Thaumatin-like Gene TLP1b Confers to Seed Oil Content and Resistance to Sclerotinia sclerotiorum in Arabidopsis
Abstract
1. Introduction
2. Results
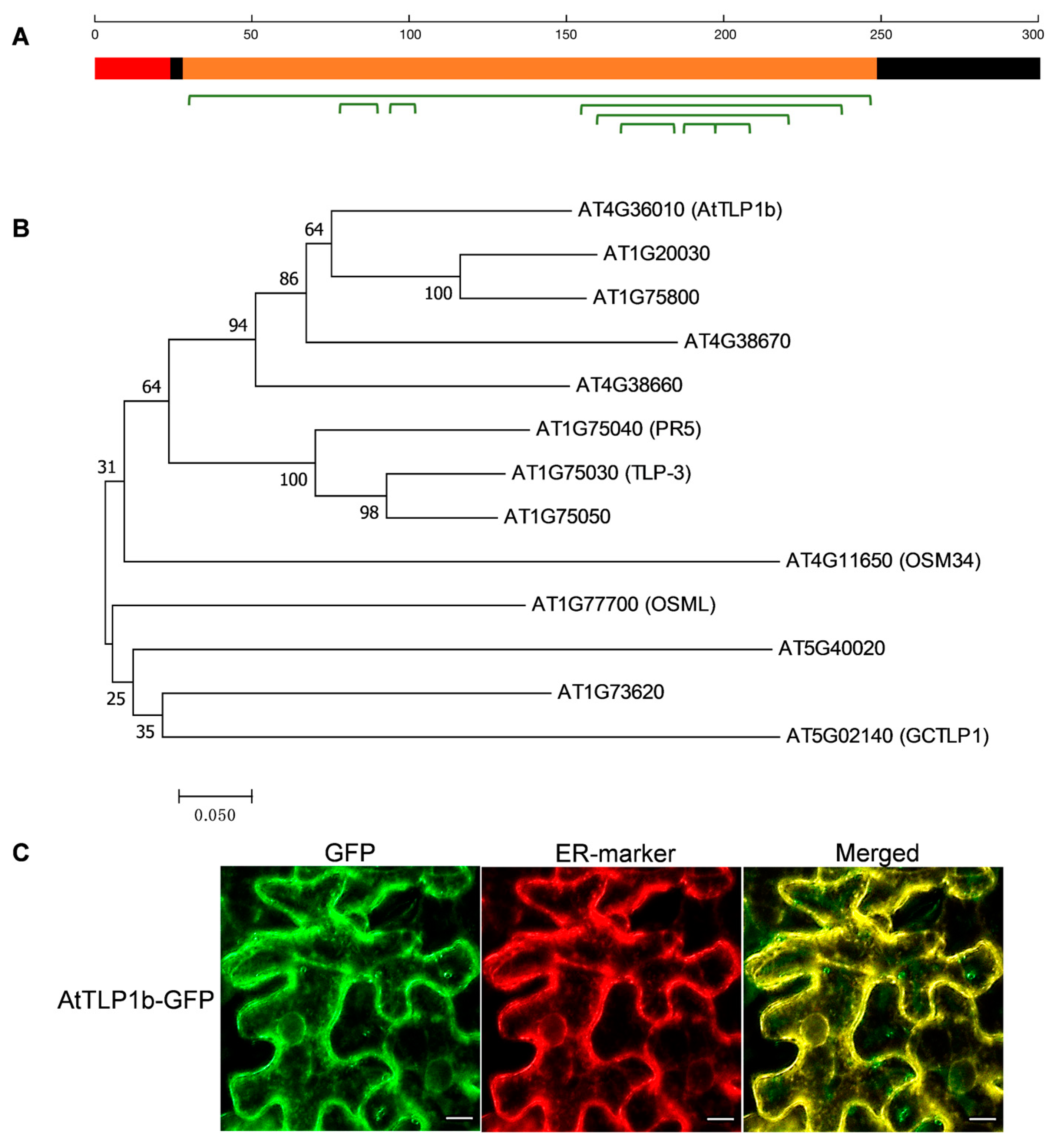
2.1. In Silico Analysis and Subcellular Localization Analysi of AtTLP1b
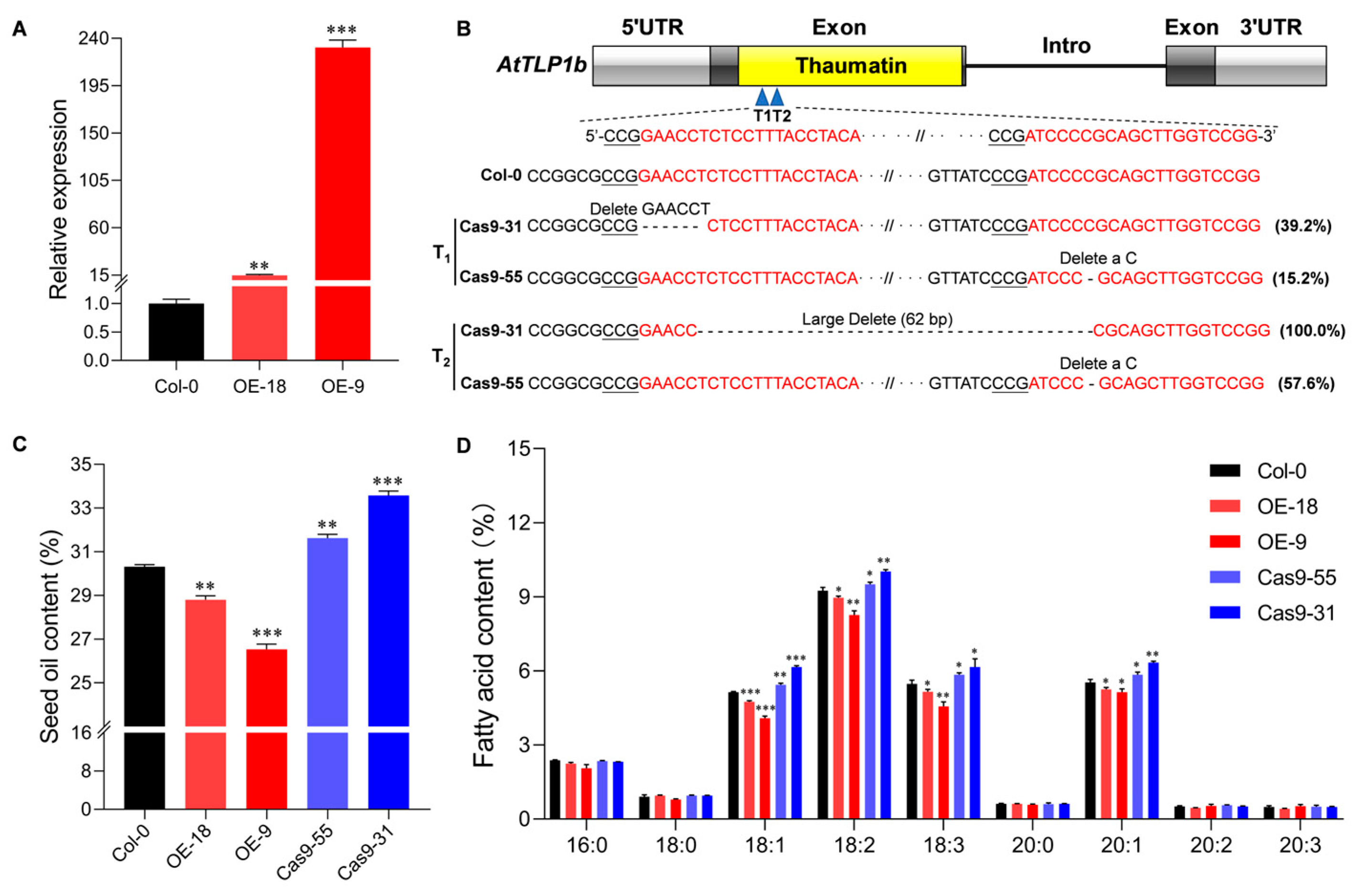
2.2. AtTLP1b Is a Negative Regulator of Seed Oil Content in Arabidopsis
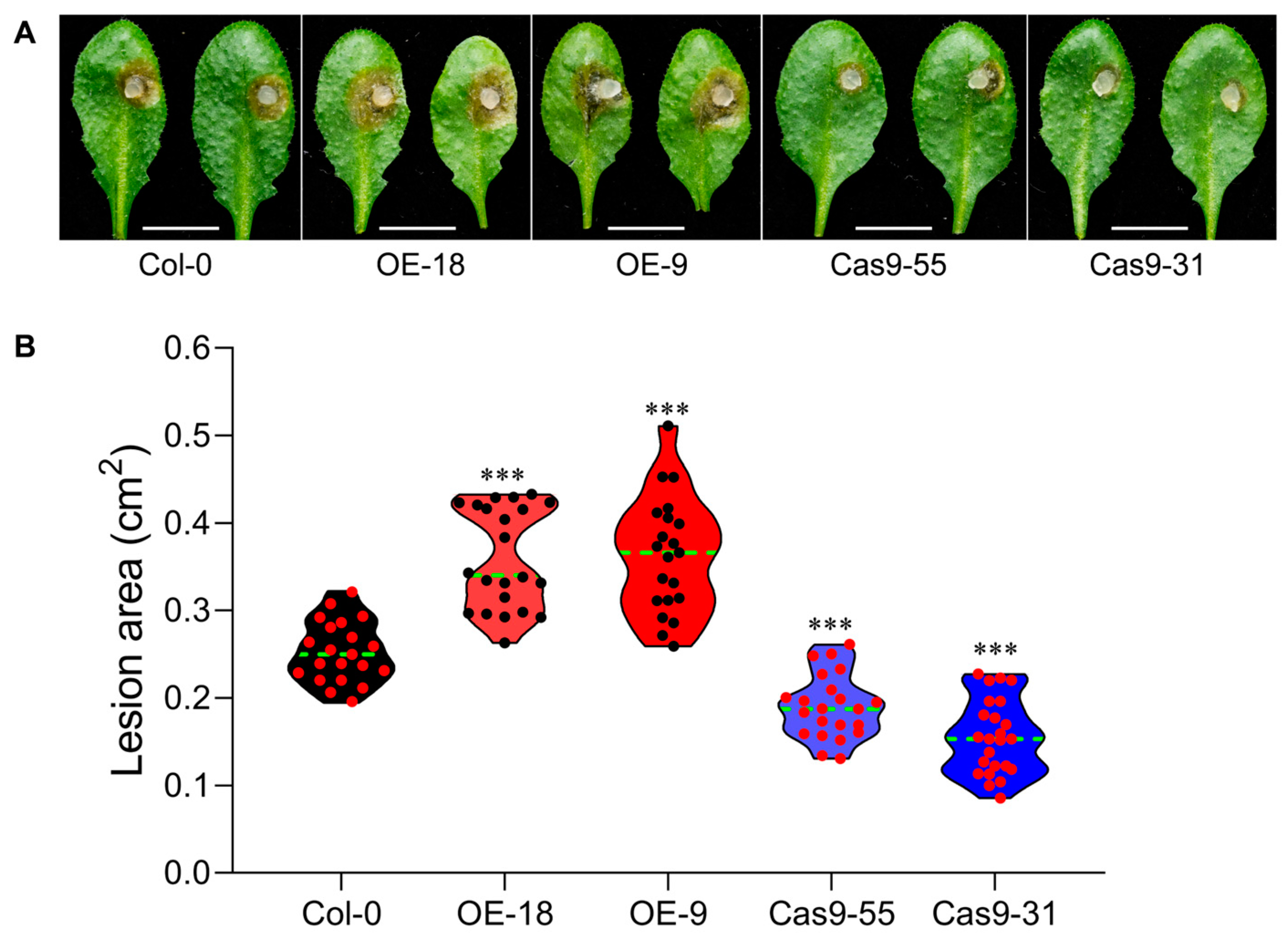
2.3. AtTLP1b Negatively Regulated the Resistance to S. sclerotiorum
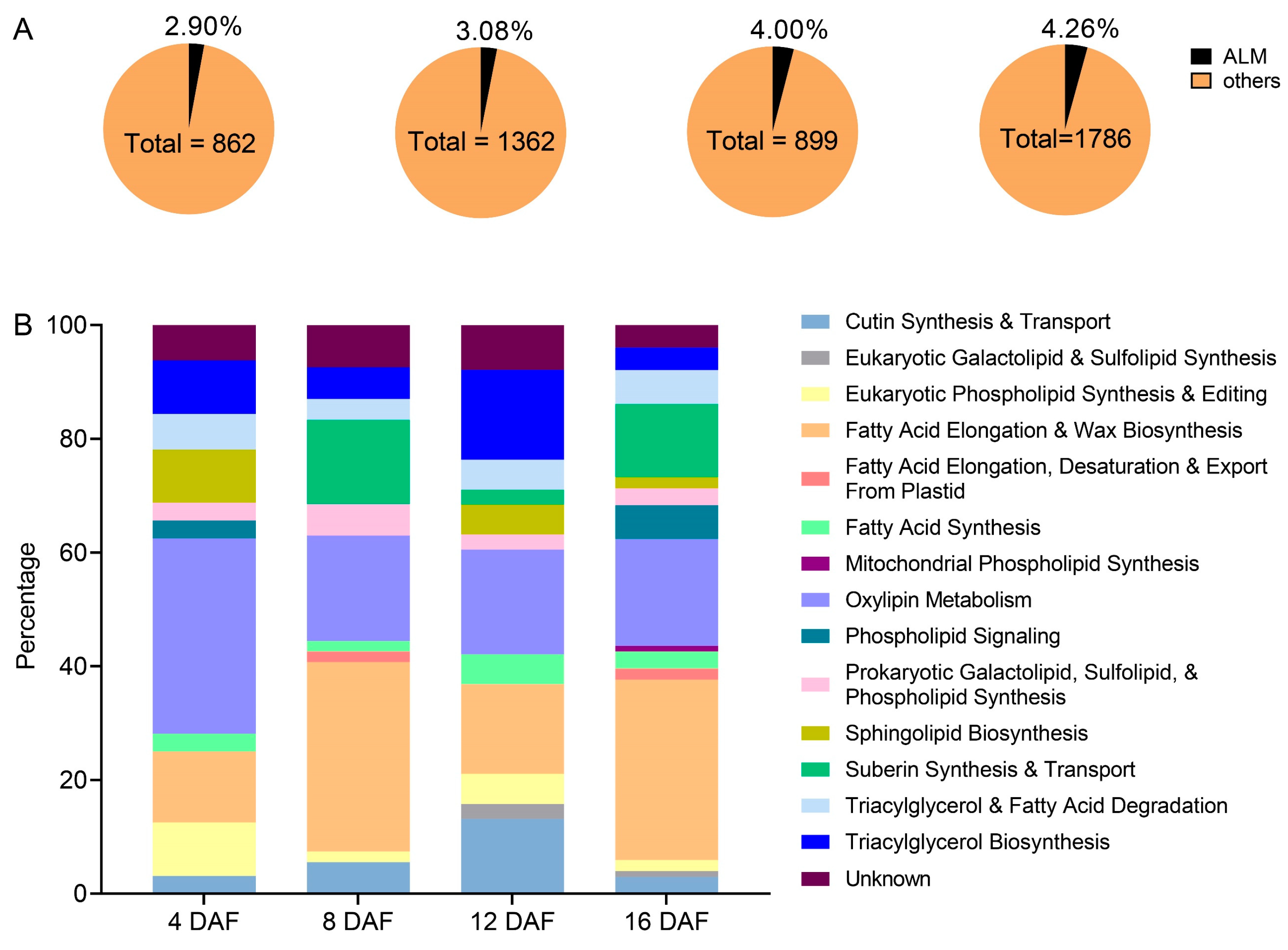
2.4. Most Acyl-Lipid Metabolism (ALM) Genes Are Related to the Synthesis of Very-Long-Chain Fatty Acid Derivatives
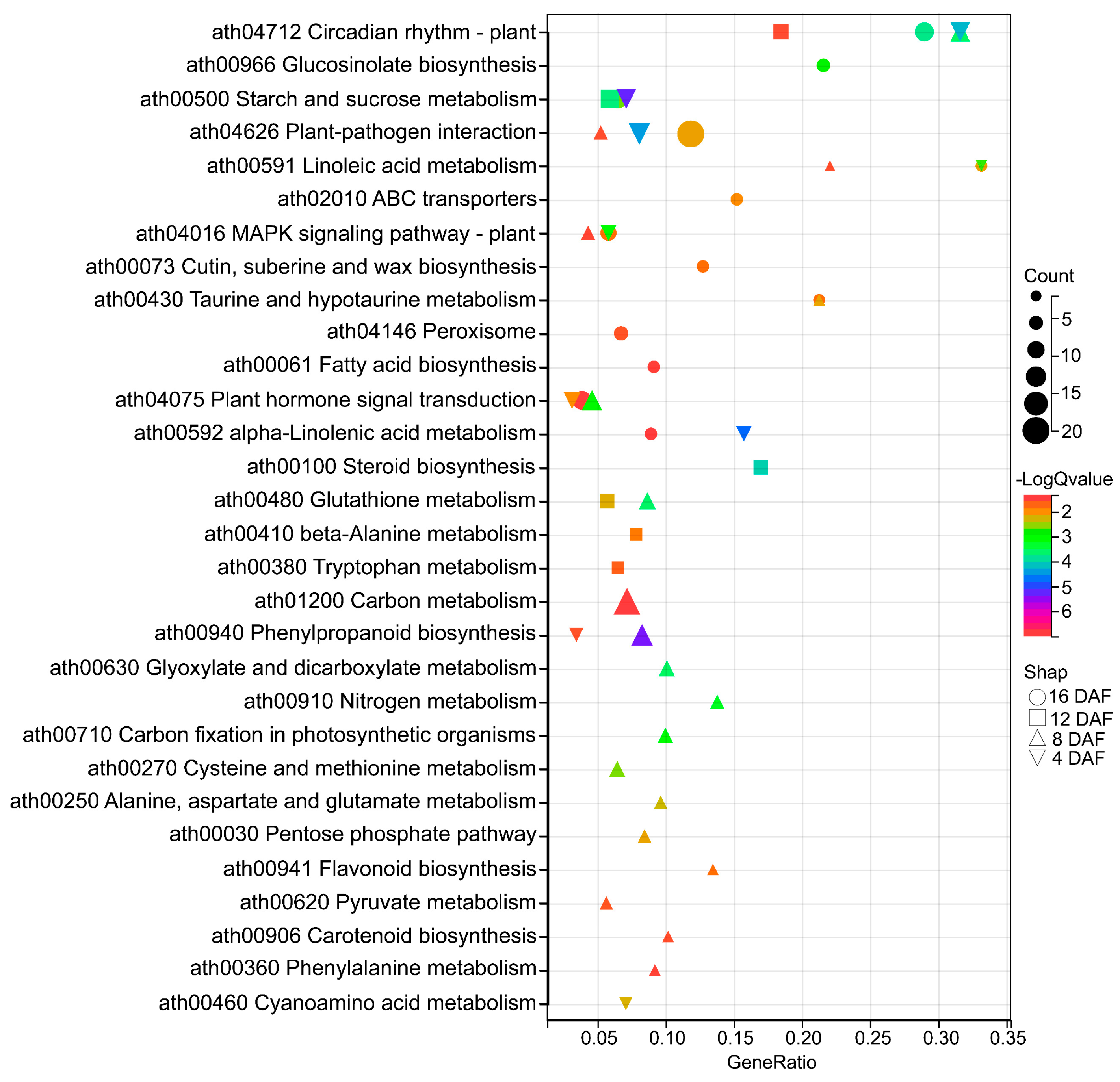
2.5. KEGG Enrichment Analysis of the Overlapping DEGs of Two Overexpressing Lines Versus Col-0
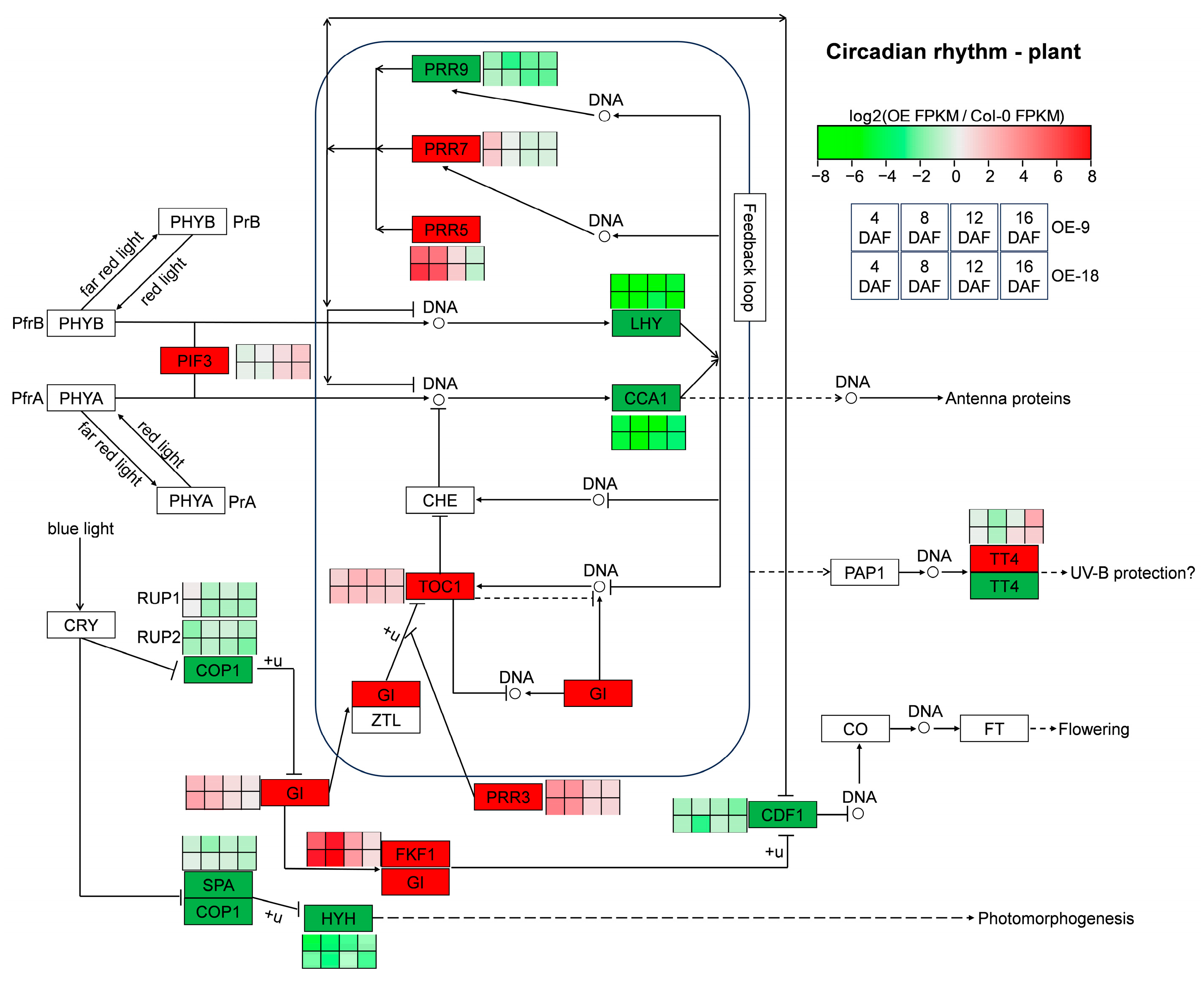
2.6. Overexpression of AtTLP1b Reprogramed the Circadian Rhythm-Plant Pathway
3. Discussion
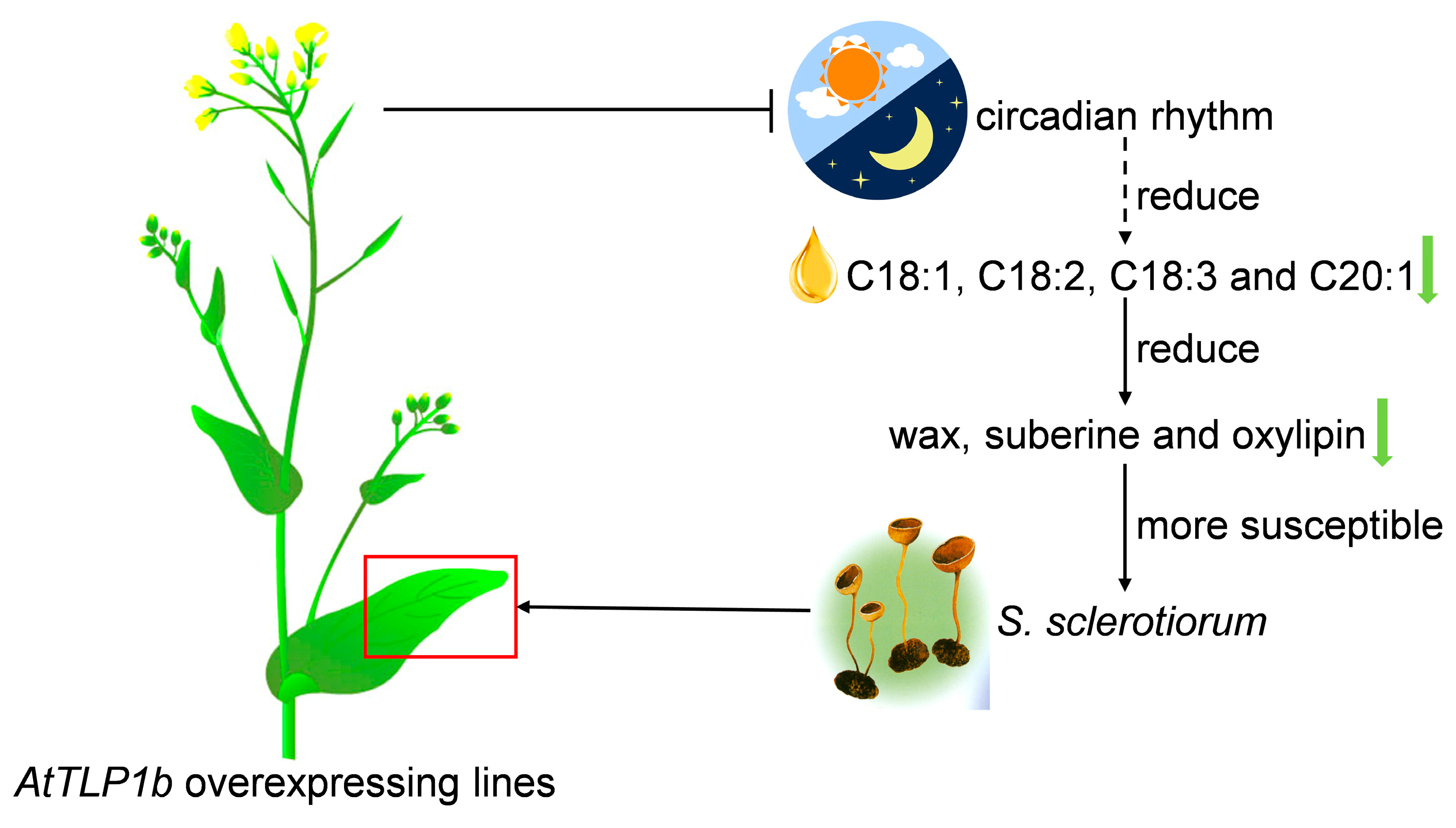
3.1. AtTLP1b Is a Potential Target to Enhance Seed Oil Content and Resistance to S. sclerotiorum
3.2. AtTLP1b Regulates Synthesis and Metabolism of Wax, Suberin and Oxylipin Through the Circadian Rhythm–Plant Pathway to Influence Resistance to S. sclerotiorum
4. Materials and Methods
4.1. Vector Constructs and Plant Transformation
4.2. Plant Materials and Growth Conditions
4.3. RNA Isolation and RT-qPCR Analysis
4.4. Subcellular Localization Analysis
4.5. Seed Oil Content and FA Analysis
4.6. Identification of Resistance to S. sclerotiorum in Transgenic Arabidopsis
4.7. RNA Sequencing and Data Analysis
4.8. Phylogenetic Tree Analysis of the TLP Family in Arabidopsis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, Y.; Kong, Q.; Lim, A.R.Q.; Lu, S.; Zhao, H.; Guo, L.; Yuan, L.; Ma, W. Transcriptional Regulation of Oil Biosynthesis in Seed Plants: Current Understanding, Applications, and Perspectives. Plant Commun. 2022, 3, 100328. [Google Scholar] [CrossRef] [PubMed]
