ZnO Nanorods Create a Hypoxic State with Induction of HIF-1 and EPAS1, Autophagy, and Mitophagy in Cancer and Non-Cancer Cells
Abstract
1. Introduction
2. Results
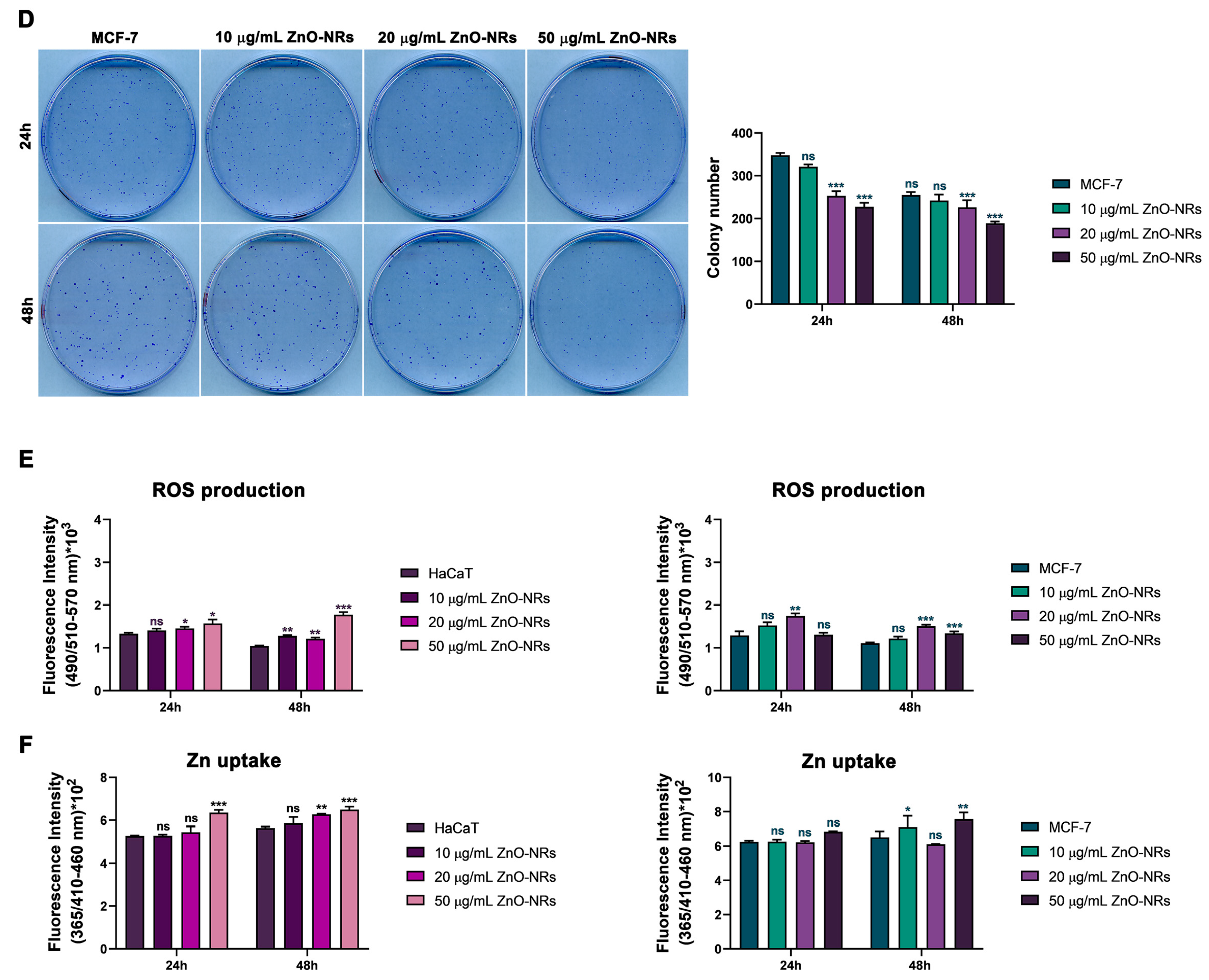
2.1. ZnO-NRs Reduce Cell Vitality and Colony Formation
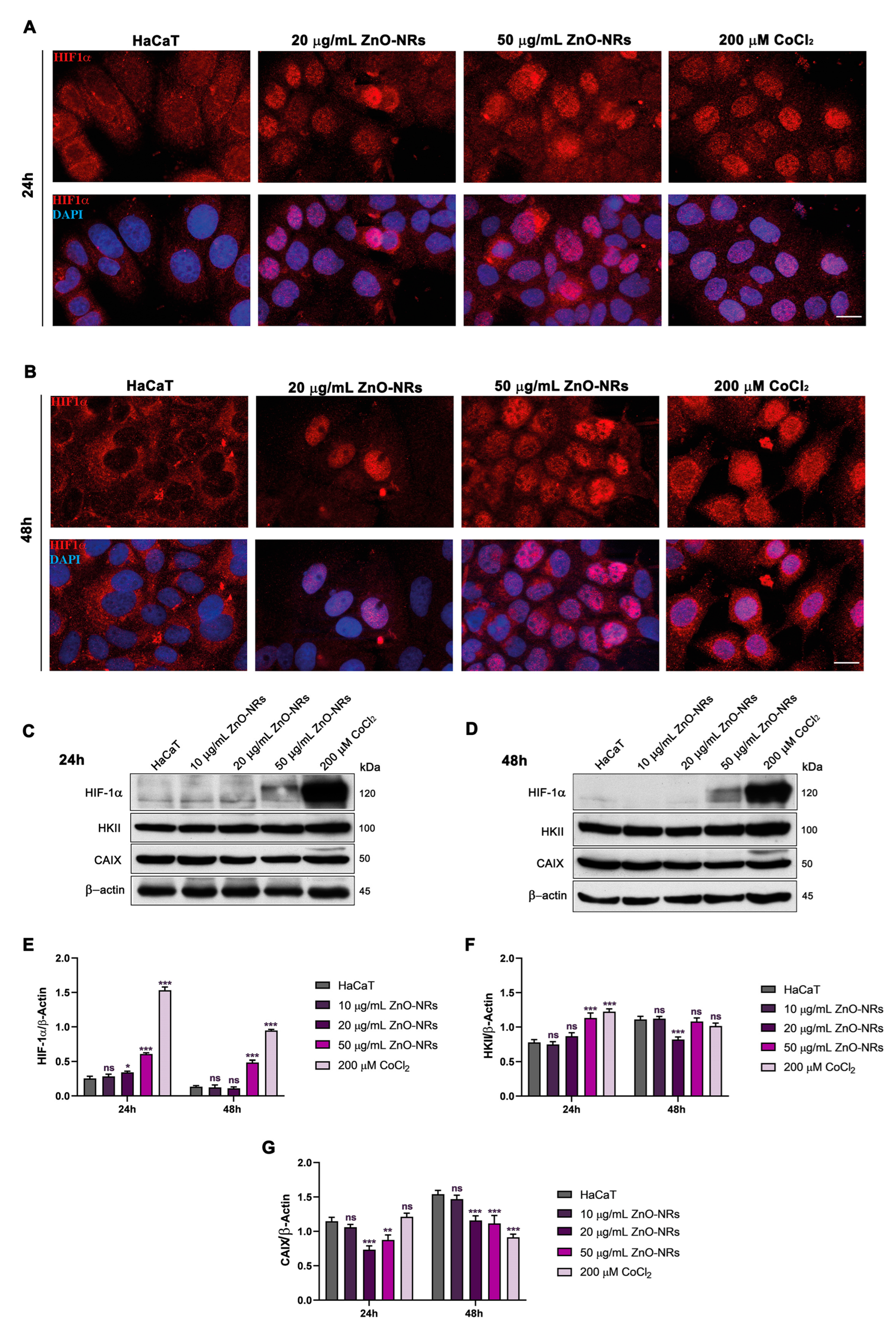
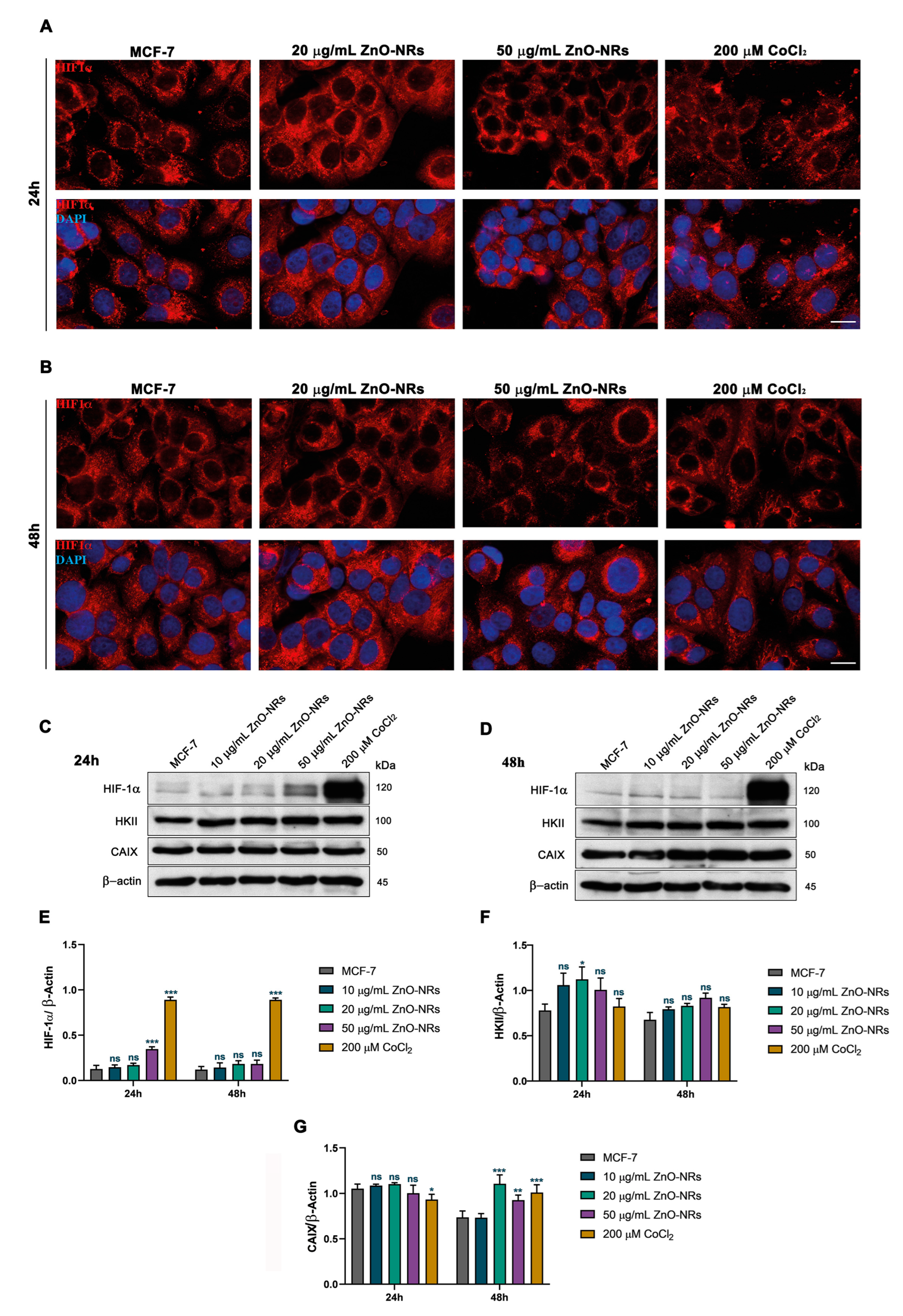
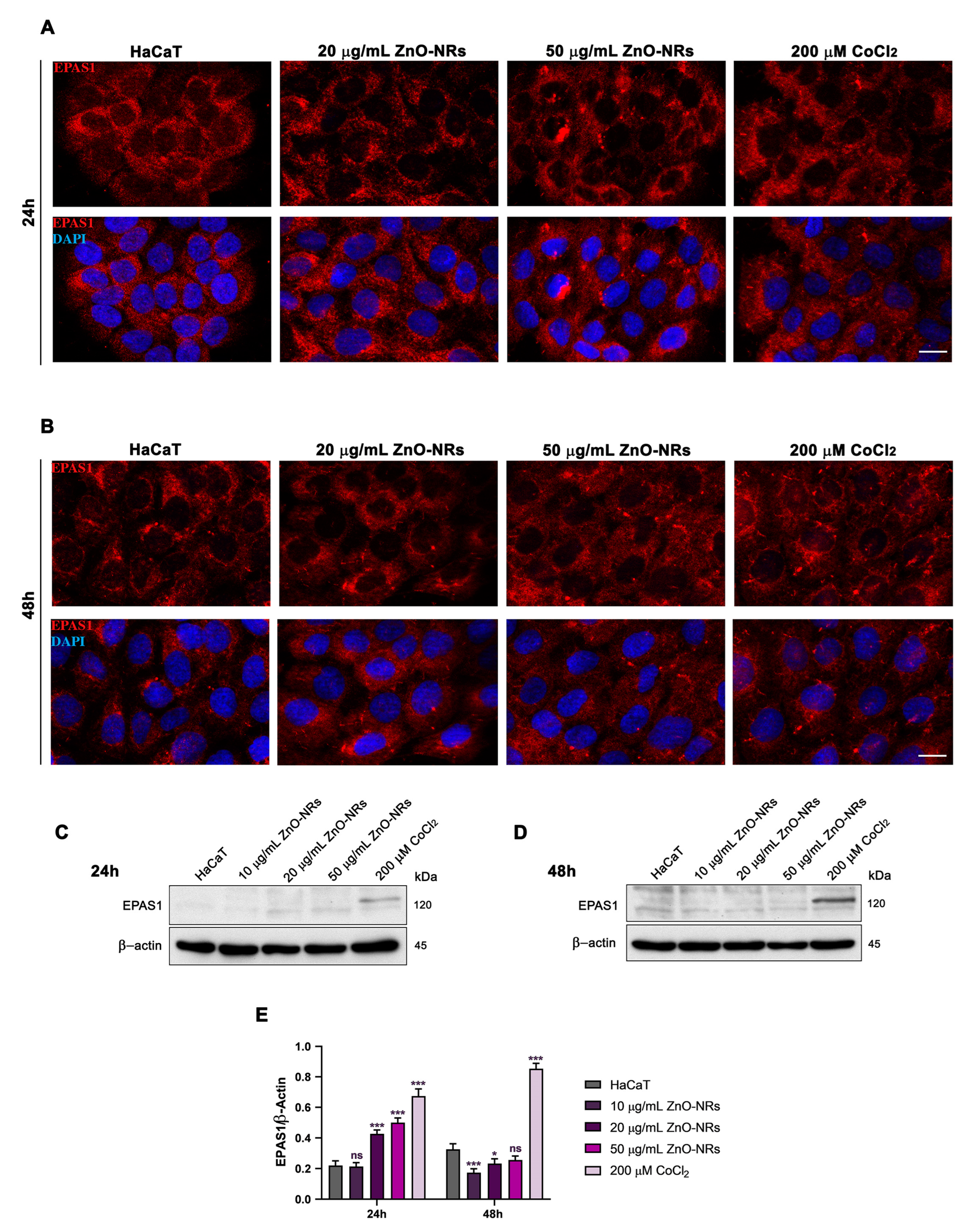
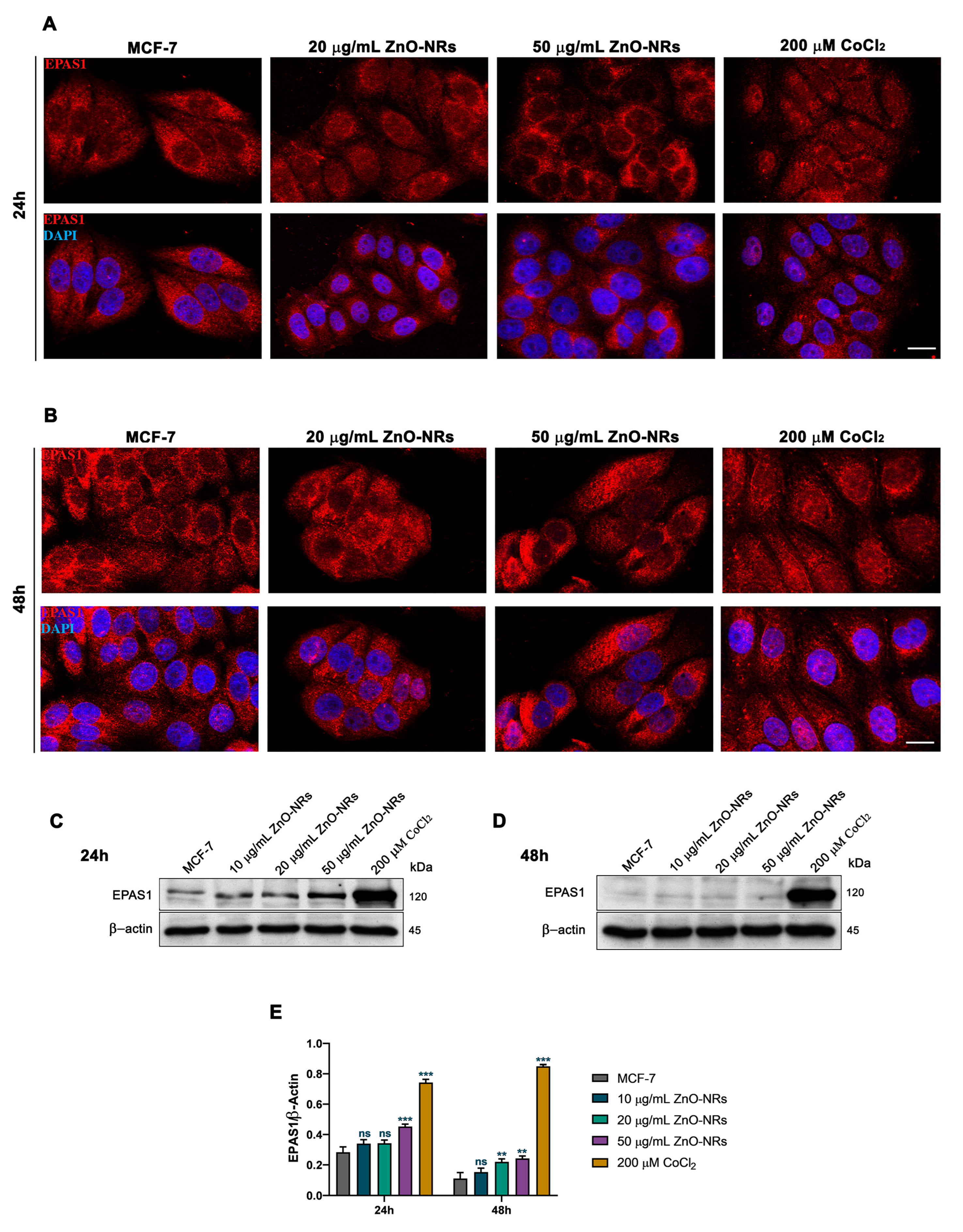
2.2. ZnO-NRs Create a Hypoxic State with HIF-1 and EPAS1 Activation
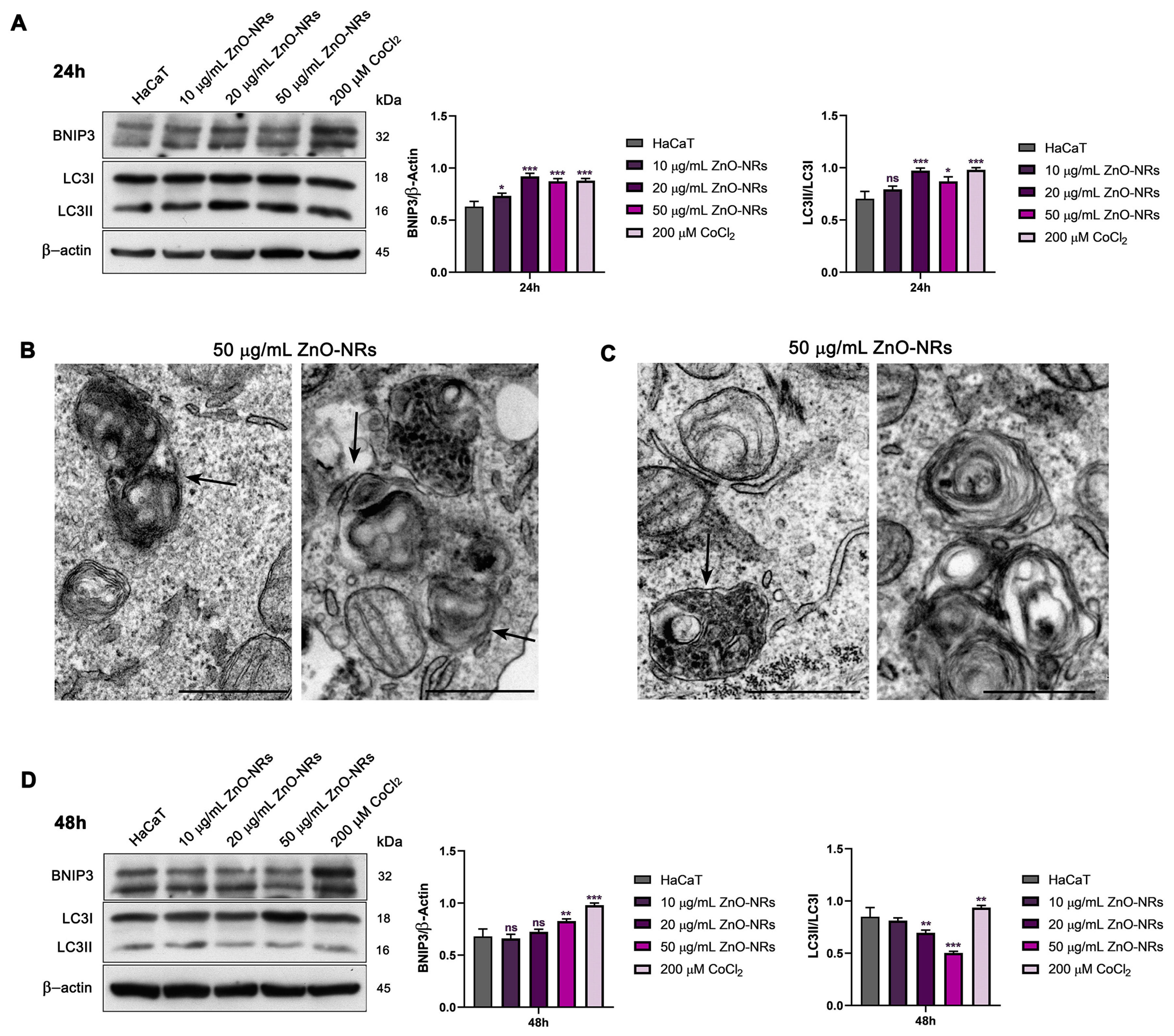
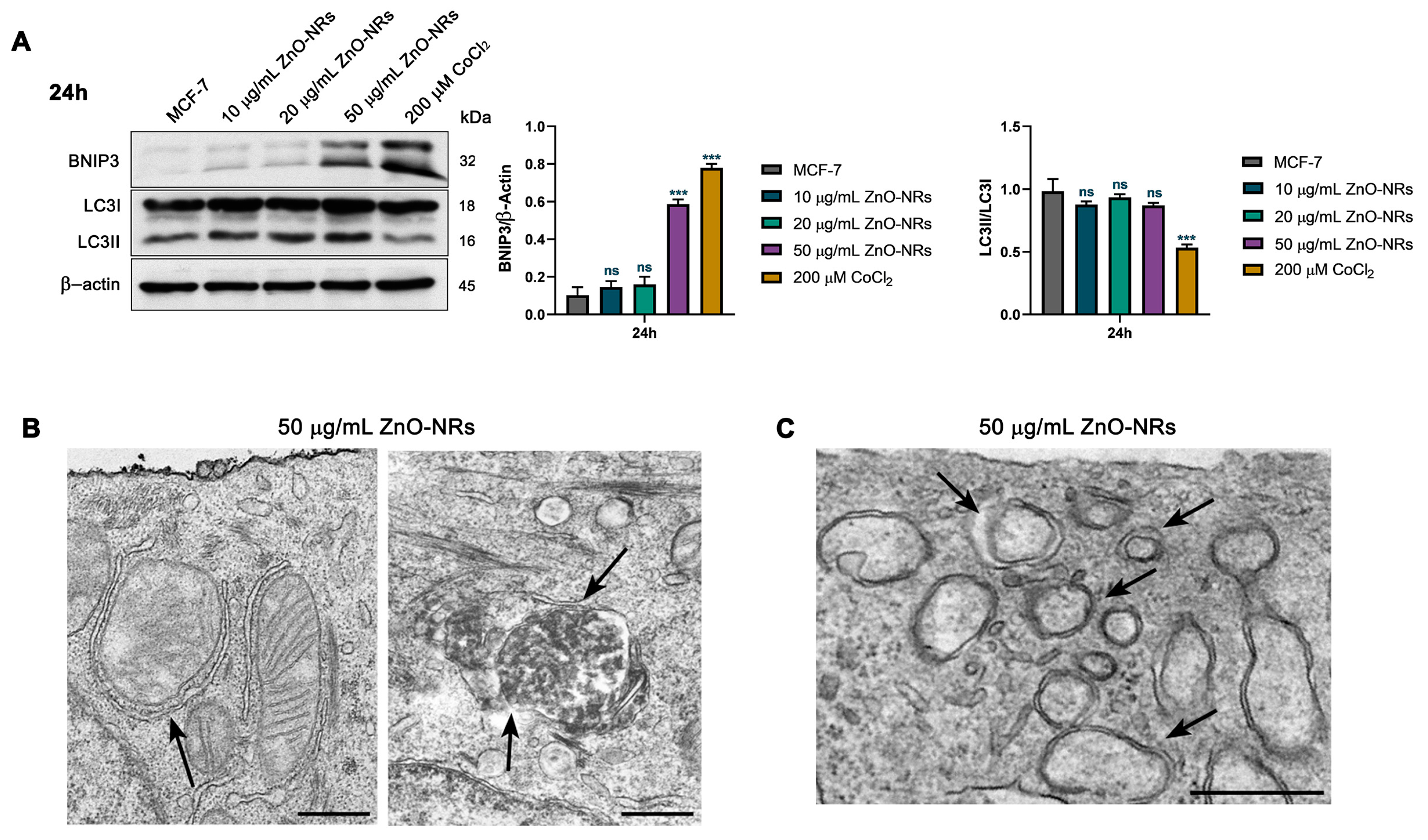
2.3. Autophagy and Mitophagy Regulation by ZnO-NRs
3. Discussion
4. Materials and Methods
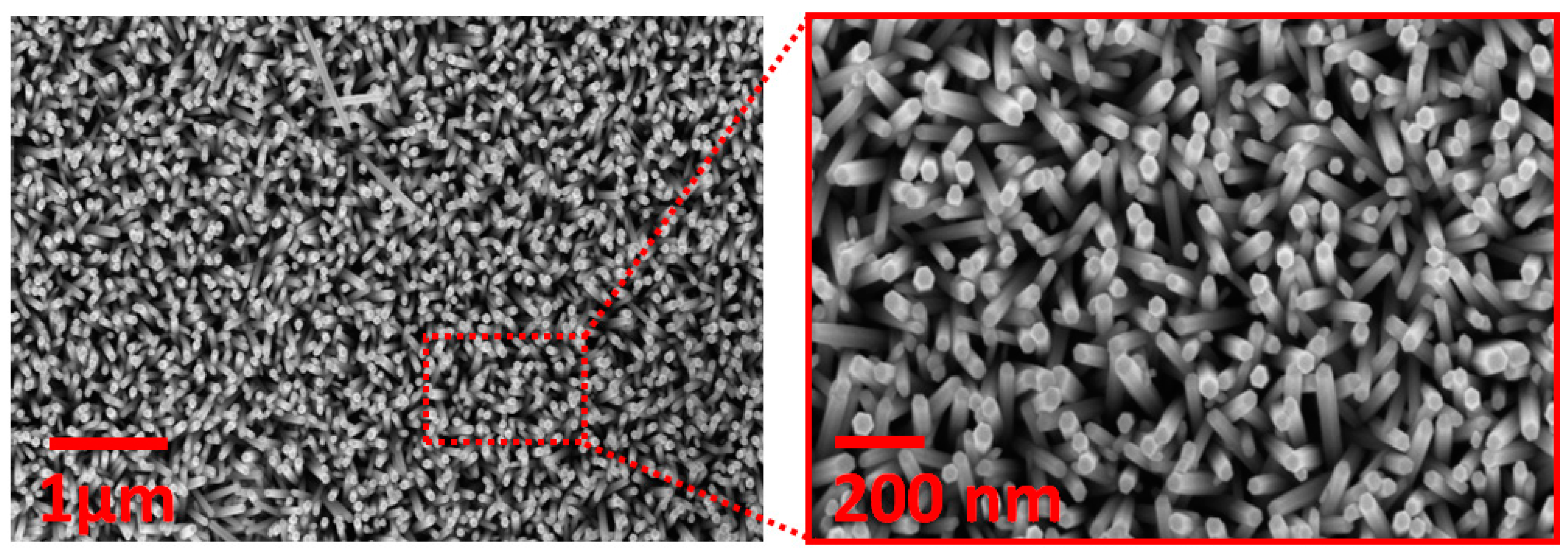
4.1. ZnO-NR Synthesis and Characterization
4.2. Cell Culture and Treatments
4.3. Cell Viability Assay
4.4. Clonogenic Assay
4.5. Zn2+ Accumulation
4.6. Measurement of Reactive Oxygen Species
4.7. Western Blot Analysis
4.8. Immunofluorescence Microscopy Analysis
4.9. TEM
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mo, R.; Jiang, T.; Gu, Z. Recent progress in multidrug delivery to cancer cells by liposomes. Nanomedicine 2014, 9, 1117–1120. [Google Scholar] [CrossRef]
- Ravi, S.; Bajpai, V. Recent advances in ZnO nanostructures and their future perspective. Adv. Nano Res. 2021, 11, 37–54. [Google Scholar] [CrossRef]
