Effects of Darbepoetin Alfa and Ferric Derisomaltose Plus Darbepoetin Alfa in Functional Iron-Deficiency Anemia
Abstract
:1. Introduction
2. Results
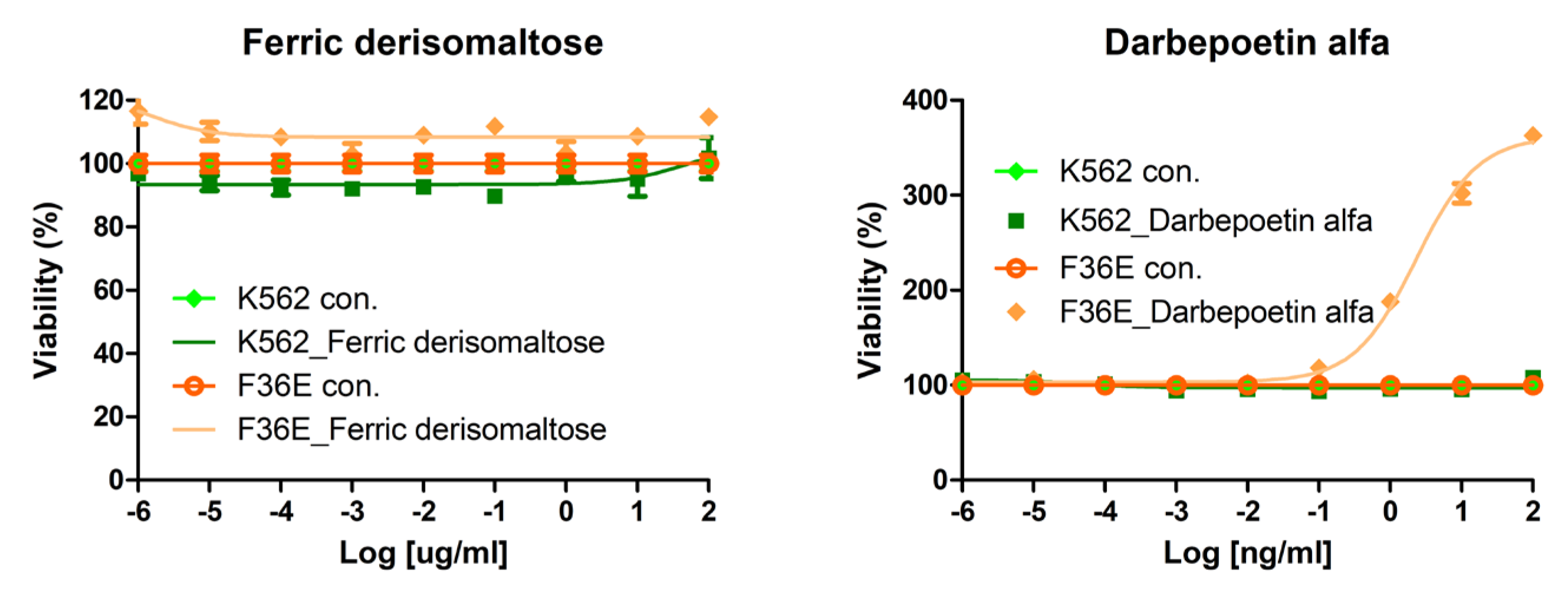
2.1. Effects of Darbepoetin Alfa and Ferric Derisomaltose on Acute Myeloid and Erythroid Leukemia Cell Lines
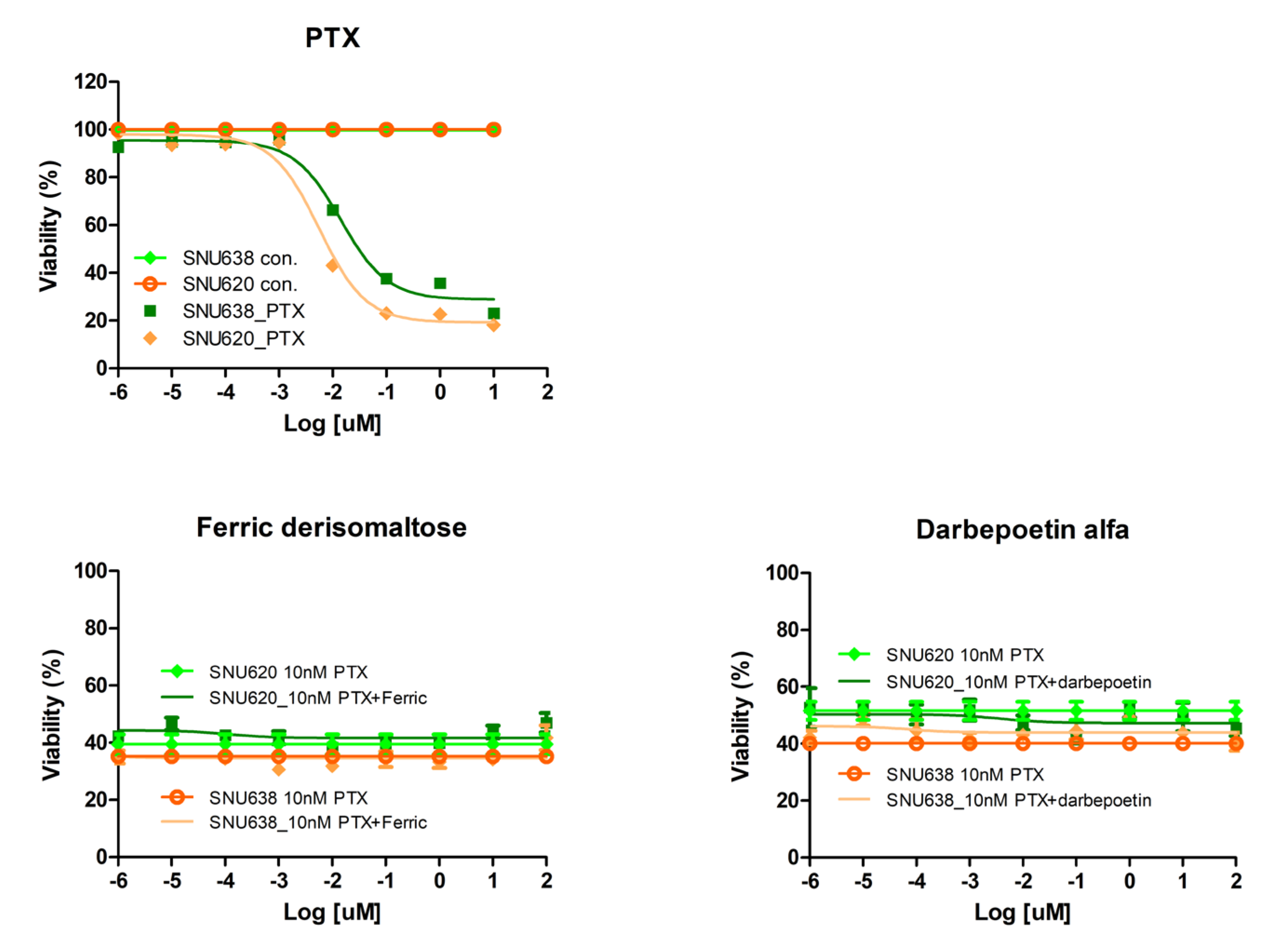
2.2. Effects of Darbepoetin Alfa and Ferric Derisomaltose on Gastric Cancer Cell Lines
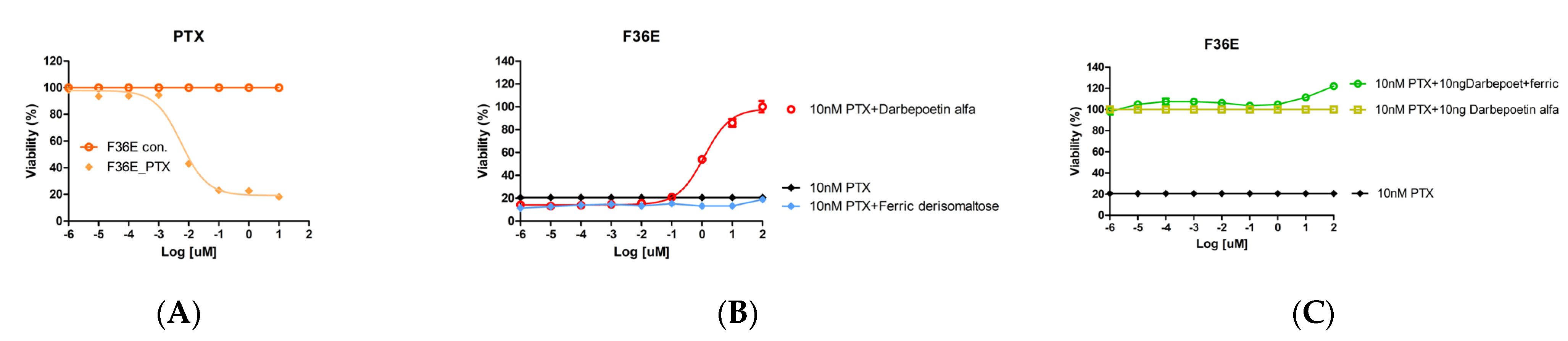
2.3. Effects of Ferric Derisomaltose and Darbepoetin Alfa on PTX-Induced Apoptosis of Erythroid Cells
2.4. Effects of Ferric Derisomaltose and Darbepoetin Alfa on the Efficacy of PTX in Gastric Cancer Cell Lines
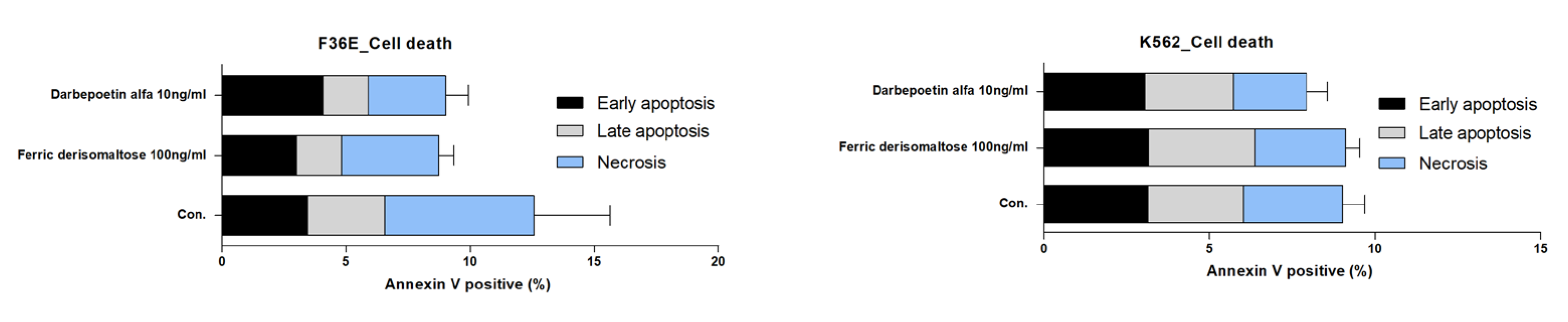
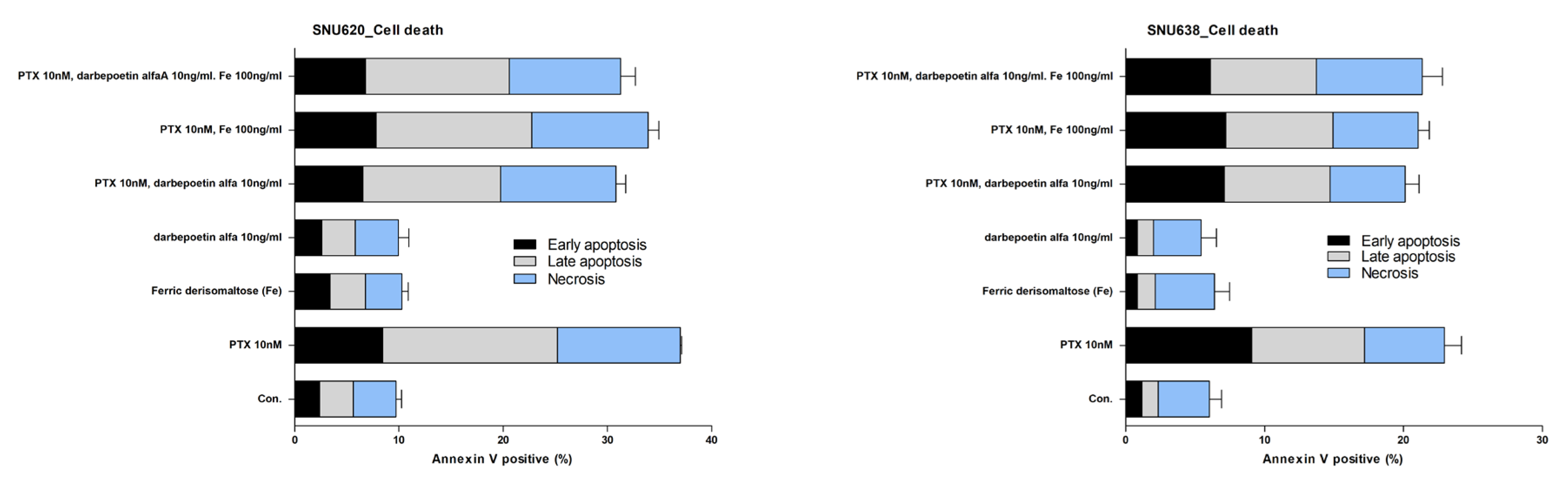
2.5. Effects of Ferric Derisomaltose and Darbepoetin Alfa on Apoptosis of Myeloid and Erythroid Cell Lines
2.6. Effects of Ferric Derisomaltose and Darbepoetin Alfa on PTX-Induced Apoptosis in GC Cell Lines
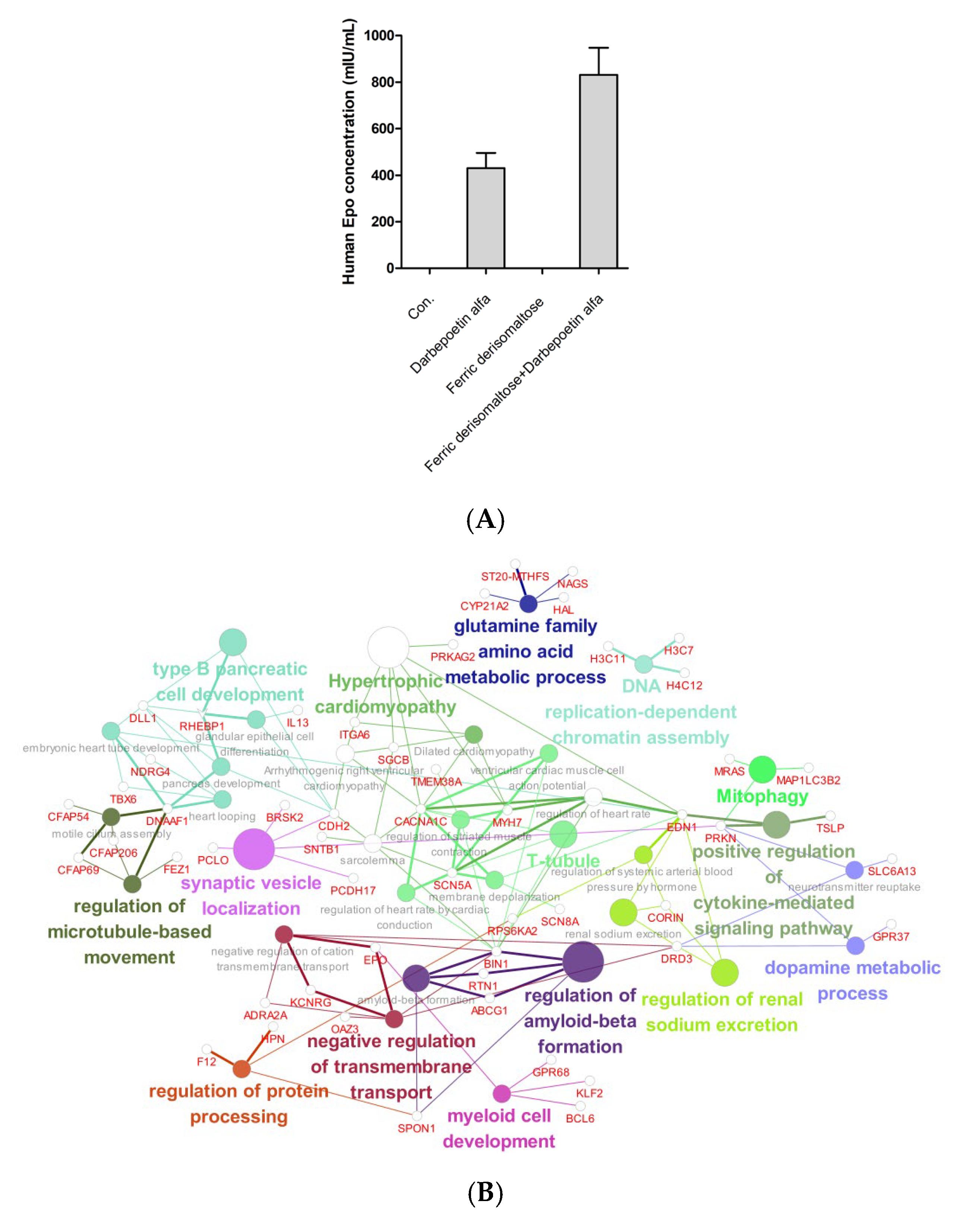
2.7. Effects of Darbepoetin Alfa vs. Ferric Derisomaltose Plus Darbepoetin Alfa on F36E Erythroid Cells
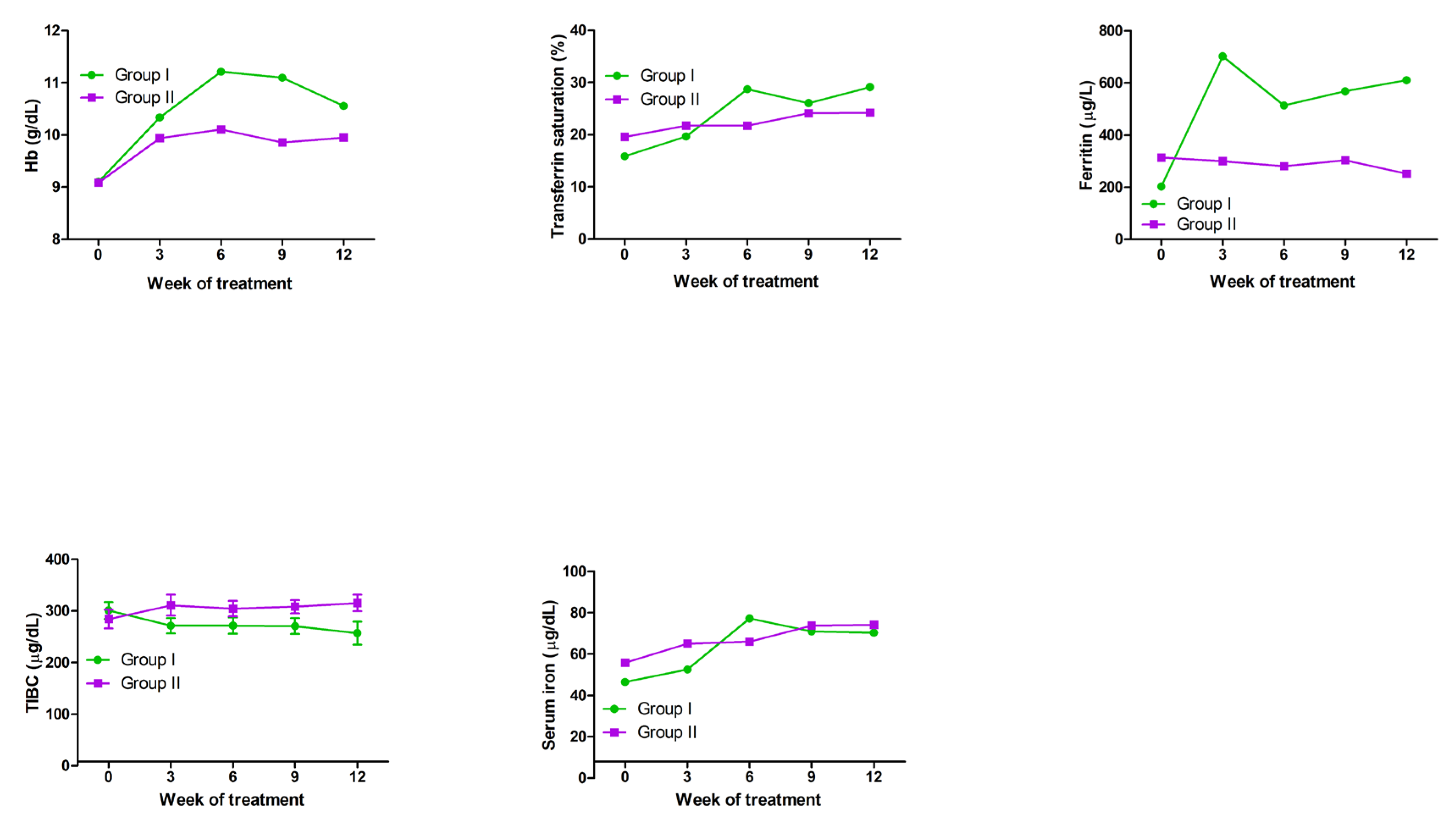
