Generation and Characterization of Human iPSC-Derived Astrocytes with Potential for Modeling X-Linked Adrenoleukodystrophy Phenotypes
Abstract
:1. Introduction
2. Results
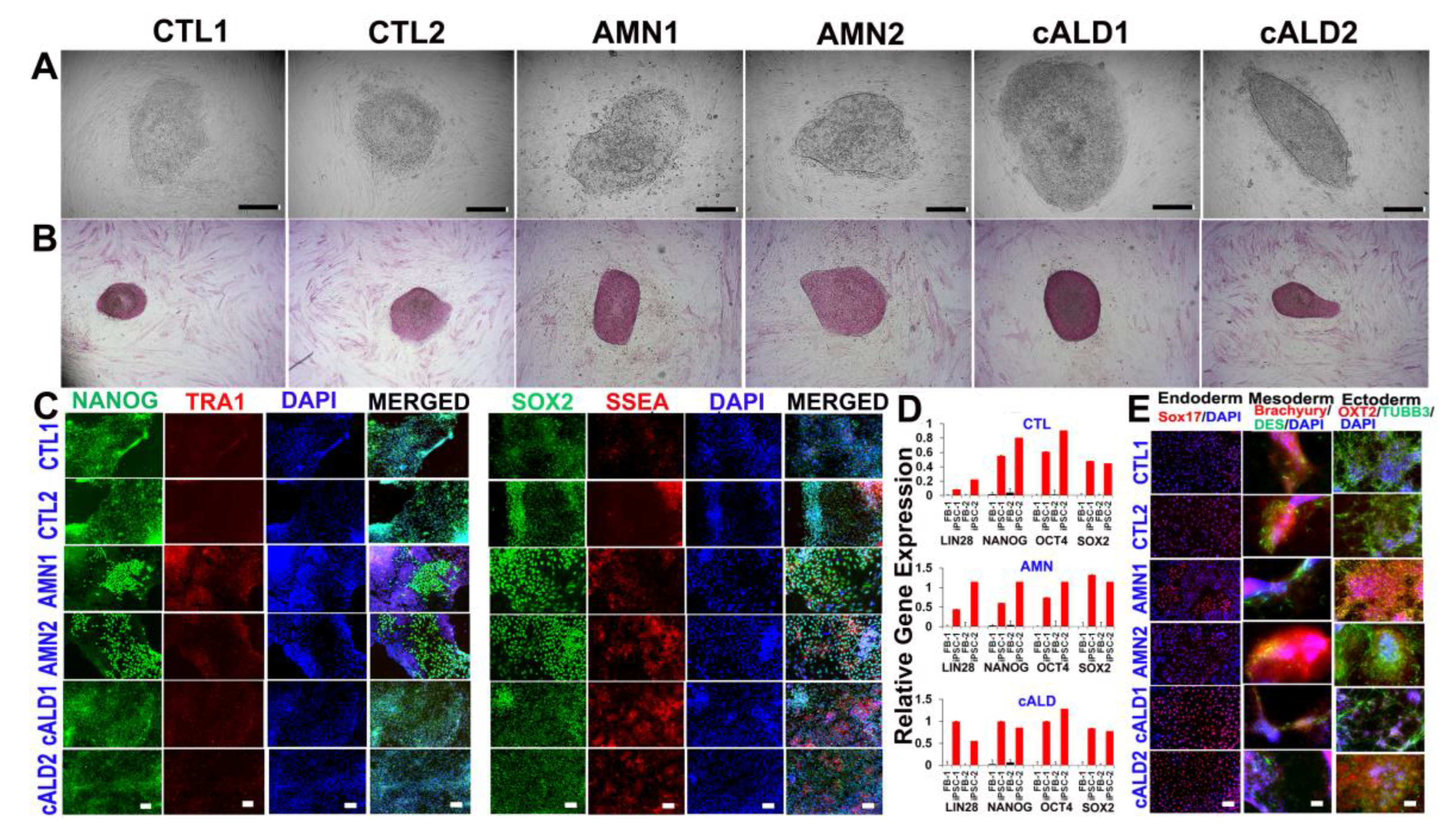
2.1. CTL, AMN, and cALD Patient Fibroblast-Derived iPSCs Are Positive for AP and Expressed Pluripotency Markers
2.2. Expression of Pluripotency Markers in CTL, AMN, and cALD Fibroblast-Derived iPSCs
2.3. Differentiation of iPSC Colonies into Germ Layers
2.4. Astrocyte Differentiation of CTL, AMN, and cALD iPSC Colonies
2.5. ABCD1 Expression and VLCFA Levels in CTL, AMN, and cALD iPSC-Derived Astrocytes
2.6. Mycoplasma Detection in CTL, AMN, and cALD iPSC-Derived Cells
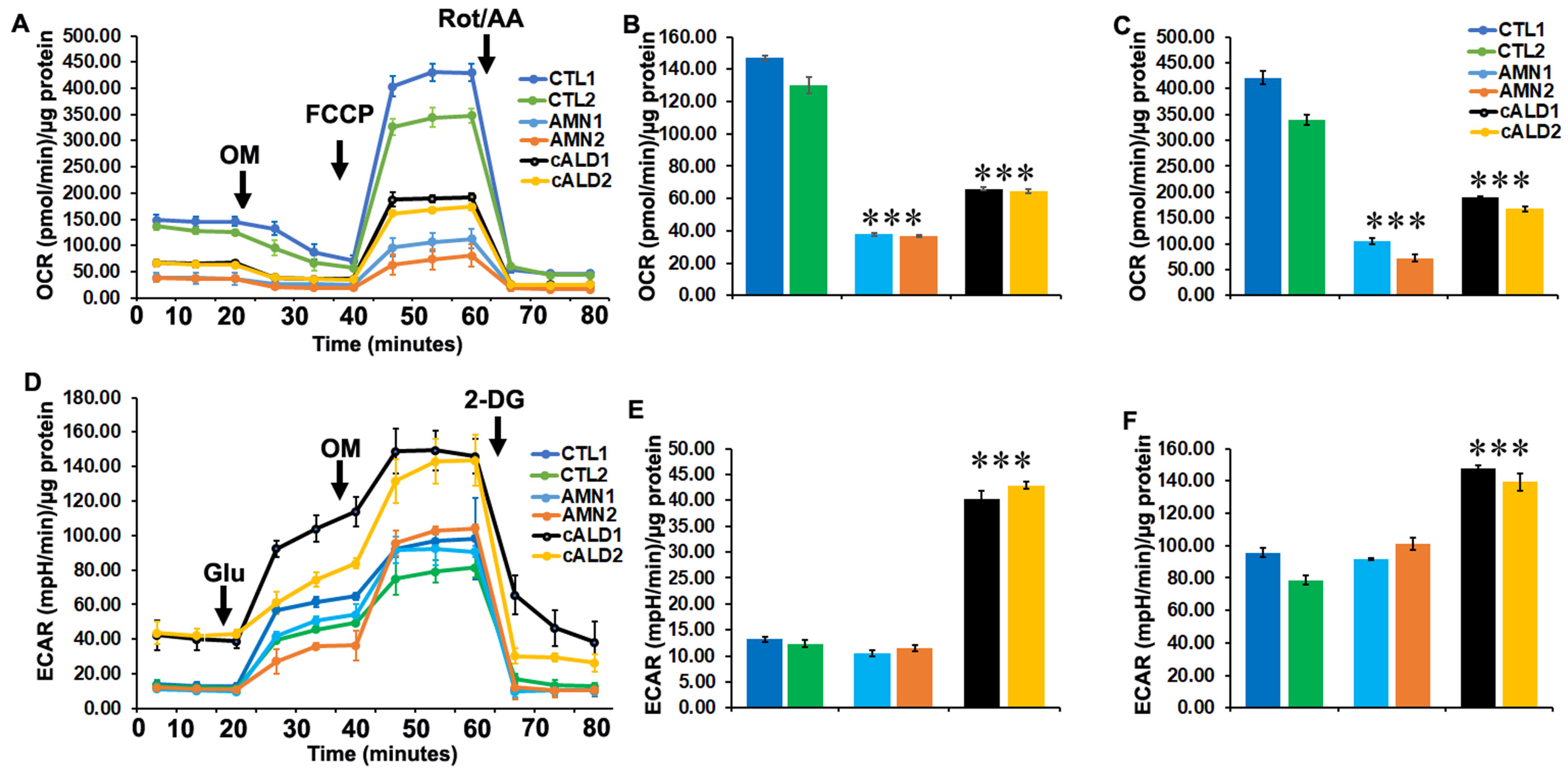
2.7. Glycolytic Rate Is Significantly Increased in cALD iPSC-Derived Astrocytes Compared to AMN Astrocytes
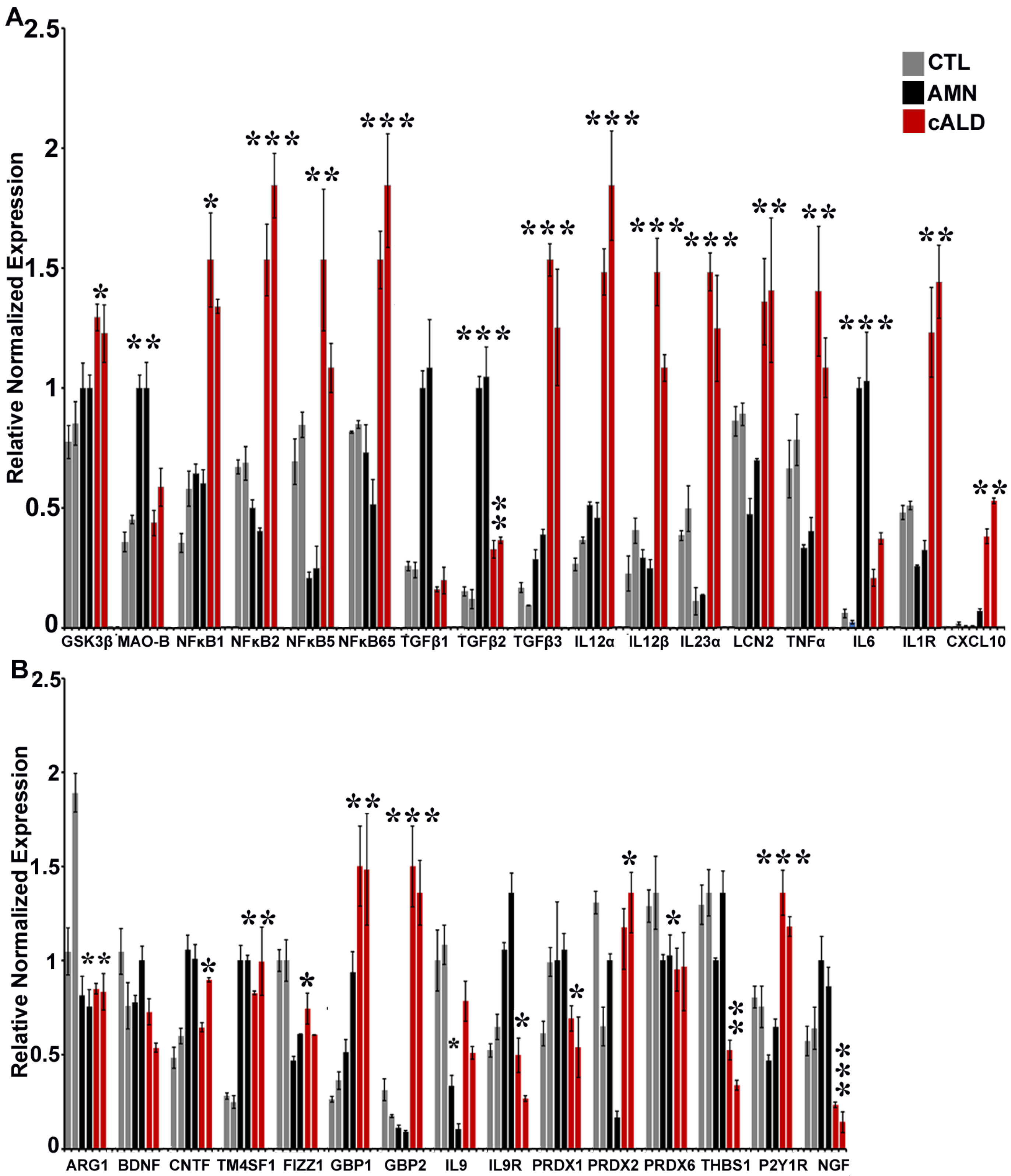
2.8. The Balance of Pro- and Anti-Inflammatory Cytokine Pathway Genes Is Altered Between AMN and cALD Astrocytes
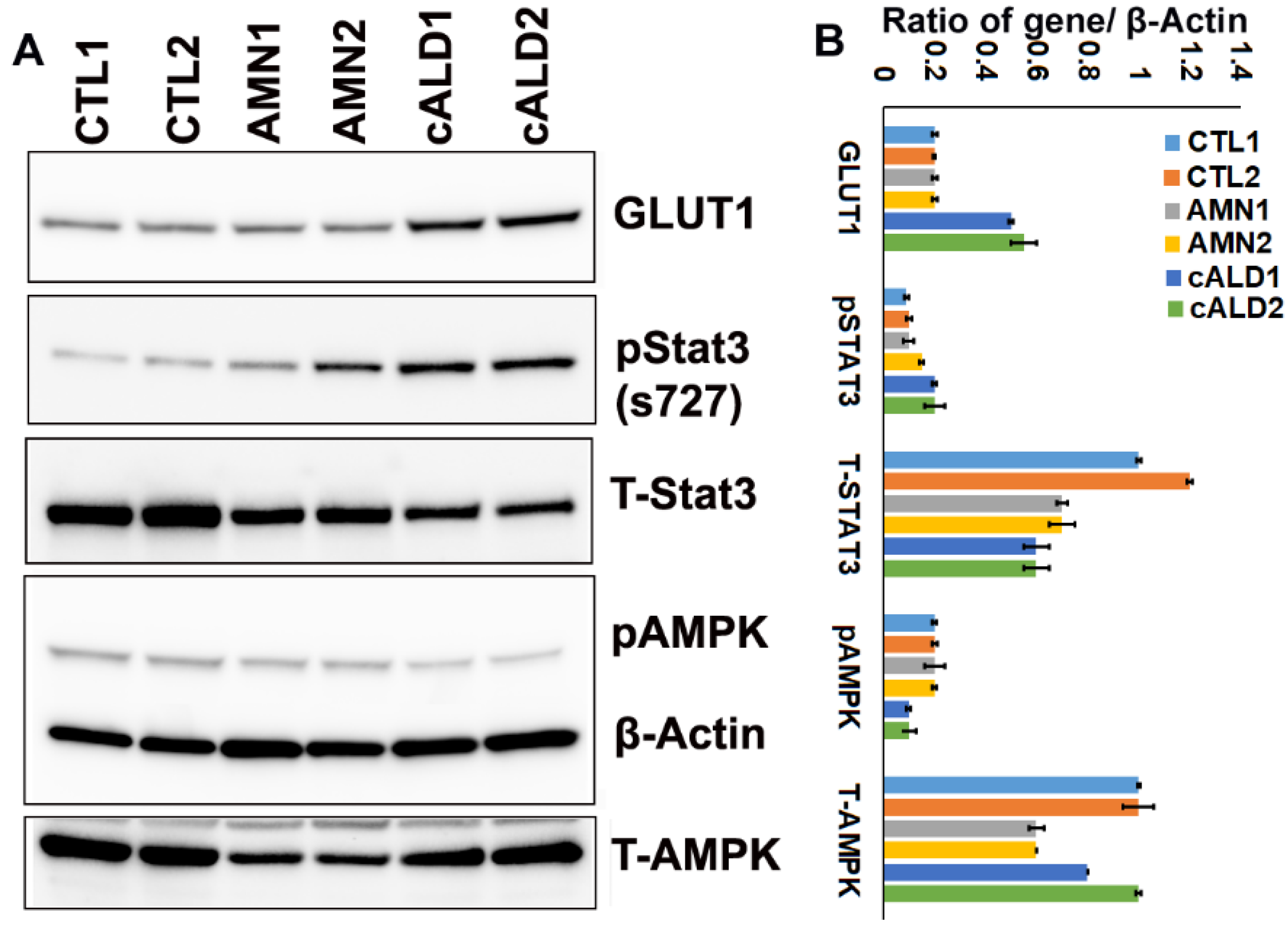
2.9. cALD Astrocytes Have Increased STAT3 Phosphorylation and Decreased AMPK Levels
3. Discussion
4. Material and Methods
4.1. Ethics Approval
4.2. Human Fibroblasts
4.3. Derivation of iPSCs and Differentiation into Astrocytes
4.4. Mycoplasma Detection
4.5. Culturing of Mature iPSC-Derived Astrocytes
4.6. VLCFA Analysis
4.7. Mitochondrial Oxygen Consumption and Glycolytic Function Measurement
4.8. Quantitative Real-Time Polymerase Chain Reaction Gene Expression
4.9. Immunofluorescence Staining
4.10. Western Blot Analysis
4.11. Karyotyping
4.12. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. List of Human Real-Time Primers Used
| Name | Sequence |
| OCT4-F | GAAACCCACACTGCAGATCA |
| OCT4-R | GGTTACAGAACCACACTCG |
| NANOG-F | AGATGCCTCACACGGAGACT |
| NANOG-R | TTTGCGACACTCTTCTCTGC |
| SOX2-F | TGCTGCCTCTTTAAGACTAGGAC |
| SOX2-R | CCTGGGGCTCAAACTTCTCT |
| LIN28-F | GGCAGTGGAGTTCACCTTTAAGA |
| LIN28-R | AGCTTGCATTCCTTGGCATGATGA |
| L27-F | TGGACAAAACTGTCGTCAATAAGG |
| L27-R | AGAACCACTTGTTCTTGCCTGTC |
| TM4SF1-F | GGCTACTGTGTCATTGTGGCAG |
| TM4SF1-R | ACTCGGACCATGTGGAGGTATC |
| THBS1-F | GCTGGAAATGTGGTGCTTGTCC |
| THBS1-R | CTCCATTGTGGTTGAAGCAGGC |
| THBS2-F | CAGTCTGAGCAAGTGTGACACC |
| THBS2-R | TTGCAGAGACGGATGCGTGTGA |
| IL9-F | GACCAGTTGTCTCTGTTTGGGC |
| IL9-R | TTTCACCCGACTGAAAATCAGTGG |
| IL-9R-F | ATCAGTCCTGCCTTGGAGCCAA |
| IL-9R-R | CCGACAATGTGATCCCTGTGCT |
| MAO-F | GTGAAGCAGTGTGGAGGCACAA |
| MAO-R | TTCACTCGGTCTCCAAGGAGGT |
| NFkB1-F | GCAGCACTACTTCTTGACCACC |
| NFkB1-R | TCTGCTCCTGAGCATTGACGTC |
| NFkB2-F | GGCAGACCAGTGTCATTGAGCA |
| NFkB2-R | CAGCAGAAAGCTCACCACACTC |
| NFkB65-F | TGAACCGAAACTCTGGCAGCTG |
| NFkB65-R | CATCAGCTTGCGAAAAGGAGCC |
| GBP1-F | TAGCAGACTTCTGTTCCTACATCT |
| GBP1-R | CCACTGCTGATGGCATTGACGT |
| GBP2-F | GTTCCTACATCCTCAGCCATTCC |
| GBP2-R | CCACTGCTGATGGCATTGACGT |
| PRDX1-F | CTGCCAAGTGATTGGTGCTTCTG |
