Rapid Determination of Colistin Susceptibility by Flow Cytometry Directly from Positive Urine Samples—Preliminary Results
Abstract
:1. Introduction
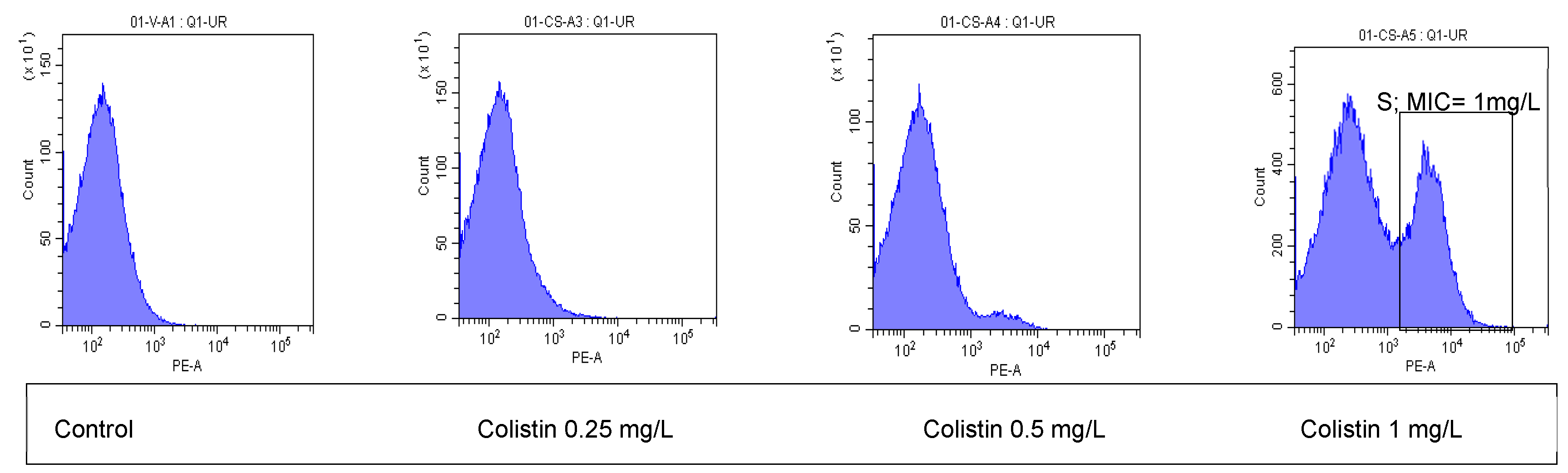
2. Results
3. Discussion
4. Material and Methods
4.1. Urine Samples
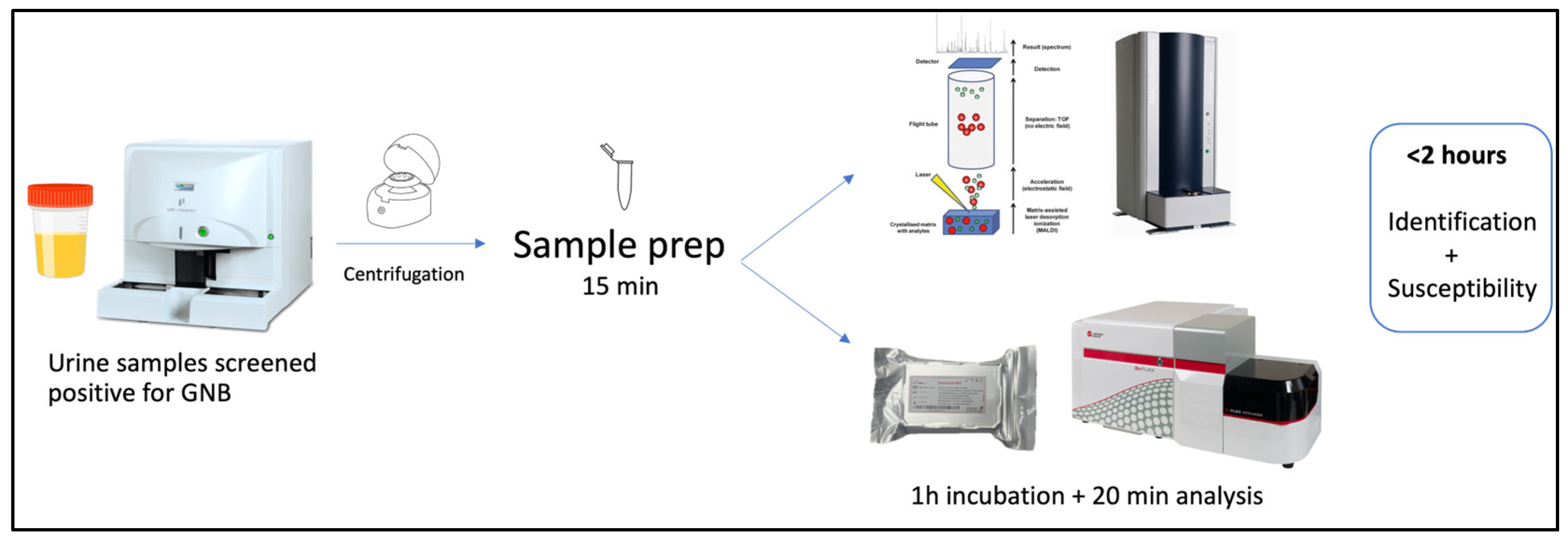
4.2. Sample Preparation and Identification
4.3. Incubation with Colistin and Fluorescent Probe
4.4. Flow Cytometry Analysis
4.5. Reference Method for Colistin Susceptibility
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCann, E.; Sung, A.H.; Ye, G.; Vankeepuram, L.; Tabak, Y.P. Contributing Factors to the Clinical and Economic Burden of Patients with Laboratory-Confirmed Carbapenem-Nonsusceptible Gram-Negative Urinary Tract Infections. Clin. Outcomes Res. 2020, 12, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, G.; Midiri, A.; Gerace, E.; Marra, M.; Zummo, S.; Biondo, C. Urinary Tract Infections: The Current Scenario and Future Prospects. Pathogens 2023, 12, 623. [Google Scholar] [CrossRef] [PubMed]
- Bavanandan, S.; Keita, N. Urinary Tract Infection Prevention and Treatment. Semin. Nephrol. 2024, 43, 151468. [Google Scholar] [CrossRef] [PubMed]
- Bologna, E.; Licari, L.C.; Manfredi, C.; Ditonno, F.; Cirillo, L.; Fusco, G.M.; Abate, M.; Passaro, F.; Di Mauro, E.; Crocetto, F.; et al. Carbapenem-Resistant Enterobacteriaceae in Urinary Tract Infections: From Biological Insights to Emerging Therapeutic Alternatives. Medicina 2024, 60, 214. [Google Scholar] [CrossRef] [PubMed]
- Andrade, F.F.; Silva, D.; Rodrigues, A.; Pina-Vaz, C. Colistin Update on Its Mechanism of Action and Resistance, Present and Future Challenges. Microorganisms 2020, 8, 1716. [Google Scholar] [CrossRef] [PubMed]
- GBD 2021 Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef] [PubMed]
- Binsker, U.; Käsbohrer, A.; Hammerl, J.A. Global colistin use: A review of the emergence of resistant Enterobacterales and the impact on their genetic basis. FEMS Microbiol. Rev. 2021, 46, fuab049. [Google Scholar] [CrossRef] [PubMed]
- Pietropaolo, A.; Jones, P.; Moors, M.; Birch, B.; Somani, B.K. Use and Effectiveness of Antimicrobial Intravesical Treatment for Prophylaxis and Treatment of Recurrent Urinary Tract Infections (UTIs): A Systematic Review. Curr. Urol. Rep. 2018, 19, 78. [Google Scholar] [CrossRef] [PubMed]
- Leshaba, T.M.S.; Mbelle, N.M.; Sekyere, J.O. Current and emerging polymyxin resistance diagnostics: A systematic review of established and novel detection methods. J. Appl. Microbiol. 2021, 132, 8–30. [Google Scholar] [CrossRef] [PubMed]