- Dhankher, O.P.; Foyer, C.H. Climate Resilient Crops for Improving Global Food Security and Safety. Plant Cell Environ. 2018, 41, 877–884. [Google Scholar] [CrossRef]
- Li Beisson, Y.; Shorrosh, B.; Beisson, F.; Andersson, M.X.; Arondel, V.; Bates, P.D.; Baud, S.; Bird, D.; Debono, A.; Durrett, T.P.; et al. Acyl-Lipid Metabolism. Arab. Book 2013, 11, e0161. [Google Scholar] [CrossRef]
- Cui, Y.; Zeng, X.; Xiong, Q.; Wei, D.; Liao, J.; Xu, Y.; Chen, G.; Zhou, Y.; Dong, H.; Wan, H.; et al. Combining Quantitative Trait Locus and Co-Expression Analysis Allowed Identification of New Candidates for Oil Accumulation in Rapeseed. J. Exp. Bot. 2021, 72, 1649–1660. [Google Scholar] [CrossRef]
- De Jesús Pires, C.; Ferreira Neto, J.R.C.; Pacifico Bezerra Neto, J.; Kido, E.A.; De Oliveira Silva, R.L.; Pandolfi, V.; Wanderley Nogueira, A.C.; Binneck, E.; Da Costa, A.F.; Pio Ribeiro, G.; et al. Plant Thaumatin-like Proteins: Function, Evolution and Biotechnological Applications. Curr. Protein Pept. Sci. 2020, 21, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Lv, Y.; Hou, Z.; Li, X.; Ding, L. Expansion and Evolution of Thaumatin-like Protein (TLP) Gene Family in Six Plants. Plant Growth Regul. 2016, 79, 299–307. [Google Scholar] [CrossRef]
- Petre, B.; Major, I.; Rouhier, N.; Duplessis, S. Genome-Wide Analysis of Eukaryote Thaumatin-like Proteins (TLPs) with an Emphasis on Poplar. BMC Plant Biol. 2011, 11, 33. [Google Scholar] [CrossRef]
- Park, E.J.; Kim, T.H. Thaumatin-like Genes Function in the Control of Both Biotic Stress Signaling and ABA Signaling Pathways. Biochem. Biophys. Res. Commun. 2021, 567, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xie, Y.; Jiang, Y.; Nadeem, H.; Wang, Y.; Yang, N.; Zhu, H.; Tang, C. GhTLP1, a Thaumatin-like Protein 1, Improves Verticillium Wilt Resistance in Cotton via JA, ABA and MAPK Signaling Pathway-Plant Pathways. Int. J. Biol. Macromol. 2023, 253, 127388. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, H.; Rajput, R.; Pandey, A.; Upadhyay, S.K. Molecular Characterization Revealed the Role of Thaumatin-Like Proteins of Bread Wheat in Stress Response. Front. Plant Sci. 2022, 12, 807448. [Google Scholar] [CrossRef] [PubMed]
