Recent Insights on the Role of Nuclear Receptors in Alzheimer’s Disease: Mechanisms and Therapeutic Application
Abstract
:1. Introduction
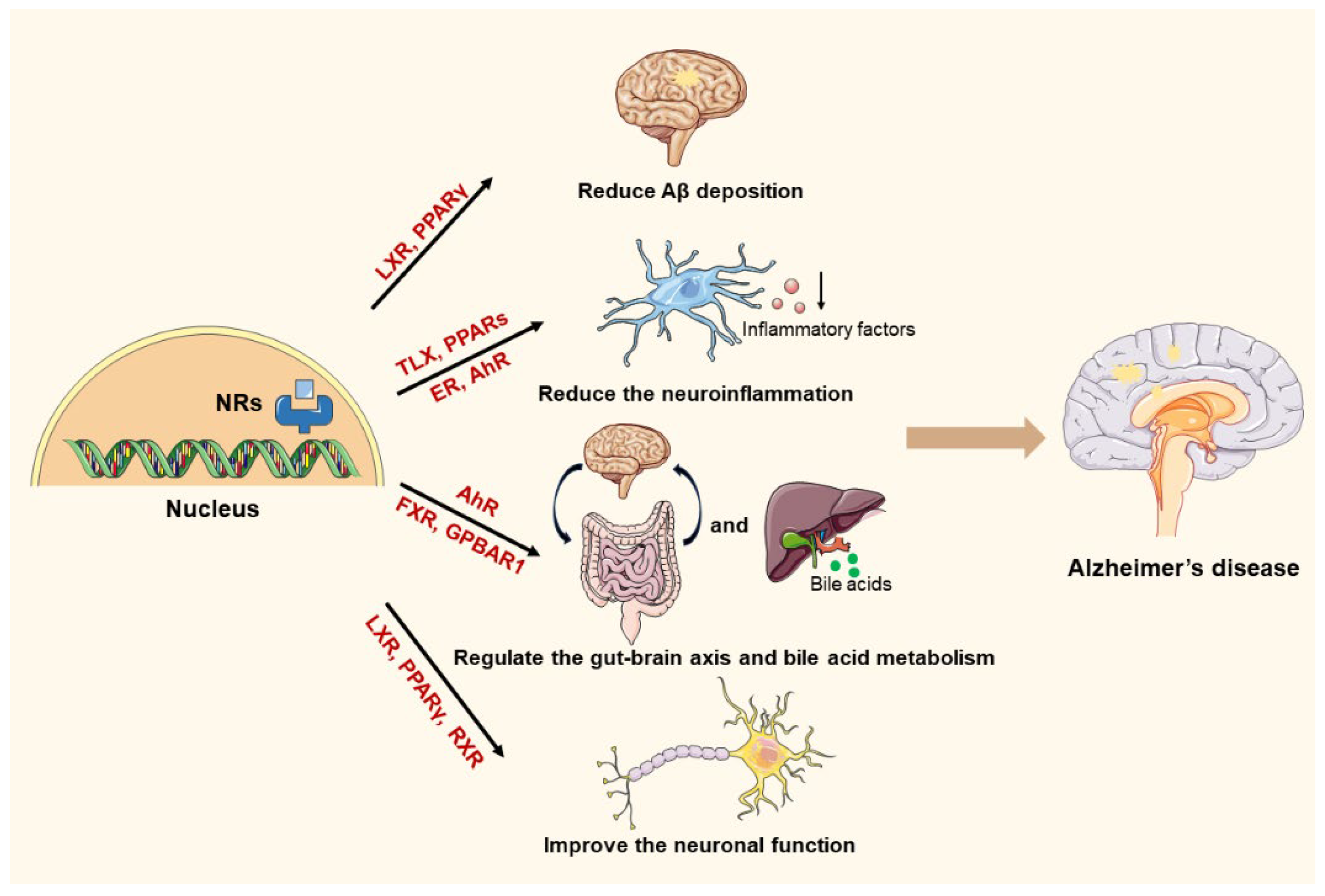
2. Structure and Function of NRs and Their Role in AD Pathogenesis
3. The Pathogenesis of AD and the Mechanistic Involvement of NRs
3.1. The Involvement of NRs in Aβ Regulation
3.2. The Involvement of NRs in Regulating Inflammation
3.2.1. Activation of Microglia
3.2.2. Astrocyte Dysfunction and Reactive Gliosis
3.2.3. Inflammatory Mediators and Their Impact on Neurons
3.2.4. The Vicious Cycle of Neuroinflammation
3.2.5. NRs Involved in the Neuroinflammation in AD
3.3. The Involvement of NRs in Regulating Metabolism
3.3.1. Regulating the Gut–Brain Axis
3.3.2. Regulating Bile Acid Metabolism
3.4. The Involvement of NRs in Improving Neuronal Dysfunction
3.4.1. The Neuronal Dysfunction in AD
3.4.2. The Role of NRs in the Improvement of Neuronal Function in AD
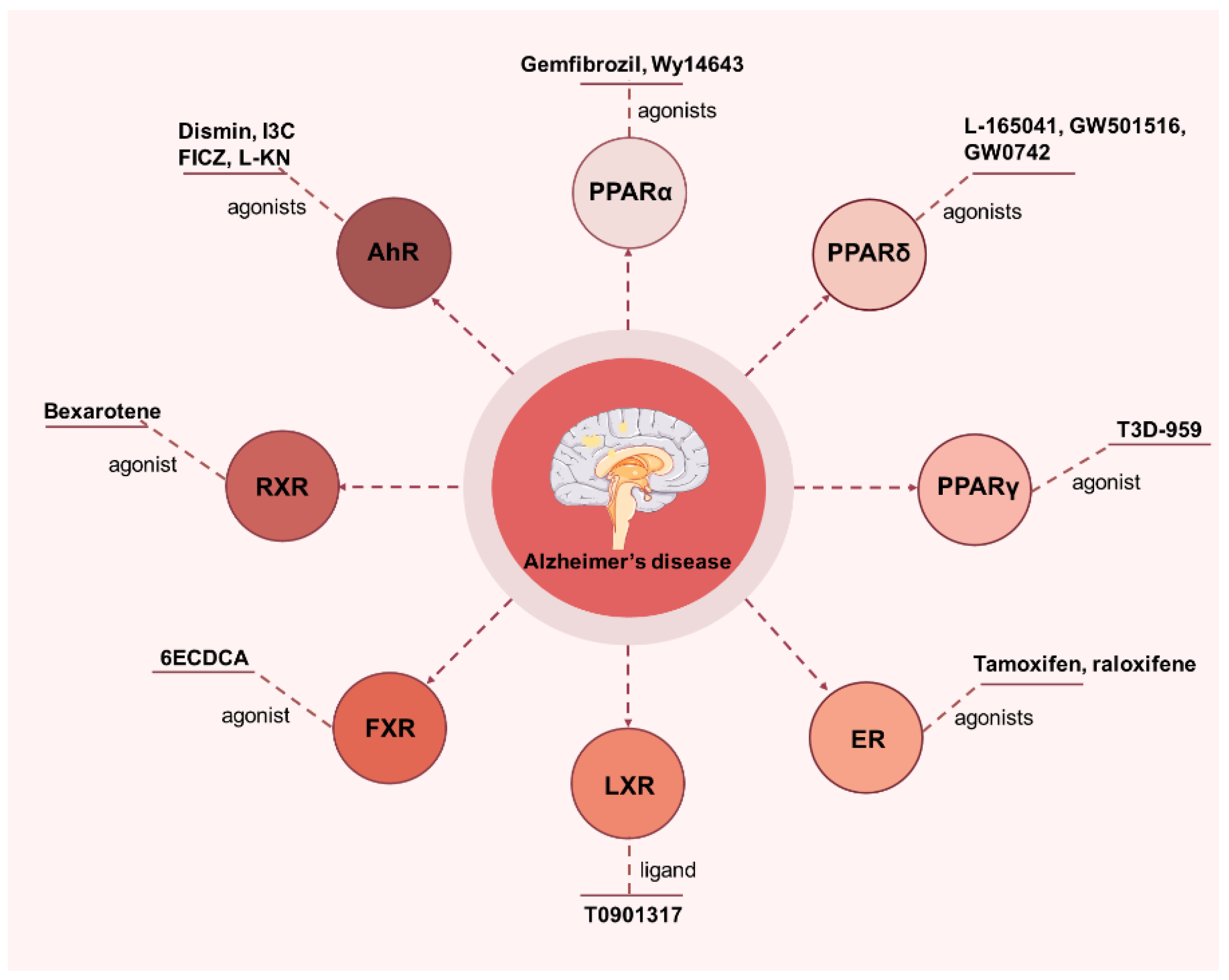
4. Applications of NRs in AD
4.1. PPAR
4.2. Estrogen Receptor (ER)
4.3. Liver X Receptor (LXR)
4.4. Farnesoid X Receptor (FXR)
4.5. Retinoid X Receptor (RXR)
4.6. Aryl Hydrocarbon Receptor (AhR)
4.7. Other
5. Conclusions and Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Smith, D.; Rapp, P.; McKay, H.; Roberts, J.; Tuszynski, M. Memory impairment in aged primates is associated with focal death of cortical neurons and atrophy of subcortical neurons. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 4373–4381. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.; Cummings, J.; van der Flier, W. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Guo, H.; Niu, L.; Yin, Q.; Zhang, Y.; Zhuang, P. Clinical value-oriented research paradigm about inheritance and innovation development of TCM dominant diseases. Chin. Herb. Med. 2023, 15, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ospitalieri, S.; Robberechts, T.; Hofmann, L.; Schmid, C.; Rijal Upadhaya, A.; Koper, M.; von Arnim, C.; Kumar, S.; Willem, M.; et al. Seeding, maturation and propagation of amyloid β-peptide aggregates in Alzheimer’s disease. Brain A J. Neurol. 2022, 145, 3558–3570. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, X.; Ma, T.; Xiao, J.; Chen, X.; Tang, M.; Zhang, L.; Zhang, T.; Fan, M.; Liao, J.; et al. Late-life physical activity, midlife-to-late-life activity patterns, APOE ε4 genotype, and cognitive impairment among Chinese older adults: A population-based observational study. Int. J. Behav. Nutr. Phys. Act. 2025, 22, 5. [Google Scholar] [CrossRef]
- Upadhyay, A.; Chhangani, D.; Rao, N.; Kofler, J.; Vassar, R.; Rincon-Limas, D.; Savas, J. Amyloid fibril proteomics of AD brains reveals modifiers of aggregation and toxicity. Mol. Neurodegener. 2023, 18, 61. [Google Scholar] [CrossRef]
- Jauregi-Zinkunegi, A.; Gleason, C.; Bendlin, B.; Okonkwo, O.; Hermann, B.; Blennow, K.; Zetterberg, H.; Hogervorst, E.; Johnson, S.; Langhough, R.; et al. Menopausal hormone therapy is associated with worse levels of Alzheimer’s disease biomarkers in APOE ε4-carrying women: An observational study. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2025, 1–13. [Google Scholar] [CrossRef]
- Katsipis, G.; Lavrentiadou, S.; Geromichalos, G.; Tsantarliotou, M.; Halevas, E.; Litsardakis, G.; Pantazaki, A. Evaluation of the Anti-Amyloid and Anti-Inflammatory Properties of a Novel Vanadium(IV)-Curcumin Complex in Lipopolysaccharides-Stimulated Primary Rat Neuron-Microglia Mixed Cultures. Int. J. Mol. Sci. 2024, 26, 282. [Google Scholar] [CrossRef]
- Ward, A.; Crean, S.; Mercaldi, C.; Collins, J.; Boyd, D.; Cook, M.; Arrighi, H. Prevalence of apolipoprotein E4 genotype and homozygotes (APOE e4/4) among patients diagnosed with Alzheimer’s disease: A systematic review and meta-analysis. Neuroepidemiology 2012, 38, 1–17. [Google Scholar] [CrossRef]
- Foley, K.; Winder, Z.; Sudduth, T.; Martin, B.; Nelson, P.; Jicha, G.; Harp, J.; Weekman, E.; Wilcock, D. Alzheimer’s disease and inflammatory biomarkers positively correlate in plasma in the UK-ADRC cohort. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2024, 20, 1374–1386. [Google Scholar] [CrossRef]
- Ravichandran, S.; Snyder, P.; Alber, J.; Murchison, C.; Chaby, L.; Jeromin, A.; Arthur, E. Association and multimodal model of retinal and blood-based biomarkers for detection of preclinical Alzheimer’s disease. Alzheimer’s Res. Ther. 2025, 17, 19. [Google Scholar] [CrossRef] [PubMed]
- Namba, Y.; Tomonaga, M.; Kawasaki, H.; Otomo, E.; Ikeda, K. Apolipoprotein E immunoreactivity in cerebral amyloid deposits and neurofibrillary tangles in Alzheimer’s disease and kuru plaque amyloid in Creutzfeldt-Jakob disease. Brain Res. 1991, 541, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Xing, H.; Kant, S.; Kanuparthy, M.; Harris, D.; Stone, C.; Broadwin, M.; Zhang, Z.; Pearson, E.; Hu, J.; Sauer, A.; et al. Impaired cerebral microvascular reactivity and endothelial SK channel activity in a streptozotocin-treated mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. JAD 2025, 13872877241309120. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, C.; Walsh, M.; Reddy, V.; Donthireddy, V.; Mahmood, F.; Bode, A.; Turner, J.; Jacober, S.; Sowers, J. Altered myosin light-chain phosphorylation in resting platelets from premenopausal women with diabetes. Metab. Clin. Exp. 2001, 50, 151–156. [Google Scholar] [CrossRef]
