Changes to the Autophagy-Related Muscle Proteome Following Short-Term Treatment with Ectoine in the Duchenne Muscular Dystrophy Mouse Model mdx
Abstract
1. Introduction
2. Results
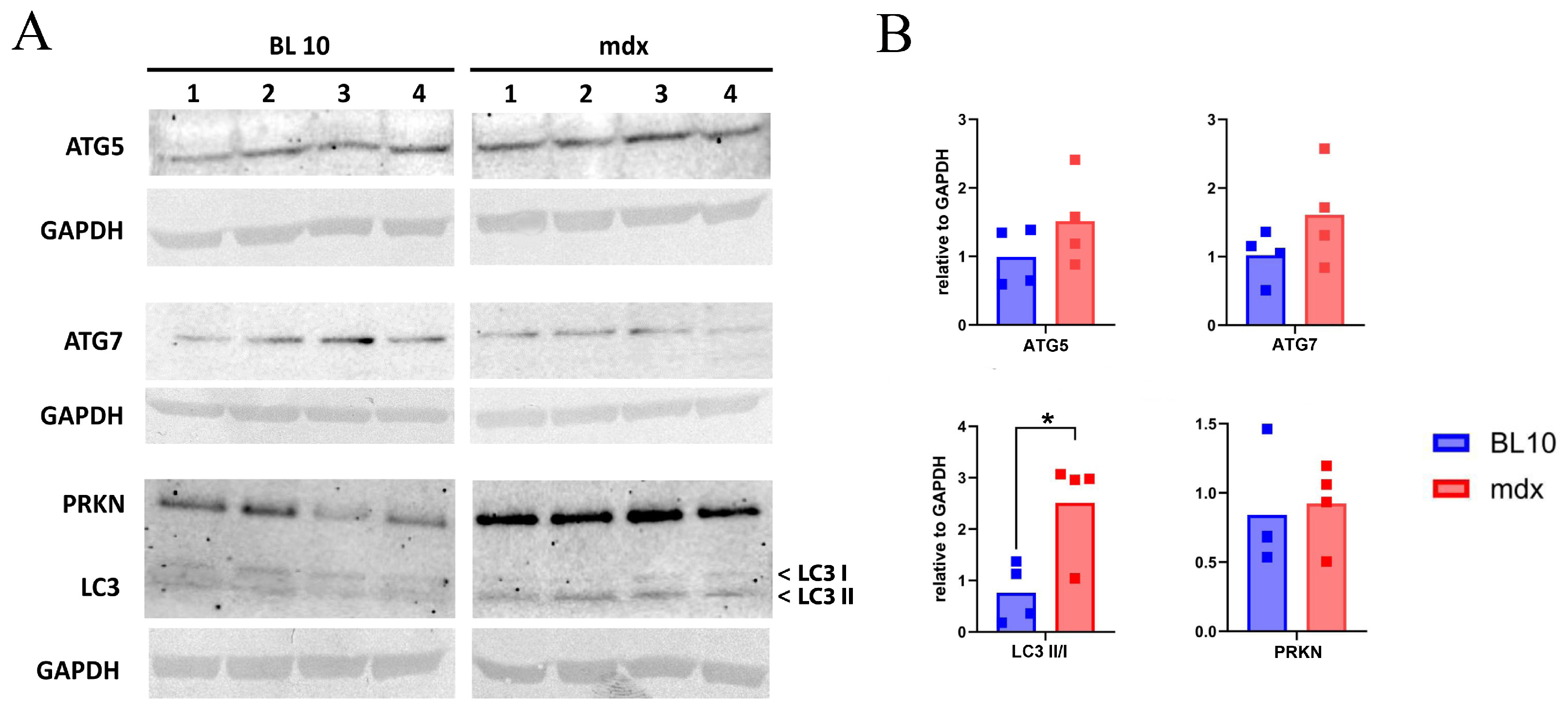
2.1. Levels of Autophagy-Related Proteins of Skeletal Muscle of mdx Compared to Wild Type
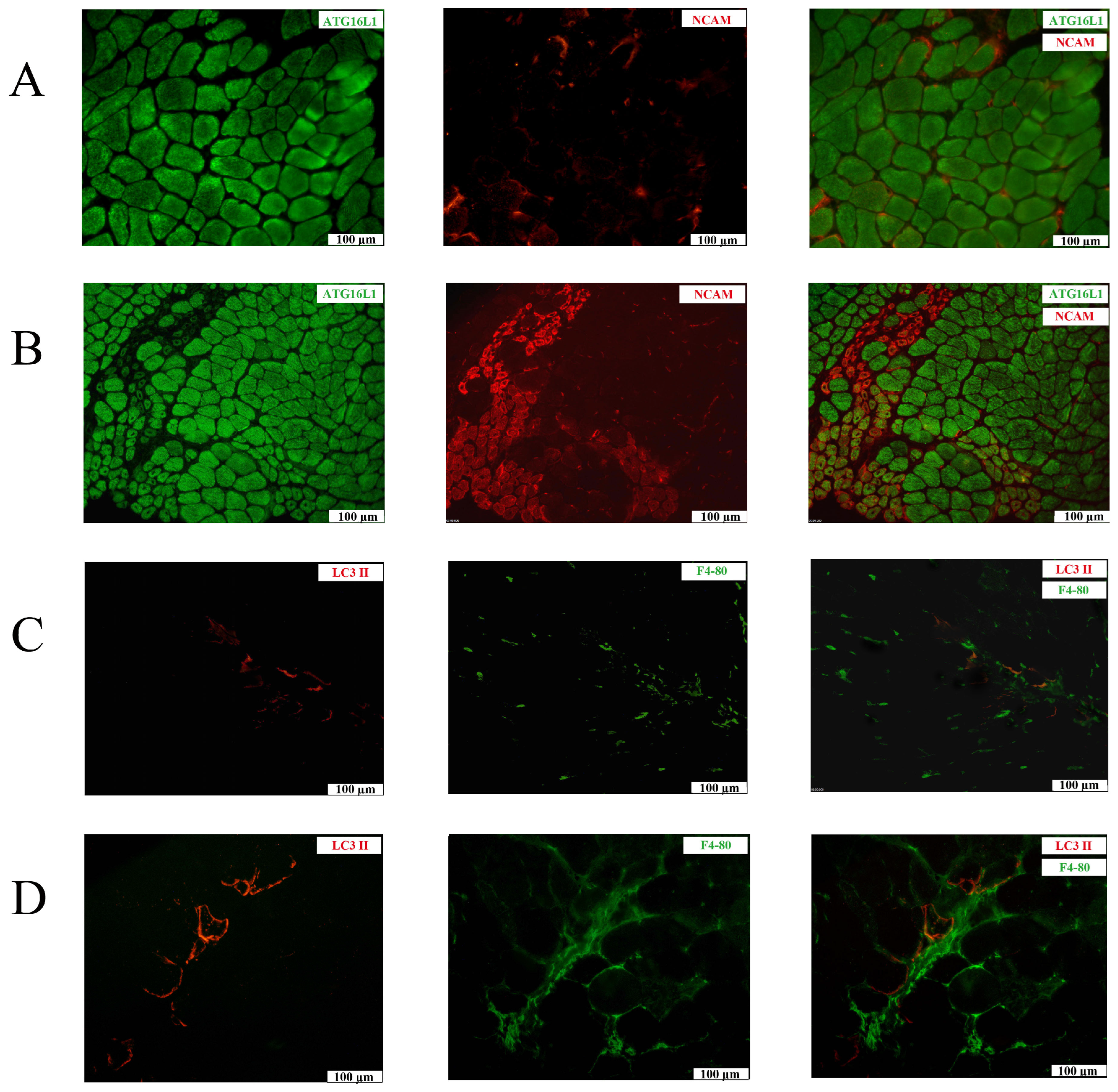
2.2. Tissue Expression Patterns of Autophagy-Related Proteins
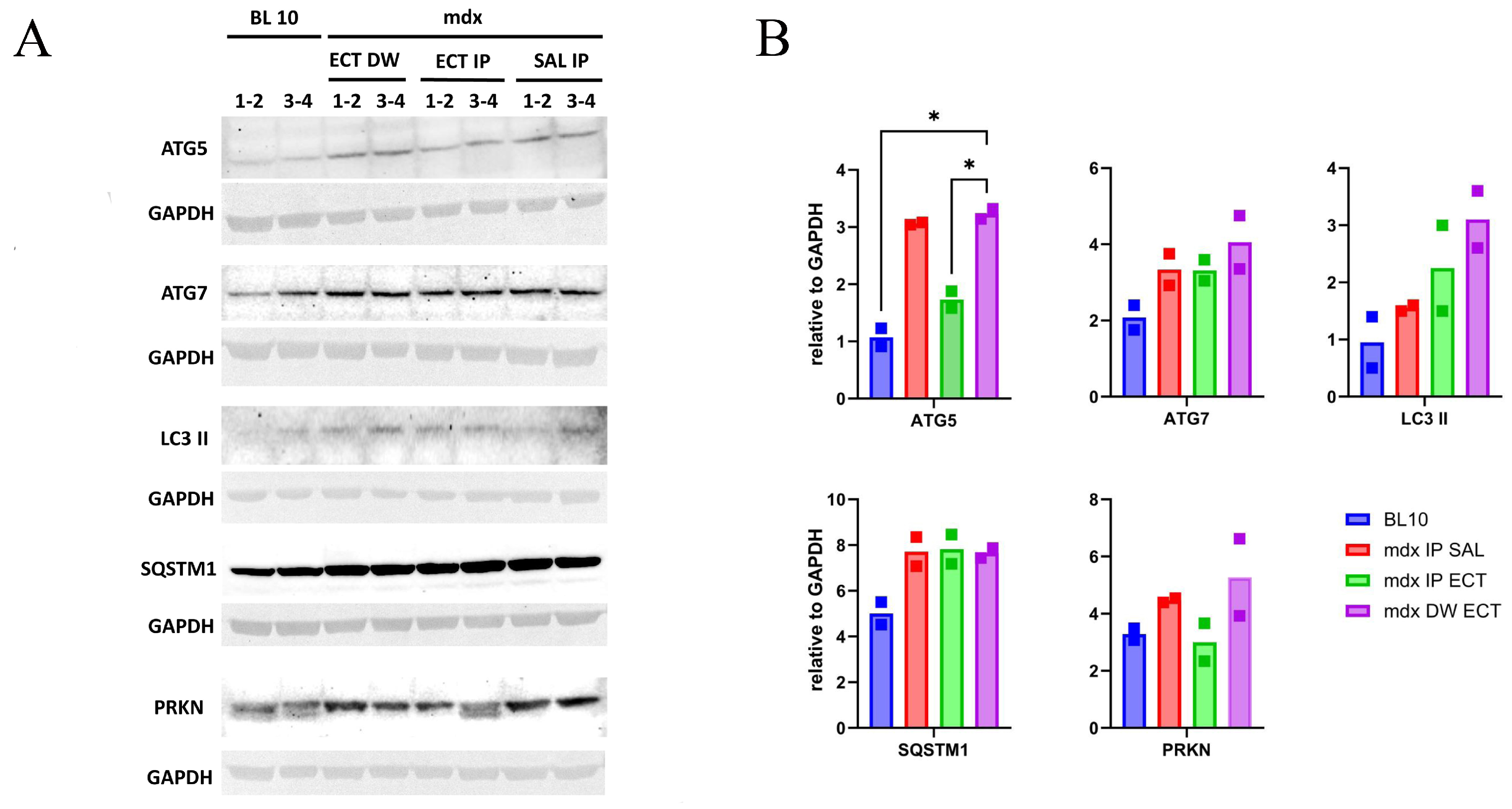
2.3. The Impact of Ectoine Treatment on the Levels of Autophagy-Related Proteins
2.4. The Impact of Ectoine on the Tissue Expression Patterns of Autophagy-Related Proteins
2.5. Impact of Ectoine Treatment on Autophagy-Related Protein Levels in Mitochondrial Fractions
3. Discussion
4. Materials and Methods
4.1. Animal Care, Treatment and Muscle Sampling
4.2. Protein Sample Preparation
4.3. Western Blotting
4.4. Statistical Analyses
4.5. Immunofluorescence Staining
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aartsma-Rus, A.; Ginjaar, I.B.; Bushby, K. The importance of genetic diagnosis for Duchenne muscular dystrophy. J. Med. Genet. 2016, 53, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Andrews, J.G.; Wahl, R.A. Duchenne and Becker muscular dystrophy in adolescents: Current perspectives. Adolesc. Health Med. Ther. 2018, 9, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Suthar, R.; Sankhyan, N. Duchenne Muscular Dystrophy: A Practice Update. Indian J. Pediatr. 2018, 85, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Constantin, B. Dystrophin complex functions as a scaffold for signalling proteins. Biomembranes 2014, 1838, 635–642. [Google Scholar] [CrossRef]
- Soblechero-Martín, P.; Albiasu-Arteta, E.; Anton-Martinez, A.; de la Puente-Ovejero, L.; Garcia-Jimenez, I.; González-Iglesias, G.; Larrañaga-Aiestaran, I.; López-Martínez, A.; Poyatos-García, J.; Ruiz-Del-Yerro, E.; et al. Duchenne muscular dystrophy cell culture models created by CRISPR/Cas9 gene editing and their application in drug screening. Sci. Rep. 2021, 11, 18188. [Google Scholar] [CrossRef] [PubMed]
- Grounds, M.D.; Terrill, J.R.; Al-Mshhdani, B.A.; Duong, M.N.; Radley-Crabb, H.G.; Arthur, P.G. Biomarkers for Duchenne muscular dystrophy: Myonecrosis, inflammation and oxidative stress. Dis. Model Mech. 2020, 13, dmm043638. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Li, H.; He, J.; Liang, J.; Liu, Y.; Zhang, W. TRIM72 Alleviates Muscle Inflammation in mdx Mice via Promoting Mitophagy-Mediated NLRP3 Inflammasome Inactivation. Oxid. Med. Cell. Longev. 2023, 2023, 8408574. [Google Scholar] [CrossRef]