- Rai, R.S.; P, G.J.; Bajpai, V.; Khan, M.I.; Elboughdiri, N.; Shanableh, A.; Luque, R. An eco-friendly approach on green synthesis, bio-engineering applications, and future outlook of ZnO nanomaterial: A critical review. Environ. Res. 2023, 221, 114807. [Google Scholar] [CrossRef] [PubMed]
- Król, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 2017, 249, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Fouda, A.; Hassan, E.-D.; Salem, S.S.; Shaheen, T.I. In-Vitro cytotoxicity, antibacterial, and UV protection properties of the biosynthesized Zinc oxide nanoparticles for medical textile applications. Microb. Pathog. 2018, 125, 252–261. [Google Scholar] [CrossRef]
- Plum, L.M.; Rink, L.; Haase, H. The essential toxin: Impact of zinc on human health. Int. J. Environ. Res. Public Health 2010, 7, 1342–1365. [Google Scholar] [CrossRef]
- Peng, H.; Fangli, Y.; Liuyang, B.; Jinlin, L.; Yunfa, C. Plasma Synthesis of Large Quantities of Zinc Oxide Nanorods. J. Phys. Chem. C 2007, 111, 194–200. [Google Scholar] [CrossRef]
- Willander, M.; Nur, O.; Zhao, Q.; Yang, L.; Lorenz, M.; Cao, B.; Pérez, J.Z.; Czekalla, C.; Zimmermann, G.; Grundmann, M.; et al. Zinc oxide nanorod based photonic devices: Recent progress in growth, light emitting diodes and lasers. Nanotechnology 2009, 20, 332001. [Google Scholar] [CrossRef]
