Intestinal Permeability in Patients Early after Kidney Transplantation Treated with Two Different Formulations of Once-Daily Tacrolimus
Abstract
1. Introduction
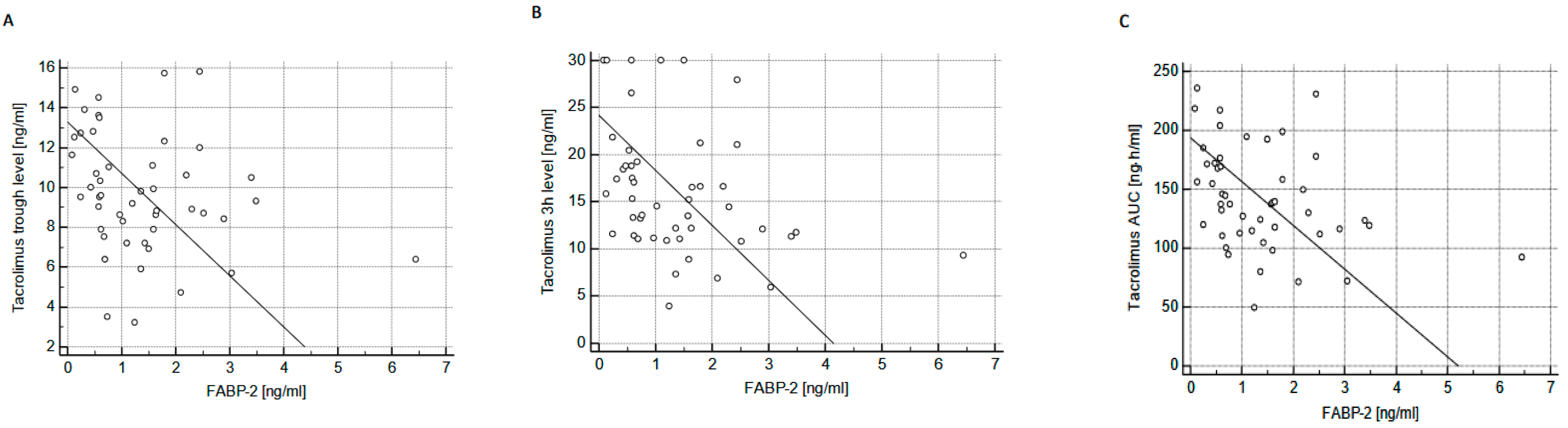
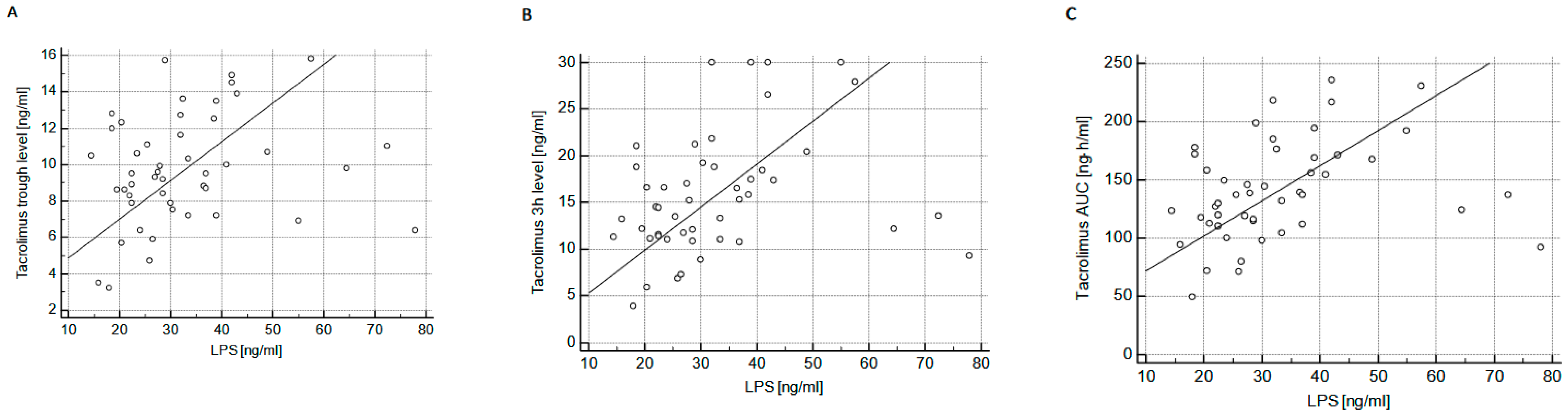
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Group
4.2. Immunosuppression Protocol
4.3. Laboratory and Hormonal Parameters
4.4. Data and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stratta, P.; Quaglia, M.; Cena, T.; Antoniotti, R.; Fenoglio, R.; Menegotto, A.; Ferrante, D.; Genazzani, A.; Terrazzino, S.; Magnani, C. The interactions of age, sex, body mass index, genetics, and steroid weight-based doses on tacrolimus dosing requirement after adult kidney transplantation. Eur. J. Clin. Pharmacol. 2012, 68, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Krzyżowska, K.; Kolonko, A.; Giza, P.; Chudek, J.; Więcek, A. Which kidney transplant recipients can benefit from the initial tacrolimus dose reduction? BioMed Res. Int. 2018, 2018, 4573452. [Google Scholar] [CrossRef] [PubMed]
- Vavic, N.; Rancic, N.; Dragojevic-Simic, V.; Draskovic-Pavlovic, B.; Boonjic, D.; Ignjatovic, L.; Mikov, M. The influence of comedication on tacrolimus blood concentration in patients subjected to kidney transplantation: A retrospective study. Eur. J. Drug. Metabol. Pharmacokinet. 2014, 39, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Amada, N.; Sato, T.; Miura, S.; Ohashi, Y.; Sekiguchi, S.; Satomi, S.; Okazaki, H. Severe elevations of FK506 blood concentrations due to diarrhea in renal transplant recipients. Clin. Transplant. 2004, 18, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Staatz, C.E.; Tett, S.E. Clinical pharmacokinetics of once-daily tacrolimus in solid-organ transplantation. Clin. Pharmacokin. 2015, 54, 993–1025. [Google Scholar] [CrossRef] [PubMed]
- Fernandez Rivera, C.F.; Calvo Rodriguez, M.C.; Poveda, J.L.; Pascual, J.; Crespo, M.; Gomez, G.; Cabello Pelegrin, S.; Paul, J.; Lauzurica, R.; Perez Mir, M.; et al. Better study. Bioavailability of once-dailyBioavailability of once-daily tacrolimus formulations used in clinical practice in the management of de novo kidney transplant recipients: The better study. Clin. Transplant. 2022, 36, e14550. [Google Scholar] [CrossRef] [PubMed]
- Glander, P.; Waiser, J.; Kasbohm, S.; Friedersdorff, F.; Peters, R.; Rudolph, B.; Wu, K.; Budde, K.; Liefeldt, L. Bioavailability and costs of once-daily and twice-daily tacrolimus formulation in de novo kidney transplantation. Clin. Transplant. 2018, 32, e13311. [Google Scholar] [CrossRef]
