Role of the Rumen Epithelium and Associated Changes Under High-Concentrate Diets
Abstract
1. Introduction
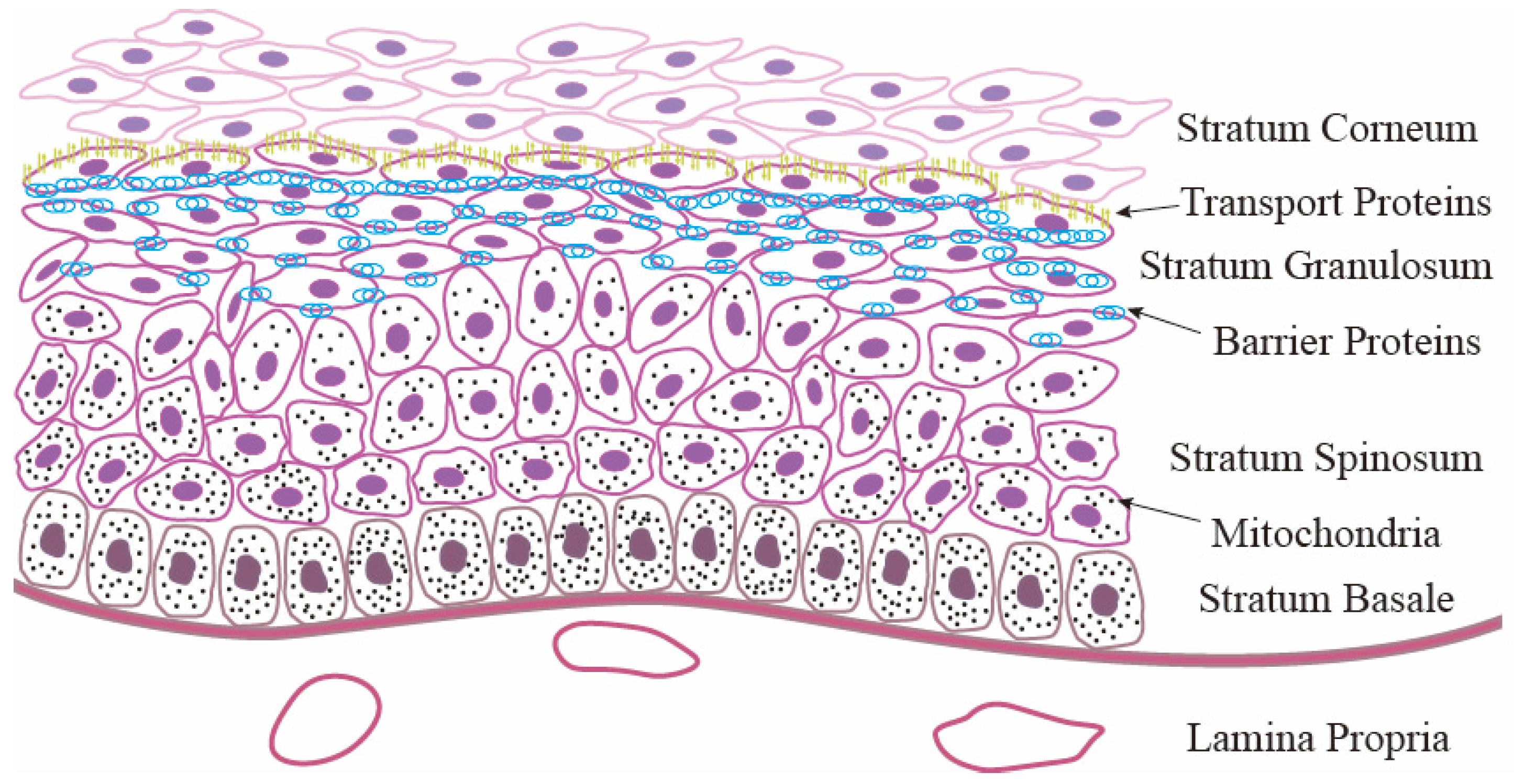
2. Structure and Functions of the RE
3. Responses of the RE Under HCD
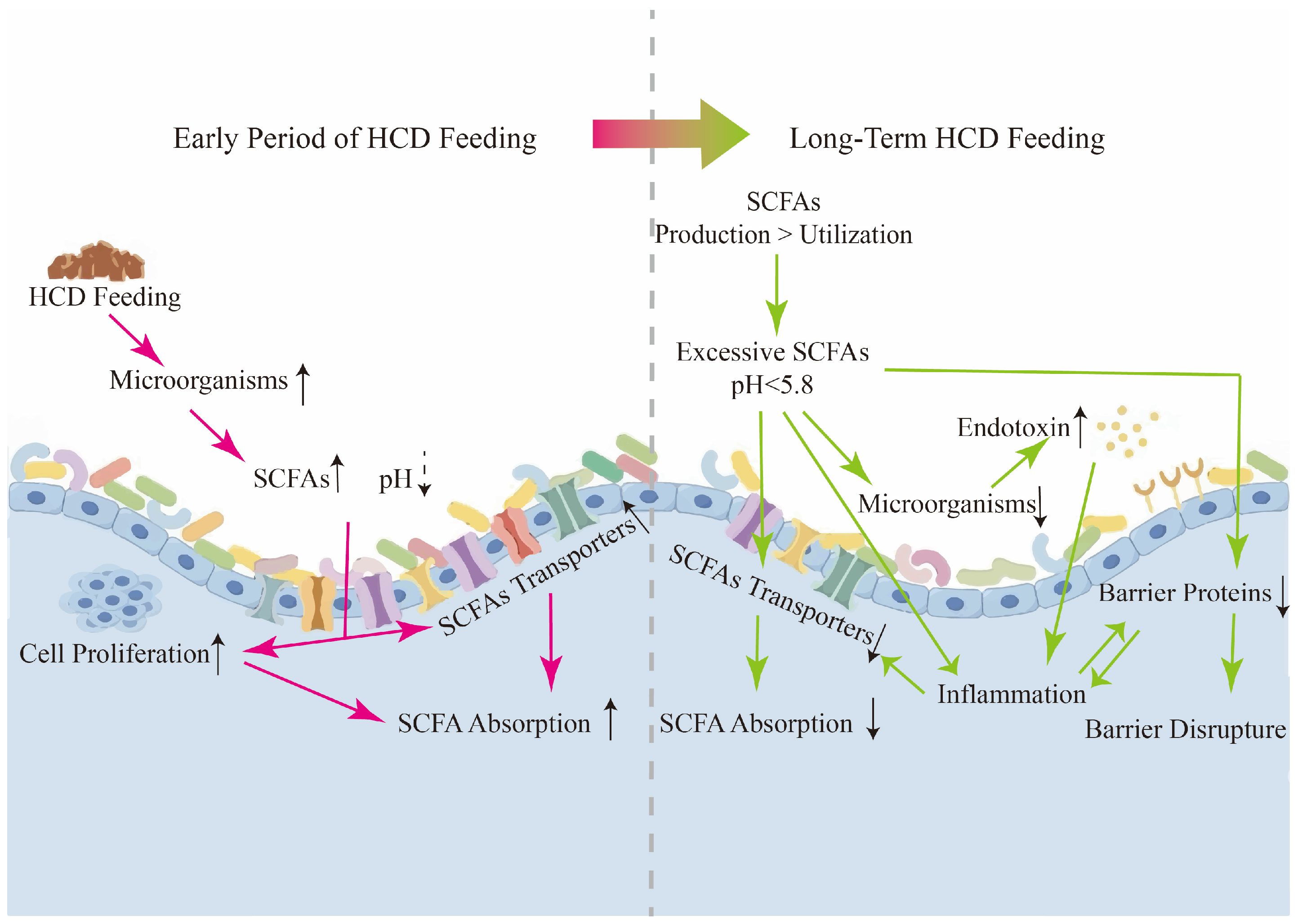
3.1. RE Proliferation
3.2. RE Absorption and Metabolism Changes
3.3. RE Barrier Disruption
3.4. RE Inflammation
4. Future Directions
- (i)
- Studies on RE barrier disruption under SARA are mainly focused on the mechanism. It remains unclear whether the recovery of RE barrier function may be delayed compared to the morphological recovery after changing the HCD. Understanding this mechanism can optimize the barrier function of the RE and mitigate the detrimental effects of abnormal metabolites associated with HCD on the host.
- (ii)
- As one of the important intermediate products of HCD in rumen fermentation, lactate has pleiotropic properties in the pathogenesis of SARA. Owing to the conversion of lactate into SCFAs in the rumen, its effects on SARA are easily overlooked. Lactic acidosis, which has been proposed thus far, has focused mainly on the impact of lactate on the microbial community in the rumen under HCD. However, some studies conducted on cattle and monogastric omnivores have shown that lactate can cause inflammation in both the gastrointestinal tract and various parts of the body [106,107]. Other studies have shown that lactate can control the differentiation and function of immune cells under inflammatory conditions and inhibit the inflammatory response [108]. Interestingly, our study demonstrated that RECs cultured with varying concentrations of lactate