- Asif, M.H.; Ali, S.M.U.; Nur, O.; Willander, M.; Brännmark, C.; Stralfors, P.; Englund, U.H.; Elinder, F.; Danielsson, B. Functionalised ZnO-nanorod-based selective electrochemical sensor for intracellular glucose. Biosens. Bioelectron. 2010, 25, 2205–2211. [Google Scholar] [CrossRef]
- Muruganandham, M.; Zhang, Y.; Suri, R.; Lee, G.-J.; Chen, P.-K.; Hsieh, S.-H.; Sillanpää, M.; Wu, J.J. Environmental Applications of ZnO Materials. J. Nanosci. Nanotechnol. 2015, 15, 6900–6913. [Google Scholar] [CrossRef]
- Gojova, A.; Guo, B.; Kota, R.S.; Rutledge, J.C.; Kennedy, I.M.; Barakat, A.I. Induction of inflammation in vascular endothelial cells by metal oxide nanoparticles: Effect of particle composition. Environ. Health Perspect. 2007, 115, 403–409. [Google Scholar] [CrossRef]
- Sadhukhan, P.; Kundu, M.; Rana, S.; Kumar, R.; Das, J.; Sil, P.C. Microwave induced synthesis of ZnO nanorods and their efficacy as a drug carrier with profound anticancer and antibacterial properties. Toxicol. Rep. 2019, 6, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Li, N.; Sheng, W.; Ji, X.; Liang, X.; Kong, B.; Yin, P.; Li, Y.; Zhang, X.; Liu, K. Toxicity of different zinc oxide nanomaterials and dose-dependent onset and development of Parkinson’s disease-like symptoms induced by zinc oxide nanorods. Environ. Int. 2021, 146, 106179. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wu, W.; Hu, G.; Wu, H.; Cui, S. Hydrothermal synthesis of ZnO nanorod arrays with the addition of polyethyleneimine. Mater. Res. Bull. 2008, 43, 2113–2118. [Google Scholar] [CrossRef]