- Lee, J.R.; Muthukumar, T.; Dadhania, D.; Taur, Y.; Jenq, R.R.; Toussaint, N.C.; Ling, L.; Pamer, E.; Suthanthiran, M. Gut microbiota and tacrolimus dosing in kidney transplantation. PLoS ONE 2015, 10, e0122399. [Google Scholar] [CrossRef]
- Coilly, A.; Calmus, Y.; Chermak, F.; Dumortier, J.; Duvoux, C.; Guillaud, O.; Houssel-Debry, P.; Neau-Cransac, M.; Stocco, J. Once-daily prolonged relase tacrolimus in liver transplantation: Expert’s literature review and recommendations. Liver Transplant. 2015, 21, 1312–1321. [Google Scholar] [CrossRef]
- Gabe, S.M.; Bjarnason, I.; Tolou-Ghamari, Z.; Tredger, J.M.; Johnson, P.G.; Barclay, G.R.; Williams, R.; Silk, D.B. The effect of tacrolimus (FK506) on intestinal barrier function and cellular energy production in humans. Gastroenterology 1998, 115, 67–74. [Google Scholar] [CrossRef]
- Parrilli, G.; Abazia, C.; Sarnelli, G.; Corsaro, M.M.; Coccoli, P.; Viglione, L.; Cuomo, R.; Budillon, G. Effect of chronic administration of tacrolimus and cyclosporine on human gastrointestinal permeability. Liver Transplant. 2003, 9, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.R.; Muthukumar, T.; Dadhania, D.; Toussaint, N.C.; Ling, L.; Pamer, E.; Suthanthiran, M. Gut microbial community structure and complications after kidney transplantation: A pilot study. Transplantation 2014, 98, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Wegh, C.A.M.; de Roos, N.M.; Hovenier, R.; Meijerink, J.; Besseling-van der Vaart, I.; van Hemert, S.; Witteman, B.J.M. Intestinal permeability measured by urinary sucrose excretion correlates with serum zonulin and faecal calprotectin concentrations in UC patients in remission. J. Nutr. Metabol. 2019, 2019, 2472754. [Google Scholar] [CrossRef] [PubMed]
- Mokkala, K.; Röytiö, H.; Munukka, E.; Pietilä, S.; Ekblad, U.; Rönnemaa, T.; Eerola, E.; Laiho, A.; Laitinen, K. Gut microbiota richness and composition and dietary intake of overweight pregnant women are related to serum zonulin concentration, a marker for intestinal permeability. J. Nutr. 2016, 146, 1694–1700. [Google Scholar] [CrossRef]
- Cinkajzlová, A.; Lacinová, Z.; Kloučková, J.; Kaválková, P.; Kratochvílová, H.; Křížová, J.; Trachta, P.; Mráz, M.; Haluzík, M. Increased intestinal permeability in patients with short bowel syndrome is not affected by parental nutrition. Physiol. Res. 2019, 68, 817–825. [Google Scholar] [CrossRef]
- Guo, S.; Al-Sadi, R.; Said, H.M.; Ma, T.Y. Lipopolysaccharide causes an increase in intestinal tight junction permeability in vitro and in vivo by inducing enterocyte membrane expression and localization of TLR-4 and CD14. Am. J. Pathol. 2013, 182, 375–387. [Google Scholar] [CrossRef]
