Extended Photoperiod Facilitated the Restoration of the Expression of GH-IGF Axis Genes in Submerged Rainbow Trout (Oncorhynchus mykiss)
Abstract
1. Introduction
2. Results
2.1. Comparative Analysis of Growth Parameters of Rainbow Trout in Various Environments
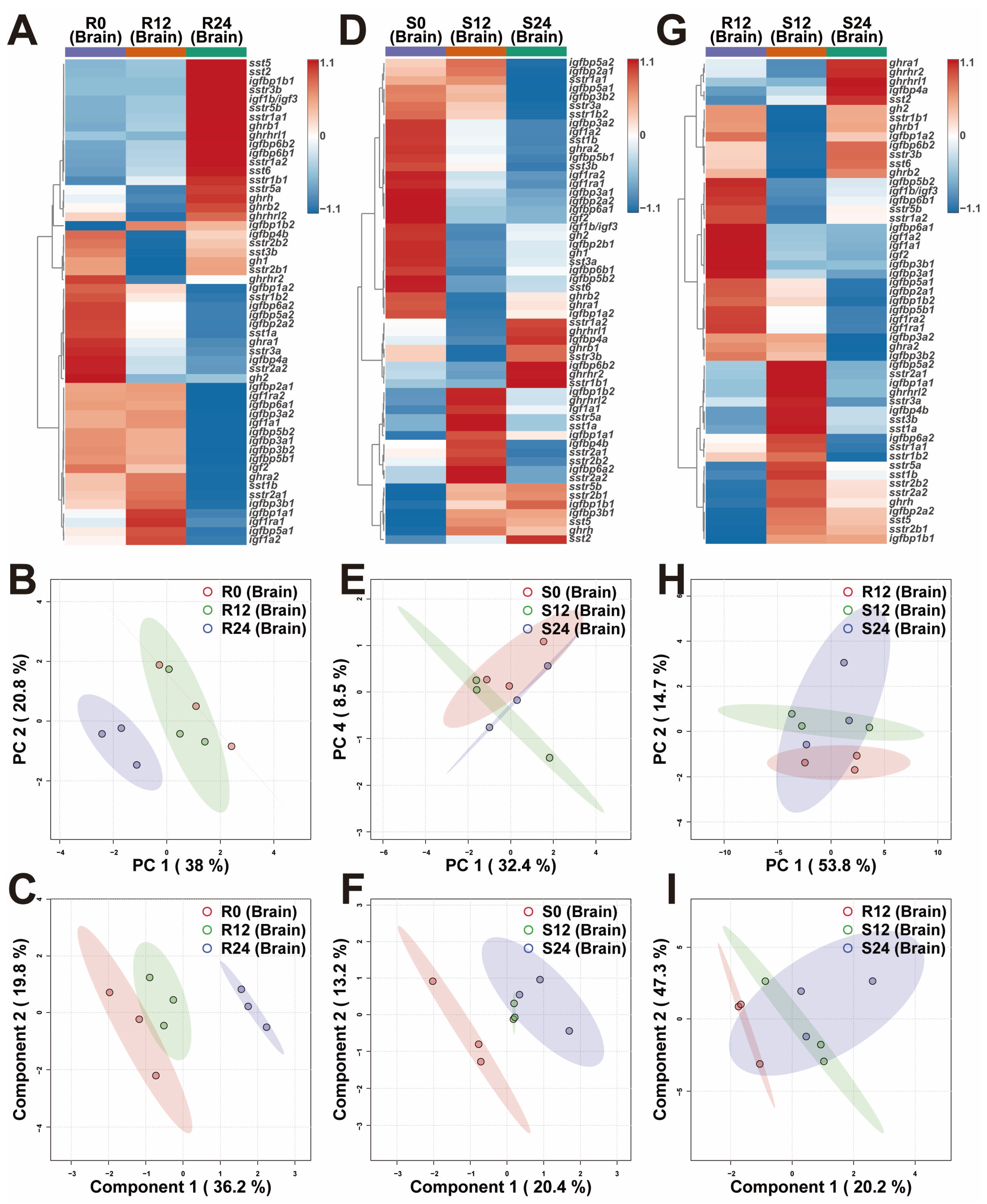
2.2. Transcriptional Differences of Brain GH-IGF Axis Genes in Various Environments
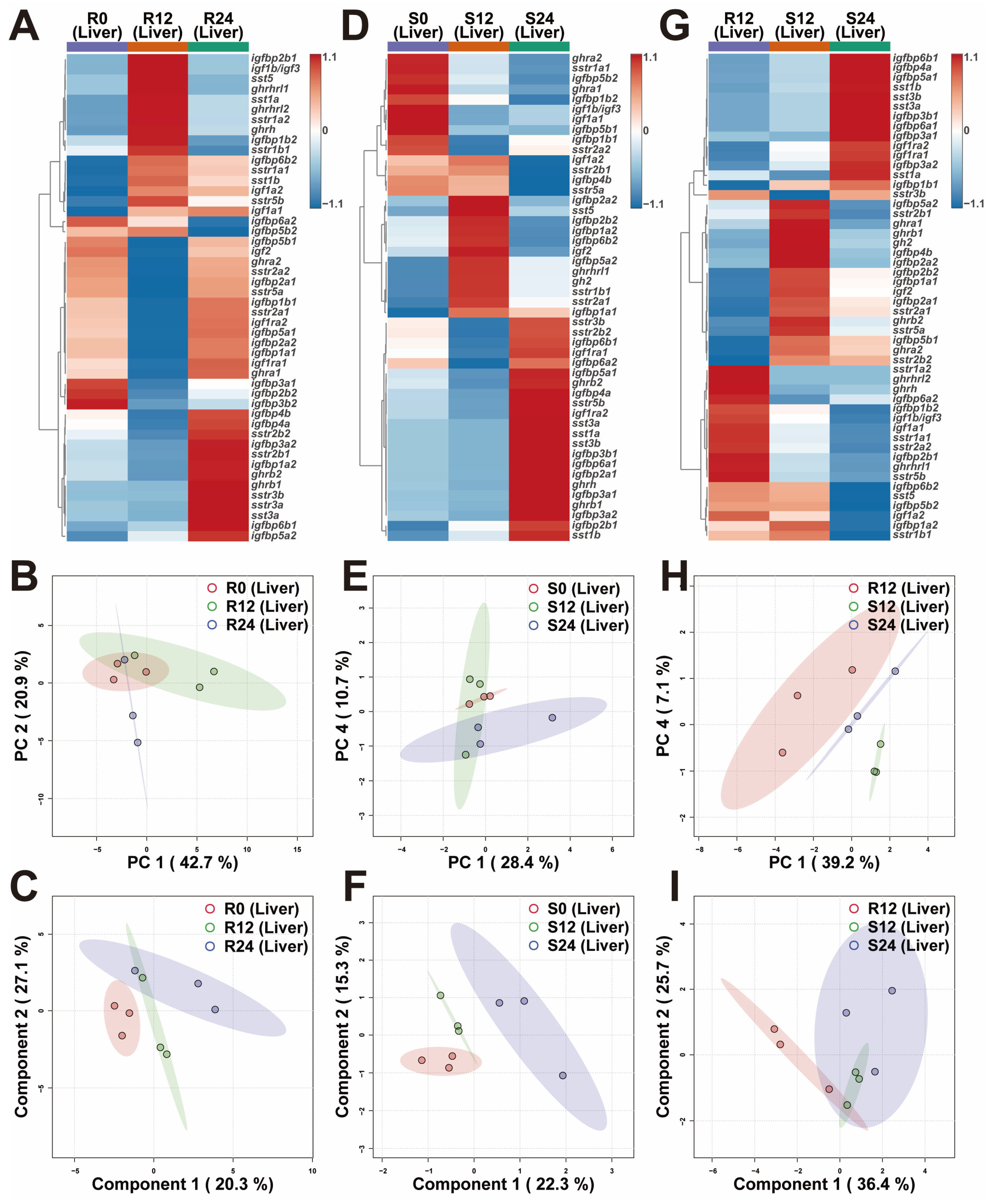
