Sex-Specific Improvements in Myocardial Function and Angiogenesis with SGLT-2 Inhibitor Canagliflozin in a Swine Model of Metabolic Syndrome
Abstract
1. Introduction
2. Results
2.1. Functional Results
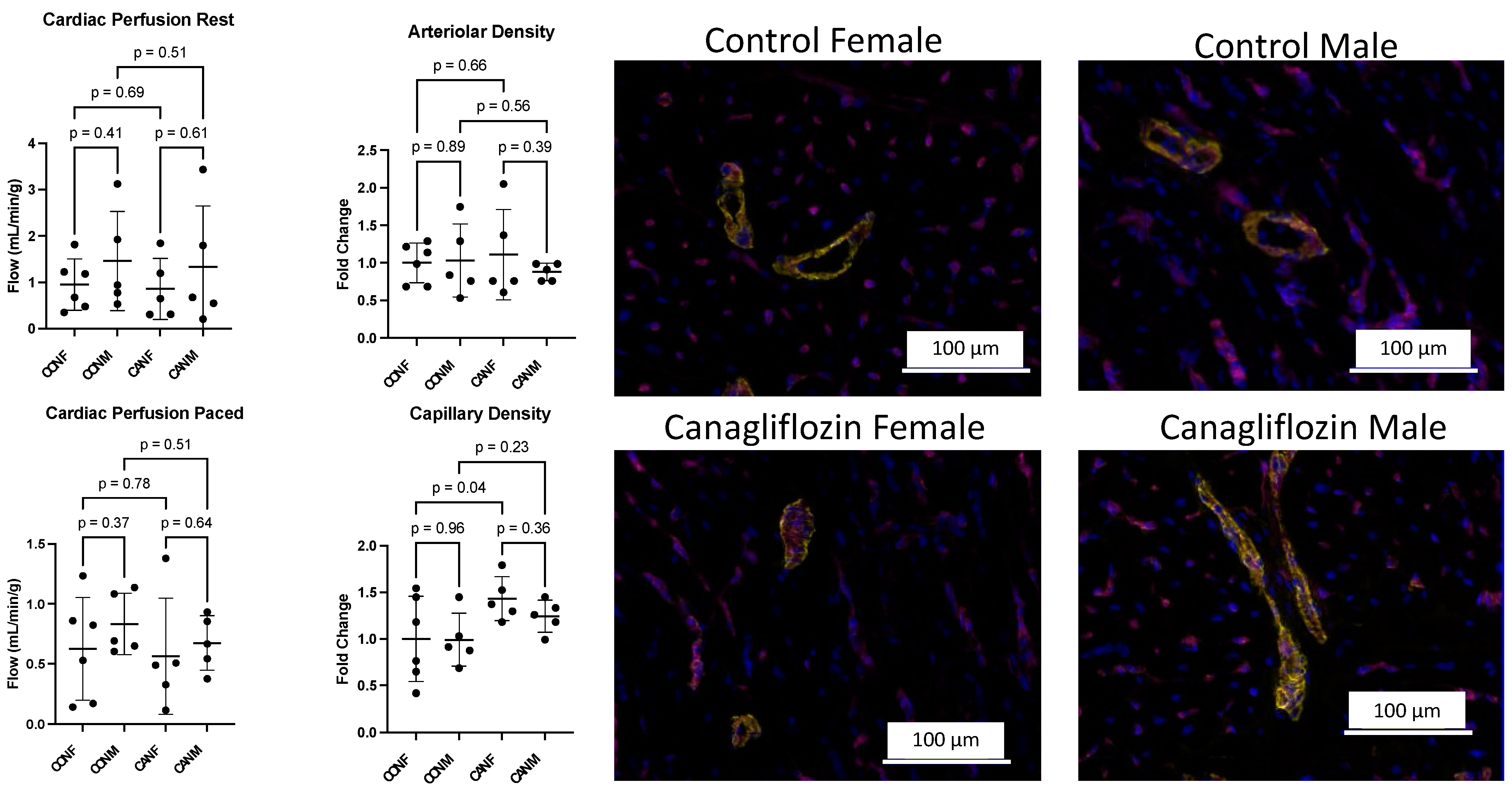
2.2. Myocardial Angiogenesis and Perfusion
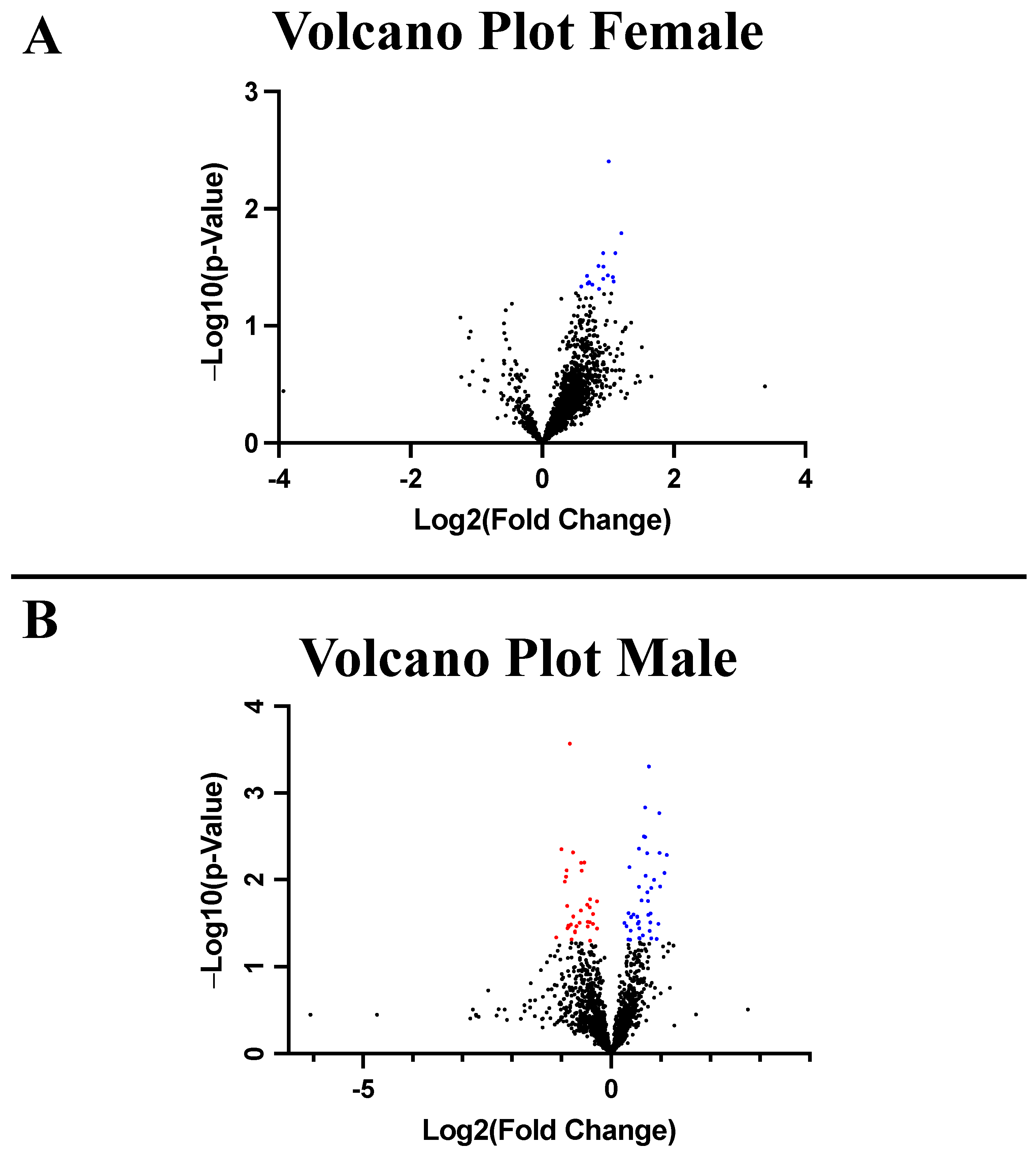
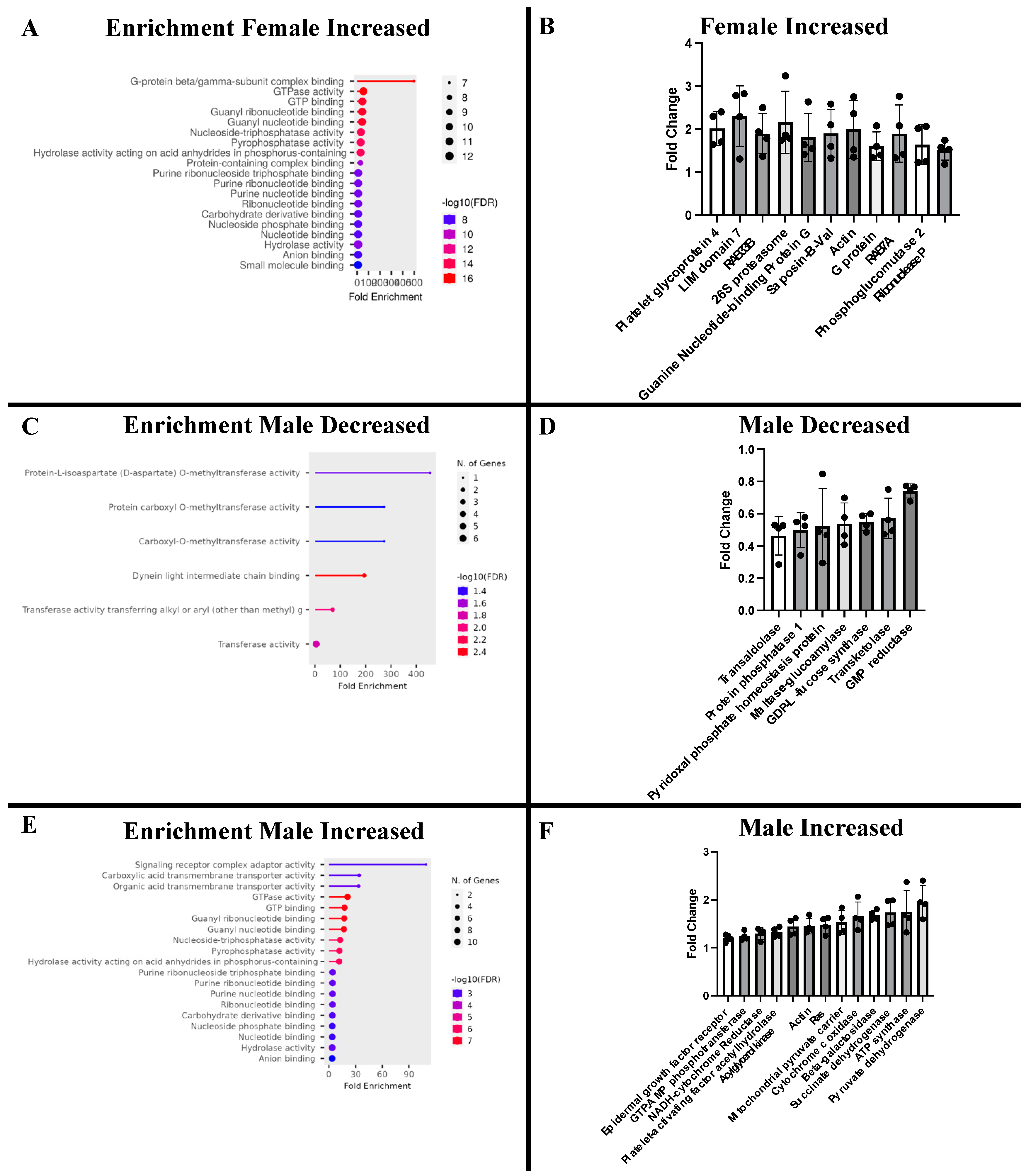
2.3. Proteomic Analysis
3. Discussion
4. Methods
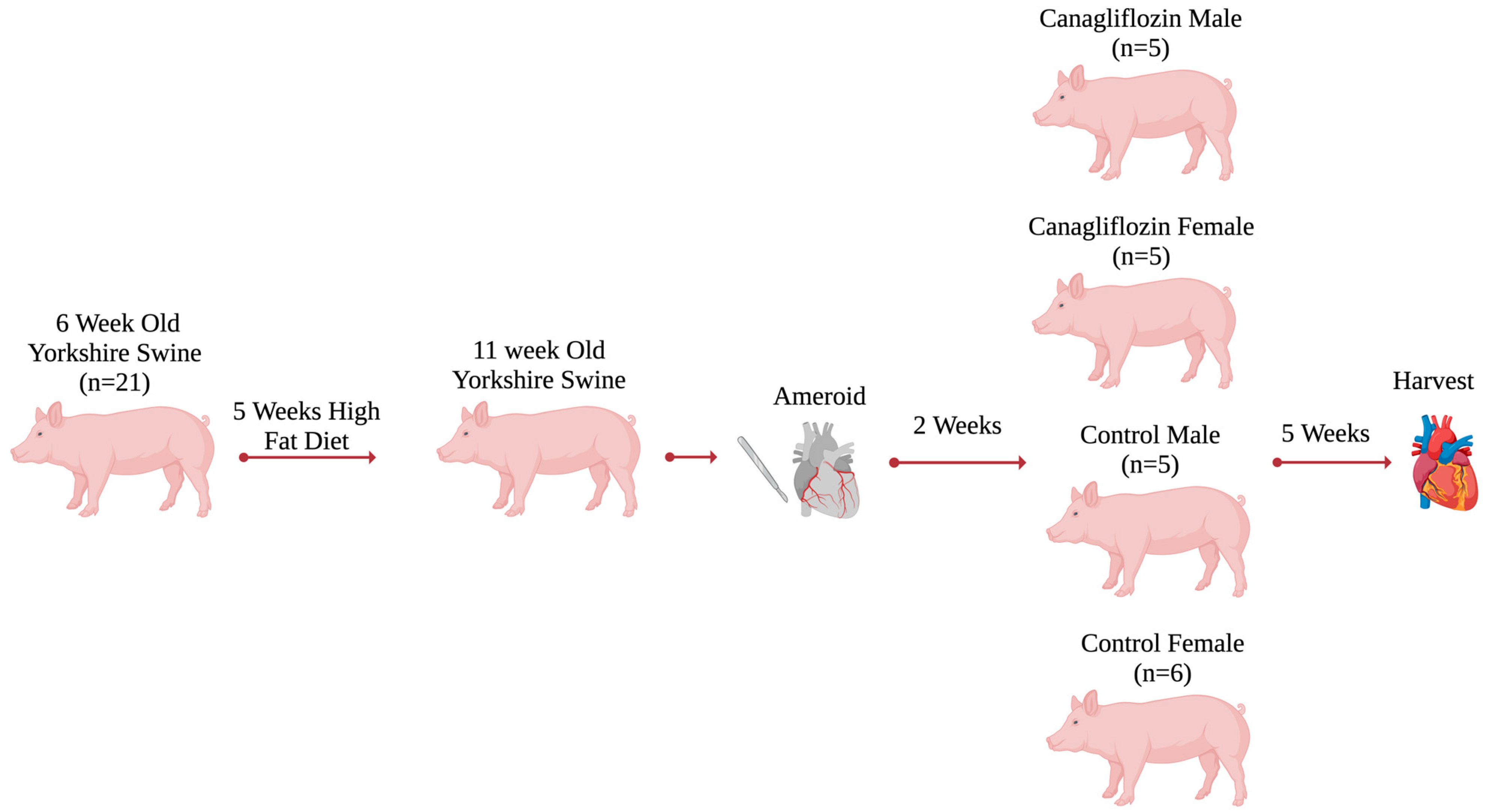
