Synthesis of Novel Benzofuran Spiro-2-Pyrrolidine Derivatives via [3+2] Azomethine Ylide Cycloadditions and Their Antitumor Activity
Abstract
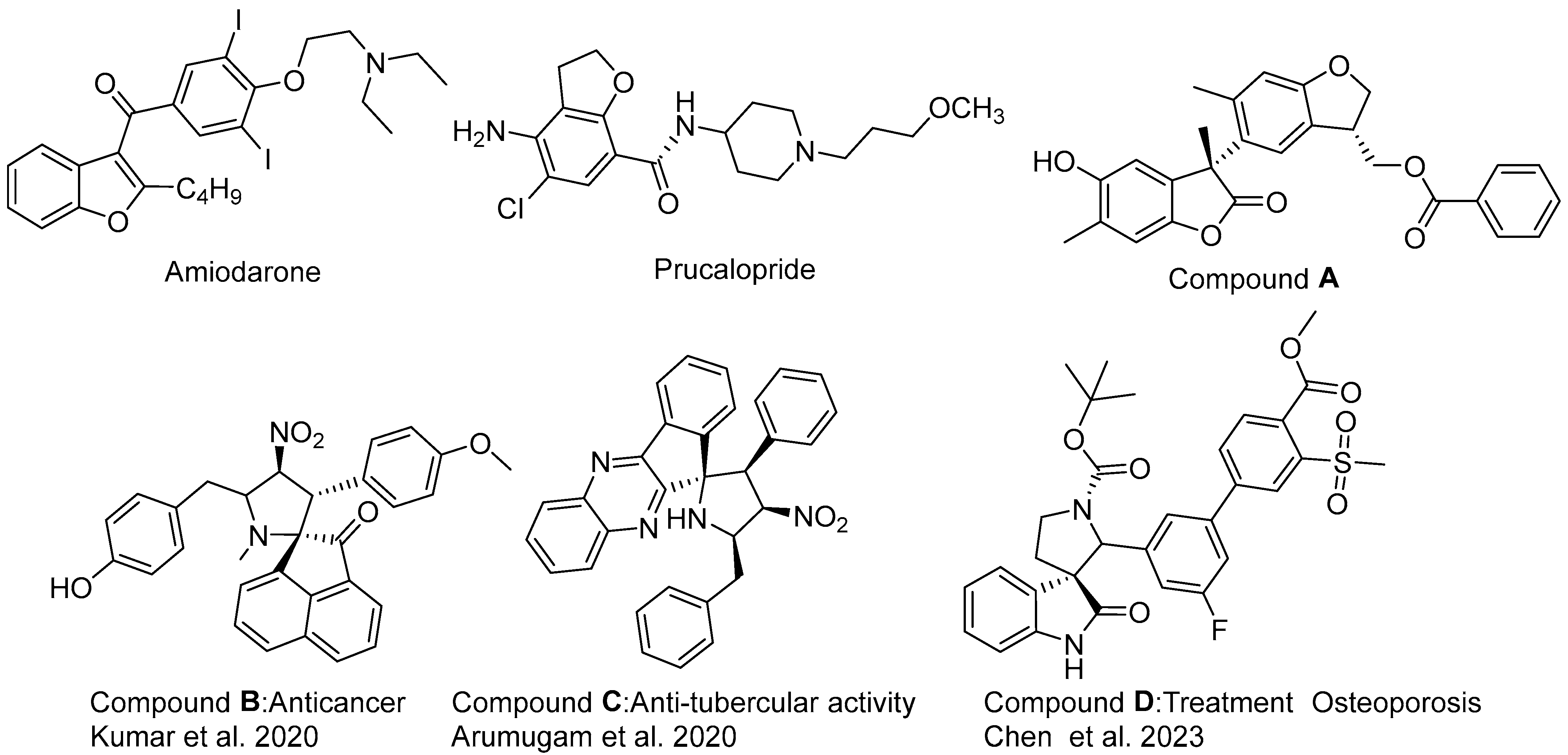
:1. Introduction
2. Results and Discussion
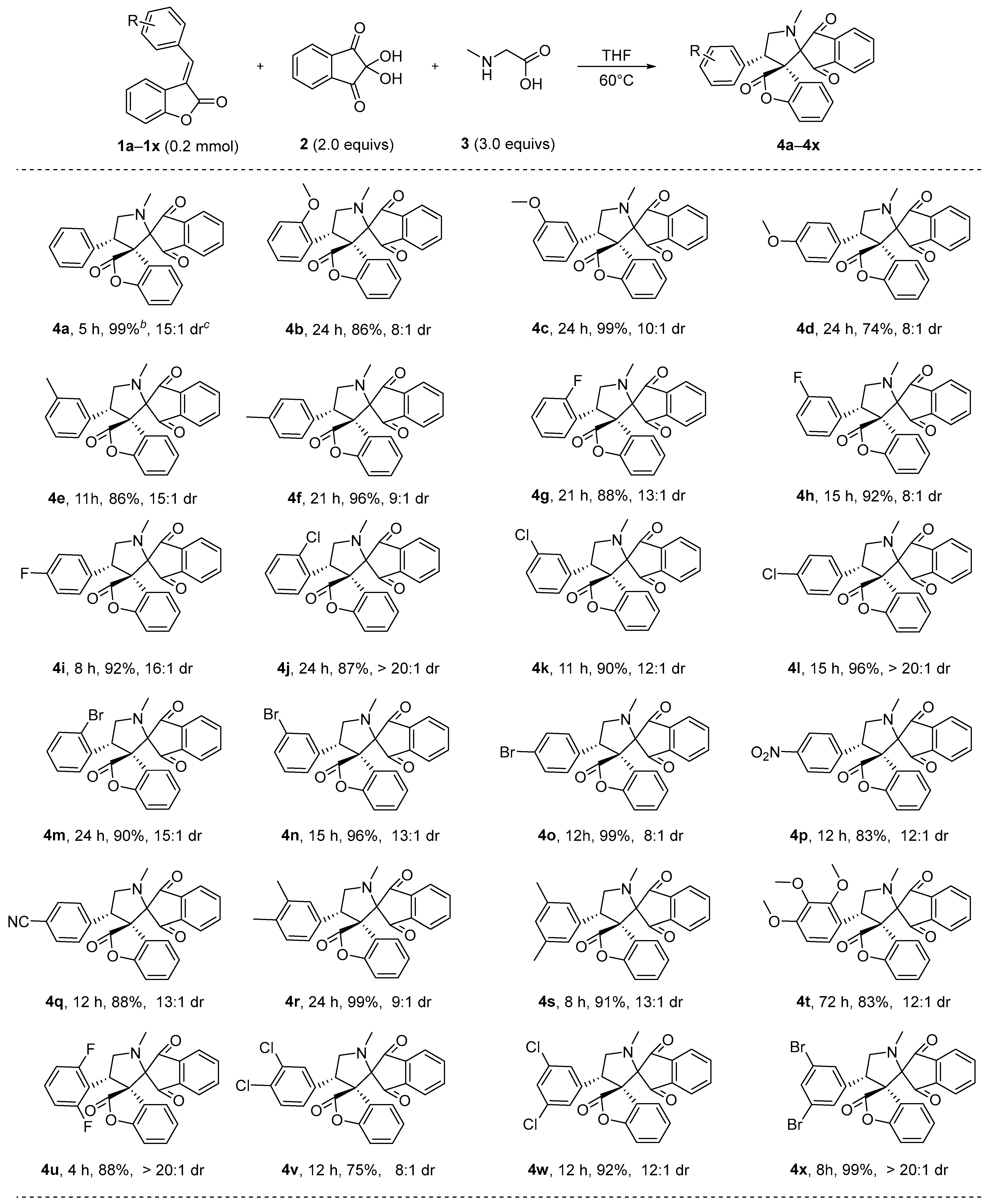
2.1. Synthesis of Spiro-Pyrrolidine Compounds
2.2. In Vitro Effect on Viability of Cancer Cells
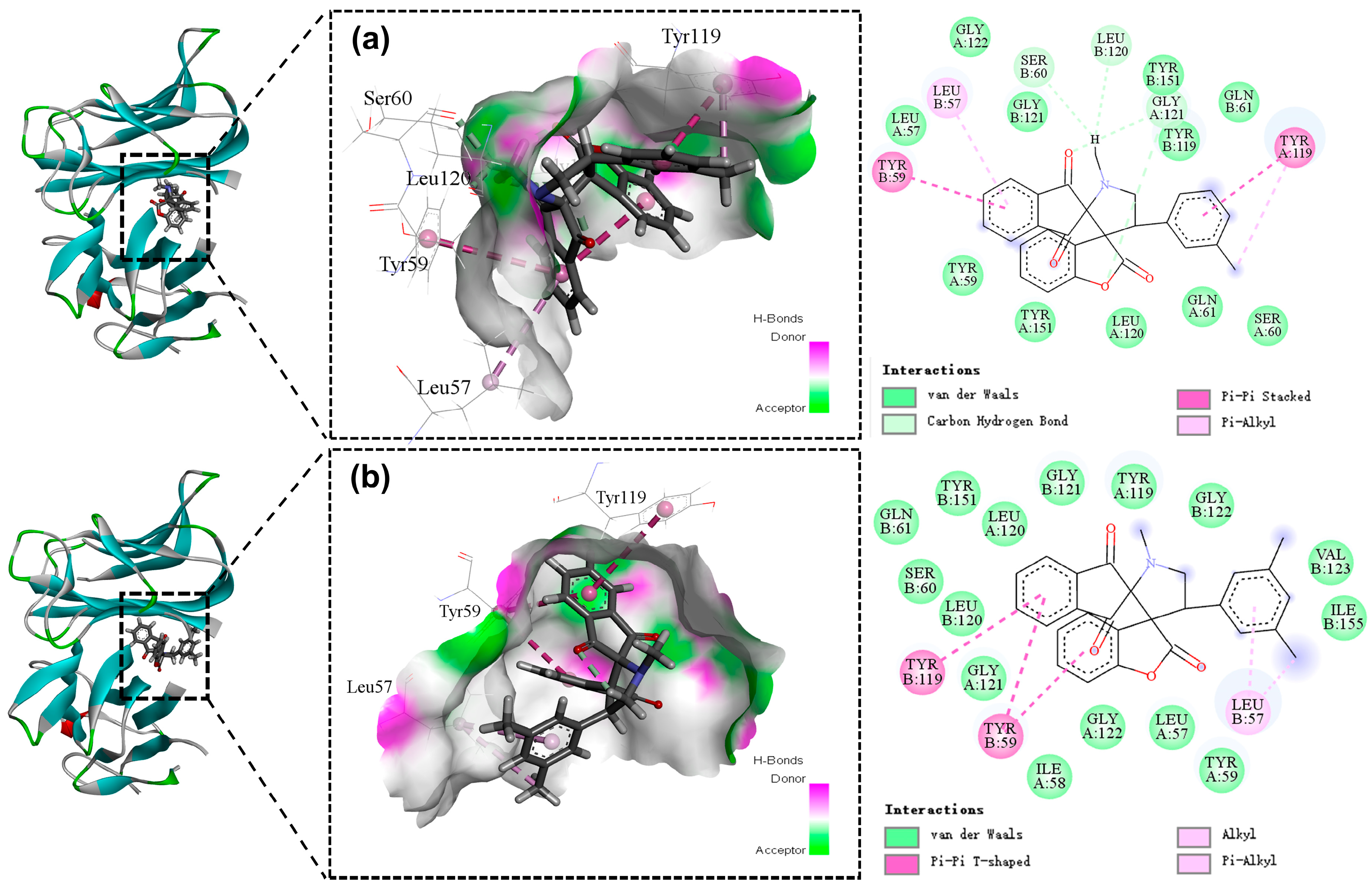
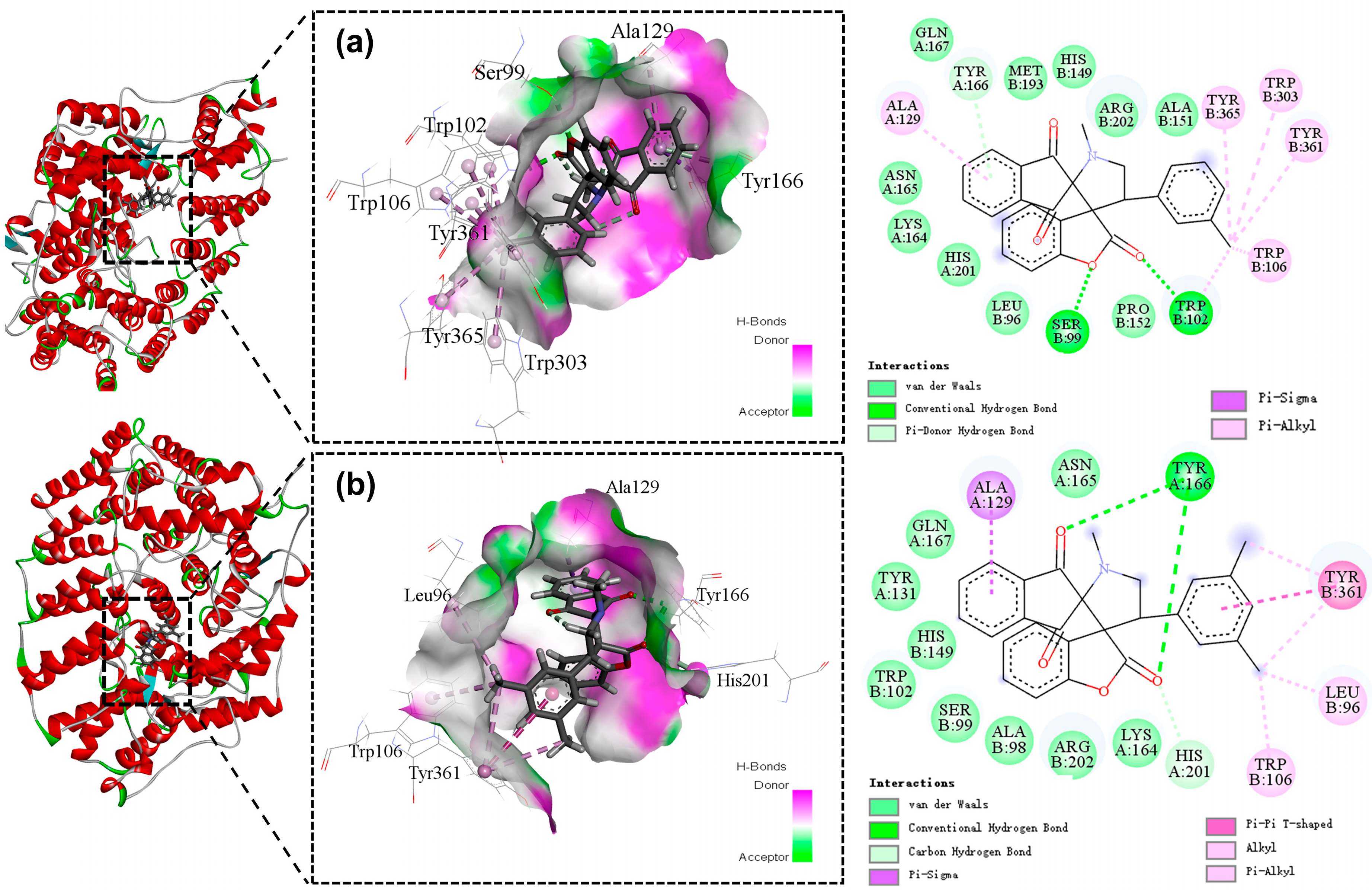
2.3. Molecular Docking
3. Materials and Methods
3.1. General Information
3.2. Synthesis of the Compounds 4a-4x and 1a-1x
3.3. Characterization Data
3.4. Cell Culture
3.5. MTT Assay
3.6. Docking Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The Ever-Increasing Importance of Cancer as a Leading Cause of Premature Death Worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Abu-Khudir, R.; Ismail, G.A.; Diab, T. Antimicrobial, Antioxidant, and Anti-Tumor Activities of Sargassum Linearifolium and Cystoseira Crinita from Egyptian Mediterranean Coast. Nutr. Cancer 2021, 73, 829–844. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Hu, W.; Yan, J. Nano-Chemotherapy Synergize with Immune Checkpoint Inhibitor—A Better Option? Front. Immunol. 2022, 13, 963533. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Duan, L.; Tong, L.; Pu, P.; Wei, L.; Wang, L.; Hu, D.; Tang, H. Research Progress on Flavonoids in Traditional Chinese Medicine to Counteract Cardiotoxicity Associated with Anti-Tumor Drugs. Rev. Cardiovasc. Med. 2024, 25, 74. [Google Scholar] [CrossRef] [PubMed]