2.3. Transcriptional Differences of Liver GH-IGF Axis Genes in Various Environments
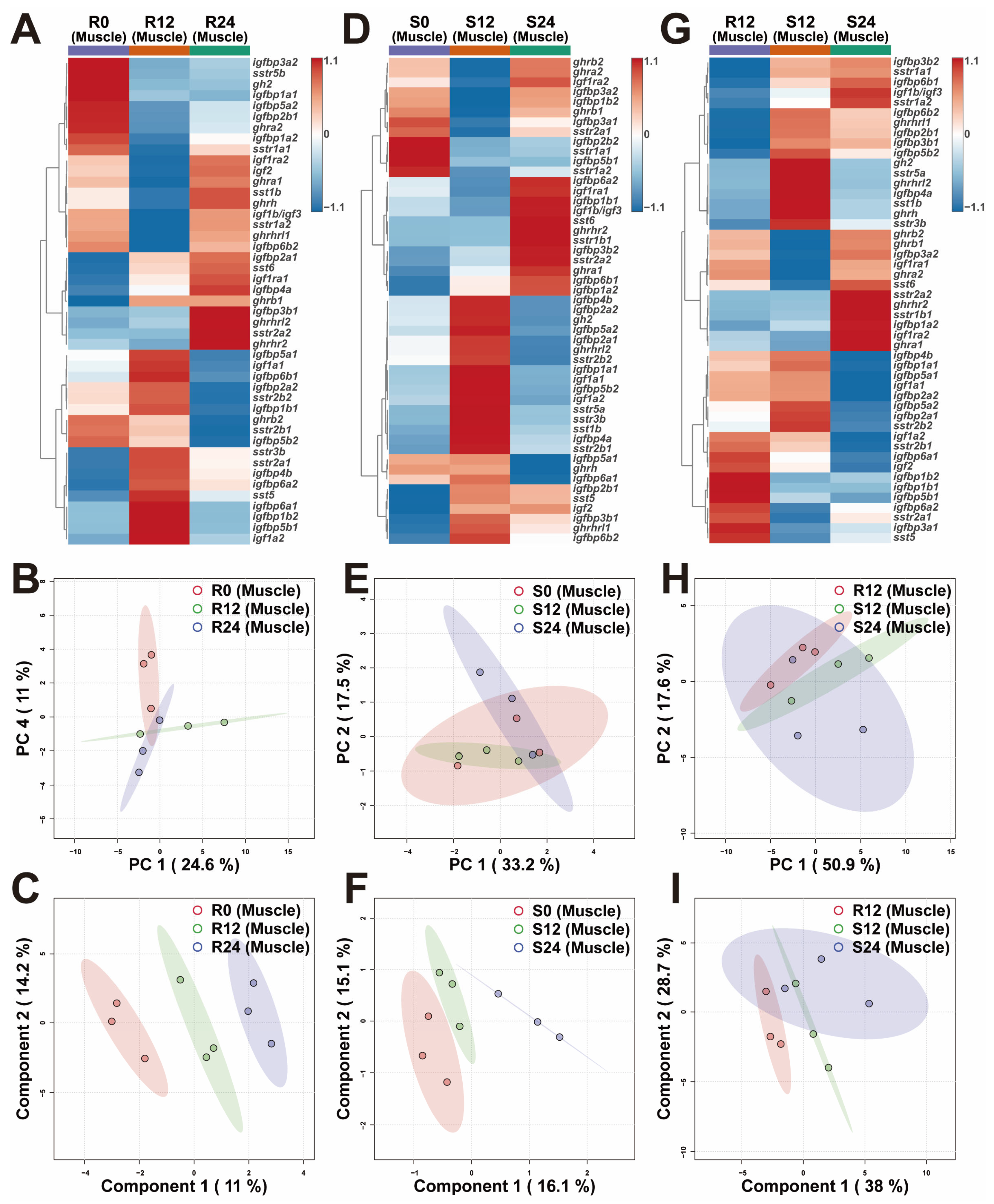
2.4. Transcriptional Differences of Muscle GH-IGF Axis Genes in Various Environments
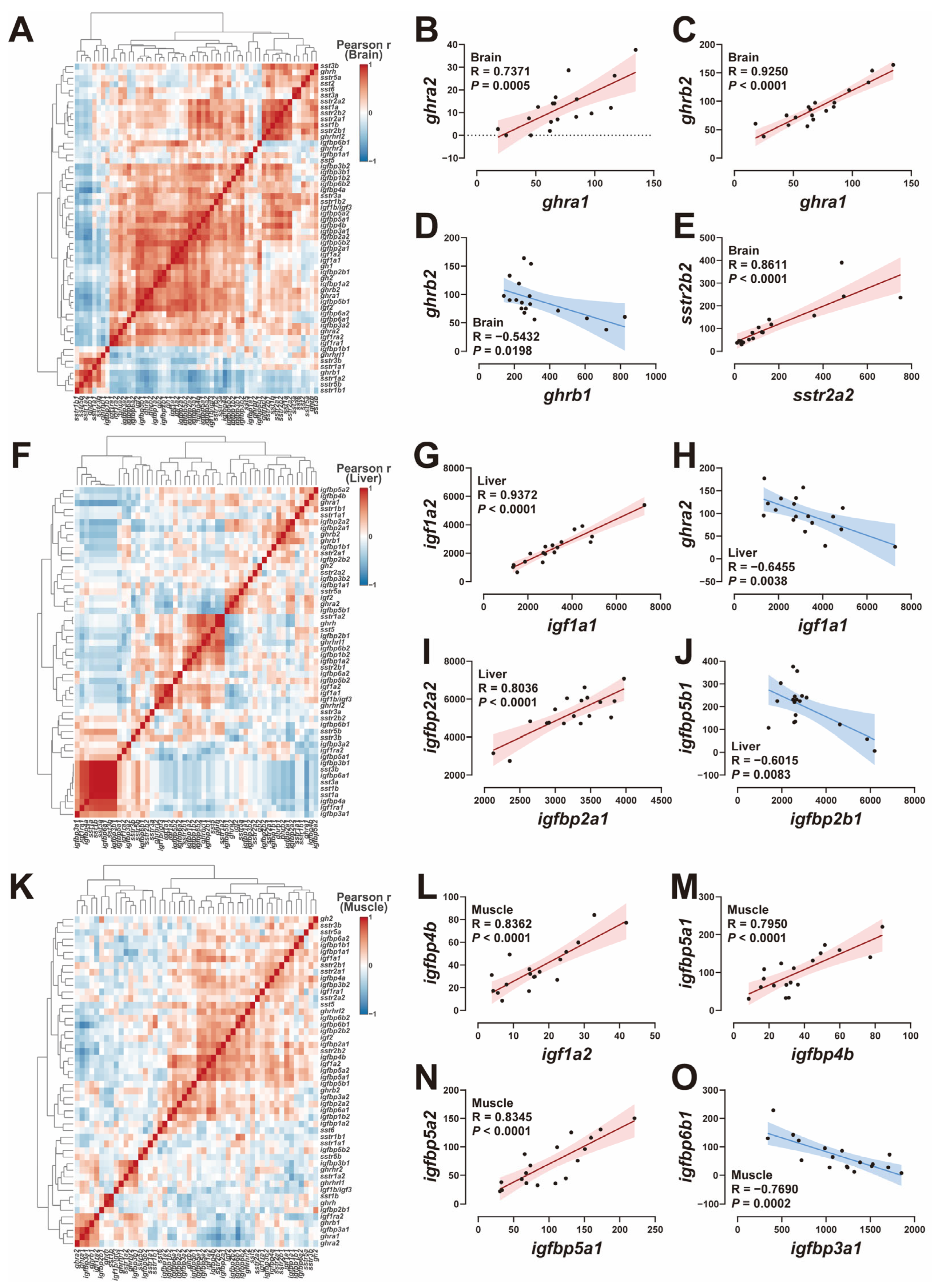
2.5. Correlation Analysis of GH-IGF Axis Genes
3. Discussion
3.1. Extended Photoperiod Partially Mitigated the Adverse Effects of Long-Term Submergence on Rainbow Trout Growth
3.2. In Response to the Variations in Photoperiod, the GH-IGF Axis Genes Exhibited Significant Transcriptional Differences