exhibited damage under the pH condition of acidosis, suggesting that lactate contributes to RECs injury. Quiroga et al. also showed that D-lactate induces the secretion of proinflammatory cytokines from bovine fibroblast-like synoviocytes via the PI3K/Akt/HIF-1 and GSK-3β axes and triggers the release of DNA extracellular traps in bovine polymorphonuclear neutrophils, which can lead to inflammation [109,110]. Current research on the role of lactate in inducing damage to the RE is limited.
- (iii)
- An increasing number of studies are investigating the mechanisms of SARA using cell culture techniques. However, significant structural differences between tissues and monolayer cell cultures may obscure some research results. To circumvent the limitations inherent in conventional cell culture methods, three-dimensional cell cultures and organoid cultures should be promoted and applied. The first organoid model of sheep RECs was successfully established by Xu [111]. The first study using rumen organoids was conducted by Zhang et al. [62]. Rumen organoids with internal lumen are expected to facilitate a more accurate simulation of rumen physiology. However, organoid technology has not been widely used in rumen-related research due to technical difficulties, including issues with the integrity of the simulated in vivo environment, the repeatability of the technique, and the lack of a standardized culture process [112,113,114]. Nevertheless, there is still potential for advances in rumen organoid technology. Exploiting further biomaterials to build three-dimensional structures and organoids may be one of our future endeavors.