- Smolarska, A.; Pruszynska, I.; Wasylko, W.; Godlewska, K.; Markowska, M.; Rybak, A.; Botther, J.; Kucharzewska, P.; Nowakowska, J.; Szeliga, J.; et al. Targeted Therapies for Glioblastoma Treatment. J. Physiol. Pharmacol. 2023, 74, 251–261. [Google Scholar]
- Liu, X.J.; Zhao, H.C.; Hou, S.J.; Zhang, H.J.; Cheng, L.; Yuan, S.; Zhang, L.R.; Song, J.; Zhang, S.Y.; Chen, S.W. Recent Devel-opment of Multi-Target Vegfr-2 Inhibitors for the Cancer Therapy. Bioorg. Chem. 2023, 133, 106425. [Google Scholar] [CrossRef]
- Nair, H.G.; Jurkiewicz, A.; Graczyk, D. Inhibition of Rna Polymerase Iii Augments the Anti-Cancer Properties of Tnfα. Cancers 2023, 15, 1495. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, S.; Fan, T.; Hai, Y.; Gao, Y.; He, J. Reigniting Hope in Cancer Treatment: The Promise and Pitfalls of Il-2 and Il-2r Targeting Strategies. Mol. Cancer 2023, 22, 121. [Google Scholar] [CrossRef] [PubMed]
- Al-Jassas, R.M.; Islam, M.S.; Al-Majid, A.M.; Nafie, M.S.; Haukka, M.; Rahman, A.M.; Alayyaf, A.M.A.; Barakat, A. Synthesis and Sars Study of Novel Spiro-Oxindoles as Potent Antiproliferative Agents with Cdk-2 Inhibitory Activities. Arch. Pharm. 2023, 356, 2300185. [Google Scholar] [CrossRef]