- Hayashi, M.; Tomita, M. Mechanistic analysis for drug permeation through intestinal membrane. Drug. Metab. Pharmacokinet. 2007, 22, 67–77. [Google Scholar] [CrossRef]
- Seethaler, B.; Basrai, M.; Neyrinck, A.M.; Nazare, J.A.; Walter, J.; Delzenne, N.M.; Bischoff, S.C. Biomarkers for assessment of intestinal permeability in clinical practice. Am. J. Physiol. Gastriointest Liver Physiol. 2021, 321, G11–G17. [Google Scholar] [CrossRef]
- Funaoka, H.; Kanda, T.; Fujii, H. Intestinal fatty acid-binding protein (I-FABP) as a new biomarker for intestinal disease. Rinsho Byori 2010, 58, 162–168. [Google Scholar]
- Guthmann, F.; Borchers, T.; Wolfrum, C.; Wustrack, T.; Bartholomaus, S.; Spener, F. Plasma concentration of intestinal- and liver-FABP in neonates suffering from necrotizing enterocolitis and in healthy preterm neonates. Mol. Cell. Biochem. 2002, 239, 227–234. [Google Scholar] [CrossRef]
- Cox, A.J.; Zhang, P.; Bowden, D.W.; Devereaux, B.; Davoren, P.M.; Cripps, A.W.; West, N.P. Increased intestinal permeability as a risk factor for type 2 diabetes. Diabetes Metab. 2017, 43, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, B.; Roth, B.; Larsson, E.; Hoglund, P. Calprotectin in serum and zonulin in serum and feces are elevated after introduction of a diet with lower carbohydrate content and higher fiber, fat and protein contents. Biomed. Rep. 2017, 6, 411–422. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Prasad, R.; Feng, D.; Beli, E.; Li Calzi, S.; Longhini, A.L.F.; Lamendella, R.; Floyd, J.L.; Dupont, M.; Noothi, S.K.; et al. Bone marrow-derived cells restore functional integrity of the gut epithelial and vascular barriers in a model of diabetes and ACE2 deficiency. Circ. Res. 2019, 125, 969–988. [Google Scholar] [CrossRef] [PubMed]
- Carron, C.; de Barros, J.P.P.; Gaiffe, E.; Deckert, V.; Adda-Rezig, H.; Roubiou, C.; Laheurte, C.; Masson, D.; Simula-Faivre, D.; Louvat, P.; et al. End-stage renal disease-associated gut bacterial translocation: Evolution and impact on chronic inflammation and acute rejection after renal transplantation. Front. Immunol. 2019, 10, 1630. [Google Scholar] [CrossRef]
- Nighot, M.; Al-Sadi, R.; Guo, S.; Rawat, M.; Nighot, P.; Watterson, M.D.; Ma, T.Y. Lipopolysaccharide-induced increase in intestinal epithelial tight permeability is mediated by toll-like receptor 4/myeloid differentiation primary response 88 (MyD88) activation of myosin light chain kinase expression. Am. J. Pathol. 2017, 187, 2698–2710. [Google Scholar] [CrossRef]
