Lanatoside C Inhibits Proliferation and Induces Apoptosis in Human Prostate Cancer Cells Through the TNF/IL-17 Signaling Pathway
Abstract
1. Introduction
2. Results
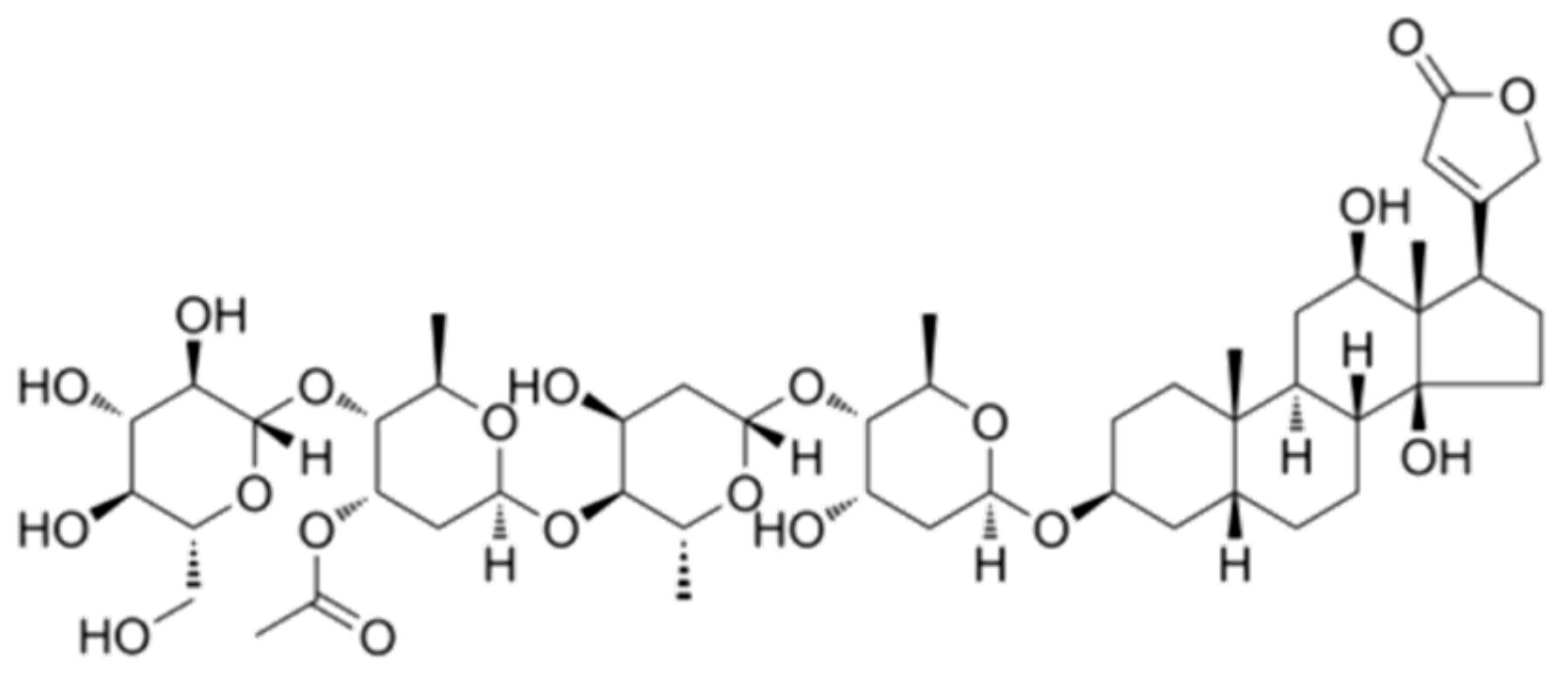
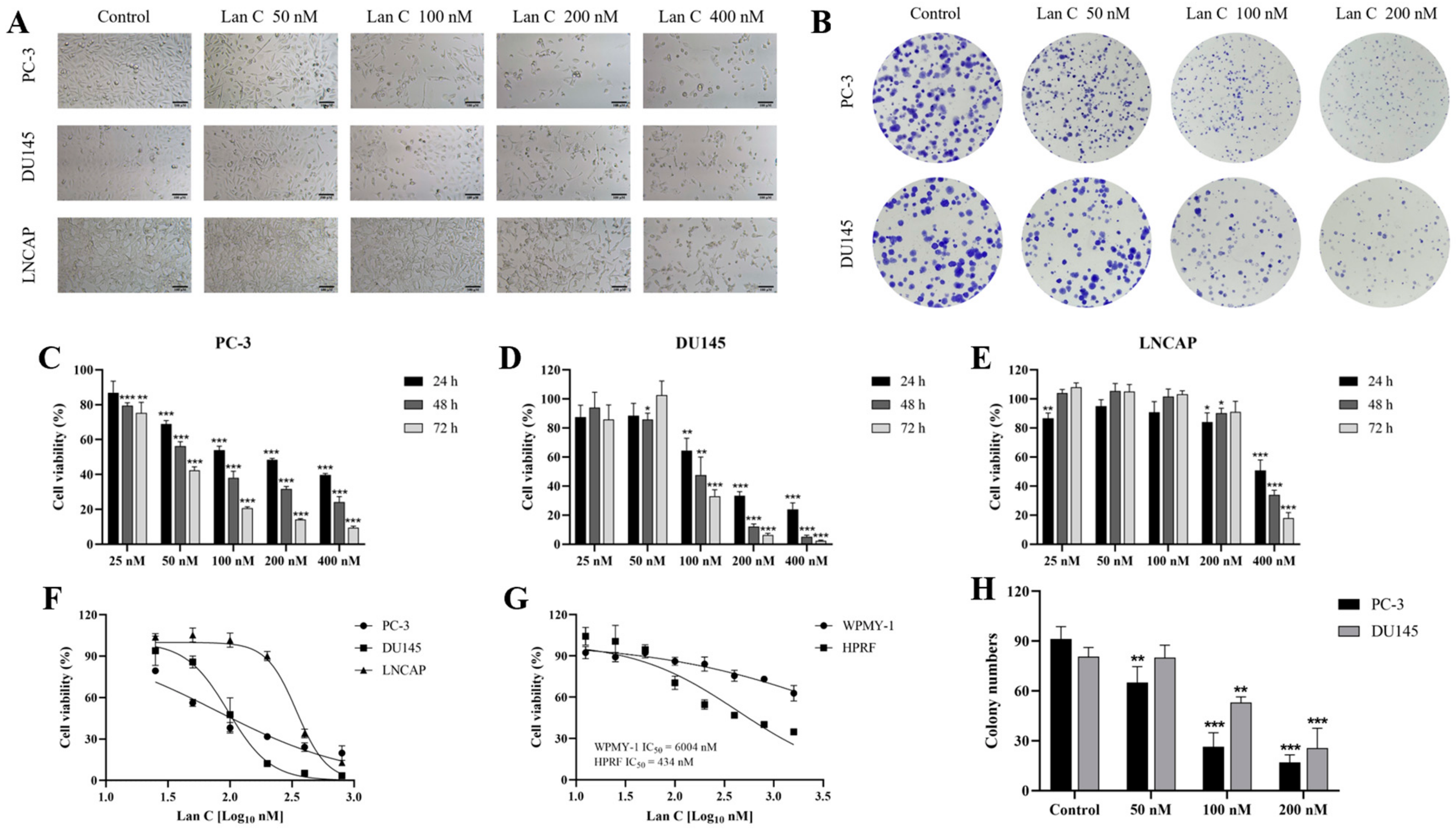
2.1. Effect of Lan C on the Morphology of Prostate Cancer Cells
2.2. Lan C Inhibits the Growth of Prostate Cancer Cells
2.3. Effect of Lan C on the Growth of Normal Prostate Cells
2.4. Lan C Inhibits Colony Formation in Prostate Cancer Cells
2.5. Lan C Promotes Apoptosis in Prostate Cancer Cells
2.6. Lan C Induces Cell Cycle Arrest in Prostate Cancer Cells
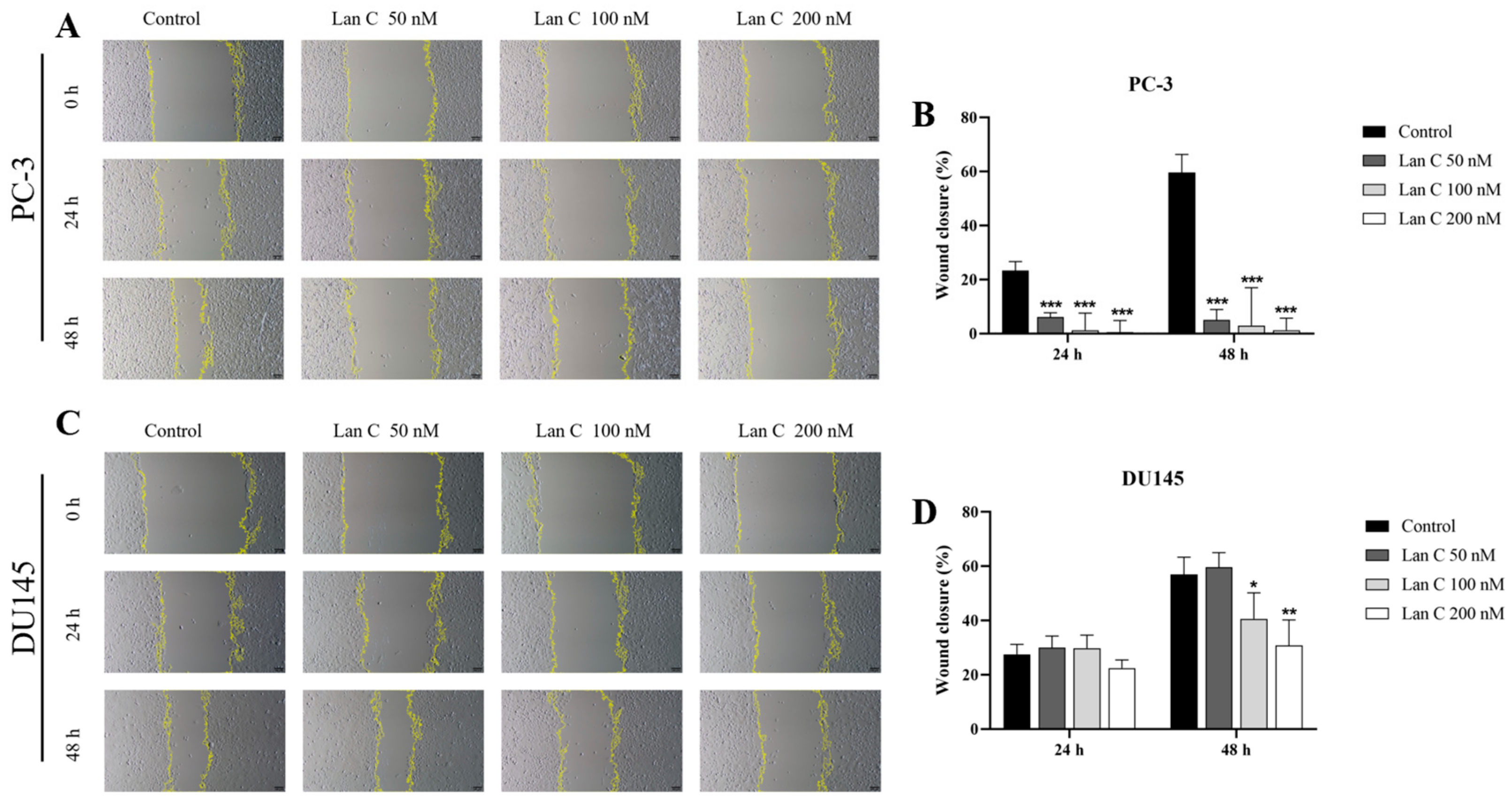
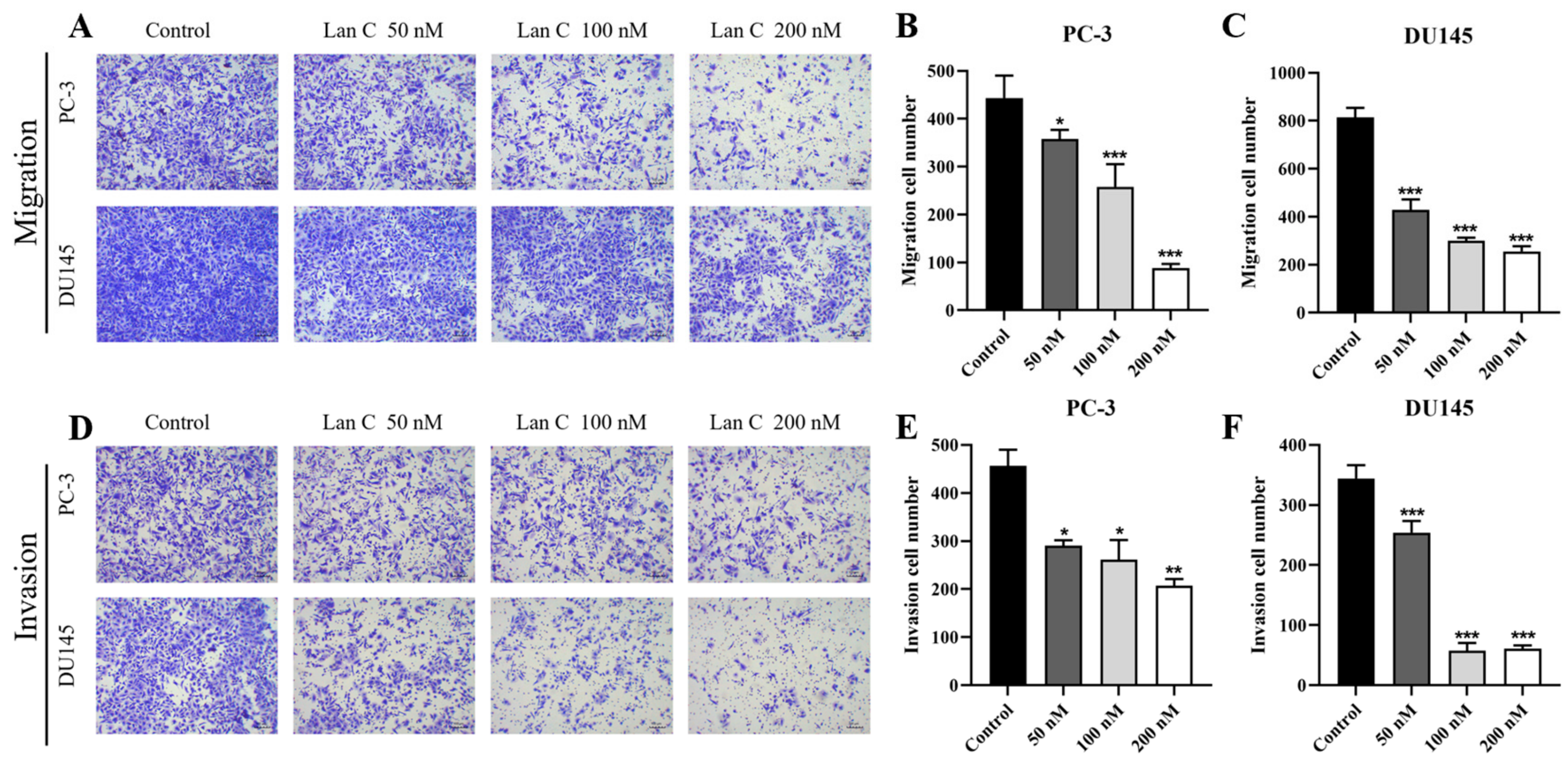
2.7. Lan C Inhibits the Migration Ability of Prostate Cancer Cells
2.8. Lan C Inhibits Prostate Cancer Cell Invasion
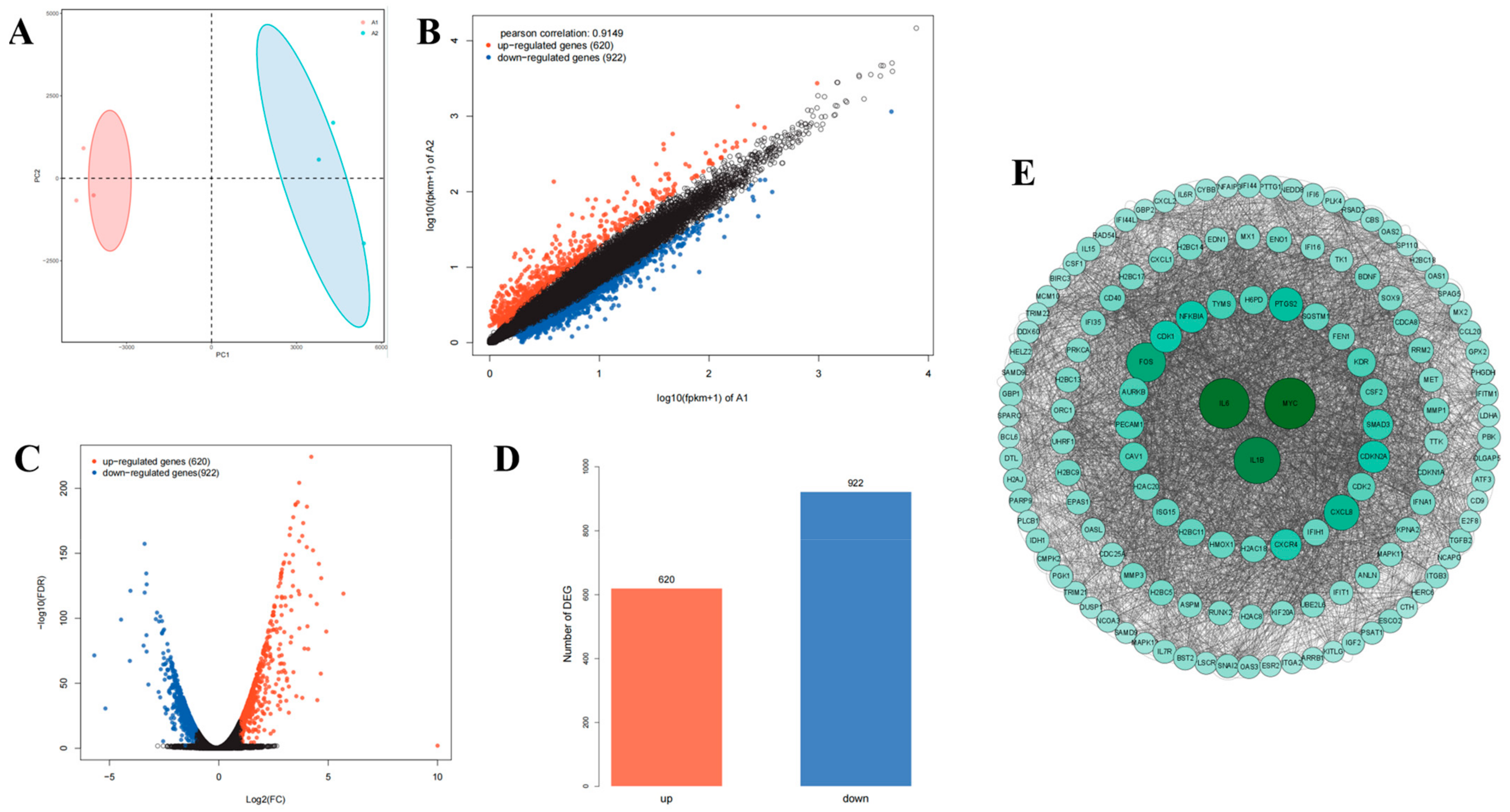
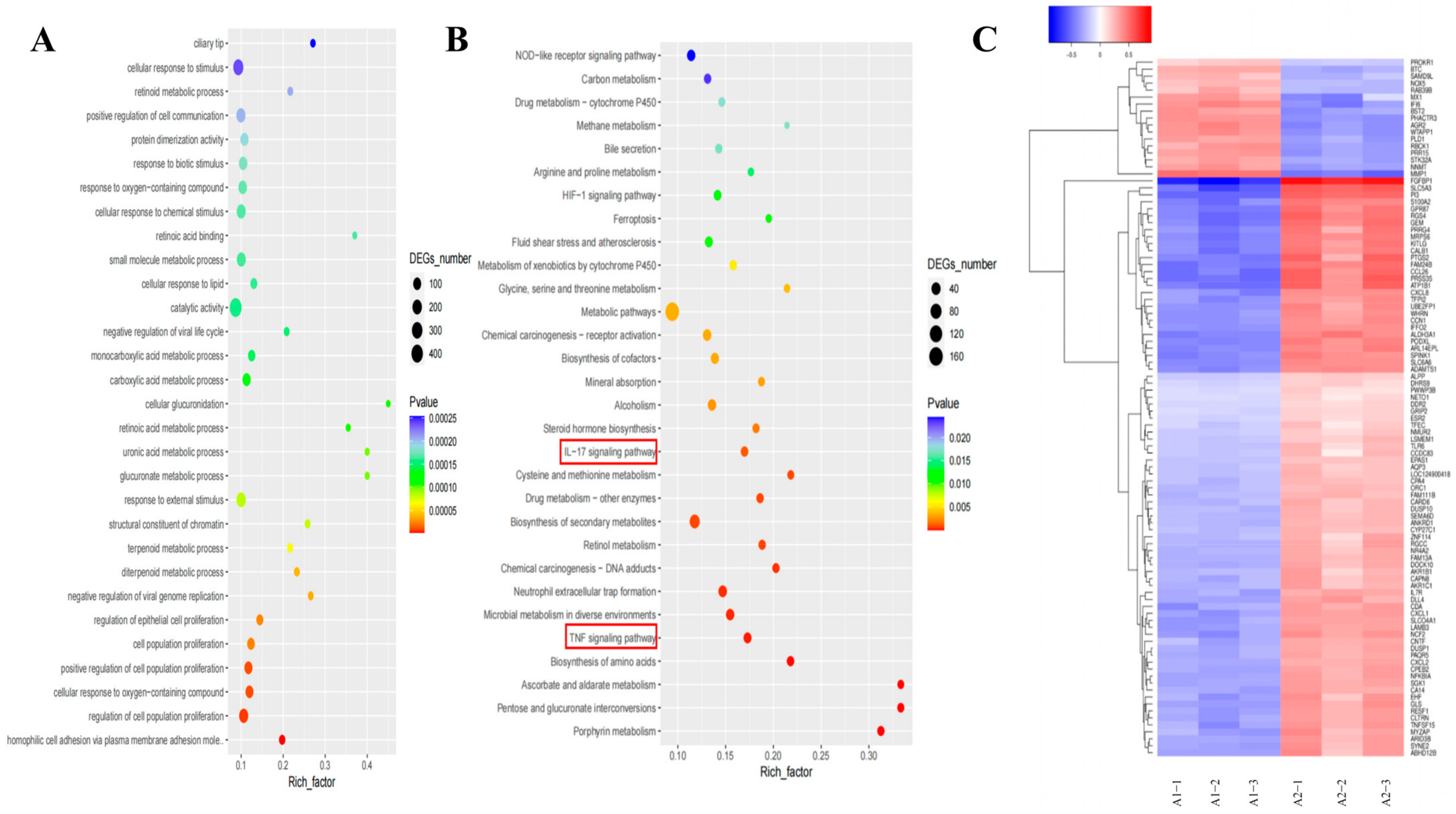
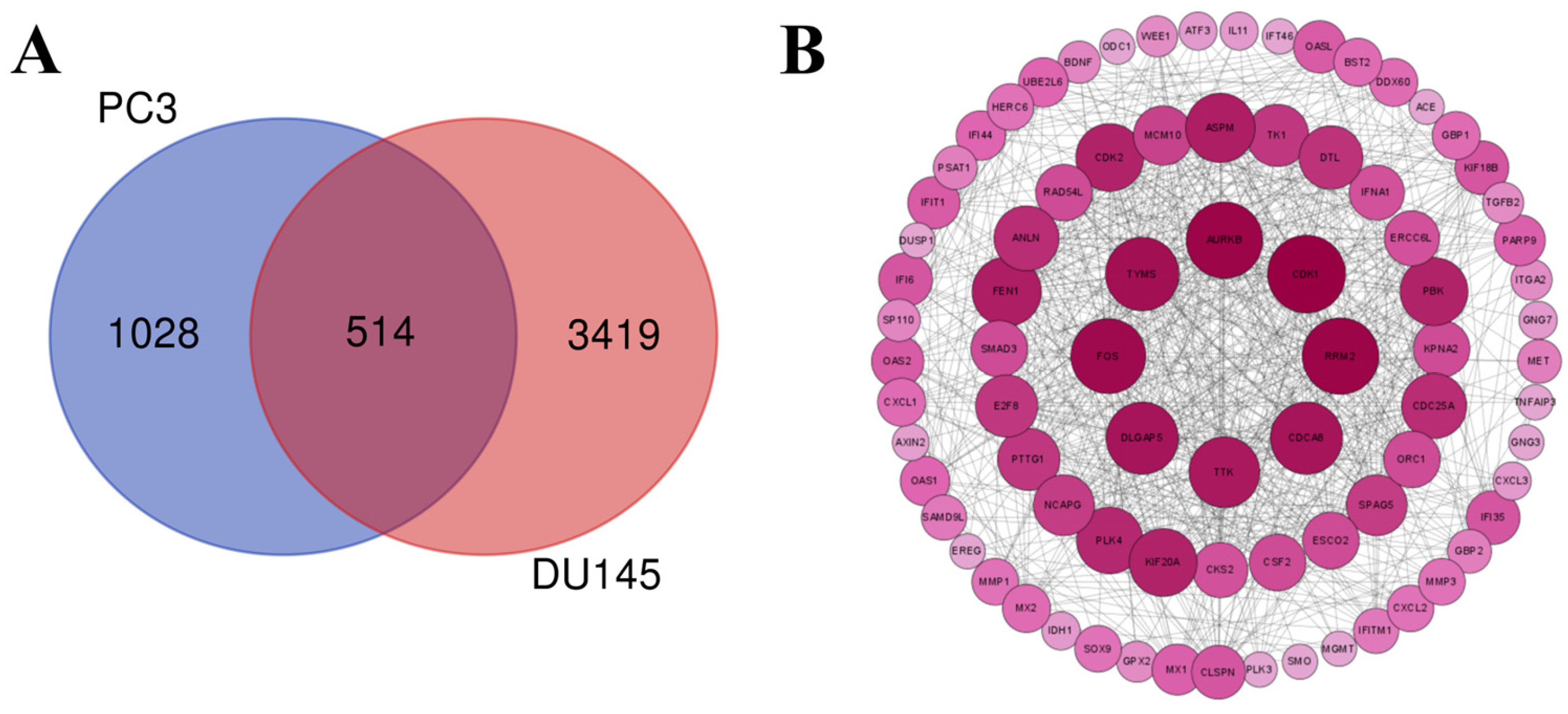
2.9. Transcriptomic Analysis Reveals That Lan C Modulates the TNF/IL-17 Signaling Pathway in PC-3 Cells
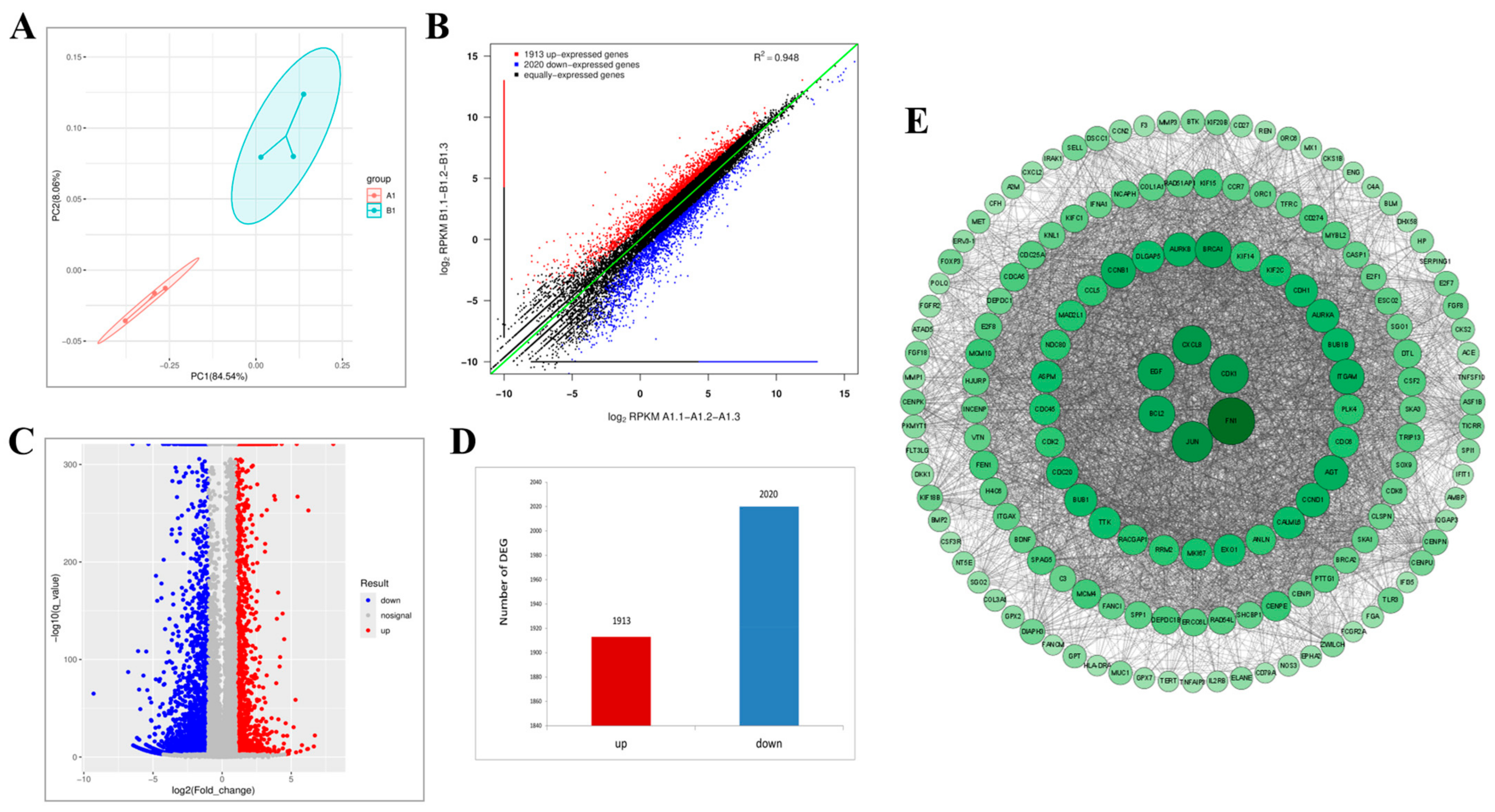
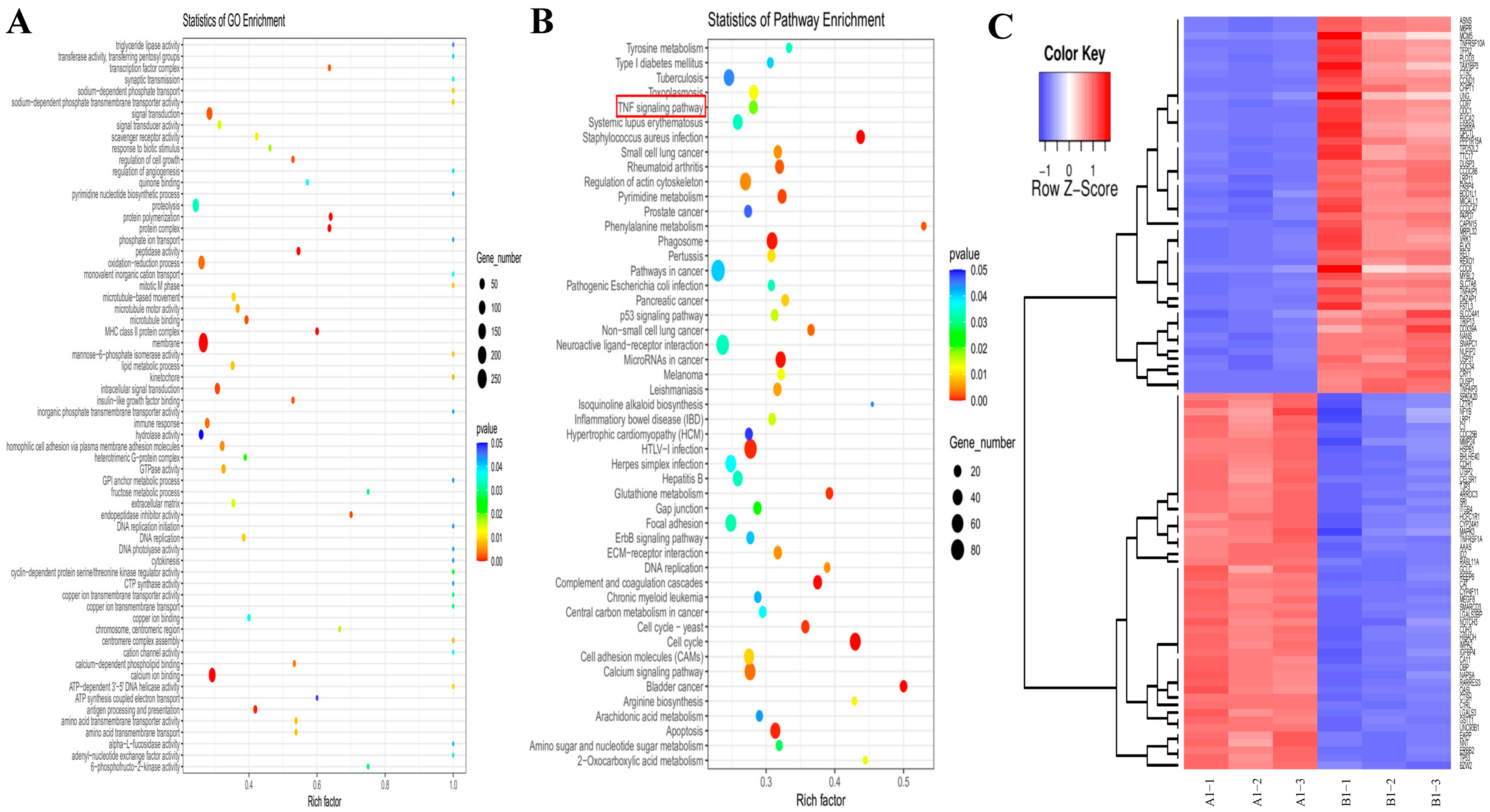
2.10. Transcriptomic Analysis Reveals That Lan C Modulates the TNF/IL-17 Signaling Pathway in DU145 Cells
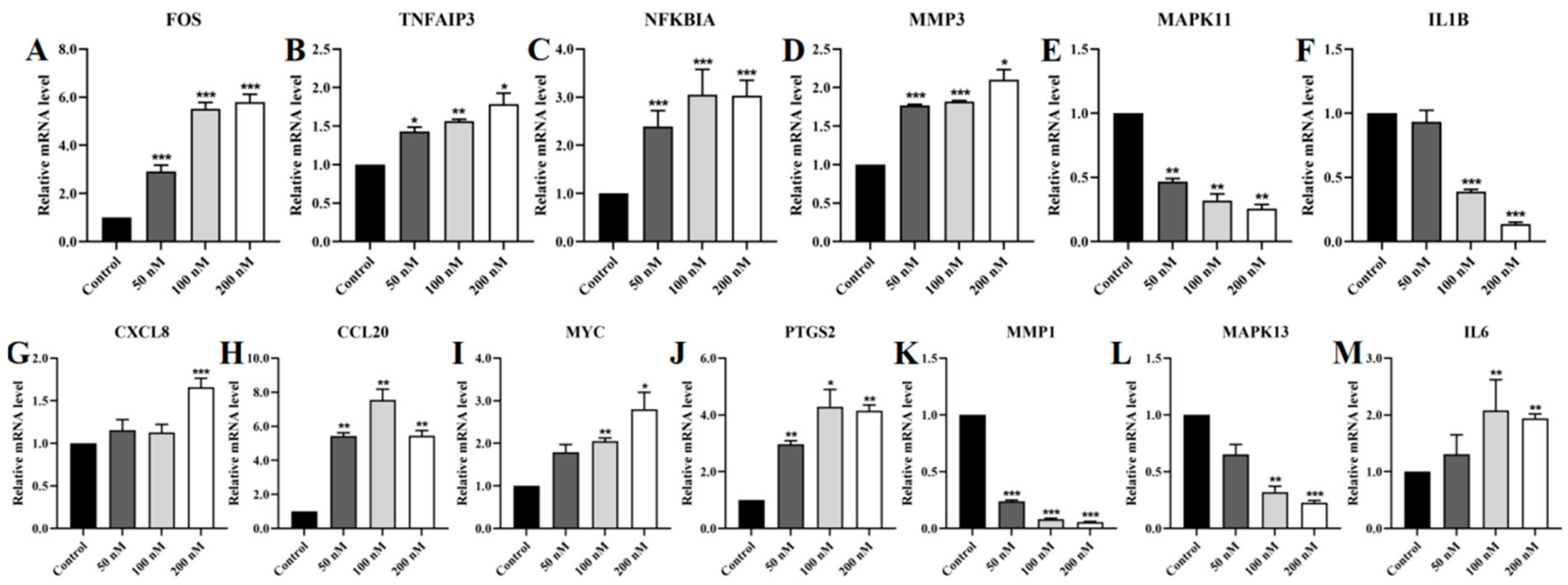
2.11. Regulation of TNF/IL-17 Signaling Pathway Gene Expression by Lan C in PC-3 Cells
2.12. Regulation of TNF/IL-17 Signaling Pathway Gene Expression by Lan C in DU145 Cells
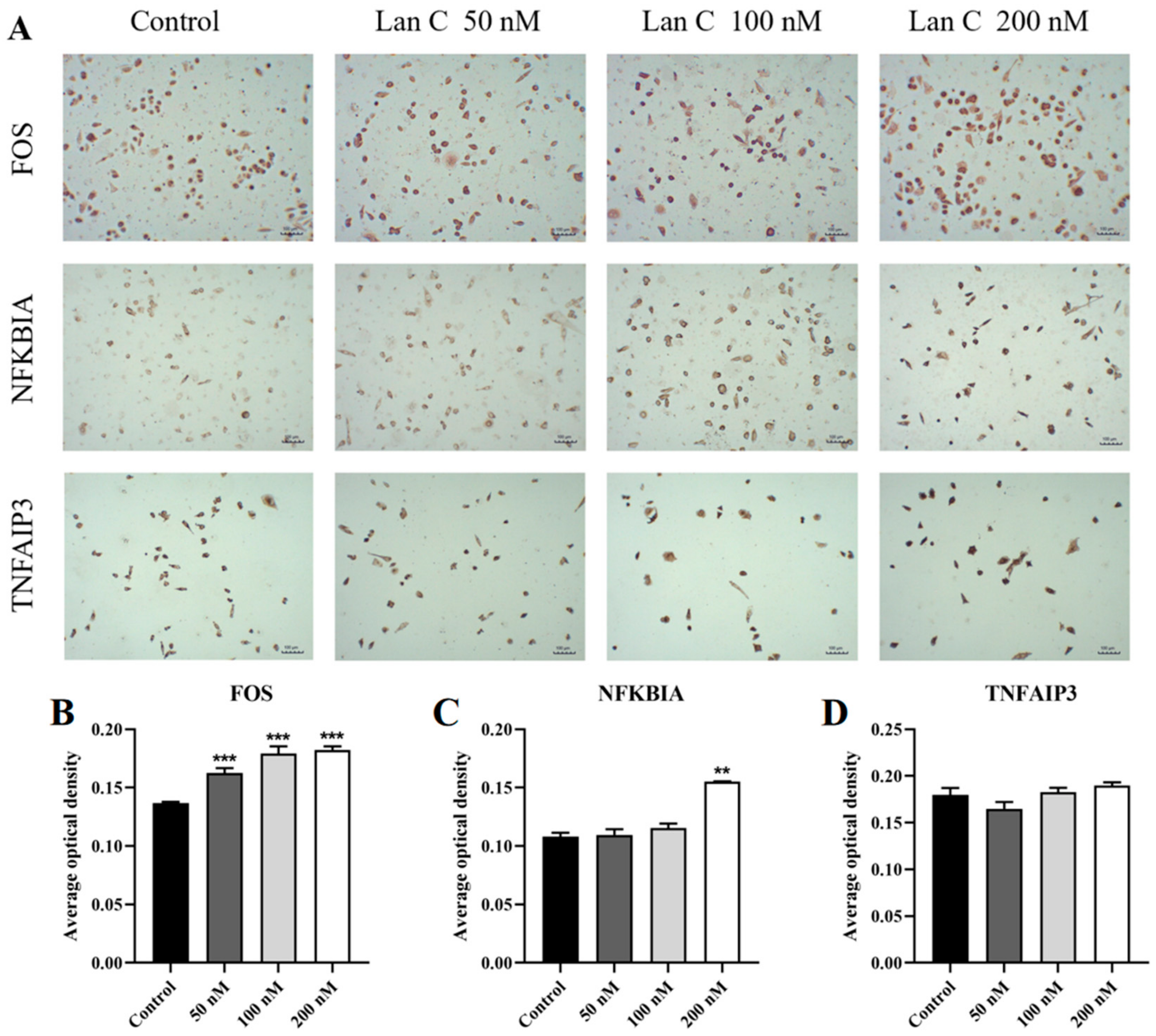
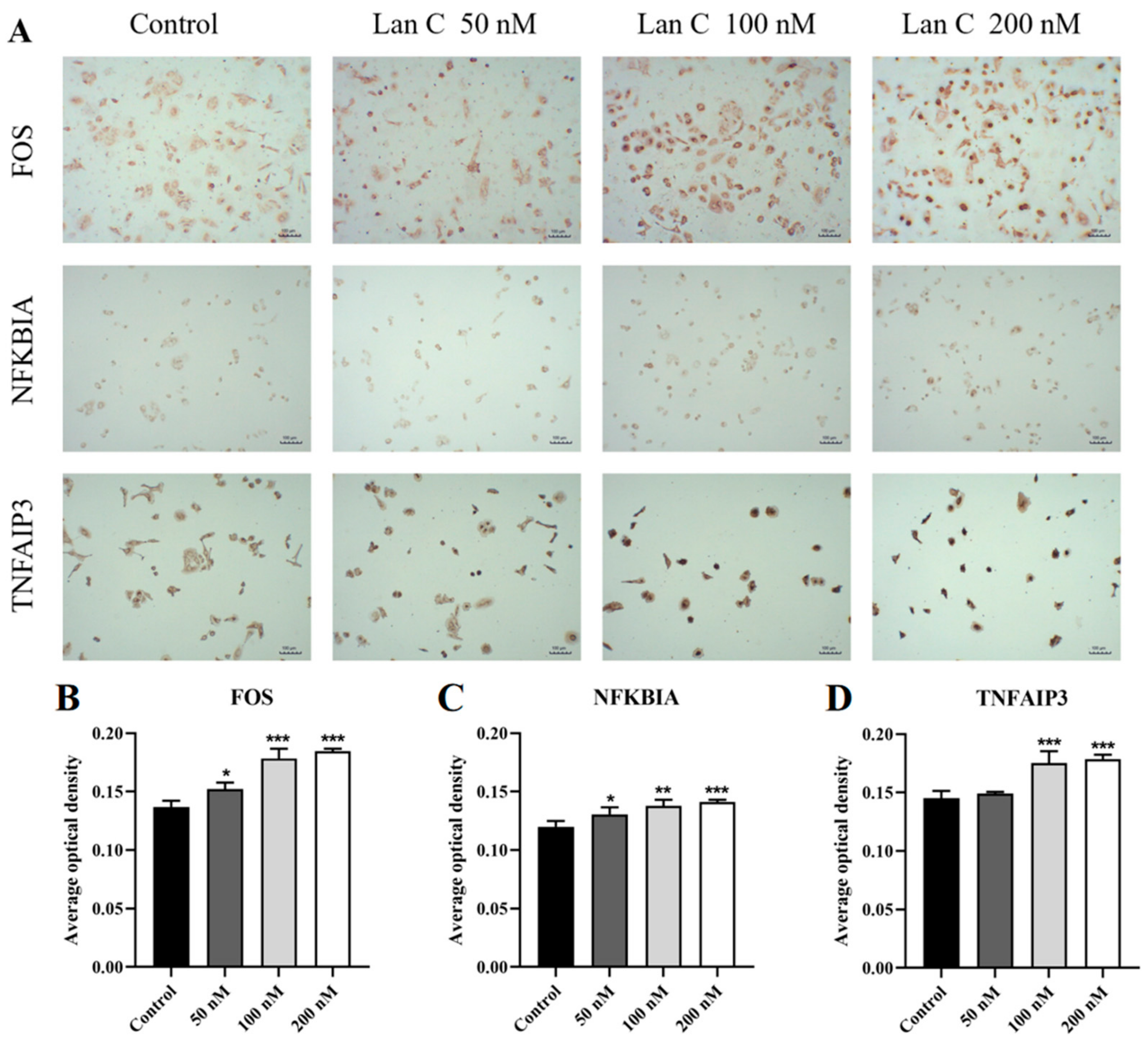
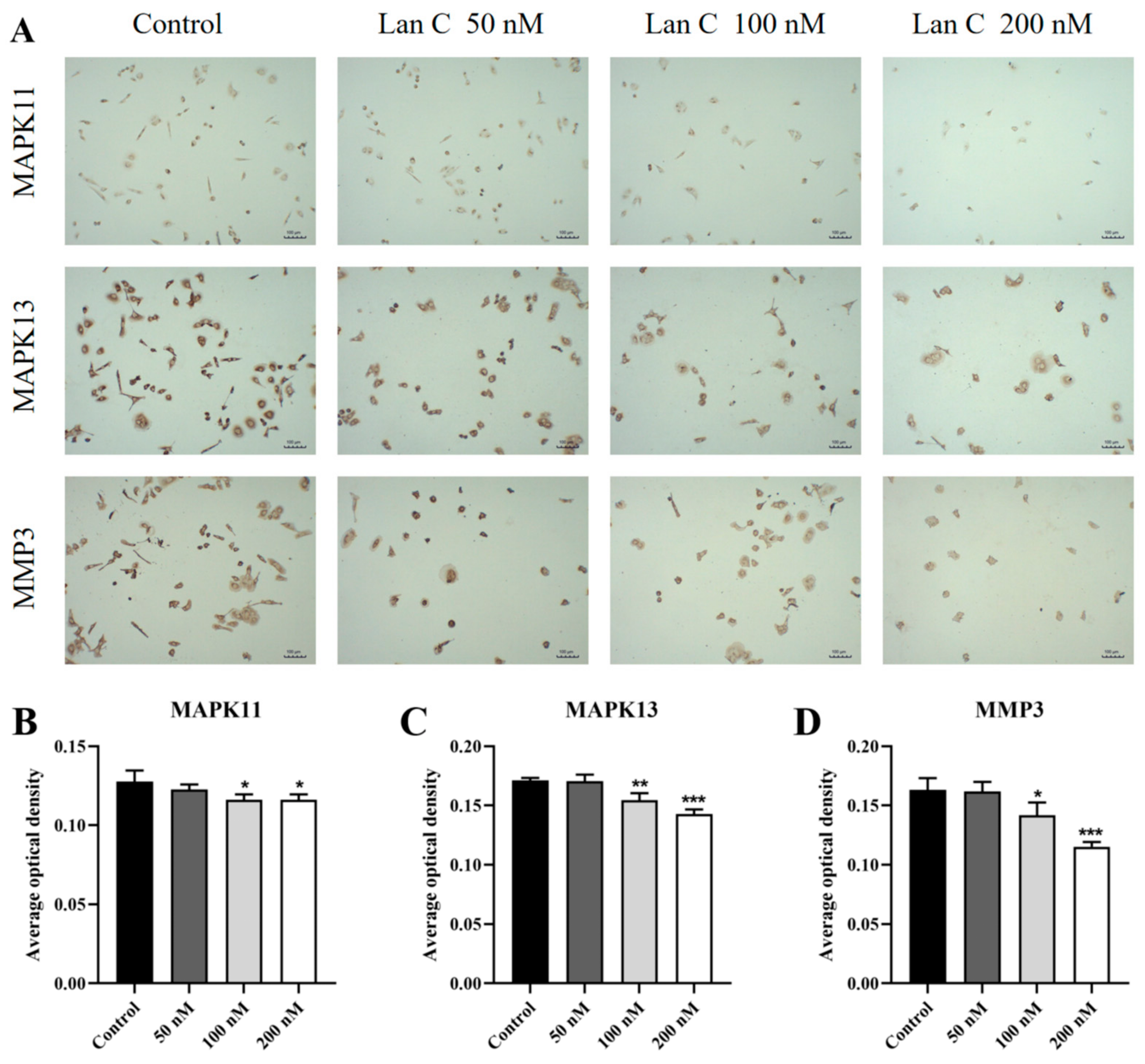
2.13. Lan C Upregulates the Expression of FOS and NFKBIA Proteins and Downregulates the Expression of MAPK11, MAPK13, and MMP3 Proteins in PC-3 Cells
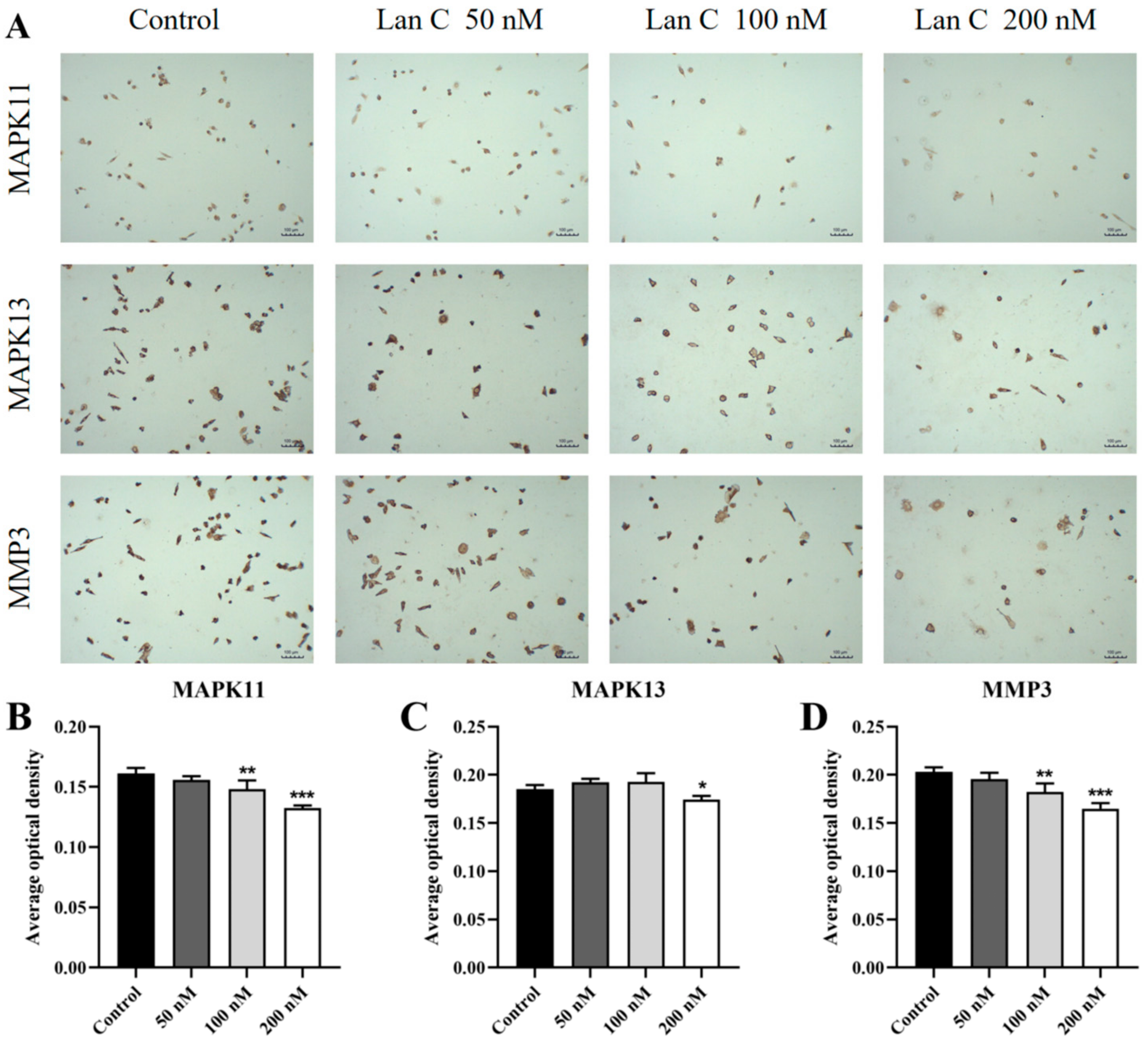
2.14. Lan C Modulates the Expression of FOS, NFKBIA, and TNFAIP3 Proteins and Downregulates MAPK11, MAPK13, and MMP3 Proteins in DU145 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Cell Culture
4.3. Cellular Morphological Observation
4.4. Cell Viability Assay
4.5. Colony Formation Assay
4.6. AnnexinV-FITC/PI Double Staining for Apoptosis Detection
4.7. Cell Cycle Analysis by PI Staining
4.8. Cell Migration and Invasion Assays
4.9. Transcriptome Sequencing
4.9.1. Total RNA Extraction and Quality Control
4.9.2. mRNA Purification
4.9.3. cDNA Library Construction
4.9.4. Library Quality Control
4.9.5. Sequencing Workflow
4.10. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