| PRDX1-R | AATGGTGCGCTTCGGGTCTGAT |
| PRDX2-F | CCTTCCAGTACACAGACGAGCA |
| PRDX2-R | CTCACTATCCGTTAGCCAGCCT |
| PRDX6-F | CAGCTACCACTGGCAGGAACTT |
| PRDX6-R | GGAAGGACCATCACACTATCCC |
| LCN2-F | GTGAGCACCAACTACAACCAGC |
| LCN2-R | GTTCCGAAGTCAGCTCCTTGGT |
| TGFb1-F | TACCTGAACCCGTGTTGCTCTC |
| TGFb1-R | GTTGCTGAGGTATCGCCAGGAA |
| TGFb2-F | AAGAAGCGTGCTTTGGATGCGG |
| TGFb2-R | ATGCTCCAGCACAGAAGTTGGC |
| TGFb3-F | CTAAGCGGAATGAGCAGAGGATC |
| TGFb3-R | TCTCAACAGCCACTCACGCACA |
| GLUT1-F | TTGCAGGCTTCTCCAACTGGAC |
| GLUT1-R | CAGAACCAGGAGCACAGTGAAG |
| IL12α-F | TGCCTTCACCACTCCCAAAACC |
| IL12α-R | CAATCTCTTCAGAAGTGCAAGGG |
| IL12β-F | GACATTCTGCGTTCAGGTCCAG |
| IL12β-R | CATTTTTGCGGCAGATGACCGTG |
| IL23α-F | GAGCCTTCTCTGCTCCCTGATA |
| IL23α-R | GACTGAGGCTTGGAATCTGCTG |
| IL1R-F | GTGCTTTGGTACAGGGATTCCTG |
| IL1R-R | CACAGTCAGAGGTAGACCCTTC |
| TNFα-F | CTCTTCTGCCTGCTGCACTTTG |
| TNFα-R | ATGGGCTACAGGCTTGTCACTC |
| ARG1-F | TCATCTGGGTGGATGCTCACAC |
| ARG1-R | GAGAATCCTGGCACATCGGGAA |
| FIZZ1-F | GCAAGAAGCTCTCGTGTGCTAG |
| FIZZ1-R | AACATCCCACGAACCACAGCCA |
| BDNF-F | CATCCGAGGACAAGGTGGCTTG |
| BDNF-R | GCCGAACTTTCTGGTCCTCATC |
| CNTF-F | TCAGACCTGACTGCTCTTACGG |
| CNTF-R | TTGGAGTCGCTCTGCCTCGGT |
| NGF-F | ACCCGCAACATTACTGTGGACC |
| NGF-R | GACCTCGAAGTCCAGATCCTGA |
| P2RY1-F | GCCATCTGGATGTTCGTCTTCC |
| P2RY1-R | TGGCAGAGTCAGCACGTACAAG |
Appendix B. List of Antibodies Used
| Catalog No | Antibody Details |
| 5685S | EAAT1 (D20D5) RABBIT mAb |
| 59678S | AQP4(D1F8E) XP RABBIT mAb |
| 85828S | ALDH1L1(E712Q) RABBIT mAb |
| G3893 | MONOCLONAL ANTI-GLIAL FIBRILLARY ACID PROTEIN (GFAP) |
| S2532 | MONOCLONAL ANTI-S-100B |
| AB53521–1001 | Anti-A2B5 antibody |
| 5568S | BETA-3-TUBULIN |
| 5332S | Desmin (D93F5) XP® Rabbit mAb |
| 963121 | Goat anti-human SOX17 |
| 963273 | Goat anti-human Otx2 |
| 963427 | Goat anti-human Brachyury |
| 73349S | NESTIN |
| AF2018 | Human/Mouse/Rat SOX2 Affinity Purified Polyclonal Ab |
| 60064AD | Anti-Human TRA-1–60 Antibody, Clone TRA-1–60R, Alexa Fluor® 488 |
| 60062PE | Anti-Human SSEA-4 Antibody, Clone MC-813–70, PE |
| 2840S | Oct-4A Rabbit mAb |
| AF1997 | Human Nanog Antibody |
| MAB2018 | Human/mouse/rat Sox2 |
| 2840S | Oct-4A Rabbit mAb |
| AF-1759 | Human/Mouse/Oct-3/4 Antibody |
| 23064S | Sox2 (D9B8N) Rabbit mAb |
| ab197013 | ABCD1/ALD antibody [EPR15929] |
| Secondary Antibodies | |
| A21206 | Alexa Fluor 488 Donkey Anti Rabbit |
| A21042 | Alexa Fluor 488 Goat anti mouse |
| A11055 | Alexa Fluor 488 Donkey anti goat |
| 705–605–147 | Alexa Fluor 647 Donkey Anti Goat |
| 715–605–150 | Alexa Fluor 647 Donkey Anti mouse |
| 711–605–152 | Alexa Fluor 647 Donkey Anti Rabbit |
| Manufacturer | Dilution |
| CELL SIGNALING | 1/200 |
| CELL SIGNALING | 1/400 |
| CELL SIGNALING | 2/100 |
| SIGMA | 1/100 |
| SIGMA | 1/100 |
| ABCAM | 1/100 |
| Cell Signaling | 1/200 |
| Cell Signaling | 1/100 |
| R&D | 1/100 |
| R&D | 1/100 |