- Fasano, A. Zonulin, regulation of tight junctions, and autoimmune diseases. Ann. N. Y. Acad. Sci. 2012, 1258, 25–33. [Google Scholar] [CrossRef]
- Talley, N.J.; Holtmann, G.J.; Jones, M.; Koloski, N.A.; Walker, M.M.; Burns, G.; Potter, M.D.E.; Shah, A.; Keely, S. Zonulin in serum as a biomarker fails to identify the IBS, functional dyspepsia and non-coeliac wheat sensitivity. Gut 2020, 69, 1719–1722. [Google Scholar] [CrossRef]
- Scheffler, L.; Crane, A.; Heyne, H.; Tönjes, A.; Schleinitz, D.; Ihling, C.H.; Stumvoll, M.; Freire, R.; Fiorentino, M.; Fasano, A.; et al. Widely used commercial ELISA does not detect precursor of haptoglobin2, but recognizes properdin as a potential second member of the zonulin family. Front. Endocrinol. 2018, 9, 22. [Google Scholar] [CrossRef]
- Kamar, N.; Cassuto, E.; Piotti, G.; Govoni, M.; Ciurlia, G.; Geraci, S.; Poli, G.; Nicolini, G.; Mariat, C.; Essig, M.; et al. Pharmacokinetics of prolonged-release once-daily formulations of tacrolimus in de novo kidney transplant recipients: A randomized, parallel-group, open-label, multicenter study. Adv. Ther. 2019, 36, 462–477. [Google Scholar] [CrossRef]
- Liu, X.X.; Xu, B.M.; Chen, H.; Song, Y.Y.; Yang, W.H.; Chen, B. Limited sampling strategy for the estimation of tacrolimus area under the curve concentration-time curve in Chinese adult liver transplant patients. Pharmacology 2016, 98, 229–241. [Google Scholar] [CrossRef]
| Advagraf N = 49 | Envarsus N = 49 | p | |
|---|---|---|---|
| Patient | |||
| Age [years] | 52 (39–59) | 50 (42–58) | 0.97 |
| Sex [M/F] | 28/21 | 28/81 | 1.00 |
| BMI [kg/m2] | 25.2 (22.9–28.9) | 25.9 (23.3–29.0) | 0.51 |
| Dialysis vintage [months] * | 31 (20–44) | 31 (24–50) | 0.58 |
| Residual diuresis [mL] * | 500 (100–2000) | 500 (150–1000) | 0.96 |
| Pre-transplant diabetes [n (%)] | 6 (12.2) | 6 (12.2) | 1.0 |
| Early post-transplant diabetes [n (%)] | 2 (4.1) | 2 (4.1) | 1.0 |
| Transplant procedure | |||
| Retransplant [n, %] | 6/43 | 3/46 | 0.30 |
| HLA class I mismatch * | 2.49 (2.25–2.73) | 2.31 (2.04–2.57) | 0.31 |
| HLA class II mismatch * | 0.65 (0.50–0.80) | 0.61 (0.46–0.77) | 0.70 |
| Cold ischemia time [h] | 17.2 (15.5–18.9) | 18.9 (17.2–20.7) | 0.14 |
| Induction therapy | |||
| Basiliximab [n, %] | 35 (71.4) | 33 (67.3) | 0.66 |
| Antithymocyte globulin [n, %] | 14 (28.6) | 16 (32.7) | |
| Delayed graft function [n, %] | 10 (20.4) | 9 (18.4) | 0.80 |
| Tacrolimus dosing and exposure prior to discharge | |||
| Tacrolimus dose [mg/d] * | 7.5 (6.0–11.0) | 4.75 (3.25–7.0) | <0.001 |