- Muoki, R.C.; Paul, A.; Kaachra, A.; Kumar, S. Membrane Localized Thaumatin-like Protein from Tea (CsTLP) Enhanced Seed Yield and the Plant Survival under Drought Stress in Arabidopsis thaliana. Plant Physiol. Biochem. 2021, 163, 36–44. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Furutera, A.; Seki, K.; Toyoda, Y.; Tanaka, K.; Sugimoto, Y. Malondialdehyde Generated from Peroxidized Linolenic Acid Causes Protein Modification in Heat-Stressed Plants. Plant Physiol. Biochem. 2008, 46, 786–793. [Google Scholar] [CrossRef]
- Wilson, R.A.; Calvo, A.M.; Chang, P.K.; Keller, N.P. Characterization of the Aspergillus Parasiticus Delta12-Desaturase Gene: A Role for Lipid Metabolism in the Aspergillus-Seed Interaction. Microbiology 2004, 150, 2881–2888. [Google Scholar] [CrossRef]
- Xue, H.Q.; Upchurch, R.G.; Kwanyuen, P. Ergosterol as a Quantifiable Biomass Marker for Diaporthe haseolorum and Cercospora kikuchi. Plant Dis. 2006, 90, 1395–1398. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, J.; Hu, Y.; Zhao, T.; Huang, C.; Chen, J.; He, L.; Dai, F.; Chen, S.; Wang, L.; Jin, S.; et al. Comparative Transcriptomic Analysis Provides Insights into the Genetic Networks Regulating Oil Differential Production in Oil Crops. BMC Biol. 2024, 22, 110. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Bao, L.L.; Zhao, F.Y.; Tang, M.Q.; Chen, T.; Li, Y.; Wang, B.X.; Fu, B.; Fang, H.; Li, G.Y.; et al. BnaMPK3 Is a Key Regulator of Defense Responses to the Devastating Plant Pathogen Sclerotinia sclerotiorum in Oilseed Rape. Front. Plant Sci. 2019, 10, 91. [Google Scholar] [CrossRef] [PubMed]
- Mosblech, A.; Feussner, I.; Heilmann, I. Oxylipins: Structurally Diverse Metabolites from Fatty Acid Oxidation. Plant Physiol. Biochem. 2009, 47, 511–517. [Google Scholar] [CrossRef]
