Targeting Bacterial Cell Division with Benzodioxane–Benzamide FtsZ Inhibitors as a Novel Strategy to Fight Gram-Positive Ovococcal Pathogens
Abstract
:1. Introduction
2. Results
2.1. Chemistry
2.2. Antimicrobial Activity on S. pneumoniae
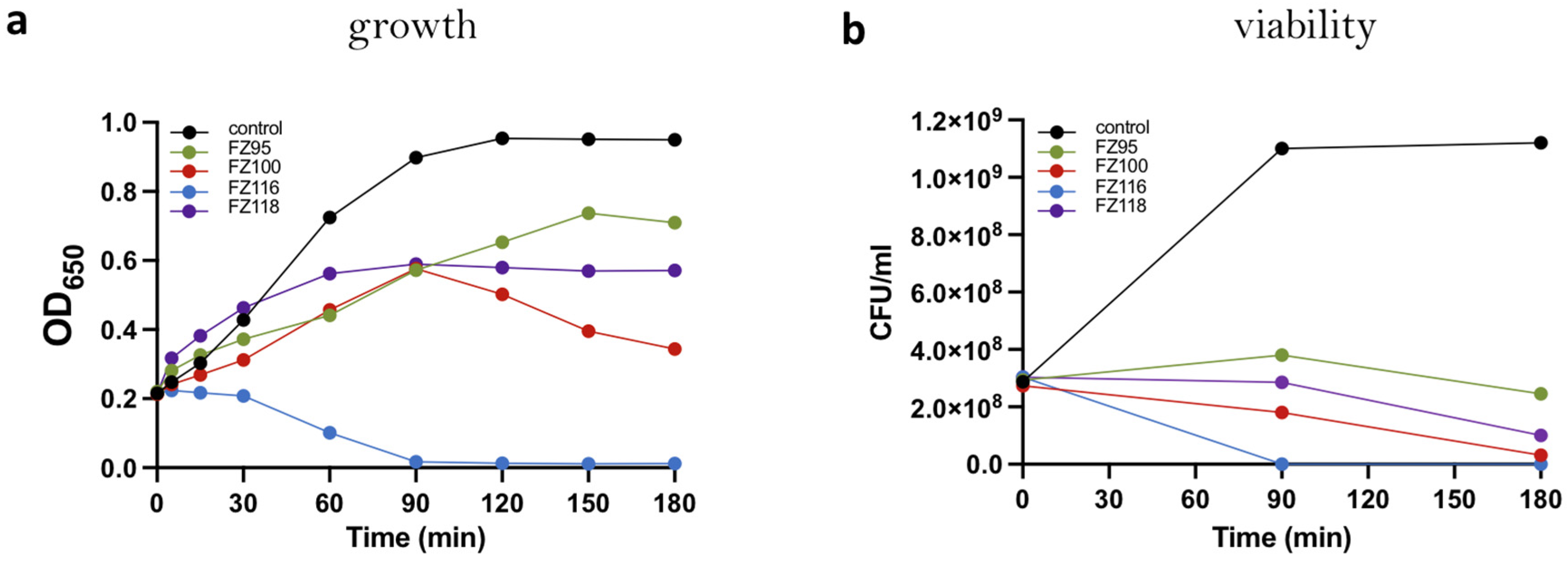
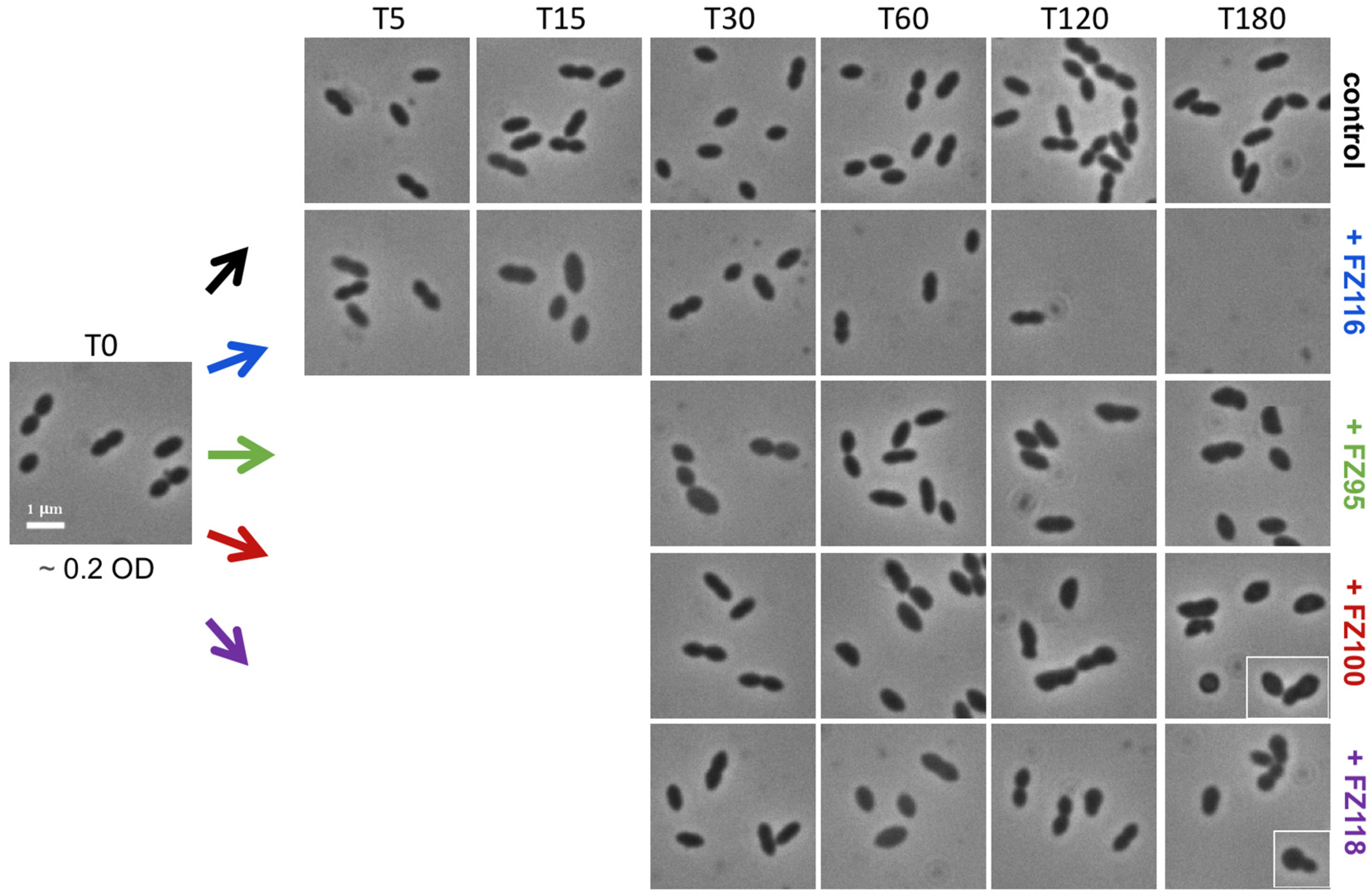