- Zheng, Y.; Comaills, V.; Burr, R.; Boulay, G.; Miyamoto, D.T.; Wittner, B.S.; Emmons, E.; Sil, S.; Koulopoulos, M.W.; Broderick, K.T.; et al. COX-2 Mediates Tumor-Stromal Prolactin Signaling to Initiate Tumorigenesis. Proc. Natl. Acad. Sci. USA 2019, 116, 5223–5232. [Google Scholar] [CrossRef]
- Hongnak, S.; Gust, R. Structure-Activity Relationship Study to Improve Cytotoxicity and Selectivity of Lonafarnib against Breast Cancer. Arch. Pharm. 2023, 356, 2200263. [Google Scholar] [CrossRef]
- Pomella, S.; Cassandri, M.; Braghini, M.R.; Marampon, F.; Alisi, A.; Rota, R. New Insights on the Nuclear Functions and Targeting of Fak in Cancer. Int. J. Mol. Sci. 2022, 23, 1988. [Google Scholar] [CrossRef] [PubMed]
- Khanam, H.; Shamsuzzaman. Bioactive Benzofuran Derivatives: A Review. Eur. J. Med. Chem. 2015, 97, 483–504. [Google Scholar] [CrossRef]
- Zuo, L.; Sun, Z.; Wang, Z.; Du, S.; Kong, X.; Li, L.; Yang, J.; Kang, J.; Zhang, X. Pharmacokinetics and Tissue Distribution Study of Prucalopride in Rats by Ultra High Performance Liquid Chromatography with Tandem Mass Spectrometry. J. Pharm. Biomed. Anal. 2016, 131, 246–255. [Google Scholar] [CrossRef]
- Meng, Q.; Tong, S.; Zhao, Y.; Peng, X.; Li, Z.; Feng, T.; Liu, J. New Phenolic Dimers from Plant Paeonia Suffruticosa and Their Cytotoxicity and No Production Inhibition. Molecules 2023, 28, 4590. [Google Scholar] [CrossRef]
