Role of SLC7A11/xCT in Ovarian Cancer
Abstract
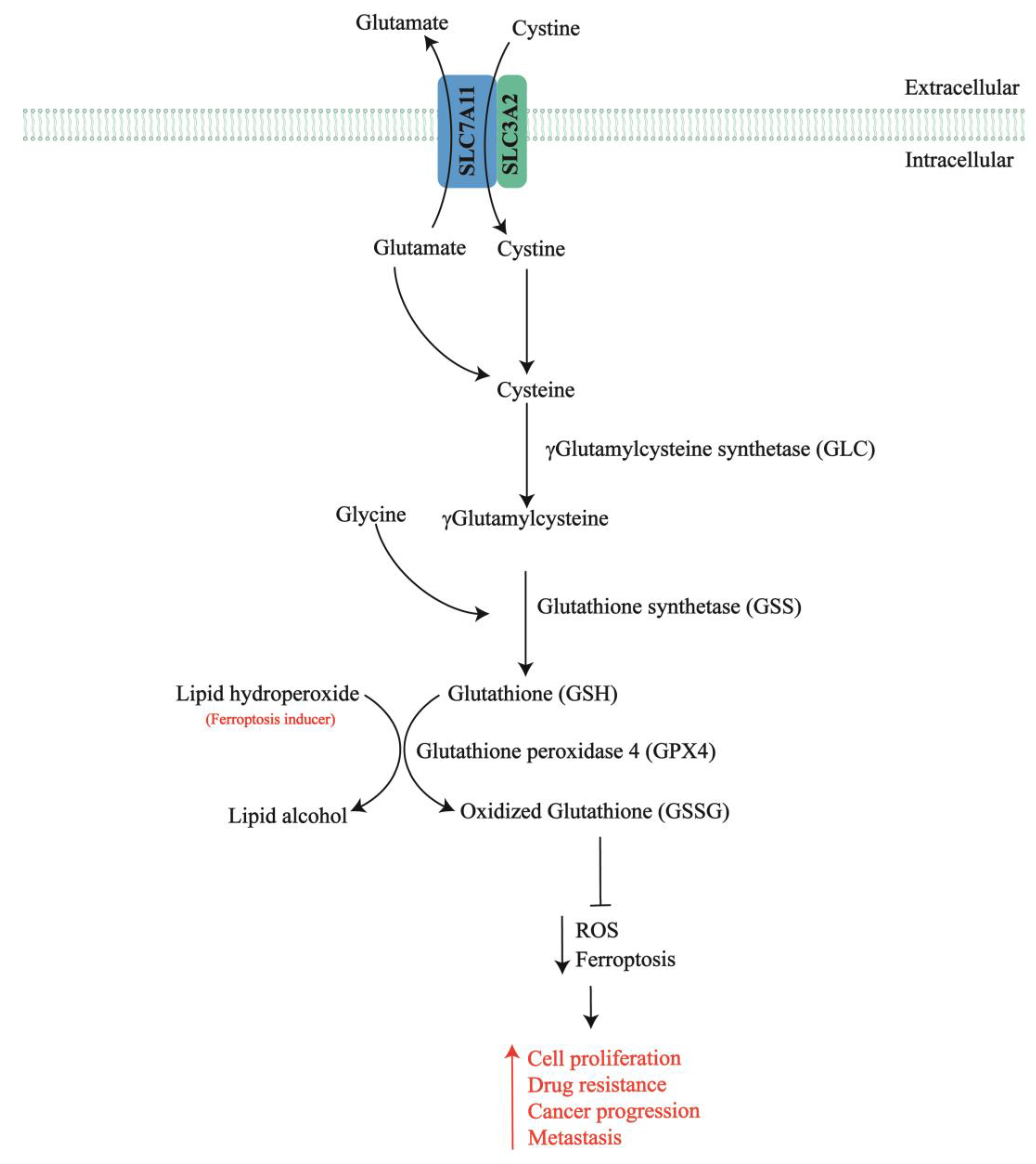
1. Introduction
2. SLC7A11 in Chemotherapy-Resistant Ovarian Cancer
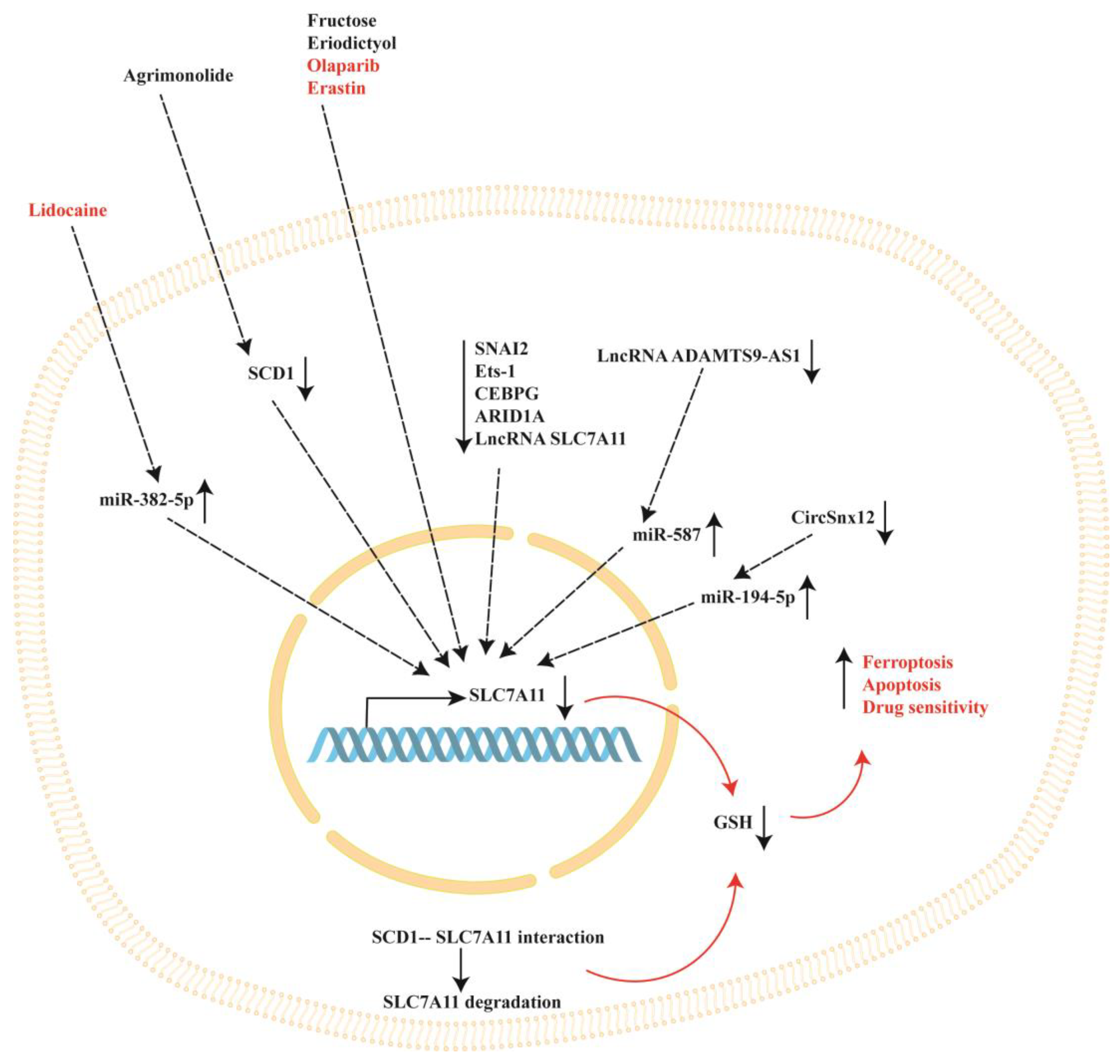
3. SLC7A11 Modulation by Natural and Synthetic Compounds in Ovarian Cancer
4. Intracellular Modulators of SLC7A11 in Ovarian Cancer
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giannice, R.; Foti, E.; Poerio, A.; Marana, E.; Mancuso, S.; Scambia, G. Perioperative morbidity and mortality in elderly gynecological oncological patients (≥70 Years) by the American Society of Anesthesiologists physical status classes. Ann. Surg. Oncol. 2004, 11, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Momenimovahed, Z.; Tiznobaik, A.; Taheri, S.; Salehiniya, H. Ovarian cancer in the world: Epidemiology and risk factors. Int. J. Womens Health 2019, 11, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.Y.; Park, K.; Song, J.M.; Pyeon, S.Y.; Lee, S.H.; Chung, Y.S.; Lee, J.M. Risk Factors and Prognosis of Stroke in Gynecologic Cancer Patients. Cancers 2023, 15, 4895. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, B.; Huang, Y.; Wu, M.; Li, Y.; Li, Y.; Zhu, X.; Wu, M. Role of the Nrf2 Signaling Pathway in Ovarian Aging: Potential Mechanism and Protective Strategies. Int. J. Mol. Sci. 2023, 24, 13327. [Google Scholar] [CrossRef] [PubMed]
- Zamwar, U.M.; Anjankar, A.P. Aetiology, Epidemiology, Histopathology, Classification, Detailed Evaluation, and Treatment of Ovarian Cancer. Cureus 2022, 14, e30561. [Google Scholar] [CrossRef] [PubMed]
- Kobel, M.; Kang, E.Y. The Evolution of Ovarian Carcinoma Subclassification. Cancers 2022, 14, 416. [Google Scholar] [CrossRef]
- Damia, G.; Broggini, M. Platinum Resistance in Ovarian Cancer: Role of DNA Repair. Cancers 2019, 11, 119. [Google Scholar] [CrossRef]
- Kleih, M.; Bopple, K.; Dong, M.; Gaissler, A.; Heine, S.; Olayioye, M.A.; Aulitzky, W.E.; Essmann, F. Direct impact of cisplatin on mitochondria induces ROS production that dictates cell fate of ovarian cancer cells. Cell Death Dis. 2019, 10, 851. [Google Scholar] [CrossRef]
- Abal, M.; Andreu, J.M.; Barasoain, I. Taxanes: Microtubule and centrosome targets, and cell cycle dependent mechanisms of action. Curr. Cancer Drug Targets 2003, 3, 193–203. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Fojo, T.; Bates, S.E. Multidrug resistance in cancer: Role of ATP-dependent transporters. Nat. Rev. Cancer 2002, 2, 48–58. [Google Scholar] [CrossRef]
- Robey, R.W.; Pluchino, K.M.; Hall, M.D.; Fojo, A.T.; Bates, S.E.; Gottesman, M.M. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat. Rev. Cancer 2018, 18, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Wu, J.; Chen, Y.; Nie, J.; Chen, C. Activation of PI3K/AKT/mTOR Pathway Causes Drug Resistance in Breast Cancer. Front. Pharmacol. 2021, 12, 628690. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Kolluri, S.K.; Lin, F.; Liu, W.; Han, Y.H.; Cao, X.; Dawson, M.I.; Reed, J.C.; Zhang, X.K. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell 2004, 116, 527–540. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Sasaki, C.Y.; Hardwick, J.M.; Longo, D.L. Bcl-2-mediated drug resistance: Inhibition of apoptosis by blocking nuclear factor of activated T lymphocytes (NFAT)-induced Fas ligand transcription. J. Exp. Med. 1999, 190, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Campagna, R.; Pozzi, V.; Spinelli, G.; Sartini, D.; Milanese, G.; Galosi, A.B.; Emanuelli, M. The Utility of Nicotinamide N-Methyltransferase as a Potential Biomarker to Predict the Oncological Outcomes for Urological Cancers: An Update. Biomolecules 2021, 11, 1214. [Google Scholar] [CrossRef]
- Togni, L.; Mascitti, M.; Sartini, D.; Campagna, R.; Pozzi, V.; Salvolini, E.; Offidani, A.; Santarelli, A.; Emanuelli, M. Nicotinamide N-Methyltransferase in Head and Neck Tumors: A Comprehensive Review. Biomolecules 2021, 11, 1594. [Google Scholar] [CrossRef]
- Emanuelli, M.; Sartini, D.; Molinelli, E.; Campagna, R.; Pozzi, V.; Salvolini, E.; Simonetti, O.; Campanati, A.; Offidani, A. The Double-Edged Sword of Oxidative Stress in Skin Damage and Melanoma: From Physiopathology to Therapeutical Approaches. Antioxidants 2022, 11, 612. [Google Scholar] [CrossRef] [PubMed]
- Bacchetti, T.; Campagna, R.; Sartini, D.; Cecati, M.; Morresi, C.; Bellachioma, L.; Martinelli, E.; Rocchetti, G.; Lucini, L.; Ferretti, G.; et al. C. spinosa L. subsp. rupestris Phytochemical Profile and Effect on Oxidative Stress in Normal and Cancer Cells. Molecules 2022, 27, 6488. [Google Scholar] [CrossRef]