4.1. Swine Model
4.2. Sample Size and Dosing
4.3. Animal Care
4.4. Ameroid Constrictor Procedure
4.5. Harvest Procedure
4.6. Immunofluorescence
4.7. Data Analysis and Statistics
4.8. Proteomics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ESPVR | End-systolic pressure-volume relationship |
| CAN | Canagliflozin |
| CAN-F | Canagliflozin females |
| CAN-M | Canagliflozin males |
| CMI | Chronic myocardial ischemia |
| CON-F | Control females |
| CON-M | Control males |
| PRSW | Preload recruitable stroke work |
References
- Whelton, P.K.; Carey, R.M.; Aronow, W.S.; Casey, D.E., Jr.; Collins, K.J.; Himmelfarb, C.D.; DePalma, S.M.; Gidding, S.; Jamerson, K.A.; Jones, D.W.; et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: Executive Summary: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2018, 138, e426–e483. [Google Scholar] [CrossRef]
- Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; Don, C.W.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e18–e114. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 ACC/AHA/HFSA Guideline for the Management of Heart Failure. J. Card. Fail. 2022, 28, e1–e167. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Padda, I.S.; Mahtani, A.U.; Parmar, M. Sodium-Glucose Transport Protein 2 (SGLT2) Inhibitors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK576405/ (accessed on 17 January 2024).