- Brendel, M.; Jaworska, A.; Overhoff, F.; Blume, T.; Probst, F.; Gildehaus, F.; Bartenstein, P.; Haass, C.; Bohrmann, B.; Herms, J.; et al. Efficacy of chronic BACE1 inhibition in PS2APP mice depends on the regional Aβ deposition rate and plaque burden at treatment initiation. Theranostics 2018, 8, 4957–4968. [Google Scholar] [CrossRef]
- Rojas-Solé, C.; Lillo-Moya, J.; Rodrigo, R. Chapter 8—Oxidative stress biomarkers in human health and disease. In Modulation of Oxidative Stress; Academic Press: London, UK, 2023. [Google Scholar]
- Schultzberg, M.; Lindberg, C.; Aronsson, A.; Hjorth, E.; Spulber, S.; Oprica, M. Inflammation in the nervous system--physiological and pathophysiological aspects. Physiol. Behav. 2007, 92, 121–128. [Google Scholar] [CrossRef]
- Hu, M.; Ying, X.; Zheng, M.; Wang, C.; Li, Q.; Gu, L.; Zhang, X. Therapeutic potential of natural products against Alzheimer’s disease via autophagic removal of Aβ. Brain Res. Bull. 2024, 206, 110835. [Google Scholar] [CrossRef]
- Szychowski, K.; Skóra, B. The elastin-derived peptide (VGVAPG) activates autophagy in neuroblastoma (SH-SY5Y) cells via peroxisome proliferator-activated receptor gamma (PPARγ). Mol. Cell. Neurosci. 2023, 127, 103902. [Google Scholar] [CrossRef]
- He, Z.; Li, X.; Wang, Z.; Cao, Y.; Han, S.; Li, N.; Cai, J.; Cheng, S.; Liu, Q. Protective effects of luteolin against amyloid beta-induced oxidative stress and mitochondrial impairments through peroxisome proliferator-activated receptor γ-dependent mechanism in Alzheimer’s disease. Redox Biol. 2023, 66, 102848. [Google Scholar] [CrossRef]
- Schupp, M.; Lazar, M. Endogenous ligands for nuclear receptors: Digging deeper. J. Biol. Chem. 2010, 285, 40409–40415. [Google Scholar] [CrossRef]
- Abd El Fattah, M.; Abdelhamid, Y.; Elyamany, M.; Badary, O.; Heikal, O. Rice Bran Extract Protected against LPS-Induced Neuroinflammation in Mice through Targeting PPAR-γ Nuclear Receptor. Mol. Neurobiol. 2021, 58, 1504–1516. [Google Scholar] [CrossRef] [PubMed]
- Bwayi, M.; Garcia-Maldonado, E.; Chai, S.; Xie, B.; Chodankar, S.; Huber, A.; Wu, J.; Annu, K.; Wright, W.; Lee, H.; et al. Molecular basis of crosstalk in nuclear receptors: Heterodimerization between PXR and CAR and the implication in gene regulation. Nucleic Acids Res. 2022, 50, 3254–3275. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Ko, S. Anti-Inflammatory and Anticancer Effects of Kaurenoic Acid in Overcoming Radioresistance in Breast Cancer Radiotherapy. Nutrients 2024, 16, 4320. [Google Scholar] [CrossRef] [PubMed]
- Maglich, J.; Sluder, A.; Guan, X.; Shi, Y.; McKee, D.; Carrick, K.; Kamdar, K.; Willson, T.; Moore, J. Comparison of complete nuclear receptor sets from the human, Caenorhabditis elegans and Drosophila genomes. Genome Biol. 2001, 2, RESEARCH0029. [Google Scholar] [CrossRef] [PubMed]
- Gronemeyer, H.; Gustafsson, J.; Laudet, V. Principles for modulation of the nuclear receptor superfamily. Nat. Rev. Drug Discov. 2004, 3, 950–964. [Google Scholar] [CrossRef]
- Ye, H.; Lin, J.; Zhang, H.; Wang, J.; Fu, Y.; Zeng, Z.; Zheng, J.; Tao, J.; Qiu, J. Nuclear receptor 4A1 Regulates Mitochondrial Homeostasis in Cardiac Post-Ischemic Injury by Controlling Mitochondrial Fission 1 Protein-Mediated Fragmentation and Parkin-Dependent Mitophagy. Int. J. Biol. Sci. 2025, 21, 400–414. [Google Scholar] [CrossRef]
- Hazarika, S.; Yu, T.; Biswas, A.; Dube, N.; Villalona, P.; Okafor, C. Nuclear Receptor Interdomain Communication is Mediated by the Hinge with Ligand Specificity. J. Mol. Biol. 2024, 436, 168805. [Google Scholar] [CrossRef]
- Obst, J.; Tien, A.; Setiawan, J.; Deneault, L.; Sadar, M. Inhibitors of the transactivation domain of androgen receptor as a therapy for prostate cancer. Steroids 2024, 210, 109482. [Google Scholar] [CrossRef]
- Katsu, Y.; Zhang, J.; Baker, M. Novel Evolution of Mineralocorticoid Receptor in Humans Compared to Chimpanzees, Gorillas, and Orangutans. Genes 2024, 15, 767. [Google Scholar] [CrossRef]
- Annalora, A.; Marcus, C.; Iversen, P. Alternative Splicing in the Nuclear Receptor Superfamily Expands Gene Function to Refine Endo-Xenobiotic Metabolism. Drug Metab. Dispos. Biol. Fate Chem. 2020, 48, 272–287. [Google Scholar] [CrossRef]
- Safe, S.; Shrestha, R.; Mohankumar, K. Orphan nuclear receptor 4A1 (NR4A1) and novel ligands. Essays Biochem. 2021, 65, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, Y.; Liu, W.; Schlenk, D.; Liu, J. Glucocorticoid and mineralocorticoid receptors and corticosteroid homeostasis are potential targets for endocrine-disrupting chemicals. Environ. Int. 2019, 133, 105133. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.; Mangelsdorf, D. Nuclear Receptors, RXR, and the Big Bang. Cell 2014, 157, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, Y.; Hao, W.; You, J.; Wu, L. Non-canonical hepatic androgen receptor mediates glucagon sensitivity in female mice through the PGC1α/ERRα/mitochondria axis. Cell Rep. 2025, 44, 115188. [Google Scholar] [CrossRef]
- Annam, S.; Neal, W.; Pandey, P.; Avula, B.; Katragunta, K.; Husain, I.; Khan, S.; Koturbash, I.; Gurley, B.; Khan, I.; et al. A Combined Approach for Rapid Dereplication of Herb-Drug Interaction Causative Agents in Botanical Extracts-A Molecular Networking Strategy To Identify Potential Pregnane X Receptor (PXR) Modulators in Yohimbe. ACS Omega 2024, 9, 51394–51407. [Google Scholar] [CrossRef]
- Sakellakis, M. Orphan receptors in prostate cancer. Prostate 2022, 82, 1016–1024. [Google Scholar] [CrossRef]