| R&D | 1/100 |
| CELL SIGNALING | 1/400 |
| R&D | 5 µg/mL |
| STEM CELL TECH | 2/100 |
| STEM CELL TECH | 2/100 |
| Cell Signaling | 1/100 |
| R&D | 10 µg/mL |
| R&D | 8 µg/mL |
| Cell Signaling | 1/100 |
| R&D | 10 µg/mL |
| Cell Signaling | 1/100 |
| Abcam | 1/1000 |
| Invitrogen | 1/200 |
| Invitrogen | 1/200 |
| Invitrogen | 1/200 |
| JacksonImmunoResearch | 1.5/100 |
| JacksonImmunoResearch | 1.5/100 |
| JacksonImmunoResearch | 1.5/100 |
References
- Moser, H.W.; Mahmood, A.; Raymond, G.V. X-linked adrenoleukodystrophy. Nat. Clin. Pract. Neurol. 2007, 3, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Moser, H.W.; Moser, A.B.; Frayer, K.K.; Chen, W.; Schulman, J.D.; O’Neill, B.P.; Kishimoto, Y. Adrenoleukodystrophy: Increased plasma content of saturated very long chain fatty acids. Neurology 1981, 31, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Kemp, S.; Wanders, R. Biochemical aspects of X-linked adrenoleukodystrophy. Brain Pathol. 2010, 20, 831–837. [Google Scholar] [CrossRef]
- Singh, I.; Pujol, A. Pathomechanisms underlying X-adrenoleukodystrophy: A three-hit hypothesis. Brain Pathol. 2010, 20, 838–844. [Google Scholar] [CrossRef]
- Moser, H.W.; Moser, A.B.; Smith, K.D.; Bergin, A.; Borel, J.; Shankroff, J.; Stine, O.C.; Merette, C.; Ott, J.; Krivit, W.; et al. Adrenoleukodystrophy: Phenotypic variability and implications for therapy. J. Inherit. Metab. Dis. 1992, 15, 645–664. [Google Scholar] [CrossRef]
- Turk, B.R.; Theda, C.; Fatemi, A.; Moser, A.B. X-linked adrenoleukodystrophy: Pathology, pathophysiology, diagnostic testing, newborn screening and therapies. Int. J. Dev. Neurosci. 2020, 80, 52–72. [Google Scholar] [CrossRef]
- Forss-Petter, S.; Werner, H.; Berger, J.; Lassmann, H.; Molzer, B.; Schwab, M.H.; Bernheimer, H.; Zimmermann, F.; Nave, K.A. Targeted inactivation of the X-linked adrenoleukodystrophy gene in mice. J. Neurosci. Res. 1997, 50, 829–843. [Google Scholar] [CrossRef]
- Kobayashi, T.; Shinnoh, N.; Kondo, A.; Yamada, T. Adrenoleukodystrophy protein-deficient mice represent abnormality of very long chain fatty acid metabolism. Biochem. Biophys. Res. Commun. 1997, 232, 631–636. [Google Scholar] [CrossRef]
- Lu, J.F.; Lawler, A.M.; Watkins, P.A.; Powers, J.M.; Moser, A.B.; Moser, H.W.; Smith, K.D. A mouse model for X-linked adrenoleukodystrophy. Proc. Natl. Acad. Sci. USA 1997, 94, 9366–9371. [Google Scholar] [CrossRef]
- Fourcade, S.; Lopez-Erauskin, J.; Galino, J.; Duval, C.; Naudi, A.; Jove, M.; Kemp, S.; Villarroya, F.; Ferrer, I.; Pamplona, R.; et al. Early oxidative damage underlying neurodegeneration in X-adrenoleukodystrophy. Hum. Mol. Genet. 2008, 17, 1762–1773. [Google Scholar] [CrossRef]
