An In Vitro Cell Model of Intestinal Barrier Function Using a Low-Cost 3D-Printed Transwell Device and Paper-Based Cell Membrane
Abstract
1. Introduction
2. Results
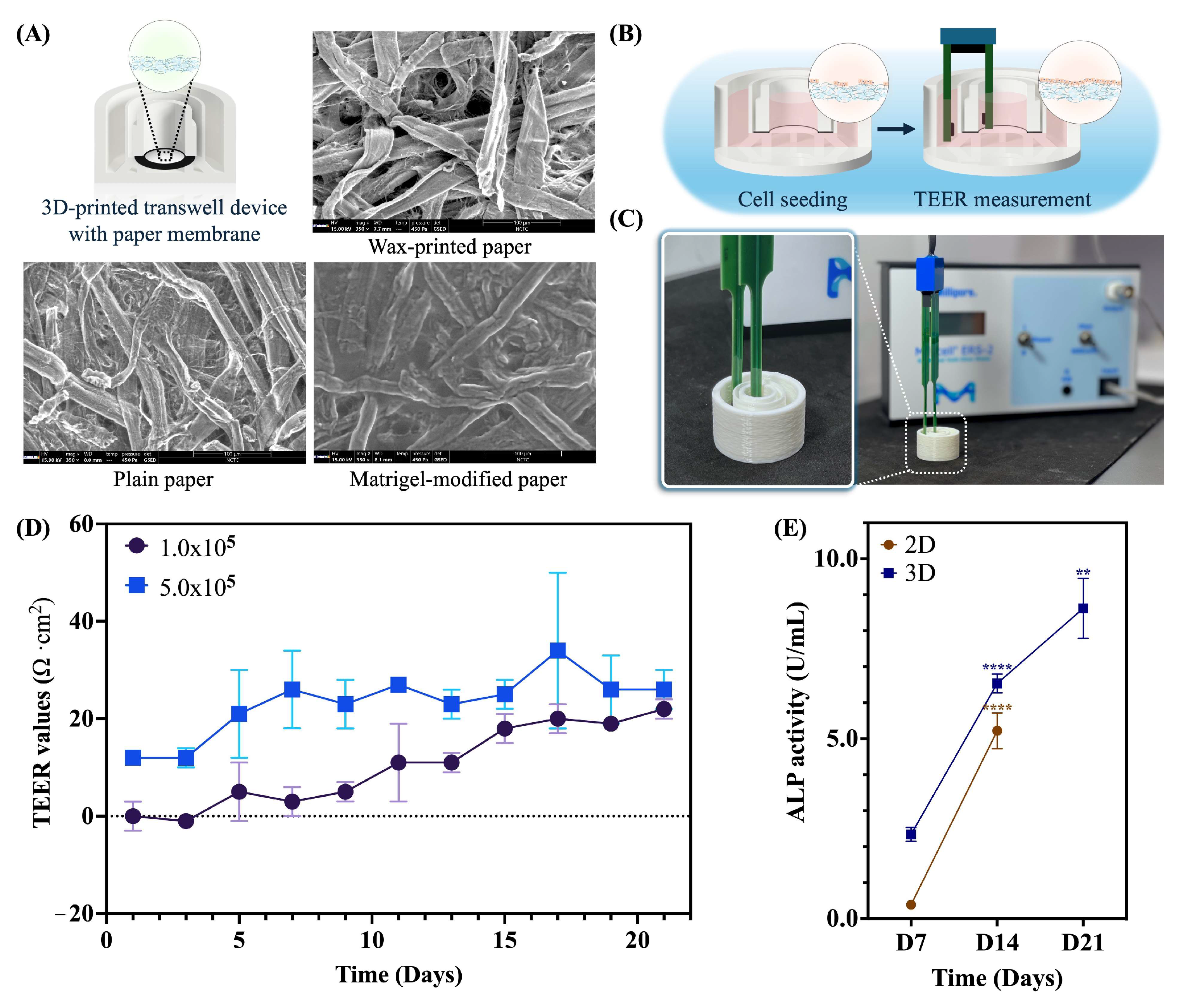
2.1. Assessment of a Paper-Based Intestinal Barrier Membrane
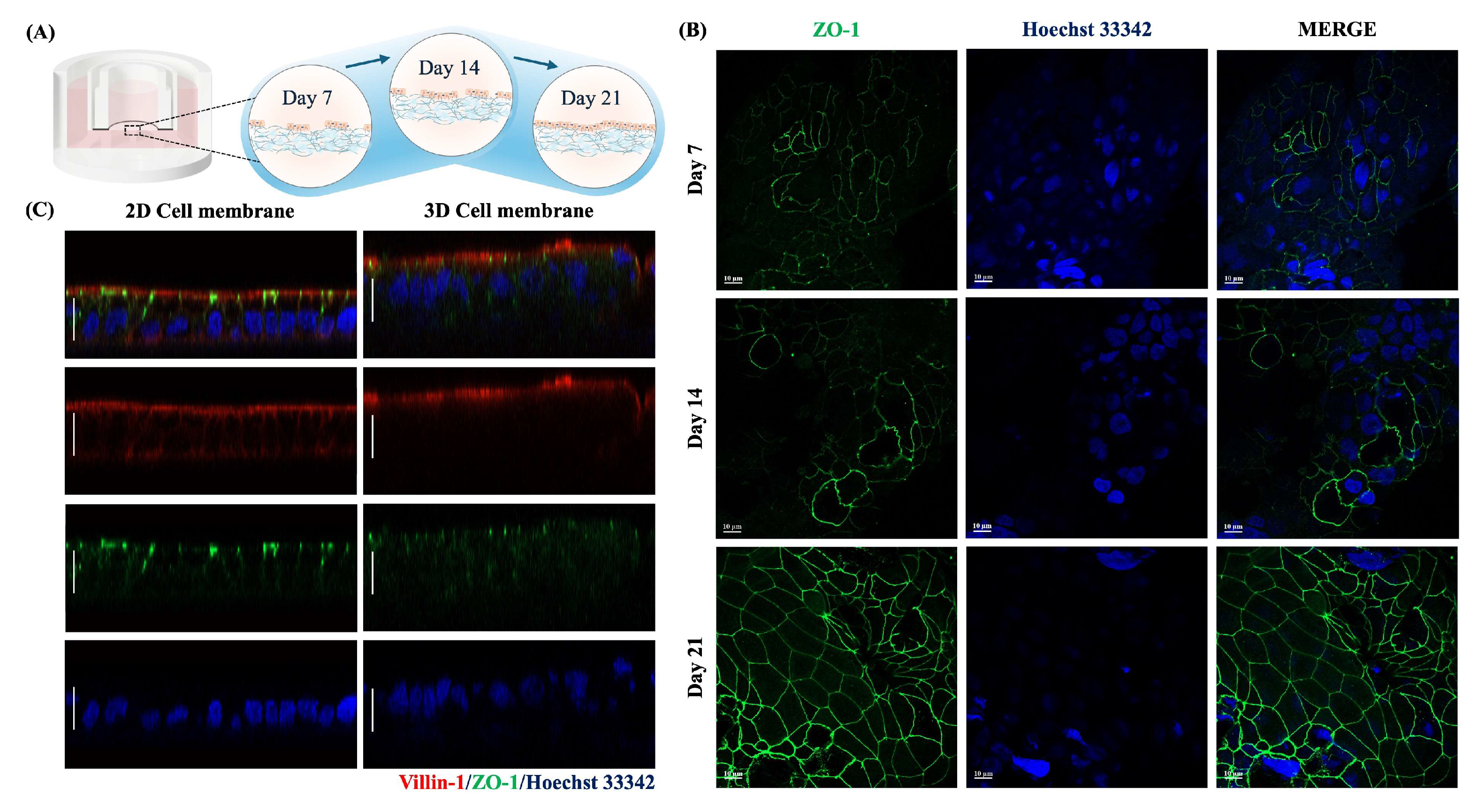
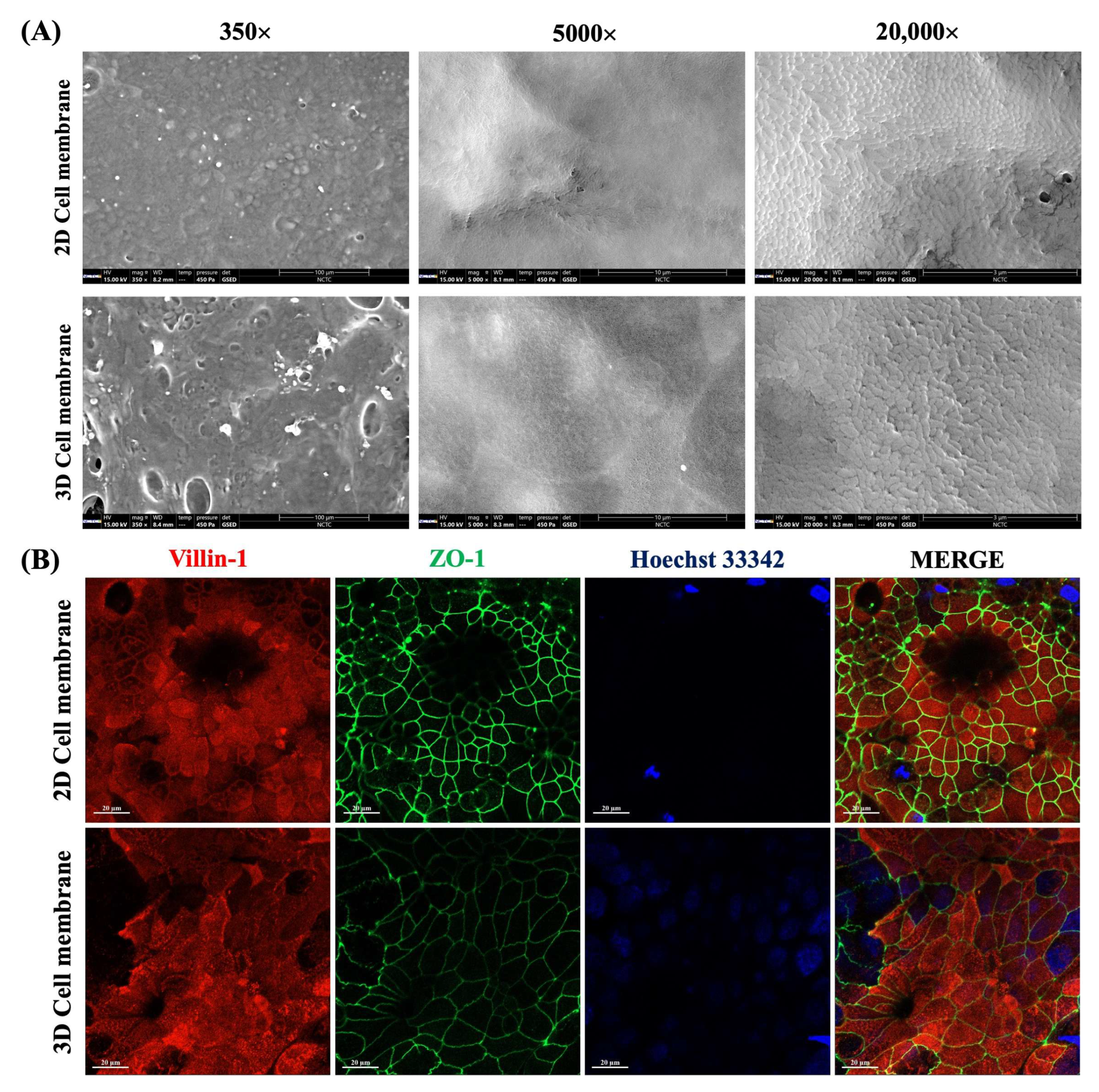
2.2. Cell Characteristics of a Paper-Based Intestinal Barrier Membrane
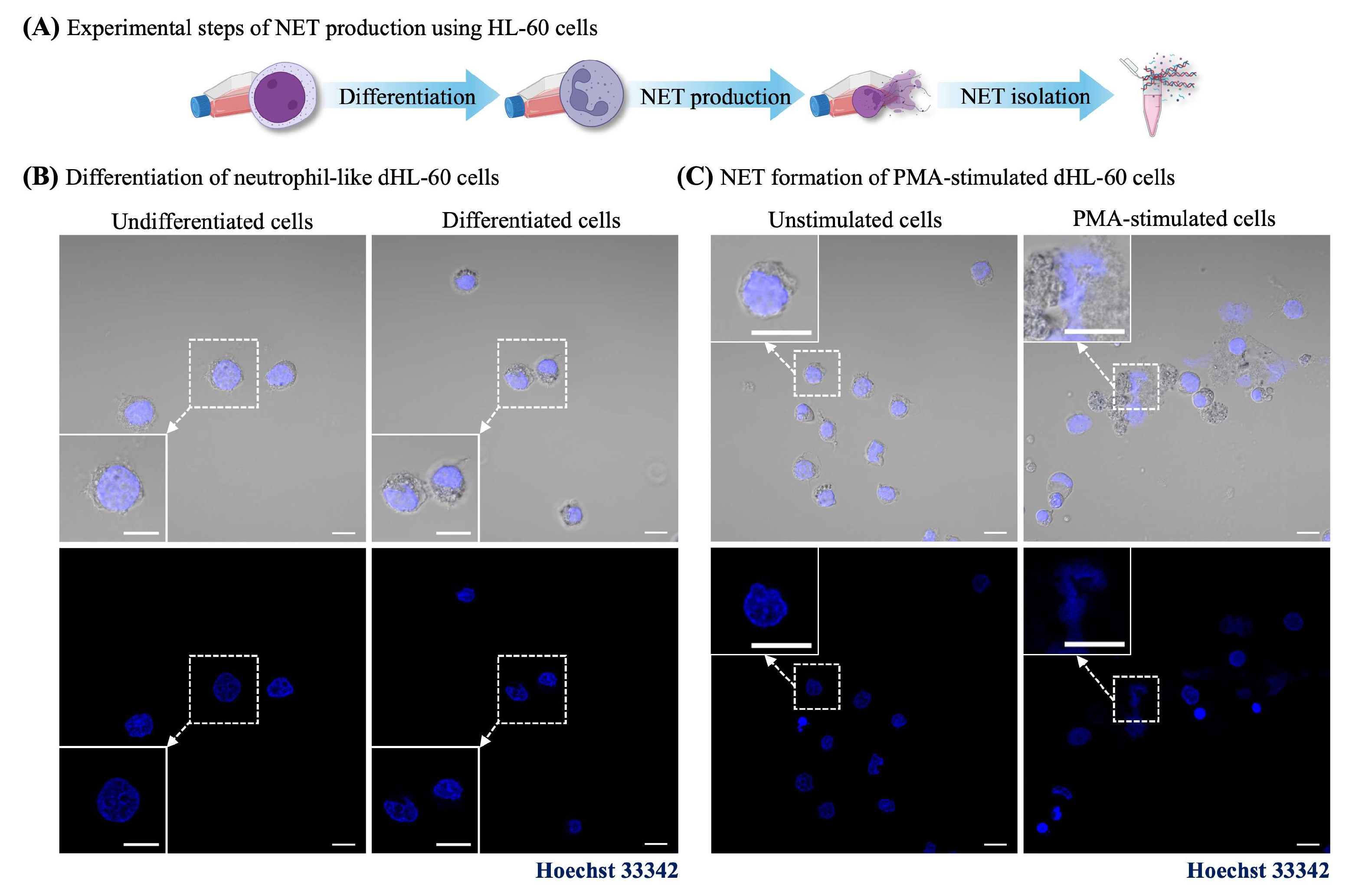
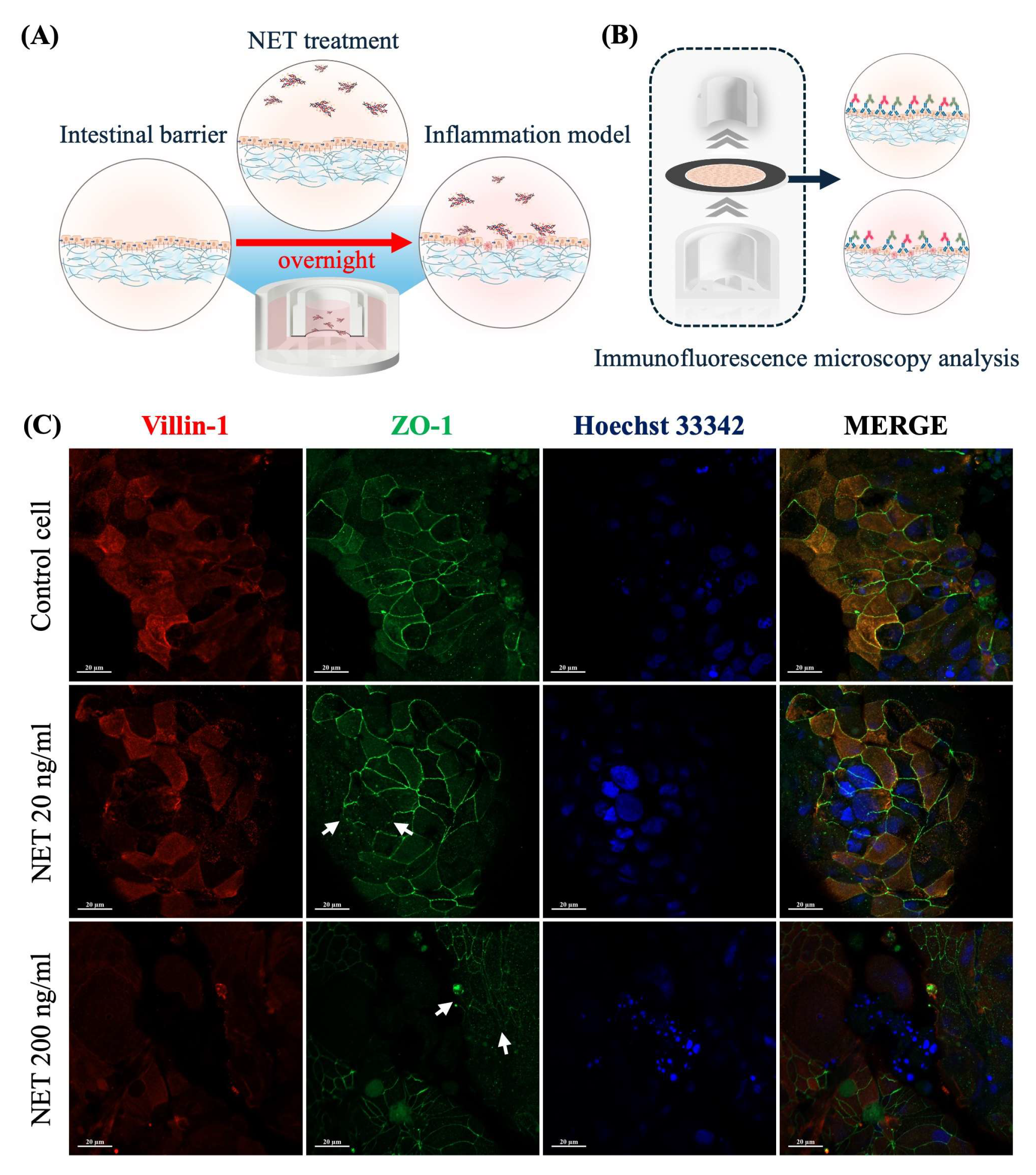
2.3. NET-Induced Intestinal Barrier Dysfunction
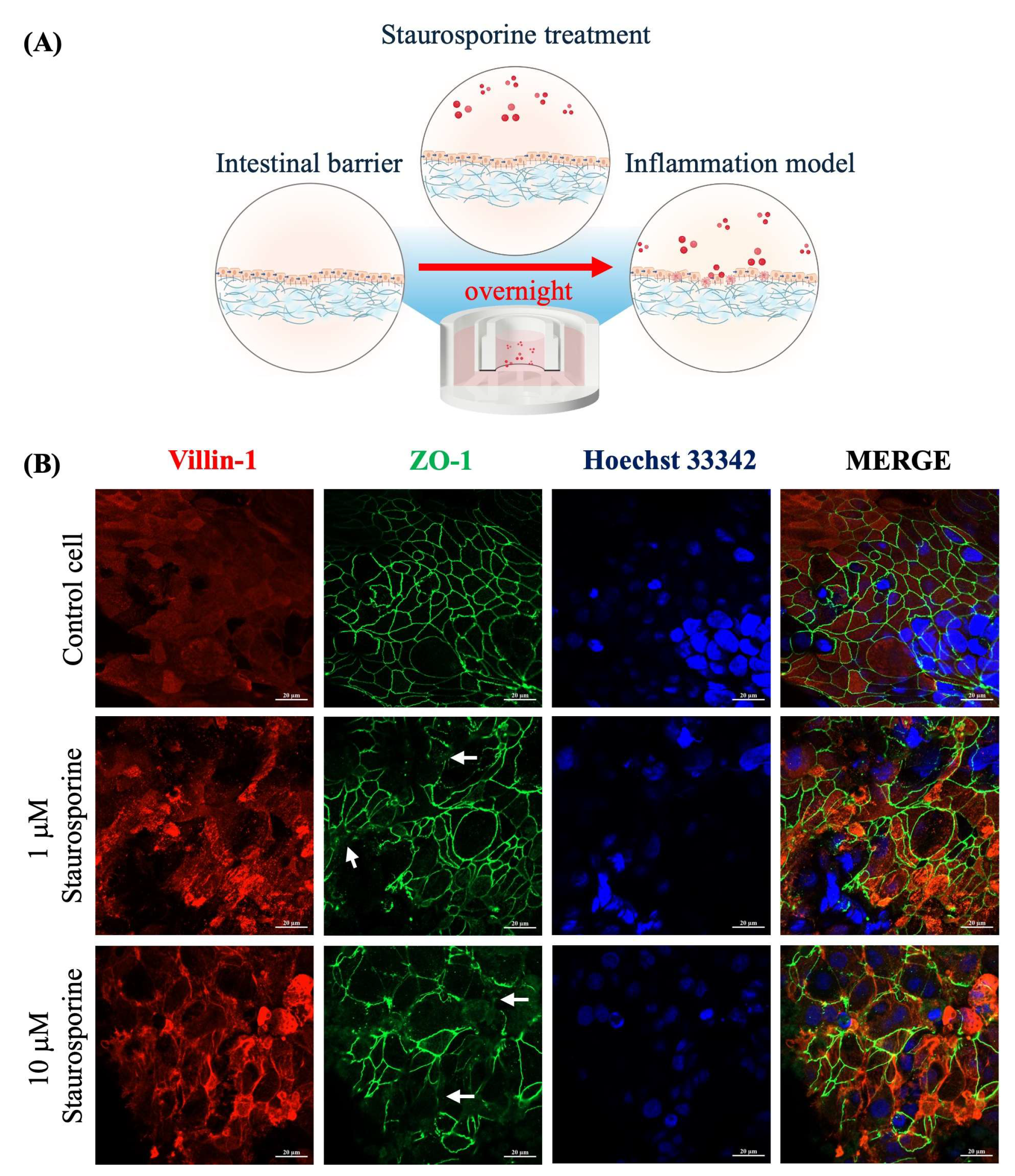
2.4. Staurosporine-Induced Intestinal Barrier Dysfunction
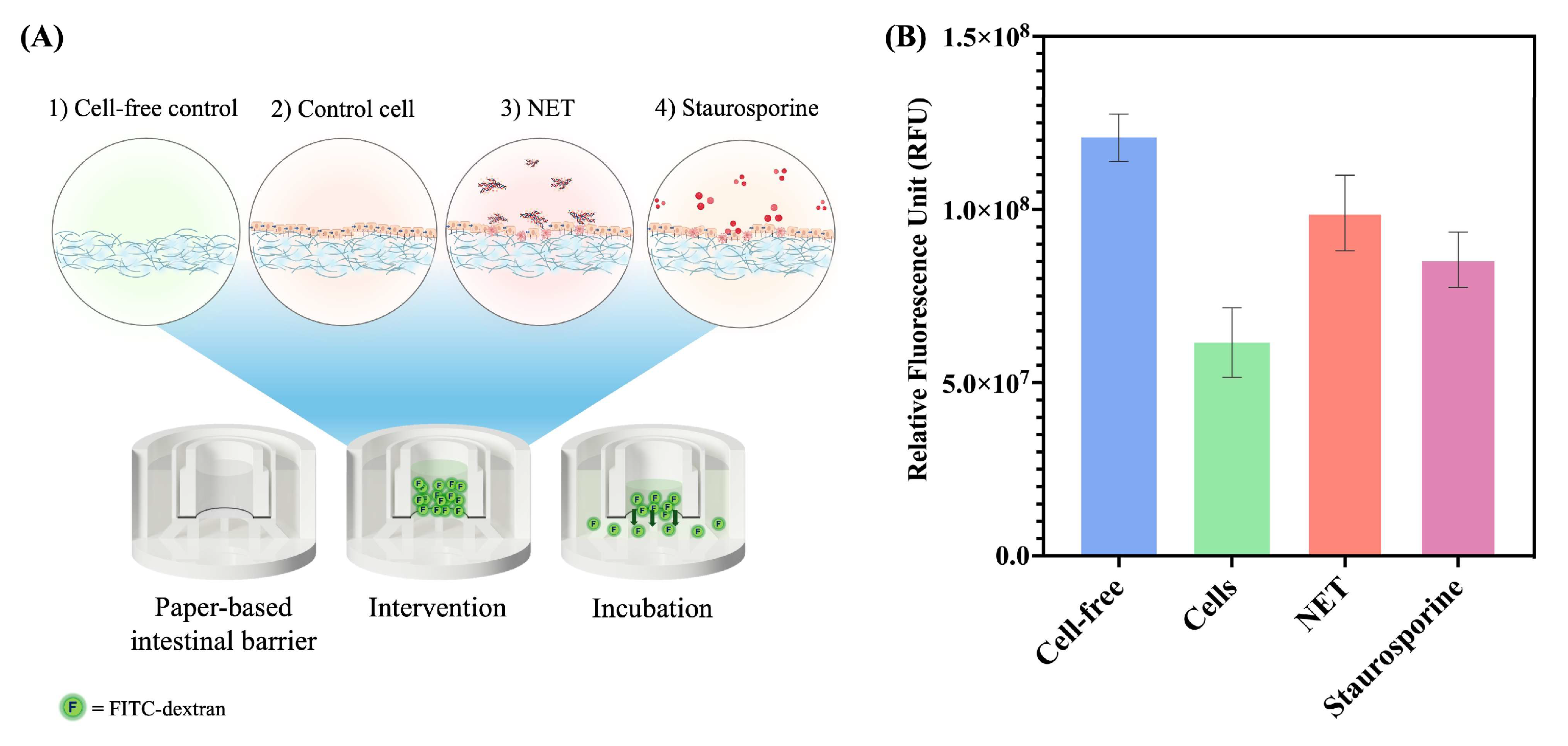
2.5. Permeability Assay of a Paper-Based Intestinal Barrier Membrane
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture and Differentiation
4.3. Transwell Device and Paper Membrane Preparation
4.4. TEER Measurements
4.5. Alkaline Phosphatase (ALP) Activity Assessment
4.6. NET Formation
4.7. Interventions for Intestinal Barrier Dysfunction
4.8. Fluorescence and Immunofluorescence Analysis
4.9. Permeability Assay
4.10. Scanning Electron Microscopy (SEM)
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, E.Y.-H.; Lai, H.-J.; Cheng, Y.-K.; Leong, K.-Q.; Cheng, L.-C.; Chou, Y.-C.; Peng, Y.-C.; Hsu, Y.-H.; Chiang, H.-S. Neutrophil Extracellular Traps Impair Intestinal Barrier Function During Experimental Colitis. Biomedicines 2020, 8, 275. [Google Scholar] [CrossRef] [PubMed]
- Chelakkot, C.; Ghim, J.; Ryu, S.H. Mechanisms regulating intestinal barrier integrity and its pathological implications. Exp. Mol. Med. 2018, 50, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Whitley, C.S.; Haribabu, B.; Jala, V.R. Regulation of Intestinal Barrier Function by Microbial Metabolites. Cell Mol. Gastroenterol. Hepatol. 2021, 11, 1463–1482. [Google Scholar] [CrossRef]
- Torras, N.; García-Díaz, M.; Fernández-Majada, V.; Martínez, E. Mimicking Epithelial Tissues in Three-Dimensional Cell Culture Models. Front. Bioeng. Biotechnol. 2018, 6, 197. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim. Sci. J. 2020, 91, e13357. [Google Scholar] [CrossRef]