- Simons, S.S.; Kumar, R. Variable steroid receptor responses: Intrinsically disordered AF1 is the key. Mol. Cell. Endocrinol. 2013, 376, 81–84. [Google Scholar] [CrossRef]
- Vicent, G.; Meliá, M.; Beato, M. Asymmetric binding of histone H1 stabilizes MMTV nucleosomes and the interaction of progesterone receptor with the exposed HRE. J. Mol. Biol. 2002, 324, 501–517. [Google Scholar] [CrossRef]
- Shukla, N.; Shah, K.; Rathore, D.; Soni, K.; Shah, J.; Vora, H.; Dave, H. Androgen receptor: Structure, signaling, function and potential drug discovery biomarker in different breast cancer subtypes. Life Sci. 2024, 348, 122697. [Google Scholar] [CrossRef]
- Yu, X.; Yi, P.; Hamilton, R.A.; Shen, H.; Chen, M.; Foulds, C.E.; Mancini, M.A.; Ludtke, S.J.; Wang, Z.; O’Malley, B.W. Structural Insights of Transcriptionally Active, Full-Length Androgen Receptor Coactivator Complexes. Mol. Cell 2020, 79, 812–823. [Google Scholar] [CrossRef]
- Lei, N.; Ying-Nai, W.; Jung-Mao, H.; Junwei, H.; Yu-Yi, C.; Li-Chuan, C.; Longfei, H.; Yongkun, W.; Rong, D.; Jun, T.; et al. Nuclear export signal mutation of epidermal growth factor receptor enhances malignant phenotypes of cancer cells. Am. J. Cancer Res. 2023, 13, 1209–1239. [Google Scholar]
- Hugo, C.-P.; Jennifer, M.; Dan, B.K.; Isabel, P.; Ingrid, K.-K.; Lutz, A.; Jon Petter, G. Binding of per- and polyfluoroalkyl substances (PFASs) by organic soil materials with different structural composition—Charge- and concentration-dependent sorption behavior. Chemosphere 2022, 297, 134167. [Google Scholar]
- Longying, J.; Xueke, L.; Xujun, L.; Shuyan, D.; Hudie, W.; Ming, G.; Zhuchu, C.; Desheng, X.; Yongheng, C. Structural basis of the farnesoid X receptor/retinoid X receptor heterodimer on inverted repeat DNA. Comput. Struct. Biotechnol. J. 2023, 21, 3149–3157. [Google Scholar]
- Bulynko, Y.A.; O’Malley, B.W. Nuclear Receptor Coactivators: Structural and Functional Biochemistry. Biochemistry 2011, 50, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Saijo, K.; Winner, B.; Carson, C.T.; Collier, J.G.; Boyer, L.; Rosenfeld, M.G.; Gage, F.H.; Glass, C.K. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell 2009, 137, 47–59. [Google Scholar] [CrossRef]
- Kiran, T.; Heena, K.; Amarjot Kaur, G.; Thakur Gurjeet, S. Nuclear orphan receptors: A novel therapeutic agent in neuroinflammation. Int. Immunopharmacol. 2023, 124, 110845. [Google Scholar]
- Todd, E.G. Alzheimer’s disease—The journey of a healthy brain into organ failure. Mol. Neurodegener. 2022, 17, 18. [Google Scholar]
- Citron, M. Alzheimer’s disease: Strategies for disease modification. Nat. Rev. Drug Discov. 2010, 9, 387–398. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Bax, F.; van Veluw, S.J. Amyloid-related imaging abnormalities: Manifestations, metrics and mechanisms. Nat. Rev. Neurol. 2025, 1–11. [Google Scholar] [CrossRef]
- Suren, A.T. Challenges and hopes for Alzheimer’s disease. Drug Discov. Today 2022, 27, 1027–1043. [Google Scholar]
- Anika, S.; Oliver, W. Endogenous Apolipoprotein E (ApoE) Fragmentation Is Linked to Amyloid Pathology in Transgenic Mouse Models of Alzheimer’s Disease. Mol. Neurobiol. 2016, 54, 319–327. [Google Scholar]
- Jiang, Q.; Lee, C.D.; Mandrekar, S.; Wilkinson, B.; Cramer, P.; Zelcer, N.; Mann, K.; Lamb, B.; Willson, T.M.; Collins, J.L.; et al. ApoE promotes the proteolytic degradation of Abeta. Neuron 2008, 58, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Katrin, B.; Jennifer, H.S.; Loukia, K.; Miriam, R.; Malcolm, G.P.; Mark, C.; Magdalena, S. The nuclear cofactor receptor interacting protein-140 (RIP140) regulates the expression of genes involved in Aβ generation. Neurobiol. Aging 2016, 47, 180–191. [Google Scholar]
- Ping-Chih, H.; Ya-Shan, C.; Chen-Hsiang, H.; Li-Na, W. Cytoplasmic receptor-interacting protein 140 (RIP140) interacts with perilipin to regulate lipolysis. Cell Signal. 2011, 23, 1396–1403. [Google Scholar]
- Sneham, T.; Venkata, A.; Ajeet, K.; Adriana, Y.; Madhavan, N. Alzheimer’s disease: Pathogenesis, diagnostics, and therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554. [Google Scholar]
- Valeria, C.; Paul, E. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimers Dement. 2016, 12, 719–732. [Google Scholar]
- Sujata, T.; Rishika, D.; Phulen, S.; Bikash, M.; Dibbanti HariKrishna, R. Neuroinflammation in Alzheimer’s Disease: Current Progress in Molecular Signaling and Therapeutics. Inflammation 2022, 46, 1–17. [Google Scholar]
- Yaoxue, G.; Junli, Z.; Xing, L.; Pu, L.; Furu, L.; Xueyan, W.; Jing, W.; Yan, H. Ghrelin Induces Ferroptosis Resistance and M2 Polarization of Microglia to Alleviate Neuroinflammation and Cognitive Impairment in Alzheimer’s Disease. J. Neuroimmune Pharmacol. 2025, 20, 6. [Google Scholar]
- Tianchi, W.; Chunkai, W. SMOC2 promotes microglia activity and neuroinflammation in Alzheimer’s disease. J. Alzheimers Dis. 2025, 20, 1–22. [Google Scholar]
- Yoonsu, K.; Jinkyu, L.; Jisun, O. Taming neuroinflammation in Alzheimer’s disease: The protective role of phytochemicals through the gut-brain axis. Biomed. Pharmacother. 2024, 178, 117277. [Google Scholar]
- Hyunuk, K.; Hui, Z.; Yushan, Y.; Jiangfan, Y.; Zhonghua, L.; Puming, H.; Bo, L.; Yuanyuan, W.; Yaomin, W.; Youying, T. Tieguanyin Oolong Tea Extracts Alleviate Behavioral Abnormalities by Modulating Neuroinflammation in APP/PS1 Mouse Model of Alzheimer’s Disease. Foods 2022, 11, 81. [Google Scholar]
