanol, 5-ethenyltetrahydro-.α.,.α.,5-trimethyl-, cis- | Alcohols | 0.45 ± 0a | 0.24 ± 0b | 0.51 ± 0.1a | 0.25 ± 0b | n.d. | n.d. |
| 12 | Benzenemethanol, .α.,.α.,4-trimethyl- | Alcohols | 0.45 ± 0 | 0.37 ± 0 | 0.54 ± 0.1a | 0.34 ± 0b | 0.59 ± 0.2 | 0.49 ± 0.24 |
| 13 | p-Mentha-1,8-dien-7-ol | Alcohols | n.d. | 0.52 ± 0.1 | 0.42 ± 0a | 0.22 ± 0b | n.d. | 0.21 ± 0 |
| 14 | 1,3-Cyclohexadiene-1-methanol, 4-(1-methylethyl)- | Alcohols | 0.44 ± 0 | n.d. | 0.42 ± 0.1 | 0.33 ± 0 | 1.66 ± 0.44 | 1.29 ± 0.2 |
| 15 | trans-Ascaridol glycol | Alcohols | 0.96 ± 0.24a | 0.25 ± 0b | 0.35 ± 0 | n.d. | 0.34 ± 0.1 | n.d. |
| 16 | 1,3-Cyclohexadiene, 5-(1,5-dimethyl-4-hexenyl)-2-methyl-, [S-(R*,S*)]- | Alcohols | 1.35 ± 0.71a | 0.17 ± 0.1b | 0.31 ± 0.1 | n.d. | 0.51 ± 0.1 | n.d. |
| 17 | Carotol | Alcohols | 0.85 ± 0.36a | 0.32 ± 0b | 0.31 ± 0.1a | 0.13 ± 0b | 0.39 ± 0.1a | 0.19 ± 0b |
| 18 | Phenylethyl Alcohol | Alcohols | n.d. | 0.08 ± 0 | 0.12 ± 0 | n.d. | 0.45 ± 0.14a | 0.17 ± 0b |
| 19 | p-Menth-8-en-1-ol, stereoisomer | Alcohols | 5.71 ± 0.32 | n.d. | n.d. | 3.32 ± 0.37 | n.d. | 12.22 ± 0.39 |
| 20 | 1-Octanol | Alcohols | 0.21 ± 0 | n.d. | n.d. | n.d. | 0.92 ± 0.22a | 0.4 ± 0b |
| 21 | 1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl-, (E)- | Alcohols | 0.05 ± 0a | 0.03 ± 0b | n.d. | n.d. | 0.03 ± 0 | n.d. |
| 22 | (S)-(−)-(4-Isopropenyl-1-cyclohexenyl)methanol | Alcohols | 1.15 ± 0.36 | n.d. | n.d. | n.d. | 0.43 ± 0.14 | n.d. |