- Li, C.; Liu, X.J.; Yan, Y.; Alam, M.S.; Liu, Z.; Yang, Z.K.; Tao, R.F.; Yue, E.K.; Duan, M.H.; Xu, J.H. OsLHY Is Involved in Regulating Flowering through the Hd1- and Ehd1- Mediated Pathways in Rice (Oryza sativa L.). Plant Sci. 2022, 315, 111145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ren, Z.; Zhou, Y.; Ma, Z.; Ma, Y.; Hou, D.; Xu, Z.; Huang, X. NPR1 and Redox Rhythmx: Connections, between Circadian Clock and Plant Immunity. Int. J. Mol. Sci. 2019, 20, 1211. [Google Scholar] [CrossRef]
- Song, X.; Meng, X.; Guo, H.; Cheng, Q.; Jing, Y.; Chen, M.; Liu, G.; Wang, B.; Wang, Y.; Li, J.; et al. Targeting a Gene Regulatory Element Enhances Rice Grain Yield by Decoupling Panicle Number and Size. Nat. Biotechnol. 2022, 40, 1403–1411. [Google Scholar] [CrossRef]
- Büschges, R.; Hollricher, K.; Panstruga, R.; Simons, G.; Wolter, M.; Frijters, A.; van Daelen, R.; van der Lee, T.; Diergaarde, P.; Groenendijk, J.; et al. The Barley Mlo Gene: A Novel Control Element of Plant Pathogen Resistance. Cell 1997, 88, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Consonni, C.; Humphry, M.E.; Hartmann, H.A.; Livaja, M.; Durner, J.; Westphal, L.; Vogel, J.; Lipka, V.; Kemmerling, B.; Schulze Lefert, P.; et al. Conserved Requirement for a Plant Host Cell Protein in Powdery Mildew Pathogenesis. Nat. Genet. 2006, 38, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Sharp, G.L.; Martin, J.M.; Lanning, S.P.; Blake, N.K.; Brey, C.W.; Sivamani, E.; Qu, R.; Talbert, L.E. Field Evaluation of Transgenic and Classical Sources of Wheat Streak Mosaic Virus Resistance. Crop Sci. 2002, 42, 105–110. [Google Scholar] [CrossRef]
- Wang, X.; Kong, L.; Zhi, P.; Chang, C. Update on Cuticular Wax Biosynthesis and Its Roles in Plant Disease Resistance. Int. J. Mol. Sci. 2020, 21, 5514. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.Y.; Gao, H.N.; Jiang, H.; Liu, C.; Li, Y.Y. MdKCS2 Increased Plant Drought Resistance by Regulating Wax Biosynthesis. Plant Cell Rep. 2021, 40, 2357–2368. [Google Scholar] [CrossRef]