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A Review on Recent Advances in Nitrogen-Containing Molecules and Their Biological Applications. Molecules 2020, 25, 1909. [Google Scholar] [CrossRef]
- Heravi, M.M.; Zadsirjan, V. Prescribed Drugs Containing Nitrogen Heterocycles: An Overview. RSC Adv. 2020, 10, 44247–44311. [Google Scholar] [CrossRef]
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. Fda Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- Kathirvelan, D.; Haribabu, J.; Reddy, B.; Balachandran, C.; Duraipandiyan, V. Facile and Diastereoselective Synthesis of 3,2′-Spiropyrrolidine-Oxindoles Derivatives, Their Molecular Docking and Antiproliferative Activities. Bioorganic Med. Chem. Lett. 2015, 25, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.S.; Almansour, A.I.; Arumugam, N.; Mohammad, F.; Kumar, R.R. In Vitro Mechanistic Exploration of Novel Spiropyrrolidine Heterocyclic Hybrids as Anticancer Agents. Front. Chem. 2020, 8, 465. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, N.; Almansour, A.I.; Kumar, R.S.; Alaqeel, S.I.; Krishna, V.S.; Sriram, D. Anti-Tubercular Activity of Novel Class of Spiropyrrolidine Tethered Indenoquinoxaline Heterocyclic Hybrids. Bioorg. Chem. 2020, 99, 103799. [Google Scholar] [CrossRef]
- Hassaneen, H.M.; Eid, E.M.; Eid, H.A.; Farghaly, T.A.; Mabkhot, Y.N. Facial Regioselective Synthesis of Novel Bioactive Spiropyrrolidine/Pyrrolizine-Oxindole Derivatives Via a Three Components Reaction as Potential Antimicrobial Agents. Molecules 2017, 22, 357. [Google Scholar] [CrossRef]