- Powers, J.M.; Pei, Z.; Heinzer, A.K.; Deering, R.; Moser, A.B.; Moser, H.W.; Watkins, P.A.; Smith, K.D. Adreno-leukodystrophy: Oxidative stress of mice and men. J. Neuropathol. Exp. Neurol. 2005, 64, 1067–1079. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Giri, S. Loss of AMP-activated protein kinase in X-linked adrenoleukodystrophy patient-derived fibroblasts and lymphocytes. Biochem. Biophys. Res. Commun. 2014, 445, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Khan, M.; Singh, I. Silencing of Abcd1 and Abcd2 genes sensitizes astrocytes for inflammation: Implication for X-adrenoleukodystrophy. J. Lipid Res. 2009, 50, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Suhail, H.; Giri, S. Loss of AMP-activated protein kinase induces mitochondrial dysfunction and proinflammatory response in unstimulated Abcd1-knockout mice mixed glial cells. Mediators Inflamm. 2015, 2015, 176983. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Parasar, P.; Kaur, N.; Singh, J. IPSC-Derived Astrocytes to Model Neuroinflammatory and Metabolic Responses in X-linked Adrenoleukodystrophy. J. Biotech. Biomed. 2023, 6, 281–293. [Google Scholar] [CrossRef]
- Singh, J.; Olle, B.; Suhail, H.; Felicella, M.M.; Giri, S. Metformin-induced mitochondrial function and ABCD2 up-regulation in X-linked adrenoleukodystrophy involves AMP-activated protein kinase. J. Neurochem. 2016, 138, 86–100. [Google Scholar] [CrossRef]
- Paintlia, A.S.; Gilg, A.G.; Khan, M.; Singh, A.K.; Barbosa, E.; Singh, I. Correlation of very long chain fatty acid accumulation and inflammatory disease progression in childhood X-ALD: Implications for potential therapies. Neurobiol. Dis. 2003, 14, 425–439. [Google Scholar] [CrossRef]
- Khan, M.; Singh, J.; Singh, I. Plasmalogen deficiency in cerebral adrenoleukodystrophy and its modulation by lovastatin. J. Neurochem. 2008, 106, 1766–1779. [Google Scholar] [CrossRef]
- Singh, J.; Khan, M.; Singh, I. Caffeic acid phenethyl ester induces adrenoleukodystrophy (Abcd2) gene in human X-ALD fibroblasts and inhibits the proinflammatory response in Abcd1/2 silenced mouse primary astrocytes. Biochim. Biophys. Acta 2013, 1831, 747–758. [Google Scholar] [CrossRef] [PubMed]
- Baarine, M.; Khan, M.; Singh, A.; Singh, I. Functional Characterization of IPSC-Derived Brain Cells as a Model for X-Linked Adrenoleukodystrophy. PLoS ONE 2015, 10, e0143238. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Kang, H.C.; Kim, H.S.; Kim, J.Y.; Huh, Y.J.; Kim, D.S.; Yoo, J.E.; Lee, J.A.; Lim, B.; Lee, J.; et al. Induced pluripotent stem cell models from X-linked adrenoleukodystrophy patients. Ann. Neurol. 2011, 70, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Bernheimer, H.; Fae, I.; Braun, A.; Roscher, A.; Molzer, B.; Fischer, G. Association of X-linked adrenoleukodystrophy with HLA DRB1 alleles. Biochem. Biophys. Res. Commun. 1995, 216, 447–451. [Google Scholar] [CrossRef]
- Singh, J.; Khan, M.; Pujol, A.; Baarine, M.; Singh, I. Histone deacetylase inhibitor upregulates peroxisomal fatty acid oxidation and inhibits apoptotic cell death in abcd1-deficient glial cells. PLoS ONE 2013, 8, e70712. [Google Scholar] [CrossRef]