- Campagna, R.; Bacchetti, T.; Salvolini, E.; Pozzi, V.; Molinelli, E.; Brisigotti, V.; Sartini, D.; Campanati, A.; Ferretti, G.; Offidani, A.; et al. Paraoxonase-2 Silencing Enhances Sensitivity of A375 Melanoma Cells to Treatment with Cisplatin. Antioxidants 2020, 9, 1238. [Google Scholar] [CrossRef]
- Sartini, D.; Campagna, R.; Lucarini, G.; Pompei, V.; Salvolini, E.; Mattioli-Belmonte, M.; Molinelli, E.; Brisigotti, V.; Campanati, A.; Bacchetti, T.; et al. Differential immunohistochemical expression of paraoxonase-2 in actinic keratosis and squamous cell carcinoma. Hum. Cell 2021, 34, 1929–1931. [Google Scholar] [CrossRef]
- Combs, J.A.; DeNicola, G.M. The Non-Essential Amino Acid Cysteine Becomes Essential for Tumor Proliferation and Survival. Cancers 2019, 11, 678. [Google Scholar] [CrossRef] [PubMed]
- Stipanuk, M.H.; Dominy, J.E., Jr.; Lee, J.I.; Coloso, R.M. Mammalian cysteine metabolism: New insights into regulation of cysteine metabolism. J. Nutr. 2006, 136, 1652S–1659S. [Google Scholar] [CrossRef] [PubMed]
- Koppula, P.; Zhuang, L.; Gan, B. Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 2021, 12, 599–620. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Chio, I.I.C.; Tuveson, D.A. ROS in Cancer: The Burning Question. Trends Mol. Med. 2017, 23, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Kandasamy, P.; Gyimesi, G.; Kanai, Y.; Hediger, M.A. Amino acid transporters revisited: New views in health and disease. Trends Biochem. Sci. 2018, 43, 752–789. [Google Scholar] [CrossRef]
- Lewerenz, J.; Hewett, S.J.; Huang, Y.; Lambros, M.; Gout, P.W.; Kalivas, P.W.; Massie, A.; Smolders, I.; Methner, A.; Pergande, M.; et al. The cystine/glutamate antiporter system xc− in health and disease: From molecular mechanisms to novel therapeutic opportunities. Antioxid. Redox Signal. 2013, 18, 522–555. [Google Scholar] [CrossRef]
- Jyotsana, N.; Ta, K.T.; DelGiorno, K.E. The Role of Cystine/Glutamate Antiporter SLC7A11/xCT in the Pathophysiology of Cancer. Front. Oncol. 2022, 12, 858462. [Google Scholar] [CrossRef]
- Endale, H.T.; Tesfaye, W.; Mengstie, T.A. ROS induced lipid peroxidation and their role in ferroptosis. Front. Cell Dev. Biol. 2023, 11, 1226044. [Google Scholar] [CrossRef]
- Tang, X.; Chen, W.; Liu, H.; Liu, N.; Chen, D.; Tian, D.; Wang, J. Research progress on SLC7A11 in the regulation of cystine/cysteine metabolism in tumors. Oncol. Lett. 2022, 23, 47. [Google Scholar] [CrossRef]
- Qin, K.; Zhang, F.; Wang, H.; Wang, N.; Qiu, H.; Jia, X.; Gong, S.; Zhang, Z. circRNA circSnx12 confers Cisplatin chemoresistance to ovarian cancer by inhibiting ferroptosis through a miR-194-5p/SLC7A11 axis. BMB Rep. 2023, 56, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Yi, S.; Wei, L.; Zhao, B.; Li, J.; Cai, X.; Dong, C.; Liu, X. Microarray-based identification of genes associated with prognosis and drug resistance in ovarian cancer. J. Cell. Biochem. 2019, 120, 6057–6070. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Shen, S.; Qin, J.; Fei, W.; Fan, F.; Gu, J.; Shen, T.; Zhang, T.; Cheng, X. High co-expression of SLC7A11 and GPX4 as a predictor of platinum resistance and poor prognosis in patients with epithelial ovarian cancer. BJOG 2022, 129 (Suppl. S2), 40–49. [Google Scholar] [CrossRef] [PubMed]
- Tossetta, G. Metformin Improves Ovarian Cancer Sensitivity to Paclitaxel and Platinum-Based Drugs: A Review of In Vitro Findings. Int. J. Mol. Sci. 2022, 23, 12893. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhong, B.; Yang, Y.; Wang, Y.; Pan, Z. ceRNA networks in gynecological cancers progression and resistance. J. Drug Target. 2023, 31, 920–930. [Google Scholar] [CrossRef]
- Ke, Y.; Chen, X.; Su, Y.; Chen, C.; Lei, S.; Xia, L.; Wei, D.; Zhang, H.; Dong, C.; Liu, X.; et al. Low Expression of SLC7A11 Confers Drug Resistance and Worse Survival in Ovarian Cancer via Inhibition of Cell Autophagy as a Competing Endogenous RNA. Front. Oncol. 2021, 11, 744940. [Google Scholar] [CrossRef] [PubMed]
- Schulte, T.W.; Akinaga, S.; Soga, S.; Sullivan, W.; Stensgard, B.; Toft, D.; Neckers, L.M. Antibiotic radicicol binds to the N-terminal domain of Hsp90 and shares important biologic activities with geldanamycin. Cell Stress Chaperones 1998, 3, 100–108. [Google Scholar] [CrossRef]
- Creagan, E.T.; Long, H.J.; Ahmann, D.L.; Green, S.J. Phase II evaluation of L-alanosine (NSC-153353) for patients with disseminated malignant melanoma. Am. J. Clin. Oncol. 1984, 7, 543–544. [Google Scholar] [CrossRef]
