TGF-β Induces the Secretion of Extracellular Vesicles Enriched with CD39 and CD73 from Cervical Cancer Cells
Abstract
1. Introduction
2. Results
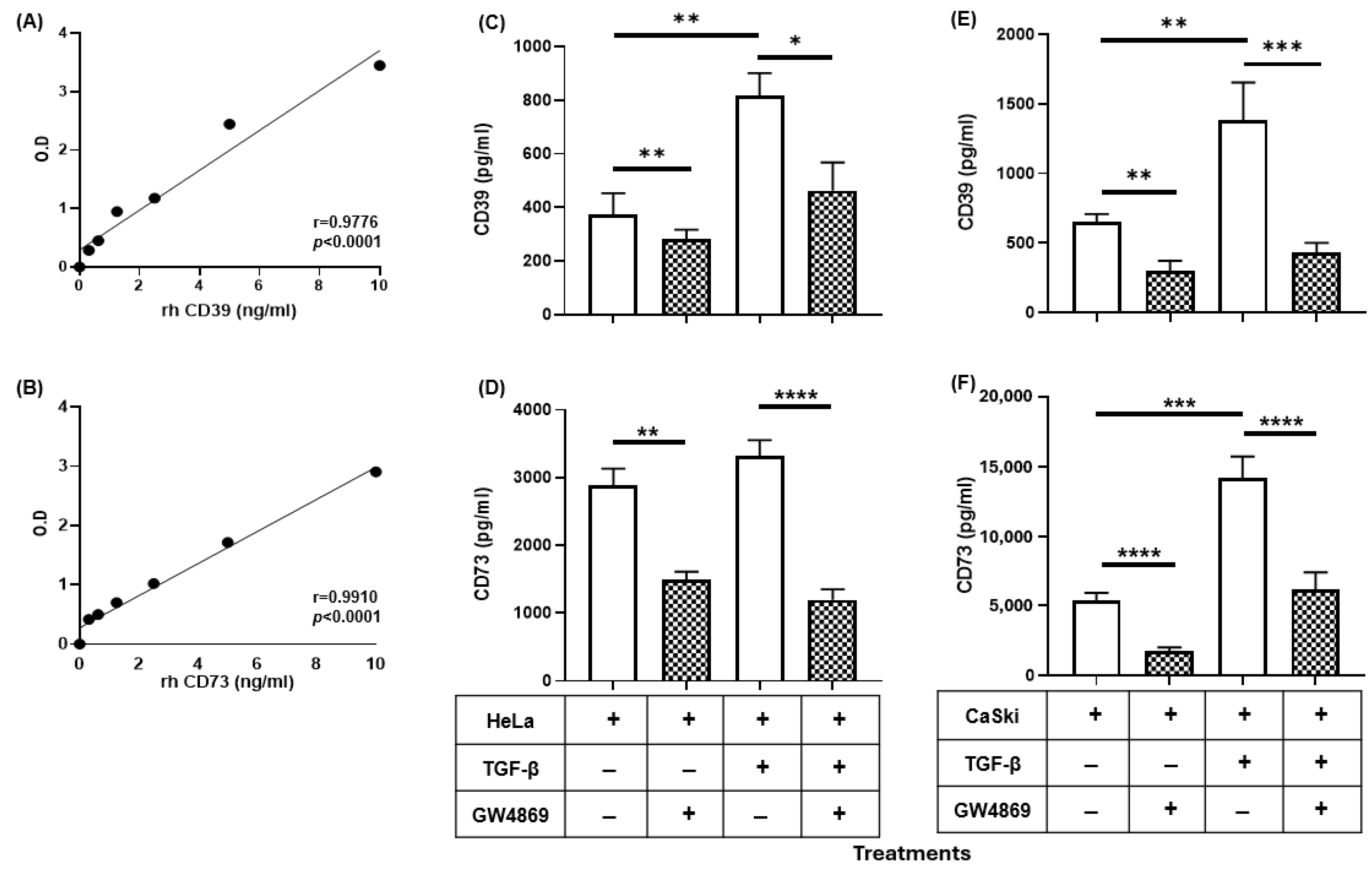
2.1. TGF-β Induced the Expression of CD39 and CD73 in CC Cells
2.2. GW4869 Abrogated the Increase in the Release of CD39 and CD73 Induced by TGF-β in CC Cells

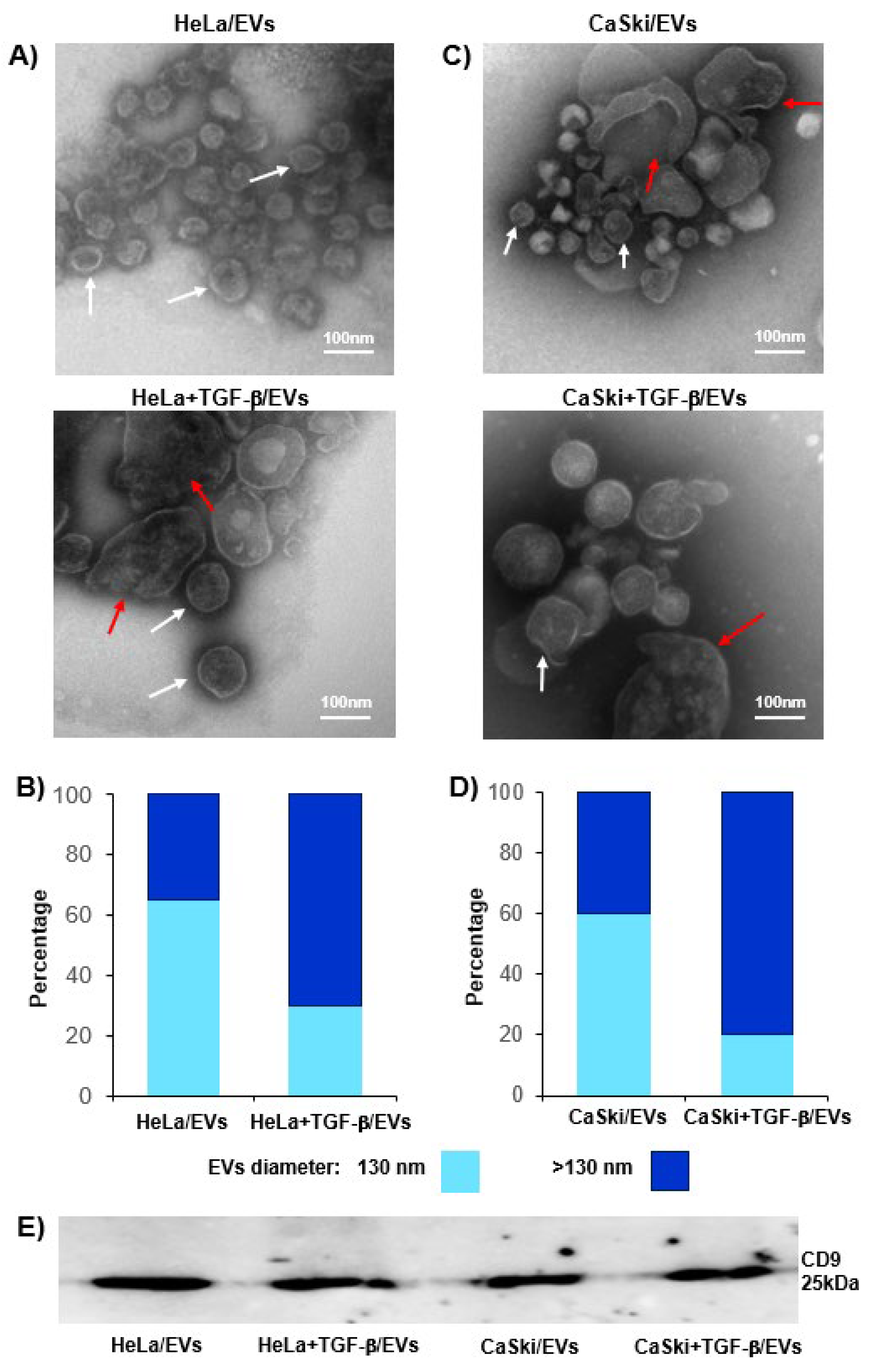
2.3. TGF-β Increased the Release of CD39- and CD73-Positive EVs from CC Cells
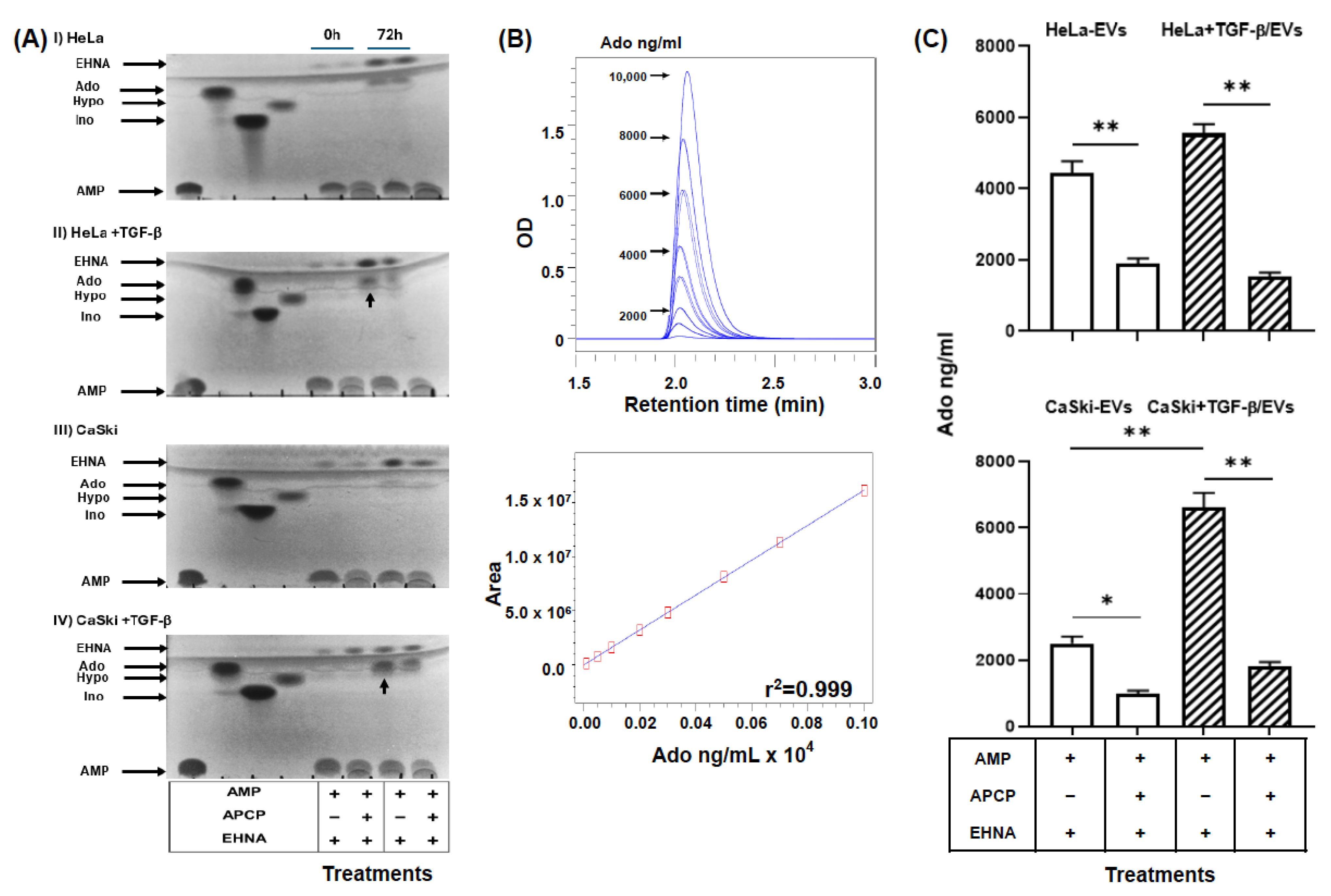
2.4. EVs Were Secreted by TGF-β-Stimulated CC Cells and Had a High Capacity to Generate Ado
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Expression of CD39 and CD73
4.3. Isolation of EVs
4.4. Analysis of EVs
4.5. Western Blot Analysis
4.6. Detection and Quantification of CD39 and CD73
4.7. Evaluation of CD73 Hydrolytic Activity in EVs
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ado | adenosine |
| ADA | Adenosine deaminase |
| ADP | adenosine diphosphate |
| AMP | adenosine monophosphate |
| APCP | adenosine 5′-(α,β-methylene)diphosphate |
| ARs | adenosine receptors |
| ATP | adenosine triphosphate |
| CC | cervical cancer |
| CD39 | ectonucleoside triphosphate diphosphohydrolase-1 |
| CD73 | 5′-ectonucleotidase |
| EHNA | erythro-9-(2-hydroxy-3-nonyl) adenine |
| ELISA | enzyme-linked immunosorbent assay |
| EVs | extracellular vesicles |
| HR-HPV | high-risk human papillomavirus |
| MFI | mean fluorescence intensity |
| MRS1754 | A2BR antagonist |
| PD-1 | programmed cell death-1 |
| PD-L1 | programmed death-ligand 1 |
| TGF-β1 | transforming growth factor-beta 1 |
| TLC | thin-layer chromatography |
| TME | tumor microenvironment |
| UPLC | ultra-performance liquid chromatography |
| ZM241385 | A2AR antagonist |
References
- Filho, A.M.; Laversanne, M.; Ferlay, J.; Colombet, M.; Piñeros, M.; Znaor, A.; Parkin, D.M.; Soerjomataram, I.; Bray, F. The GLOBOCAN 2022 cancer estimates: Data sources, methods, and a snapshot of the cancer burden worldwide. Int. J. Cancer 2025, 156, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Georges, D.; Man, I.; Baussano, I.; Clifford, G.M. Causal attribution of human papillomavirus genotypes to invasive cervical cancer worldwide: A systematic analysis of the global literature. Lancet 2024, 404, 435–444. [Google Scholar] [CrossRef]
- Cicchini, L.; Chakraborty, A.; Kong, Y.; Ketkar, A. Suppression of antitumor immune responses by human papillomavirus through epigenetic downregulation of CXCL14. mBio 2016, 7, e00270-16. [Google Scholar] [CrossRef]