- (iv)
- In the investigation of gene expression related to rumen function, most studies have predominantly focused on assessing RNA expression levels. Given that proteins perform functional roles within the organism and that RNA must undergo a series of biological processes to be translated into proteins, changes in RNA expression cannot fully represent changes in protein expression levels. Therefore, despite challenges in finding ruminant-specific antibodies, it is imperative that researchers endeavor to measure protein expression levels to enhance the reliability and validity of their results.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Newbold, C.J.; Ramos-Morales, E. Review: Ruminal microbiome and microbial metabolome: Effects of diet and ruminant host. Animal 2020, 14, s78–s86. [Google Scholar] [CrossRef] [PubMed]
- Aschenbach, J.R.; Penner, G.B.; Stumpff, F.; Gäbel, G. Ruminant Nutrition Symposium: Role of fermentation acid absorption in the regulation of ruminal pH. J. Anim. Sci. 2011, 89, 1092–1107. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Sanabria, E.; Goonewardene, L.A.; Wang, Z.Q.; Durunna, O.N.; Moore, S.S.; Guan, L.L. Impact of Feed Efficiency and Diet on Adaptive Variations in the Bacterial Community in the Rumen Fluid of Cattle. Appl. Environ. Microbiol. 2012, 78, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Khafipour, E.; Li, S.; Tun, H.M.; Derakhshani, H.; Moossavi, S.; Plaizier, J.C. Effects of grain feeding on microbiota in the digestive tract of cattle. Anim. Front. 2016, 6, 13–19. [Google Scholar] [CrossRef]
- Nocek, J.E. Bovine acidosis: Implications on laminitis. J. Dairy Sci. 1997, 80, 1005–1028. [Google Scholar] [CrossRef]
- AlZahal, O.; Kebreab, E.; France, J.; McBride, B.W. A mathematical approach to predicting biological values from ruminal pH measurements. J. Dairy Sci. 2007, 90, 3777–3785. [Google Scholar] [CrossRef]
- Penner, G.B.; Steele, M.A.; Aschenbach, J.R.; McBride, B.W. Ruminant Nutrition Symposium: Molecular adaptation of ruminal epithelia to highly fermentable diets. J. Anim. Sci. 2011, 89, 1108–1119. [Google Scholar] [CrossRef]
- Guo, J.F.; Chang, G.J.; Zhang, K.; Xu, L.; Jin, D.; Bilal, M.S.; Shen, X.Z. Rumen-derived lipopolysaccharide provoked inflammatory injury in the liver of dairy cows fed a high-concentrate diet. Oncotarget 2017, 8, 46769–46780. [Google Scholar] [CrossRef]
- Steele, M.A.; Penner, G.B.; Chaucheyras-Durand, F.; Guan, L.L. Development and physiology of the rumen and the lower gut: Targets for improving gut health. J. Dairy Sci. 2016, 99, 4955–4966. [Google Scholar] [CrossRef]
- Baldwin, R.L., VI; Jesse, B.W. Technical note: Isolation and characterization of sheep ruminal epithelial cells. J. Anim. Sci. 1991, 69, 3603–3609. [Google Scholar] [CrossRef]
- Graham, C.; Simmons, N.L. Functional organization of the bovine rumen epithelium. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R173–R181. [Google Scholar] [CrossRef] [PubMed]
- Baaske, L.; Gaebel, G.; Dengler, F. Ruminal epithelium: A checkpoint for cattle health. J. Dairy Sci. 2020, 87, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Penner, G.B.; Oba, M.; Gäbel, G.; Aschenbach, J.R. A single mild episode of subacute ruminal acidosis does not affect ruminal barrier function in the short term. J. Dairy Sci. 2010, 93, 4838–4845. [Google Scholar] [CrossRef] [PubMed]
- Bannink, A.; France, J.; Lopez, S.; Gerrits, W.J.J.; Kebreab, E.; Tamminga, S.; Dijkstra, J. Modelling the implications of feeding strategy on rumen fermentation and functioning of the rumen wall. Anim. Feed Sci. Technol. 2008, 143, 3–26. [Google Scholar] [CrossRef]
- Gui, H.; Shen, Z. Concentrate diet modulation of ruminal genes involved in cell proliferation and apoptosis is related to combined effects of short-chain fatty acid and pH in rumen of goats. J. Dairy Sci. 2016, 99, 6627–6638. [Google Scholar] [CrossRef]
- Onaga, T.; Shimizu, Y.; Hayashi, H.; Tsuji, M.; Endoh, D.; Okada, H. Localization and secretion of epidermal growth factor in the parotid gland and its intragastric kinetics in sheep. Life Sci. 2006, 79, 1616–1629. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Eriksen, L.; Norgaard, P.; Rode, L.M. Short communication: Salivary secretion during meals in lactating dairy cattle. J. Dairy Sci. 2008, 91, 2077–2081. [Google Scholar] [CrossRef]
- Gorka, P.; Kowalski, Z.M.; Pietrzak, P.; Kotunia, A.; Kiljanczyk, R.; Flaga, J.; Holst, J.J.; Guilloteau, P.; Zabielski, R. Effect of sodium butyrate supplementation in milk replacer and starter diet on rumen development in calves. J. Physiol. Pharmacol. 2009, 60, 47–53. [Google Scholar]
- Shen, Z.M.; Seyfert, H.M.; Löhrke, B.; Schneider, F.; Zitnan, R.; Chudy, A.; Kuhla, S.; Hammon, H.M.; Blum, J.W.; Martens, H.; et al. An energy-rich diet causes rumen papillae proliferation associated with more IGF type 1 receptors and increased plasma IGF-1 concentrations in young goats. J. Nutr. 2004, 134, 11–17. [Google Scholar] [CrossRef]
