The Induction of Disease Resistance by Scopolamine and the Application of Datura Extract Against Potato (Solanum tuberosum L.) Late Blight
Abstract
:1. Introduction
2. Results
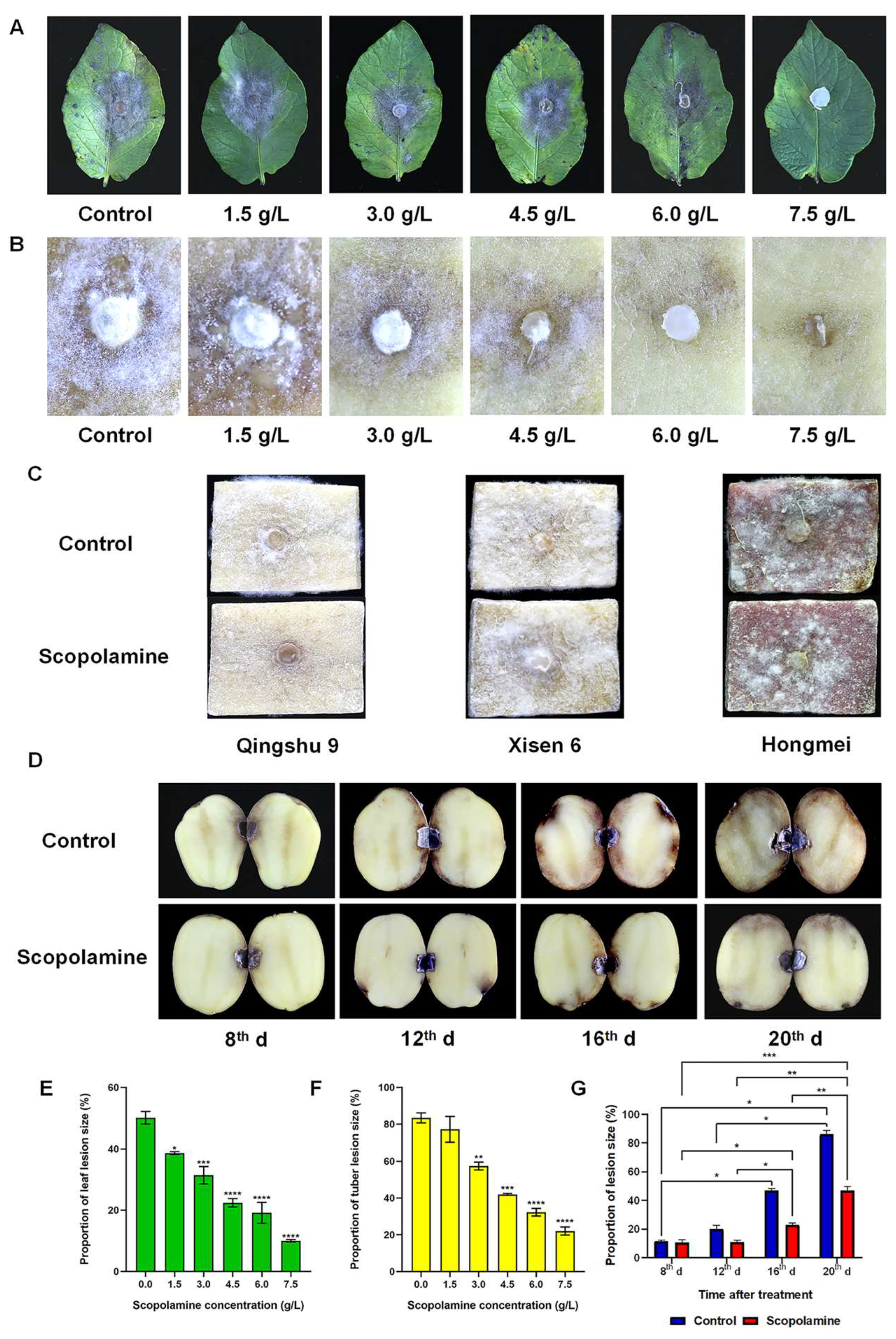
2.1. Scopolamine Enhances Potato Resistance to Late Blight In Vitro and In Vivo
2.2. Scopolamine Affects Disease-Resistant Substances of Potato Tubers
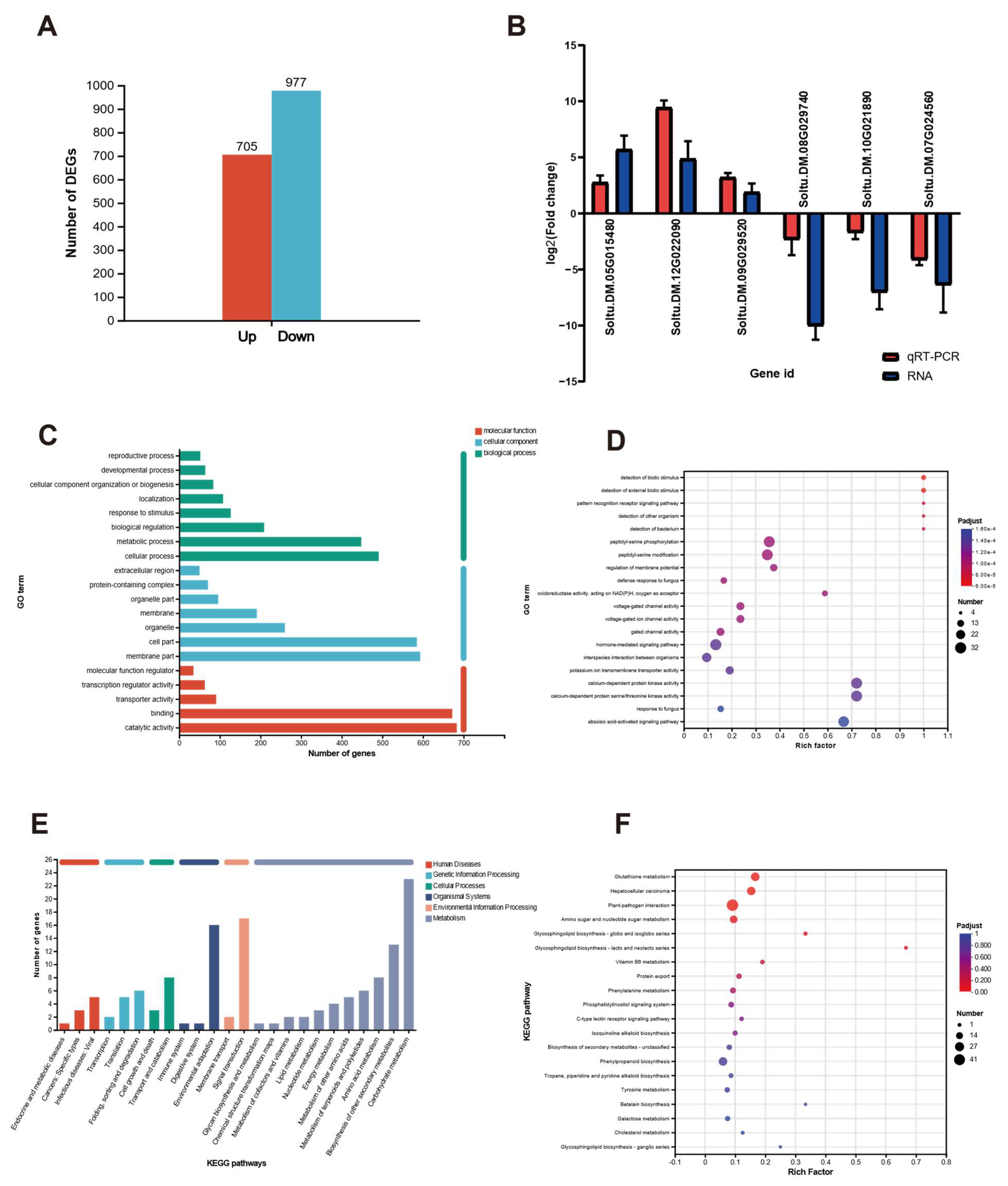
2.3. RNA-Seq Analysis
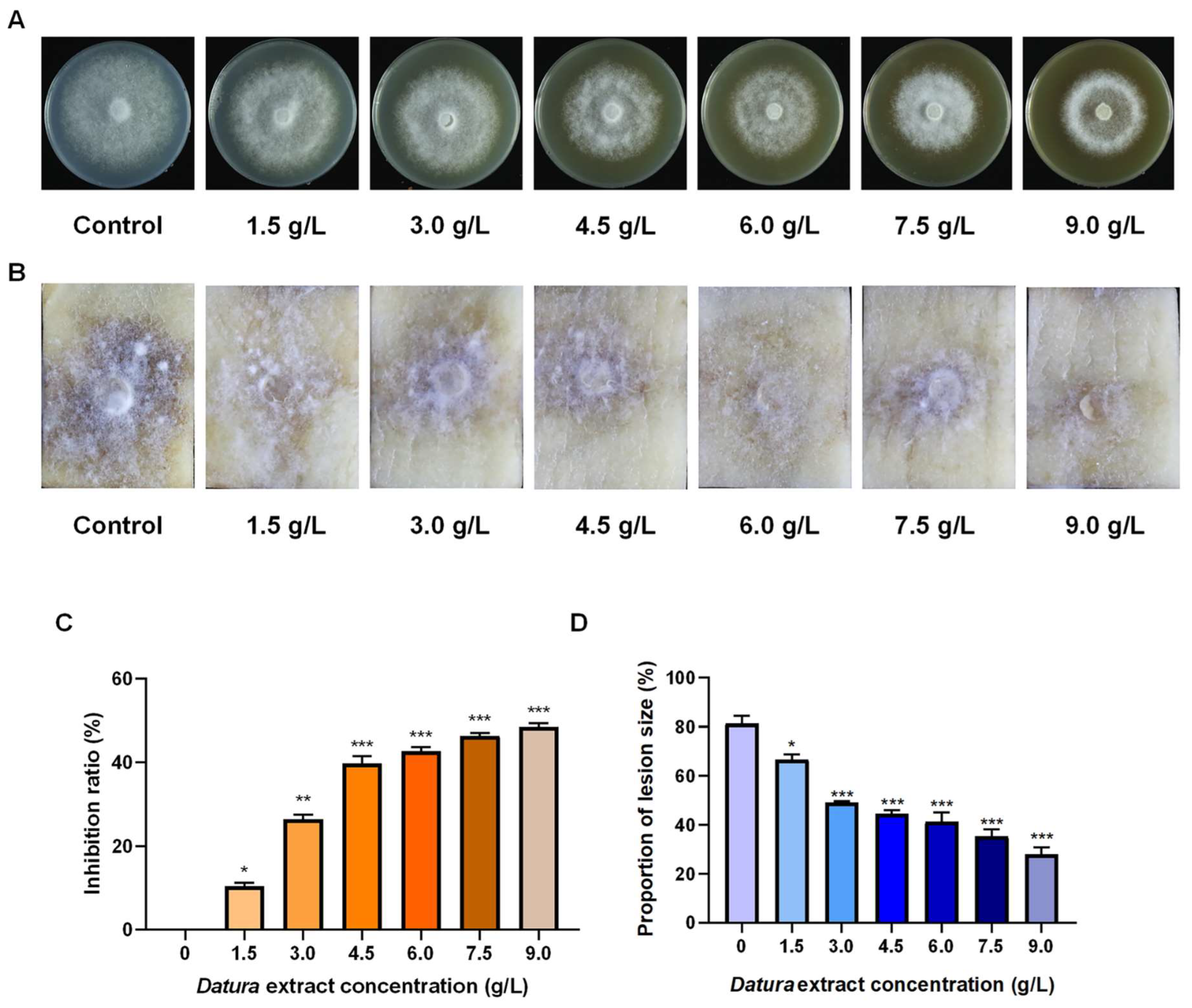
2.4. Datura Extract Has Dual Effects Against Potato Late Blight
2.5. Joint Effect of Datura Extract and Infinito in the Field Test
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. The Effect of Scopolamine on Inducing the Resistance of Potato Pieces, Leaves, and Whole Potatoes to Late Blight
4.3. Assays of Disease-Resistant Substances in Potato Tubers
4.4. The Effect of H2O2 on Inducing the Resistance of Potato Pieces
4.5. RNA Sequencing
4.6. Quantitative Real-Time PCR Analysis
4.7. Effect of Datura Extract on Mycelia Growth of P. infestans
4.8. Effect of Datura Extract on Induction of Late Blight Resistance in Potato Pieces
4.9. Field Test
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elfikrie, N.; Ho, Y.B.; Zaidon, S.Z.; Juahir, H.; Tan, E.S.S. Occurrence of pesticides in surface water, pesticides removal efficiency in drinking water treatment plant and potential health risk to consumers in Tengi River Basin, Malaysia. Sci. Total Environ. 2020, 712, 136540. [Google Scholar] [CrossRef]
- Kumbar, B.; Mahmood, R.; Nagesha, S.N.; Nagaraja, M.S.; Prashant, D.G.; Kerima, O.Z.; Karosiya, A.; Chavan, M. Field application of Bacillus subtilis isolates for controlling late blight disease of potato caused by Phytophthora infestans. Biocatal. Agric. Biotechnol. 2019, 22, 101366. [Google Scholar] [CrossRef]
- Lastochkina, O.; Pusenkova, L.; Garshina, D.; Kasnak, C.; Palamutoglu, R.; Shpirnaya, I.; Mardanshin, I.d.; Maksimov, I. Improving the biocontrol potential of endophytic bacteria Bacillus subtilis with salicylic acid against Phytophthora infestans-caused postharvest potato tuber late blight and impact on stored tubers quality. Horticulturae 2022, 8, 117. [Google Scholar] [CrossRef]