| Total | 623.15 ± 141.79a | 306.47 ± 46.44b | 315.82 ± 43.21a | 48.16 ± 5.86b | 285.72 ± 63.67 | 315.52 ± 32.12 | ||
| 23 | Nonanal | Aldehydes | 0.53 ± 0.1 | 0.38 ± 0.1 | n.d. | n.d. | n.d. | n.d. |
| 24 | .α.-Campholenal | Aldehydes | 0.22 ± 0 | 0.14 ± 0 | n.d. | 0.1 ± 0 | n.d. | n.d. |
| 25 | 3-p-Menthen-7-al | Aldehydes | 80.27 ± 2.92 | 78.8 ± 15.24 | 168.04 ± 23.41a | 112.08 ± 11.01b | 184.19 ± 50.11 | 124 ± 14.67 |
| 26 | 6-Octenal, 7-methyl-3-methylene- | Aldehydes | 0.34 ± 0a | 3.26 ± 0.46b | 0.44 ± 0a | 0.2 ± 0b | 0.24 ± 0 | n.d. |
| 27 | Benzaldehyde, 4-(1-methylethyl)- | Aldehydes | 355.96 ± 118.03 | 357.18 ± 54.28 | 596.57 ± 83.92a | 347.05 ± 33.99b | 527.31 ± 139 | 326.2 ± 37.69 |
| 28 | 1-Cyclohexene-1-carboxaldehyde, 4-(1-methylethyl)- | Aldehydes | 8.08 ± 0.92 | n.d. | 29.33 ± 4.73a | 17.58 ± 2.8b | 38.67 ± 11.69 | 26.61 ± 3.58 |
| 29 | 2-Propenal, 2-methyl-3-phenyl- | Aldehydes | 0.4 ± 0.14 | 0.32 ± 0.1 | 0.94 ± 0.14a | 0.49 ± 0b | 0.9 ± 0.22a | 0.53 ± 0b |
| Total | 445.79 ± 119.93 | 440.08 ± 69.99 | 795.32 ± 112.26a | 477.49 ± 47.66b | 751.3 ± 200.87 | 477.34 ± 55.82 | ||
| 31 | Bicyclo[3.1.1]heptane, 6,6-dimethyl-2-methylene-, (1S)- | Alkanes | 1687.35 ± 635.85a | 357.67 ± 46.72b | 1069.14 ± 50.97 | 874.69 ± 131.91 | n.d. | |
| 32 | (1S,2R,4R,7R)-4-Isopropyl-7-methyl-3,8-dioxatricyclo[5.1.0.02,4]octane | Alkanes | 1.4 ± 0.17a | 0.61 ± 0.1b | 1.01 ± 0.22a | 0.37 ± 0b | 0.86 ± 0.3 | 0.35 ± 0 |