- Huang, Y.; Dai, Z.; Barbacioru, C.; Sadee, W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance. Cancer Res. 2005, 65, 7446–7454. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Marzioni, D.; Mazzucchelli, R. Role of Natural and Synthetic Compounds in Modulating NRF2/KEAP1 Signaling Pathway in Prostate Cancer. Cancers 2023, 15, 3037. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Piani, F.; Crescimanno, C.; Ciavattini, A.; Giannubilo, S.R.; Marzioni, D. Modulation of NRF2/KEAP1 Signaling in Preeclampsia. Cells 2023, 12, 1545. [Google Scholar] [CrossRef] [PubMed]
- Tossetta, G.; Fantone, S.; Goteri, G.; Giannubilo, S.R.; Ciavattini, A.; Marzioni, D. The Role of NQO1 in Ovarian Cancer. Int. J. Mol. Sci. 2023, 24, 7839. [Google Scholar] [CrossRef] [PubMed]
- Tossetta, G.; Marzioni, D. Targeting the NRF2/KEAP1 pathway in cervical and endometrial cancers. Eur. J. Pharmacol. 2023, 941, 175503. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Licini, C.; Marzioni, D.; Mattioli-Belmonte, M. The multifaced role of HtrA1 in the development of joint and skeletal disorders. Bone 2022, 157, 116350. [Google Scholar] [CrossRef] [PubMed]
- Tossetta, G.; Fantone, S.; Giannubilo, S.R.; Marzioni, D. The Multifaced Actions of Curcumin in Pregnancy Outcome. Antioxidants 2021, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Marinelli Busilacchi, E.; Costantini, A.; Mancini, G.; Tossetta, G.; Olivieri, J.; Poloni, A.; Viola, N.; Butini, L.; Campanati, A.; Goteri, G.; et al. Nilotinib Treatment of Patients Affected by Chronic Graft-versus-Host Disease Reduces Collagen Production and Skin Fibrosis by Downmodulating the TGF-beta and p-SMAD Pathway. Biol. Blood Marrow Transplant. 2020, 26, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Teng, H.; Fang, T.; Xiao, J. Agrimonolide from Agrimonia pilosa suppresses inflammatory responses through down-regulation of COX-2/iNOS and inactivation of NF-kappaB in lipopolysaccharide-stimulated macrophages. Phytomedicine 2016, 23, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Huang, Q.; Chen, L. Inhibition of cell proliferation and triggering of apoptosis by agrimonolide through MAP kinase (ERK and p38) pathways in human gastric cancer AGS cells. Food Funct. 2016, 7, 4605–4613. [Google Scholar] [CrossRef]
- Wang, C.; Qi, C.; Liu, M.; Wang, L.; Cheng, G.; Li, L.; Xing, Y.; Zhao, X.; Liu, J. Protective effects of agrimonolide on hypoxia-induced H9c2 cell injury by maintaining mitochondrial homeostasis. J. Cell Biochem. 2022, 123, 306–321. [Google Scholar] [CrossRef]
- Yu, L.; Gai, Y. Elucidating the Mechanism of Agrimonolide in Treating Colon Cancer Based on Network Pharmacology. Drug Drug Des. Dev. Ther. 2023, 17, 2209–2222. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Wang, H.; Ding, P.; Wang, C. Agrimonolide inhibits cancer progression and induces ferroptosis and apoptosis by targeting SCD1 in ovarian cancer cells. Phytomedicine 2022, 101, 154102. [Google Scholar] [CrossRef] [PubMed]
- Tracz-Gaszewska, Z.; Dobrzyn, P. Stearoyl-CoA Desaturase 1 as a Therapeutic Target for the Treatment of Cancer. Cancers 2019, 11, 948. [Google Scholar] [CrossRef] [PubMed]
- Tesfay, L.; Paul, B.T.; Konstorum, A.; Deng, Z.; Cox, A.O.; Lee, J.; Furdui, C.M.; Hegde, P.; Torti, F.M.; Torti, S.V. Stearoyl-CoA Desaturase 1 Protects Ovarian Cancer Cells from Ferroptotic Cell Death. Cancer Res. 2019, 79, 5355–5366. [Google Scholar] [CrossRef] [PubMed]
- Najafi, M.; Farhood, B.; Mortezaee, K. Cancer stem cells (CSCs) in cancer progression and therapy. J. Cell. Physiol. 2019, 234, 8381–8395. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Monian, P.; Pan, Q.; Zhang, W.; Xiang, J.; Jiang, X. Ferroptosis is an autophagic cell death process. Cell Res. 2016, 26, 1021–1032. [Google Scholar] [CrossRef] [PubMed]
- Muhoberac, B.B.; Vidal, R. Iron, Ferritin, Hereditary Ferritinopathy, and Neurodegeneration. Front. Neurosci. 2019, 13, 1195. [Google Scholar] [CrossRef]
- Sohn, E.J.; Kim, J.H.; Oh, S.O.; Kim, J.Y. Regulation of self-renewal in ovarian cancer stem cells by fructose via chaperone-mediated autophagy. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2023, 1869, 166723. [Google Scholar] [CrossRef]
- Deng, Z.; Hassan, S.; Rafiq, M.; Li, H.; He, Y.; Cai, Y.; Kang, X.; Liu, Z.; Yan, T. Pharmacological Activity of Eriodictyol: The Major Natural Polyphenolic Flavanone. Evid. Based Complement. Altern. Med. 2020, 2020, 6681352. [Google Scholar] [CrossRef]
- Islam, A.; Islam, M.S.; Rahman, M.K.; Uddin, M.N.; Akanda, M.R. The pharmacological and biological roles of eriodictyol. Arch. Pharmacal Res. 2020, 43, 582–592. [Google Scholar] [CrossRef]