2.8. Effects of Ferric Derisomaltose Plus Darbepoetin Alfa and Darbepoetin Alfa Monotherapy in Patients with Chemotherapy-Induced Functional Iron-Deficiency Anemia
3. Discussion
4. Materials and Methods
4.1. Drug Preparation
4.2. Cell Lines and Cell Culture
4.3. Cell Viability Assays
4.4. Apoptosis Analysis
4.5. Erythropoietin Concentration
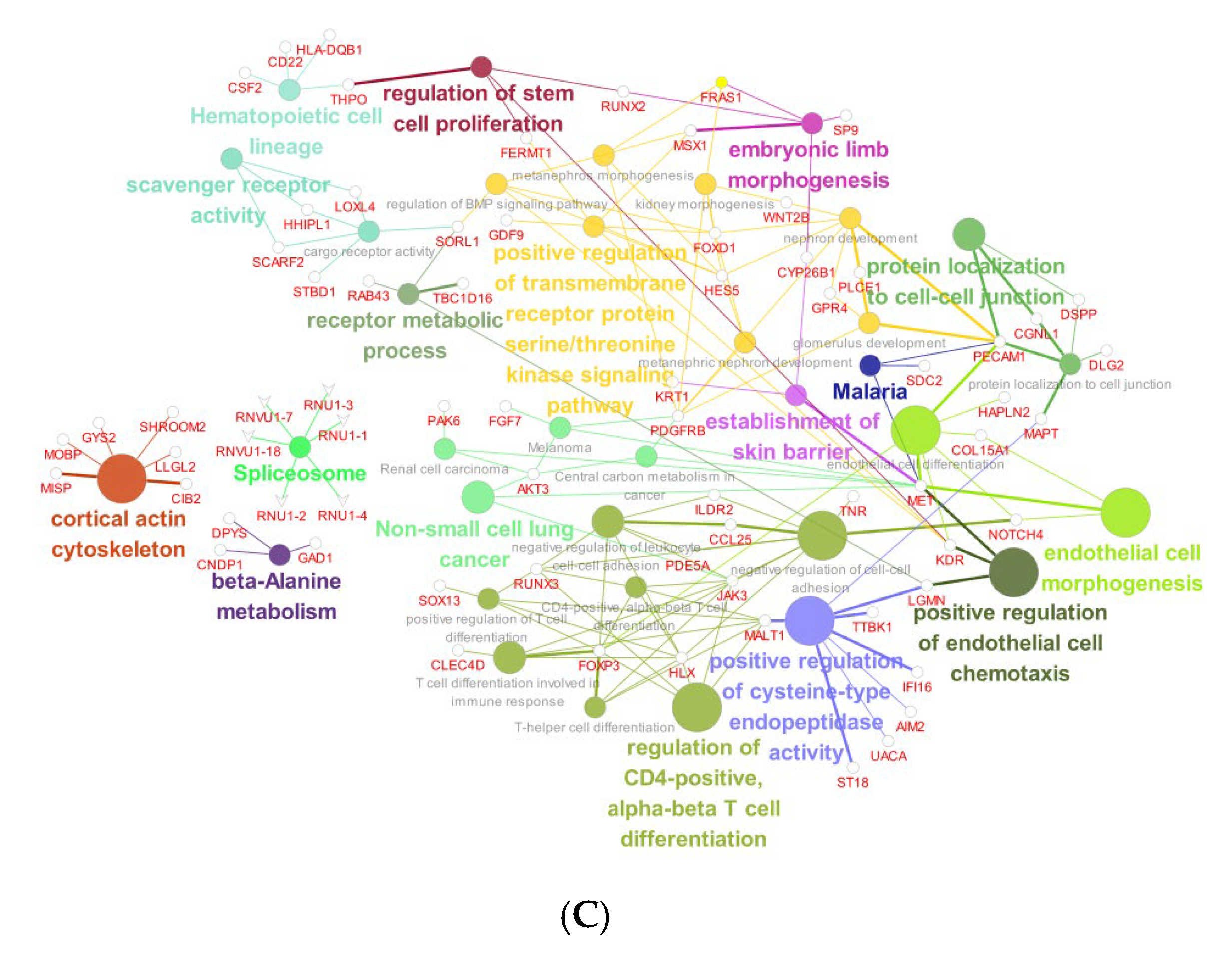
4.6. RNA-Sequencing Analysis
4.7. Study Subjects and Blood Collection
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FIDA | Functional iron-deficiency anemia |
| PTX | Paclitaxel |
| AML | Acute myeloid leukemia |
| CIA | Chemotherapy-induced anemia |
| RBCs | Red blood cells |
| ESAs | Erythropoiesis-stimulating agents |
| EPO | Erythropoietin |
| IDA | Iron-deficiency anemia |
| AIDA | Absolute iron-deficiency anemia |
References
- Cella, D.; Kallich, J.; McDermott, A.; Xu, X. The longitudinal relationship of hemoglobin, fatigue and quality of life in anemic cancer patients: Results from five randomized clinical trials. Ann. Oncol. 2004, 15, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, H.; Van Belle, S.; Barrett-Lee, P.; Birgegard, G.; Bokemeyer, C.; Gascon, P.; Kosmidis, P.; Krzakowski, M.; Nortier, J.; Olmi, P.; et al. The European Cancer Anaemia Survey (ECAS): A large, multinational, prospective survey defining the prevalence, incidence, and treatment of anaemia in cancer patients. Eur. J. Cancer 2004, 40, 2293–2306. [Google Scholar] [CrossRef] [PubMed]