- di Vito, R.; Conte, C.; Traina, G. A Multi-Strain Probiotic Formulation Improves Intestinal Barrier Function by the Modulation of Tight and Adherent Junction Proteins. Cells 2022, 11, 2617. [Google Scholar] [CrossRef]
- Di Tommaso, N.; Gasbarrini, A.; Ponziani, F.R. Intestinal Barrier in Human Health and Disease. Int. J. Environ. Res. Public Health 2021, 18, 12836. [Google Scholar] [CrossRef]
- Nesci, A.; Carnuccio, C.; Ruggieri, V.; D’Alessandro, A.; Di Giorgio, A.; Santoro, L.; Gasbarrini, A.; Santoliquido, A.; Ponziani, F.R. Gut Microbiota and Cardiovascular Disease: Evidence on the Metabolic and Inflammatory Background of a Complex Relationship. Int. J. Mol. Sci. 2023, 24, 9087. [Google Scholar] [CrossRef]
- Schoultz, I.; Keita, Å.V. The Intestinal Barrier and Current Techniques for the Assessment of Gut Permeability. Cells 2020, 9, 1909. [Google Scholar] [CrossRef]
- Drury, B.; Hardisty, G.; Gray, R.D.; Ho, G.T. Neutrophil Extracellular Traps in Inflammatory Bowel Disease: Pathogenic Mechanisms and Clinical Translation. Cell Mol. Gastroenterol. Hepatol. 2021, 12, 321–333. [Google Scholar] [CrossRef]
- Sorvillo, N.; Cherpokova, D.; Martinod, K.; Wagner, D.D. Extracellular DNA NET-Works with Dire Consequences for Health. Circ. Res. 2019, 125, 470–488. [Google Scholar] [CrossRef] [PubMed]
- Kenny, E.F.; Herzig, A.; Krüger, R.; Muth, A.; Mondal, S.; Thompson, P.R.; Brinkmann, V.; Bernuth, H.v.; Zychlinsky, A. Diverse stimuli engage different neutrophil extracellular trap pathways. eLife 2017, 6, e24437. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; He, J.; Morita, H.; Yokoyama, K.; Kaji, H.; Tanaka, C.; Suemori, S.-i.; Tohyama, K.; Tohyama, Y. Rab27a Is Essential for the Formation of Neutrophil Extracellular Traps (NETs) in Neutrophil-Like Differentiated HL60 Cells. PLoS ONE 2014, 9, e84704. [Google Scholar] [CrossRef] [PubMed]
- Linz, G.; Djeljadini, S.; Steinbeck, L.; Köse, G.; Kiessling, F.; Wessling, M. Cell barrier characterization in transwell inserts by electrical impedance spectroscopy. Biosens. Bioelectron. 2020, 165, 112345. [Google Scholar] [CrossRef]
- Zeiringer, S.; Wiltschko, L.; Glader, C.; Reiser, M.; Absenger-Novak, M.; Fröhlich, E.; Roblegg, E. Development and Characterization of an In Vitro Intestinal Model Including Extracellular Matrix and Macrovascular Endothelium. Mol. Pharm. 2023, 20, 5173–5184. [Google Scholar] [CrossRef]