- Trdan, S.; Vučajnk, F.; Bohinc, T.; Vidrih, M. The effect of a mixture of two plant growth-promoting bacteria from Argentina on the yield of potato, and occurrence of primary potato diseases and pest—Short communication. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2018, 69, 89–94. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Hou, Y.; Xia, X.; Zhu, Z.; Huang, A.; Feng, S.; Li, P.; Shi, L.; Dong, P. Isolation and screening of antagonistic endophytes against Phytophthora infestans and preliminary exploration on anti-oomycete mechanism of Bacillus velezensis 6-5. Plants 2023, 12, 909. [Google Scholar] [CrossRef] [PubMed]
- Purwantisari, S.; Priyatmojo, A.; Sancayaningsih, R.; Kasiamdari, R.; Budihardjo, K. Systemic inducing resistance against late blight by applying antagonist Trichoderma viride. J. Phys. Conf. Ser. 2018, 1025, 012053. [Google Scholar] [CrossRef]
- Yao, Y.; Li, Y.; Chen, Z.; Zheng, B.; Zhang, L.; Niu, B.; Meng, J.; Li, A.; Zhang, J.; Wang, Q. Biological control of potato late blight using isolates of Trichoderma. Am. J. Potato Res. 2016, 93, 33–42. [Google Scholar] [CrossRef]
- Fu, X.; Liu, S.; Ru, J.; Tang, B.; Zhai, Y.; Wang, Z.; Wang, L. Biological control of potato late blight by Streptomyces sp. FXP04 and potential role of secondary metabolites. Biol. Control 2022, 169, 104891. [Google Scholar] [CrossRef]
- Deng, S.; Guo, Q.; Gao, Y.; Li, J.; Xu, Z. Induced resistance to rice sheath blight (Rhizoctonia solani Kühn) by β-amino-butyric acid conjugate of phenazine-1-carboxylic acid. Pestic. Biochem. Physiol. 2023, 194, 105502. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Liang, J.; Wang, L.; Gao, W.; Jiang, J.; Chang, R. Surfactin and fengycin B extracted from Bacillus pumilus W-7 provide protection against potato late blight via distinct and synergistic mechanisms. Appl. Microbiol. Biotechnol. 2020, 104, 7467–7481. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, X.; Reiter, R.; Feng, S.; Wang, Y.; Liu, S.; Jin, L.; Li, Z.; Datla, R.; Ren, M. Melatonin attenuates potato late blight by disrupting cell growth, stress tolerance, fungicide susceptibility and homeostasis of gene expression in Phytophthora infestans. Front. Plant Sci. 2017, 8, 1993. [Google Scholar] [CrossRef] [PubMed]