4.11. Immunocytochemistry
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Endzelins, E.; Melne, V.; Kalnina, Z.; Lietuvietis, V.; Riekstina, U.; Llorente, A.; Line, A. Diagnostic, prognostic and predictive value of cell-free miRNAs in prostate cancer: A systematic review. Mol. Cancer 2016, 15, 41. [Google Scholar] [CrossRef] [PubMed]
- Desai, K.; McManus, J.M.; Sharifi, N. Hormonal Therapy for Prostate Cancer. Endocr. Rev. 2021, 42, 354–373. [Google Scholar] [CrossRef] [PubMed]
- Teo, M.Y.; Rathkopf, D.E.; Kantoff, P. Treatment of Advanced Prostate Cancer. Annu. Rev. Med. 2019, 70, 479–499. [Google Scholar] [CrossRef] [PubMed]
- Alabi, B.R.; Liu, S.; Stoyanova, T. Current and emerging therapies for neuroendocrine prostate cancer. Pharmacol. Ther. 2022, 238, 108255. [Google Scholar] [CrossRef]
- Ge, R.; Wang, Z.; Montironi, R.; Jiang, Z.; Cheng, M.; Santoni, M.; Huang, K.; Massari, F.; Lu, X.; Cimadamore, A.; et al. Epigenetic modulations and lineage plasticity in advanced prostate cancer. Ann. Oncol. 2020, 31, 470–479. [Google Scholar] [CrossRef]
- Botelho, A.F.M.; Pierezan, F.; Soto-Blanco, B.; Melo, M.M. A review of cardiac glycosides: Structure, toxicokinetics, clinical signs, diagnosis and antineoplastic potential. Toxicon 2019, 158, 63–68. [Google Scholar] [CrossRef]
- Calderon-Montano, J.M.; Burgos-Moron, E.; Orta, M.L.; Maldonado-Navas, D.; Garcia-Dominguez, I.; Lopez-Lazaro, M. Evaluating the cancer therapeutic potential of cardiac glycosides. Biomed. Res. Int. 2014, 2014, 794930. [Google Scholar] [CrossRef]
- Ren, J.; Gao, X.; Guo, X.; Wang, N.; Wang, X. Research Progress in Pharmacological Activities and Applications of Cardiotonic Steroids. Front. Pharmacol. 2022, 13, 902459. [Google Scholar] [CrossRef]
- Skubnik, J.; Pavlickova, V.; Rimpelova, S. Cardiac Glycosides as Immune System Modulators. Biomolecules 2021, 11, 659. [Google Scholar] [CrossRef]
- Jansson, D.; Dieriks, V.B.; Rustenhoven, J.; Smyth, L.C.D.; Scotter, E.; Aalderink, M.; Feng, S.; Johnson, R.; Schweder, P.; Mee, E.; et al. Cardiac glycosides target barrier inflammation of the vasculature, meninges and choroid plexus. Commun. Biol. 2021, 4, 260. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Khalifa, S.A.M.; Taher, E.A.; Farag, M.A.; Saeed, A.; Gamal, M.; Hegazy, M.F.; Youssef, D.; Musharraf, S.G.; Alajlani, M.M.; et al. Cardenolides: Insights from chemical structure and pharmacological utility. Pharmacol. Res. 2019, 141, 123–175. [Google Scholar] [CrossRef] [PubMed]