- Jessica, S.S.; Michael, R.O.D.; Philip, H.; Taitea, D.; Arline, F.; Shane, A.L. Astrocytes and oligodendrocytes undergo subtype-specific transcriptional changes in Alzheimer’s disease. Neuron 2022, 110, 1788–1805. [Google Scholar]
- Fakhoury, M. Microglia and Astrocytes in Alzheimer’s Disease: Implications for Therapy. Curr. Neuropharmacol. 2018, 16, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Preman, P.; Alfonso-Triguero, M.; Alberdi, E.; Verkhratsky, A.; Arranz, A.M. Astrocytes in Alzheimer’s Disease: Pathological Significance and Molecular Pathways. Cells 2021, 10, 540. [Google Scholar] [CrossRef] [PubMed]
- Borislav, D.; Tiffany, W.; Ming-Chi, T.; David, G.; Vineela, D.G.; Christopher, M.R.; Corey, E.B.; Hai, N.; Yuanyuan, W.; Shristi, P.; et al. Complement C1q-dependent excitatory and inhibitory synapse elimination by astrocytes and microglia in Alzheimer’s disease mouse models. Nat. Aging 2023, 2, 837–850. [Google Scholar]
- Melika, A.; Mandana, A. The neuroinflammatory role of microglia in Alzheimer’s disease and their associated therapeutic targets. CNS Neurosci. Ther. 2024, 30, e14856. [Google Scholar]
- Cuicui, W.; Shuai, Z.; Xiaolin, C.; Xueying, W.; Shuang, W.; Le, W.; Yingchao, L.; Zhiming, L. The effects of microglia-associated neuroinflammation on Alzheimer’s disease. Front. Immunol. 2023, 14, 1117172. [Google Scholar]
- Xiao, L.; Qian, Z.; Jia-He, Z.; Ke-Yong, W.; Takashi, S.; Takaomi, C.S.; Xiaoying, W.; Xiumei, G.; Kagaku, A. Microglia-Based Sex-Biased Neuropathology in Early-Stage Alzheimer’s Disease Model Mice and the Potential Pharmacologic Efficacy of Dioscin. Cells 2021, 10, 3261. [Google Scholar] [CrossRef]
- Rishika, D.; Subhendu Shekhar, H.; Phulen, S.; Anusuya, B.; Bikash, M.; Dibbanti HariKrishna, R. Recent advances in molecular pathways and therapeutic implications targeting neuroinflammation for Alzheimer’s disease. Inflammopharmacology 2021, 29, 1669–1681. [Google Scholar]
- Xiaoqing, S.; Jinying, W.; Yu, Z.; Xinqian, D.; Qibing, Q. Protective effect of hydroxysafflor yellow A on MSCs against senescence induced by d-galactose. Chin. Herb. Med. 2023, 15, 86–93. [Google Scholar]
- Amanda, M.; Mathew, B.-J. Microglia in Alzheimer’s Disease: Exploring How Genetics and Phenotype Influence Risk. J. Mol. Biol. 2019, 431, 1805–1817. [Google Scholar]
- Sneha, K.; Rishika, D.; Prajjwal, S.; Sunil, K.S.; Dibbanti HariKrishna, R. Implicative role of Cytokines in Neuroinflammation mediated AD and associated signaling pathways: Current Progress in molecular signaling and therapeutics. Ageing Res. Rev. 2024, 102098. [Google Scholar]
- Pascoal, T.A.; Benedet, A.L.; Ashton, N.J.; Kang, M.S.; Therriault, J.; Chamoun, M.; Savard, M.; Lussier, F.Z.; Tissot, C.; Karikari, T.K.; et al. Publisher Correction: Microglial activation and tau propagate jointly across Braak stages. Nat. Med. 2021, 27, 2048–2049. [Google Scholar] [CrossRef]
- Titus, C.; Hoque, M.T.; Bendayan, R. PPAR agonists for the treatment of neuroinflammatory diseases. Trends Pharmacol. Sci. 2023, 45, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Jihong, K.; Serge, R. Lipid metabolism and neuroinflammation in Alzheimer’s disease: A role for liver X receptors. Endocr. Rev. 2012, 33, 715–746. [Google Scholar]
- Qu, C.; Qu, C.; Xu, L.; Shen, J.; Lv, D.; Li, Y.; Song, H.; Li, T.; Zheng, J.; Zhang, J. Nuclear receptor TLX may be through regulating the SIRT1/NF-κB pathway to ameliorate cognitive impairment in chronic cerebral hypoperfusion. Brain Res. Bull. 2021, 166, 142–149. [Google Scholar] [CrossRef]
- Chung, S.W.; Kang, B.Y.; Kim, S.H.; Pak, Y.K.; Cho, D.; Trinchieri, G.; Kim, T.S. Oxidized low density lipoprotein inhibits interleukin-12 production in lipopolysaccharide-activated mouse macrophages via direct interactions between peroxisome proliferator-activated receptor-gamma and nuclear factor-kappa B. J. Biol. Chem. 2000, 275, 32681–32687. [Google Scholar] [CrossRef]
- Biswas, D.; Singh, S.; Shi, Q.; Pardee, A.; Iglehart, J. Crossroads of estrogen receptor and NF-kappaB signaling. Sci. STKE Signal Transduct. Knowl. Environ. 2005, 2005, pe27. [Google Scholar]
- Yang, A.; Bagit, A.; MacPherson, R. Resveratrol, Metabolic Dysregulation, and Alzheimer’s Disease: Considerations for Neurogenerative Disease. Int. J. Mol. Sci. 2021, 22, 4628. [Google Scholar] [CrossRef]
- Leblhuber, F.; Ehrlich, D.; Steiner, K.; Geisler, S.; Fuchs, D.; Lanser, L.; Kurz, K. The Immunopathogenesis of Alzheimer’s Disease Is Related to the Composition of Gut Microbiota. Nutrients 2021, 13, 361. [Google Scholar] [CrossRef]
- Bosco, D.; Fava, A.; Plastino, M.; Montalcini, T.; Pujia, A. Possible implications of insulin resistance and glucose metabolism in Alzheimer’s disease pathogenesis. J. Cell. Mol. Med. 2011, 15, 1807–1821. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Hai, W.; Chen, S.; Zhang, M.; Jiang, X.; Tang, H. Multi-omics data reveals aberrant gut microbiota-host glycerophospholipid metabolism in association with neuroinflammation in APP/PS1 mice. Gut Microbes 2023, 15, 2282790. [Google Scholar] [CrossRef] [PubMed]
- Bhupinder, K.; Pratim, B.; Monica, G.; Pooja, R.; Reena, G. Gut microbiome and Alzheimer’s disease: What we know and what remains to be explored. Ageing Res. Rev. 2024, 102, 102570. [Google Scholar]
- Maren, L.S.; James, B.W.; Jennifer, W.; Jasmohan, S.B. Gut microbiome-brain-cirrhosis axis. Hepatology 2023, 80, 465–485. [Google Scholar]