- Hasan, U.A.; Bates, E.; Takeshita, F.; Biliato, A.; Accardi, R.; Landsman, D. The human papillomavirus type 16 E7 oncoprotein induces a transcriptional repressor complex on the toll-like receptor 9 promoter. J. Exp. Med. 2013, 210, 1369–1387. [Google Scholar] [CrossRef] [PubMed]
- Georgopoulos, N.T.; Proffitt, J.L.; Blair, G.E. Transcriptional regulation of the major histocompatibility complex (MHC) class I heavy chain, TAP1, and LMP2 genes by the human papillomavirus (HPV) type 6b, 16, and 18 E7 oncoproteins. Oncogene 2000, 19, 4930–4935. [Google Scholar] [CrossRef] [PubMed]
- Piersma, S.J.; Jordanova, E.S.; van Poelgeest, M.I.E.; Kwappenberg, K.M.C.; van der Hulst, J.M.; Drijfhout, J.W.; Melief, C.J.M.; Kenter, G.G. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Cancer Res. 2007, 67, 354–361. [Google Scholar] [CrossRef]
- Gu, M.; Liu, J.; Li, S.; Wang, J.; Xu, L. Single-cell RNA sequencing reveals multiple pathways, and the tumor microenvironment could lead to chemotherapy resistance in cervical cancer. Front. Oncol. 2021, 11, 753386. [Google Scholar] [CrossRef]
- Bahreyni, A.; Khazaei, M.; ShahidSales, S.; Ghayour-Mobarhan, M.; Maftouh, M.; Hassanian, S.M.; Avan, A. Adenosine: An endogenous mediator in the pathogenesis of gynecological cancer. J. Cell. Physiol. 2018, 233, 2715–2722. [Google Scholar] [CrossRef]
- Pfaffenzeller, M.S.; Babinska, A.; Shibata, Y.; Robson, S.C.; Burnstock, G. Purinergic signaling and tumor microenvironment in cervical cancer. Purinergic Signal. 2020, 16, 123–135. [Google Scholar] [CrossRef]
- Linden, J. Molecular approach to adenosine receptors: Receptor-mediated mechanisms of tissue protection. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 775–787. [Google Scholar] [CrossRef]
- Allard, B.; Beavis, P.A.; Darcy, P.K.; Stagg, J. Immunosuppressive activities of adenosine in cancer. Curr. Opin. Pharmacol. 2016, 29, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Allard, B.; Longhi, M.S.; Robson, S.C.; Stagg, J. The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets. Immunol. Rev. 2017, 276, 121–144. [Google Scholar] [CrossRef] [PubMed]
- Ryzhov, S.; Novitskiy, S.V.; Goldstein, D.M.; Carbone, D.P.; Biaggioni, I.; Feoktistov, I. Role of TGF-β signaling in generation of CD39+CD73+ myeloid cells in tumors. J. Immunol. 2014, 193, 3155–3164. [Google Scholar] [CrossRef]
- Giraulo, C.; Polverino, F.; Napolitano, A.; Di Lorenzo, G. The CD73 is induced by TGF-β1 triggered by nutrient deprivation and highly expressed in dedifferentiated human melanoma. Biomed. Pharmacother. 2023, 165, 115225. [Google Scholar] [CrossRef]