- Ainembabazi, D.; Zhang, Y.; Turchi, J.J. The mechanistic role of cardiac glycosides in DNA damage response and repair signaling. Cell. Mol. Life Sci. 2023, 80, 250. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Wang, S.; Lin, X.; Yang, S.; Ba, X.; Xiong, D.; Xiao, L.; Li, R. Lanatoside C inhibits herpes simplex virus 1 replication by regulating NRF2 distribution within cells. Phytomedicine 2024, 124, 155308. [Google Scholar] [CrossRef] [PubMed]
- Nie, Y.; Zhang, D.; Jin, Z.; Li, B.; Wang, X.; Che, H.; You, Y.; Qian, X.; Zhang, Y.; Zhao, P.; et al. Lanatoside C protects mice against bleomycin-induced pulmonary fibrosis through suppression of fibroblast proliferation and differentiation. Clin. Exp. Pharmacol. Physiol. 2019, 46, 575–586. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, H.Y.; Gao, L.; Bai, M.Z.; Fu, W.K.; Huang, C.F.; Mi, N.N.; Ma, H.D.; Lu, Y.W.; Jiang, N.Z.; et al. Lanatoside C decelerates proliferation and induces apoptosis through inhibition of STAT3 and ROS-mediated mitochondrial membrane potential transformation in cholangiocarcinoma. Front. Pharmacol. 2023, 14, 1098915. [Google Scholar] [CrossRef]
- Ha, D.P.; Tsai, Y.L.; Lee, A.S. Suppression of ER-stress induction of GRP78 as an anti-neoplastic mechanism of the cardiac glycoside Lanatoside C in pancreatic cancer: Lanatoside C suppresses GRP78 stress induction. Neoplasia 2021, 23, 1213–1226. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, T.; Deng, S.; Li, L.; Li, J.; Zhang, F.; He, S.; Yuan, W.; Wu, D.; Xu, Y. Lanatoside C induces ferroptosis in non-small cell lung cancer in vivo and in vitro by regulating SLC7A11/GPX4 signaling pathway. Transl. Cancer Res. 2024, 13, 2295–2307. [Google Scholar] [CrossRef]
- Duan, Y.; Chen, L.; Shao, J.; Jiang, C.; Zhao, Y.; Li, Y.; Ke, H.; Zhang, R.; Zhu, J.; Yu, M. Lanatoside C inhibits human cervical cancer cell proliferation and induces cell apoptosis by a reduction of the JAK2/STAT6/SOCS2 signaling pathway. Oncol. Lett. 2021, 22, 740. [Google Scholar] [CrossRef]
- Reddy, D.; Kumavath, R.; Ghosh, P.; Barh, D. Lanatoside C Induces G2/M Cell Cycle Arrest and Suppresses Cancer Cell Growth by Attenuating MAPK, Wnt, JAK-STAT, and PI3K/AKT/mTOR Signaling Pathways. Biomolecules 2019, 9, 792. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, K.; Wang, G.; Zhang, D.; Shi, C.; Ding, Y.; Hong, D.; Zhang, D.; He, H.; Sun, L.; et al. Lanatoside C inhibits cell proliferation and induces apoptosis through attenuating Wnt/beta-catenin/c-Myc signaling pathway in human gastric cancer cell. Biochem. Pharmacol. 2018, 150, 280–292. [Google Scholar] [CrossRef]