- Van Putten, M.; Putker, K.; Overzier, M.; Adamzek, W.A.; Pasteuning-Vuhman, S.; Plomp, J.J.; Aartsma-Rus, A. Natural disease history of the D2-mdx mouse model for Duchenne muscular dystrophy. FASEB J. 2019, 33, 8110–8124. [Google Scholar] [CrossRef] [PubMed]
- Mucha, O.; Kaziród, K.; Podkalicka, P.; Rusin, K.; Dulak, J.; Łoboda, A. Dysregulated Autophagy and Mitophagy in a Mouse Model of Duchenne Muscular Dystrophy Remain Unchanged Following Heme Oxygenase-1 Knockout. Int. J. Mol. Sci. 2021, 23, 470. [Google Scholar] [CrossRef]
- Frudd, K.; Burgoyne, T.; Burgoyne, J.R. Oxidation of Atg3 and Atg7 mediates inhibition of autophagy. Nat. Commun. 2018, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Collier, J.J.; Suomi, F.; Oláhová, M.; McWilliams, T.G.; Taylor, R.W. Emerging roles of ATG7 in human health and disease. EMBO Mol. Med. 2021, 13, e14824. [Google Scholar] [CrossRef] [PubMed]
- Gammoh, N. The multifaceted functions of ATG16L1 in autophagy and related processes. J. Cell Sci. 2020, 133, jcs249227. [Google Scholar] [CrossRef]
- McWilliams, T.G.; Barini, E.; Pohjolan-Pirhonen, R.; Brooks, S.P.; Singh, F.; Burel, S.; Balk, K.; Kumar, A.; Montava-Garriga, L.; Prescott, A.R.; et al. Phosphorylation of Parkin at serine 65 is essential for its activation in vivo. Open Biol. 2018, 8, 180108. [Google Scholar] [CrossRef] [PubMed]
- Sebori, R.; Kuno, A.; Hosoda, R.; Hayashi, T.; Horio, Y. Resveratrol Decreases Oxidative Stress by Restoring Mitophagy and Improves the Pathophysiology of Dystrophin-Deficient mdx Mice. Oxid. Med. Cell. Longev. 2018, 2018, 9179270. [Google Scholar] [CrossRef] [PubMed]
- McGreevy, J.W.; Hakim, C.H.; McIntosh, M.A.; Duan, D. Animal models of Duchenne muscular dystrophy: From basic mechanisms to gene therapy. Dis. Model Mech. 2015, 8, 195–213. [Google Scholar] [CrossRef] [PubMed]
- Willmann, R.; Possekel, S.; Dubach-Powell, J.; Meier, T.; Ruegg, M.A. Mammalian animal models for Duchenne muscular dystrophy. Neuromuscul. Disord. 2009, 19, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Wells, D.J. Tracking progress: An update on animal models for Duchenne muscular dystrophy. Dis. Model Mech. 2018, 11, dmm035774. [Google Scholar] [CrossRef] [PubMed]
- Grounds, M.D.; Radley, H.G.; Lynch, G.S.; Nagaraju, K.; De Luca, A. Towards developing standard operating procedures for pre-clinical testing in the mdx mouse model of Duchenne muscular dystrophy. Neurobiol. Dis. 2008, 31, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Panza, E.; Vellecco, V.; Iannotti, F.A.; Paris, D.; Manzo, O.L.; Smimmo, M.; Mitilini, N.; Boscaino, A.; de Dominicis, G.; Bucci, M.; et al. Duchenne’s muscular dystrophy involves a defective transsulfuration pathway activity. Redox Biol. 2021, 45, 102040. [Google Scholar] [CrossRef]