- Antfolk, M.; Jensen, K.B. A bioengineering perspective on modelling the intestinal epithelial physiology in vitro. Nat. Commun. 2020, 11, 6244. [Google Scholar] [CrossRef]
- Fang, G.; Chen, Y.-C.; Lu, H.; Jin, D. Advances in Spheroids and Organoids on a Chip. Adv. Funct. Mater. 2023, 33, 2215043. [Google Scholar] [CrossRef]
- Alnemari, R.; Sukumar, P.; Deliorman, M.; Qasaimeh, M.A. Paper-Based Cell Cryopreservation. Adv. Biosyst. 2020, 4, 1900203. [Google Scholar] [CrossRef]
- Tao, F.F.; Xiao, X.; Lei, K.F.; Lee, I.C. Paper-based cell culture microfluidic system. BioChip J. 2015, 9, 97–104. [Google Scholar] [CrossRef]
- Ng, K.; Gao, B.; Yong, K.W.; Li, Y.; Shi, M.; Zhao, X.; Li, Z.; Zhang, X.; Pingguan-Murphy, B.; Yang, H.; et al. Paper-based cell culture platform and its emerging biomedical applications. Mater. Today 2017, 20, 32–44. [Google Scholar] [CrossRef]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef] [PubMed]
- De Dios Andres, P.; Westensee, I.N.; Brodszkij, E.; Ramos-Docampo, M.A.; Gal, N.; Städler, B. Evaluation of Hybrid Vesicles in an Intestinal Cell Model Based on Structured Paper Chips. Biomacromolecules 2021, 22, 3860–3872. [Google Scholar] [CrossRef] [PubMed]
- Pupinyo, N.; Chatatikun, M.; Chiabchalard, A.; Laiwattanapaisal, W. In situ paper-based 3D cell culture for rapid screening of the anti-melanogenic activity. Analyst 2019, 144, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Badea, M.A.; Balas, M.; Hermenean, A.; Ciceu, A.; Herman, H.; Ionita, D.; Dinischiotu, A. Influence of Matrigel on Single- and Multiple-Spheroid Cultures in Breast Cancer Research. SLAS Discov. 2019, 24, 563–578. [Google Scholar] [CrossRef]
- Law, A.M.K.; Rodriguez de la Fuente, L.; Grundy, T.J.; Fang, G.; Valdes-Mora, F.; Gallego-Ortega, D. Advancements in 3D Cell Culture Systems for Personalizing Anti-Cancer Therapies. Front. Oncol. 2021, 11, 782766. [Google Scholar] [CrossRef]
- Supjaroen, P.; Niamsi, W.; Thirabowonkitphithan, P.; Thummarati, P.; Laiwattanapaisal, W. A customizable and low-cost 3D-printed transwell device coupled with 3D cell culture for permeability assay. HardwareX 2024, 20, e00603. [Google Scholar] [CrossRef]