- Marzioni, D.; Mazzucchelli, R.; Fantone, S.; Tossetta, G. NRF2 modulation in TRAMP mice: An in vivo model of prostate cancer. Mol. Biol. Rep. 2023, 50, 873–881. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Marzioni, D.; Mazzucchelli, R. Cellular Modulators of the NRF2/KEAP1 Signaling Pathway in Prostate Cancer. Front. Biosci. 2023, 28, 143. [Google Scholar] [CrossRef] [PubMed]
- Tossetta, G.; Marzioni, D. Natural and synthetic compounds in Ovarian Cancer: A focus on NRF2/KEAP1 pathway. Pharmacol. Res. 2022, 183, 106365. [Google Scholar] [CrossRef] [PubMed]
- Tossetta, G.; Fantone, S.; Montanari, E.; Marzioni, D.; Goteri, G. Role of NRF2 in Ovarian Cancer. Antioxidants 2022, 11, 663. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Yang, H.; Son, G.W.; Park, H.R.; Park, C.S.; Jin, Y.H.; Park, Y.S. Eriodictyol Protects Endothelial Cells against Oxidative Stress-Induced Cell Death through Modulating ERK/Nrf2/ARE-Dependent Heme Oxygenase-1 Expression. Int. J. Mol. Sci. 2015, 16, 14526–14539. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, W.J.; Zheng, X.R.; Liu, Q.L.; Du, Q.; Lai, Y.J.; Liu, S.Q. Eriodictyol ameliorates cognitive dysfunction in APP/PS1 mice by inhibiting ferroptosis via vitamin D receptor-mediated Nrf2 activation. Mol. Med. 2022, 28, 11. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, J.; Tie, H.; Tian, W.; Zhao, Y.; Qin, L.; Guo, S.; Li, Q.; Bao, C. Eriodictyol regulated ferroptosis, mitochondrial dysfunction, and cell viability via Nrf2/HO-1/NQO1 signaling pathway in ovarian cancer cells. J. Biochem. Mol. Toxicol. 2023, 37, e23368. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Tian, S.; Chen, Y.; Miao, C.; Zhao, Y.; Wang, R.; Zhang, Q. Ferroptosis-Related Gene Model to Predict Overall Survival of Ovarian Carcinoma. J. Oncol. 2021, 2021, 6687391. [Google Scholar] [CrossRef]
- Yang, J.; Wang, C.; Cheng, S.; Zhang, Y.; Jin, Y.; Zhang, N.; Wang, Y. Construction and validation of a novel ferroptosis-related signature for evaluating prognosis and immune microenvironment in ovarian cancer. Front. Genet. 2022, 13, 1094474. [Google Scholar] [CrossRef]
- Krishnakumar, R.; Kraus, W.L. The PARP side of the nucleus: Molecular actions, physiological outcomes, and clinical targets. Mol. Cell 2010, 39, 8–24. [Google Scholar] [CrossRef]
- Campagna, R.; Vignini, A. NAD+ Homeostasis and NAD+-Consuming Enzymes: Implications for Vascular Health. Antioxidants 2023, 12, 376. [Google Scholar] [CrossRef]
- Campagna, R.; Pozzi, V.; Sartini, D.; Salvolini, E.; Brisigotti, V.; Molinelli, E.; Campanati, A.; Offidani, A.; Emanuelli, M. Beyond Nicotinamide Metabolism: Potential Role of Nicotinamide N-Methyltransferase as a Biomarker in Skin Cancers. Cancers 2021, 13, 4943. [Google Scholar] [CrossRef] [PubMed]
- Alsop, K.; Fereday, S.; Meldrum, C.; deFazio, A.; Emmanuel, C.; George, J.; Dobrovic, A.; Birrer, M.J.; Webb, P.M.; Stewart, C.; et al. BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: A report from the Australian Ovarian Cancer Study Group. J. Clin. Oncol. 2012, 30, 2654–2663. [Google Scholar] [CrossRef] [PubMed]
- Audeh, M.W.; Carmichael, J.; Penson, R.T.; Friedlander, M.; Powell, B.; Bell-McGuinn, K.M.; Scott, C.; Weitzel, J.N.; Oaknin, A.; Loman, N.; et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: A proof-of-concept trial. Lancet 2010, 376, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Schiavoni, V.; Campagna, R.; Pozzi, V.; Cecati, M.; Milanese, G.; Sartini, D.; Salvolini, E.; Galosi, A.B.; Emanuelli, M. Recent Advances in the Management of Clear Cell Renal Cell Carcinoma: Novel Biomarkers and Targeted Therapies. Cancers 2023, 15, 3207. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Kon, N.; Li, T.; Wang, S.J.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 2015, 520, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Stordal, B.; Timms, K.; Farrelly, A.; Gallagher, D.; Busschots, S.; Renaud, M.; Thery, J.; Williams, D.; Potter, J.; Tran, T.; et al. BRCA1/2 mutation analysis in 41 ovarian cell lines reveals only one functionally deleterious BRCA1 mutation. Mol. Oncol. 2013, 7, 567–579. [Google Scholar] [CrossRef]
- Hong, T.; Lei, G.; Chen, X.; Li, H.; Zhang, X.; Wu, N.; Zhao, Y.; Zhang, Y.; Wang, J. PARP inhibition promotes ferroptosis via repressing SLC7A11 and synergizes with ferroptosis inducers in BRCA-proficient ovarian cancer. Redox Biol. 2021, 42, 101928. [Google Scholar] [CrossRef]
- Guler, S.; Konemann, H.; Wolfes, J.; Guner, F.; Ellermann, C.; Rath, B.; Frommeyer, G.; Lange, P.S.; Kobe, J.; Reinke, F.; et al. Lidocaine as an anti-arrhythmic drug: Are there any indications left? Clin. Transl. Sci. 2023, 16, 2429–2437. [Google Scholar] [CrossRef]