| 33 | Pentadecane | Alkanes | n.d. | 0.29 ± 0 | 0.44 ± 0.35 | n.d. | n.d. | n.d. |
| 34 | Dodecane | Alkanes | 0.67 ± 0.3 | n.d. | n.d. | n.d. | 0.65 ± 0 | n.d. |
| 35 | Tridecane | Alkanes | n.d. | n.d. | n.d. | 0.33 ± 0b | 0.32 ± 0 | n.d. |
| Total | 1689.41 ± 635.49a | 358.57 ± 46.84b | 1070.59 ± 50.98a | 0.7 ± 0b | 876.52 ± 132.22a | 0.35 ± 0b | ||
| 36 | Bicyclo[2.2.1]heptan-2-ol, 1,7,7-trimethyl-, acetate, (1S-endo)- | Esters | n.d. | 3 ± 0.14 | 5.38 ± 0.87 | n.d. | 5.6 ± 1.62 | n.d. |
| 37 | Isopropyl palmitate | Esters | 1.9 ± 0.41 | n.d. | 1.23 ± 0.2a | 0.58 ± 0b | 1.25 ± 0.28a | 0.6 ± 0b |
| 38 | Hexadecanoic acid, methyl ester | Esters | 0.21 ± 0 | 0.19 ± 0 | 0.13 ± 0 | n.d. | 0.12 ± 0 | n.d. |
| 39 | Butyrolactone | Esters | n.d. | n.d. | n.d. | 0.39 ± 0.1 | n.d. | 0.41 ± 0.1 |
| 40 | Acetic acid, 1,7,7-trimethyl-bicyclo[2.2.1]hept-2-yl ester | Esters | n.d. | n.d. | n.d. | 2.15 ± 0 | n.d. | 2.18 ± 0.1 |
| 41 | Cyclohexanol, 1-methyl-4-(1-methylethylidene)-, acetate | Esters | 0.77 ± 0.14a | 0.23 ± 0b | n.d. | n.d. | n.d. | |
| Total | 2.88 ± 0.58 | 3.42 ± 0.17 | 6.74 ± 1.09a | 3.11 ± 0.17b | 6.97 ± 1.91a | 3.19 ± 0.2b | ||
| 42 | Pinocarvone | Ketones | 1.61 ± 0 | 1.4 ± 0 | n.d. | n.d. | ||
| 43 | 2-Cyclohexen-1-one, 4-(1-methylethyl)- | Ketones | 1.75 ± 0.14 | 1.59 ± 0.2 | 1.67 ± 1.12 | 2.16 ± 0.39 | n.d. | |
| Total | 3.36 ± 0.14a | 1.59 ± 0.2b | 1.67 ± 1.12 | 1.4 ± 0 | 2.16 ± 0.39 | n.d. | ||