- Jian, Y.; Feng, S.; Huang, A.; Zhu, Z.; Zhang, J.; Tang, S.; Jin, L.; Ren, M.; Dong, P. Integrative mRNA and microRNA analysis exploring the inducing effect and mechanism of diallyl trisulfide (DATS) on potato against late blight. Int. J. Mol. Sci. 2023, 24, 3474. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; You, Z.; Luo, Y.; Yang, C.; Ren, J.; Liu, Y.; Wei, G.; Dong, P.; Ren, M. Antifungal activity of chitosan against Phytophthora infestans, the pathogen of potato late blight. Int. J. Biol. Macromol. 2021, 166, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Xiong, Z.; Zou, W.; Shi, Z.; Li, S.; Zhang, X.; Liu, S.; Liu, Y.; Luo, X.; Ren, J.; et al. Anti-oomycete ability of scopolamine against Phytophthora infestans, a terrible pathogen of potato late blight. J. Sci. Food Agric. 2023, 103, 6416–6428. [Google Scholar] [CrossRef]
- Kamle, M.; Borah, R.; Bora, H.; Jaiswal, A.K.; Singh, R.K.; Kumar, P. Systemic Acquired Resistance (SAR) and Induced Systemic Resistance (ISR): Role and Mechanism of Action Against Phytopathogens. In Fungal Biotechnology and Bioengineering; Hesham, A.E.-L., Upadhyay, R.S., Sharma, G.D., Manoharachary, C., Gupta, V.K., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 457–470. [Google Scholar]
- Sorokan, A.; Benkovskaya, G.; Burkhanova, G.; Blagova, D.; Maksimov, I. Endophytic Strain Bacillus subtilis 26DCryChS Producing Cry1Ia Toxin from Bacillus thuringiensis Promotes Multifaceted Potato Defense against Phytophthora infestans (Mont.) de Bary and Pest Leptinotarsa decemlineata Say. Plants 2020, 9, 1115. [Google Scholar] [CrossRef]
- Tomar, S.; Singh, B.P.; Khan, M.A.; Kumar, S.; Sharma, S.; Lal, M. Identification of Pseudomonas aeruginosa strain producing biosurfactant with antifungal activity against Phytophthora infestans. Potato J. 2013, 40, 155–163. [Google Scholar]
- Jaafar, F.R.; Ajeena, S.J.; Mehdy, S.S. Anti-inflammatory impacts and analgesiac activity of aqueous extract Datura innoxia leaves against induced pain and inflammation in mice. J. Entomol. Zool. Stud. 2018, 6, 1894–1899. [Google Scholar]