- Caracas, H.C.; Maciel, J.V.; Martins, P.M.; de Souza, M.M.; Maia, L.C. The use of lidocaine as an anti-inflammatory substance: A systematic review. J. Dent. 2009, 37, 93–97. [Google Scholar] [CrossRef]
- Adler, D.M.T.; Damborg, P.; Verwilghen, D.R. The antimicrobial activity of bupivacaine, lidocaine and mepivacaine against equine pathogens: An investigation of 40 bacterial isolates. Vet. J. 2017, 223, 27–31. [Google Scholar] [CrossRef]
- Liu, C.; Yu, M.; Li, Y.; Wang, H.; Xu, C.; Zhang, X.; Li, M.; Guo, H.; Ma, D.; Guo, X. Lidocaine inhibits the metastatic potential of ovarian cancer by blocking Na(V) 1.5-mediated EMT and FAK/Paxillin signaling pathway. Cancer Med. 2021, 10, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Li, Y.C.; Zhang, X.Y. Lidocaine Promoted Ferroptosis by Targeting miR-382-5p/SLC7A11 Axis in Ovarian and Breast Cancer. Front. Pharmacol. 2021, 12, 681223. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, P.; De Souza, R.; Huynh, L.; Piquette-Miller, M.; Allen, C. Combination drug delivery strategy for the treatment of multidrug resistant ovarian cancer. Mol. Pharm. 2011, 8, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Ambudkar, S.V.; Dey, S.; Hrycyna, C.A.; Ramachandra, M.; Pastan, I.; Gottesman, M.M. Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 361–398. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, V.S.; Ryan, M.J.; Dhruv, H.D.; Gill, S.; Eichhoff, O.M.; Seashore-Ludlow, B.; Kaffenberger, S.D.; Eaton, J.K.; Shimada, K.; Aguirre, A.J.; et al. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature 2017, 547, 453–457. [Google Scholar] [CrossRef]
- Schinkel, A.H.; Jonker, J.W. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: An overview. Adv. Drug Deliv. Rev. 2003, 55, 3–29. [Google Scholar] [CrossRef]
- Gottesman, M.M.; Ling, V. The molecular basis of multidrug resistance in cancer: The early years of P-glycoprotein research. FEBS Lett. 2006, 580, 998–1009. [Google Scholar] [CrossRef]
- Christie, E.L.; Pattnaik, S.; Beach, J.; Copeland, A.; Rashoo, N.; Fereday, S.; Hendley, J.; Alsop, K.; Brady, S.L.; Lamb, G.; et al. Multiple ABCB1 transcriptional fusions in drug resistant high-grade serous ovarian and breast cancer. Nat. Commun. 2019, 10, 1295. [Google Scholar] [CrossRef]
- Sajid, A.; Rahman, H.; Ambudkar, S.V. Advances in the structure, mechanism and targeting of chemoresistance-linked ABC transporters. Nat. Rev. Cancer 2023, 23, 762–779. [Google Scholar] [CrossRef]
- Seborova, K.; Vaclavikova, R.; Soucek, P.; Elsnerova, K.; Bartakova, A.; Cernaj, P.; Bouda, J.; Rob, L.; Hruda, M.; Dvorak, P. Association of ABC gene profiles with time to progression and resistance in ovarian cancer revealed by bioinformatics analyses. Cancer Med. 2019, 8, 606–616. [Google Scholar] [CrossRef]
- Hamidovic, A.; Hahn, K.; Kolesar, J. Clinical significance of ABCB1 genotyping in oncology. J. Oncol. Pharm. Pract. 2010, 16, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Parekh, H.; Wiesen, K.; Simpkins, H. Acquisition of taxol resistance via P-glycoprotein- and non-P-glycoprotein-mediated mechanisms in human ovarian carcinoma cells. Biochem. Pharmacol. 1997, 53, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Kusumi, R.; Hamashima, S.; Kobayashi, S.; Sasaki, S.; Komiyama, Y.; Izumikawa, T.; Conrad, M.; Bannai, S.; Sato, H. The ferroptosis inducer erastin irreversibly inhibits system x(c)- and synergizes with cisplatin to increase cisplatin’s cytotoxicity in cancer cells. Sci. Rep. 2018, 8, 968. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.H.; Chen, X.; Cai, L.Y.; Nan, X.W.; Chen, J.H.; Chen, X.X.; Yang, Y.; Xing, Z.H.; Wei, M.N.; Li, Y.; et al. Erastin Reverses ABCB1-Mediated Docetaxel Resistance in Ovarian Cancer. Front. Oncol. 2019, 9, 1398. [Google Scholar] [CrossRef] [PubMed]
- Campagna, R.; Belloni, A.; Pozzi, V.; Salvucci, A.; Notarstefano, V.; Togni, L.; Mascitti, M.; Sartini, D.; Giorgini, E.; Salvolini, E.; et al. Role Played by Paraoxonase-2 Enzyme in Cell Viability, Proliferation and Sensitivity to Chemotherapy of Oral Squamous Cell Carcinoma Cell Lines. Int. J. Mol. Sci. 2022, 24, 338. [Google Scholar] [CrossRef]
- Campagna, R.; Pozzi, V.; Giorgini, S.; Morichetti, D.; Goteri, G.; Sartini, D.; Serritelli, E.N.; Emanuelli, M. Paraoxonase-2 is upregulated in triple negative breast cancer and contributes to tumor progression and chemoresistance. Hum. Cell 2023, 36, 1108–1119. [Google Scholar] [CrossRef]