- Zhang, Y.L.; Zhang, C.L.; Wang, G.L.; Wang, Y.X.; Qi, C.H.; Zhao, Q.; You, C.X.; Li, Y.Y.; Hao, Y.J. The R2R3 MYB Transcription Factor MdMYB30 Modulates Plant Resistance against Pathogens by Regulating Cuticular Wax Biosynthesis. BMC Plant Biol. 2019, 19, 362. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, A.; Huang, H.; Zheng, M.; Zaman, W.; Li, D.; Saqib, S.; Zhao, H.; Lü, S. Molecular Cloning and Functional Analysis of GmLACS2-3 Reveals Its Involvement in Cutin and Suberin Biosynthesis along with Abiotic Stress Tolerance. Int. J. Mol. Sci. 2021, 22, 9175. [Google Scholar] [CrossRef] [PubMed]
- Basheer, J.; Vadovič, P.; Šamajová, O.; Melicher, P.; Komis, G.; Křenek, P.; Králová, M.; Pechan, T.; Ovečka, M.; Takáč, T.; et al. Knockout of MITOGEN-ACTIVATED PROTEIN KINASE 3 Causes Barley Root Resistance against Fusarium graminearum. Plant Physiol. 2022, 190, 2847–2867. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, P.; Shockey, J.; Lévesque, C.A.; Cook, R.J.; Browse, J. A Role for Jasmonate in Pathogen Defense of Arabidopsis. Proc. Natl. Acad. Sci. USA 1998, 95, 7209–7214. [Google Scholar] [CrossRef]
- Thomma, B.P.; Eggermont, K.; Penninckx, I.A.; Mauch Mani, B.; Vogelsang, R.; Cammue, B.P.; Broekaert, W.F. Separate Jasmonate-Dependent and Salicylate-Dependent Defense-Response Pathways in Arabidopsis Are Essential for Resistance to Distinct Microbial Pathogens. Proc. Natl. Acad. Sci. USA 1998, 95, 15107–15111. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Xie, D. Jasmonate in Plant Defence: Sentinel or Double Agent? Plant Biotechnol. J. 2015, 13, 1233–1240. [Google Scholar] [CrossRef]
- Raffaele, S.; Leger, A.; Roby, D. Very Long Chain Fatty Acid and Lipid Signaling in the Response of Plants to Pathogens. Plant Signal Behav. 2009, 4, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Karapetyan, S.; Dong, X. Redox and the Circadian Clock in Plant Immunity: A Balancing Act. Free Radic. Biol. Med. 2018, 119, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Oravec, M.W.; Greenham, K. The Adaptive Nature of the Plant Circadian Clock in Natural Environments. Plant Physiol. 2022, 190, 968–980. [Google Scholar] [CrossRef]