- Markati, T.; Oskoui, M.; Farrar, M.A.; Duong, T.; Goemans, N.; Servais, L. Emerging therapies for Duchenne muscular dystrophy. Lancet Neurol. 2022, 21, 814–829. [Google Scholar] [CrossRef] [PubMed]
- Reid, A.L.; Alexander, M.S. The Interplay of Mitophagy and Inflammation in Duchenne Muscular Dystrophy. Life 2021, 11, 648. [Google Scholar] [CrossRef] [PubMed]
- Menke, A.; Jockusch, H. Decreased osmotic stability of dystrophin-less muscle cells from the mdx mouse. Nature 1991, 349, 69–71. [Google Scholar] [CrossRef]
- Menke, A.; Jockusch, H. Extent of shock-induced membrane leakage in human and mouse myotubes depends on dystrophin. J. Cell Sci. 1995, 108, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Herbelet, S.; De Paepe, B.; De Bleecker, J.L. Abnormal NFAT5 Physiology in Duchenne Muscular Dystrophy Fibroblasts as a Putative Explanation for the Permanent Fibrosis Formation in Duchenne Muscular Dystrophy. Int. J. Mol. Sci. 2020, 21, 7888. [Google Scholar] [CrossRef] [PubMed]
- Merckx, C.; De Paepe, B. The Role of Taurine in Skeletal Muscle Functioning and Its Potential as a Supportive Treatment for Duchenne Muscular Dystrophy. Metabolites 2022, 12, 193. [Google Scholar] [CrossRef] [PubMed]
- Merckx, C.; Zschüntzsch, J.; Meyer, S.; Raedt, R.; Verschuere, H.; Schmidt, J.; De Paepe, B.; De Bleecker, J.L. Exploring the Therapeutic Potential of Ectoine in Duchenne Muscular Dystrophy: Comparison with Taurine, a Supplement with Known Beneficial Effects in the mdx Mouse. Int. J. Mol. Sci. 2022, 23, 9567. [Google Scholar] [CrossRef]
- Czech, L.; Hermann, L.; Stöveken, N.; Richter, A.A.; Höppner, A.; Smits, S.H.J.; Heider, J.; Bremer, E. Role of the Extremolytes Ectoine and Hydroxyectoine as Stress Protectants and Nutrients: Genetics, Phylogenomics, Biochemistry, and Structural Analysis. Genes 2018, 9, 177. [Google Scholar] [CrossRef] [PubMed]
- Bownik, A.; Stępniewska, Z. Ectoine as a promising protective agent in humans and animals. Arh. Hig. Rada. Toksikol. 2016, 67, 260–265. [Google Scholar] [CrossRef]
- Barbiera, A.; Sorrentino, S.; Lepore, E.; Carfì, A.; Sica, G.; Dobrowolny, G.; Scicchitano, B.M. Taurine Attenuates Catabolic Processes Related to the Onset of Sarcopenia. Int. J. Mol. Sci. 2020, 21, 8865. [Google Scholar] [CrossRef]
- Rouschop, K.M.; van den Beucken, T.; Dubois, L.; Niessen, H.; Bussink, J.; Savelkouls, K.; Keulers, T.; Mujcic, H.; Landuyt, W.; Voncken., J.W.; et al. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J. Clin. Investig. 2010, 120, 127–141. [Google Scholar] [CrossRef]
