Relationship Between Lifestyle and Physical Fitness Among Older Women with Sarcopenia
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
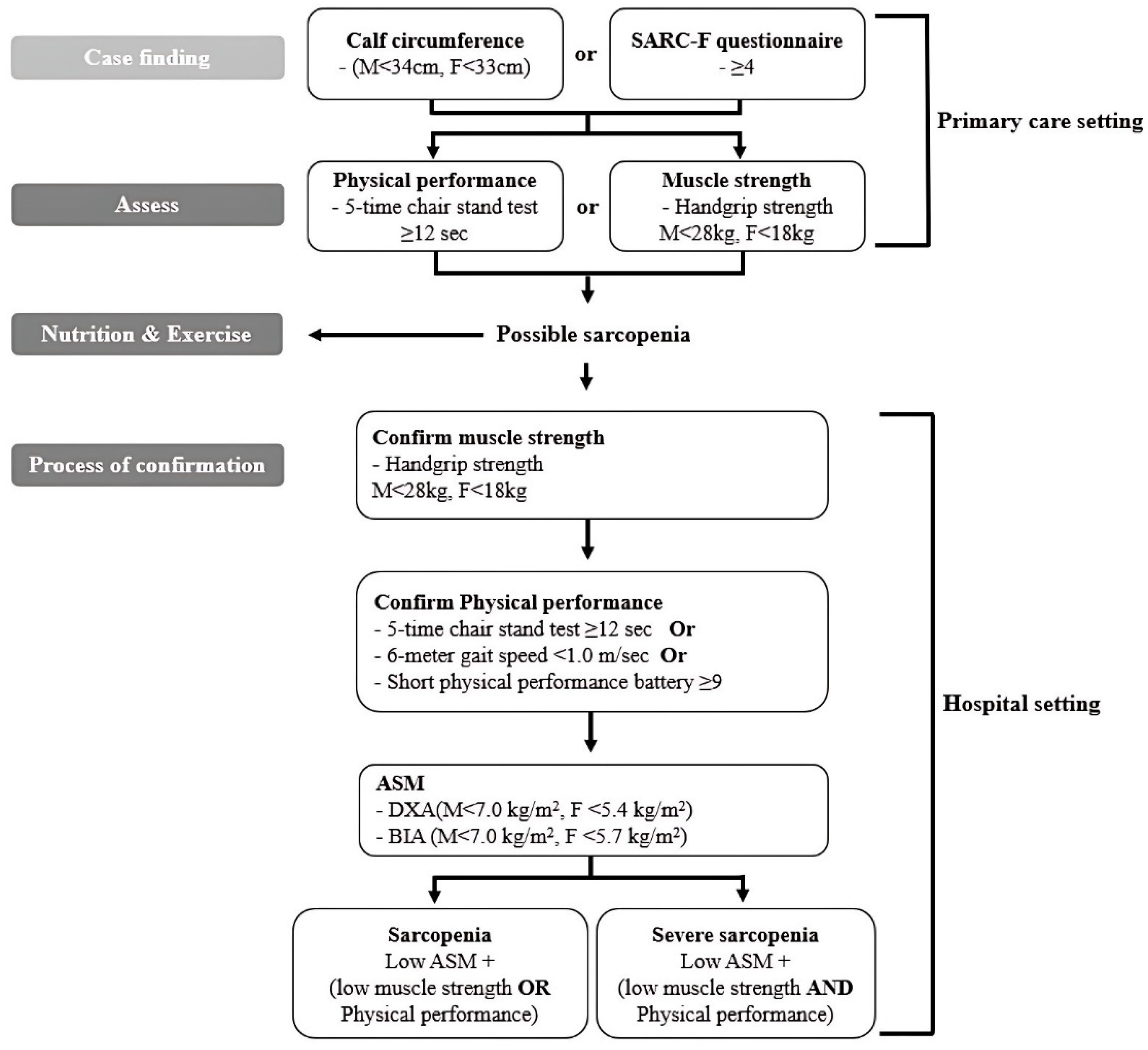
4.1. Participants
4.2. Measurement of Health and Physical Fitness Factors
- HG: To measure HG, we utilized a hand dynamometer. We instructed the participant to exert maximal force on the dynamometer for approximately three seconds, ensuring that the arm was positioned at a right angle with the elbow close to the body.