- Katsiki, N.; Mikhailidis, D.P.; Theodorakis, M.J. Sodium-glucose Cotransporter 2 Inhibitors (SGLT2i): Their Role in Cardiometabolic Risk Management. Curr. Pharm. Des. 2017, 23, 1522–1532. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Verma, S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl. Sci. 2020, 5, 632–644. [Google Scholar] [CrossRef]
- McGuire, D.K.; Shih, W.J.; Cosentino, F.; Charbonnel, B.; Cherney, D.Z.I.; Dagogo-Jack, S.; Pratley, R.; Greenberg, M.; Wang, S.; Huyck, S.; et al. Association of SGLT2 Inhibitors with Cardiovascular and Kidney Outcomes in Patients with Type 2 Diabetes. JAMA Cardiol. 2021, 6, 148–158. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Lim, V.G.; Bell, R.M.; Arjun, S.; Kolatsi-Joannou, M.; Long, D.A.; Yellon, D.M. SGLT2 Inhibitor, Canagliflozin, Attenuates Myocardial Infarction in the Diabetic and Nondiabetic Heart. JACC: Basic Transl. Sci. 2019, 4, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Vaduganathan, M.; Docherty, K.F.; Claggett, B.L.; Jhund, P.S.; A de Boer, R.; Hernandez, A.F.; E Inzucchi, S.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. SGLT2 inhibitors in patients with heart failure: A comprehensive meta-analysis of five randomised controlled trials. Lancet 2022, 400, 757–767. [Google Scholar] [CrossRef]
- Mariani, M.V.; Manzi, G.; Pierucci, N.; Laviola, D.; Piro, A.; D’Amato, A.; Filomena, D.; Matteucci, A.; Severino, P.; Miraldi, F.; et al. SGLT2i effect on atrial fibrillation: A network meta-analysis of randomized controlled trials. J. Cardiovasc. Electrophysiol. 2024, 35, 1754–1765. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wei, Y.; Li, D.; Pu, J.; Ding, H.; Zhang, X. Mechanisms of SGLT2 Inhibitors in Heart Failure and Their Clinical Value. J. Cardiovasc. Pharmacol. 2023, 81, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Al Thani, N.A.; Hasan, M.; Yalcin, H.C. Use of Animal Models for Investigating Cardioprotective Roles of SGLT2 Inhibitors. J. Cardiovasc. Transl. Res. 2023, 16, 975–986. [Google Scholar] [CrossRef]
- Pennig, J.; Scherrer, P.; Gissler, M.C.; Anto-Michel, N.; Hoppe, N.; Füner, L.; Härdtner, C.; Stachon, P.; Wolf, D.; Hilgendorf, I.; et al. Glucose lowering by SGLT2-inhibitor empagliflozin accelerates atherosclerosis regression in hyperglycemic STZ-diabetic mice. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Schönberger, E.; Mihaljević, V.; Steiner, K.; Šarić, S.; Kurevija, T.; Majnarić, L.T.; Ćurčić, I.B.; Canecki-Varžić, S. Immunomodulatory Effects of SGLT2 Inhibitors—Targeting Inflammation and Oxidative Stress in Aging. Int. J. Environ. Res. Public Heal. 2023, 20, 6671. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhang, J.; Xue, M.; Li, X.; Han, F.; Liu, X.; Xu, L.; Lu, Y.; Cheng, Y.; Li, T.; et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc. Diabetol. 2019, 18, 1–13. [Google Scholar] [CrossRef]