2.3. Effect on Growth, Viability, and Morphology of S. pneumoniae Rx1 of Selected Benzodioxane–Benzamides
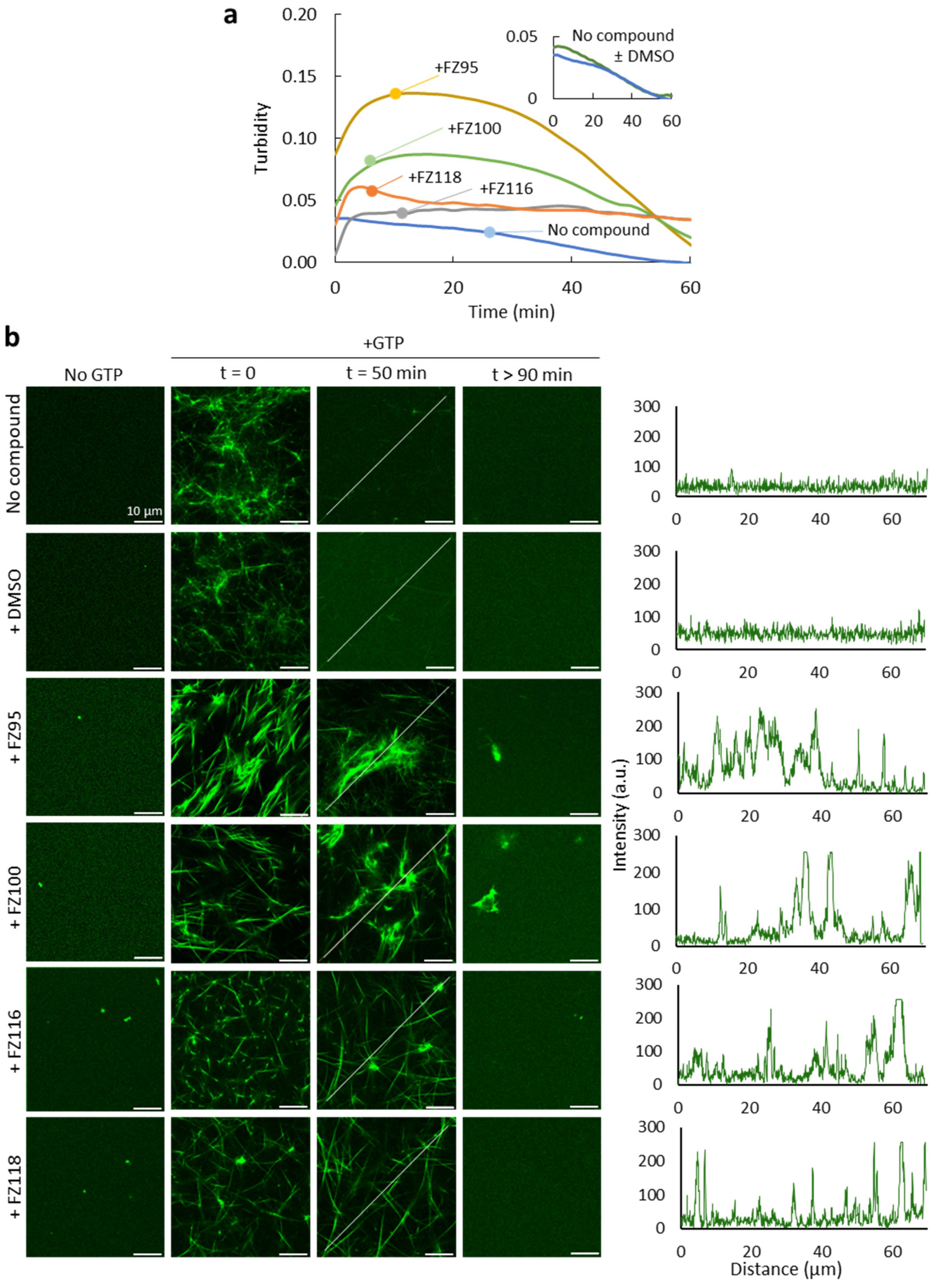
2.4. Effect of Benzodioxane–Benzamides on FtsZ Assembly in Crowding Conditions
3. Discussion
4. Materials and Methods
4.1. Antimicrobial Activity on S. pneumoniae
4.2. In Vitro Activity of Benzodioxane–Benzamides Under Crowded Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- FDA. New Drugs at FDA. Available online: https://www.fda.gov/drugs/development-approval-process-drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products (accessed on 10 November 2024).