- Groopman, J.E.; Itri, L.M. Chemotherapy-induced anemia in adults: Incidence and treatment. J. Natl. Cancer Inst. 1999, 91, 1616–1634. [Google Scholar] [CrossRef] [PubMed]
- Mhaskar, R.; Wao, H.; Miladinovic, B.; Kumar, A.; Djulbegovic, B. The role of iron in the management of chemotherapy-induced anemia in cancer patients receiving erythropoiesis-stimulating agents. Cochrane Database Syst. Rev. 2016, 2, CD009624. [Google Scholar] [CrossRef]
- Tiotiu, A.; Clement-Duchene, C.; Martinet, Y. Management of chemotherapy-induced anemia in lung cancer. Rev. Mal. Respir. 2015, 32, 809–821. [Google Scholar] [CrossRef]
- Aapro, M.; Beguin, Y.; Bokemeyer, C.; Dicato, M.; Gascon, P.; Glaspy, J.; Hofmann, A.; Link, H.; Littlewood, T.; Ludwig, H.; et al. Management of anaemia and iron deficiency in patients with cancer: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29, iv271. [Google Scholar] [CrossRef]
- Egrie, J.C.; Dwyer, E.; Browne, J.K.; Hitz, A.; Lykos, M.A. Darbepoetin alfa has a longer circulating half-life and greater in vivo potency than recombinant human erythropoietin. Exp. Hematol. 2003, 31, 290–299. [Google Scholar] [CrossRef]