- Torres-Poveda, K.; Bahena-Román, M.; Madrid-González, C.; Burguete-García, A.I.; Bermúdez-Morales, V.H.; Peralta-Zaragoza, O.; Madrid-Marina, V. Role of IL-10 and TGF-beta1 in local immunosuppression in HPV-associated cervical neoplasia. World J. Clin. Oncol. 2014, 5, 753–763. [Google Scholar] [CrossRef]
- Zhu, H.; Luo, H.; Zhang, W.; Shen, Z. Transforming growth factor-beta1 in carcinogenesis, progression, and therapy in cervical cancer. Tumor Biol. 2016, 37, 7075–7083. [Google Scholar] [CrossRef]
- García-Rocha, R.; Monroy-García, A.; Hernández-Montes, J.; Weiss-Steider, B.; Gutiérrez-Serrano, V.; Mora-García, M.L. Cervical cancer cells produce TGF-β1 through the CD73-adenosine pathway and maintain CD73 expression through the autocrine activity of TGF-β1. Cytokine 2019, 118, 71–79. [Google Scholar] [CrossRef]
- Clayton, A.; Al-Taei, S.; Webber, J.; Mason, M.D.; Tabi, Z. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J. Immunol. 2011, 187, 676–683. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MIDSEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Yokoi, A.; Matsuzaki, J. Exosomes and extracellular vesicles: Rethinking the essential values in cancer biology. Semin. Cancer Biol. 2021, 74, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.; Lam, T.K.; Hebert, E.; Divi, R.L. Extracellular vesicles: Potential applications in cancer diagnosis, prognosis, and epidemiology. BMC Clin. Pathol. 2015, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I.; Karin, M.; Wieland, H. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Bao, Q.; Ren, Z.; Hu, L.; Cheng, S.; Zhang, Z. Tumor-derived extracellular vesicles regulate cancer progression in the tumor microenvironment. Front. Mol. Biosci. 2022, 8, 796385. [Google Scholar] [CrossRef]
- Bonowicz, K.; Sadej, R.; Kozlowska, E. Mechanism of extracellular vesicle secretion associated with TGF-β-dependent inflammatory response in the tumor microenvironment. Int. J. Mol. Sci. 2022, 23, 15335. [Google Scholar] [CrossRef]
- Kang, J.H.; Kim, S.H.; Lee, S.W.; Lee, E.J.; Kim, J.W.; Song, H.S. Transforming growth factor beta induces fibroblasts to express and release the immunomodulatory protein PD-L1 into extracellular vesicles. FASEB J. 2020, 34, 2213–2226. [Google Scholar] [CrossRef]
- Chatterjee, S.; Azad, S.; Nair, S.; Manna, S. Transforming growth factor beta orchestrates PD-L1 enrichment in tumor-derived exosomes and mediates CD8 T-cell dysfunction regulating early phosphorylation of TCR signalome in breast cancer. Carcinogenesis 2021, 42, 38–47. [Google Scholar] [CrossRef]