- Pyo, J.O.; Yoo, S.M.; Ahn, H.H.; Nah, J.; Hong, S.H.; Kam, T.I.; Jung, S.; Jung, Y.K. Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nat. Commun. 2013, 4, 2300. [Google Scholar] [CrossRef]
- Sin, J.; Andres, A.M.; Taylor, D.J.R.; Weston, T.; Hiraumi, Y.; Stotland, A.; Kim, B.J.; Huang, C.; Doran, K.S.; Gottlieb, R.A. Mitophagy is required for mitochondrial biogenesis and myogenic differentiation of C2C12 myoblasts. Autophagy 2016, 12, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Paolini, A.; Omairi, S.; Mitchell, R.; Vaughan, D.; Matsakas, A.; Vaiyapuri, S.; Ricketts, T.; Rubinsztein, D.C.; Patel, K. Attenuation of autophagy impacts on muscle fibre development, starvation induced stress and fibre regeneration following acute injury. Sci. Rep. 2018, 8, 9062. [Google Scholar] [CrossRef] [PubMed]
- Hamaoui, D.; Subtil, A. ATG16L1 functions in cell homeostasis beyond autophagy. FEBS J. 2022, 289, 1779–1800. [Google Scholar] [CrossRef]
- Rodriguez-Outeiriño, L.; Hernandez-Torres, F.; Ramirez de Acuña, F.; Rastrojo, A.; Creus, C.; Carvajal, A.; Salmeron, L.; Montolio, M.; Soblechero-Martin, P.; Arechavala-Gomeza, V.; et al. miR-106b is a novel target to promote muscle regeneration and restore satellite stem cell function in injured Duchenne dystrophic muscle. Mol. Ther. Nucleic Acids 2022, 29, 769–786. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Velasco, E.; Vallejo, D.; Esteban, F.J.; Doherty, C.; Hernández-Torres, F.; Franco, D.; Aránega, A.E. A Pitx2-MicroRNA Pathway Modulates Cell Proliferation in Myoblasts and Skeletal-Muscle Satellite Cells and Promotes Their Commitment to a Myogenic Cell Fate. Mol. Cell. Biol. 2015, 35, 2892–2909. [Google Scholar] [CrossRef]
- Aránega, A.E.; Lozano-Velasco, E.; Rodriguez-Outeiriño, L.; Ramírez de Acuña, F.; Franco, D.; Hernández-Torres, F. MiRNAs and Muscle Regeneration: Therapeutic Targets in Duchenne Muscular Dystrophy. Int. J. Mol. Sci. 2021, 22, 4236. [Google Scholar] [CrossRef]
- Zhai, Z.; Wu, F.; Chuang, A.Y.; Kwon, J.H. miR-106b fine tunes ATG16L1 expression and autophagic activity in intestinal epithelial HCT116 cells. Inflamm. Bowel Dis. 2013, 19, 2295–2301. [Google Scholar] [CrossRef] [PubMed]
- Cosemans, G.; Merckx, C.; De Bleecker, J.L.; De Paepe, B. Inducible Heat Shock Protein 70 Levels in Patients and the mdx Mouse Affirm Regulation during Skeletal Muscle Regeneration in Muscular Dystrophy. Front. Biosci. 2022, 14, 19. [Google Scholar] [CrossRef]