- Qunibi, W.Y. The efficacy and safety of current intravenous iron preparations for the management of iron-deficiency anaemia: A review. Arzneimittelforschung 2010, 60, 399–412. [Google Scholar] [CrossRef]
- Steinmetz, T.; Tschechne, B.; Harlin, O.; Klement, B.; Franzem, M.; Wamhoff, J.; Tesch, H.; Rohrberg, R.; Marschner, N. Clinical experience with ferric carboxymaltose in the treatment of cancer- and chemotherapy-associated anaemia. Ann. Oncol. 2013, 24, 475–482. [Google Scholar] [CrossRef]
- Toledano, A.; Luporsi, E.; Morere, J.F.; Scotte, F.; Laribi, K.; Barriere, J.; Huot-Marchand, P.; Duvillie, L.; Concas, V.H.; Bugat, R. Clinical use of ferric carboxymaltose in patients with solid tumours or haematological malignancies in France. Support Care Cancer 2016, 24, 67–75. [Google Scholar] [CrossRef]
- Joy, M.S. Darbepoetin alfa: A novel erythropoiesis-stimulating protein. Ann. Pharmacother. 2002, 36, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Coccia, M.A.; Cooke, K.; Stoney, G.; Pistillo, J.; Del Castillo, J.; Duryea, D.; Tarpley, J.E.; Molineux, G. Novel erythropoiesis stimulating protein (darbepoetin alfa) alleviates anemia associated with chronic inflammatory disease in a rodent model. Exp. Hematol. 2001, 29, 1201–1209. [Google Scholar] [CrossRef]