- Lopez-Erauskin, J.; Galino, J.; Ruiz, M.; Cuezva, J.M.; Fabregat, I.; Cacabelos, D.; Boada, J.; Martinez, J.; Ferrer, I.; Pamplona, R.; et al. Impaired mitochondrial oxidative phosphorylation in the peroxisomal disease X-linked adrenoleukodystrophy. Hum. Mol. Genet. 2013, 22, 3296–3305. [Google Scholar] [CrossRef]
- Pamies, D.; Sartori, C.; Schvartz, D.; Gonzalez-Ruiz, V.; Pellerin, L.; Nunes, C.; Tavel, D.; Maillard, V.; Boccard, J.; Rudaz, S.; et al. Neuroinflammatory Response to TNFalpha and IL1beta Cytokines Is Accompanied by an Increase in Glycolysis in Human Astrocytes In Vitro. Int. J. Mol. Sci. 2021, 22, 4065. [Google Scholar] [CrossRef]
- Suhail, H.; Nematullah, M.; Rashid, F.; Sajad, M.; Fatma, M.; Singh, J.; Zahoor, I.; Cheung, W.L.; Tiwari, N.; Ayasolla, K.; et al. An early glycolysis burst in microglia regulates mitochondrial dysfunction in oligodendrocytes under neuroinflammation. iScience 2023, 26, 107921. [Google Scholar] [CrossRef]
- Kim, H.; Leng, K.; Park, J.; Sorets, A.G.; Kim, S.; Shostak, A.; Embalabala, R.J.; Mlouk, K.; Katdare, K.A.; Rose, I.V.L.; et al. Reactive astrocytes transduce inflammation in a blood-brain barrier model through a TNF-STAT3 signaling axis and secretion of alpha 1-antichymotrypsin. Nat. Commun. 2022, 13, 6581. [Google Scholar] [CrossRef]
- Carroll, K.C.; Viollet, B.; Suttles, J. AMPKalpha1 deficiency amplifies proinflammatory myeloid APC activity and CD40 signaling. J. Leukoc. Biol. 2013, 94, 1113–1121. [Google Scholar] [CrossRef]
- Galic, S.; Fullerton, M.D.; Schertzer, J.D.; Sikkema, S.; Marcinko, K.; Walkley, C.R.; Izon, D.; Honeyman, J.; Chen, Z.P.; van Denderen, B.J.; et al. Hematopoietic AMPK beta1 reduces mouse adipose tissue macrophage inflammation and insulin resistance in obesity. J. Clin. Investig. 2011, 121, 4903–4915. [Google Scholar] [CrossRef] [PubMed]
- Mounier, R.; Theret, M.; Arnold, L.; Cuvellier, S.; Bultot, L.; Goransson, O.; Sanz, N.; Ferry, A.; Sakamoto, K.; Foretz, M.; et al. AMPKalpha1 regulates macrophage skewing at the time of resolution of inflammation during skeletal muscle regeneration. Cell Metab. 2013, 18, 251–264. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.; Hardie, D.G. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 2013, 493, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Nath, N.; Khan, M.; Rattan, R.; Mangalam, A.; Makkar, R.S.; de Meester, C.; Bertrand, L.; Singh, I.; Chen, Y.; Viollet, B.; et al. Loss of AMPK exacerbates experimental autoimmune encephalomyelitis disease severity. Biochem. Biophys. Res. Commun. 2009, 386, 16–20. [Google Scholar] [CrossRef]
- Viollet, B.; Athea, Y.; Mounier, R.; Guigas, B.; Zarrinpashneh, E.; Horman, S.; Lantier, L.; Hebrard, S.; Devin-Leclerc, J.; Beauloye, C.; et al. AMPK: Lessons from transgenic and knockout animals. Front. Biosci. (Landmark Ed.) 2009, 14, 19–44. [Google Scholar] [CrossRef]