- Chen, H.; Hua, P.; Huang, D.; Zhang, Y.; Zhou, H.; Xu, J.; Gu, Q. Discovery of Spiro[Pyrrolidine-3,3′-Oxindole] Lxrβ Agonists for the Treatment of Osteoporosis. J. Med. Chem. 2023, 66, 752–765. [Google Scholar] [CrossRef]
- Kumar, R.S.; Almansour, A.I.; Arumugam, N.; Kotresha, D.; Manohar, T.S.; Venketesh, S. Cholinesterase Inhibitory Activity of Highly Functionalized Fluorinated Spiropyrrolidine Heterocyclic Hybrids. Saudi J. Biol. Sci. 2021, 28, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Hugentobler, K.M.; Carreira, E.M. Discovery and Surprises with Cyclizations, Cycloadditions, Fragmentations, and Rearrangements in Complex Settings. Acc. Chem. Res. 2021, 54, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Acherar, S.; Colombeau, L.; Frochot, C.; Vanderesse, R. Synthesis of Porphyrin, Chlorin and Phthalocyanine Derivatives by Azide-Alkyne Click Chemistry. Curr. Med. Chem. 2015, 22, 3217–3254. [Google Scholar] [CrossRef] [PubMed]
- Doraghi, F.; Serajian, A.; Karimian, S.; Ghanbarlou, M.; Moradkhani, F.; Larijani, B.; Mahdavi, M. Decarboxylative 1,3-Dipolar Cycloadditions of L-Proline. RSC Adv. 2024, 14, 8481–8501. [Google Scholar] [CrossRef] [PubMed]