- World Health Organization. 2021 Antibacterial Agents in Clinical and Preclinical Development: An Overview and Analysis; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Walsh, C.T.; Wencewicz, T.A. Prospects for new antibiotics: A molecule-centered perspective. J Antibiot 2014, 67, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Fischbach, M.A.; Walsh, C.T. Antibiotics for emerging pathogens. Science 2009, 325, 1089–1093. [Google Scholar] [CrossRef] [PubMed]
- Silver, L.L. Challenges of antibacterial discovery. Clin. Microbiol. Rev. 2011, 24, 71–109. [Google Scholar] [CrossRef]
- Vicente, M.; Hodgson, J.; Massidda, O.; Tonjum, T.; Henriques-Normark, B.; Ron, E.Z. The fallacies of hope: Will we discover new antibiotics to combat pathogenic bacteria in time? FEMS Microbiol. Rev. 2006, 30, 841–852. [Google Scholar] [CrossRef]
- Lock, R.L.; Harry, E.J. Cell-division inhibitors: New insights for future antibiotics. Nat. Rev. Drug Discov. 2008, 7, 324–338. [Google Scholar] [CrossRef]
- de Boer, P.A.J. Advances in understanding E. coli cell fission. Curr. Opin. Microbiol. 2010, 13, 730–737. [Google Scholar] [CrossRef]
- Lutkenhaus, J.; Pichoff, S.; Du, S. Bacterial cytokinesis: From Z ring to divisome. Cytoskeleton 2012, 69, 778–790. [Google Scholar] [CrossRef]
- Egan, A.J.F.; Vollmer, W. The physiology of bacterial cell division. Ann. N. Y. Acad. Sci. 2013, 1277, 8–28. [Google Scholar] [CrossRef]
- Ortiz, C.; Natale, P.; Cueto, L.; Vicente, M. The keepers of the ring: Regulators of FtsZ assembly. FEMS Microbiol. Rev. 2016, 40, 57–67. [Google Scholar] [CrossRef]
- Haeusser, D.P.; Margolin, W. Splitsville: Structural and functional insights into the dynamic bacterial Z ring. Nat. Rev. Microbiol. 2016, 14, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Zapun, A.; Vernet, T.; Pinho, M.G. The different shapes of cocci. FEMS Microbiol. Rev. 2008, 32, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Massidda, O.; Nováková, L.; Vollmer, W. From models to pathogens: How much have we learned about Streptococcus pneumoniae cell division? Environ. Microbiol. 2013, 15, 3133–3157. [Google Scholar] [CrossRef] [PubMed]
- Pinho, M.G.; Kjos, M.; Veening, J.-W. How to get (a)round: Mechanisms controlling growth and division of coccoid bacteria. Nat. Rev. Microbiol. 2013, 11, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Vollmer, W.; Massidda, O.; Tomasz, A. The Cell Wall of Streptococcus pneumoniae. Microbiol. Spectr. 2019, 7, 1–24. [Google Scholar] [CrossRef]
- Briggs, N.S.; Bruce, K.E.; Naskar, S.; Winkler, M.E.; Roper, D.I. The Pneumococcal Divisome: Dynamic Control of Streptococcus pneumoniae Cell Division. Front. Microbiol. 2021, 12, 737396. [Google Scholar] [CrossRef]