- Yokoi, A.; Ukai, M.; Yasui, T.; Inokuma, Y.; Hyeon-Deuk, K.; Matsuzaki, J.; Yoshida, K.; Kitagawa, M.; Chattrairat, K.; Iida, M.; et al. Identifying high-grade serous ovarian carcinoma-specific extracellular vesicles by polyketone-coated nanowires. Sci. Adv. 2023, 7, eade6958. [Google Scholar] [CrossRef]
- Nagao, Y.; Yokoi, A.; Yoshida, K.; Kitagawa, M.; Asano-Inami, E.; Kato, T.; Ishikawa, M.; Yamamoto, Y.; Kajiyama, H. ; Yoshida, K.; Kitagawa, M.; Asano-Inami, E.; Kato, T.; Ishikawa, M.; Yamamoto, Y.; Kajiyama, H. Uterine leiomyosarcoma cell-derived extracellular vesicles induce the formation of cancer-associated fibroblasts. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167103. [Google Scholar] [CrossRef]
- Perez, G.I.; Bernard, M.P.; Vocelle, D.; Zarea, A.A.; Saleh, N.A.; Gagea, M.A.; Schneider, D.; Bauzon, M.; Hermiston, T.; Kanada, M. Phosphatidylserine-Exposing Annexin A1-Positive Extracellular Vesicles: Potential Cancer Biomarkers. Vaccines 2023, 11, 639. [Google Scholar] [CrossRef] [PubMed]
- Bebelman, M.P.; Smit, M.J.; Pegtel, D.M.; Baglio, S.R. Biogenesis and function of extracellular vesicles in cancer. Pharmacol. Ther. 2018, 188, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Clancy, J.W.; Sedgwick, A.; Rosse, C.; Muralidharan-Chari, V.; Raposo, G. Tumor-derived extracellular vesicles: Multifunctional entities in the tumor microenvironment. Annu. Rev. Pathol. Mech. Dis. 2023, 18, 205–229. [Google Scholar] [CrossRef]
- Robbins, P.D.; Morelli, A.E. Regulation of immune responses by extracellular vesicles. Nat. Rev. Immunol. 2014, 14, 195–208. [Google Scholar] [CrossRef]
- Abusamra, A.J.; Zhong, Z.; Zheng, X.; Li, M.; Ichim, T.E.; Min, W.P. Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol. Dis. 2005, 35, 169–173. [Google Scholar] [CrossRef]
- Wieckowski, E.U.; Visus, C.; Szajnik, M.; Szczepanski, M.J.; Storkus, W.J.; Whiteside, T.L. Tumor-derived microvesicles promote regulatory T cell expansion and induce apoptosis in tumor-reactive activated CD8+ T lymphocytes. J. Immunol. 2009, 183, 3720–3730. [Google Scholar] [CrossRef]
- Ludwig, S.; Sharma, P.; Theodoraki, M.N.; Pietrowska, M.; Yerneni, S.S.; Lang, S.; Ferrone, S.; Whiteside, T.L. Molecular and functional profiles of exosomes from HPV(+) and HPV(-) head and neck cancer cell lines. Front. Oncol. 2018, 8, 445. [Google Scholar] [CrossRef]