- Deng, Z.; Luo, C.; Liu, B.; Chen, L.; Tan, J. Development and utilization of medicinal value on Datura stramonium L. Prog. Mod. Biomed. 2011, 11, 1394–1398. [Google Scholar]
- Zhu, H.; Feng, R.; Hu, L. Preliminary antifungal research of Datura stramonium on Phytopathogens. J. Henan Inst. Sci. Technol. 2015, 43, 30–32. [Google Scholar]
- Akila, R.; Rajendran, L.; Harish, S.; Saveetha, K.; Raguchander, T.; Samiyappan, R. Combined application of botanical formulations and biocontrol agents for the management of Fusarium oxysporum f. sp. cubense (Foc) causing Fusarium wilt in banana. Biol. Control 2011, 57, 175–183. [Google Scholar]
- Javaid, A.; Saddique, A. Control of charcoal rot fungus Macrophomina phaseolina by extracts of Datura metel. Nat. Prod. Res. 2012, 26, 1715–1720. [Google Scholar] [CrossRef] [PubMed]
- Kagale, S.; Marimuthu, T.; Thayumanavan, B.; Nandakumar, R.; Samiyappan, R. Antimicrobial activity and induction of systemic resistance in rice by leaf extract of Datura metel against Rhizoctonia solani and Xanthomonas oryzae pv. oryzae. Physiol. Mol. Plant Pathol. 2004, 65, 91–100. [Google Scholar] [CrossRef]
- Vega-Ceja, J.; Jiménez-Amezcua, R.M.; Hernández, J.; Silva-Guzmán, J.; Torres Rendon, J.; Lomeli Ramirez, M.G.; García-Enriquez, S. Antifungal activity of Datura stramonium L. extractives against Xylophagous Fungi. Forests 2022, 13, 1222. [Google Scholar] [CrossRef]
- Cai, S.L.; Mu, X.Q. Allelopathic potential of aqueous leaf extracts of Datura stramonium L. on seed germination, seedling growth and root anatomy of Glycine max (L.) Merrill. Allelopath. J. 2012, 30, 235–246. [Google Scholar]
- Ali, A.; Ahmad, F.; Biondi, A.; Wang, Y.; Desneux, N. Potential for using Datura alba leaf extracts against two major stored grain pests, the khapra beetle Trogoderma granarium and the rice weevil Sitophillus oryzae. J. Pest Sci. 2012, 85, 359–366. [Google Scholar] [CrossRef]
- Shi, Z.; Zou, W.; Zhu, Z.; Xiong, Z.; Li, S.; Dong, P.; Zhu, Z. Tropane alkaloids (hyoscyamine, scopolamine and atropine) from genus Datura: Extractions, contents, syntheses and effects. Ind. Crops Prod. 2022, 186, 115283. [Google Scholar] [CrossRef]