- PF: To measure PF, we used an isokinetic dynamometer. We instructed the participant to perform maximal plantar flexion against the resistance provided by the device, ensuring that the knee was extended, and the foot was positioned correctly on the footplate.
- DF: To measure DF, we used an isokinetic dynamometer. We instructed the participant to perform maximal dorsiflexion against the resistance provided by the device, ensuring that the knee was extended, and the foot was properly aligned on the footplate.
- SPPB: The SPPB was used to assess lower extremity function using three components: balance tests, a gait speed test, and a chair stand test. Each component was scored from 0 to 4, with higher scores indicating better performance and the total score ranging from 0 to 12.
- TUG: The TUG test was used to measure mobility and balance. The participant began seated in a chair, stood up, walked a distance of three meters, turned around, walked back to the chair, and sat down. The time taken to complete the task was recorded, with shorter times indicating better functional mobility.
- 2-min walking test: The 2-min walking test was used to measure the distance the participant could walk in two minutes. Without moving forward, their knees should rise to a specific marking height to recognize the walking cadence.
4.3. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LMM | Low muscle mass |
| LMS | Low muscle strength |
| ASM | Appendicular skeletal muscle |
| BMI | Body mass index |
| CC | Calf circumference |
| HG | Hand grip |
| PF | Plantar flexion |
| DF | Dorsalflexion |
| SPPB | Short physical performance battery |
| TUG | Timed up-and-go |
| ANOVA | Analysis of variance |
| SPARC | Secreted Protein Acidic and Rich in Cysteine |
| PI3K | phosphatidylinositol 4,5-bisphosphate 3-kinase |
| IGF-1 | Insulin-like Growth Factor 1 |
| IL-6 | interleukin 6 |
| BDNF | Brain-derived neurotrophic factor |
| TNF-α | Tumor necrosis factor-α |
References
- United Nations Department of Economic and Social Affairs. World Population Ageing 2019. Available online: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Report.pdf (accessed on 3 March 2024).
- Bilski, J.; Pierzchalski, P.; Szczepanik, M.; Bonior, J.; Zoladz, J.A. Multifactorial mechanism of sarcopenia and sarcopenic obesity. Role of physical exercise, microbiota and myokines. Cells 2022, 11, 160. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Bache, R.J. Muscle and Exercise Physiology; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- He, N.; Zhang, Y.; Zhang, L.; Zhang, S.; Ye, H. Relationship Between Sarcopenia and Cardiovascular Diseases in the Elderly: An Overview. Front. Cardiovasc. Med. 2021, 8, 743710. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Morley, J.E.; von Haehling, S. Welcome to the ICD-10 code for sarcopenia. J. Cachexia Sarcopenia Muscle 2016, 7, 512–514. [Google Scholar] [CrossRef]