- Poisson, L.M.; Kaur, N.; Felicella, M.M.; Singh, J. System-based integrated metabolomics and microRNA analysis identifies potential molecular alterations in human X-linked cerebral Adrenoleukodystrophy brain. Hum. Mol. Genet. 2023, 32, 3249–3262. [Google Scholar] [CrossRef]
- Di Biase, A.; Merendino, N.; Avellino, C.; Cappa, M.; Salvati, S. Th 1 cytokine production by peripheral blood mononuclear cells in X-linked adrenoleukodystrophy. J. Neurol. Sci. 2001, 182, 161–165. [Google Scholar] [CrossRef]
- Ranea-Robles, P.; Launay, N.; Ruiz, M.; Calingasan, N.Y.; Dumont, M.; Naudi, A.; Portero-Otin, M.; Pamplona, R.; Ferrer, I.; Beal, M.F.; et al. Aberrant regulation of the GSK-3beta/NRF2 axis unveils a novel therapy for adrenoleukodystrophy. EMBO Mol. Med. 2018, 10, e8604. [Google Scholar] [CrossRef]
- Ding, Z.B.; Song, L.J.; Wang, Q.; Kumar, G.; Yan, Y.Q.; Ma, C.G. Astrocytes: A double-edged sword in neurodegenerative diseases. Neural Regen. Res. 2021, 16, 1702–1710. [Google Scholar] [CrossRef]
- Marchetti, D.P.; Donida, B.; Jacques, C.E.; Deon, M.; Hauschild, T.C.; Koehler-Santos, P.; de Moura Coelho, D.; Coitinho, A.S.; Jardim, L.B.; Vargas, C.R. Inflammatory profile in X-linked adrenoleukodystrophy patients: Understanding disease progression. J. Cell Biochem. 2018, 119, 1223–1233. [Google Scholar] [CrossRef]
- Schluter, A.; Espinosa, L.; Fourcade, S.; Galino, J.; Lopez, E.; Ilieva, E.; Morato, L.; Asheuer, M.; Cook, T.; McLaren, A.; et al. Functional genomic analysis unravels a metabolic-inflammatory interplay in adrenoleukodystrophy. Hum. Mol. Genet. 2012, 21, 1062–1077. [Google Scholar] [CrossRef] [PubMed]
- Nitsch, L.; Petzinna, S.; Zimmermann, J.; Schneider, L.; Krauthausen, M.; Heneka, M.T.; Getts, D.R.; Becker, A.; Muller, M. Astrocyte-specific expression of interleukin 23 leads to an aggravated phenotype and enhanced inflammatory response with B cell accumulation in the EAE model. J. Neuroinflammation 2021, 18, 101. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Park, J.Y.; Lee, W.H.; Kim, H.; Park, H.C.; Mori, K.; Suk, K. Lipocalin-2 is an autocrine mediator of reactive astrocytosis. J. Neurosci. 2009, 29, 234–249. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Balashov, K.E.; Rottman, J.B.; Weiner, H.L.; Hancock, W.W. CCR5+ and CXCR3+ T cells are increased in multiple sclerosis and their ligands MIP-1alpha and IP-10 are expressed in demyelinating brain lesions. Proc. Natl. Acad. Sci. USA 1999, 96, 6873–6878. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef]
- Martin, M.; Rehani, K.; Jope, R.S.; Michalek, S.M. Toll-like receptor-mediated cytokine production is differentially regulated by glycogen synthase kinase 3. Nat. Immunol. 2005, 6, 777–784. [Google Scholar] [CrossRef]