3.3. Extended Photoperiod Facilitated the Restoration of the Expression of GH-IGF Axis Genes in Submerged Rainbow Trout Towards Routine Levels
3.4. The Correlation of GH-IGF Axis Genes
4. Materials and Methods
4.1. Ethics Statement
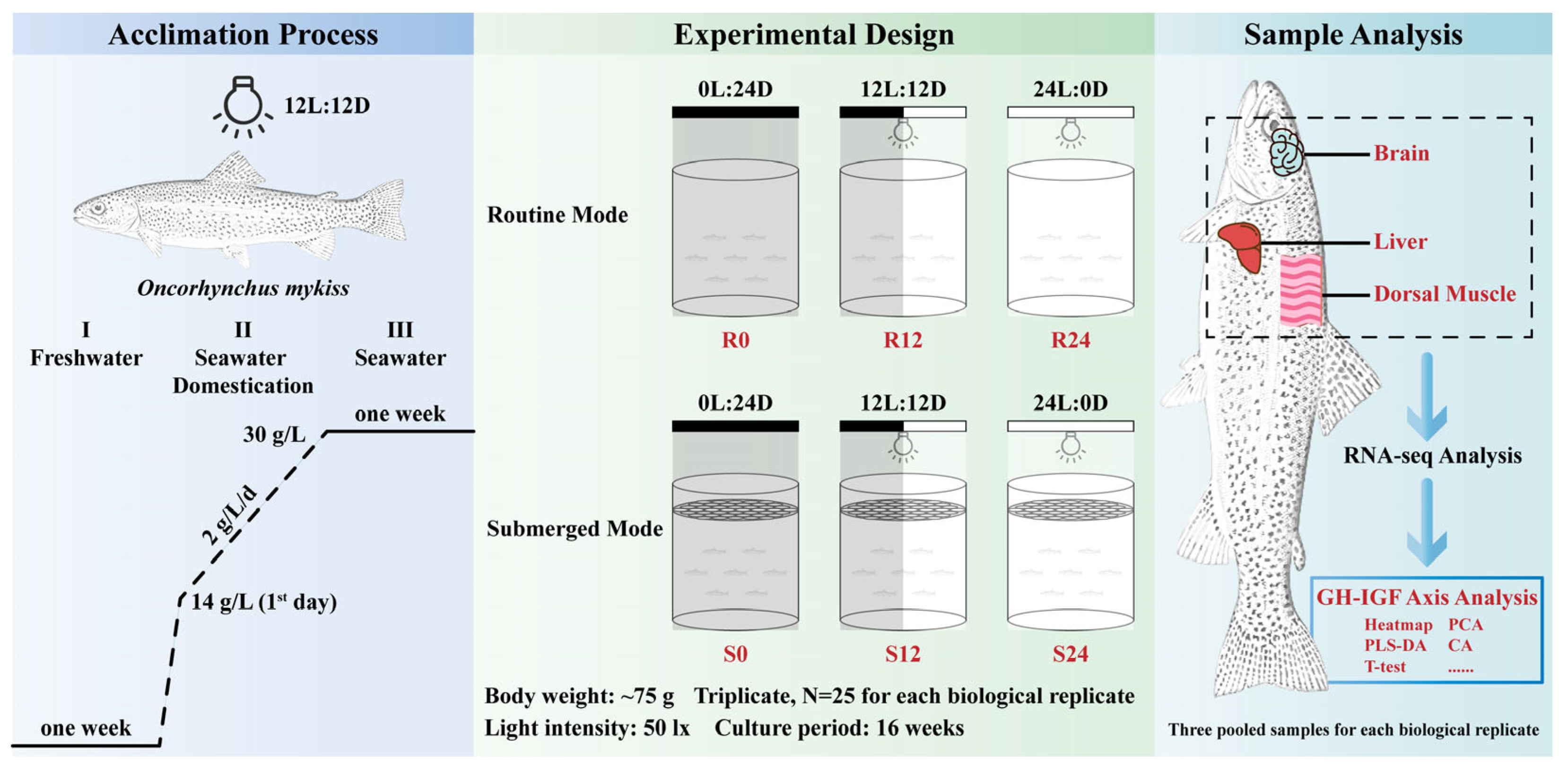
4.2. Fish Stock and Acclimation
- (I)
- They were initially reared in a freshwater system for 1 week to acclimatize to a new environment.