- Harden, M.E.; Munger, K. Human papillomavirus 16 E6 and E7 oncoprotein expression alters microRNA expression in extracellular vesicles. Virology 2017, 508, 63–69. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Meng, L.; Li, W.; Li, C.; Xu, S. Changes of miRNA expression profiles from cervical-vaginal fluid-derived exosomes in response to HPV16 infection. Biomed. Res. Int. 2020, 2020, 7046894. [Google Scholar] [CrossRef]
- Gezer, U.; Özgür, E.; Cetinkaya, M.; Isin, M.; Dalay, N. Long non-coding RNAs with low expression levels in cells are enriched in secreted exosomes. Cell Biol. Int. 2014, 38, 1076–1079. [Google Scholar] [CrossRef]
- Wang, H.; Wei, M.; Kang, Y.; Xing, J.; Zhao, Y. Circular RNA circ_PVT1 induces epithelial-mesenchymal transition to promote metastasis of cervical cancer. Aging 2020, 12, 20139–20151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhou, Q.; Wei, Y.; Da, M.; Zhang, C.; Shen, J. The exosome-mediated PI3k/Akt/mTOR signaling pathway in cervical cancer. Int. J. Clin. Exp. Pathol. 2019, 12, 2474–2484. [Google Scholar] [PubMed]
- Mata-Rocha, M.; Rodríguez-Hernández, R.M.; Chávez-Olmos, P.; Garrido, E.; Robles-Vázquez, C.; Aguilar-Ruiz, S.; Romero-Tlalolini, M.L. Presence of HPV DNA in extracellular vesicles from HeLa cells and cervical samples. Enferm. Infecc. Microbiol. Clín. Engl. Ed. 2020, 38, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, N.; Gillespie, D.G.; Reichert, T.E.; Jackson, E.K.; Whiteside, T.L. Purine metabolites in tumor-derived exosomes may facilitate immune escape of head and neck squamous cell carcinoma. Cancers 2020, 12, 1602. [Google Scholar] [CrossRef]
- Lin, H.; Huang, C.C.; Ou, Y.C.; Huang, E.Y.; Changchien, C.C.; Tseng, C.W.; Fu, H.C.; Wu, C.H.; Li, C.J.; Ma, Y.Y. High immunohistochemical expression of TGF-β1 predicts a poor prognosis in cervical cancer patients who harbor enriched endoglin microvessel density. Int. J. Gynecol. Pathol. 2012, 31, 482–489. [Google Scholar] [CrossRef]
- Comerci, J.T., Jr.; Runowicz, C.D.; Flanders, K.C.; De Victoria, C.; Fields, A.L.; Kadish, A.S.; Goldberg, G.L. Altered expression of transforming growth factor-beta 1 in cervical neoplasia as an early biomarker in carcinogenesis of the uterine cervix. Cancer 1996, 77, 1107–1114. [Google Scholar] [CrossRef]
- Mora-García, M.L.; Ávila-Ibarra, L.R.; García-Rocha, R.; Weiss-Steider, B.; Hernandez-Montes, J.; Don-López, C.A.; Gutiérrez-Serrano, V.; Titla-Vilchis, I.J.; Fuentes-Castaneda, M.C.; Monroy-Mora, A.; et al. Cervical cancer cells suppress effector functions of cytotoxic T cells through the adenosinergic pathway. Cell. Immunol. 2017, 320, 46–55. [Google Scholar] [CrossRef]