- Zaveri, T.D.; Dolgova, N.V.; Chu, B.H.; Lee, J.; Wong, J.; Lele, T.P.; Ren, F.; Keselowsky, B.G. Contributions of surface topography and cytotoxicity to the macrophage response to zinc oxide nanorods. Biomaterials 2010, 31, 2999–3007. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Prox, J.D.; Yan, B.; Wu, Y.; Argall, A.D.; Guo, L. Zinc oxide nanorod array as an inhibitory biointerface. MRS Commun. 2018, 8, 1381–1386. [Google Scholar] [CrossRef]
- Liang, X.; Xu, S.; Zhang, J.; Li, J.; Shen, Q. Cascade Amplifiers of Intracellular Reactive Oxygen Species Based on Mitochondria-Targeted Core-Shell ZnO-TPP@D/H Nanorods for Breast Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 38749–38759. [Google Scholar] [CrossRef]
- He, G.; Pan, X.; Liu, X.; Zhu, Y.; Ma, Y.; Du, C.; Liu, X.; Mao, C. HIF-1α-Mediated Mitophagy Determines ZnO Nanoparticle-Induced Human Osteosarcoma Cell Death both In Vitro and In Vivo. ACS Appl. Mater. Interfaces 2020, 12, 48296–48309. [Google Scholar] [CrossRef]
- He, G.; Nie, J.-J.; Liu, X.; Ding, Z.; Luo, P.; Liu, Y.; Zhang, B.-W.; Wang, R.; Liu, X.; Hai, Y.; et al. Zinc oxide nanoparticles inhibit osteosarcoma metastasis by downregulating β-catenin via HIF-1α/BNIP3/LC3B-mediated mitophagy pathway. Bioact. Mater. 2022, 19, 690–702. [Google Scholar] [CrossRef]