- Sadowski, M.; Kula, K. Unexpected Course of Reaction between (1e,3e)-1,4-Dinitro-1,3-Butadiene and N-Methyl Azomethine Ylide—A Comprehensive Experimental and Quantum-Chemical Study. Molecules 2024, 29, 5066. [Google Scholar] [CrossRef]
- Jasiński, R.; Dresler, E. On the Question of Zwitterionic Intermediates in the [3+2] Cycloaddition Reactions: A Critical Review. Organics 2020, 1, 49–69. [Google Scholar] [CrossRef]
- Zhang, H.; He, X.; Wang, X.; Yu, B.; Zhao, S.; Jiao, P.; Jin, H.; Liu, Z.; Wang, K.; Zhang, L.; et al. Design, synthesis and biological activities of piperidinespirooxadiazole derivatives as α7 nicotinic receptor antagonists. Eur. J. Med. Chem. 2020, 207, 112774–112790. [Google Scholar] [CrossRef]
- Ramesh, D.; Joji, A.; Vijayakumar, B.G.; Sethumadhavan, A.; Mani, M.; Kannan, T. Indole Chalcones: Design, Synthesis, In vitro and in Silico Evaluation against Mycobacterium Tuberculosis. Eur. J. Med. Chem. 2020, 198, 112358. [Google Scholar] [CrossRef] [PubMed]

 | |||||
| Entry | Solvent | t (°C) | Time (h) | Yield (%) b | dr c |
| 1 | Acetonitrile | 40 | 16 | 82 | 9:1 |
| 2 | DCE | 40 | 45 | 84 | 8:1 |
| 3 | EtOAc | 40 | 24 | 86 | 8:1 |
| 4 | EtOH | 40 | 16 | 88 | 6:1 |
| 5 | Toluene | 40 | 46 | 82 | 10:1 |
| 6 d | H2O | 40 | 24 | trace | / |
| 7 | THF | 40 | 21 | 90 | 15:1 |
| 8 | THF | 60 | 5 | 99 | 15:1 |
| 9 | THF | 80 | 5 | 88 | 15:1 |
| 10 e | THF | 60 | 5 | 84 | 15:1 |
| Compound | IC50 (μM) a | Compound | IC50 (μM) | ||