- Wang, S.; Guo, P.; Feng, M.; Qian, M.; Shen, X.; Wang, G. The efficacy of scopolamine for patients with Parkinson’s disease and depression: Two case reports. Asian J. Psychiatry 2020, 52, 102107. [Google Scholar] [CrossRef]
- Abdel-Motaal, F.; El-Zayat, S.; Kosaka, Y.; El-Sayed, M.; Kashima, R.; Maeda, Y.; Nassar, M.; Ito, S.-i. Antifungal activities of hyoscyamine and scopolamine against two major rice pathogens: Magnaporthe oryzae and Rhizoctonia solani. J. Gen. Plant Pathol. 2010, 76, 102–111. [Google Scholar] [CrossRef]
- Ibrahim, M.; Siddique, S.; Rehman, K.; Husnain, M.; Hussain, A.; Akash, M.S.H.; Azam, F. Comprehensive analysis of phytochemical constituents and ethnopharmacological investigation of genus Datura. Crit. Rev. Eukaryot. Gene Expr. 2018, 28, 223–283. [Google Scholar] [CrossRef]
- Wang, X.; Zheng, K.; Cheng, W.; Li, J.; Liang, X.; Shen, J.; Dou, D.; Yin, M.; Yan, S. Field application of star polymer-delivered chitosan to amplify plant defense against potato late blight. Chem. Eng. J. 2021, 417, 129327. [Google Scholar] [CrossRef]
- Song, Y.; Ren, Y.; Xue, Y.; Lu, D.; Yan, T.; He, J. Putrescine (1,4-Diaminobutane) enhances antifungal activity in postharvest mango fruit against Colletotrichum gloeosporioides through direct fungicidal and induced resistance mechanisms. Pestic. Biochem. Physiol. 2023, 195, 105581. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Fan, L.; Xia, X.; Wang, Z.; Yin, Y.; Cheng, Y.; Li, Z. Melatonin decreases resistance to postharvest green mold on citrus fruit by scavenging defense-related reactive oxygen species. Postharvest Biol. Technol. 2019, 153, 21–30. [Google Scholar] [CrossRef]
- Zhang, T.; Shen, Y.; Zhu, R.; Shan, W.; Li, Y.; Yan, M.; Zhang, Y. Benzo [a] pyrene exposure promotes RIP1-mediated necroptotic death of osteocytes and the JNK/IL-18 pathway activation via generation of reactive oxygen species. Toxicology 2022, 476, 153244. [Google Scholar] [CrossRef] [PubMed]