- Lu, J.; Zhao, H.; Xu, J.; Zhang, L.; Yan, L.; Shen, Z. Elevated cyclin D1 expression is governed by plasma IGF-1 through Ras/Raf/MEK/ERK pathway in rumen epithelium of goats supplying a high metabolizable energy diet. J. Anim. Physiol. Anim. Nutr. 2013, 97, 1170–1178. [Google Scholar] [CrossRef]
- Steele, M.A.; Dionissopoulos, L.; AlZahal, O.; Doelman, J.; McBride, B.W. Rumen epithelial adaptation to ruminal acidosis in lactating cattle involves the coordinated expression of insulin-like growth factor-binding proteins and a cholesterolgenic enzyme. J. Dairy Sci. 2012, 95, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Ricci, S.; Pacífico, C.; Kreuzer-Redmer, S.; Castillo-Lopez, E.; Rivera-Chacon, R.; Sener-Aydemir, A.; Rossi, G.; Galosi, L.; Biagini, L.; Schwartz-Zimmermann, H.E.; et al. Integrated microbiota-host-metabolome approaches reveal adaptive ruminal changes to prolonged high-grain feeding and phytogenic supplementation in cattle. FEMS Microbiol. Ecol. 2024, 100, fiae006. [Google Scholar] [CrossRef] [PubMed]
- Ferry, R.J.; Katz, L.E.L.; Grimberg, A.; Cohen, P.; Weinzimer, S.A. Cellular actions of insulin-like growth factor binding proteins. Horm. Metab. Res. 1999, 31, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.C. Signalling pathways involved in antiproliferative effects of IGFBP-3: A review. Mol. Pathol. 2001, 54, 145–148. [Google Scholar] [CrossRef]
- Firth, S.M.; Baxter, R.C. Cellular actions of the insulin-like growth factor binding proteins. Endocr. Rev. 2002, 23, 824–854. [Google Scholar] [CrossRef]
- Steele, M.A.; Croom, J.; Kahler, M.; AlZahal, O.; Hook, S.E.; Plaizier, K.; McBride, B.W. Bovine rumen epithelium undergoes rapid structural adaptations during grain-induced subacute ruminal acidosis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 300, R1515–R1523. [Google Scholar] [CrossRef]
- Lu, Z.; Gui, H.; Yao, L.; Yan, L.; Martens, H.; Aschenbach, J.R.; Shen, Z. Short-chain fatty acids and acidic pH upregulate UT-B, GPR41, and GPR4 in rumen epithelial cells of goats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R283–R293. [Google Scholar] [CrossRef]
- Meng, Z.T.; Tan, D.J.; Cheng, Z.Q.; Jiang, M.C.; Zhan, K. GPR41 Regulates the Proliferation of BRECs via the PIK3-AKT-mTOR Pathway. Int. J. Mol. Sci. 2023, 24, 4203. [Google Scholar] [CrossRef]
- Yang, B.; Xu, Z.; Qin, Y.; Peng, Y.; Luo, Y.; Wang, J. Exploring the effects of Hippo signaling pathway on rumen epithelial proliferation. BMC Vet. Res. 2024, 20, 186. [Google Scholar] [CrossRef]
- Hadjiagapiou, C.; Schmidt, L.; Dudeja, P.K.; Layden, T.J.; Ramaswamy, K. Mechanism(s) of butyrate transport in Caco-2 cells: Role of monocarboxylate transporter 1. Am. J. Physiol. Gastrointest. Liver. Physiol. 2000, 279, G775–G780. [Google Scholar] [CrossRef]
- Penner, G.B.; Aschenbach, J.R.; Gäbel, G.; Rackwitz, R.; Oba, M. Epithelial capacity for apical uptake of short chain fatty acids is a key determinant for intraruminal pH and the susceptibility to subacute ruminal acidosis in sheep. J. Nutr. 2009, 139, 1714–1720. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, B.; Shen, Z. Dietary modulation of the expression of genes involved in short-chain fatty acid absorption in the rumen epithelium is related to short-chain fatty acid concentration and pH in the rumen of goats. J. Dairy Sci. 2014, 97, 5668–5675. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.; Xi, Z.; Nasr, S.M.; He, F.; Han, M.; Yin, J.; Ge, L.; Chen, Y.; Wang, Y.; Wei, W.; et al. Multi-Omics Reveals the Impact of Exogenous Short-Chain Fatty Acid Infusion on Rumen Homeostasis: Insights into Crosstalk between the Microbiome and the Epithelium in a Goat Model. Microbiol. Spectr. 2023, 11, e0534322. [Google Scholar] [CrossRef]
- Nakamura, S.; Haga, S.; Kimura, K.; Matsuyama, S. Propionate and butyrate induce gene expression of monocarboxylate transporter 4 and cluster of differentiation 147 in cultured rumen epithelial cells derived from preweaning dairy calves. J. Anim. Sci. 2018, 96, 4902–4911. [Google Scholar] [CrossRef]
- Sehested, J.; Andersen, J.B.; Aaes, O.; Kristensen, N.B.; Diernæs, L.; Moller, P.D.; Skadhauge, E. Feed-induced changes in the transport of butyrate, sodium and chloride ions across the isolated bovine rumen epithelium. Acta Agric. Scand. Sect. A Anim. Sci. 2000, 50, 47–55. [Google Scholar] [CrossRef]
- Lodemann, U.; Martens, H. Effects of diet and osmotic pressure on Na+ transport and tissue conductance of sheep isolated rumen epithelium. Exp. Physiol. 2006, 91, 539–550. [Google Scholar] [CrossRef]
- Müller, F.; Aschenbach, J.R.; Gäbel, G. Role of Na+/H+ exchange and HCO3− transport in pHi recovery from intracellular acid load in cultured epithelial cells of sheep rumen. J. Comp. Physiol. B 2000, 170, 337–343. [Google Scholar] [CrossRef]
- Missner, A.; Kügler, P.; Antonenko, Y.N.; Pohl, P. Passive transport across bilayer lipid membranes: Overton continues to rule. Proc. Natl. Acad. Sci. USA 2008, 105, E123. [Google Scholar] [CrossRef]
- Allen, M.S. Relationship between fermentation acid production in the rumen and the requirement for physically effective fiber. J. Dairy Sci. 1997, 80, 1447–1462. [Google Scholar] [CrossRef]