- Kang, M.A.; Kim, M.S.; Kim, W.; Um, J.H.; Shin, Y.J.; Song, J.Y.; Jeong, J.H. Lanatoside C suppressed colorectal cancer cell growth by inducing mitochondrial dysfunction and increased radiation sensitivity by impairing DNA damage repair. Oncotarget 2016, 7, 6074–6087. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, N.M.; Gucalp, A.; Dannenberg, A.J.; Hudis, C.A. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J. Clin. Oncol. 2016, 34, 4270–4276. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Dai, J.; Wang, J.; Feng, Q.; Wang, Y.; Han, T.; Wu, C. RNF167-mediated ubiquitination of Tollip inhibits TNF-alpha-triggered NF-kappaB and MAPK activation. FASEB J. 2023, 37, e23089. [Google Scholar] [CrossRef]
- Giampietri, C.; Petrungaro, S.; Padula, F.; D’Alessio, A.; Marini, E.S.; Facchiano, A.; Filippini, A.; Ziparo, E. Autophagy modulators sensitize prostate epithelial cancer cell lines to TNF-alpha-dependent apoptosis. Apoptosis 2012, 17, 1210–1222. [Google Scholar] [CrossRef]
- Wu, N.; Wang, Y.; Wang, K.; Zhong, B.; Liao, Y.; Liang, J.; Jiang, N. Cathepsin K regulates the tumor growth and metastasis by IL-17/CTSK/EMT axis and mediates M2 macrophage polarization in castration-resistant prostate cancer. Cell Death Dis. 2022, 13, 813. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, S.; Parajuli, K.R.; Zhang, W.; Zhang, K.; Mo, Z.; Liu, J.; Chen, Z.; Yang, S.; Wang, A.R.; et al. Interleukin-17 promotes prostate cancer via MMP7-induced epithelial-to-mesenchymal transition. Oncogene 2017, 36, 687–699. [Google Scholar] [CrossRef]
- Yan, R.; Dai, W.; Mao, Y.; Yu, G.; Li, W.; Shu, M.; Xu, B. Melittin inhibits tumor cell migration and enhances cisplatin sensitivity by suppressing IL-17 signaling pathway gene LCN2 in castration-resistant prostate cancer. Prostate 2023, 83, 1430–1445. [Google Scholar] [CrossRef]
- López-Lázaro, M. Digitoxin as an anticancer agent with selectivity for cancer cells: Possible mechanisms involved. Expert Opin. Ther. Targets 2007, 11, 1043–1053. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Zhao, Y.; Xu, N.; Lv, E.; Ci, C.; Li, X. 1,25-dihydroxyvitamin D3 ameliorates lupus nephritis through inhibiting the NF-kappaB and MAPK signalling pathways in MRL/lpr mice. BMC Nephrol. 2022, 23, 243. [Google Scholar] [CrossRef]