- Żamojć, K.; Zdrowowicz, M.; Jacewicz, D.; Wyrzykowski, D.; Chmurzyński, L. Fluorescent probes used for detection of hydrogen peroxide under biological conditions. Crit. Rev. Anal. Chem. 2016, 46, 171–200. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Wang, X.; Jiao, L.; Jiang, H.; Yuan, S.; Zhang, L.; Shi, Z.; Fan, Z.; Meng, D. Epsilon-poly-l-lysine alleviates brown blotch disease of postharvest Agaricus bisporus mushrooms by directly inhibiting Pseudomonas tolaasii and inducing mushroom disease resistance. Pestic. Biochem. Physiol. 2024, 199, 105759. [Google Scholar] [CrossRef]
- Saleem, D.; Zuhra, Z.; Akhtar, W.; Koiwa, H.; Mahmood, T. Salicylic Acid and H2O2 Induce PPO Derived GUS Expression in Arabidopsis. Russ. J. Plant Physiol. 2020, 67, 822–826. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Q.; Yi, L.; Song, X.; Li, L.; Deng, G.; Liang, J.; Chen, F.; Yu, M.; Long, H. PAL-mediated SA biosynthesis pathway contributes to nematode resistance in wheat. Plant J. 2021, 107, 698–712. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, P.; Wu, J.; Wang, P.; Chen, W.; Chen, C.; Wang, J.; Li, M.; Yu, A.; Zhang, D.; et al. Changes in phenylalanine ammonia-lyase activity of different susceptible soybean varieties treated with Phytophthora soya root rot toxin. J. Crops 2008, 1, 47–49. [Google Scholar]
- Brady, J.D.; Fry, S. Formation of Di-isodityrosine and loss of isodityrosine in the cell walls of tomato cell-suspension cultures treated with fungal elicitors or H2O2. Plant Physiol. 1997, 115, 87–92. [Google Scholar] [CrossRef]
- Mohnike, L.; Rekhter, D.; Huang, W.; Feussner, K.; Tian, H.; Herrfurth, C.; Zhang, Y.; Feussner, I. The glycosyltransferase UGT76B1 modulates N-hydroxy-pipecolic acid homeostasis and plant immunity. Plant Cell 2021, 33, 735–749. [Google Scholar] [CrossRef]
- Yang, Z.; Xia, J.; Hong, J.; Zhang, C.; Wei, H.; Ying, W.; Sun, C.; Sun, L.; Mao, Y.; Gao, Y.; et al. Structural insights into auxin recognition and efflux by Arabidopsis PIN1. Nature 2022, 609, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Sun, Y.; Guo, T.; Shi, C.; Zhang, Y.; Kan, Y.; Xiang, Y.; Zhang, H.; Yang, Y.; Li, Y.; et al. UDP-glucosyltransferase regulates grain size and abiotic stress tolerance associated with metabolic flux redirection in rice. Nat. Commun. 2020, 11, 2629. [Google Scholar] [CrossRef] [PubMed]