- Maekawa, M.; Beauchemin, K.A.; Christensen, D.A. Effect of concentrate level and feeding management on chewing activities, saliva production, and ruminal pH of lactating dairy cows. J. Dairy Sci. 2002, 85, 1165–1175. [Google Scholar] [CrossRef]
- Rackwitz, R.; Gäbel, G. Effects of dissolved carbon dioxide on the integrity of the rumen epithelium: An agent in the development of ruminal acidosis. J. Anim. Physiol. Anim. Nutr. 2018, 102, e345–e352. [Google Scholar] [CrossRef] [PubMed]
- Simmons, N.L.; Chaudhry, A.S.; Graham, C.; Scriven, E.S.; Thistlethwaite, A.; Smith, C.P.; Stewart, G.S. Dietary regulation of ruminal bovine UT-B urea transporter expression and localization. J. Anim. Sci. 2009, 87, 3288–3299. [Google Scholar] [CrossRef] [PubMed]
- Etschmann, B.; Suplie, A.; Martens, H. Change of ruminal sodium transport in sheep during dietary adaptation. Arch. Anim. Nutr. 2009, 63, 26–38. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, Y.; Peng, Z.; Sun, X.; Sun, G.; Yuan, X.; Li, X.; Liu, G. Subacute ruminal acidosis suppressed the expression of MCT1 in rumen of cows. J. Cell Physiol. 2019, 234, 11734–11745. [Google Scholar] [CrossRef]
- Zhao, C.; Bobe, G.; Wang, Y.; Zhang, X.; Zhao, Z.; Zhang, S.; Sun, G.; Yuan, X.; Li, X.; Liu, G. Potential Role of SLC5A8 Expression in the Etiology of Subacute Ruminal Acidosis. Front. Vet. Sci. 2020, 7, 394. [Google Scholar] [CrossRef]
- Xue, M.; Wu, J.; Xie, Y.; Zhu, S.; Zhong, Y.; Liu, J.; Sun, H. Investigation of fiber utilization in the rumen of dairy cows based on metagenome-assembled genomes and single-cell RNA sequencing. Microbiome 2022, 10, 11. [Google Scholar] [CrossRef]
- Goodlad, R.A. Some effects of diet on the mitotic index and the cell cycle of the ruminal epithelium of sheep. Q. J. Exp. Physiol. 1981, 66, 487–499. [Google Scholar] [CrossRef]
- Kleemann, R.; Kooistra, T. HMG-CoA reductase inhibitors: Effects on chronic subacute inflammation and onset of atherosclerosis induced by dietary cholesterol. Curr. Drug Targets Cardiovasc. Haematol. Disord. 2005, 5, 441–453. [Google Scholar] [CrossRef]
- Omoigui, S. Cholesterol synthesis is the trigger and isoprenoid dependent interleukin-6 mediated inflammation is the common causative factor and therapeutic target for atherosclerotic vascular disease and age-related disorders including osteoporosis and type 2 diabetes. Med. Hypotheses 2005, 65, 559–569. [Google Scholar] [CrossRef]
- Gensure, R.H.; Zeidel, M.L.; Hill, W.G. Lipid raft components cholesterol and sphingomyelin increase H+/OH− permeability of phosphatidylcholine membranes. Biochem. J. 2006, 398, 485–495. [Google Scholar] [CrossRef]
- Gao, X.; Oba, M. Characteristics of dairy cows with a greater or lower risk of subacute ruminal acidosis: Volatile fatty acid absorption, rumen digestion, and expression of genes in rumen epithelial cells. J. Dairy Sci. 2016, 99, 8733–8745. [Google Scholar] [CrossRef]
- Mu, Y.; Qi, W.; Zhang, T.; Zhang, J.; Mao, S. Multi-omits Analysis Revealed Coordinated Responses of Rumen Microbiome and Epithelium to High-Grain-Induced Subacute Rumen Acidosis in Lactating Dairy Cows. Msystems 2022, 7, e0149021. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Larsen, A.; Fregulia, P. Investigating the impact of feed-induced, subacute ruminal acidosis on rumen epimural transcriptome and metatranscriptome in young calves at 8-and 17-week of age. Front. Vet. Sci. 2024, 11, 1328539. [Google Scholar] [CrossRef] [PubMed]
- Steele, M.A.; Vandervoort, G.; AlZahal, O.; Hook, S.E.; Matthews, J.C.; McBride, B.W. Rumen epithelial adaptation to high-grain diets involves the coordinated regulation of genes involved in cholesterol homeostasis. Physiol. Genom. 2011, 43, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.Y.; Qi, W.P.; Zhang, T.; Zhang, J.Y.; Li, M.; Mao, S.Y. Changes in rumen epithelial morphology and transcriptome, rumen metabolome, and blood biochemical parameters in lactating dairy cows with subacute rumen acidosis following rumen content transplantation. J. Dairy Sci. 2024, 107, 7960–7972. [Google Scholar] [CrossRef]
- Yang, B.; Xu, Z.; Chen, H.; Ma, T.; Zhao, Y.; Pang, M.; Wang, J. YAP1/TAZ Mediates Rumen Epithelial Cell Proliferation but Not Short-Chain Fatty Acid Metabolism In Vitro. Animals 2024, 14, 922. [Google Scholar] [CrossRef]
- Gao, J.; Xu, Q.; Wang, M.; Ouyang, J.; Tian, W.; Feng, D.; Liang, Y.; Jiang, B.; Loor, J.J. Ruminal epithelial cell proliferation and short-chain fatty acid transporters in vitro are associated with abundance of period circadian regulator 2 (PER2). J. Dairy Sci. 2020, 103, 12091–12103. [Google Scholar] [CrossRef]
- Froy, O.; Chapnik, N.; Miskin, R. Long-lived αMUPA transgenic mice exhibit pronounced circadian rhythms. Am. J. Physiol. Endocrinol. Metab. 2006, 291, E1017–E1024. [Google Scholar] [CrossRef]
- Liu, J.H.; Xu, T.T.; Liu, Y.J.; Zhu, W.Y.; Mao, S.Y. A high-grain diet causes massive disruption of ruminal epithelial tight junctions in goats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, R232–R241. [Google Scholar] [CrossRef]