- Williams, G.R.; Dunne, R.F.; Giri, S.; Shachar, S.S.; Caan, B.J. Sarcopenia in the Older Adult with Cancer. J. Clin. Oncol. 2021, 39, 2068–2078. [Google Scholar] [CrossRef]
- Agarwal, E.; Miller, M.; Yaxley, A.; Isenring, E. Malnutrition in the elderly: A narrative review. Maturitas 2013, 76, 296–302. [Google Scholar] [CrossRef]
- Calvani, R.; Picca, A.; Coelho-Júnior, H.J.; Tosato, M.; Marzetti, E.; Landi, F. Diet for the prevention and management of sarcopenia. Metabolism 2023, 146, 155637. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.R.; Lee, S.; Song, S.K. A Review of Sarcopenia Pathophysiology, Diagnosis, Treatment and Future Direction. J. Korean Med. Sci. 2022, 37, e146. [Google Scholar] [CrossRef] [PubMed]
- Bahat, G.; Yilmaz, O.; Kılıç, C.; Oren, M.M.; Karan, M.A. Performance of SARC-F in Regard to Sarcopenia Definitions, Muscle Mass and Functional Measures. J. Nutr. Health Aging 2018, 22, 898–903. [Google Scholar] [CrossRef]
- Ishii, S.; Tanaka, T.; Shibasaki, K.; Ouchi, Y.; Kikutani, T.; Higashiguchi, T.; Obuchi, S.P.; Ishikawa-Takata, K.; Hirano, H.; Kawai, H.; et al. Development of a simple screening test for sarcopenia in older adults. Geriatr. Gerontol. Int. 2014, 14, 93–101. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.K.; Fielding, R.A.; Martin, F.C.; Michel, J.P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef]
- Kwon, H.J.; Ha, Y.C.; Park, H.M. Prevalence of Sarcopenia in the Korean Woman Based on the Korean National Health and Nutritional Examination Surveys. J. Bone Metab. 2016, 23, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.C. How to Diagnose Sarcopenia in Korean Older Adults? Ann. Geriatr. Med. Res. 2018, 22, 73–79. [Google Scholar] [CrossRef]
- Park, H.M. Current Status of Sarcopenia in Korea: A Focus on Korean Geripausal Women. Ann. Geriatr. Med. Res. 2018, 22, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Sung, J.-Y.; Kim, J. Effect of Low-Intensity High-Repetition Versus High-Intensity Low-Repetition Elastic Band Resistance Training on Functional Physical Fitness and Myokine Levels in Older Adults. Appl. Sci. 2025, 15, 757. [Google Scholar] [CrossRef]
- Gomarasca, M.; Banfi, G.; Lombardi, G. Myokines: The endocrine coupling of skeletal muscle and bone. Adv. Clin. Chem. 2020, 94, 155–218. [Google Scholar]
- Ghanemi, A.; Melouane, A.; Yoshioka, M.; St-Amand, J. Secreted protein acidic and rich in cysteine (Sparc) KO Leads to an accelerated ageing phenotype which is improved by exercise whereas SPARC overexpression mimics exercise effects in mice. Metabolites 2022, 12, 125. [Google Scholar] [CrossRef]
- Mathes, S.; Fahrner, A.; Luca, E.; Krützfeldt, J. Growth hormone/IGF-I-dependent signaling restores decreased expression of the myokine SPARC in aged skeletal muscle. J. Mol. Med. 2022, 100, 1647–1658. [Google Scholar] [CrossRef]