- (II)
- Next, the trout underwent a process of seawater domestication, where the freshwater in the rearing system was gradually replaced with deep-well seawater, which had a salinity of approximately 30 g/L. Specifically, on the first day of this process, a substantial amount of seawater was introduced into the rearing system, immediately raising the salinity to 14 g/L. Thereafter, seawater was incrementally added, increasing the rearing water’s salinity by 2 g/L each day, until the rearing system was completely filled with seawater.
- (III)
- Finally, the trout were retained in seawater for a further week to ensure complete adjustment.
4.3. Experimental Design and Sample Collection
4.4. Calculation of Growth Parameters
4.5. RNA-Seq Analysis
4.6. qPCR Validation of the Transcriptome
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mills, D.; Crawford, L.M. Salmonids. In Encyclopedia of Ocean Sciences, 3rd ed.; Cochran, J.K., Bokuniewicz, H.J., Yager, P.L., Eds.; Academic Press: Oxford, UK, 2019; Volume 2, pp. 297–306. [Google Scholar] [CrossRef]
- Moxness Reksten, A.; Ho, Q.T.; Nøstbakken, O.J.; Wik Markhus, M.; Kjellevold, M.; Bøkevoll, A.; Hannisdal, R.; Frøyland, L.; Madsen, L.; Dahl, L. Temporal variations in the nutrient content of Norwegian farmed Atlantic salmon (Salmo salar), 2005–2020. Food Chem. 2022, 373, 131445. [Google Scholar] [CrossRef] [PubMed]
- Sprague, M.; Dick, J.R.; Tocher, D.R. Impact of sustainable feeds on omega-3 long-chain fatty acid levels in farmed Atlantic salmon, 2006–2015. Sci. Rep. 2016, 6, 21892. [Google Scholar] [CrossRef] [PubMed]
- Jensen, I.J.; Mæhre, H.K.; Tømmerås, S.; Eilertsen, K.E.; Olsen, R.L.; Elvevoll, E.O. Farmed Atlantic salmon (Salmo salar L.) is a good source of long chain omega-3 fatty acids. Nutr. Bull. 2012, 37, 25–29. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef]
- Aas, T.S.; Åsgård, T.; Ytrestøyl, T. Chemical composition of whole body and fillet of slaughter sized Atlantic salmon (Salmo salar) and rainbow trout (Oncorhynchus mykiss) farmed in Norway in 2020. Aquac. Rep. 2022, 25, 101252. [Google Scholar] [CrossRef]
- Pilon, G.; Ruzzin, J.; Rioux, L.-E.; Lavigne, C.; White, P.J.; Frøyland, L.; Jacques, H.; Bryl, P.; Beaulieu, L.; Marette, A. Differential effects of various fish proteins in altering body weight, adiposity, inflammatory status, and insulin sensitivity in high-fat–fed rats. Metabolism 2011, 60, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Zhang, Y.Y.; Huang, X.H.; Xin, R.; Dong, X.P.; Konno, K.; Zhu, B.W.; Fisk, I.; Qin, L. Dynamic sensations of fresh and roasted salmon (Salmo salar) during chewing. Food Chem. 2022, 368, 130844. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture; FAO: Rome, Italy, 2022; Volume 3. [Google Scholar] [CrossRef]
- Abolofia, J.; Asche, F.; Wilen, J.E. The cost of lice: Quantifying the impacts of parasitic sea lice on farmed salmon. J. Agric. Econ. 2017, 32, 329–349. [Google Scholar] [CrossRef]
- Barker, S.E.; Bricknell, I.R.; Covello, J.; Purcell, S.; Fast, M.D.; Wolters, W.; Bouchard, D.A. Sea lice, Lepeophtheirus salmonis (Krøyer 1837), infected Atlantic salmon (Salmo salar L.) are more susceptible to infectious salmon anemia virus. PLoS ONE 2019, 14, e0209178. [Google Scholar] [CrossRef]
- Vargas-Chacoff, L.; Muñoz, J.L.P.; Saravia, J.; Oyarzún, R.; Pontigo, J.P.; González, M.P.; Mardones, O.; Hawes, C.; Pino, J.; Wadsworth, S.; et al. Neuroendocrine stress response in Atlantic salmon (Salmo salar) and Coho salmon (Oncorhynchus kisutch) during sea lice infestation. Aquaculture 2019, 507, 329–340. [Google Scholar] [CrossRef]
- Godwin, S.C.; Dill, L.M.; Krkošek, M.; Price, M.H.H.; Reynolds, J.D. Reduced growth in wild juvenile sockeye salmon Oncorhynchus nerka infected with sea lice. J. Fish Biol. 2017, 91, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Sogn-Grundvåg, G.; Tveterås, R. The impact of parasitic sea lice on harvest quantities and sizes of farmed salmon. Aquaculture 2023, 576, 739884. [Google Scholar] [CrossRef]