|---|---|---|---|---|---|
| Hela | CT26 | Hela | CT26 | ||
| 4a | 65.55 ± 3.45 | 34.42 ± 1.82 | 4n | 77.04 ± 3.16 | 89.72 ± 4.84 |
| 4b | 15.14 ± 1.33 | 23.58 ± 1.34 | 4o | >100 | >100 |
| 4c | 10.26 ± 0.87 | 18.39 ±1.01 | 4p | 55.18 ± 3.29 | >100 |
| 4d | 45.49 ± 2.42 | 21.96 ± 2.08 | 4q | 37.15 ± 2.36 | 23.32 ± 1.36 |
| 4e | 26.75 ± 1.53 | 8.31 ± 0.64 | 4r | 24.18 ± 1.81 | 19.48 ± 1.35 |
| 4f | 33.62 ± 1.68 | 25.67 ± 1.38 | 4s | 17.48 ± 1.36 | 5.28 ± 0.72 |
| 4g | 41.36 ± 2.48 | 36.42 ± 2.47 | 4t | 46.53 ± 2.49 | 16.15 ± 1.06 |
| 4h | 35.77 ± 2.34 | 58.66 ± 3.64 | 4u | 30.46 ± 1.71 | 46.62 ± 3.63 |
| 4i | 22.06 ± 1.22 | 49.17 ± 2.73 | 4v | >100 | 14.34 ± 0.65 |
| 4j | >100 | 26.54 ± 1.39 | 4w | 71.39 ± 3.18 | 15.44 ± 1.12 |
| 4k | >100 | 53.41 ± 3.25 | 4x | >100 | 39.02± 2.02 |
| 4l | 60.93 ± 3.48 | >100 | Cisplatin b | 15.91 ± 1.09 | 10.27 ± 0.71 |
| 4m | >100 | >100 | |||
| Compound | Docking Score | |||||||
|---|---|---|---|---|---|---|---|---|
| 1YSI | 1Y6B | 2AZ5 | 1M48 | 2BHE | 6COX | 4GTM | 4D4S | |
| (Bcl-x1) | (VEGFR2) | (TNFα) | (IL-2) | (CDK-2) | (Cox-2) | (FTase) | (FAK) | |
| 4b | −5.537 | −3.168 | −4.919 | −1.833 | −2.016 | −2.782 | −4.457 | −2.067 |
| 4c | −6.842 | −2.382 | −7.053 | −1.394 | −1.053 | −2.245 | −5.760 | −2.397 |
| 4e | −6.130 | −6.596 | −8.793 | −3.203 | −4.031 | −4.806 | −7.858 | −2.711 |