- Di Rosa, M.C.; Zimbone, S.; Saab, M.W.; Tomasello, M.F. The pleiotropic potential of BDNF beyond Neurons: Implication for a healthy mind in a healthy body. Life 2021, 11, 1256. [Google Scholar] [CrossRef]
- Marzetti, E.; Calvani, R.; Tosato, M.; Cesari, M.; Di Bari, M.; Cherubini, A.; Collamati, A.; D’Angelo, E.; Pahor, M.; Bernabei, R.; et al. Sarcopenia: An overview. Aging Clin. Exp. Res. 2017, 29, 11–17. [Google Scholar] [CrossRef]
- Gao, Q.; Hu, K.; Yan, C.; Zhao, B.; Mei, F.; Chen, F.; Zhao, L.; Shang, Y.; Ma, Y.; Ma, B. Associated Factors of Sarcopenia in Community-Dwelling Older Adults: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 4291. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.J.; Kim, Y.H. Factors Affecting Sarcopenia in Korean Adults by Age Groups. Osong Public Health Res. Perspect. 2017, 8, 169–178. [Google Scholar] [CrossRef]
- Chang, K.V.; Hsu, T.H.; Wu, W.T.; Huang, K.C.; Han, D.S. Is sarcopenia associated with depression? A systematic review and meta-analysis of observational studies. Age Ageing 2017, 46, 738–746. [Google Scholar] [CrossRef] [PubMed]
- da Rocha, A.Q.; Lobo, P.C.B.; Pimentel, G.D. Muscle Function Loss and Gain of Body Weight during the COVID-19 Pandemic in Elderly Women: Effects of One Year of Lockdown. J. Nutr. Health Aging 2021, 25, 1028–1029. [Google Scholar] [CrossRef]
- Scott, D.; Sanders, K.M.; Aitken, D.; Hayes, A.; Ebeling, P.R.; Jones, G. Sarcopenic obesity and dynapenic obesity: 5-year associations with falls risk in middle-aged and older adults. Obesity 2014, 22, 1568–1574. [Google Scholar] [CrossRef]
- Dent, E.; Morley, J.E.; Cruz-Jentoft, A.J.; Arai, H.; Kritchevsky, S.B.; Guralnik, J.; Bauer, J.M.; Pahor, M.; Clark, B.C.; Cesari, M.; et al. International Clinical Practice Guidelines for Sarcopenia (ICFSR): Screening, Diagnosis and Management. J. Nutr. Health Aging 2018, 22, 1148–1161. [Google Scholar] [CrossRef]
- Lee, R.C.; Wang, Z.; Heo, M.; Ross, R.; Janssen, I.; Heymsfield, S.B. Total-body skeletal muscle mass: Development and cross-validation of anthropometric prediction models. Am. J. Clin. Nutr. 2000, 72, 796–803. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
| Variables | Total n (%) | Normal n (%) | Possible n (%) | Sarcopenia n (%) | Severe n (%) | F | p | |
|---|---|---|---|---|---|---|---|---|
| Age (years) | 60–69 | 101 (19.7) | 77 (39.1) | 19 (12.8) | 3 (4.3) | 2 (2) | 76.058 | <0.001 *** |
| 70–79 | 165 (32.2) | 85 (43.1) | 45 (30.4) | 22 (31.8) | 13 (13.3) | |||
| 80–89 | 204 (39.9) | 32 (16.2) | 72 (48.6) | 35 (50.8) | 65 (66.3) | |||
| >90 | 42 (8.2) | 3 (1.5) | 12 (8.1) | 9(13.1) | 18 (18.4) | |||
| Height (cm) | <149.9 | 191 (37.3) | 36 (18.3) | 68 (45.9) | 31 (44.9) | 56 (57.1) | 38.027 | <0.001 *** |