- Wilson, L.A.; Yamamoto, H.; Singh, G. Role of the transcription factor Ets-1 in cisplatin resistance. Mol. Cancer Ther. 2004, 3, 823–832. [Google Scholar] [CrossRef]
- Verschoor, M.L.; Singh, G. Ets-1 regulates intracellular glutathione levels: Key target for resistant ovarian cancer. Mol. Cancer 2013, 12, 138. [Google Scholar] [CrossRef]
- Lekstrom-Himes, J.; Xanthopoulos, K.G. Biological role of the CCAAT/enhancer-binding protein family of transcription factors. J. Biol. Chem. 1998, 273, 28545–28548. [Google Scholar] [CrossRef]
- Zhang, X.; Zheng, X.; Ying, X.; Xie, W.; Yin, Y.; Wang, X. CEBPG suppresses ferroptosis through transcriptional control of SLC7A11 in ovarian cancer. J. Transl. Med. 2023, 21, 334. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Gesuita, R.; Montironi, R.; Marzioni, D.; Mazzucchelli, R. AT-rich interactive domain 1A (ARID1A) cannot be considered a morphological marker for prostate cancer progression: A pilot study. Acta Histochem. 2022, 124, 151847. [Google Scholar] [CrossRef] [PubMed]
- Fantone, S.; Mazzucchelli, R.; Giannubilo, S.R.; Ciavattini, A.; Marzioni, D.; Tossetta, G. AT-rich interactive domain 1A protein expression in normal and pathological pregnancies complicated by preeclampsia. Histochem. Cell Biol. 2020, 154, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Tang, C. The Role of ARID1A in Tumors: Tumor Initiation or Tumor Suppression? Front. Oncol. 2021, 11, 745187. [Google Scholar] [CrossRef] [PubMed]
- Fontana, B.; Gallerani, G.; Salamon, I.; Pace, I.; Roncarati, R.; Ferracin, M. ARID1A in cancer: Friend or foe? Front. Oncol. 2023, 13, 1136248. [Google Scholar] [CrossRef] [PubMed]
- Ogiwara, H.; Takahashi, K.; Sasaki, M.; Kuroda, T.; Yoshida, H.; Watanabe, R.; Maruyama, A.; Makinoshima, H.; Chiwaki, F.; Sasaki, H.; et al. Targeting the Vulnerability of Glutathione Metabolism in ARID1A-Deficient Cancers. Cancer Cell 2019, 35, 177–190.e178. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Gross, K.M.; Kuperwasser, C. Molecular regulation of Snai2 in development and disease. J. Cell Sci. 2019, 132, jcs235127. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, X.; Xu, S.; Jin, P.; Li, X.; Wei, X.; Liu, D.; Huang, K.; Long, S.; Wang, Y.; et al. Reprogramming of stromal fibroblasts by SNAI2 contributes to tumor desmoplasia and ovarian cancer progression. Mol. Cancer 2017, 16, 163. [Google Scholar] [CrossRef]
- Li, J.; Li, S.; Chen, R.; Lu, X. Increased risk of poor survival in ovarian cancer patients with high expression of SNAI2 and lymphovascular space invasion. Oncotarget 2017, 8, 9672–9685. [Google Scholar] [CrossRef]
- Jin, Y.; Chen, L.; Li, L.; Huang, G.; Huang, H.; Tang, C. SNAI2 promotes the development of ovarian cancer through regulating ferroptosis. Bioengineered 2022, 13, 6451–6463. [Google Scholar] [CrossRef]
- Karamali, N.; Ebrahimnezhad, S.; Khaleghi Moghadam, R.; Daneshfar, N.; Rezaiemanesh, A. HRD1 in human malignant neoplasms: Molecular mechanisms and novel therapeutic strategy for cancer. Life Sci. 2022, 301, 120620. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Zhang, W. HRD1 functions as a tumor suppressor in ovarian cancer by facilitating ubiquitination-dependent SLC7A11 degradation. Cell Cycle 2023, 22, 1116–1126. [Google Scholar] [CrossRef] [PubMed]
- Licini, C.; Avellini, C.; Picchiassi, E.; Mensa, E.; Fantone, S.; Ramini, D.; Tersigni, C.; Tossetta, G.; Castellucci, C.; Tarquini, F.; et al. Pre-eclampsia predictive ability of maternal miR-125b: A clinical and experimental study. Transl. Res. 2021, 228, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xun, C.; Yu, C.H. Role of microRNA-regulated cancer stem cells in recurrent hepatocellular carcinoma. World J. Hepatol. 2022, 14, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Shen, J.; Wang, Q.; Ke, Y.; Yan, Q.; Li, H.; Zhang, D.; Duan, S. LINC00324 in cancer: Regulatory and therapeutic implications. Front. Oncol. 2022, 12, 1039366. [Google Scholar] [CrossRef] [PubMed]
- Rysz, J.; Konecki, T.; Franczyk, B.; Lawinski, J.; Gluba-Brzozka, A. The Role of Long Noncoding RNA (lncRNAs) Biomarkers in Renal Cell Carcinoma. Int. J. Mol. Sci. 2022, 24, 643. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, Y.; Lv, X.; Zhou, J.; Cang, S.; Song, Y. LncRNA ADAMTS9-AS1 inhibits the stemness of lung adenocarcinoma cells by regulating miR-5009-3p/NPNT axis. Genomics 2023, 115, 110596. [Google Scholar] [CrossRef] [PubMed]
- Javanmard, A.R.; Jahanbakhshi, A.; Nemati, H.; Mowla, S.J.; Soltani, B.M. ADAMTS9-AS1 Long Non-coding RNA Sponges miR-128 and miR-150 to Regulate Ras/MAPK Signaling Pathway in Glioma. Cell. Mol. Neurobiol. 2023, 43, 2309–2322. [Google Scholar] [CrossRef]