- Mora-García, M.L.; López-Cisneros, S.; Gutiérrez-Serrano, V.; García-Rocha, R.; Weiss-Steider, B.; Hernández-Montes, J.; Sánchez-Peña, H.I.; Ávila-Ibarra, L.R.; Don-López, C.A.; Muñóz-Godínez, R.; et al. HPV-16 infection is associated with a high content of CD39 and CD73 ectonucleotidases in cervical samples from patients with CIN-1. Mediat. Inflamm. 2019, 2019, 4651627. [Google Scholar] [CrossRef]
- Muñóz-Godínez, R.; Mora-García, M.L.; Weiss-Steider, B.; Montesinos-Montesinos, J.J.; Aguilar-Lemarroy, A.C.; García-Rocha, R.; Hernández-Montes, J.; Don-López, C.A.; Ávila-Ibarra, L.R.; Torres-Pineda, D.B.; et al. Detection of CD39 and a highly glycosylated isoform of soluble CD73 in the plasma of patients with cervical cancer: Correlation with disease progression. Mediators Inflamm. 2020, 2020, 1678780. [Google Scholar] [CrossRef]
- Cristiana, I.I.; Santin, B.A.P.; Beckenkamp, L.R.; Lopes, C.M.E.; Maria-Engler, S.S.; Wink, M.R. Adenosinergic Signalling in Cervical Cancer Microenvironment. Expert. Rev. Mol. Med. 2025, 27, e5. [Google Scholar] [CrossRef]
- Iancu, I.V.; Botezatu, A.; Goia-Rusanu, C.D.; Stanescu, A.; Huica, I.; Nistor, E.; Anton, G.; Plesa, A. TGF-beta signalling pathway factors in HPV-induced cervical lesions, Roum. Arch. Microbiol. Immunol. 2010, 69, 113–118. [Google Scholar]
- Daniel, C.W.; Robinson, S.D. Regulation of mammary growth and function by TGFbeta. Mol. Reprod. Dev. 1992, 32, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, L.; Chen, X.; Li, L.; Li, Y.; Ping, Y.; Huang, L.; Yue, D.; Zhang, Z.; Wang, F.; et al. CD39/CD73 upregulation on myeloid-derived suppressor cells via TGF-β-mTOR-HIF-1 signaling in patients with non-small cell lung cancer. Oncoimmunology 2017, 6, e1320011. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Liao, X.; Li, L.; Lv, L.; Zhi, X.; Yu, J.; Zhou, P. A preliminary study of the role of extracellular 5′-nucleotidase in breast cancer stem cells and epithelial-mesenchymal transition. In Vitro Cell. Dev. Biol. Anim. 2017, 53, 132–140. [Google Scholar] [CrossRef]
- Leclerc, B.G.; Charlebois, R.; Chouinard, G.; Allard, B.; Pommey, S.; Saad, F.; Stagg, J. CD73 expression is an independent prognostic factor in prostate cancer. Clin. Cancer Res. 2016, 22, 158–166. [Google Scholar] [CrossRef]
- Fausther, M.; Sheung, N.; Saiman, Y.; Bansal, M.B.; Dranoff, J.A. Activated hepatic stellate cells upregulate transcription of ecto-5′-nucleotidase/CD73 via specific SP1 and SMAD promoter elements. Am. J. Physiol.-Gastroint. Liver Physiol. 2012, 303, G904–G914. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Köhler, D.; Eckle, T.; Kong, T.; Robson, S.C.; Colgan, S.P. Central role of Sp1-regulated CD39 in hypoxia/ischemia protection. Blood 2009, 113, 224–232. [Google Scholar] [CrossRef]