- Yu, K.-N.; Yoon, T.-J.; Minai-Tehrani, A.; Kim, J.-E.; Park, S.J.; Jeong, M.S.; Ha, S.-W.; Lee, J.-K.; Kim, J.S.; Cho, M.-H. Zinc oxide nanoparticle induced autophagic cell death and mitochondrial damage via reactive oxygen species generation. Toxicol. In Vitro 2013, 27, 1187–1195. [Google Scholar] [CrossRef]
- Helczynska, K.; Larsson, A.-M.; Mengelbier, L.H.; Bridges, E.; Fredlund, E.; Borgquist, S.; Landberg, G.; Pahlman, S.; Jirström, K. Hypoxia-inducible factor-2alpha correlates to distant recurrence and poor outcome in invasive breast cancer. Cancer Res. 2008, 68, 9212–9220. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Peng, Y.; Zou, J.; Wang, J.; Lu, S.; Fu, T.; Jiang, L.; Zhang, C.; Zhang, J. Hypoxia Inducible Factor-1α Is a Regulator of Autophagy in Osteoarthritic Chondrocytes. Cartilage 2021, 13 (Suppl. 2), 1030S–1040S. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Sun, X.; Zheng, C.; Xue, C.; Jin, Y.; Zhou, N.; Sun, S. The evolutionarily conserved hif-1/bnip3 pathway promotes mitophagy and mitochondrial fission in crustacean testes during hypoxia. Am. J. Physiol. Integr. Comp. Physiol. 2023, 324, R128–R142. [Google Scholar] [CrossRef] [PubMed]