| 44 | Hydroxylamine | Others | 13.93 ± 3.69a | 22.73 ± 4.37b | 19.27 ± 2.48a | 29.09 ± 4.66b | 17.14 ± 3.2a | 30.24 ± 1.3b |
| 45 | 1-Isopropyl-4-methoxymethylbenzene | Others | n.d. | 0.26 ± 0 | 0.18 ± 0 | n.d. | 0.18 ± 0 | n.d. |
| 46 | Ammonium acetate | Others | 1.05 ± 0 | n.d. | n.d. | 0.43 ± 0.1 | n.d. | 0.38 ± 0.1 |
| 47 | Pyridine, 3,4-dimethyl- | Others | n.d. | 0.89 ± 0 | n.d. | 0.92 ± 0.1 | ||
| Total | 14.98 ± 3.68 | 22.99 ± 4.4 | 20.33 ± 2.52a | 29.52 ± 4.77b | 18.23 ± 3.23a | 30.62 ± 1.38b | ||
| 48 | Phenol, 3-(1-methylethyl)- | Phenols | 0.99 ± 0.22a | 0.47 ± 0b | 0.78 ± 0 | n.d. | 0.66 ± 0.2 | |
| 49 | Phenol, 2-methyl-5-(1-methylethyl)- | Phenols | 0.84 ± 0.14a | 0.28 ± 0b | 0.67 ± 0.14a | 0.12 ± 0b | 0.76 ± 0.36a | 0.22 ± 0b |
| 50 | Thymol | Phenols | 0.11 ± 0 | 0.14 ± 0 | 0.11 ± 0 | n.d. | 0.18 ± 0 | n.d. |
| 51 | p-Cumenol | Phenols | n.d. | n.d. | n.d. | 0.26 ± 0 | n.d. | 0.29 ± 0 |
| Total | 1.93 ± 0.35a | 0.88 ± 0.1b | 1.56 ± 0.17a | 0.39 ± 0b | 1.6 ± 0.6a | 0.51 ± 0b | ||
| .γ.-Terpinene | Terpenes | 710.86 ± 176.97 | 430.93 ± 64.05 | 849.95 ± 99.06a | 416.68 ± 4.12b | 664.54 ± 47.39a | 271.54 ± 97.75b | |
| 52 | p-Cymene | Terpenes | 284.75 ± 6.24 | n.d. | 336.14 ± 37.96a | 134.43 ± 14.1b | 299.92 ± 57.11a | 99.82 ± 10.23b |
| 53 | .β.-Myrcene | Terpenes | 304.76 ± 146.99a | 47.94 ± 6b | 183.25 ± 21.33a | 51.08 ± 4.85b | 176.09 ± 27.77a | 33.39 ± 3.34b |