- Spychala, J.; Kitajewski, J. Wnt and beta-catenin signaling target the expression of ecto-5′-nucleotidase and increase extracellular adenosine generation. Exp. Cell Res. 2004, 296, 99–108. [Google Scholar] [CrossRef]
- Catalano, M.; O’Driscoll, L. Inhibiting extracellular vesicles formation and release: A review of EV inhibitors. J. Extracell. Vesicles 2019, 9, 1703244. [Google Scholar] [CrossRef]
- Schneider, E.; Wyss, M.; Hackenhaar, F.S.; Fecher-Trost, C.; Woehleke, C.; Smola, S.; Schneider, B. CD73-mediated adenosine production by CD8 T cell-derived extracellular vesicles constitutes an intrinsic mechanism of immune suppression. Nat. Commun. 2021, 12, 5911. [Google Scholar] [CrossRef]
- Ciardiello, C.; Migliorino, R.; Leone, A.; Budillon, A. Large extracellular vesicles: Size matters in tumor progression. Cytokine Growth Factor. Rev. 2020, 51, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Fricke, F.; Mussack, V.; Buschmann, D.; Hausser, I.; Pfaffl, M.W.; Kopitz, J.; Gebert, J. TGFBR2-dependent alterations of microRNA profiles in extracellular vesicles and parental colorectal cancer cells. Int. J. Oncol. 2019, 55, 925–937. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues-Junior, D.M.; Tsirigoti, C.; Psatha, K.; Kletsas, D.; Aivaliotis, M.; Heldin, C.H.; Moustakas, A. TGF-β Induces Cholesterol Accumulation to Regulate the Secretion of Tumor-Derived Extracellular Vesicles. J. Exp. Clin. Cancer Res. 2025, 44, 42. [Google Scholar] [CrossRef]
- Rodrigues-Junior, D.M.; Tsirigoti, C.; Lim, S.K.; Heldin, C.H.; Moustakas, A. Extracellular Vesicles and Transforming Growth Factor β Signaling in Cancer. Front. Cell Dev. Biol. 2022, 10, 849938. [Google Scholar] [CrossRef]
- Ran, Z.; Liu, C.; Zhou, Z. Advances in exosome biomarkers for cervical cancer. Cancer Med. 2022, 11, 4966–4978. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, S.; Zhang, L. Application of extracellular vesicles in gynecologic cancer treatment. Bioengineering 2022, 9, 740. [Google Scholar] [CrossRef]
- Mora-Garcia, M.L.; Garcia-Rocha, R.; Morales-Ramirez, O.; Montesinos, J.J.; Weiss-Steider, B.; Hernandez-Montes, J.; Avila-Ibarra, L.R.; Don-Lopez, C.A.; Velasco-Velazquez, M.A.; Gutierrez-Serrano, V.; et al. Mesenchymal stromal cells derived from cervical cancer produce high amounts of adenosine to suppress cytotoxic T lymphocyte functions. J. Transl. Med. 2016, 14, 302. [Google Scholar] [CrossRef]