- Zhang, B.; He, Y.; Ma, G.; Zhang, L.; Qi, P.; Han, D.; Yue, Z.; Shang, P. Identification of stemness index-related long noncoding RNA SNHG12 in human bladder cancer based on WGCNA. Mol. Cell Probes 2022, 66, 101867. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, L.; Zou, W.; Wang, R.; Wang, X.; Chen, Z. ADAMTS9-AS1 Constrains Breast Cancer Cell Invasion and Proliferation via Sequestering miR-301b-3p. Front. Cell Dev. Biol. 2021, 9, 719993. [Google Scholar] [CrossRef]
- Cai, L.; Hu, X.; Ye, L.; Bai, P.; Jie, Y.; Shu, K. Long non-coding RNA ADAMTS9-AS1 attenuates ferroptosis by Targeting microRNA-587/solute carrier family 7 member 11 axis in epithelial ovarian cancer. Bioengineered 2022, 13, 8226–8239. [Google Scholar] [CrossRef]
- Yuan, J.; Liu, Z.; Song, R. Antisense lncRNA As-SLC7A11 suppresses epithelial ovarian cancer progression mainly by targeting SLC7A11. Pharmazie 2017, 72, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, H.; Burnett, J.C.; Rossi, J.J. The role of antisense long noncoding RNA in small RNA-triggered gene activation. RNA 2014, 20, 1916–1928. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.Y.; Cai, Z.R.; Liu, J.; Wang, D.S.; Ju, H.Q.; Xu, R.H. Circular RNA: Metabolism, functions and interactions with proteins. Mol. Cancer 2020, 19, 172. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Qadir, J.; Yang, B.B. CircRNA perspective: New strategies for RNA therapy. Trends Mol. Med. 2022, 28, 343–344. [Google Scholar] [CrossRef]
- Liu, T.; Jiang, L.; Tavana, O.; Gu, W. The Deubiquitylase OTUB1 Mediates Ferroptosis via Stabilization of SLC7A11. Cancer Res. 2019, 79, 1913–1924. [Google Scholar] [CrossRef]
- Feng, H.; Stockwell, B.R. Unsolved mysteries: How does lipid peroxidation cause ferroptosis? PLoS Biol. 2018, 16, e2006203. [Google Scholar] [CrossRef]
- Roh, J.L.; Kim, E.H.; Jang, H.; Shin, D. Aspirin plus sorafenib potentiates cisplatin cytotoxicity in resistant head and neck cancer cells through xCT inhibition. Free. Radic. Biol. Med. 2017, 104, 1–9. [Google Scholar] [CrossRef]
| Modulator | Model | Result | Ref. |
|---|---|---|---|
Agrimonolide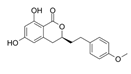 | A2780 and SKOV-3 cells | Agrimonolide inhibited proliferation, migration, and invasion and promoted apoptosis. Agrimonolide induced ferroptosis, downregulated SCD1, SLC7A11 and GPX4 expression and increased ROS levels, total iron levels and Fe2+. SCD1 overexpression increased SLC7A11 and GPX4 protein expression. Agrimonolide acts as an apoptosis- and ferroptosis-inducing agent, inducing ferroptosis through the modulation of the SCD1/ SLC7A11/ GPX4 axis. | [51] |
Fructose | A2780 and SKOV3 CSCs in spheroids | Fructose treatment decreased SLC7A11 expression while increasing FTH expression, resulting in an enrichment of the ferroptosis pathway (demonstrated using KEGG functional analysis). | [57] |
Eriodictyol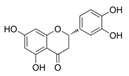 | CaoV3 A2780 | Eriodictyol suppressed cell viability and induced cell apoptosis. Eriodictyol increased the Fe2+ content and ROS production while reducing SLC7A11 and GPX4. Eriodictyol downregulated NRF2 expression in tumor tissues in mice xenograft models. | [66] |
Olaparib | HEY and A2780 ovarian cancer cells (which carry wild-type BRCA1/2) and p53-deficient ovarian cancer cell line SKOV3 | Olaparib downregulated SLC7A1 and GPX4 and increased p53 expression, promoting ferroptosis and DNA damage in HEY and A2780 ovarian cancer cells and leading to apoptosis. Treatment of SKOV3 cells with olaparib reduced PARP1 expression without affecting SLC7A11 protein levels. In addition, olaparib downregulated SLC7A11 expression in HEY control cells carrying the p53 protein but not in p53 knockout cells. Olaparib represses SLC7A11 transcription through the upregulation of p53 expression. | [77] |
Lidocaine | SKOV-3 ovarian cancer cells | Lidocaine induced the accumulation of Fe2+ and increased ROS levels, which suppressed proliferation, invasion and migration and promoted apoptosis. Lidocaine downregulated SLC7A11 expression by enhancing miR-382-5p levels. The overexpression of miR-382-5p decreased SLC7A11 expression, while the inhibition of miR-382-5p blocked lidocaine-induced ferroptosis in SKOV-3 cells. Lidocaine promotes ferroptosis by modulating the miR-382-5p/SLC7A11 axis. | [82] |