- Supjaroen, P.; Niamsi, W.; Thummarati, P.; Laiwattanapaisal, W. Development of a paper-based stacking co-culture cell model for an alternative in vitro intestinal permeability assay. Microchem. J. 2025, 210, 113001. [Google Scholar] [CrossRef]
- Ramiah Rajasekaran, P.; Chapin, A.A.; Quan, D.N.; Herberholz, J.; Bentley, W.E.; Ghodssi, R. 3D-Printed electrochemical sensor-integrated transwell systems. Microsyst. Nanoeng. 2020, 6, 100. [Google Scholar] [CrossRef]
- Dogan, A.A.; Dufva, M. Customized 3D-printed stackable cell culture inserts tailored with bioactive membranes. Sci. Rep. 2022, 12, 3694. [Google Scholar] [CrossRef]
- Cenhrang, K.; Robart, L.; Castiaux, A.D.; Martin, R.S. 3D printed devices with integrated collagen scaffolds for cell culture studies including transepithelial/transendothelial electrical resistance (TEER) measurements. Anal. Chim. Acta 2022, 1221, 340166. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Q.; Cao, P.; Zhang, J.; Wu, D.; Xu, R.; Zhang, Y.; Xu, Y.; Liang, T.; Chen, W.; et al. A biomimetic helical robot actuated by rotating magnetic field for targeted navigation and in situ prodrug activation to treat intestinal diseases. Device 2023, 1, 100064. [Google Scholar] [CrossRef]
- Alonso-Fernández, I.; Haugen, H.J.; López-Peña, M.; González-Cantalapiedra, A.; Muñoz, F. Use of 3D-printed polylactic acid/bioceramic composite scaffolds for bone tissue engineering in preclinical in vivo studies: A systematic review. Acta BioMater. 2023, 168, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, Y.; Wu, B.; Cui, C.; Guo, Y.; Yan, C. A critical review of fused deposition modeling 3D printing technology in manufacturing polylactic acid parts. J. Adv. Manuf. Technol. 2019, 102, 2877–2889. [Google Scholar] [CrossRef]
- Yang, Y.; Luo, R.; Chao, S.; Xue, J.; Jiang, D.; Feng, Y.H.; Guo, X.D.; Luo, D.; Zhang, J.; Li, Z.; et al. Improved pharmacodynamics of epidermal growth factor via microneedles-based self-powered transcutaneous electrical stimulation. Nat. Commun. 2022, 13, 6908. [Google Scholar] [CrossRef]