- Zanni, E.; De Palma, S.; Chandraiahgari, C.R.; De Bellis, G.; Cialfi, S.; Talora, C.; Palleschi, C.; Sarto, M.S.; Uccelletti, D.; Mancini, P. In vitro toxicity studies of zinc oxide nano- and microrods on mammalian cells: A comparative analysis. Mater. Lett. 2016, 179, 90–94. [Google Scholar] [CrossRef]
- Choudhury, S.R.; Ordaz, J.; Lo, C.L.; Damayanti, N.P.; Zhou, F.; Irudayaraj, J. From the Cover: Zinc oxide Nanoparticles-Induced Reactive Oxygen Species Promotes Multimodal Cyto- and Epigenetic Toxicity. Toxicol. Sci. 2017, 156, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-F.; Chiu, I.-J.; Cheng, F.-Y.; Lee, Y.-H.; Wang, Y.-J.; Hsu, Y.-H.; Chiu, H.-W. The role of hypoxia-inducible factor-1α in zinc oxide nanoparticle-induced nephrotoxicity in vitro and in vivo. Part. Fibre Toxicol. 2016, 13, 52. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeong, M.S.; Kim, D.Y.; Her, S.; Wie, M.B. Zinc oxide nanoparticles induce lipoxygenase-mediated apoptosis and necrosis in human neuroblastoma SH-SY5Y cells. Neurochem. Int. 2015, 90, 204–214. [Google Scholar] [CrossRef]
- Condello, M.; De Berardis, B.; Ammendolia, M.G.; Barone, F.; Condello, G.; Degan, P.; Meschini, S. ZnO nanoparticle tracking from uptake to genotoxic damage in human colon carcinoma cells. Toxicol. In Vitro 2016, 35, 169–179. [Google Scholar] [CrossRef]