- Sabe, S.A.; Xu, C.M.; Sabra, M.; Harris, D.D.; Malhotra, A.; Aboulgheit, A.; Stanley, M.; Abid, M.R.; Sellke, F.W. Canagliflozin Improves Myocardial Perfusion, Fibrosis, and Function in a Swine Model of Chronic Myocardial Ischemia. J. Am. Hear. Assoc. 2023, 12. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, D.; Sabe, S.A.; Xing, H.; Xu, C.; Sabra, M.; Harris, D.D.; Broadwin, M.; Abid, M.R.; Usheva, A.; Feng, J.; et al. Canagliflozin improves coronary microvascular vasodilation and increases absolute blood flow to the myocardium independent of angiogenesis. J. Thorac. Cardiovasc. Surg. 2023, 166, e535–e550. [Google Scholar] [CrossRef]
- Harris, D.D.; Sabe, S.A.; Xu, C.M.; Sabra, M.; Broadwin, M.; Malhotra, A.; Li, J.W.; Abid, M.R.; Sellke, F.W. Sodium-glucose co-transporter 2 inhibitor canagliflozin modulates myocardial metabolism and inflammation in a swine model for chronic myocardial ischemia. Surgery 2023, 175, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Harris, D.D.; Broadwin, M.; Sabe, S.A.; Stone, C.; Kanuparthy, M.; Nho, J.-W.; Bellam, K.; Banerjee, D.; Abid, M.R.; Sellke, F.W. Effects of diet-induced metabolic syndrome on cardiac function and angiogenesis in response to the sodium-glucose cotransporter-2 inhibitor canagliflozin. J. Thorac. Cardiovasc. Surg. 2024, 168, e183–e199. [Google Scholar] [CrossRef] [PubMed]
- Clayton, J.A.; Gaugh, M.D. Sex as a Biological Variable in Cardiovascular Diseases. Circ. 2022, 79, 1388–1397. [Google Scholar] [CrossRef] [PubMed]
- Connelly, P.J.; Jandeleit-Dahm, K.A.; Delles, C. Sex and gender aspects in vascular pathophysiology. Clin. Sci. 2020, 134, 2203–2207. [Google Scholar] [CrossRef] [PubMed]
- Regitz-Zagrosek, V.; Kararigas, G. Mechanistic Pathways of Sex Differences in Cardiovascular Disease. Physiol. Rev. 2017, 97, 1–37. [Google Scholar] [CrossRef] [PubMed]
- Connelly, P.J.; Freel, E.M.; Perry, C.; Ewan, J.; Touyz, R.M.; Currie, G.; Delles, C. Gender-Affirming Hormone Therapy, Vascular Health and Cardiovascular Disease in Transgender Adults. Hypertension 2019, 74, 1266–1274. [Google Scholar] [CrossRef]
- Man, J.J.; Beckman, J.A.; Jaffe, I.Z. Sex as a Biological Variable in Atherosclerosis. Circ. Res. 2020, 126, 1297–1319. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Clapier, R.; Piquereau, J.; Garnier, A.; Mericskay, M.; Lemaire, C.; Crozatier, B. Gender issues in cardiovascular diseases. Focus on energy metabolism. Biochim. et Biophys. Acta (BBA) Mol. Basis Dis. 2020, 1866, 165722. [Google Scholar] [CrossRef]
- Rivera, F.B.; Tang, V.A.S.; De Luna, D.V.; Lerma, E.V.; Vijayaraghavan, K.; Kazory, A.; Shah, N.S.; Volgman, A.S. Sex differences in cardiovascular outcomes of SGLT-2 inhibitors in heart failure randomized controlled trials: A systematic review and meta-analysis. Am. Hear. J. Plus Cardiol. Res. Pr. 2023, 26. [Google Scholar] [CrossRef]