- Moreau, R.A.; Rawa, D. Phospholipase activity in cultures of Phytophthora infestans and in infected potato leaves. Physiol. Plant Pathol. 1984, 24, 187–199. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Luo, X.; Wu, X.; Ren, J.; Huang, X.; Feng, S.; Lin, X.; Ren, M.; Dong, P. Antifungal activity and mechanism of thymol against Fusarium oxysporum, a pathogen of potato dry rot, and its potential application. Postharvest Biol. Technol. 2022, 192, 112025. [Google Scholar] [CrossRef]
- Klopfenstein, D.; Zhang, L.; Pedersen, B.; Ramírez, F.; Warwick Vesztrocy, A.; Naldi, A.; Mungall, C.; Yunes, J.; Botvinnik, O.; Weigel, M.; et al. GOATOOLS: A Python library for Gene Ontology analyses. Sci. Rep. 2018, 8, 10872. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.-Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39 (Suppl. 2), W316–W322. [Google Scholar] [CrossRef]
- Jin, Z.J. About the evaluation of drug combination. Acta Pharmacol. Sin. 2004, 25, 146–147. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Liu, S.; Liu, Y.; Zhang, X.; Shi, Z.; Liu, S.; Zhu, Z.; Dong, P. The Induction of Disease Resistance by Scopolamine and the Application of Datura Extract Against Potato (Solanum tuberosum L.) Late Blight. Int. J. Mol. Sci. 2024, 25, 13442. https://doi.org/10.3390/ijms252413442
Zhu Z, Liu S, Liu Y, Zhang X, Shi Z, Liu S, Zhu Z, Dong P. The Induction of Disease Resistance by Scopolamine and the Application of Datura Extract Against Potato (Solanum tuberosum L.) Late Blight. International Journal of Molecular Sciences. 2024; 25(24):13442. https://doi.org/10.3390/ijms252413442
Chicago/Turabian StyleZhu, Zhiming, Shicheng Liu, Yi Liu, Xinze Zhang, Zhiwen Shi, Shuting Liu, Zhenglin Zhu, and Pan Dong. 2024. "The Induction of Disease Resistance by Scopolamine and the Application of Datura Extract Against Potato (Solanum tuberosum L.) Late Blight" International Journal of Molecular Sciences 25, no. 24: 13442. https://doi.org/10.3390/ijms252413442
APA StyleZhu, Z., Liu, S., Liu, Y., Zhang, X., Shi, Z., Liu, S., Zhu, Z., & Dong, P. (2024). The Induction of Disease Resistance by Scopolamine and the Application of Datura Extract Against Potato (Solanum tuberosum L.) Late Blight. International Journal of Molecular Sciences, 25(24), 13442. https://doi.org/10.3390/ijms252413442