- Kuhlmann, T.; Remington, L.; Cognet, I.; Bourbonniere, L.; Zehntner, S.; Guilhot, F.; Herman, A.; Guay-Giroux, A.; Antel, J.P.; Owens, T.; et al. Continued administration of ciliary neurotrophic factor protects mice from inflammatory pathology in experimental autoimmune encephalomyelitis. Am. J. Pathol. 2006, 169, 584–598. [Google Scholar] [CrossRef]
- Modi, K.K.; Sendtner, M.; Pahan, K. Up-regulation of ciliary neurotrophic factor in astrocytes by aspirin: Implications for remyelination in multiple sclerosis. J. Biol. Chem. 2013, 288, 18533–18545. [Google Scholar] [CrossRef]
- Prencipe, G.; Minnone, G.; Strippoli, R.; De Pasquale, L.; Petrini, S.; Caiello, I.; Manni, L.; De Benedetti, F.; Bracci-Laudiero, L. Nerve growth factor downregulates inflammatory response in human monocytes through TrkA. J. Immunol. 2014, 192, 3345–3354. [Google Scholar] [CrossRef]
- Boehm, U.; Guethlein, L.; Klamp, T.; Ozbek, K.; Schaub, A.; Futterer, A.; Pfeffer, K.; Howard, J.C. Two families of GTPases dominate the complex cellular response to IFN-gamma. J. Immunol. 1998, 161, 6715–6723. [Google Scholar] [CrossRef] [PubMed]
- Dozio, V.; Sanchez, J.C. Profiling the proteomic inflammatory state of human astrocytes using DIA mass spectrometry. J. Neuroinflammation 2018, 15, 331. [Google Scholar] [CrossRef]
- Hartmann, K.; Sepulveda-Falla, D.; Rose, I.V.L.; Madore, C.; Muth, C.; Matschke, J.; Butovsky, O.; Liddelow, S.; Glatzel, M.; Krasemann, S. Complement 3+-astrocytes are highly abundant in prion diseases, but their abolishment led to an accelerated disease course and early dysregulation of microglia. Acta Neuropathol. Commun. 2019, 7, 83. [Google Scholar] [CrossRef]
- Noelle, R.J.; Nowak, E.C. Cellular sources and immune functions of interleukin-9. Nat. Rev. Immunol. 2010, 10, 683–687. [Google Scholar] [CrossRef]
- Ding, X.; Cao, F.; Cui, L.; Ciric, B.; Zhang, G.X.; Rostami, A. IL-9 signaling affects central nervous system resident cells during inflammatory stimuli. Exp. Mol. Pathol. 2015, 99, 570–574. [Google Scholar] [CrossRef]
- Li, H.; Nourbakhsh, B.; Ciric, B.; Zhang, G.X.; Rostami, A. Neutralization of IL-9 ameliorates experimental autoimmune encephalomyelitis by decreasing the effector T cell population. J. Immunol. 2010, 185, 4095–4100. [Google Scholar] [CrossRef]
- Kim, S.; Lee, W.; Jo, H.; Sonn, S.K.; Jeong, S.J.; Seo, S.; Suh, J.; Jin, J.; Kweon, H.Y.; Kim, T.K.; et al. The antioxidant enzyme Peroxiredoxin-1 controls stroke-associated microglia against acute ischemic stroke. Redox Biol. 2022, 54, 102347. [Google Scholar] [CrossRef]
- Chang, R.Y.; Etheridge, N.; Dodd, P.R.; Nouwens, A.S. Targeted quantitative analysis of synaptic proteins in Alzheimer’s disease brain. Neurochem. Int. 2014, 75, 66–75. [Google Scholar] [CrossRef]
- Voigt, D.; Scheidt, U.; Derfuss, T.; Bruck, W.; Junker, A. Expression of the Antioxidative Enzyme Peroxiredoxin 2 in Multiple Sclerosis Lesions in Relation to Inflammation. Int. J. Mol. Sci. 2017, 18, 760. [Google Scholar] [CrossRef]