- Steele, M.A.; AlZahal, O.; Hook, S.E.; Croom, J.; McBride, B.W. Ruminal acidosis and the rapid onset of ruminal parakeratosis in a mature dairy cow: A case report. Acta Vet. Scand. 2009, 51, 39. [Google Scholar] [CrossRef]
- Luo, D.; Peng, Z.; Yang, L.; Qu, M.; Xiong, X.; Xu, L.; Zhao, X.-H.; Pan, K.; Ouyang, K. Niacin Protects against Butyrate-Induced Apoptosis in Rumen Epithelial Cells. Oxidative Med. Cell. Longev. 2019, 2019, 2179738. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhang, Y.; Qin, J.; Zhu, H.; Liu, N.; Sun, D.; Yin, Y.; Mao, S.; Zhu, W.; Huang, Z.; et al. Early concentrate starter introduction induces rumen epithelial parakeratosis by blocking keratinocyte differentiation with excessive ruminal butyrate accumulation. J. Adv. Res. 2024, 66, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Yang, S.; Cheng, M.; Song, L.; Xu, M.; Gao, M.; Yu, Z. Long-term effect of subacute ruminal acidosis on the morphology and function of rumen epithelial barrier in lactating goats. J. Integr. Agric. 2022, 21, 3302–3313. [Google Scholar] [CrossRef]
- Wang, M.Y.; Li, Y.; Gao, M.; Song, L.W.; Xu, M.; Zhao, X.L.; Jia, Y.; Zhao, M.; Sun, Y.Y.; Hu, H.L. Effects of subacute ruminal acidosis on colon epithelial morphological structure, permeability, and expression of key tight junction proteins in dairy goats. J. Dairy Sci. 2021, 104, 4260–4270. [Google Scholar] [CrossRef]
- Dai, H.; Ma, N.; Chang, G.; Aabdin, Z.U.; Shen, X. Long-term high-concentrate diet feeding induces apoptosis of rumen epithelial cells and inflammation of rumen epithelium in dairy cows. Anim. Biotechnol. 2022, 33, 289–296. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, C.; Elmhadi, M.; Zhang, H.; Han, Y.; Shen, B.; He, B.L.; Liu, X.Y.; Wang, H.R. Thiamine ameliorates metabolic disorders induced by a long-term high-concentrate diet and promotes rumen epithelial development in goats. J. Dairy Sci. 2021, 104, 11522–11536. [Google Scholar] [CrossRef]
- Greco, G.; Hagen, F.; Meißner, S.; Shen, Z.; Lu, Z.; Amasheh, S.; Aschenbach, J.R. Effect of individual SCFA on the epithelial barrier of sheep rumen under physiological and acidotic luminal pH conditions. J. Anim. Sci. 2018, 96, 126–142. [Google Scholar] [CrossRef]
- Meissner, S.; Hagen, F.; Deiner, C.; Günzel, D.; Greco, G.; Shen, Z.; Aschenbach, J.R. Key role of short-chain fatty acids in epithelial barrier failure during ruminal acidosis. J. Dairy Sci. 2017, 100, 6662–6675. [Google Scholar] [CrossRef]
- Khafipour, E.; Krause, D.O.; Plaizier, J.C. A grain-based subacute ruminal acidosis challenge causes translocation of lipopolysaccharide and triggers inflammation. J. Dairy Sci. 2009, 92, 1060–1070. [Google Scholar] [CrossRef]
- Zhang, K.; Meng, M.; Gao, L.; Tu, Y.; Bai, Y. Rumen-derived lipopolysaccharide induced ruminal epithelium barrier damage in goats fed a high-concentrate diet. Microb. Pathog. 2019, 131, 81–86. [Google Scholar] [CrossRef]
- Reisinger, N.; Wendner, D.; Schauerhuber, N.; Mayer, E. Effect of Lipopolysaccharides (LPS) and Lipoteichoic Acid (LTA) on the Inflammatory Response in Rumen Epithelial Cells (REC) and the Impact of LPS on Claw Explants. Animals 2021, 11, 2058. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Youssef, M.; Rawat, M.; Guo, S.; Dokladny, K.; Haque, M.; Watterson, M.D.; Ma, T.Y. MMP-9-induced increase in intestinal epithelial tight permeability is mediated by p38 kinase signaling pathway activation of MLCK gene. Am. J. Physiol. Gastrointest. Liver. Physiol. 2019, 316, G278–G290. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhula, A.; Liu, W.; Lu, Z.; Shen, Z.; Penner, G.B.; Ma, L.; Bu, D. Direct effect of lipopolysaccharide and histamine on permeability of the rumen epithelium of steers ex vivo. J. Anim. Sci. 2022, 100, skac005. [Google Scholar] [CrossRef] [PubMed]
- Crawford, C.K.; Lopez Cervantes, V.; Quilici, M.L.; Armién, A.G.; Questa, M.; Matloob, M.S.; Huynh, L.D.; Beltran, A.; Karchemskiy, S.J.; Crakes, K.R.; et al. Inflammatory cytokines directly disrupt the bovine intestinal epithelial barrier. Sci. Rep. 2022, 12, 14578. [Google Scholar] [CrossRef]
- Gao, Z.; Li, Y.; Xu, C.; Luo, D.; Qiu, Q.; Pan, K.; Xiong, X.; Qu, M.; Ouyang, K. Niacin mitigates rumen epithelial damage in vivo by inhibiting rumen epithelial cell apoptosis on a high concentrate diet. Vet. Res. Commun. 2022, 46, 699–709. [Google Scholar] [CrossRef]
- Yang, T.; Lu, Z.; Song, H.; Chen, Y.; Jiang, M.; Zhan, K.; Zhao, G. Knockout of hexokinase 2 regulates mitochondrial dysfunction and activates the NLRP3 signal pathway in the rumen epithelial cells of dairy cows. Int. J. Biol. Macromol. 2025, 289, 138831. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, H.; Xie, W.; Meng, M.; Wang, Y.; Ma, N.; Chang, G.; Shen, X. Subacute ruminal acidosis induces pyroptosis via the mitophagy-mediated NLRP3 inflammasome activation in the livers of dairy cows fed a high-grain diet. J. Dairy Sci. 2024, 107, 4092–4107. [Google Scholar] [CrossRef]
- Bauman, D.E.; Brown, R.E.; Davis, C.L. Pathways of fatty acid synthesis and reducing equivalent generation in mammary gland of rat, sow, and cow. Arch. Biochem. Biophys. 1970, 140, 237–244. [Google Scholar] [CrossRef]