- Guerrache, A.; Micheau, O. TNF-Related Apoptosis-Inducing Ligand: Non-Apoptotic Signalling. Cells 2024, 13, 521. [Google Scholar] [CrossRef]
- Sekhoacha, M.; Riet, K.; Motloung, P.; Gumenku, L.; Adegoke, A.; Mashele, S. Prostate Cancer Review: Genetics, Diagnosis, Treatment Options, and Alternative Approaches. Molecules 2022, 27, 5730. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.; Narwani, D.; Notta, S.; Ghaffar, D.; Mardhekar, N.; Quadri, S.S.A. Oxidative stress and redox signaling in CRPC progression: Therapeutic potential of clinically-tested Nrf2-activators. Cancer Drug Resist. 2021, 4, 96–124. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chen, X.; Wang, X.; Li, H.; Zhu, Y.; Li, X.; Xiao, Z.; Zi, T.; Qin, X.; Zhao, Y.; et al. Glycolysis related lncRNA SNHG3/miR-139-5p/PKM2 axis promotes castration-resistant prostate cancer (CRPC) development and enzalutamide resistance. Int. J. Biol. Macromol. 2024, 260, 129635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Kan, D.; Zhang, B.; Chen, X.; Wang, C.; Chen, S.; Gao, W.; Yang, Z.; Li, Y.; Chen, Y.; et al. P300/SP1 complex mediating elevated METTL1 regulates CDK14 mRNA stability via internal m7G modification in CRPC. J. Exp. Clin. Cancer Res. 2023, 42, 215. [Google Scholar] [CrossRef]
- Skubnik, J.; Bejcek, J.; Pavlickova, V.S.; Rimpelova, S. Repurposing Cardiac Glycosides: Drugs for Heart Failure Surmounting Viruses. Molecules 2021, 26, 5627. [Google Scholar] [CrossRef]
- Benderska, N.; Chakilam, S.; Hugle, M.; Ivanovska, J.; Gandesiri, M.; Schulze-Luhrmann, J.; Bajbouj, K.; Croner, R.; Schneider-Stock, R. Apoptosis signalling activated by TNF in the lower gastrointestinal tract-review. Curr. Pharm. Biotechnol. 2012, 13, 2248–2258. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Y.; Luan, J.; Duan, H.; Zhang, F.; Yagasaki, K.; Zhang, G. Effects of bufalin on the proliferation of human lung cancer cells and its molecular mechanisms of action. Cytotechnology 2010, 62, 573–583. [Google Scholar] [CrossRef]
- Elbaz, H.A.; Stueckle, T.A.; Wang, H.Y.; O’Doherty, G.A.; Lowry, D.T.; Sargent, L.M.; Wang, L.; Dinu, C.Z.; Rojanasakul, Y. Digitoxin and a synthetic monosaccharide analog inhibit cell viability in lung cancer cells. Toxicol. Appl. Pharmacol. 2012, 258, 51–60. [Google Scholar] [CrossRef]