| 150–159.9 | 287 (56.1) | 134 (68.0) | 76 (51.4) | 37 (53.6) | 40 (40.8) | |||
| >160 | 34 (6.6) | 27 (13.7) | 4 (2.7) | 1 (1.5) | 2 (2.1) | |||
| Weight (kg) | <44.9 | 50 (9.8) | 8 (4.1) | 0 (0) | 10 (14.5) | 32 (32.7) | 109.464 | <0.001 *** |
| 45–49.9 | 66 (12.9) | 20 (10.2) | 2 (1.4) | 18 (26.1) | 26 (26.5) | |||
| 50–54.9 | 123 (24) | 50 (25.4) | 14 (9.5) | 36 (52.2) | 23 (23.5) | |||
| >55 | 273 (53.3) | 119 (60.3) | 132 (89.1) | 5 (7.2) | 17 (17.3) | |||
| BFM (%) | <19.9 | 12 (2.3) | 2 (1) | 0 (0) | 6 (8.7) | 4 (4.1) | 92.899 | <0.001 *** |
| 20–29.9 | 88 (17.2) | 39 (19.8) | 5 (3.4) | 15 (21.7) | 29 (29.6) | |||
| 30–39.9 | 271 (52.9) | 122 (61.9) | 60 (40.5) | 40 (58) | 49 (50) | |||
| >40 | 141 (27.6) | 34 (17.3) | 83 (56.1) | 8 (11.6) | 16 (16.3) | |||
| BMI (kg/m2) | <18.5 | 33 (6.5) | 16 (8.1) | 0 (0) | 6 (8.7) | 11 (11.2) | 93.037 | <0.001 *** |
| 18.6–24.9 | 197 (38.5) | 108 (54.9) | 20 (13.5) | 4 (5.8) | 65 (66.3) | |||
| 25–29.9 | 189 (36.9) | 63 (31.9) | 91 (61.5) | 13 (18.8) | 22 (22.5) | |||
| >30 | 93 (18.1) | 10 (5.1) | 37 (25) | 46 (66.7) | 0 (0) | |||
| ASM | <5.9 | 262 (51.1) | 93 (47.2) | 37 (25) | 65 (94.2) | 97 (98.9) | 137.385 | <0.001 *** |
| 6–8.9 | 222 (48.9) | 103 (52.2) | 111 (75) | 4 (5.8) | 1 (1.1) | |||
| >9 | 1 | 1 (0.6) | 0 (0) | 0 (0) | 0 (0) | |||
| Disease | Yes | 301 (58.8) | 103(52.2) | 89 (60.1) | 40 (58) | 69 (97.9) | 3.701 | 0.012 * |
| No | 211 (31.2) | 94 (47.8) | 59 (39.9) | 29 (42) | 29 (2.1) | |||
| Total | 512 | 197 | 148 | 69 | 98 | |||
| Variable | 60 s | 70 s | 80 s | 90 s | F | p | |||
|---|---|---|---|---|---|---|---|---|---|
| 1 | ASM | Normal | 6.37 ± 0.08 | 5.99 ± 0.08 | 5.83 ± 0.12 | 7.19 ± 0.41 | A: | 3.687 | 0.012 + |
| Possible | 7.04 ± 0.16 | 6.53 ± 0.10 | 6.41 ± 0.08 | 6.26 ± 0.20 | S: | 62.439 | <0.001 ### | ||
| Sarcopenia | 5.16 ± 0.49 | 5.34 ± 0.15 | 4.93 ± 4.68 | 4.42 ± 0.29 | A × S: | 2.272 | 0.017 * | ||
| Severe | 5.12 ± 0.49 | 5.15 ± 0.19 | 4.87 ± 0.09 | 4.55 ± 0.17 | |||||
| CC | Normal | 34.66 ± 0.28 | 34.03 ± 0.27 | 32.93 ± 0.43 | 34.00 ± 1.41 | A: | 1.148 | 0.329 | |
| Possible | 35.52 ± 0.56 | 34.13 ± 0.37 | 34.43 ± 0.30 | 34.50 ± 0.70 | S: | 35.216 | <0.001 ### | ||
| Sarcopenia | 31.50 ± 1.41 | 32.05 ± 0.52 | 30.91 ± 0.44 | 28.50 ± 1.01 | A × S: | 1.828 | 0.061 | ||
| Severe | 28.50 ± 1.73 | 30.36 ± 0.62 | 30.27 ± 0.31 | 30.81 ± 0.58 | |||||
| SPPB | Normal | 11.83 ± 0.21 | 11.53 ± 0.20 | 11.29 ± 0.36 | 11.00 ± 1.85 | A: | 7.927 | <0.001 +++ | |
| Possible | 9.78 ± 0.42 | 9.47 ± 0.28 | 7.31 ± 0.26 | 8.25 ± 0.92 | S: | 23.961 | <0.001 ### | ||
| Sarcopenia | 10.67 ± 1.07 | 9.77 ± 0.39 | 8.67 ± 0.38 | 6.50 ± 1.31 | A × S: | 2.808 | 0.003 ** | ||
| Severe | 8.50 ± 1.31 | 6.62 ± 0.51 | 6.44 ± 0.28 | 5.50 ± 0.58 | |||||
| TUG | Normal | 7.44 ± 0.55 | 7.52 ± 0.52 | 9.11 ± 0.92 | 12.13 ± 4.82 | A: | 6.012 | 0.001 +++ | |