- Fernández-Fernández, M.R.; Valpuesta, J.M. Hsp70 chaperone: A master player in protein homeostasis. F1000Res 2018, 7, F1000 Faculty Rev-1497. [Google Scholar] [CrossRef]
- Leu, J.I.; Barnoud, T.; Zhang, G.; Tian, T.; Wei, Z.; Herlyn, M.; Murphy, M.E.; George, D.L. Inhibition of stress-inducible HSP70 impairs mitochondrial proteostasis and function. Oncotarget 2017, 8, 45656–45669. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.M.; Lin, A.J.; Strumwasser, A.R.; Cory, K.; Whitney, K.; Ho, T.; Ho, T.; Lee, J.L.; Rucker, D.H.; Nguyen, C.Q.; et al. Mitochondrial Dysfunction Is an Early Consequence of Partial or Complete Dystrophin Loss in mdx Mice. Front. Physiol. 2020, 11, 690. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.; Spaulding, H.R.; Quindry, T.S.; Hudson, M.B.; Quindry, J.C.; Selsby, J.T. Indices of Defective Autophagy in Whole Muscle and Lysosome Enriched Fractions From Aged D2-mdx Mice. Front. Physiol. 2021, 12, 691245. [Google Scholar] [CrossRef] [PubMed]
- Spaulding, H.R.; Ballmann, C.; Quindry, J.C.; Hudson, M.B.; Selsby, J.T. Autophagy in the heart is enhanced and independent of disease progression in mus musculus dystrophinopathy models. JRSM Cardiovasc. Dis. 2019, 8, 2048004019879581. [Google Scholar] [CrossRef] [PubMed]
- Kuno, A.; Hosoda, R.; Sebori, R.; Hayashi, T.; Sakuragi, H.; Tanabe, M.; Horio, Y. Resveratrol Ameliorates Mitophagy Disturbance and Improves Cardiac Pathophysiology of Dystrophin-deficient mdx Mice. Sci. Rep. 2018, 8, 15555. [Google Scholar] [CrossRef]
- Nakashima, R.; Hosoda, R.; Tatekoshi, Y.; Iwahara, N.; Saga, Y.; Kuno, A. Transcriptional dysregulation of autophagy in the muscle of a mouse model of Duchenne muscular dystrophy. Sci. Rep. 2024, 14, 1365. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Badr, M.A.; Kyrychenko, V.; Eskelinen, E.L.; Shirokova, N. Deficit in PINK1/PARKIN-mediated mitochondrial autophagy at late stages of dystrophic cardiomyopathy. Cardiovasc. Res. 2018, 114, 90–102. [Google Scholar] [CrossRef] [PubMed]
- De Palma, C.; Morisi, F.; Cheli, S.; Pambianco, S.; Cappello, V.; Vezzoli, M.; Rovere-Querini, P.; Moggio, M.; Ripolone, M.; Francolini, M.; et al. Autophagy as a new therapeutic target in Duchenne muscular dystrophy. Cell Death Dis. 2012, 3, e418. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Lei, S.; She, Y.; Shi, H.; Li, Y.; Zhou, X.; Chen, R. Running improves muscle mass by activating autophagic flux and inhibiting ubiquitination degradation in mdx mice. Gene 2024, 899, 148136. [Google Scholar] [CrossRef] [PubMed]