- e Silva, D.F.; Andrade, F.F.; Gomes, R.; Silva-Dias, A.; Martins-Oliveira, I.; Pérez-Viso, B.; Ramos, M.H.; Rodrigues, A.G.; Cantón, R.; Pina-Vaz, C. Ultra-rapid flow cytometry assay for colistin MIC determination in Enterobacterales, Pseudomonas ae-ruginosa and Acinetobacter baumannii. Clin. Microbiol. Infect. 2020, 26, 1559.e1–1559.e4. [Google Scholar] [CrossRef] [PubMed]
- e Silva, D.F.; Silva-Dias, A.; Gomes, R.; Martins-Oliveira, I.; Ramos, M.H.; Rodrigues, A.G.; Cantón, R.; Pina-Vaz, C. Evaluation of rapid colistin susceptibility directly from positive blood cultures using flow cytometry assay. Int. J. Antimicrob. Agents 2019, 54, 820–823. [Google Scholar] [CrossRef] [PubMed]
- Hattab, S.; Ma, A.H.; Tariq, Z.; Prado, I.V.; Drobish, I.; Lee, R.; Yee, R. Rapid Phenotypic and Genotypic Antimicrobial Susceptibility Testing Approaches for Use in the Clinical Laboratory. Antibiotics 2024, 13, 786. [Google Scholar] [CrossRef] [PubMed]
- Broeren, M.A.C.; Bahçeci, S.; Vader, H.L.; Arents, N.L.A. Screening for Urinary Tract Infection with the Sysmex UF-1000i Urine Flow Cytometer. J. Clin. Microbiol. 2011, 49, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.S.D.; Yang, C.C.; Chen, Y.S.; Chang, S.J. A performance comparison of the fully automated urine particle analyzer UF-5000 with UF-1000i and Gram staining in predicting bacterial growth patterns in women with uncomplicated urinary tract infections. BMC Urol. 2021, 21, 24. [Google Scholar] [CrossRef] [PubMed]
- Cruz, S.; Abreu, D.; Gomes, R.; Martins-Oliveira, I.; Silva-Dias, A.; Perez-Viso, B.; Cantón, R.; Pina-Vaz, C. An improved protocol for bacteria identification by MALDI-TOF MS directly from positive blood cultures. Eur. J. Clin. Microbiol. Infect. Dis. 2023, 43, 605–610. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters, version 14.0; EUCAST: Växjö, Sweden, 2024. [Google Scholar]
- Pina-Vaz, C.; Silva-Dias, A.; Martins-Oliveira, I.; Gomes, R.; Perez-Viso, B.; Cruz, S.; Rodrigues, A.G.; Sarmento, A.; Cantón, R. A multisite validation of a two hours antibiotic susceptibility flow cytometry assay directly from positive blood cultures. BMC Microbiol. 2024, 24, 187. [Google Scholar] [CrossRef] [PubMed]
- Aragão, F.; Palos, C.; Mimoso, C.; Neves, I.; Janeiro, M.J.; Pina-Vaz, C.; Paiva, J.A. Incremental Value of the Ultra Rapid Phenotypic Antimicrobial Susceptibility Testing in Patients with Bloodstream Infection: A modeling approach. In Proceedings of the ESCMID/ASM, Porto, Portugal, 17–20 September 2024. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 34th ed.; CLSI 100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2024. [Google Scholar]