- Zheng, M.; Ye, H.; Yang, X.; Shen, L.; Dang, X.; Liu, X.; Gong, Y.; Wu, Q.; Wang, L.; Ge, X.; et al. Probiotic Clostridium butyricum ameliorates cognitive impairment in obesity via the microbiota-gut-brain axis. Brain Behav. Immun. 2024, 115, 565–587. [Google Scholar] [CrossRef]
- Zhan, Q.; Kong, F.; Shao, S.; Zhang, B.; Huang, S. Pathogenesis of Depression in Alzheimer’s Disease. Neurochem. Res. 2024, 49, 548–556. [Google Scholar] [CrossRef]
- Wang, J.; Li, W.; Yu, S.; Xie, H.; Han, H. Catalpol regulates function of hypothalamic-pituitary-adrenocortical-axis in an Alzheimer’s disease rat model. Die Pharm. 2014, 69, 688–693. [Google Scholar]
- Bonaz, B.; Bazin, T.; Pellissier, S. The Vagus Nerve at the Interface of the Microbiota-Gut-Brain Axis. Front. Neurosci. 2018, 12, 49. [Google Scholar] [CrossRef]
- Dong, F.; Hao, F.; Murray, I.; Smith, P.; Koo, I.; Tindall, A.; Kris-Etherton, P.; Gowda, K.; Amin, S.; Patterson, A.; et al. Intestinal microbiota-derived tryptophan metabolites are predictive of Ah receptor activity. Gut Microbes 2020, 12, 1788899. [Google Scholar] [CrossRef]
- Yin, J.; Zhang, Y.; Liu, X.; Li, W.; Hu, Y.; Zhang, B.; Wang, S. Gut Microbiota-Derived Indole Derivatives Alleviate Neurodegeneration in Aging through Activating GPR30/AMPK/SIRT1 Pathway. Mol. Nutr. Food Res. 2023, 67, e2200739. [Google Scholar] [CrossRef]
- Xu, H.; Fang, F.; Wu, K.; Song, J.; Li, Y.; Lu, X.; Liu, J.; Zhou, L.; Yu, W.; Yu, F.; et al. Gut microbiota-bile acid crosstalk regulates murine lipid metabolism via the intestinal FXR-FGF19 axis in diet-induced humanized dyslipidemia. Microbiome 2023, 11, 262. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, M.; Robbins, D.; Chen, T. Targeting xenobiotic receptors PXR and CAR in human diseases. Drug Discov. Today 2015, 20, 618–628. [Google Scholar] [CrossRef] [PubMed]
- Yasamineh, S.; Mehrabani, F.J.; Derafsh, E.C. RenizoForood, Amir Mohammad KarimiSoltani, SiamakHadi, MeeadGholizadeh, Omid, Potential Use of the Cholesterol Transfer Inhibitor U18666A as a Potent Research Tool for the Study of Cholesterol Mechanisms in Neurodegenerative Disorders. Mol. Neurobiol. 2024, 61, 3503–3527. [Google Scholar] [CrossRef] [PubMed]
- Copple, B.L.; Li, T. Pharmacology of bile acid receptors: Evolution of bile acids from simple detergents to complex signaling molecules. Pharmacol. Res. 2016, 104, 9–21. [Google Scholar] [CrossRef]
- Marksteiner, J.; Blasko, I.; Kemmler, G.; Koal, T.; Humpel, C. Bile acid quantification of 20 plasma metabolites identifies lithocholic acid as a putative biomarker in Alzheimer’s disease. Metabolomics 2018, 14, 1. [Google Scholar] [CrossRef]
- Samanta, S.; Akhter, F.; Roy, A.; Chen, D.; Turner, B.; Wang, Y.; Clemente, N.; Wang, C.; Swerdlow, R.; Battaile, K.; et al. New cyclophilin D inhibitor rescues mitochondrial and cognitive function in Alzheimer’s disease. Brain A J. Neurol. 2024, 147, 1710–1725. [Google Scholar] [CrossRef]
- Vossel, K.; Ranasinghe, K.; Beagle, A.; La, A.; Ah Pook, K.; Castro, M.; Mizuiri, D.; Honma, S.; Venkateswaran, N.; Koestler, M.; et al. Effect of Levetiracetam on Cognition in Patients With Alzheimer Disease With and Without Epileptiform Activity: A Randomized Clinical Trial. JAMA Neurol. 2021, 78, 1345–1354. [Google Scholar] [CrossRef]
- Weller, J.; Budson, A. Current understanding of Alzheimer’s disease diagnosis and treatment. F1000 Res. 2018, 7, 1161. [Google Scholar] [CrossRef]
- Sandoval-Hernández, A.G.; Hernández, H.G.; Restrepo, A.; Muñoz, J.I.; Bayon, G.F.; Fernández, A.F.; Fraga, M.F.; Cardona-Gómez, G.P.; Arboleda, H.; Arboleda, G.H. Liver X Receptor Agonist Modifies the DNA Methylation Profile of Synapse and Neurogenesis-Related Genes in the Triple Transgenic Mouse Model of Alzheimer’s Disease. J. Mol. Neurosci. 2016, 58, 243–253. [Google Scholar] [CrossRef]
- Elisa, M.-J.; Itzia, J.-F.C.; Martha, P.-E.; Cristina, E.R.-S.; Lourdes, Á.-A.; Javier, C.-M.; Maribel, H.-R.; Enrique, J.-F.; Alejandro, Z.; Jaime, T.; et al. Malva parviflora extract ameliorates the deleterious effects of a high fat diet on the cognitive deficit in a mouse model of Alzheimer’s disease by restoring microglial function via a PPAR-γ-dependent mechanism. J. Neuroinflammation 2019, 16, 143. [Google Scholar]
- Denner, L.A.; Rodriguez-Rivera, J.; Haidacher, S.J.; Jahrling, J.B.; Carmical, J.R.; Hernandez, C.M.; Zhao, Y.; Sadygov, R.G.; Starkey, J.M.; Spratt, H. Cognitive Enhancement with Rosiglitazone Links the Hippocampal PPAR and ERK MAPK Signaling Pathways. J. Neurosci. 2012, 32, 16725–16735. [Google Scholar] [CrossRef] [PubMed]
- Mariani, M.M.; Malm, T.; Lamb, R.; Jay, T.R.; Neilson, L.; Casali, B.; Medarametla, L.; Landreth, G.E. Neuronally-directed effects of RXR activation in a mouse model of Alzheimer’s disease. Sci. Rep. 2017, 7, 42270. [Google Scholar] [CrossRef] [PubMed]
- Rachmian, N.; Medina, S.; Cherqui, U.; Akiva, H.; Deitch, D.; Edilbi, D.; Croese, T.; Salame, T.M.; Ramos, J.M.P.; Cahalon, L. Identification of senescent, TREM2-expressing microglia in aging and Alzheimer’s disease model mouse brain. Nat. Neurosci. 2024, 27, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Mirza, A.Z.; Althagafi, I.I.; Shamshad, H. Role of PPAR receptor in different diseases and their ligands: Physiological importance and clinical implications. Eur. J. Med. Chem. Chim. Ther. 2019, 166, 502–513. [Google Scholar] [CrossRef]
- Diradourian, C.; Girard, J.; Pegorier, J.P. Phosphorylation of PPARs: From molecular characterization to physiological relevance. Biochimie 2005, 87, 33–38. [Google Scholar] [CrossRef]