- Miller, J.L. Iron deficiency anemia: A common and curable disease. Cold Spring Harb. Perspect. Med. 2013, 3, a011866. [Google Scholar] [CrossRef] [PubMed]
- Auerbach, M.; Henry, D.; DeLoughery, T.G. Intravenous ferric derisomaltose for the treatment of iron deficiency anemia. Am. J. Hematol. 2021, 96, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Broxmeyer, H.E. Erythropoietin: Multiple targets, actions, and modifying influences for biological and clinical consideration. J. Exp. Med. 2013, 210, 205–208. [Google Scholar] [CrossRef]
- Ueda, N.; Takasawa, K. Impact of Inflammation on Ferritin, Hepcidin and the Management of Iron Deficiency Anemia in Chronic Kidney Disease. Nutrients 2018, 10, 1173. [Google Scholar] [CrossRef]
- Bastit, L.; Vandebroek, A.; Altintas, S.; Gaede, B.; Pinter, T.; Suto, T.S.; Mossman, T.W.; Smith, K.E.; Vansteenkiste, J.F. Randomized, multicenter, controlled trial comparing the efficacy and safety of darbepoetin alpha administered every 3 weeks with or without intravenous iron in patients with chemotherapy-induced anemia. J. Clin. Oncol. 2008, 26, 1611–1618. [Google Scholar] [CrossRef]