- Wu, Z.; Mirza, H.; Teo, J.D.W.; Tan, K.S.W. Strain-Dependent Induction of Human Enterocyte Apoptosis by Blastocystis Disrupts Epithelial Barrier and ZO-1 Organization in a Caspase 3- and 9-Dependent Manner. Biomed. Res. Int. 2014, 2014, 209163. [Google Scholar] [CrossRef]
- Pupinyo, N.; Heiskanen, A.; Chailapakul, O.; Gorton, L.; Emnéus, J.; Laiwattanapaisal, W. Impedimetric melanoma invasion assay device using a simple paper membrane and stencil-printed electrode on PMMA substrate. Sens. Biosens. Res. 2020, 29, 100354. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, Y.; Lu, X. An Integrated Paper Microfluidic Device Based on Isothermal Amplification for Simple Sample-to-Answer Detection of Campylobacter jejuni. Appl. Environ. Microbiol. 2023, 89, e00695-23. [Google Scholar] [CrossRef]
- Kuo, W.-T.; Odenwald, M.A.; Turner, J.R.; Zuo, L. Tight junction proteins occludin and ZO-1 as regulators of epithelial proliferation and survival. Ann. N. Y. Acad. Sci. 2022, 1514, 21–33. [Google Scholar] [CrossRef]
- Shin, W.; Kim, H.J. 3D in vitro morphogenesis of human intestinal epithelium in a gut-on-a-chip or a hybrid chip with a cell culture insert. Nat. Protoc. 2022, 17, 910–939. [Google Scholar] [CrossRef]
- Rudolph, S.E.; Longo, B.N.; Tse, M.W.; Houchin, M.R.; Shokoufandeh, M.M.; Chen, Y.; Kaplan, D.L. Crypt-Villus Scaffold Architecture for Bioengineering Functional Human Intestinal Epithelium. ACS BioMater. Sci. Eng. 2022, 8, 4942–4955. [Google Scholar] [CrossRef]
- Sitte, Z.R.; Miranda Buzetta, A.A.; Jones, S.J.; Lin, Z.-W.; Whitman, N.A.; Lockett, M.R. Paper-Based Coculture Platform to Evaluate the Effects of Fibroblasts on Estrogen Signaling in ER+ Breast Cancers. ACS Meas. Sci. Au 2023, 3, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.J.; Doan, H.T.; Lin, E.Y.; Chiu, Y.L.; Cheng, Y.K.; Lin, Y.H.; Chiang, H.S. Histones of Neutrophil Extracellular Traps Directly Disrupt the Permeability and Integrity of the Intestinal Epithelial Barrier. Inflamm. Bowel Dis. 2023, 29, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Duan, Z.; Wang, X.; Chu, C.; Yang, C.; Chen, F.; Wang, D.; Wang, C.; Li, Q.; Ding, W. Neutrophil extracellular traps impair intestinal barrier functions in sepsis by regulating TLR9-mediated endoplasmic reticulum stress pathway. Cell Death Dis. 2021, 12, 606. [Google Scholar] [CrossRef]