| 54 | (1R)-2,6,6-Trimethylbicyclo[3.1.1]hept-2-ene | Terpenes | 219.63 ± 78.01a | 26.09 ± 3.68b | 133.97 ± 18.17 | n.d. | 89.87 ± 10.28 | n.d. |
| 55 | d-Limonene | Terpenes | 49.5 ± 26.68a | 13.13 ± 2.5b | 35.39 ± 3.87a | 11.06 ± 0.1b | 32.76 ± 8.18a | 7.64 ± 1.68b |
| 56 | (1R,4R,5S)-1,8-Dimethyl-4-(prop-1-en-2-yl)spiro[4.5]dec-7-ene | Terpenes | 13.89 ± 1.57a | 6.96 ± 1.14b | 11.98 ± 1.39a | 4.1 ± 0.63b | 16.45 ± 3.72a | 5.77 ± 0.61b |
| 57 | (+)-4-Carene | Terpenes | n.d. | 3.95 ± 0.62 | 8.08 ± 1.15 | n.d. | 8.09 ± 1.59 | n.d. |
| 58 | (E)-.β.-Famesene | Terpenes | 14.88 ± 0.98a | 5.36 ± 0.94b | 9.95 ± 2.71 | n.d. | 12.66 ± 3.38 | n.d. |
| 59 | (+)-3-Carene | Terpenes | 10.16 ± 3.92a | 1.38 ± 0.14b | 3.34 ± 2.51 | n.d. | 6.96 ± 0.99 | n.d. |
| 60 | Caryophyllene | Terpenes | 5.11 ± 0.59a | 2.71 ± 0.33b | 3.71 ± 0.51 | n.d. | 4.74 ± 1.19 | n.d. |
| 61 | Bicyclo[3.1.0]hex-2-ene, 2-methyl-5-(1-methylethyl)- | Terpenes | n.d. | n.d. | 0.17 ± 0 | n.d. | 0.15 ± 0 | n.d. |
| 62 | Bicyclo[4.1.0]hept-2-ene, 3,7,7-trimethyl-, (1S-cis)- | Terpenes | 10.25 ± 0.28 | n.d. | n.d. | 2.43 ± 0.2 | n.d. | 2 ± 0.24 |
| 63 | Bicyclo[7.2.0]undec-4-ene, 4,11,11-trimethyl-8-methylene-,[1R-(1R*,4Z,9S*)]- | Terpenes | n.d. | n.d. | n.d. | 2.35 ± 0.2 | n.d. | 2.98 ± 0.57 |
| 64 | cis-.β.-Farnesene | Terpenes | n.d. | n.d. | n.d. | 3.47 ± 0.66 | n.d. | 4.81 ± 0.61 |
| 65 | .β.-Phellandrene | Terpenes | n.d. | n.d. | n.d. | 325.02 ± 22.06 | n.d. | 21.36 ± 2.9 |