| 4s | −5.807 | −7.403 | −8.331 | −2.652 | −3.263 | −7.685 | −8.226 | −5.711 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, B.; Wang, T.; Zheng, L.; Dong, Z.; Liu, L.; Liu, X.; Feng, T.; Zhou, Y.; Shi, Y. Synthesis of Novel Benzofuran Spiro-2-Pyrrolidine Derivatives via [3+2] Azomethine Ylide Cycloadditions and Their Antitumor Activity. Int. J. Mol. Sci. 2024, 25, 13580. https://doi.org/10.3390/ijms252413580
Pan B, Wang T, Zheng L, Dong Z, Liu L, Liu X, Feng T, Zhou Y, Shi Y. Synthesis of Novel Benzofuran Spiro-2-Pyrrolidine Derivatives via [3+2] Azomethine Ylide Cycloadditions and Their Antitumor Activity. International Journal of Molecular Sciences. 2024; 25(24):13580. https://doi.org/10.3390/ijms252413580
Chicago/Turabian StylePan, Bowen, Tao Wang, Liangliang Zheng, Zhangchao Dong, Lijuan Liu, Xiongwei Liu, Tingting Feng, Ying Zhou, and Yang Shi. 2024. "Synthesis of Novel Benzofuran Spiro-2-Pyrrolidine Derivatives via [3+2] Azomethine Ylide Cycloadditions and Their Antitumor Activity" International Journal of Molecular Sciences 25, no. 24: 13580. https://doi.org/10.3390/ijms252413580
APA StylePan, B., Wang, T., Zheng, L., Dong, Z., Liu, L., Liu, X., Feng, T., Zhou, Y., & Shi, Y. (2024). Synthesis of Novel Benzofuran Spiro-2-Pyrrolidine Derivatives via [3+2] Azomethine Ylide Cycloadditions and Their Antitumor Activity. International Journal of Molecular Sciences, 25(24), 13580. https://doi.org/10.3390/ijms252413580