- Mucha, O.; Myszka, M.; Podkalicka, P.; Świderska, B.; Malinowska, A.; Dulak, J.; Łoboda, A. Proteome Profiling of the Dystrophic mdx Mice Diaphragm. Biomolecules 2023, 13, 1648. [Google Scholar] [CrossRef]
- Spaulding, H.R.; Kelly, E.M.; Quindry, J.C.; Sheffield, J.B.; Hudson, M.B.; Selsby, J.T. Autophagic dysfunction and autophagosome escape in the mdx mus musculus model of Duchenne muscular dystrophy. Acta Physiol. 2018, 222, e12944. [Google Scholar] [CrossRef] [PubMed]
- Herhaus, L. TBK1 (TANK-binding kinase 1)-mediated regulation of autophagy in health and disease. Matrix Biol. 2021, 100, 84–98. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.M.; Ordureau, A.; Paulo, J.A.; Rinehart, J.; Harper, J.W. The PINK1-PARKIN Mitochondrial Ubiquitylation Pathway Drives a Program of OPTN/NDP52 Recruitment and TBK1 Activation to Promote Mitophagy. Mol. Cell 2015, 60, 7–20. [Google Scholar] [CrossRef]
- Hu, L.; Xie, H.; Liu, X.; Potjewyd, F.; James, L.I.; Wilkerson, E.M.; Herring, L.E.; Xie, L.; Chen, X.; Cabrera, J.C.; et al. TBK1 Is a Synthetic Lethal Target in Cancer with VHL Loss. Cancer Discov. 2020, 10, 460–475. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, L.; Zhang, S.Y.; Casanova, J.L.; Sancho-Shimizu, V. Human TBK1: A Gatekeeper of Neuroinflammation. Trends Mol. Med. 2016, 22, 511–527. [Google Scholar] [CrossRef] [PubMed]
- Pizon, V.; Rybina, S.; Gerbal, F.; Delort, F.; Vicart, P.; Baldacci, G.; Karsenti, E. MURF2B, a novel LC3-binding protein, participates with MURF2A in the switch between autophagy and ubiquitin proteasome system during differentiation of C2C12 muscle cells. PLoS ONE 2013, 8, e76140. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdel-Aziz, A.K.; Abdelfatah, S.; Abdellatif, M.; Abdoli, A.; Abel, S.; Abeliovich, H.; Abildgaard, M.H.; Abudu, Y.P.; Acevedo-Arozena, A.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition)1. Autophagy 2021, 17, 1–382. [Google Scholar] [CrossRef] [PubMed]
- Heras-Sandoval, D.; Pérez-Rojas, J.M.; Hernández-Damián, J.; Pedraza-Chaverri, J. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell. Signal. 2014, 26, 2694–2701. [Google Scholar] [CrossRef]
- Kim, M.H.; Kay, D.I.; Rudra, R.T.; Chen, B.; Hsu, N.; Izumiya, Y.; Martinez, L.; Spencer, M.J.; Walsh, K.; Grinnell, A.D.; et al. Myogenic Akt signaling attenuates muscular degeneration, promotes myofiber regeneration and improves muscle function in dystrophin-deficient mdx mice. Hum. Molec. Genet. 2011, 20, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Abu Shelbayeh, O.; Arroum, T.; Morris, S.; Busch, K.B. PGC-1α Is a Master Regulator of Mitochondrial Lifecycle and ROS Stress Response. Antioxidants 2023, 12, 1075. [Google Scholar] [CrossRef] [PubMed]