| Tacrolimus dose per kg [mg/kg] * | 0.12 (0.08–0.16) | 0.07 (0.05–0.10) | <0.001 |
| Tacrolimus trough level [ng/mL] * | 8.4 (7.5–9.6) | 9.5 (7.9–11.6) | <0.05 |
| Tacrolimus 3h after dose [ng/mL] * | 20.8 (16.9–23.7) | 14.5 (11.4–18.8) | <0.001 |
| Tacrolimus AUC [ng·h/mL] * | 157.6 (134.3–171.6) | 137.1 (114.6–170.8) | <0.05 |
| Tacrolimus C/D ratio * | 1.17 (0.73–1.60) | 1.98 (1.29–3.02) | <0.001 |
| Advagraf N = 49 | Envarsus N = 49 | p | |
|---|---|---|---|
| eGFR [mL/min/1.73 m2] | 50.4 (33.6–70.3) | 53.5 (39.4–70.0) | 0.88 |
| C-reactive protein [mg/L] | 3.1 (1.0–5.0) | 3.3 (1.3–7.0) | 0.31 |
| Interleukin-6 [pg/mL] | 3.2 (2.1–5.1) | 4.2 (2.5–7.8) | 0.13 |
| ALT max [IU/L] | 34 (24–54) | 35 (19–55) | 0.48 |
| ALT at discharge [IU/L] | 28 (17–40) | 25 (17–36) | 0.71 |
| GGT max [IU/L] | 54 (26–94) | 48 (29–103) | 0.91 |
| GGT at discharge [IU/L] | 42 (23–85) | 37 (29–56) | 0.91 |
| Zonulin [ng/mL] | 6.2 (5.4–7.1) | 6.6 (5.2–7.6) | 0.22 |
| Calprotectin [μg/mL] | 1.73 (1.37–2.61) | 1.91 (1.62–3.06) | 0.16 |
| LPS [ng/mL] | 29.0 (22.5–36.0) | 29.0 (22.5–39.0) | 0.87 |
| LBP [μg/mL] | 5.07 (3.00–7.03) | 4.50 (3.62–6.55) | 0.76 |
| FABP-2 [ng/mL] | 1.42 (0.83–2.05) | 1.15 (0.59–1.79) | 0.15 |
| CD-14 [pg/mL] | 1.68 (1.47–1.83) × 106 | 1.51 (1.36–1.8) × 106 | 0.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolonko, A.; Słabiak-Błaż, N.; Pokora, P.; Piecha, G.; Więcek, A. Intestinal Permeability in Patients Early after Kidney Transplantation Treated with Two Different Formulations of Once-Daily Tacrolimus. Int. J. Mol. Sci. 2023, 24, 8344. https://doi.org/10.3390/ijms24098344
Kolonko A, Słabiak-Błaż N, Pokora P, Piecha G, Więcek A. Intestinal Permeability in Patients Early after Kidney Transplantation Treated with Two Different Formulations of Once-Daily Tacrolimus. International Journal of Molecular Sciences. 2023; 24(9):8344. https://doi.org/10.3390/ijms24098344
Chicago/Turabian StyleKolonko, Aureliusz, Natalia Słabiak-Błaż, Patrycja Pokora, Grzegorz Piecha, and Andrzej Więcek. 2023. "Intestinal Permeability in Patients Early after Kidney Transplantation Treated with Two Different Formulations of Once-Daily Tacrolimus" International Journal of Molecular Sciences 24, no. 9: 8344. https://doi.org/10.3390/ijms24098344
APA StyleKolonko, A., Słabiak-Błaż, N., Pokora, P., Piecha, G., & Więcek, A. (2023). Intestinal Permeability in Patients Early after Kidney Transplantation Treated with Two Different Formulations of Once-Daily Tacrolimus. International Journal of Molecular Sciences, 24(9), 8344. https://doi.org/10.3390/ijms24098344