- Typas, A.; Banzhaf, M.; Gross, C.A.; Vollmer, W. From the regulation of peptidoglycan synthesis to bacterial growth and morphology. Nat. Rev. Microbiol. 2011, 10, 123–136. [Google Scholar] [CrossRef]
- Erickson, H.P.; Anderson, D.E.; Osawa, M. FtsZ in bacterial cytokinesis: Cytoskeleton and force generator all in one. Microbiol. Mol. Biol. Rev. 2010, 74, 504–528. [Google Scholar] [CrossRef]
- Monterroso, B.; Margolin, W.; Boersma, A.J.; Rivas, G.; Poolman, B.; Zorrilla, S. Macromolecular Crowding, Phase Separation, and Homeostasis in the Orchestration of Bacterial Cellular Functions. Chem. Rev. 2024, 124, 1899–1949. [Google Scholar] [CrossRef]
- González, J.M.; Jiménez, M.; Vélez, M.; Mingorance, J.; Andreu, J.M.; Vicente, M.; Rivas, G. Essential cell division protein FtsZ assembles into one monomer-thick ribbons under conditions resembling the crowded intracellular environment. J. Biol. Chem. 2003, 278, 37664–37671. [Google Scholar] [CrossRef]
- Coltharp, C.; Buss, J.; Plumer, T.M.; Xiao, J. Defining the rate-limiting processes of bacterial cytokinesis. Proc. Natl. Acad. Sci. USA 2016, 113, E1044–E1053. [Google Scholar] [CrossRef] [PubMed]
- Kusuma, K.D.; Payne, M.; Ung, A.T.; Bottomley, A.L.; Harry, E.J. FtsZ as an Antibacterial Target: Status and Guidelines for Progressing This Avenue. ACS Infect. Dis. 2019, 5, 1279–1294. [Google Scholar] [CrossRef] [PubMed]
- Araújo-Bazán, L.; Ruiz-Avila, L.B.; Andreu, D.; Huecas, S.; Andreu, J.M. Cytological Profile of Antibacterial FtsZ Inhibitors and Synthetic Peptide MciZ. Front. Microbiol. 2016, 7, 1558. [Google Scholar] [CrossRef] [PubMed]
- Casiraghi, A.; Suigo, L.; Valoti, E.; Straniero, V. Targeting Bacterial Cell Division: A Binding Site-Centered Approach to the Most Promising Inhibitors of the Essential Protein FtsZ. Antibiotics 2020, 9, 69. [Google Scholar] [CrossRef] [PubMed]
- Haydon, D.J.; Stokes, N.R.; Ure, R.; Galbraith, G.; Bennett, J.M.; Brown, D.R.; Baker, P.J.; Barynin, V.V.; Rice, D.W.; Sedelnikova, S.E.; et al. An inhibitor of FtsZ with potent and selective anti-staphylococcal activity. Science 2008, 321, 1673–1675. [Google Scholar] [CrossRef]
- Adams, D.W.; Wu, L.J.; Czaplewski, L.G.; Errington, J. Multiple effects of benzamide antibiotics on FtsZ function. Mol. Microbiol. 2011, 80, 68–84. [Google Scholar] [CrossRef]
- Stokes, N.R.; Baker, N.; Bennett, J.M.; Berry, J.; Collins, I.; Czaplewski, L.G.; Logan, A.; Macdonald, R.; Macleod, L.; Peasley, H.; et al. An improved small-molecule inhibitor of FtsZ with superior in vitro potency, drug-like properties, and in vivo efficacy. Antimicrob. Agents Chemother. 2013, 57, 317–325. [Google Scholar] [CrossRef]