- Hedenus, M.; Birgegard, G.; Nasman, P.; Ahlberg, L.; Karlsson, T.; Lauri, B.; Lundin, J.; Larfars, G.; Osterborg, A. Addition of intravenous iron to epoetin beta increases hemoglobin response and decreases epoetin dose requirement in anemic patients with lymphoproliferative malignancies: A randomized multicenter study. Leukemia 2007, 21, 627–632. [Google Scholar] [CrossRef]
- Auerbach, M.; Ballard, H.; Trout, J.R.; McIlwain, M.; Ackerman, A.; Bahrain, H.; Balan, S.; Barker, L.; Rana, J. Intravenous iron optimizes the response to recombinant human erythropoietin in cancer patients with chemotherapy-related anemia: A multicenter, open-label, randomized trial. J. Clin. Oncol. 2004, 22, 1301–1307. [Google Scholar] [CrossRef]
- Grebien, F.; Kerenyi, M.A.; Kovacic, B.; Kolbe, T.; Becker, V.; Dolznig, H.; Pfeffer, K.; Klingmuller, U.; Muller, M.; Beug, H.; et al. Stat5 activation enables erythropoiesis in the absence of EpoR and Jak2. Blood 2008, 111, 4511–4522. [Google Scholar] [CrossRef]
- Witthuhn, B.A.; Quelle, F.W.; Silvennoinen, O.; Yi, T.; Tang, B.; Miura, O.; Ihle, J.N. JAK2 associates with the erythropoietin receptor and is tyrosine phosphorylated and activated following stimulation