- Browse, J.; Roughan, P.G.; Slack, C.R. Light Control of Fatty Acid Synthesis and Diurnal Fluctuations of Fatty Acid Composition in Leaves. Biochem. J. 1981, 196, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Ekman, Å.; Bülow, L.; Stymne, S. Elevated Atmospheric CO(2) Concentration and Diurnal Cycle Induce Changes in Lipid Composition in Arabidopsis thaliana. New Phytol. 2007, 174, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Maatta, S.; Scheu, B.; Roth, M.R.; Tamura, P.; Li, M.; Williams, T.D.; Wang, X.; Welti, R. Levels of Arabidopsis thaliana Leaf Phosphatidic Acids, Phosphatidylserines, and Most Trienoate-Containing Polar Lipid Molecular Species Increase during the Dark Period of the Diurnal Cycle. Front. Plant Sci. 2012, 3, 49. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Andrés, F.; Kanehara, K.; Liu, Y.; Coupland, G.; Dörmann, P. Diurnal and Circadian Expression Profiles of Glycerolipid Biosynthetic Genes in Arabidopsis. Plant Signal Behav. 2014, 9, e29715. [Google Scholar] [CrossRef] [PubMed]
- Rikin, A.; Dillwith, J.W.; Bergman, D.K. Correlation between the Circadian Rhythm of Resistance to Extreme Temperatures and Changes in Fatty Acid Composition in Cotton Seedlings. Plant Physiol. 1993, 101, 31–36. [Google Scholar] [CrossRef]
- McClung, C.R. The Plant Circadian Oscillator. Biology 2019, 8, 14. [Google Scholar] [CrossRef]
- Kim, S.C.; Edgeworth, K.N.; Nusinow, D.A.; Wang, X. Circadian Clock Factors Regulate the First Condensation Reaction of Fatty Acid Synthesis in Arabidopsis. Cell Rep. 2023, 42, 113483. [Google Scholar] [CrossRef] [PubMed]