- Kumavath, R.; Paul, S.; Pavithran, H.; Paul, M.K.; Ghosh, P.; Barh, D.; Azevedo, V. Emergence of Cardiac Glycosides as Potential Drugs: Current and Future Scope for Cancer Therapeutics. Biomolecules 2021, 11, 1275. [Google Scholar] [CrossRef]
- Geng, X.; Wang, F.; Tian, D.; Huang, L.; Streator, E.; Zhu, J.; Kurihara, H.; He, R.; Yao, X.; Zhang, Y.; et al. Cardiac glycosides inhibit cancer through Na/K-ATPase-dependent cell death induction. Biochem. Pharmacol. 2020, 182, 114226. [Google Scholar] [CrossRef]
- Pathak, S.; Multani, A.S.; Narayan, S.; Kumar, V.; Newman, R.A. Anvirzel, an extract of Nerium oleander, induces cell death in human but not murine cancer cells. Anticancer Drugs 2000, 11, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Mekhail, T.; Kaur, H.; Ganapathi, R.; Budd, G.T.; Elson, P.; Bukowski, R.M. Phase 1 trial of Anvirzel in patients with refractory solid tumors. Investig. New Drugs 2006, 24, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Winnicka, K.; Bielawski, K.; Bielawska, A. Cardiac glycosides in cancer research and cancer therapy. Acta Pol. Pharm. 2006, 63, 109–115. [Google Scholar]
- Sohrab, S.S.; Raj, R.; Nagar, A.; Hawthorne, S.; Paiva-Santos, A.C.; Kamal, M.A.; El-Daly, M.M.; Azhar, E.I.; Sharma, A. Chronic Inflammation’s Transformation to Cancer: A Nanotherapeutic Paradigm. Molecules 2023, 28, 4413. [Google Scholar] [CrossRef]
- Fernandes, Q.; Inchakalody, V.P.; Bedhiafi, T.; Mestiri, S.; Taib, N.; Uddin, S.; Merhi, M.; Dermime, S. Chronic inflammation and cancer; the two sides of a coin. Life Sci. 2024, 338, 122390. [Google Scholar] [CrossRef]
- Afify, S.M.; Hassan, G.; Seno, A.; Seno, M. Cancer-inducing niche: The force of chronic inflammation. Br. J. Cancer 2022, 127, 193–201. [Google Scholar] [CrossRef]
- Fraser, B.; Maranchuk, R.A.; Foley, E. A high-content RNAi screen identifies ubiquitin modifiers that regulate TNF-dependent nuclear accumulation of NF-κB. Front. Immunol. 2014, 5, 322. [Google Scholar] [CrossRef]
- Mehta, A.K.; Gracias, D.T.; Croft, M. TNF activity and T cells. Cytokine 2018, 101, 14–18. [Google Scholar] [CrossRef]
- Preedy, M.K.; White, M.R.H.; Tergaonkar, V. Cellular heterogeneity in TNF/TNFR1 signalling: Live cell imaging of cell fate decisions in single cells. Cell Death Dis. 2024, 15, 202. [Google Scholar] [CrossRef]
- Takahashi, H.; Yoshimatsu, G.; Faustman, D.L. The Roles of TNFR2 Signaling in Cancer Cells and the Tumor Microenvironment and the Potency of TNFR2 Targeted Therapy. Cells 2022, 11, 1952. [Google Scholar] [CrossRef]