- Harris, D.D.; A Sabe, S.; Broadwin, M.; Stone, C.; Malhotra, A.; Xu, C.M.; Sabra, M.; Abid, M.R.; Sellke, F.W.M. Proteomic Analysis and Sex-Specific Changes in Chronically Ischemic Swine Myocardium Treated with Sodium-Glucose Cotransporter-2 Inhibitor Canagliflozin. J. Am. Coll. Surg. 2024, 238, 1045–1055. [Google Scholar] [CrossRef]
- Swarup, S.; Goyal, A.; Grigorova, Y.; Zeltser, R. Metabolic Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459248/ (accessed on 7 March 2024).
- Karimi, M.; Pavlov, V.I.; Ziegler, O.; Sriram, N.; Yoon, S.-Y.; Agbortoko, V.; Alexandrova, S.; Asara, J.; Sellke, F.W.; Sturek, M.; et al. Robust effect of metabolic syndrome on major metabolic pathways in the myocardium. PLOS ONE 2019, 14, e0225857. [Google Scholar] [CrossRef]
- Nikolaou, P.-E.; Lambrinidis, G.; Georgiou, M.; Karagiannis, D.; Efentakis, P.; Bessis-Lazarou, P.; Founta, K.; Kampoukos, S.; Konstantin, V.; Palmeira, C.M.; et al. Hydrolytic Activity of Mitochondrial F1FO-ATP Synthase as a Target for Myocardial Ischemia–Reperfusion Injury: Discovery and In Vitro and In Vivo Evaluation of Novel Inhibitors. J. Med. Chem. 2023, 66, 15115–15140. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Zhang, H.; Wang, X.; Liu, Z.; Nie, T.; Li, L.; Su, J.; Zhu, Y.; Ma, C.; Huang, Y.; et al. Rg3 regulates myocardial pyruvate metabolism via P300-mediated dihydrolipoamide dehydrogenase 2-hydroxyisobutyrylation in TAC-induced cardiac hypertrophy. Cell Death Dis. 2022, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Serdar, C.C.; Cihan, M.; Yücel, D.; A Serdar, M. Sample size, power and effect size revisited: Simplified and practical approaches in pre-clinical, clinical and laboratory studies. Biochem. Medica 2021, 31, 27–53. [Google Scholar] [CrossRef] [PubMed]
- Ching, T.; Huang, S.; Garmire, L.X. Power analysis and sample size estimation for RNA-Seq differential expression. RNA 2014, 20, 1684–1696. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Simpson, K.L.; Lancashire, L.J.; Walker, M.J.; Dawson, M.J.; Unwin, R.D.; Rembielak, A.; Price, P.; West, C.; Dive, C.; et al. Statistical Considerations of Optimal Study Design for Human Plasma Proteomics and Biomarker Discovery. J. Proteome Res. 2012, 11, 2103–2113. [Google Scholar] [CrossRef] [PubMed]
- Stone, C.R.; Harris, D.D.; Broadwin, M.; Kanuparthy, M.; Sabe, S.A.; Xu, C.; Feng, J.; Abid, M.R.; Sellke, F.W. Crafting a Rigorous, Clinically Relevant Large Animal Model of Chronic Myocardial Ischemia: What Have We Learned in 20 Years? Methods Protoc. 2024, 7, 17. [Google Scholar] [CrossRef]
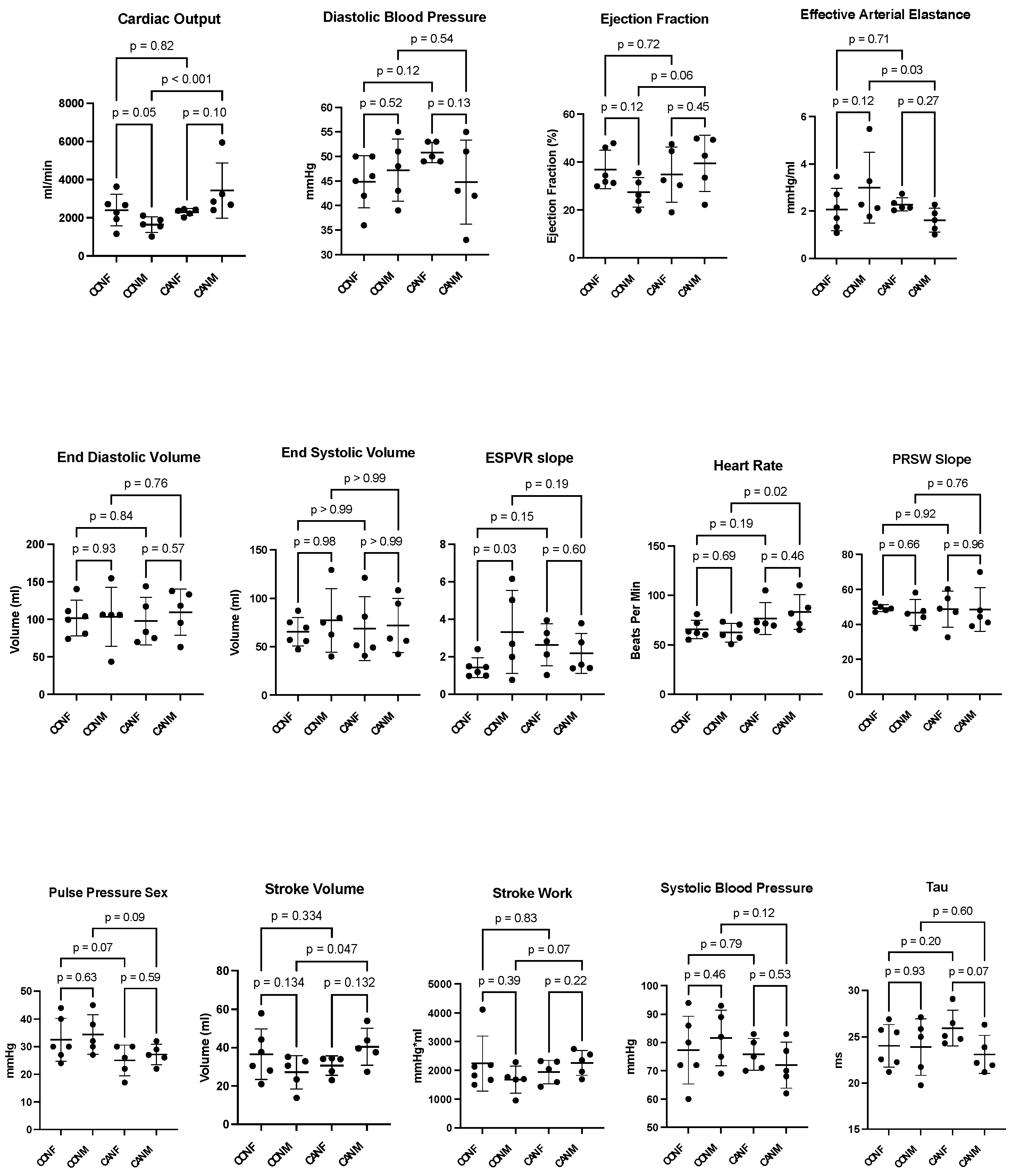
| Functional Data | Control Females | Control Males | Canagliflozin Females | Canagliflozin Males |
|---|---|---|---|---|
| Cardiac Output (mL/min, mean ± SD) | 2397 ± 830 | 1643 ± 412 | 2295 ± 177 | 3426 ± 1440 |
| Diastolic Blood Pressure (mmHg, mean ± SD) | 44.8 ± 5.3 | 47.2 ± 6.3 | 50.8 ± 2.0 | 44.8 ± 8.6 |
| Ejection Fraction (%, mean ± SD) | 36.9 ± 8.0 | 27.4 ± 6.1 | 34.7 ± 11.5 | 39.5 ± 11.7 |