- Kalinin, S.; Richardson, J.C.; Feinstein, D.L. PPARdelta agonist reduces amyloid burden and brain inflammation in a transgenic mouse model of Alzheimer’s disease. Curr. Alzheimer Res. 2009, 6, 431–437. [Google Scholar]
- Schnegg, C.; Robbins, M. Neuroprotective Mechanisms of PPARδ: Modulation of Oxidative Stress and Inflammatory Processes. PPAR Res. 2011, 2011, 373560. [Google Scholar] [CrossRef]
- Agarwal, S.; Yadav, A.; Chaturvedi, R.K. Peroxisome proliferator-activated receptors (PPARs) as therapeutic target in neurodegenerative disorders. Biochem. Biophys. Res. Commun. 2017, 483, 1166–1177. [Google Scholar] [CrossRef]
- Mangelsdorf, D.J.; Thummel, C.; Beato, M.; Herrlich, P.; Schütz, G.; Umesono, K.; Blumberg, B.; Kastner, P.; Mark, M.; Chambon, P.; et al. The nuclear receptor superfamily: The second decade. Cell 1995, 83, 835–839. [Google Scholar] [CrossRef]
- Abel, T.; Kandel, E. Positive and negative regulatory mechanisms that mediate long-term memory storage. Brain Res. Rev. 1998, 26, 360–378. [Google Scholar] [CrossRef]
- Roy, A.; Jana, M.; Corbett, G.T.; Ramaswamy, S.; Kordower, J.H.; Gonzalez, F.J.; Pahan, K. Regulation of Cyclic AMP Response Element Binding and Hippocampal Plasticity-Related Genes by Peroxisome Proliferator-Activated Receptor α. Cell Rep. 2013, 4, 724–737. [Google Scholar] [CrossRef] [PubMed]
- Pahan, K. Lipid-lowering drugs. Cell. Mol. Life Sci. Cmls 2006, 63, 1165. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Jana, M.; Kundu, M.; Corbett, G.; Rangaswamy, S.; Mishra, R.; Luan, C.H.; Gonzalez, F.; Pahan, K. HMG-CoA Reductase Inhibitors Bind to PPARα to Upregulate Neurotrophin Expression in the Brain and Improve Memory in Mice. Cell Metab. 2015, 22, 253–265. [Google Scholar] [CrossRef]
- Domingues, C.; da Cruz e Silva, O.A.B.; Henriques, A.G. Impact of Cytokines and Chemokines on Alzheimer’s Disease Neuropathological Hallmarks. Curr. Alzheimer Res. 2017, 14, 870–882. [Google Scholar] [CrossRef]
- Luo, R.; Su, L.Y.; Li, G.; Yang, J.; Liu, Q.; Yang, L.X.; Zhang, D.F.; Zhou, H.; Xu, M.; Fan, Y. Activation of PPARA-mediated autophagy reduces Alzheimer disease-like pathology and cognitive decline in a murine model. Autophagy 2020, 16, 52–69. [Google Scholar] [CrossRef]
- Qian, B.; Wang, C.; Ma, X.L.M.D.S.W.L.K. PPARβ/δ activation protects against hepatic ischaemia–reperfusion injury. Liver Int. 2023, 43, 2808–2823. [Google Scholar] [CrossRef]
- Hu, P.; Li, K.; Peng, X.; Kan, Y.; Li, H.; Zhu, Y.; Wang, Z.; Li, Z.; Liu, H.Y.; Cai, D. Nuclear Receptor PPARα as a Therapeutic Target in Diseases Associated with Lipid Metabolism Disorders. Nutrients 2023, 15, 4772. [Google Scholar] [CrossRef]
- Iwashita, A.; Muramatsu, Y.; Yamazaki, T.; Muramoto, M.; Kita, Y.; Yamazaki, S.; Mihara, K.; Moriguchi, A.; Matsuoka, N. Neuroprotective Efficacy of the Peroxisome Proliferator-Activated Receptor δ-Selective Agonists in Vitro and in Vivo. J. Pharmacol. Exp. Ther. 2007, 320, 1087–1096. [Google Scholar] [CrossRef]
- An, Y.Q.; Zhang, C.T.; Du, Y.; Zhang, M.; Tang, S.S.; Hu, M.; Long, Y.; Sun, H.B.; Hong, H. PPARδ agonist GW0742 ameliorates Aβ1-42-induced hippocampal neurotoxicity in mice. Metab. Brain Dis. 2016, 31, 663–671. [Google Scholar] [CrossRef]
- Chamberlain, S.; Gabriel, H.; Strittmatter, W.; Didsbury, J. An Exploratory Phase IIa Study of the PPAR delta/gamma Agonist T3D-959 Assessing Metabolic and Cognitive Function in Subjects with Mild to Moderate Alzheimer’s Disease. J. Alzheimer’s Dis. JAD 2020, 73, 1085–1103. [Google Scholar] [CrossRef]
- Khorasani, A.; Abbasnejad, M.; Esmaeili-Mahani, S. Phytohormone abscisic acid ameliorates cognitive impairments in streptozotocin-induced rat model of Alzheimer’s disease through PPARβ/δ and PKA signaling. Int. J. Neurosci. 2019, 129, 1053–1065. [Google Scholar] [CrossRef] [PubMed]
- Kershaw, E.E.; Schupp, M.; Guan, H.P.; Gardner, N.P.; Lazar, M.A.; Flier, J.S. PPARγ regulates adipose triglyceride lipase in adipocytes in vitro and in vivo. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1736–E1745. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Mei, X.F. Naringin may promote functional recovery following spinal cord injury by modulating microglial polarization through the PPAR-γ/NF-κB signaling pathway. Brain Res. 2023, 1821, 148563. [Google Scholar] [CrossRef] [PubMed]
- Kölsch, H.; Jessen, F.; Wiltfang, J.; Lewczuk, P.; Dichgans, M.; Teipel, S.J.; Kornhuber, J.; Frölich, L.; Heuser, I.; Peters, O.; et al. Association of SORL1 gene variants with Alzheimer’s disease. Brain Res. 2009, 1264, 1–6. [Google Scholar] [CrossRef]
- Zhang, Z.; Cui, Y.; Su, V.; Wang, D.; Tol, M.; Cheng, L.; Wu, X.; Kim, J.; Rajbhandari, P.; Zhang, S.; et al. A PPARγ/long noncoding RNA axis regulates adipose thermoneutral remodeling in mice. J. Clin. Investig. 2023, 133. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, W.; Hua, L.; Wang, Y.; Li, J.; Bai, H.; Wang, S.; Du, M.; Ma, X.; Xu, C.; et al. Interaction between PPAR γ and SORL1 gene with Late-Onset Alzheimer’s disease in Chinese Han Population. Oncotarget 2017, 8, 48313–48320. [Google Scholar] [CrossRef]
- Pocevičiūtė, D.; Roth, B.; Schultz, N.; Nuñez-Diaz, C.; Janelidze, S.; The Netherlands Brain Bank; Olofsson, A.; Hansson, O.; Wennström, M. Plasma IAPP-Autoantibody Levels in Alzheimer’s Disease Patients Are Affected by APOE4 Status. Int. J. Mol. Sci. 2023, 24, 3776. [Google Scholar] [CrossRef]