- Sheng, Y.Q.; Li, F.; Qin, Z.H. TNF Receptor 2 Makes Tumor Necrosis Factor a Friend of Tumors. Front. Immunol. 2018, 9, 1170. [Google Scholar] [CrossRef] [PubMed]
- Vanamee, E.S.; Faustman, D.L. TNFR2: A Novel Target for Cancer Immunotherapy. Trends Mol. Med. 2017, 23, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Medler, J.; Wajant, H. Tumor necrosis factor receptor-2 (TNFR2): An overview of an emerging drug target. Expert Opin. Ther. Targets 2019, 23, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liu, C.; Qian, W.; Han, Y.; Li, X.; Deng, J. Crystal structure of IL-17 receptor B SEFIR domain. J. Immunol. 2013, 190, 2320–2326. [Google Scholar] [CrossRef]
- Huang, G.H.; Wang, Y.Y.; Vogel, P.; Chi, H.B. Control of IL-17 receptor signaling and tissue inflammation by the p38α-MKP-1 signaling axis in a mouse model of multiple sclerosis. Sci. Signal. 2015, 8, ra24. [Google Scholar] [CrossRef]
- Zhong, Z.; Su, G.; Kijlstra, A.; Yang, P. Activation of the interleukin-23/interleukin-17 signalling pathway in autoinflammatory and autoimmune uveitis. Prog. Retin. Eye Res. 2021, 80, 100866. [Google Scholar] [CrossRef]
- Shen, F.; Maitra, A.; Hanel, W.; Mossman, K.; Tocker, J.; Swart, D.; Gaffen, S.L. Distinct functional motifs within the IL-17 receptor regulate signal transduction and target gene expression. Cytokine 2007, 39, 38. [Google Scholar] [CrossRef]
- Shen, F.; Hu, Z.H.; Goswami, J.; Gaffen, S.L. Identification of common transcriptional regulatory elements in interleukin-17 target genes. J. Biol. Chem. 2006, 281, 24138–24148. [Google Scholar] [CrossRef]
- Liu, H.; Yuan, S.; Zheng, K.; Liu, G.; Li, J.; Ye, B.; Yin, L.; Li, Y. IL-17 signaling pathway: A potential therapeutic target for reducing skeletal muscle inflammation. Cytokine 2024, 181, 156691. [Google Scholar] [CrossRef]
- Zhang, X.R.; Li, B.J.; Lan, T.; Chiari, C.; Ye, X.Y.; Wang, K.P.; Chen, J. The role of interleukin-17 in inflammation-related cancers. Front. Immunol. 2025, 15, 1479505. [Google Scholar] [CrossRef]


| Cell Lines | IC50 (24 h) | IC50 (48 h) | IC50 (72 h) |
|---|---|---|---|
| PC-3 | 208.10 | 79.72 | 45.43 |
| DU145 | 151.30 | 96.62 | 96.43 |
| LNCAP | 565.50 | 344.80 | 304.60 |
| Cell Lines | IC50 (48 h) | Selectivity Index (WPMY-1) a | Selectivity Index (HPRF) b |
|---|---|---|---|
| PC-3 | 79.72 nM | 75.3 | 5.4 |
| DU145 | 96.62 nM | 62.1 | 4.5 |
| LNCAP | 344.80 nM | 17.4 | 1.3 |
| Cell Lines | Source | Migration Ability | Response to Androgen |