| Possible | 10.87 ± 1.11 | 10.13 ± 0.72 | 12.53 ± 0.68 | 16.62 ± 2.40 | S: | 8.400 | <0.001 ### | ||
| Sarcopenia | 8.25 ± 2.78 | 9.46 ± 1.03 | 10.54 ± 0.98 | 14.80 ± 3.41 | A × S: | 2.288 | 0.016 * | ||
| Severe | 9.90 ± 3.41 | 15.48 ± 1.37 | 15.95 ± 0.71 | 15.50 ± 1.54 | |||||
| 2 | HG | Normal | 24.81 ± 0.45 | 22.71 ± 0.43 | 20.72 ± 0.76 | 20.39 ± 3.94 | A: | 1.671 | 0.173 |
| Possible | 19.63 ± 0.90 | 19.05 ± 0.93 | 16.69 ± 0.55 | 14.18 ± 1.97 | S: | 16.829 | <0.001 ### | ||
| Sarcopenia | 17.93 ± 2.27 | 19.47 ± 0.84 | 18.70 ± 0.80 | 16.64 ± 2.79 | A × S: | 1.084 | 0.373 | ||
| Severe | 10.85 ± 2.79 | 14.47 ± 1.09 | 14.53 ± 0.59 | 13.27 ± 1.25 | |||||
| PF | Normal | 17.89 ± 0.44 | 15.82 ± 0.42 | 13.77 ± 0.75 | 15.10 ± 3.89 | A: | 4.092 | 0.007 ++ | |
| Possible | 16.18 ± 0.89 | 12.78 ± 0.58 | 10.68 ± 0.53 | 10.06 ± 1.94 | S: | 10.986 | <0.001 ### | ||
| Sarcopenia | 16.70 ± 2.24 | 13.71 ± 0.83 | 12.35 ± 0.79 | 11.56 ± 2.75 | A × S: | 1.268 | 0.252 | ||
| Severe | 7.60 ± 2.75 | 9.20 ± 1.08 | 10.05 ± 0.58 | 7.87 ± 1.23 | |||||
| DF | Normal | 15.27 ± 0.38 | 13.64 ± 0.36 | 11.04 ± 0.64 | 14.20 ± 3.34 | A: | 3.408 | 0.018 + | |
| Possible | 13.27 ± 0.76 | 9.62 ± 0.49 | 7.89 ± 0.46 | 5.99 ± 1.67 | S: | 15.413 | <0.001 ### | ||
| Sarcopenia | 12.00 ± 1.92 | 10.25 ± 0.71 | 9.37 ± 0.68 | 7.54 ± 2.36 | A × S: | 2.664 | 0.005 ** | ||
| Severe | 4.40 ± 2.36 | 6.31 ± 0.93 | 7.88 ± 0.50 | 5.66 ± 1.06 | |||||
| 2 min | Normal | 97.70 ± 2.85 | 98.25 ± 2.71 | 83.66 ± 4.81 | 46.00 ± 24.99 | A: | 9.132 | <0.001 ### | |
| Possible | 81.47 ± 5.73 | 68.73 ± 3.73 | 47.31 ± 3.47 | 30.00 ± 12.49 | S: | 4.904 | 0.002 ++ | ||
| Sarcopenia | 79.00 ± 14.43 | 75.50 ± 5.33 | 68.63 ± 5.10 | 28.00 ± 17.67 | A × S: | 1.947 | 0.044 * | ||
| Severe | 55.50 ± 17.67 | 51.84 ± 6.93 | 48.53 ± 3.73 | 29.60 ± 7.90 | |||||
| Independent Variable | Model 1 | Model 2 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| B | β | t | p | B | β | t | p | ||
| A | (Constant) | −3.222 | −3.951 | <0.001 *** | −2.902 | −2.739 | 0.007 ** | ||
| Age | 0.054 | 0.420 | 6.271 | <0.001 *** | 0.045 | 0.346 | 4.973 | <0.001 *** | |
| Residential area | −0.042 | −0.142 | −2.214 | 0.028 * | −0.044 | −0.149 | −2.338 | 0.020 * | |
| Religion | 0.043 | 0.053 | 1.035 | 0.302 | 0.054 | 0.066 | 1.276 | 0.203 | |
| Education level | −0.083 | −0.082 | −1.415 | 0.158 | −0.030 | −0.030 | −0.480 | 0.631 | |
| Regular physical activity | 0.154 | 0.044 | 0.844 | 0.400 | 0.056 | 0.016 | 0.300 | 0.764 | |
| Type of physical activity | 0.072 | 0.138 | 2.500 | 0.013 * | 0.063 | 0.120 | 2.159 | 0.032 * | |
| Family | 0.013 | 0.014 | 0.275 | 0.783 | |||||
| House | 0.012 | 0.008 | 0.168 | 0.867 | |||||
| Education level of family | 0.006 | 0.006 | 0.114 | 0.910 | |||||
| Income | −0.110 | −0.156 | −2.517 | 0.013 * | |||||
| Drinking frequency | 0.095 | 0.143 | 2.724 | 0.007 ** | |||||