- Zanni, E.; Chandraiahgari, C.R.; De Bellis, G.; Montereali, M.R.; Armiento, G.; Ballirano, P.; Polimeni, A.; Sarto, M.S.; Uccelletti, D. Zinc Oxide Nanorods-Decorated Graphene Nanoplatelets: A Promising Antimicrobial Agent against the Cariogenic Bacterium Streptococcus mutans. Nanomaterials 2016, 6, 179. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Zinc in Cellular Regulation: The Nature and Significance of “Zinc Signals”. Int. J. Mol. Sci. 2017, 18, 2285. [Google Scholar] [CrossRef]
- Rago, I.; Chandraiahgari, C.R.; Bracciale, M.P.; De Bellis, G.; Zanni, E.; Cestelli Guidi, M.; Sali, D.; Broggi, A.; Palleschi, C.; Sarto, M.S.; et al. Zinc oxide microrods and nanorods: Different antibacterial activity and their mode of action against Gram-positive bacteria. RSC Adv. 2014, 4, 56031–56040. [Google Scholar] [CrossRef]
- Bossù, M.; Mancini, P.; Bruni, E.; Uccelletti, D.; Preziosi, A.; Rulli, M.; Relucenti, M.; Donfrancesco, O.; Iaculli, F.; Di Giorgio, G.; et al. Biocompatibility and Antibiofilm Properties of Calcium Silicate-Based Cements: An In Vitro Evaluation and Report of Two Clinical Cases. Biology 2021, 10, 470. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aventaggiato, M.; Preziosi, A.; Cheraghi Bidsorkhi, H.; Schifano, E.; Vespa, S.; Mardente, S.; Zicari, A.; Uccelletti, D.; Mancini, P.; Lotti, L.V.; et al. ZnO Nanorods Create a Hypoxic State with Induction of HIF-1 and EPAS1, Autophagy, and Mitophagy in Cancer and Non-Cancer Cells. Int. J. Mol. Sci. 2023, 24, 6971. https://doi.org/10.3390/ijms24086971