- Selsby, J.T.; Morine, K.J.; Pendrak, K.; Barton, E.R.; Sweeney, H.L. Rescue of dystrophic skeletal muscle by PGC-1α involves a fast to slow fiber type shift in the mdx mouse. PLoS ONE 2012, 7, e30063. [Google Scholar] [CrossRef]
- Dimauro, I.; Pearson, T.; Caporossi, D.; Jackson, M.J. A simple protocol for the subcellular fractionation of skeletal muscle cells and tissue. BMC Res. Notes 2012, 5, 513. [Google Scholar] [CrossRef]
- Sun, Y.; Vashisht, A.A.; Tchieu, J.; Wohlschlegel, J.A.; Dreier, L. Voltage-dependent Anion Channels (VDACs) Recruit Parkin to Defective Mitochondria to Promote Mitochondrial Autophagy. J. Biol. Chem. 2012, 287, 40652–40660. [Google Scholar] [CrossRef]
- Castets, P.; Frank, S.; Sinnreich, M.; Rüegg, M.A. “Get the Balance Right”: Pathological Significance of Autophagy Perturbation in Neuromuscular Disorders. J. Neuromuscul. Dis. 2016, 3, 127–155. [Google Scholar] [CrossRef]
- Grumati, P.; Coletto, L.; Sabatelli, P.; Cescon, M.; Angelin, A.; Bertaggia, E.; Blaauw, B.; Urciuolo, A.; Tiepolo, T.; Merlini, L.; et al. Autophagy is defective in collagen VI muscular dystrophies, and its reactivation rescues myofiber degeneration. Nat. Med. 2010, 16, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Fanin, M.; Nascimbeni, A.C.; Angelini, C. Muscle atrophy, ubiquitin-proteasome, and autophagic pathways in dysferlinopathy. Muscle Nerve 2014, 50, 340–347. [Google Scholar] [CrossRef] [PubMed]




| Marker | Muscle | Age | Regulation | Reference |
|---|---|---|---|---|
| LC3 I | quadriceps | 11 weeks | mdx > BL 10 | [42] |
| gastrocnemius | 11 months | D2-mdx > BL 10 | [43] | |
| diaphragm | 11 months | D2-mdx > BL 10 | [43] | |
| heart | 7 weeks | mdx ≅ BL 10 | [44] | |
| heart | 5.5 months | mdx > BL 10 | [45] | |
| heart | 14 months | mdx ≅ BL 10 | [44] | |
| heart | 17 months | mdx ≅ BL 10 | [44] | |
| LC3 II | quadriceps | 11 weeks | mdx > BL 10 | [42] |
| tibialis anterior | 5.5 months | mdx > BL 10 | [46] | |
| extensor digitorum longus | 5.5 weeks | mdx* ≅ BL 10 | this study | |
| gastrocnemius | 5.5 weeks | mdx* ≅BL 10 | this study | |
| gastrocnemius | 11 months | D2-mdx ≅ BL 10 | [43] | |
| diaphragm | 11 months | D2-mdx ≅ BL 10 | [43] | |
| heart | 7 weeks | mdx ≅ BL 10 | [44] | |
| heart | 5.5 months | mdx > BL 10 | [45] | |
| heart | 12 months | mdx > BL 10 | [47] | |
| heart | 14 months | mdx < BL 10 | [44] | |
| heart | 17 months | mdx < BL 10 | [44] | |
| LC3 II/I | quadriceps | 11 weeks | mdx ≅ BL 10 | [42] |
| quadriceps | 5.5 months | mdx > BL 10 | [14] | |