- d’Abramo, C.; Massone, S.; Zingg, J.; Pizzuti, A.; Marambaud, P.; Dalla Piccola, B.; Azzi, A.; Marinari, U.; Pronzato, M.; Ricciarelli, R. Role of peroxisome proliferator-activated receptor gamma in amyloid precursor protein processing and amyloid beta-mediated cell death. Biochem. J. 2005, 391, 693–698. [Google Scholar] [CrossRef]
- Moutard, L.; Goudin, C.; Jaeger, C.; Duparc, C.; Louiset, E.; Pereira, T.; Fraissinet, F.; Delessard, M.; Saulnier, J.; Rives-Feraille, A.; et al. Steroidogenesis and androgen/estrogen signaling pathways are altered in in vitro matured testicular tissues of prepubertal mice. eLife 2023, 12, RP85562. [Google Scholar] [CrossRef]
- Vita, B.; Ken, K.Y.H. MECHANISMS IN ENDOCRINOLOGY: Paracrine and endocrine control of the growth hormone axis by estrogen. Eur. J. Endocrinol. 2021, 184, R269–R278. [Google Scholar]
- Micheline, M.; Ami, P.R. The peri-menopause in a woman’s life: A systemic inflammatory phase that enables later neurodegenerative disease. J. Neuroinflammation 2020, 17, 317. [Google Scholar]
- Hua, X.; Lei, M.; Ding, J.; Han, Q.; Hu, G.; Xiao, M. Pathological and biochemical alterations of astrocytes in ovariectomized rats injected with D-galactose: A potential contribution to Alzheimer’s disease processes. Exp. Neurol. 2008, 210, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Wenke, H.; Sen, Z.; Zhengtang, Q.; Weina, L. Unveiling the potential of estrogen: Exploring its role in neuropsychiatric disorders and exercise intervention. Pharmacol. Res. 2024, 204, 107201. [Google Scholar]
- Cyr, M.; Calon, F.; Morissette, M.; Grandbois, M.; Callier, S.; Paolo, T.D. Drugs with estrogen-like potency and brain activity: Potential therapeutic application for the CNS. Curr. Pharm. Des. 2000, 6, 1287–1312. [Google Scholar] [CrossRef]
- Ahmed, B.; Grant, C.H.; Rebecca, E.K.M. Exercise and estrogen: Common pathways in Alzheimer’s disease pathology. Am. J. Physiol. Endocrinol. Metab. 2021, 321, E164–E168. [Google Scholar]
- Graham, J.D.; Bain, D.L.; Richer, J.K.; Jackson, T.A.; Tung, L.; Horwitz, K.B. Nuclear receptor conformation, coregulators, and tamoxifen-resistant breast cancer. Steroids 2000, 65, 579–584. [Google Scholar] [CrossRef]
- Kuan-Fu, L.; Cheng-Li, L.; Shih-Wei, L. Nationwide Case-Control Study Examining the Association between Tamoxifen Use and Alzheimer’s Disease in Aged Women with Breast Cancer in Taiwan. Front. Pharmacol. 2017, 8, 612. [Google Scholar]
- Zsombor, K.; Rachel, Y.C. Targeting the non-classical estrogen pathway in neurodegenerative diseases and brain injury disorders. Front. Endocrinol. 2022, 13, 999236. [Google Scholar]
- Gao, J.; Littman, R.; Diamante, G.; Xiao, X.; Ahn, I.S.; Yang, X.; Cole, T.A.; Tontonoz, P. Therapeutic IDOL Reduction Ameliorates Amyloidosis and Improves Cognitive Function in APP/PS1 Mice. Mol. Cell Biol. 2020, 40, e00518-19. [Google Scholar] [CrossRef]
- Kevin, M.; Aleksandra, C.; Anne, P.; Jovana, K.; William, C.; Cédric, R.; Serge, L. Regulation of Brain Cholesterol: What Role Do Liver X Receptors Play in Neurodegenerative Diseases? Int. J. Mol. Sci. 2019, 20, 3858. [Google Scholar] [CrossRef]
- Noam, Z.; Negar, K.; Ryan, C.; Qingguang, J.; Erin, G.R.-G.; Gary, E.L.; Harry, V.V.; Peter, T. Attenuation of neuroinflammation and Alzheimer’s disease pathology by liver x receptors. Proc. Natl. Acad. Sci. USA 2007, 104, 10601–10606. [Google Scholar]
- Yu, S.; Jun, Y.; Tae-Wan, K.; Alan, R.T. Expression of liver X receptor target genes decreases cellular amyloid beta peptide secretion. J. Biol. Chem. 2003, 278, 27688–27694. [Google Scholar]
- Cui, W.; Sun, Y.; Wang, Z.; Xu, C.; Peng, Y.; Li, R. Liver X receptor activation attenuates inflammatory response and protects cholinergic neurons in APP/PS1 transgenic mice. Neuroscience 2012, 210, 200–210. [Google Scholar] [CrossRef]
- Radosveta, P.K.; Iliya, M.L.; Matthias, S.; Darren, W.; Shaohua, H.; Joseph, C.G.; Michael, W.; Michael, G.R.; John, S.L. The liver X receptor ligand T0901317 decreases amyloid beta production in vitro and in a mouse model of Alzheimer’s disease. J. Biol. Chem. 2004, 280, 4079–4088. [Google Scholar]
- Demetrios, M.; Constantinos, G.; Gerasimos, T.; Stamatios, T. Farnesoid-X Receptor (FXR) as a Promising Pharmaceutical Target in Atherosclerosis. Curr. Med. Chem. 2017, 24, 1147–1157. [Google Scholar]
- Chen, Q.; Ma, H.; Guo, X.; Liu, J.; Gui, T.; Gai, Z. Farnesoid X Receptor (FXR) Aggravates Amyloid-β-Triggered Apoptosis by Modulating the cAMP-Response Element-Binding Protein (CREB)/Brain-Derived Neurotrophic Factor (BDNF) Pathway In Vitro. Med. Sci. Monit. 2019, 25, 9335. [Google Scholar] [CrossRef]
- Ahuja, H.S.; Szanto, A.; Nagy, L.; Davies, P.J. The retinoid X receptor and its ligands: Versatile regulators of metabolic function, cell differentiation and cell death. J. Biol. Regul. Homeost. Agents 2003, 17, 29–45. [Google Scholar]
- Heike, K.; Dieter, L.; Frank, J.; Julius, P.; Frank, H.; Peter, K.; Silvia, F.; T A Wolfgang, M.; Reinhard, H. RXRA gene variations influence Alzheimer’s disease risk and cholesterol metabolism. J. Cell Mol. Med. 2009, 13, 589–598. [Google Scholar]
- Yogita, D.; Nitin, C.; Veer, G.; Mojdeh, A.; Mehdi, M.; Yuyi, Y.; Roger, C.; Stuart, L.G.; Vivek, G. Bexarotene Modulates Retinoid-X-Receptor Expression and Is Protective Against Neurotoxic Endoplasmic Reticulum Stress Response and Apoptotic Pathway Activation. Mol. Neurobiol. 2018, 55, 9043–9056. [Google Scholar]
- Brad, T.C.; Erin, G.R.-G.; Gary, E.L. Nuclear receptor agonist-driven modification of inflammation and amyloid pathology enhances and sustains cognitive improvements in a mouse model of Alzheimer’s disease. J. Neuroinflammation 2018, 15, 43. [Google Scholar]