- Pankiewicz, J.E.; Diaz, J.R.; Marta-Ariza, M.; Lizinczyk, A.M.; Franco, L.A.; Sadowski, M.J. Peroxiredoxin 6 mediates protective function of astrocytes in Abeta proteostasis. Mol. Neurodegener. 2020, 15, 50. [Google Scholar] [CrossRef]
- Christopherson, K.S.; Ullian, E.M.; Stokes, C.C.; Mullowney, C.E.; Hell, J.W.; Agah, A.; Lawler, J.; Mosher, D.F.; Bornstein, P.; Barres, B.A. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 2005, 120, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.Q.; Chen, J.Q.; Zhou, H.Y.; Luo, L.; Zhang, B.Y.; Li, M.T.; He, H.Y.; Chen, C.; Guo, Y. Thrombospondin1 mimics rapidly relieve depression via Shank3 dependent uncoupling between dopamine D1 and D2 receptors. iScience 2023, 26, 106488. [Google Scholar] [CrossRef] [PubMed]
- Doyen, V.; Rubio, M.; Braun, D.; Nakajima, T.; Abe, J.; Saito, H.; Delespesse, G.; Sarfati, M. Thrombospondin 1 is an autocrine negative regulator of human dendritic cell activation. J. Exp. Med. 2003, 198, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Grimbert, P.; Bouguermouh, S.; Baba, N.; Nakajima, T.; Allakhverdi, Z.; Braun, D.; Saito, H.; Rubio, M.; Delespesse, G.; Sarfati, M. Thrombospondin/CD47 interaction: A pathway to generate regulatory T cells from human CD4+ CD25− T cells in response to inflammation. J. Immunol. 2006, 177, 3534–3541. [Google Scholar] [CrossRef]
- Delekate, A.; Fuchtemeier, M.; Schumacher, T.; Ulbrich, C.; Foddis, M.; Petzold, G.C. Metabotropic P2Y1 receptor signalling mediates astrocytic hyperactivity in vivo in an Alzheimer’s disease mouse model. Nat. Commun. 2014, 5, 5422. [Google Scholar] [CrossRef]
- Luo, S.; Tamada, A.; Saikawa, Y.; Wang, Y.; Yu, Q.; Hisatsune, T. P2Y1R silencing in Astrocytes Protected Neuroinflammation and Cognitive Decline in a Mouse Model of Alzheimer’s Disease. Aging Dis. 2024, 15, 1969–1988. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaur, N.; Singh, J. Generation and Characterization of Human iPSC-Derived Astrocytes with Potential for Modeling X-Linked Adrenoleukodystrophy Phenotypes. Int. J. Mol. Sci. 2025, 26, 1576. https://doi.org/10.3390/ijms26041576
Kaur N, Singh J. Generation and Characterization of Human iPSC-Derived Astrocytes with Potential for Modeling X-Linked Adrenoleukodystrophy Phenotypes. International Journal of Molecular Sciences. 2025; 26(4):1576. https://doi.org/10.3390/ijms26041576
Chicago/Turabian StyleKaur, Navtej, and Jaspreet Singh. 2025. "Generation and Characterization of Human iPSC-Derived Astrocytes with Potential for Modeling X-Linked Adrenoleukodystrophy Phenotypes" International Journal of Molecular Sciences 26, no. 4: 1576. https://doi.org/10.3390/ijms26041576
APA StyleKaur, N., & Singh, J. (2025). Generation and Characterization of Human iPSC-Derived Astrocytes with Potential for Modeling X-Linked Adrenoleukodystrophy Phenotypes. International Journal of Molecular Sciences, 26(4), 1576. https://doi.org/10.3390/ijms26041576