with erythropoietin. Cell 1993, 74, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ha Chung, B.; Joo, K.W.; Shin, S.K.; Kim, Y.L.; Na, K.Y.; Do, J.Y.; Park, S.K.; Shin, B.C.; Lee, J.S.; et al. Efficacy and safety of CKD-11101 (darbepoetin-alfa proposed biosimilar) compared with NESP in anaemic chronic kidney disease patients not on dialysis. Curr. Med. Res. Opin. 2019, 35, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Skopek, R.; Palusinska, M.; Kaczor-Keller, K.; Pingwara, R.; Papierniak-Wygladala, A.; Schenk, T.; Lewicki, S.; Zelent, A.; Szymanski, L. Choosing the Right Cell Line for Acute Myeloid Leukemia (AML) Research. Int. J. Mol. Sci. 2023, 24, 5377. [Google Scholar] [CrossRef] [PubMed]

| Group | Subject No. | Sex/Age |
|---|---|---|
| Group 1 | s-001-001 | M/87 |
| s-001-005 | F/70 | |
| s-001-009 | M/64 | |
| s001-010 | M/67 | |
| s001-012 | M/73 | |
| s001-013 | M/76 | |
| s001-018 | F/55 | |
| s001-020 | F/81 | |
| s001-022 | M/68 | |
| s001-024 | M/58 | |
| s001-026 | M/61 | |
| s001-027 | F/64 | |
| s001-031 | M/53 | |
| s001-032 | F/60 | |
| Group 2 | s-001-008 | F/67 |
| s-001-007 | F/68 | |
| s001-011 | M/40 | |
| s001-014 | F/74 | |
| s001-016 | M/57 | |
| s001-017 | F/70 | |