Erastin | Docetaxel-resistant (A2780/Taxol) and -sensitive (A2780) ovarian cancer cells | Erastin and docetaxel cotreatment decreased cell viability, promoted cell apoptosis, and induced cell cycle arrest at the G2/M stage in A2780/Taxol cells (which overexpress the ABCB1 protein). Erastin binds ABCB1 and antagonizes its drug-efflux function, increasing the amount of intracellular docetaxel. The inhibition of SLC7A11 by erastin decreases the intracellular GSH content, further improving docetaxel efficiency. | [94] |
| Modulator | Model | Result | Reference |
|---|---|---|---|
| Ets-1 | 2008 | The overexpression of Ets-1 decreased intracellular ROS and increased intracellular GSH, GPX antioxidant activity and SLC7A11 expression and activity. Under oxidative stress, the intracellular GSH levels decreased significantly in correlation with increased Ets-1 expression following sulfasalazine (a SLC7A11 inhibitor) treatment. Ets-1 regulates the enhanced SLC7A11 activity under oxidative stress. | [98] |
| CEBPG | A2780 and Hey ovarian cancer cells | The knockdown of CEBPG decreased GPX4 and SLC7A11 expression, inhibited cancer cell proliferation and invasion and promoted ferroptosis. CEBPG inhibited ferroptosis and promoted the transcription of the SLC7A11 gene by binding its promoter region. | [100] |
| ARID1A | TOV21G ovarian cancer cells | ARID1A-deficient cells showed a decreased expression of SLC7A11 and are more sensitive to GSH inhibition treatments. The overexpression of ARID1A significantly increased SLC7A11 protein expression. | [105] |
| SNAI2 | SKOV3, A2780 and CAOV3 ovarian cancer cells | SNAI2 knockdown decreased cell migration and invasion and promoted cell apoptosis and ferroptosis. SNAI2 directly binds to the promoter of the SLC7A11 gene, decreasing its expression. SLC7A11 expression was also decreased after erastin treatment. | [109] |
| HRD1 | SKOV3 and A2780 ovarian cancer cells | The overexpression of HRD1 inhibited proliferation and promoted apoptosis and ferroptosis. HRD1 interacted with SLC7A11 and promoted its degradation. | [111] |
| LncRNA ADAMTS9-AS1 | ES-2, OVCAR3, SKOV3, CAOV-3 and normal human ovarian surface epithelial (OSE) cell line | LncRNA ADAMTS9-AS1 expression was increased in cancer cells. The knockdown of lncRNA ADAMTS9-AS1 in cancer cells inhibited cellular proliferation and migration by promoting ferroptosis, and miRNA-587 increased, while SLC7A11 expression decreased. The same results were obtained when miRNA-587 was overexpressed. LncRNA ADAMTS9-AS1 attenuates ferroptosis by targeting the miR-587/SLC7A11 axis. | [120] |
| LncRNA As-SLC7A11 | OVCA433, OVCA429, TOV112D ovarian cancer cell lines and normal human ovarian surface epithelial (OSE) cell line | LncRNA As-SLC7A11 levels were decreased in ovarian cancer cell lines compared to normal OSE cells. The silencing of lncRNA As-SLC7A11 enhanced ovarian cancer cell migration and invasion, while its overexpression induced apoptosis and decreased SLC7A11 protein expression. | [121] |
| CircSnx12 | Cisplatin-resistant SKOV3/DDP and A2780/DDP cells, and cisplatin-sensitive SKOV3 and A2780 cells. | SKOV3/DDP and A2780/DDP cells showed increased expression of SLC7A11, GPX4 and circSnx12 compared to the cisplatin-sensitive cells. The silencing of circSnx12 in SKOV3/DDP and A2780/DDP cells increased the expression of miR-194-5p and decreased SLC7A11, GPX4 and GSH levels, thus increasing cisplatin sensitivity and ferroptosis. MiR-194-5p overexpression decreased SLC7A11 expression. CircSnx12 regulates ferroptosis and chemoresistance via the miR-194-5p/SLC7A11 pathway by sponging miR-194-5p. | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fantone, S.; Piani, F.; Olivieri, F.; Rippo, M.R.; Sirico, A.; Di Simone, N.; Marzioni, D.; Tossetta, G. Role of SLC7A11/xCT in Ovarian Cancer. Int. J. Mol. Sci. 2024, 25, 587. https://doi.org/10.3390/ijms25010587
Fantone S, Piani F, Olivieri F, Rippo MR, Sirico A, Di Simone N, Marzioni D, Tossetta G. Role of SLC7A11/xCT in Ovarian Cancer. International Journal of Molecular Sciences. 2024; 25(1):587. https://doi.org/10.3390/ijms25010587
Chicago/Turabian StyleFantone, Sonia, Federica Piani, Fabiola Olivieri, Maria Rita Rippo, Angelo Sirico, Nicoletta Di Simone, Daniela Marzioni, and Giovanni Tossetta. 2024. "Role of SLC7A11/xCT in Ovarian Cancer" International Journal of Molecular Sciences 25, no. 1: 587. https://doi.org/10.3390/ijms25010587
APA StyleFantone, S., Piani, F., Olivieri, F., Rippo, M. R., Sirico, A., Di Simone, N., Marzioni, D., & Tossetta, G. (2024). Role of SLC7A11/xCT in Ovarian Cancer. International Journal of Molecular Sciences, 25(1), 587. https://doi.org/10.3390/ijms25010587