- Alabadí, D.; Oyama, T.; Yanovsky, M.J.; Harmon, F.G.; Más, P.; Kay, S.A. Reciprocal Regulation between TOC1 and LHY/CCA1 within the Arabidopsis Circadian Clock. Science 2001, 293, 880–883. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, A.S.; Haslam, R.P.; Michaelson, L.V.; Liao, P.; Napier, J.A.; Chye, M.L. Gene Expression in Plant Lipid Metabolism in Arabidopsis Seedlings. PLoS ONE 2014, 9, e107372. [Google Scholar] [CrossRef] [PubMed]
- Nagel, D.H.; Doherty, C.J.; Pruneda Paz, J.L.; Schmitz, R.J.; Ecker, J.R.; Kay, S.A. Genome-Wide Identification of CCA1 Targets Uncovers an Expanded Clock Network in Arabidopsis. Proc. Natl. Acad. Sci. USA 2015, 112, E4802–E4810. [Google Scholar] [CrossRef]
- Fauser, F.; Schiml, S.; Puchta, H. Both CRISPR/Cas-Based Nucleases and Nickases Can Be Used Efficiently for Genome Engineering in Arabidopsis thaliana. Plant J. 2014, 79, 348–359. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral Dip: A Simplified Method for Agrobacterium-Mediated Transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, W.; Ren, G.; Li, D.; Cahoon, R.E.; Chen, M.; Zhou, Y.; Yu, B.; Cahoon, E.B. Chlorophyll Synthase under Epigenetic Surveillance Is Critical for Vitamin E Synthesis, and Altered Expression Affects Tocopherol Levels in Arabidopsis. Plant Physiol. 2015, 168, 1503–1511. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, C.; Jiao, X.; Zhang, H.; Song, L.; Li, Y.; Gao, C.; Wang, K. Hi-TOM: A Platform for High-Throughput Tracking of Mutations Induced by CRISPR/Cas Systems. Sci. China Life Sci. 2019, 62, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Sparkes, I.A.; Runions, J.; Kearns, A.; Hawes, C. Rapid, Transient Expression of Fluorescent Fusion Proteins in Tobacco Plants and Generation of Stably Transformed Plants. Nat. Protoc. 2006, 1, 2019–2025. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A Fast Spliced Aligner with Low Memory Requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and Quantifying Mammalian Transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Mao, Z.; Shi, Y.; Cao, Q.; Chen, Y.; Sun, Y.; Liu, Z.; Zhang, Q.; Huang, M. Transcriptional Regulation on the Gene Expression Signature in Combined Allergic Rhinitis and Asthma Syndrome. Epigenomics 2018, 10, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Gibson, T.J.; Higgins, D.G. Multiple Sequence Alignment Using ClustalW and ClustalX. Curr. Protoc. Bioinform. 