- Chiodini, G.; Pallavicini, M.; Zanotto, C.; Bissa, M.; Radaelli, A.; Straniero, V.; Bolchi, C.; Fumagalli, L.; Ruggeri, P.; Morghen, C.D.G.; et al. Benzodioxane-benzamides as new bacterial cell division inhibitors. Eur. J. Med. Chem. 2015, 89, 252–265. [Google Scholar] [CrossRef]
- Straniero, V.; Pallavicini, M.; Chiodini, G.; Zanotto, C.; Volontè, L.; Radaelli, A.; Bolchi, C.; Fumagalli, L.; Sanguinetti, M.; Menchinelli, G.; et al. 3-(Benzodioxan-2-ylmethoxy)-2,6-difluorobenzamides bearing hydrophobic substituents at the 7-position of the benzodioxane nucleus potently inhibit methicillin-resistant Sa and Mtb cell division. Eur. J. Med. Chem. 2016, 120, 227–243. [Google Scholar] [CrossRef]
- Straniero, V.; Zanotto, C.; Straniero, L.; Casiraghi, A.; Duga, S.; Radaelli, A.; de Giuli Morghen, C.; Valoti, E. 2,6-Difluorobenzamide Inhibitors of Bacterial Cell Division Protein FtsZ: Design, Synthesis, and Structure-Activity Relationships. ChemMedChem 2017, 12, 1303–1318. [Google Scholar] [CrossRef]
- Straniero, V.; Suigo, L.; Casiraghi, A.; Sebastián-Pérez, V.; Hrast, M.; Zanotto, C.; Zdovc, I.; de Giuli Morghen, C.; Radaelli, A.; Valoti, E. Benzamide Derivatives Targeting the Cell Division Protein FtsZ: Modifications of the Linker and the Benzodioxane Scaffold and Their Effects on Antimicrobial Activity. Antibiotics 2020, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- Straniero, V.; Sebastián Pérez, V.; Hrast, M.; Zanotto, C.; Casiraghi, A.; Suigo, L.; Zdovc, I.; Radaelli, A.; de Giuli Morghen, C.; Valoti, E. Benzodioxane-benzamides as antibacterial agents: Computational and SAR studies to evaluate the influence of the 7-substitution in FtsZ interaction. ChemMedChem 2020, 2, 195–209. [Google Scholar] [CrossRef]
- Straniero, V.; Sebastián-Pérez, V.; Suigo, L.; Margolin, W.; Casiraghi, A.; Hrast, M.; Zanotto, C.; Zdovc, I.; Radaelli, A.; Valoti, E. Computational Design and Development of Benzodioxane-Benzamides as Potent Inhibitors of FtsZ by Exploring the Hydrophobic Subpocket. Antibiotics 2021, 10, 442. [Google Scholar] [CrossRef]
- Suigo, L.; Margolin, W.; Ulzurrun, E.; Hrast Rambaher, M.; Zanotto, C.; Sebastián-Pérez, V.; Campillo, N.E.; Straniero, V.; Valoti, E. Benzodioxane-Benzamides as FtsZ Inhibitors: Effects of Linker’s Functionalization on Gram-Positive Antimicrobial Activity. Antibiotics 2023, 12, 1712. [Google Scholar] [CrossRef]
- Kaul, M.; Mark, L.; Zhang, Y.; Parhi, A.K.; Lavoie, E.J.; Pilch, D.S. An FtsZ-targeting prodrug with oral antistaphylococcal efficacy in vivo. Antimicrob. Agents Chemother. 2013, 57, 5860–5869. [Google Scholar] [CrossRef]