- Stene, A.; Bang Jensen, B.; Knutsen, Ø.; Olsen, A.; Viljugrein, H. Seasonal increase in sea temperature triggers pancreas disease outbreaks in Norwegian salmon farms. J. Fish Dis. 2014, 37, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Wade, N.M.; Clark, T.D.; Maynard, B.T.; Atherton, S.; Wilkinson, R.J.; Smullen, R.P.; Taylor, R.S. Effects of an unprecedented summer heatwave on the growth performance, flesh colour and plasma biochemistry of marine cage-farmed Atlantic salmon (Salmo salar). J. Therm. Biol. 2019, 80, 64–74. [Google Scholar] [CrossRef]
- van Walraven, N.; Fjørtoft, H.B.; Stene, A. Less is more: Negative relationship between biomass density and sea lice infestation in marine salmon farming. Aquaculture 2021, 539, 736602. [Google Scholar] [CrossRef]
- Oppedal, F.; Samsing, F.; Dempster, T.; Wright, D.W.; Bui, S.; Stien, L.H. Sea lice infestation levels decrease with deeper ‘snorkel’ barriers in Atlantic salmon sea-cages. Pest Manag. Sci. 2017, 73, 1935–1943. [Google Scholar] [CrossRef]
- Stien, L.H.; Dempster, T.; Bui, S.; Glaropoulos, A.; Fosseidengen, J.E.; Wright, D.W.; Oppedal, F. ‘Snorkel’ sea lice barrier technology reduces sea lice loads on harvest-sized Atlantic salmon with minimal welfare impacts. Aquaculture 2016, 458, 29–37. [Google Scholar] [CrossRef]
- Nilsen, A.; Nielsen, K.V.; Biering, E.; Bergheim, A. Effective protection against sea lice during the production of Atlantic salmon in floating enclosures. Aquaculture 2017, 466, 41–50. [Google Scholar] [CrossRef]
- Dempster, T.; Korsoen, O.; Folkedal, O.; Juell, J.-E.; Oppedal, F. Submergence of Atlantic salmon (Salmo salar L.) in commercial scale sea-cages: A potential short-term solution to poor surface conditions. Aquaculture 2009, 288, 254–263. [Google Scholar] [CrossRef]
- Glaropoulos, A.; Stien, L.H.; Folkedal, O.; Dempster, T.; Oppedal, F. Welfare, behaviour and feasibility of farming Atlantic salmon in submerged cages with weekly surface access to refill their swim bladders. Aquaculture 2019, 502, 332–337. [Google Scholar] [CrossRef]
- Macaulay, G.; Wright, D.; Oppedal, F.; Dempster, T. Buoyancy matters: Establishing the maximum neutral buoyancy depth of Atlantic salmon. Aquaculture 2020, 519, 734925. [Google Scholar] [CrossRef]
- Korsoen, O.J.; Dempster, T.; Fjelldal, P.G.; Oppedal, F.; Kristiansen, T.S. Long-term culture of Atlantic salmon (Salmo salar L.) in submerged cages during winter affects behaviour, growth and condition. Aquaculture 2009, 296, 373–381. [Google Scholar] [CrossRef]
- Sievers, M.; Korsoen, O.; Dempster, T.; Fjelldal, P.G.; Kristiansen, T.; Folkedal, O.; Oppedal, F. Growth and welfare of submerged Atlantic salmon under continuous lighting. Aquac. Environ. Interact. 2018, 10, 501–510. [Google Scholar] [CrossRef]
- Korsoen, O.J.; Dempster, T.; Oppedal, F.; Kristiansen, T.S. Individual variation in swimming depth and growth in Atlantic salmon (Salmo salar L.) subjected to submergence in sea-cages. Aquaculture 2012, 334, 142–151. [Google Scholar] [CrossRef]
- Ytrestøyl, T.; Hjelle, E.; Kolarevic, J.; Takle, H.; Rebl, A.; Afanasyev, S.; Krasnov, A.; Brunsvik, P.; Terjesen, B.F. Photoperiod in recirculation aquaculture systems and timing of seawater transfer affect seawater growth performance of Atlantic salmon (Salmo salar). J. World Aquacult. Soc. 2023, 54, 73–95. [Google Scholar] [CrossRef]
- Noori, A.; Mojazi Amiri, B.; Mirvaghefi, A.; Rafiee, G.; Kalvani Neitali, B. Enhanced growth and retarded gonadal development of farmed rainbow trout, Oncorhynchus mykiss (Walbaum) following a long-day photoperiod. Aquac. Res. 2015, 46, 2398–2406. [Google Scholar] [CrossRef]
- Taylor, J.F.; North, B.P.; Porter, M.J.R.; Bromage, N.R.; Migaud, H. Photoperiod can be used to enhance growth and improve feeding efficiency in farmed rainbow trout, Oncorhynchus mykiss. Aquaculture 2006, 256, 216–234. [Google Scholar] [CrossRef]
- Lundova, K.; Matousek, J.; Prokesova, M.; Vanina, T.; Sebesta, R.; Urban, J.; Stejskal, V. The effects of a prolonged photoperiod and light source on growth, sexual maturation, fin condition, and vulnerability to fungal disease in brook trout Salvelinus fontinalis. Aquac. Res. 2019, 50, 256–267. [Google Scholar] [CrossRef]
- Taylor, J.; Migaud, H. Timing and duration of constant light affects rainbow trout (Oncorhynchus mykiss) growth during autumn–spring grow-out in freshwater. Aquac. Res. 2009, 40, 1551–1558. [Google Scholar] [CrossRef]
- Handeland, S.O.; Porter, M.; Björnsson, B.T.; Stefansson, S.O. Osmoregulation and growth in a wild and a selected strain of Atlantic salmon smolts on two photoperiod regimes. Aquaculture 2003, 222, 29–43. [Google Scholar] [CrossRef]