Aventaggiato M, Preziosi A, Cheraghi Bidsorkhi H, Schifano E, Vespa S, Mardente S, Zicari A, Uccelletti D, Mancini P, Lotti LV, et al. ZnO Nanorods Create a Hypoxic State with Induction of HIF-1 and EPAS1, Autophagy, and Mitophagy in Cancer and Non-Cancer Cells. International Journal of Molecular Sciences. 2023; 24(8):6971. https://doi.org/10.3390/ijms24086971
Chicago/Turabian StyleAventaggiato, Michele, Adele Preziosi, Hossein Cheraghi Bidsorkhi, Emily Schifano, Simone Vespa, Stefania Mardente, Alessandra Zicari, Daniela Uccelletti, Patrizia Mancini, Lavinia Vittoria Lotti, and et al. 2023. "ZnO Nanorods Create a Hypoxic State with Induction of HIF-1 and EPAS1, Autophagy, and Mitophagy in Cancer and Non-Cancer Cells" International Journal of Molecular Sciences 24, no. 8: 6971. https://doi.org/10.3390/ijms24086971
APA StyleAventaggiato, M., Preziosi, A., Cheraghi Bidsorkhi, H., Schifano, E., Vespa, S., Mardente, S., Zicari, A., Uccelletti, D., Mancini, P., Lotti, L. V., Sarto, M. S., & Tafani, M. (2023). ZnO Nanorods Create a Hypoxic State with Induction of HIF-1 and EPAS1, Autophagy, and Mitophagy in Cancer and Non-Cancer Cells. International Journal of Molecular Sciences, 24(8), 6971. https://doi.org/10.3390/ijms24086971