- Władysław, L.; Danuta, J.; Monika, L.; Magdalena, R.; Agnieszka, B.-K. The Vitamin D Receptor as a Potential Target for the Treatment of Age-Related Neurodegenerative Diseases Such as Alzheimer’s and Parkinson’s Diseases: A Narrative Review. Cells 2023, 12, 660. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.Y.; Misner, D.; Kempermann, G.; Schikorski, T.; Giguère, V.; Sucov, H.M.; Gage, F.H.; Stevens, C.F.; Evans, R.M. An essential role for retinoid receptors RARbeta and RXRgamma in long-term potentiation and depression. Neuron 1999, 21, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Michinori, M. ABCA1 and ABCG1 as potential therapeutic targets for the prevention of atherosclerosis. J. Pharmacol. Sci. 2022, 148, 197–203. [Google Scholar]
- Sonia, M.-N.; Pedro, M.F.-S. New Trends in Aryl Hydrocarbon Receptor Biology. Front. Cell Dev. Biol. 2016, 4, 45. [Google Scholar]
- Antero, S. Activation of aryl hydrocarbon receptor (AhR) in Alzheimer’s disease: Role of tryptophan metabolites generated by gut host-microbiota. J. Mol. Med. 2023, 101, 201–222. [Google Scholar]
- Antero, S. Aryl hydrocarbon receptor impairs circadian regulation in Alzheimer’s disease: Potential impact on glymphatic system dysfunction. Eur. J. Neurosci. 2024, 60, 3901–3920. [Google Scholar]
- Antero, S. Aryl hydrocarbon receptor (AhR) reveals evidence of antagonistic pleiotropy in the regulation of the aging process. Cell Mol. Life Sci. 2022, 79, 489. [Google Scholar]
- Cheng, Q.; Chunjie, Y.; Mengting, L.; Jiaxin, B.; Haiyan, S.; Bingquan, D.; Shensen, L.; Wenwen, L.; Mu, Z.; Changchun, C. Activating AhR alleviates cognitive deficits of Alzheimer’s disease model mice by upregulating endogenous Aβ catabolic enzyme Neprilysin. Theranostics 2021, 11, 8797. [Google Scholar]
- Puigserver, P.; Wu, Z.; Park, C.W.; Graves, R.; Wright, M.; Spiegelman, B.M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 1998, 92, 829–839. [Google Scholar] [CrossRef]
- Lehman, J.J.; Barger, P.M.; Kovacs, A.; Saffitz, J.E.; Medeiros, D.M.; Kelly, D.P. Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J. Clin. Investig. 2000, 106, 847–856. [Google Scholar] [CrossRef]
- Han, B.; Jiang, W.; Liu, H.; Wang, J.; Hao, J.; Zheng, K.; Cui, P.; Feng, Y.; Dang, C.; Bu, Y.; et al. Upregulation of neuronal PGC-1α ameliorates cognitive impairment induced by chronic cerebral hypoperfusion. Theranostics 2020, 10, 2832–2848. [Google Scholar] [CrossRef] [PubMed]
- Tippabathani, J.; Nellore, J.; Radhakrishnan, V.; Banik, S.; Kapoor, S. Identification of NURR1 (Exon 4) and FOXA1 (Exon 3) Haplotypes Associated with mRNA Expression Levels in Peripheral Blood Lymphocytes of Parkinson’s Patients in Small Indian Population. Park. Dis. 2017, 2017, 6025358. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.J.; Cheng, Y.F.; Yu, N.; Di, Q. Effects of carbamazepine on the P-gp and CYP3A expression correlated with PXR or NF-κB activity in the bEnd.3 cells. Neurosci. Lett. 2019, 690, 48–55. [Google Scholar] [CrossRef]
- Hartz, A.M.S.; Miller, D.S.; Bauer, B. Restoring blood-brain barrier P-glycoprotein reduces brain amyloid-beta in a mouse model of Alzheimer’s disease. Mol. Pharmacol. 2010, 77, 715. [Google Scholar] [CrossRef]
- Ahmed, O.M.; El-Gareib, A.W.; El-Bakry, A.M.; El-Tawab, S.M.A.; Ahmed, R.G. Thyroid hormones states and brain development interactions. Int. J. Dev. Neurosci. 2008, 26, 147–209. [Google Scholar] [CrossRef]
- Bennett, S.N.; Chang, A.B.; Rogers, F.D.; Jones, P.; Pea, C.J. Thyroid hormones mediate the impact of early-life stress on ventral tegmental area gene expression and behavior. Horm. Behav. 2024, 159, 105472. [Google Scholar] [CrossRef]
- Rotenstein, L.S.; Sheridan, M.; Garg, R.; Adler, G.K. Effect of mineralocorticoid receptor blockade on hippocampal-dependent memory in adults with obesity. Obesity 2015, 23, 1136–1142. [Google Scholar] [CrossRef]
- Chen, L.; Shi, R.; She, X.; Gu, C.; Li, R. Mineralocorticoid receptor antagonist-mediated cognitive improvement in a mouse model of Alzheimer’s type: Possible involvement of BDNF-H2 S-Nrf2 signaling. Fundam. Clin. Pharmacol. 2020, 34, 697–707. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shan, X.; Li, D.; Yin, H.; Tao, W.; Zhou, L.; Gao, Y.; Xing, C.; Zhang, C. Recent Insights on the Role of Nuclear Receptors in Alzheimer’s Disease: Mechanisms and Therapeutic Application. Int. J. Mol. Sci. 2025, 26, 1207. https://doi.org/10.3390/ijms26031207
Shan X, Li D, Yin H, Tao W, Zhou L, Gao Y, Xing C, Zhang C. Recent Insights on the Role of Nuclear Receptors in Alzheimer’s Disease: Mechanisms and Therapeutic Application. International Journal of Molecular Sciences. 2025; 26(3):1207. https://doi.org/10.3390/ijms26031207
Chicago/Turabian StyleShan, Xiaoxiao, Dawei Li, Huihui Yin, Wenwen Tao, Lele Zhou, Yu Gao, Chengjie Xing, and Caiyun Zhang. 2025. "Recent Insights on the Role of Nuclear Receptors in Alzheimer’s Disease: Mechanisms and Therapeutic Application" International Journal of Molecular Sciences 26, no. 3: 1207. https://doi.org/10.3390/ijms26031207
APA StyleShan, X., Li, D., Yin, H., Tao, W., Zhou, L., Gao, Y., Xing, C., & Zhang, C. (2025). Recent Insights on the Role of Nuclear Receptors in Alzheimer’s Disease: Mechanisms and Therapeutic Application. International Journal of Molecular Sciences, 26(3), 1207. https://doi.org/10.3390/ijms26031207