|---|---|---|---|
| LNCAP | Androgen-dependent prostate cancer | Weak | Androgen-dependent |
| PC-3 | Bone metastasis from androgen-independent prostate cancer | Moderate | Androgen-independent |
| DU145 | Brain metastasis from androgen-independent prostate cancer | Strong | Androgen-independent |
| Genes | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| GAPDH | TCCAAAATCAAGTGGGGCGA | AAATGAGCCCCAGCCTTCTC |
| MYC | TTCATAACGCGCTCTCCAAGT | CAGAGCGTGGGATGTTAGTGT |
| IL6 | CTTCGGTCCAGTTGCCTTCT | TGGAATCTTCTCCTGGGGGT |
| IL1B | GTTCTTTGAAGCTGATGGCCC | GAAGCCCTTGCTGTAGTGGT |
| FOS | CAAGCGGAGACAGACCAACT | GTGAGCTGCCAGGATGAACT |
| CXCL8 | CACTGCGCCAACACAGAAAT | TTCTCAGCCCTCTTCAAAAACTTC |
| PTGS2 | GTTCCACCCGCAGTACAGAA | AGGGCTTCAGCATAAAGCGT |
| NFKBIA | CCACTCCACTTGGCTGTGAT | TTCCTCGAAAGTCTCGGAGC |
| CCL20 | GCGAATCAGAAGCAAGCAAC | CCGTGTGAAGCCCACAATAAA |
| MMP3 | ATCCTACTGTTGCTGTGCGT | GGTTCATGCTGGTGTCCTCA |
| TNFAIP3 | TCCACAAAGCCCTCATCGAC | TTCGTTTTCAGCGCCACAAG |
| MAPK11 | AGAACGTCATCGGGCTTCT | TGGCACTTGACGATGTTGTT |
| MAPK13 | AAGACCTACGTGTCCCCGA | TCGGCTCAGCTTCTTGATGG |
| MMP1 | AGAGCAGATGTGGACCATGC | TTGTCCCGATGATCTCCCCT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Huang, D.; Jin, Y.; Shao, C.; Su, X.; Yang, R.; Jiang, J.; Wu, J. Lanatoside C Inhibits Proliferation and Induces Apoptosis in Human Prostate Cancer Cells Through the TNF/IL-17 Signaling Pathway. Int. J. Mol. Sci. 2025, 26, 2558. https://doi.org/10.3390/ijms26062558
Huang S, Huang D, Jin Y, Shao C, Su X, Yang R, Jiang J, Wu J. Lanatoside C Inhibits Proliferation and Induces Apoptosis in Human Prostate Cancer Cells Through the TNF/IL-17 Signaling Pathway. International Journal of Molecular Sciences. 2025; 26(6):2558. https://doi.org/10.3390/ijms26062558
Chicago/Turabian StyleHuang, Sisi, Dongyan Huang, Yangtao Jin, Congcong Shao, Xin Su, Rongfu Yang, Juan Jiang, and Jianhui Wu. 2025. "Lanatoside C Inhibits Proliferation and Induces Apoptosis in Human Prostate Cancer Cells Through the TNF/IL-17 Signaling Pathway" International Journal of Molecular Sciences 26, no. 6: 2558. https://doi.org/10.3390/ijms26062558
APA StyleHuang, S., Huang, D., Jin, Y., Shao, C., Su, X., Yang, R., Jiang, J., & Wu, J. (2025). Lanatoside C Inhibits Proliferation and Induces Apoptosis in Human Prostate Cancer Cells Through the TNF/IL-17 Signaling Pathway. International Journal of Molecular Sciences, 26(6), 2558. https://doi.org/10.3390/ijms26062558