- Chu, C.; Wang, X.; Chen, F.; Yang, C.; Shi, L.; Xu, W.; Wang, K.; Liu, B.; Wang, C.; Sun, D.; et al. Neutrophil extracellular traps aggravate intestinal epithelial necroptosis in ischaemia-reperfusion by regulating TLR4/RIPK3/FUNDC1-required mitophagy. Cell Prolif. 2024, 57, e13538. [Google Scholar] [CrossRef] [PubMed]
- Wen, S.-H.; Hong, Z.-W.; Chen, C.-C.; Chang, H.-W.; Fu, H.-W. Helicobacter pylori Neutrophil-Activating Protein Directly Interacts with and Activates Toll-like Receptor 2 to Induce the Secretion of Interleukin-8 from Neutrophils and ATRA-Induced Differentiated HL-60 Cells. Int. J. Mol. Sci. 2021, 22, 11560. [Google Scholar] [CrossRef]
- Chakrabarti, G.; Zhou, X.; McClane, B.A. Death pathways activated in CaCo-2 cells by Clostridium perfringens enterotoxin. Infect. Immun. 2003, 71, 4260–4270. [Google Scholar] [CrossRef]
- Belmokhtar, C.A.; Hillion, J.; Ségal-Bendirdjian, E. Staurosporine induces apoptosis through both caspase-dependent and caspase-independent mechanisms. Oncogene 2001, 20, 3354–3362. [Google Scholar] [CrossRef]
- Holthaus, D.; Kraft, M.R.; Krug, S.M.; Wolf, S.; Müller, A.; Delgado Betancourt, E.; Schorr, M.; Holland, G.; Knauf, F.; Schulzke, J.-D.; et al. Dissection of Barrier Dysfunction in Organoid-Derived Human Intestinal Epithelia Induced by Giardia duodenalis. Gastroenterology 2022, 162, 844–858. [Google Scholar] [CrossRef]
- Voetmann, L.M.; Rolin, B.; Kirk, R.K.; Pyke, C.; Hansen, A.K. The intestinal permeability marker FITC-dextran 4kDa should be dosed according to lean body mass in obese mice. Nutr. Diabetes 2023, 13, 1. [Google Scholar] [CrossRef]
- Rallabandi, H.R.; Yang, H.; Oh, K.B.; Lee, H.C.; Byun, S.J.; Lee, B.R. Evaluation of Intestinal Epithelial Barrier Function in Inflammatory Bowel Diseases Using Murine Intestinal Organoids. Tissue Eng. Regen. Med. 2020, 17, 641–650. [Google Scholar] [CrossRef]
- Li, C.; Bai, X.; Liu, X.; Zhang, Y.; Liu, L.; Zhang, L.; Xu, F.; Yang, Y.; Liu, M. Disruption of Epithelial Barrier of Caco-2 Cell Monolayers by Excretory Secretory Products of Trichinella spiralis Might Be Related to Serine Protease. Front. Microbiol. 2021, 12, 634185. [Google Scholar] [CrossRef]