- Kaul, M.; Mark, L.; Zhang, Y.; Parhi, A.K.; Lyu, Y.L.; Pawlak, J.; Saravolatz, S.; Saravolatz, L.D.; Weinstein, M.P.; Lavoie, E.J.; et al. TXA709, an FtsZ-Targeting Benzamide Prodrug with Improved Pharmacokinetics and Enhanced In Vivo Efficacy against Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2015, 59, 4845–4855. [Google Scholar] [CrossRef]
- Suigo, L.; Monterroso, B.; Sobrinos-Sanguino, M.; Alfonso, C.; Straniero, V.; Rivas, G.; Zorrilla, S.; Valoti, E.; Margolin, W. Benzodioxane-benzamides as promising inhibitors of Escherichia coli FtsZ. Int. J. Biol. Macromol. 2023, 253, 126398. [Google Scholar] [CrossRef]
- World Health Organization. WHO Publishes List of Bacteria for Which New Antibiotics are Urgently Needed. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 10 November 2024).
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Pushpakaran, A.; Battaje, R.R.; Panda, D. Vitamin K3 inhibits FtsZ assembly, disrupts the Z-ring in Streptococcus pneumoniae and displays anti-pneumococcal activity. Biochem. J. 2022, 479, 1543–1558. [Google Scholar] [CrossRef]
- Jahan, K.; Battaje, R.R.; Pratap, V.; Ahire, G.; Pushpakaran, A.; Ashtam, A.; Bharatam, P.V.; Panda, D. Identification of ethyl-6-bromo-2((phenylthio)methyl)imidazo1,2-apyridine-3-carboxylate as a narrow spectrum inhibitor of Streptococcus pneumoniae and its FtsZ. Eur. J. Med. Chem. 2024, 267, 116196. [Google Scholar] [CrossRef]
- Straniero, V.; Suigo, L.; Lodigiani, G.; Valoti, E. Obtainment of Threo and Erythro Isomers of the 6-Fluoro-3-(2,3,6,7,8,9-hexahydronaphtho[2,3-b][1,4]dioxin-2-yl)-2,3-dihydrobenzo[b][1,4]dioxine-5-carboxamide. Molbank 2023, 2023, M1559. [Google Scholar] [CrossRef]
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases. Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 2003, 9, ix. [Google Scholar] [CrossRef]
- Battaje, R.R.; Panda, D. Lessons from bacterial homolog of tubulin, FtsZ for microtubule dynamics. Endocr. -Relat. Cancer 2017, 24, T1–T21. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.J.; Villicana, J.B.; Tsui, H.-C.T.; Danforth, M.L.; Benedet, M.; Massidda, O.; Winkler, M.E. FtsZ-Ring Regulation and Cell Division Are Mediated by Essential EzrA and Accessory Proteins ZapA and ZapJ in Streptococcus pneumoniae. Front. Microbiol. 2021, 12, 780864. [Google Scholar] [CrossRef] [PubMed]
- Mura, A.; Fadda, D.; Perez, A.J.; Danforth, M.L.; Musu, D.; Rico, A.I.; Krupka, M.; Denapaite, D.; Tsui, H.-C.T.; Winkler, M.E.; et al. Roles of the Essential Protein FtsA in Cell Growth and Division in Streptococcus pneumoniae. J. Bacteriol. 2017, 199, 1–20. [Google Scholar] [CrossRef]
- Salvarelli, E.; Krupka, M.; Rivas, G.; Mingorance, J.; Gómez-Puertas, P.; Alfonso, C.; Rico, A.I. The Cell Division Protein FtsZ from Streptococcus pneumoniae Exhibits a GTPase Activity Delay. J. Biol. Chem. 2015, 290, 25081–25089. [Google Scholar] [CrossRef]