| s001-019 | M/67 | |
| s001-021 | M/66 | |
| s001-025 | F/67 | |
| s001-028 | M/58 | |
| s001-029 | M/62 | |
| s001-030 | M/87 | |
| s001-033 | M/63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sohn, S.-H.; Sul, H.; Kim, B.; Zang, D. Effects of Darbepoetin Alfa and Ferric Derisomaltose Plus Darbepoetin Alfa in Functional Iron-Deficiency Anemia. Int. J. Mol. Sci. 2025, 26, 2203. https://doi.org/10.3390/ijms26052203
Sohn S-H, Sul H, Kim B, Zang D. Effects of Darbepoetin Alfa and Ferric Derisomaltose Plus Darbepoetin Alfa in Functional Iron-Deficiency Anemia. International Journal of Molecular Sciences. 2025; 26(5):2203. https://doi.org/10.3390/ijms26052203
Chicago/Turabian StyleSohn, Sung-Hwa, Heejung Sul, Bumjun Kim, and Daeyoung Zang. 2025. "Effects of Darbepoetin Alfa and Ferric Derisomaltose Plus Darbepoetin Alfa in Functional Iron-Deficiency Anemia" International Journal of Molecular Sciences 26, no. 5: 2203. https://doi.org/10.3390/ijms26052203
APA StyleSohn, S.-H., Sul, H., Kim, B., & Zang, D. (2025). Effects of Darbepoetin Alfa and Ferric Derisomaltose Plus Darbepoetin Alfa in Functional Iron-Deficiency Anemia. International Journal of Molecular Sciences, 26(5), 2203. https://doi.org/10.3390/ijms26052203