- ISO 20776-2:2021; Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices. ISO: Geneva, Switzerland, 2021.
| Species | Patients | Inoculated | Total | MIC | |
|---|---|---|---|---|---|
| Enterobacterales | Escherichia coli | 23 | 7 | 30 | 0.125->64 |
| Klebsiella pneumoniae | 13 | 6 | 19 | 0.5–2 | |
| Klebsiella aerogenes | 2 | 1 | 3 | 1–2 | |
| Serratia marcescens | 1 | 1 | 8 | ||
| Proteus mirabilis | 1 | 2 | 3 | >64 | |
| Providencia rettgeri | 1 | 1 | 4 | ||
| Total | 39 | 18 | 57 | ||
| No fermenters | Pseudomonas aeruginosa | 30 | 30 | 0.25–4 | |
| Acinetobacter baumannii | 12 | 12 | 1–4 | ||
| Enterobacter cloacae | 1 | 1 | 2 | ||
| Total | 0 | 43 | 43 | ||
| Total studied | 39 | 61 | 100 |
| Reference method MIC (mg/L) (microdilution) | |||||||||||
| FASTcolistin MIC (mg/L) | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | >64 | |
| 0.25 | 1 | 2 | 3 | ||||||||
| 0.5 | 4 | 8 | 9 | ||||||||
| 1 | 1 | 14 | 10 | 6 | |||||||
| 2 | 15 | 17 | |||||||||
| 4 | 5 | ||||||||||
| 8 | 1 | ||||||||||
| 16 | |||||||||||
| 32 | |||||||||||
| ≥64 | 4 | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca-Silva, D.; Gomes, R.; Martins-Oliveira, I.; Silva-Dias, A.; Ramos, M.H.; Pina-Vaz, C. Rapid Determination of Colistin Susceptibility by Flow Cytometry Directly from Positive Urine Samples—Preliminary Results. Int. J. Mol. Sci. 2025, 26, 883. https://doi.org/10.3390/ijms26030883
Fonseca-Silva D, Gomes R, Martins-Oliveira I, Silva-Dias A, Ramos MH, Pina-Vaz C. Rapid Determination of Colistin Susceptibility by Flow Cytometry Directly from Positive Urine Samples—Preliminary Results. International Journal of Molecular Sciences. 2025; 26(3):883. https://doi.org/10.3390/ijms26030883
Chicago/Turabian StyleFonseca-Silva, Daniela, Rosário Gomes, Inês Martins-Oliveira, Ana Silva-Dias, Maria Helena Ramos, and Cidália Pina-Vaz. 2025. "Rapid Determination of Colistin Susceptibility by Flow Cytometry Directly from Positive Urine Samples—Preliminary Results" International Journal of Molecular Sciences 26, no. 3: 883. https://doi.org/10.3390/ijms26030883
APA StyleFonseca-Silva, D., Gomes, R., Martins-Oliveira, I., Silva-Dias, A., Ramos, M. H., & Pina-Vaz, C. (2025). Rapid Determination of Colistin Susceptibility by Flow Cytometry Directly from Positive Urine Samples—Preliminary Results. International Journal of Molecular Sciences, 26(3), 883. https://doi.org/10.3390/ijms26030883