- Oppedal, F.; Taranger, G.L.; Hansen, T. Growth performance and sexual maturation in diploid and triploid Atlantic salmon (Salmo salar L.) in seawater tanks exposed to continuous light or simulated natural photoperiod. Aquaculture 2003, 215, 145–162. [Google Scholar] [CrossRef]
- Triantaphyllopoulos, K.A.; Cartas, D.; Miliou, H. Factors influencing GH and IGF-I gene expression on growth in teleost fish: How can aquaculture industry benefit? Rev. Aquacult. 2020, 12, 1637–1662. [Google Scholar] [CrossRef]
- Chanddhini, S.; Trumboo, B.; Jose, S.; Varghese, T.; Rajesh, M.; Kumar, V.J.R. Insulin-like growth factor signalling and its significance as a biomarker in fish and shellfish research. Fish Physiol. Biochem. 2021, 47, 1011–1031. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Z.; Yan, Z.; Zhao, Z.; Zhang, C.; Gong, Q.; Du, X.; Wu, J.; Feng, Y.; Du, J.; et al. Improvement of skeletal muscle growth by GH/IGF growth-axis contributes to growth performance in commercial fleshy sturgeon. Aquaculture 2021, 543, 736929. [Google Scholar] [CrossRef]
- Iwata, M.; Kinoshita, K.; Moriyama, S.; Kurosawa, T.; Iguma, K.; Chiba, H.; Ojima, D.; Yoshinaga, T.; Arai, T. Chum salmon fry grow faster in seawater, exhibit greater activity of the GH/IGF axis, higher Na+, K+-ATPase activity, and greater gill chloride cell development. Aquaculture 2012, 362–363, 101–108. [Google Scholar] [CrossRef]
- Qian, Y.; Yan, A.; Lin, H.; Li, W. Molecular characterization of the GHRH/GHRH-R and its effect on GH synthesis and release in orange-spotted grouper (Epinephelus coioides). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2012, 163, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Yada, T.; Hirano, T. Inhibition of growth hormone synthesis by somatostatin in cultured pituitary of rainbow trout. J. Comp. Physiol. B 1992, 162, 575–580. [Google Scholar] [CrossRef]
- Muttray, A.F.; Donaldson, E.M.; Devlin, R.H. Effects of recombinant salmon type II growth hormone and bovine growth hormone on growth of coho salmon (Oncorhynchus kisutch). Fish Physiol. Biochem. 2019, 45, 1083–1090. [Google Scholar] [CrossRef]
- Pedroso, F.L.; Fukada, H.; Masumoto, T. In vivo and in vitro effect of recombinant salmon growth hormone treatment on IGF-I and IGFBPs in yellowtail Seriola quinqueradiata. Fisheries Sci. 2009, 75, 887–894. [Google Scholar] [CrossRef]
- Picha, M.E.; Turano, M.J.; Beckman, B.R.; Borski, R.J. Endocrine biomarkers of growth and applications to aquaculture: A minireview of growth hormone, insulin-like growth factor (IGF)-I, and IGF-binding proteins as potential growth indicators in fish. North Am. J. Aquacult. 2008, 70, 196–211. [Google Scholar] [CrossRef]
- Shimizu, M.; Dickhoff, W.W. Circulating insulin-like growth factor binding proteins in fish: Their identities and physiological regulation. Gen. Comp. Endocrinol. 2017, 252, 150–161. [Google Scholar] [CrossRef]
- Allard, J.B.; Duan, C. IGF-binding proteins: Why do they exist and why are there so many? Front. Endocrinol. 2018, 9, 117. [Google Scholar] [CrossRef]
- Wood, A.W.; Duan, C.; Bern, H.A. Insulin-like growth factor signaling in fish. In International Review of Cytology; Jeon, K.W., Ed.; Academic Press: Cambridge, MA, USA, 2005; Volume 243, pp. 215–285. [Google Scholar] [CrossRef]
- Fu, X.; Zou, Z.; Zhu, J.; Xiao, W.; Li, D.; Yu, J.; Chen, B.; Yang, H. Effects of different photoperiods on growth performance, daily rhythm of growth axis-related genes, and hormones in Nile tilapia (Oreochromis niloticus). Aquaculture 2022, 553, 738071. [Google Scholar] [CrossRef]
- Nordgarden, U.; Hansen, T.; Hemre, G.I.; Sundby, A.; Björnsson, T.B. Endocrine growth regulation of adult Atlantic salmon in seawater: The effects of light regime on plasma growth hormone, insulin-like growth factor-I, and insulin levels. Aquaculture 2005, 250, 862–871. [Google Scholar] [CrossRef]
- Petit, G.; Beauchaud, M.; Attia, J.; Buisson, B. Food intake and growth of largemouth bass (Micropterus salmoides) held under alternated light/dark cycle (12L:12D) or exposed to continuous light. Aquaculture 2003, 228, 397–401. [Google Scholar] [CrossRef]