- Urrutia, N.L.; Harvatine, K.J. Acetate Dose-Dependently Stimulates Milk Fat Synthesis in Lactating Dairy Cows. J. Nutr. 2017, 147, 763–769. [Google Scholar] [CrossRef]
- Zhang, T.; Mu, Y.; Zhang, R.; Xue, Y.; Guo, C.; Qi, W.; Zhang, J.; Mao, S. Responsive changes of rumen microbiome and metabolome in dairy cows with different susceptibility to subacute ruminal acidosis. Anim. Nutr. 2022, 8, 331–340. [Google Scholar] [CrossRef]
- Abdul-Razzaq, H.A.; Bickerstaffe, R. The influence of rumen volatile fatty acids on protein metabolism in growing lambs. Br. J. Nutr. 1989, 62, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Kajikawa, H.; Mitsumori, M.; Ohmomo, S. Stimulatory and Inhibitory Effects of Protein Amino Acids on Growth Rate and Efficiency of Mixed Ruminal Bacteria. J. Dairy Sci. 2002, 85, 2015–2022. [Google Scholar] [CrossRef] [PubMed]
- Aschenbach, J.R.; Gäbel, G. Effect and absorption of histamine in sheep rumen: Significance of acidotic epithelial damage. J. Anim. Sci. 2000, 78, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yuan, X.; Chen, L.; Wang, T.; Wang, Z.; Sun, G.; Li, X.; Li, X.; Liu, G. Histamine Induces Bovine Rumen Epithelial Cell Inflammatory Response via NF-κB Pathway. Cell Physiol. Biochem. 2017, 42, 1109–1119. [Google Scholar] [CrossRef]
- Mu, Y.Y.; Qi, W.P.; Zhang, T.; Zhang, J.Y.; Mao, S.Y. Gene function adjustment for carbohydrate metabolism and enrichment of rumen microbiota with antibiotic resistance genes during subacute rumen acidosis induced by a high-grain diet in lactating dairy cows. J. Dairy Sci. 2021, 104, 2087–2105. [Google Scholar] [CrossRef]
- Wang, D.; Chen, L.; Tang, G.; Yu, J.; Chen, J.; Li, Z.; Cao, Y.; Lei, X.; Deng, L.; Wu, S.; et al. Multi-omics revealed the long-term effect of ruminal keystone bacteria and the microbial metabolome on lactation performance in adult dairy goats. Microbiome 2023, 11, 215. [Google Scholar] [CrossRef]
- Yu, J.; Li, C.; Li, X.; Liu, K.; Liu, Z.; Ni, W.; Zhou, P.; Wang, L.; Hu, S. Isolation and functional analysis of acid-producing bacteria from bovine rumen. PeerJ 2023, 11, e16294. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, G.; Li, X.; Guan, Y.; Wang, Y.; Yuan, X.; Sun, G.; Wang, Z.; Li, X. Inflammatory mechanism of Rumenitis in dairy cows with subacute ruminal acidosis. BMC Vet. Res. 2018, 14, 135. [Google Scholar] [CrossRef]
- Shi, J.J.; Zhao, Y.; Wang, Y.P.; Gao, W.Q.; Ding, J.J.; Li, P.; Hu, L.Y.; Shao, F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 2014, 514, 187. [Google Scholar] [CrossRef]
- Rodríguez-Lecompte, J.C.; Kroeker, A.D.; Ceballos-Márquez, A.; Li, S.; Plaizier, J.C.; Gomez, D.E. Evaluation of the systemic innate immune response and metabolic alterations of nonlactating cows with diet-induced subacute ruminal acidosis. J. Dairy Sci. 2014, 97, 7777–7787. [Google Scholar] [CrossRef]
- Stefanska, B.; Człapa, W.; Pruszynska-Oszmałek, E.; Szczepankiewicz, D.; Fievez, V.; Komisarek, J.; Stajek, K.; Nowak, W. Subacute ruminal acidosis affects fermentation and endotoxin concentration in the rumen and relative expression of the CD14/TLR4/MD2 genes involved in lipopolysaccharide systemic immune response in dairy cows. J. Dairy Sci. 2018, 101, 1297–1310. [Google Scholar] [CrossRef] [PubMed]
- Kent-Dennis, C.; Aschenbach, J.R.; Griebel, P.J.; Penner, G.B. Effects of lipopolysaccharide exposure in primary bovine ruminal epithelial cells. J. Dairy Sci. 2020, 103, 9587–9603. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Wang, K.; Huang, Y.; Zhang, X.; Yang, T.; Zhan, K.; Zhao, G. Quercetin Alleviates Lipopolysaccharide-Induced Cell Oxidative Stress and Inflammatory Responses via Regulation of the TLR4-NF-κB Signaling Pathway in Bovine Rumen Epithelial Cells. Toxins 2023, 15, 512. [Google Scholar] [CrossRef]
- Diamond, C.E.; Khameneh, H.J.; Brough, D.; Mortellaro, A. Novel perspectives on non-canonical inflammasome activation. Immunotargets Ther. 2015, 4, 131–141. [Google Scholar] [CrossRef]
- Lee, A.J.; Ashkar, A.A. The Dual Nature of Type I and Type II Interferons. Front. Immunol. 2018, 9, 2061. [Google Scholar] [CrossRef]
- Bae, H.R.; Leung, P.S.C.; Hodge, D.L.; Fenimore, J.M.; Jeon, S.M.; Thovarai, V.; Dzutsev, A.; Welcher, A.A.; Boedigheimer, M.; Damore, M.A.; et al. Multi-omics: Differential expression of IFN-γ results in distinctive mechanistic features linking chronic inflammation, gut dysbiosis, and autoimmune diseases. J. Autoimmun. 2020, 111, 102436. [Google Scholar] [CrossRef]
- Pu, J.; Chen, D.; Tian, G.; He, J.; Zheng, P.; Mao, X.; Yu, J.; Huang, Z.; Zhu, L.; Luo, J.; et al. Protective Effects of Benzoic Acid, Bacillus Coagulans, and Oregano Oil on Intestinal Injury Caused by Enterotoxigenic Escherichia coli in Weaned Piglets. Biomed. Res. Int. 2018, 2018, 1829632. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, Y.; Zhao, X.; Liu, C.; Wang, B.; Zhou, J. Mechanistic basis and preliminary practice of butyric acid and butyrate sodium to mitigate gut inflammatory diseases: A comprehensive review. Nutr. Res. 2021, 95, 1–18. [Google Scholar] [CrossRef]