- Montesinos, J.J.; Flores-Figueroa, E.; Castillo-Medina, S.; Flores-Guzmán, P.; Fajardo-Orduña, G.; García-Ruiz, C.; Mayani, H. Human bone marrow mesenchymal stem/stromal cells exposed to an inflammatory environment increase the expression of ICAM-1 and release microvesicles enriched in this adhesive molecule: Analysis of the participation of TNF-α and IFN-γ. J. Immunol. Res. 2020, 2020, 8839625. [Google Scholar] [CrossRef]
- García-Rocha, R.; Monroy-García, A.; Carrera-Martínez, M.; Hernández-Montes, J.; Don-López, C.A.; Weiss-Steider, B.; Monroy-Mora, K.A.; Ponce-Chavero, M.L.Á.; Montesinos-Montesinos, J.J.; Escobar-Sánchez, M.L.; et al. Evidence that cervical cancer cells cultured as tumorspheres maintain high CD73 expression and increase their protumor characteristics through TGF-β production. Cell Biochem. Funct. 2022, 40, 760–772. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina-Castillo, G.; Monroy-García, A.; García-Rocha, R.; Weiss-Steider, B.; Montesinos-Montesinos, J.J.; Hernández-Montes, J.; Don-López, C.A.; Castro-Manrreza, M.E.; Escobar-Sánchez, M.L.; Mora-García, M.d.L. TGF-β Induces the Secretion of Extracellular Vesicles Enriched with CD39 and CD73 from Cervical Cancer Cells. Int. J. Mol. Sci. 2025, 26, 2413. https://doi.org/10.3390/ijms26062413
Molina-Castillo G, Monroy-García A, García-Rocha R, Weiss-Steider B, Montesinos-Montesinos JJ, Hernández-Montes J, Don-López CA, Castro-Manrreza ME, Escobar-Sánchez ML, Mora-García MdL. TGF-β Induces the Secretion of Extracellular Vesicles Enriched with CD39 and CD73 from Cervical Cancer Cells. International Journal of Molecular Sciences. 2025; 26(6):2413. https://doi.org/10.3390/ijms26062413
Chicago/Turabian StyleMolina-Castillo, Gabriela, Alberto Monroy-García, Rosario García-Rocha, Benny Weiss-Steider, Juan José Montesinos-Montesinos, Jorge Hernández-Montes, Christian Azucena Don-López, Marta Elena Castro-Manrreza, María Luisa Escobar-Sánchez, and María de Lourdes Mora-García. 2025. "TGF-β Induces the Secretion of Extracellular Vesicles Enriched with CD39 and CD73 from Cervical Cancer Cells" International Journal of Molecular Sciences 26, no. 6: 2413. https://doi.org/10.3390/ijms26062413
APA StyleMolina-Castillo, G., Monroy-García, A., García-Rocha, R., Weiss-Steider, B., Montesinos-Montesinos, J. J., Hernández-Montes, J., Don-López, C. A., Castro-Manrreza, M. E., Escobar-Sánchez, M. L., & Mora-García, M. d. L. (2025). TGF-β Induces the Secretion of Extracellular Vesicles Enriched with CD39 and CD73 from Cervical Cancer Cells. International Journal of Molecular Sciences, 26(6), 2413. https://doi.org/10.3390/ijms26062413