- Dogan, A.A.; Dufva, M. Heterogenous morphogenesis of Caco-2 cells reveals that flow induces three-dimensional growth and maturation at high initial seeding cell densities. Biotechnol. Bioeng. 2023, 120, 1667–1677. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.-x.; Zuo, P.; Ye, B.-C. A Novel Wick-Like Paper-Based Microfluidic Device for 3D Cell Culture and Anti-Cancer Drugs Screening. Biotechnol. J. 2021, 16, 2000126. [Google Scholar] [CrossRef] [PubMed]
- Manda-Handzlik, A.; Bystrzycka, W.; Wachowska, M.; Sieczkowska, S.; Stelmaszczyk-Emmel, A.; Demkow, U.; Ciepiela, O. The influence of agents differentiating HL-60 cells toward granulocyte-like cells on their ability to release neutrophil extracellular traps. Immunol. Cell Biol. 2018, 96, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Gao, F.; Wang, Q.; Wang, K.; Pan, S.; Pan, Z.; Xu, S.; Li, L.; Zhao, D. Differentiation of HL-60 cells in serum-free hematopoietic cell media enhances the production of neutrophil extracellular traps. Exp. Ther. Med. 2021, 21, 353. [Google Scholar] [CrossRef]
- Koga, T.; Morotomi-Yano, K.; Sakugawa, T.; Saitoh, H.; Yano, K.-i. Nanosecond pulsed electric fields induce extracellular release of chromosomal DNA and histone citrullination in neutrophil-differentiated HL-60 cells. Sci. Rep. 2019, 9, 8451. [Google Scholar] [CrossRef]
- Millius, A.; Weiner, O.D. Chemotaxis in Neutrophil-like HL-60 Cells. In Chemotaxis: Methods and Protocols; Jin, T., Hereld, D., Eds.; Humana Press: Totowa, NJ, USA, 2009; pp. 167–177. [Google Scholar]
- Liu, P.; Li, B.; Fu, L.; Huang, Y.; Man, M.; Qi, J.; Sun, X.; Kang, Q.; Shen, D.; Chen, L. Hybrid Three Dimensionally Printed Paper-Based Microfluidic Platform for Investigating a Cell’s Apoptosis and Intracellular Cross-Talk. ACS Sens. 2020, 5, 464–473. [Google Scholar] [CrossRef]
- Elzinga, J.; van der Lugt, B.; Belzer, C.; Steegenga, W.T. Characterization of increased mucus production of HT29-MTX-E12 cells grown under Semi-Wet interface with Mechanical Stimulation. PLoS ONE 2021, 16, e0261191. [Google Scholar] [CrossRef]