- Wei, H.; Cai, W.; Liu, H.; Han, D.; Zhu, X.; Yang, Y.; Jin, J.; Xie, S. Effects of photoperiod on growth, lipid metabolism and oxidative stress of juvenile gibel carp (Carassius auratus). J. Photochem. Photobiol. B 2019, 198, 111552. [Google Scholar] [CrossRef] [PubMed]
- Litvak, M.K.; Zadmajid, V.; Butts, I.A.E. Growth and survival of winter flounder (Pseudopleuronectes americanus) larvae reared on different photoperiod regimes from hatch to metamorphosis. Aquacult. Res. 2020, 51, 2314–2321. [Google Scholar] [CrossRef]
- Aride, P.H.R.; Gomes, M.F.S.; Azevedo, D.G.; Sangali, G.R.; Silva, A.C.F.; Lavander, H.D.; Souza, A.B.; Polese, M.F.; Mattos, D.C.; Bassul, L.A.; et al. Dusky Grouper Epinephelus marginatus Growth and Survival When Exposed to Different Photoperiods. Fishes 2021, 6, 31. [Google Scholar] [CrossRef]
- Lundova, K.; Matousek, J.; Prokesova, M.; Sebesta, R.; Policar, T.; Stejskal, V. The effect of timing of extended photoperiod on growth and maturity of brook trout (Salvelinus fontinalis). Aquacult. Res. 2019, 50, 1697–1704. [Google Scholar] [CrossRef]
- Abdollahpour, H.; Falahatkar, B.; Lawrence, C. The effect of photoperiod on growth and spawning performance of zebrafish, Danio rerio. Aquacult. Rep. 2020, 17, 100295. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, D.; Li, X.; Jiang, G.; Liu, W. Photoperiod affects blunt snout bream (Megalobrama amblycephala) growth, diel rhythm of cortisol, activities of antioxidant enzymes and mRNA expression of GH/IGF-I. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2019, 233, 4–10. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, Q.; Huang, Y.; Xu, Q.; Dai, X.; Ju, C. Cloning and characterization of two types of growth hormone receptors in tomato clownfish (Amphiprion frenatus), and their expression under different light spectra and photoperiods. Aquacult. Int. 2022, 30, 483–500. [Google Scholar] [CrossRef]
- Very, N.M.; Knutson, D.; Kittilson, J.D.; Sheridan, M.A. Somatostatin inhibits growth of rainbow trout. J. Fish Biol. 2001, 59, 157–165. [Google Scholar] [CrossRef]
- Schultz, R.B.; Adur, M.K.; Li, Y.; Kiefer, Z.E.; Gabler, N.K.; Ross, J.W. Improved Growth Performance in the F1 Heterozygous Generation of an SSTR2 Knockout Model in Swine. J. Anim. Sci. 2021, 99, 36. [Google Scholar] [CrossRef]
- Ben-Shlomo, A.; Pichurin, O.; Khalafi, R.; Zhou, C.; Chesnokova, V.; Ren, S.; Liu, N.; Melmed, S. Constitutive Somatostatin Receptor Subtype 2 Activity Attenuates GH Synthesis. Endocrinology 2013, 154, 2399–2409. [Google Scholar] [CrossRef]
- Very, N.M.; Sheridan, M.A. Somatostatin regulates hepatic growth hormone sensitivity by internalizing growth hormone receptors and by decreasing transcription of growth hormone receptor mRNAs. Am. J. Physiol. Reg. I. Comp. Physiol. 2007, 292, R1956–R1962. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, M.A.; Hagemeister, A.L. Somatostatin and somatostatin receptors in fish growth. Gen. Comp. Endocrinol. 2010, 167, 360–365. [Google Scholar] [CrossRef]
- Fukada, H.; Ozaki, Y.; Pierce, A.L.; Adachi, S.; Yamauchi, K.; Hara, A.; Swanson, P.; Dickhoff, W.W. Salmon growth hormone receptor: Molecular cloning, ligand specificity, and response to fasting. Gen. Comp. Endocrinol. 2004, 139, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Nakano, T.; Afonso, L.O.B.; Beckman, B.R.; Iwama, G.K.; Devlin, R.H. Acute Physiological Stress Down-Regulates mRNA Expressions of Growth-Related Genes in Coho Salmon. PLoS ONE 2013, 8, e71421. [Google Scholar] [CrossRef]
- Xu, W.; Yang, Q.; Wang, Y.; Tang, R.; Li, D. The growth performance, antioxidative status and muscle quality of grass carp (Ctenopharyngodon idellus) cultured in the recirculating pond aquaculture system (RPAS). Aquaculture 2023, 562, 738829. [Google Scholar] [CrossRef]
- Naya-Català, F.; Simó-Mirabet, P.; Calduch-Giner, J.; Pérez-Sánchez, J. Transcriptomic profiling of Gh/Igf system reveals a prompted tissue-specific differentiation and novel hypoxia responsive genes in gilthead sea bream. Sci. Rep. 2021, 11, 16466. [Google Scholar] [CrossRef]
- Vélez, E.J.; Azizi, S.; Millán-Cubillo, A.; Fernández-Borràs, J.; Blasco, J.; Chan, S.J.; Calduch-Giner, J.A.; Pérez-Sánchez, J.; Navarro, I.; Capilla, E.; et al. Effects of sustained exercise on GH-IGFs axis in gilthead sea bream (Sparus aurata). Am. J. Physiol. Reg. I. Comp. Physiol. 2016, 310, R313–R322. [Google Scholar] [CrossRef] [PubMed]