- Reija, B.; Monterroso, B.; Jiménez, M.; Vicente, M.; Rivas, G.; Zorrilla, S. Development of a homogeneous fluorescence anisotropy assay to monitor and measure FtsZ assembly in solution. Anal. Biochem. 2011, 418, 89–96. [Google Scholar] [CrossRef]
- Fodeke, A.A.; Minton, A.P. Quantitative characterization of polymer-polymer, protein-protein, and polymer-protein interaction via tracer sedimentation equilibrium. J. Phys. Chem. B 2010, 114, 10876–10880. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
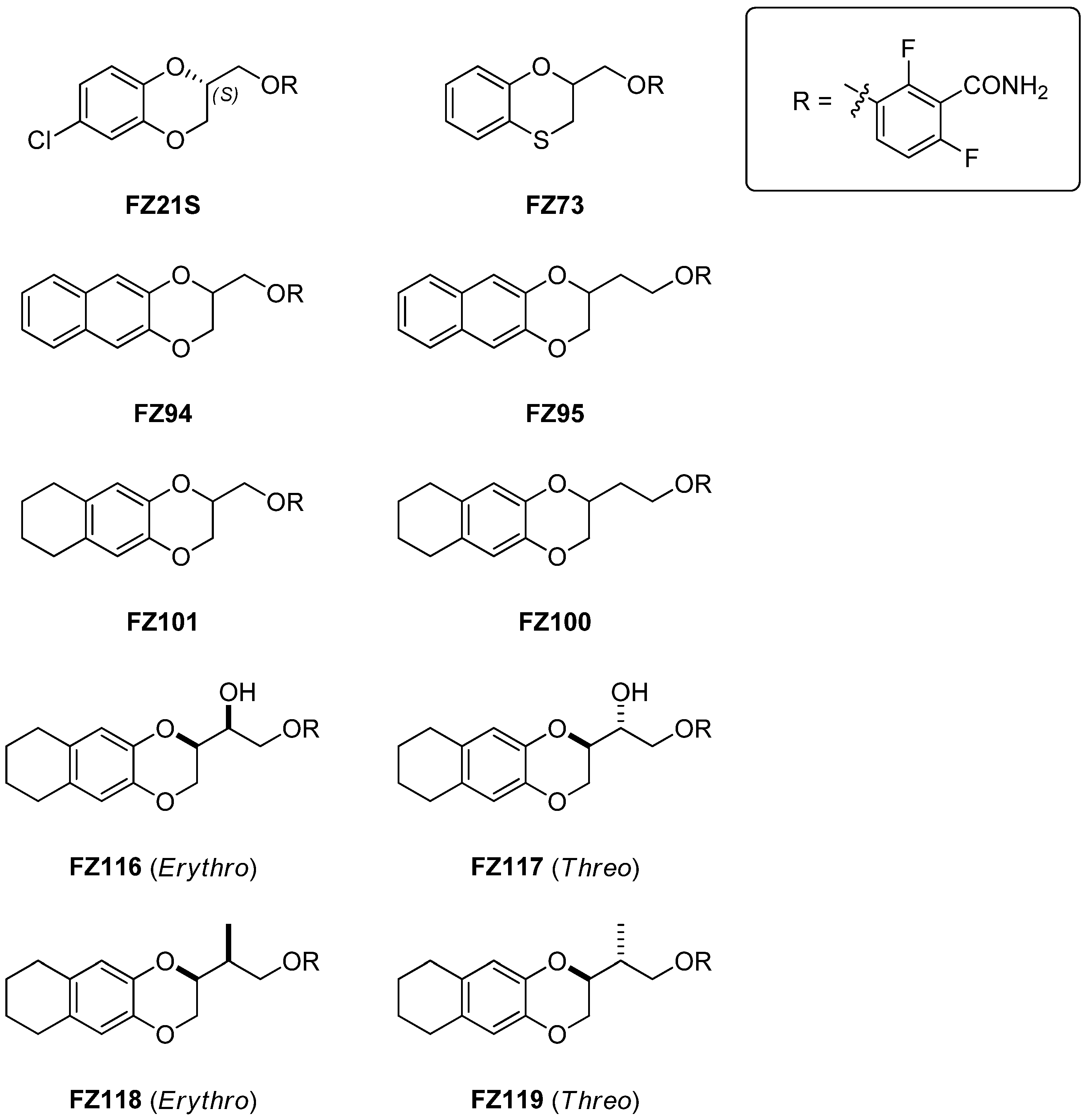
| Compound | S. pneumoniae MICs (μg/mL) | MRC-5 TD90 (μg/mL) |
|---|---|---|
| FZ21S | >100 | 600 * [30] |
| FZ73 | >100 | - |
| FZ94 | >100 | >800 [35] |
| FZ95 | 25 < MIC < 30 | 90 [35] |
| FZ100 | 25 < MIC < 30 | 75 [35] |
| FZ101 | >100 | 800 [35] |
| FZ116 | D39: 60 < MIC < 70 Rx1: 75 < MIC < 80 | 190 [36] |
| FZ117 | >100 | 190 [36] |
| FZ118 | 70 < MIC < 75 | 190 [36] |
| FZ119 | >100 | 100 [36] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furlan, B.; Sobrinos-Sanguino, M.; Sammartino, M.; Monterroso, B.; Zorrilla, S.; Lanzini, A.; Suigo, L.; Valoti, E.; Massidda, O.; Straniero, V. Targeting Bacterial Cell Division with Benzodioxane–Benzamide FtsZ Inhibitors as a Novel Strategy to Fight Gram-Positive Ovococcal Pathogens. Int. J. Mol. Sci. 2025, 26, 714. https://doi.org/10.3390/ijms26020714
Furlan B, Sobrinos-Sanguino M, Sammartino M, Monterroso B, Zorrilla S, Lanzini A, Suigo L, Valoti E, Massidda O, Straniero V. Targeting Bacterial Cell Division with Benzodioxane–Benzamide FtsZ Inhibitors as a Novel Strategy to Fight Gram-Positive Ovococcal Pathogens. International Journal of Molecular Sciences. 2025; 26(2):714. https://doi.org/10.3390/ijms26020714
Chicago/Turabian StyleFurlan, Berenice, Marta Sobrinos-Sanguino, Marcella Sammartino, Begoña Monterroso, Silvia Zorrilla, Alessia Lanzini, Lorenzo Suigo, Ermanno Valoti, Orietta Massidda, and Valentina Straniero. 2025. "Targeting Bacterial Cell Division with Benzodioxane–Benzamide FtsZ Inhibitors as a Novel Strategy to Fight Gram-Positive Ovococcal Pathogens" International Journal of Molecular Sciences 26, no. 2: 714. https://doi.org/10.3390/ijms26020714
APA StyleFurlan, B., Sobrinos-Sanguino, M., Sammartino, M., Monterroso, B., Zorrilla, S., Lanzini, A., Suigo, L., Valoti, E., Massidda, O., & Straniero, V. (2025). Targeting Bacterial Cell Division with Benzodioxane–Benzamide FtsZ Inhibitors as a Novel Strategy to Fight Gram-Positive Ovococcal Pathogens. International Journal of Molecular Sciences, 26(2), 714. https://doi.org/10.3390/ijms26020714