| Effective Arterial Elastance (mmHg/mL, mean ± SD) | 2.1 ± 0.9 | 3.1 ± 1.5 | 2.3 ± 0.3 | 1.6 ± 0.5 |
| End-Diastolic Volume (mL, mean ± SD) | 101.7 ± 23.6 | 103.3 ± 39.5 | 97.9 ± 31.8 | 109.6 ± 30.7 |
| End-Systolic Volume (mL, mean ± SD) | 65.4 ± 14.7 | 77.3 ± 32.9 | 68.7 ± 33.0 | 71.9 ± 28.0 |
| ESPVR Slope (slope, mean ± SD) | 1.4 ± 0.52 | 3.3 ± 2.2 | 2.6 ± 1.1 | 2.2 ± 1.1 |
| Heart Rate (bpm, mean ± SD) | 65.7 ± 9.3 | 62.3 ± 9.4 | 76.7 ± 16.1 | 83.2 ± 17.4 |
| PRSW Slope (slope, mean ± SD) | 49.3 ± 2.0 | 46.7 ± 7.4 | 48.7 ± 10.4 | 48.5 ± 12.5 |
| Pulse Pressure (mmHg, mean ± SD) | 32.5 ± 7.8 | 34.4 ± 7.2 | 25.0 ± 5.6 | 27.2 ± 3.7 |
| Stroke Volume (mL, mean ± SD) | 36.5 ± 13.2 | 27.2 ± 8.7 | 30.7 ± 5.1 | 40.5 ± 4.3 |
| Stroke Work (mmHg*mL, mean ± SD) | 2237 ± 958 | 1675 ± 475 | 1939 ± 408 | 2259 ± 443 |
| Sytolic Blood Pressue (mmHg, mean ± SD) | 77.3 ± 12.0 | 81.6 ± 9.8 | 75.8 ± 5.6 | 72.0 ± 8.2 |
| Tau (ms, mean ± SD) | 24.0 ± 2.3 | 23.9 ± 3.1 | 26.0 ± 1.9 | 23.1 ± 2.1 |
| Perfusion Data | ||||
| Cardiac Perfusion Rest (mL/min/g, Mean ± SD) | 0.95 ± 0.55 | 1.46 ± 1.07 | 0.86 ± 0.66 | 1.33 ± 1.32 |
| Cardiac Perfusion Paced (mL/min/g, Mean ± SD) | 0.63 ± 0.43 | 0.83 ± 0.26 | 0.56 ± 0.48 | 0.67 ± 0.23 |
| Vascular Density | ||||
| Arteriolar Density (fold change, mean ± SD) | 1.0 ± 0.2 | 1.0 ± 0.4 | 1.1 ± 0.5 | 0.9 ± 0.1 |
| Capillary Density (fold change, mean ± SD) | 1.0 ± 0.4 | 1.0 ± 0.3 | 1.4 ± 0.2 | 1.2 ± 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harris, D.D.; Broadwin, M.; Stone, C.; Sabe, S.A.; Kanuparthy, M.; Nho, J.-W.; Muir, K.C.; Abid, M.R.; Sellke, F.W. Sex-Specific Improvements in Myocardial Function and Angiogenesis with SGLT-2 Inhibitor Canagliflozin in a Swine Model of Metabolic Syndrome. Int. J. Mol. Sci. 2025, 26, 1887. https://doi.org/10.3390/ijms26051887
Harris DD, Broadwin M, Stone C, Sabe SA, Kanuparthy M, Nho J-W, Muir KC, Abid MR, Sellke FW. Sex-Specific Improvements in Myocardial Function and Angiogenesis with SGLT-2 Inhibitor Canagliflozin in a Swine Model of Metabolic Syndrome. International Journal of Molecular Sciences. 2025; 26(5):1887. https://doi.org/10.3390/ijms26051887
Chicago/Turabian StyleHarris, Dwight D., Mark Broadwin, Christopher Stone, Sharif A. Sabe, Meghamsh Kanuparthy, Ju-Woo Nho, Kelsey C. Muir, M. Ruhul Abid, and Frank W. Sellke. 2025. "Sex-Specific Improvements in Myocardial Function and Angiogenesis with SGLT-2 Inhibitor Canagliflozin in a Swine Model of Metabolic Syndrome" International Journal of Molecular Sciences 26, no. 5: 1887. https://doi.org/10.3390/ijms26051887
APA StyleHarris, D. D., Broadwin, M., Stone, C., Sabe, S. A., Kanuparthy, M., Nho, J.-W., Muir, K. C., Abid, M. R., & Sellke, F. W. (2025). Sex-Specific Improvements in Myocardial Function and Angiogenesis with SGLT-2 Inhibitor Canagliflozin in a Swine Model of Metabolic Syndrome. International Journal of Molecular Sciences, 26(5), 1887. https://doi.org/10.3390/ijms26051887