| 66 | .β.-Pinene | Terpenes | n.d. | n.d. | n.d. | 333.06 ± 67.13 | n.d. | 155.84 ± 23.19 |
| 67 | 3-Isopropyl-6,8a-dimethyl-1,2,4,5,8,8a-hexahydroazulene | Terpenes | n.d. | 13.06 ± 2.17 | 16.21 ± 2.57 | n.d. | 17.83 ± 5.15 | n.d. |
| 68 | .α.-Farnesene | Terpenes | n.d. | n.d. | 0.54 ± 0.26 | n.d. | 0.31 ± 0 | n.d. |
| Total | 1623.77 ± 129.16a | 551.51 ± 79.41b | 1592.68 ± 184.39 | 1283.68 ± 102.39 | 1330.38 ± 132.92a | 605.13 ± 116.14b | ||
| ALL Total | 4405.3 ± 600.07a | 1686.21 ± 246.01b | 3806.44 ± 383.4a | 1844.45 ± 151.42b | 3274.88 ± 532.56a | 1432.67 ± 162.43b |
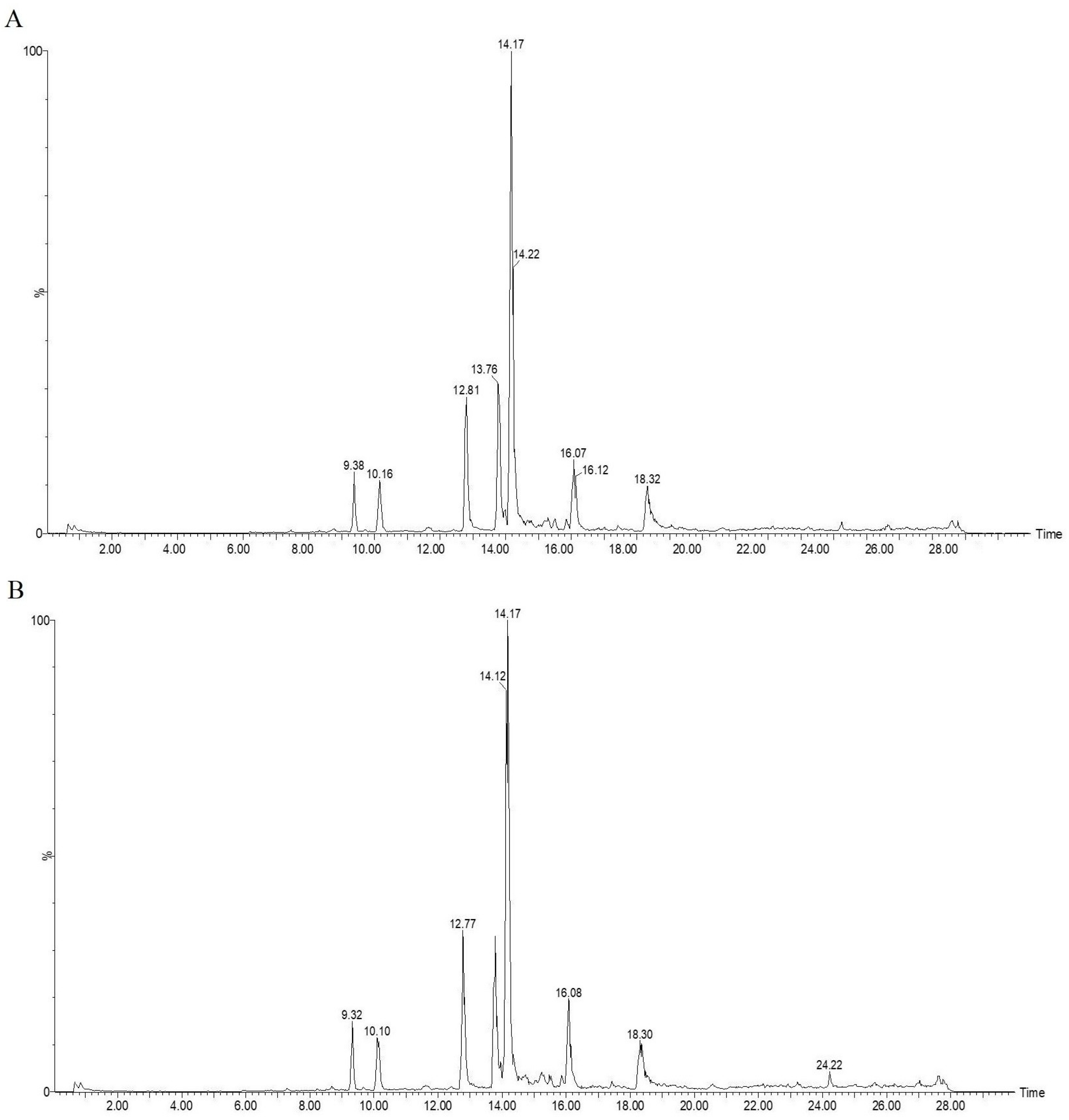
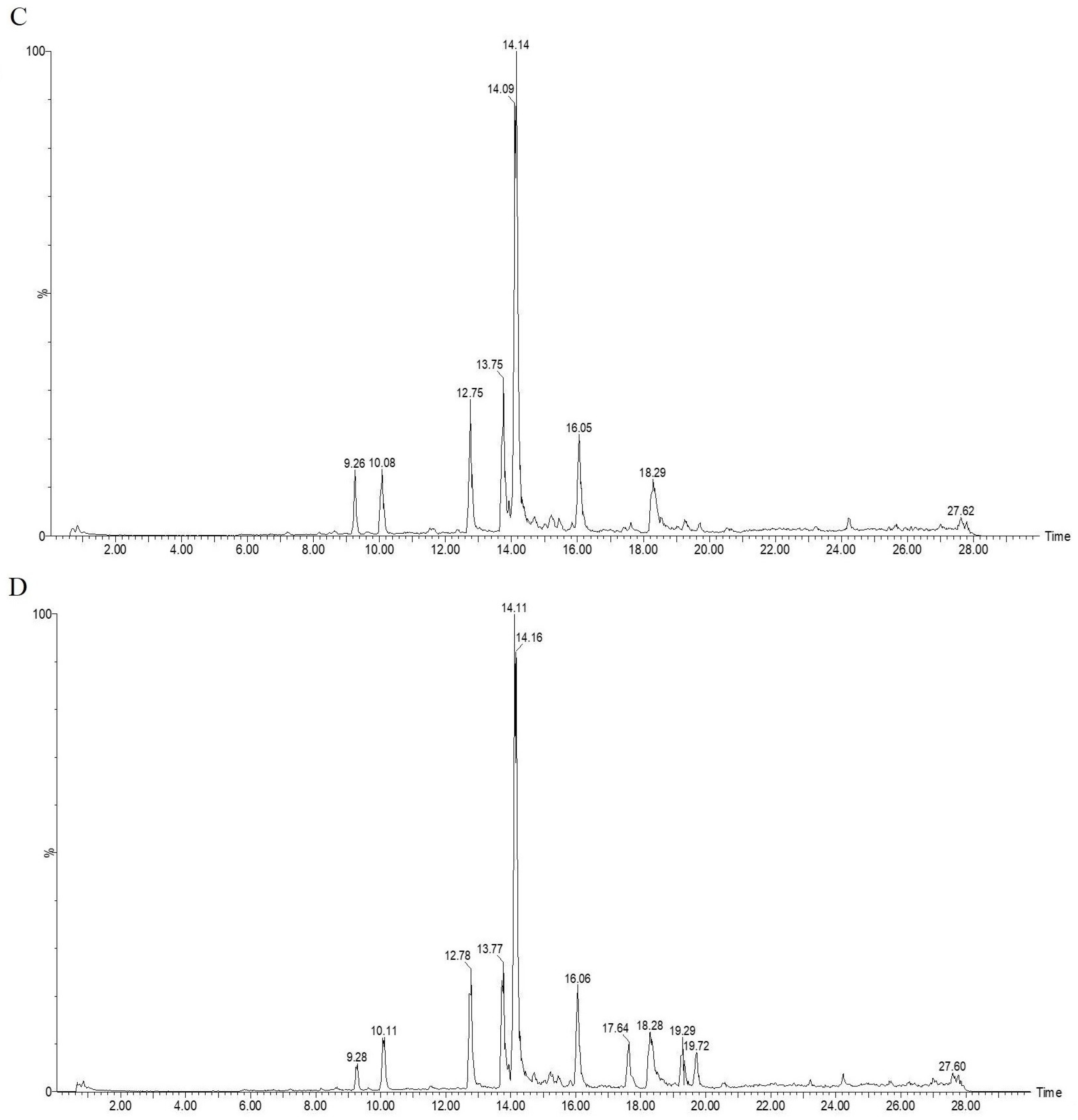
| Peak Number | Response Time | AMB | 70 °C | 100 °C | 120 °C |
|---|---|---|---|---|---|
| 1 | 9.38 | 264 | 250 | 227 | 102 |
| 2 | 10.16 | 338 | 323 | 326 | 262 |
| 3 | 12.81 | 856 | 845 | 584 | 603 |
| 4 | 13.76 | 950 | 907 | 768 | 585 |
| 5 | 14.17 | 3557 | 3487 | 3252 | 2869 |
| 6 | 16.07 | 632 | 605 | 583 | 498 |
| 7 | 17.62 | 55 | 70 | 75 | 252 |
| 8 | 18.32 | 674 | 650 | 609 | 477 |
| 9 | 19.26 | 0 | 0 | 141 | 208 |
| 10 | 19.66 | 0 | 0 | 55 | 182 |
| Peak Number | Parent Ion (m/z) | Daughter Ion (m/z) | Molecular Formula | Compound |
|---|---|---|---|---|
| 1 | 353.1074 | 191.0537 | C20H18O6 | Sesamin |
| 2 | 503.1740 | 113.0161 | C21H28O14 | 6-Caffeoylsucrose |
| 3 | 345.0989 | 96.9522 | C17H14O8 | Pobolin |
| 4 | 447.0688 | 285.2010 | C20H16O12 | Eschweilenol C |