- Saera-Vila, A.; Calduch-Giner, J.A.; Prunet, P.; Pérez-Sánchez, J. Dynamics of liver GH/IGF axis and selected stress markers in juvenile gilthead sea bream (Sparus aurata) exposed to acute confinement: Differential stress response of growth hormone receptors. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2009, 154, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Calduch-Giner, J.A.; Davey, G.; Saera-Vila, A.; Houeix, B.; Talbot, A.; Prunet, P.; Cairns, M.T.; Pérez-Sánchez, J. Use of microarray technology to assess the time course of liver stress response after confinement exposure in gilthead sea bream (Sparus aurata L.). BMC Genomics 2010, 11, 193. [Google Scholar] [CrossRef] [PubMed]
- Gabillard, J.-C.; Kamangar, B.B.; Montserrat, N. Coordinated regulation of the GH/IGF system genes during refeeding in rainbow trout (Oncorhynchus mykiss). J. Endocrinol. 2006, 191, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Saera-Vila, A.; Calduch-Giner, J.-A.; Pérez-Sánchez, J. Duplication of growth hormone receptor (GHR) in fish genome: Gene organization and transcriptional regulation of GHR type I and II in gilthead sea bream (Sparus aurata). Gen. Comp. Endocrinol. 2005, 142, 193–203. [Google Scholar] [CrossRef]
- Garcia de la Serrana, D.; Macqueen, D. Insulin-like growth factor-binding proteins of teleost fish. Front. Endocrinol. 2018, 9, 350039. [Google Scholar] [CrossRef]
- Macqueen, D.J.; Garcia de la Serrana, D.; Johnston, I.A. Evolution of ancient functions in the vertebrate insulin-like growth factor system uncovered by study of duplicated salmonid fish genomes. Mol. Biol. Evol. 2013, 30, 1060–1076. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Zhou, C.; Lin, S.; Chen, J.; Jiang, X.; Zou, S. Two grass carp (Ctenopharyngodon idella) insulin-like growth factor-binding protein 5 genes exhibit different yet conserved functions in development and growth. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2017, 204, 69–76. [Google Scholar] [CrossRef]
- Bower, N.I.; Johnston, I.A. Transcriptional regulation of the IGF signaling pathway by amino acids and insulin-like growth factors during myogenesis in Atlantic salmon. PLoS ONE 2010, 5, e11100. [Google Scholar] [CrossRef]
- Alzaid, A.; Kim, J.-H.; Devlin, R.H.; Martin, S.A.; Macqueen, D.J. Growth hormone transgenesis disrupts immune function in muscle of coho salmon (Oncorhynchus kisutch) impacting cross-talk with growth systems. bioRxiv 2017, 210104. [Google Scholar] [CrossRef]
- Wang, X.; Lu, L.; Li, Y.; Li, M.; Chen, C.; Feng, Q.; Zhang, C.; Duan, C. Molecular and functional characterization of two distinct IGF binding protein-6 genes in zebrafish. Am. J. Physiol. Reg. I. Comp. Physiol. 2009, 296, R1348–R1357. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinf. 2011, 12, 323. [Google Scholar] [CrossRef]
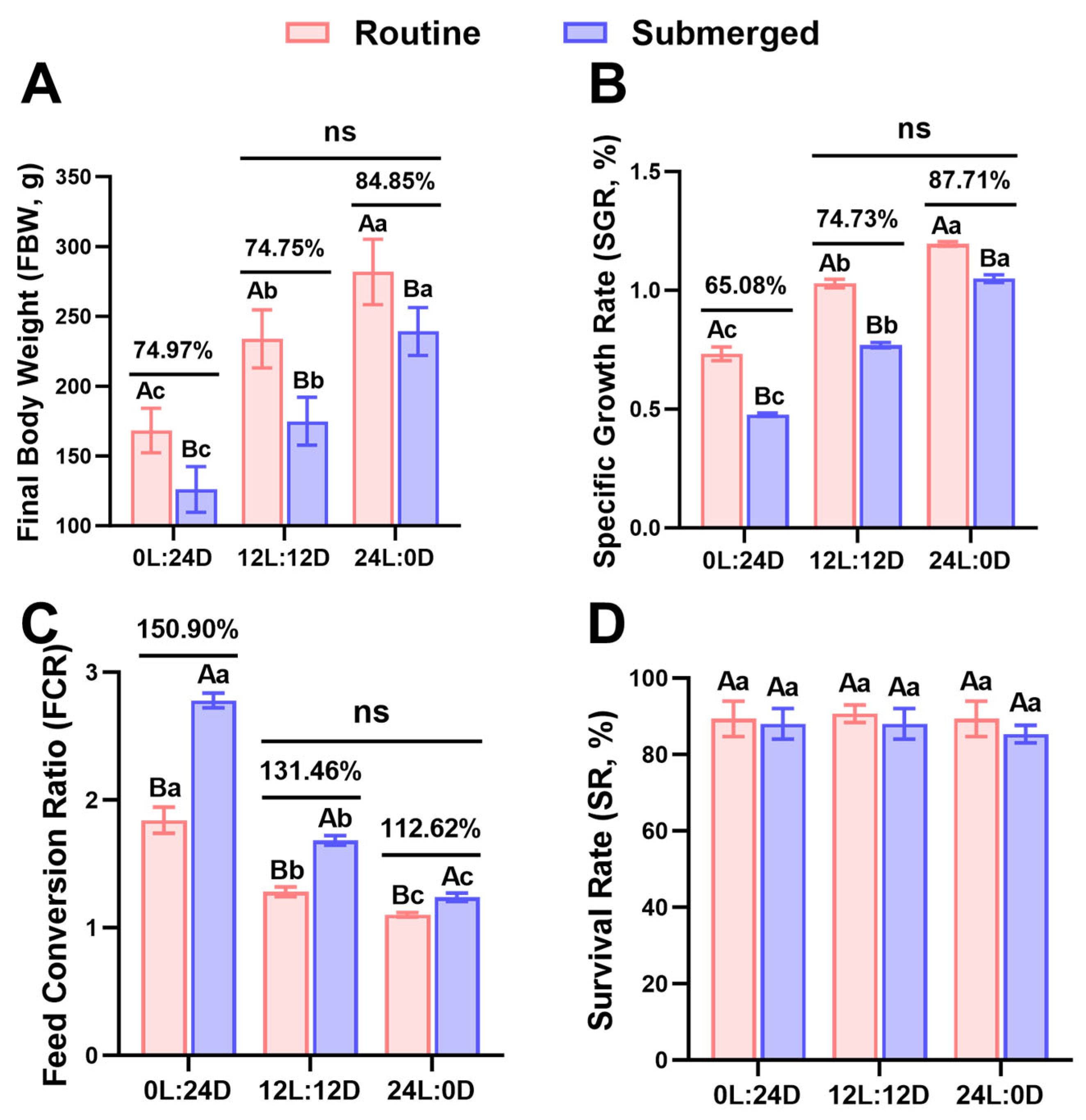
| Parameters | FBW (g) | SGR (%) | FCR | SR (%) |
|---|---|---|---|---|
| Photoperiod | 0.000 | 0.000 | 0.000 | 0.656 |
| Mode | 0.000 | 0.000 | 0.000 | 0.159 |
| Photoperiod × Mode | 0.009 | 0.000 | 0.000 | 0.831 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, K.; Hou, Z.; Li, Z.; Xu, Y.; Gao, Q. Extended Photoperiod Facilitated the Restoration of the Expression of GH-IGF Axis Genes in Submerged Rainbow Trout (Oncorhynchus mykiss). Int. J. Mol. Sci. 2024, 25, 13583. https://doi.org/10.3390/ijms252413583
Dong K, Hou Z, Li Z, Xu Y, Gao Q. Extended Photoperiod Facilitated the Restoration of the Expression of GH-IGF Axis Genes in Submerged Rainbow Trout (Oncorhynchus mykiss). International Journal of Molecular Sciences. 2024; 25(24):13583. https://doi.org/10.3390/ijms252413583
Chicago/Turabian StyleDong, Kang, Zhishuai Hou, Zhao Li, Yuling Xu, and Qinfeng Gao. 2024. "Extended Photoperiod Facilitated the Restoration of the Expression of GH-IGF Axis Genes in Submerged Rainbow Trout (Oncorhynchus mykiss)" International Journal of Molecular Sciences 25, no. 24: 13583. https://doi.org/10.3390/ijms252413583
APA StyleDong, K., Hou, Z., Li, Z., Xu, Y., & Gao, Q. (2024). Extended Photoperiod Facilitated the Restoration of the Expression of GH-IGF Axis Genes in Submerged Rainbow Trout (Oncorhynchus mykiss). International Journal of Molecular Sciences, 25(24), 13583. https://doi.org/10.3390/ijms252413583