- Blanter, M.; Gouwy, M.; Struyf, S. Studying Neutrophil Function in vitro: Cell Models and Environmental Factors. J. Inflamm. Res. 2021, 14, 141–162. [Google Scholar] [CrossRef]
- Yang, L.; Liu, L.; Zhang, R.; Hong, J.; Wang, Y.; Wang, J.; Zuo, J.; Zhang, J.; Chen, J.; Hao, H. IL-8 mediates a positive loop connecting increased neutrophil extracellular traps (NETs) and colorectal cancer liver metastasis. J. Cancer 2020, 11, 4384–4396. [Google Scholar] [CrossRef]
- Hesler, M.; Schwarz, D.H.; Dähnhardt-Pfeiffer, S.; Wagner, S.; von Briesen, H.; Wenz, G.; Kohl, Y. Synthesis and in vitro evaluation of cyclodextrin hyaluronic acid conjugates as a new candidate for intestinal drug carrier for steroid hormones. Eur. J. Pharm. Sci. 2020, 143, 105181. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, Y.; Liang, X.; Liu, S.; Cao, X.; Chen, N.; Chen, Z.; Yan, J. Monitoring the differentiation of dimethyl sulfoxide-induced human leukemia (HL-60) cells by Raman spectroscopy. J. Raman Spectrosc. 2021, 52, 1086–1094. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Supjaroen, P.; Niamsi, W.; Thummarati, P.; Laiwattanapaisal, W. An In Vitro Cell Model of Intestinal Barrier Function Using a Low-Cost 3D-Printed Transwell Device and Paper-Based Cell Membrane. Int. J. Mol. Sci. 2025, 26, 2524. https://doi.org/10.3390/ijms26062524
Supjaroen P, Niamsi W, Thummarati P, Laiwattanapaisal W. An In Vitro Cell Model of Intestinal Barrier Function Using a Low-Cost 3D-Printed Transwell Device and Paper-Based Cell Membrane. International Journal of Molecular Sciences. 2025; 26(6):2524. https://doi.org/10.3390/ijms26062524
Chicago/Turabian StyleSupjaroen, Pitaksit, Wisanu Niamsi, Parichut Thummarati, and Wanida Laiwattanapaisal. 2025. "An In Vitro Cell Model of Intestinal Barrier Function Using a Low-Cost 3D-Printed Transwell Device and Paper-Based Cell Membrane" International Journal of Molecular Sciences 26, no. 6: 2524. https://doi.org/10.3390/ijms26062524
APA StyleSupjaroen, P., Niamsi, W., Thummarati, P., & Laiwattanapaisal, W. (2025). An In Vitro Cell Model of Intestinal Barrier Function Using a Low-Cost 3D-Printed Transwell Device and Paper-Based Cell Membrane. International Journal of Molecular Sciences, 26(6), 2524. https://doi.org/10.3390/ijms26062524