- Peiró, C.; Romacho, T.; Azcutia, V.; Villalobos, L.; Fernández, E.; Bolaños, J.P.; Moncada, S.; Sánchez-Ferrer, C.F. Inflammation, glucose, and vascular cell damage: The role of the pentose phosphate pathway. Cardiovasc. Diabetol. 2016, 15, 82. [Google Scholar] [CrossRef]
- Lu, Z.; Shen, H.; Shen, Z. High-Concentrate Diet-Induced Change of Cellular Metabolism Leads to Decreases of Immunity and Imbalance of Cellular Activities in Rumen Epithelium. Cell. Physiol. Biochem. 2018, 45, 2145–2157. [Google Scholar] [CrossRef]
- Zhang, F.; Su, Q.; Gao, Z.; Wu, Z.; Ji, Q.; He, T.; Zhu, K.; Chen, X.; Zhang, Y.; Hou, S.; et al. Impact of Lysine to Methionine Ratios on Antioxidant Capacity and Immune Function in the Rumen of Tibetan Sheep: An RNA-Seq Analysis. Vet. Med. Sci. 2025, 11, e70173. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Zhang, H.; Wang, Y.; Ma, N.; Chandra, R.A.; Ye, G.; Zhuang, S.; Zhu, W.; Shen, X. Microbial community shifts elicit inflammation in the caecal mucosa via the GPR41/43 signalling pathway during subacute ruminal acidosis. BMC Vet. Res. 2019, 15, 298. [Google Scholar] [CrossRef] [PubMed]
- Zhan, K.; Gong, X.; Chen, Y.; Jiang, M.; Yang, T.; Zhao, G. Short-Chain Fatty Acids Regulate the Immune Responses via G Protein-Coupled Receptor 41 in Bovine Rumen Epithelial Cells. Front. Immunol. 2019, 10, 2042. [Google Scholar] [CrossRef]
- Kent-Dennis, C.; Penner, G.B. Effects of a proinflammatory response on metabolic function of cultured, primary ruminal epithelial cells. J. Dairy Sci. 2021, 104, 1002–1017. [Google Scholar] [CrossRef]
- Yen, W.; Wu, Y.; Wu, C.; Lin, H.; Stern, A.; Chen, S.; Shu, J.; Chiu, D.T. Impaired inflammasome activation and bacterial clearance in G6PD deficiency due to defective NOX/p38 MAPK/AP-1 redox signaling. Redox Biol. 2020, 28, 101363. [Google Scholar] [CrossRef]
- Alarcon, P.; Manosalva, C.; Conejeros, I.; Carretta, M.D.; Munoz-Caro, T.; Silva, L.M.R.; Taubert, A.; Hermosilla, C.; Hidalgo, M.A.; Burgos, R.A. D(−) Lactic Acid-Induced Adhesion of Bovine Neutrophils onto Endothelial Cells Is Dependent on Neutrophils Extracellular Traps Formation and CD11b Expression. Front. Immunol. 2017, 8, 975. [Google Scholar] [CrossRef]
- Manosalva, C.; Quiroga, J.; Teuber, S.; Cárdenas, S.; Carretta, M.D.; Morán, G.; Alarcón, P.; Hidalgo, M.A.; Burgos, R.A. D-Lactate Increases Cytokine Production in Bovine Fibroblast-Like Synoviocytes via MCT1 Uptake and the MAPK, PI3K/Akt, and NFκB Pathways. Animals 2020, 10, 2105. [Google Scholar] [CrossRef]
- Pucino, V.; Certo, M.; Bulusu, V.; Cucchi, D.; Goldmann, K.; Pontarini, E.; Haas, R.; Smith, J.; Headland, S.E.; Blighe, K.; et al. Lactate Buildup at the Site of Chronic Inflammation Promotes Disease by Inducing CD4+ T Cell Metabolic Rewiring. Cell Metab. 2019, 30, 1055. [Google Scholar] [CrossRef]
- Quiroga, J.; Alarcon, P.; Manosalva, C.; Teuber, S.; Carretta, M.D.; Burgos, R.A. D-lactate-triggered extracellular trap formation in cattle polymorphonuclear leucocytes is glucose metabolism dependent. Dev. Comp. Immunol. 2022, 135, 104492. [Google Scholar] [CrossRef]
- Quiroga, J.; Alarcon, P.; Ramirez, M.F.; Manosalva, C.; Teuber, S.; Carretta, M.D.; Burgos, R.A. D-lactate-induced ETosis in cattle polymorphonuclear leucocytes is dependent on the release of mitochondrial reactive oxygen species and the PI3K/Akt/HIF-1 and GSK-38 pathways. Dev. Comp. Immunol. 2023, 145, 104728. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, X.; Yang, B.; Mi, Y.; Wang, J. 3D sheep rumen epithelial structures driven from single cells in vitro. Vet. Res. 2023, 54, 104. [Google Scholar] [CrossRef] [PubMed]
- Beng, L.T.; Racine, V.; Beghin, A.; Guerin-Bonne, I. Organoids: Unveiling Insights with Volume Electron Microscopy. BIO Web Conf. 2024, 129, 12004. [Google Scholar] [CrossRef]
- Heinzelmann, E.; Piraino, F.; Costa, M.; Roch, A.; Norkin, M.; Garnier, V.; Homicsko, K.; Brandenberg, N. iPSC-derived and Patient-Derived Organoids: Applications and challenges in scalability and reproducibility as pre-clinical models. Curr. Res. Toxicol. 2024, 7, 100197. [Google Scholar] [CrossRef]
- Chang, L.; Li, L.; Han, Y.; Cheng, H.; Yang, L. Organoids in Haematologic Research: Advances and Future Directions. Cell Prolif. 2025. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Xia, Z.; Fu, J.; Yang, Y. Role of the Rumen Epithelium and Associated Changes Under High-Concentrate Diets. Int. J. Mol. Sci. 2025, 26, 2573. https://doi.org/10.3390/ijms26062573
Zhang L, Xia Z, Fu J, Yang Y. Role of the Rumen Epithelium and Associated Changes Under High-Concentrate Diets. International Journal of Molecular Sciences. 2025; 26(6):2573. https://doi.org/10.3390/ijms26062573
Chicago/Turabian StyleZhang, Ling, Zhenhua Xia, Jicheng Fu, and You Yang. 2025. "Role of the Rumen Epithelium and Associated Changes Under High-Concentrate Diets" International Journal of Molecular Sciences 26, no. 6: 2573. https://doi.org/10.3390/ijms26062573
APA StyleZhang, L., Xia, Z., Fu, J., & Yang, Y. (2025). Role of the Rumen Epithelium and Associated Changes Under High-Concentrate Diets. International Journal of Molecular Sciences, 26(6), 2573. https://doi.org/10.3390/ijms26062573