| Smoking frequency | 0.124 | 0.021 | 0.410 | 0.682 | |||||
| F (p) | 25.504 (<0.001 ***) | 14.415 (<0.001 ***) | |||||||
| R2 | 0.391 | 0.427 | |||||||
| adjR2 | 0.376 | 0.398 | |||||||
| Independent Variable | Model 1 | Model 2 | Model 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | β | t | p | B | β | t | p | B | β | t | p | ||
| A | (Constant) | −4.213 | −9.53 | <0.001 *** | 0.384 | 0.498 | 0.619 | 6.877 | 8.865 | <0.001 *** | |||
| Age | 0.066 | 0.495 | 11.374 | <0.001 *** | 0.045 | 0.337 | 6.846 | <0.001 *** | −0.003 | −0.019 | −0.388 | 0.698 | |
| RPA | 0.191 | 0.088 | 2.047 | 0.041 * | 0.169 | 0.079 | 1.951 | 0.052 | 0.042 | 0.020 | 0.623 | 0.534 | |
| Diabetes | 0.203 | 0.084 | 1.940 | 0.053 | 0.237 | 0.098 | 2.413 | 0.016 * | 0.049 | 0.020 | 0.632 | 0.528 | |
| Hyperlipidemia | −0.366 | −0.166 | −3.830 | <0.001 *** | −0.267 | −0.121 | −2.943 | 0.003 ** | −0.131 | −0.060 | −1.815 | 0.070 | |
| ASM | −0.364 | −0.32 | −3.913 | <0.001 *** | −0.312 | −0.274 | −4.243 | <0.001 *** | |||||
| CC | −0.047 | −0.127 | −2.055 | 0.041 * | −0.004 | −0.012 | −0.236 | 0.814 | |||||
| Percent body fat | 0.020 | 0.136 | 2.240 | 0.026 * | −0.008 | −0.056 | −1.154 | 0.249 | |||||
| HG | −0.057 | −0.28 | −6.924 | <0.001 *** | |||||||||
| PF | 0.026 | 0.116 | 2.256 | 0.025 * | |||||||||
| DF | −0.022 | −0.093 | −1.686 | 0.093 | |||||||||
| SLT | −0.007 | −0.118 | −2.711 | 0.007 ** | |||||||||
| CSR | −0.003 | −0.030 | −0.827 | 0.409 | |||||||||
| 2 min | −0.004 | −0.108 | −2.516 | 0.012 * | |||||||||
| Timed up and go | −0.024 | −0.117 | −1.862 | 0.063 | |||||||||
| Gait speed | 0.012 | 0.023 | 0.388 | 0.698 | |||||||||
| SPPB | −0.184 | −0.427 | −8.444 | <0.001 *** | |||||||||
| F (p) | 44.115 (<0.001 ***) | 38.706 (<0.001 ***) | 45.291 (<0.001 ***) | ||||||||||
| R2 | 0.318 | 0.419 | 0.664 | ||||||||||
| adjR2 | 0.311 | 0.409 | 0.650 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sung, J.-Y.; Lee, M.J.; Kim, J. Relationship Between Lifestyle and Physical Fitness Among Older Women with Sarcopenia. Int. J. Mol. Sci. 2025, 26, 2205. https://doi.org/10.3390/ijms26052205
Sung J-Y, Lee MJ, Kim J. Relationship Between Lifestyle and Physical Fitness Among Older Women with Sarcopenia. International Journal of Molecular Sciences. 2025; 26(5):2205. https://doi.org/10.3390/ijms26052205
Chicago/Turabian StyleSung, Jun-Young, Moon Jin Lee, and Jiyoun Kim. 2025. "Relationship Between Lifestyle and Physical Fitness Among Older Women with Sarcopenia" International Journal of Molecular Sciences 26, no. 5: 2205. https://doi.org/10.3390/ijms26052205
APA StyleSung, J.-Y., Lee, M. J., & Kim, J. (2025). Relationship Between Lifestyle and Physical Fitness Among Older Women with Sarcopenia. International Journal of Molecular Sciences, 26(5), 2205. https://doi.org/10.3390/ijms26052205