| tibialis anterior | 4 months | mdx > BL 10 | [48] | |
| extensor digitorum longus | 6.5 months | mdx > BL 10 | this study | |
| gastrocnemius | 11 months | D2-mdx ≅ BL 10 | [43] | |
| soleus | 3.5 months | mdx < BL 10 | [49] | |
| diaphragm | 6 weeks | mdx > BL 10 | [50] | |
| diaphragm | 3 months | mdx > BL 10 | [50] | |
| diaphragm | 4 months | mdx > BL 10 | [48] | |
| diaphragm | 11 months | D2-mdx ≅ BL 10 | [43] | |
| heart | 14 months | mdx ≅ BL 10 | [44] | |
| SQSTM1 | quadriceps | 11 weeks | mdx ≅ BL 10 | [42] |
| quadriceps | 5.5 months | mdx > BL 10 | [14] | |
| tibialis anterior | 4 months | mdx > BL 10 | [48] | |
| tibialis anterior | 5.5 months | mdx > BL 10 | [46] | |
| extensor digitorum longus | 5.5 weeks | mdx* ≅ BL 10 | this study | |
| gastrocnemius | 5.5 weeks | mdx* ≅ BL 10 | this study | |
| gastrocnemius | 11 months | D2-mdx < BL 10 | [43] | |
| soleus | 3.5 months | mdx > BL 10 | [49] | |
| diaphragm | 4 months | mdx > BL 10 | [48] | |
| diaphragm | 11 months | D2-mdx > BL 10 | [43] | |
| heart | 7 weeks | mdx ≅ BL 10 | [44] | |
| heart | 5.5 months | mdx > BL 10 | [45] | |
| heart | 12 months | mdx > BL 10 | [47] | |
| heart | 14 months | mdx ≅ BL 10 | [44] | |
| heart | 17 months | mdx ≅ BL 10 | [44] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez Armengol, E.; Merckx, C.; De Sutter, H.; De Bleecker, J.L.; De Paepe, B. Changes to the Autophagy-Related Muscle Proteome Following Short-Term Treatment with Ectoine in the Duchenne Muscular Dystrophy Mouse Model mdx. Int. J. Mol. Sci. 2025, 26, 439. https://doi.org/10.3390/ijms26020439
Gómez Armengol E, Merckx C, De Sutter H, De Bleecker JL, De Paepe B. Changes to the Autophagy-Related Muscle Proteome Following Short-Term Treatment with Ectoine in the Duchenne Muscular Dystrophy Mouse Model mdx. International Journal of Molecular Sciences. 2025; 26(2):439. https://doi.org/10.3390/ijms26020439
Chicago/Turabian StyleGómez Armengol, Eulàlia, Caroline Merckx, Hanne De Sutter, Jan L. De Bleecker, and Boel De Paepe. 2025. "Changes to the Autophagy-Related Muscle Proteome Following Short-Term Treatment with Ectoine in the Duchenne Muscular Dystrophy Mouse Model mdx" International Journal of Molecular Sciences 26, no. 2: 439. https://doi.org/10.3390/ijms26020439
APA StyleGómez Armengol, E., Merckx, C., De Sutter, H., De Bleecker, J. L., & De Paepe, B. (2025). Changes to the Autophagy-Related Muscle Proteome Following Short-Term Treatment with Ectoine in the Duchenne Muscular Dystrophy Mouse Model mdx. International Journal of Molecular Sciences, 26(2), 439. https://doi.org/10.3390/ijms26020439