2002, 2, 2.3.1–2.3.22. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The Neighbor-Joining Method: A New Method for Reconstructing Phylogenetic Trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Felsenstein, J. Confidence Limits On Phylogenies: An Approach Using The Bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Zuckerkandl, E.; Pauling, L. Evolutionary Divergence and Convergence in Proteins. In Evolving Genes and Proteins; Elsevier: Amsterdam, The Netherlands, 1965; pp. 97–166. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, J.; Qi, S.; Huang, H.; Liao, H.; Cui, Y.; Liu, Z.; Qian, W.; Dong, H. Thaumatin-like Gene TLP1b Confers to Seed Oil Content and Resistance to Sclerotinia sclerotiorum in Arabidopsis. Int. J. Mol. Sci. 2025, 26, 1930. https://doi.org/10.3390/ijms26051930
Liao J, Qi S, Huang H, Liao H, Cui Y, Liu Z, Qian W, Dong H. Thaumatin-like Gene TLP1b Confers to Seed Oil Content and Resistance to Sclerotinia sclerotiorum in Arabidopsis. International Journal of Molecular Sciences. 2025; 26(5):1930. https://doi.org/10.3390/ijms26051930
Chicago/Turabian StyleLiao, Jinghang, Shucheng Qi, Hong Huang, Hongmei Liao, Yixin Cui, Zhi Liu, Wei Qian, and Hongli Dong. 2025. "Thaumatin-like Gene TLP1b Confers to Seed Oil Content and Resistance to Sclerotinia sclerotiorum in Arabidopsis" International Journal of Molecular Sciences 26, no. 5: 1930. https://doi.org/10.3390/ijms26051930
APA StyleLiao, J., Qi, S., Huang, H., Liao, H., Cui, Y., Liu, Z., Qian, W., & Dong, H. (2025). Thaumatin-like Gene TLP1b Confers to Seed Oil Content and Resistance to Sclerotinia sclerotiorum in Arabidopsis. International Journal of Molecular Sciences, 26(5), 1930. https://doi.org/10.3390/ijms26051930