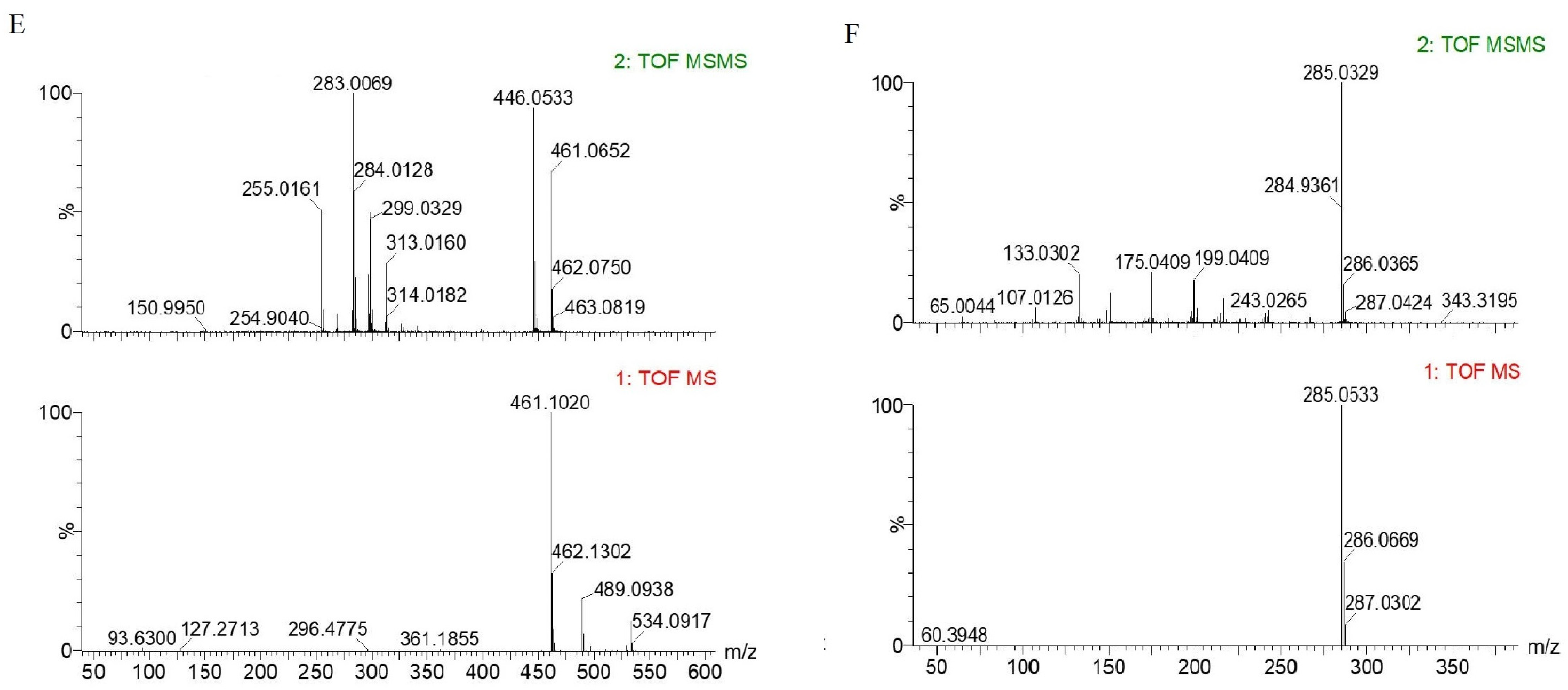
| 5 | 461.1020 | 283.0069 | C21H18O12 | Kaempferol Glucuronide |
| 6 | 285.0533 | 133.0302 | C15H10O6 | Luteolin |
| Reaction Precursor | Addition Amount |
|---|---|
| Glucose (mmol/mL) | 0.02 |
| Creatinine (mmol/mL) | 0.04 |
| Tryptophan (mmol/mL) | 0.04 |
| Cumin Ethanol Extract (g/mL) | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Lv, X.; Liu, X.; Chen, W.; Shen, X.; Chai, Z.; Zeng, M. Study on Volatile Organic Compounds and Antioxidant Polyphenols in Cumin Produced in Xinjiang. Int. J. Mol. Sci. 2025, 26, 2628. https://doi.org/10.3390/ijms26062628
Sun M, Lv X, Liu X, Chen W, Shen X, Chai Z, Zeng M. Study on Volatile Organic Compounds and Antioxidant Polyphenols in Cumin Produced in Xinjiang. International Journal of Molecular Sciences. 2025; 26(6):2628. https://doi.org/10.3390/ijms26062628
Chicago/Turabian StyleSun, Minghao, Xufang Lv, Xiuxiu Liu, Wenyu Chen, Xing Shen, Zhongping Chai, and Maomao Zeng. 2025. "Study on Volatile Organic Compounds and Antioxidant Polyphenols in Cumin Produced in Xinjiang" International Journal of Molecular Sciences 26, no. 6: 2628. https://doi.org/10.3390/ijms26062628
APA StyleSun, M., Lv, X., Liu, X., Chen, W., Shen, X., Chai, Z., & Zeng, M. (2025). Study on Volatile Organic Compounds and Antioxidant Polyphenols in Cumin Produced in Xinjiang. International Journal of Molecular Sciences, 26(6), 2628. https://doi.org/10.3390/ijms26062628







