Are There Differences in Inflammatory and Fibrotic Pathways between IPAF, CTD-ILDs, and IIPs? A Single-Center Pilot Study
Abstract
1. Introduction
2. Results
2.1. Study Group Characteristics
2.2. Comparative Analysis in Terms of Testing for Autoantibodies’ Presence and Titer
2.3. Group Membership and Cytokine Levels
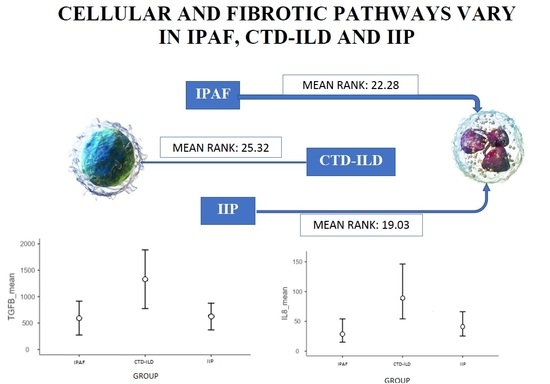
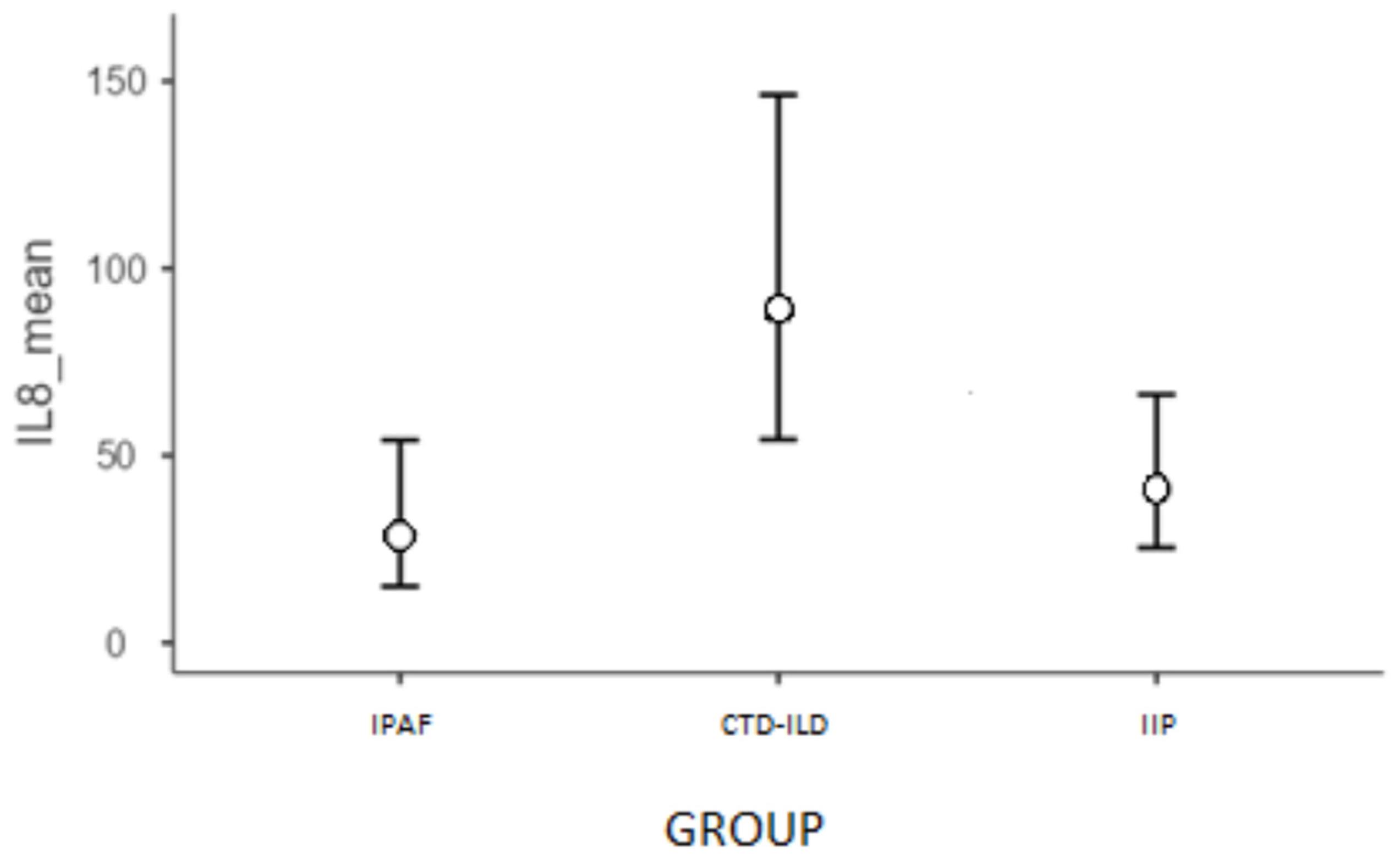
2.3.1. IL-8
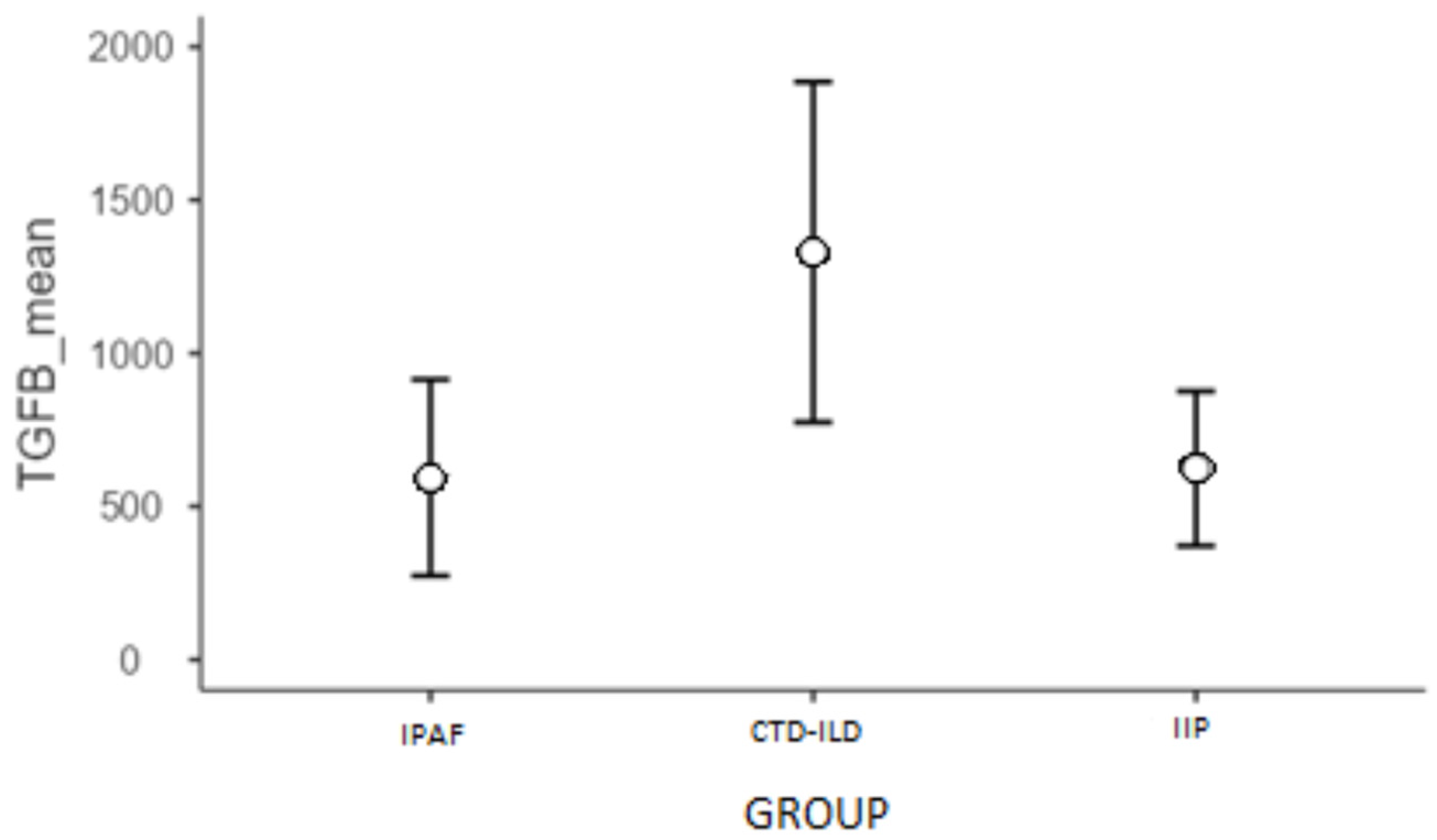
2.3.2. TGF-β1
2.3.3. SP-A, SP-D
2.3.4. CXCL1
2.4. Relationship between BAL Protein Concentration and ILD Radiological Pattern
2.5. Relationship between BAL Protein Concentration and Symptom Duration
2.6. Correlation between BAL Protein Concentration and Pulmonological Tests Parameters
2.7. Correlation between BAL Protein Concentration and Rheumatological Symptoms
3. Discussion
Study Limitations
4. Material and Methods
4.1. Study Overview
4.1.1. Serological Testing
4.1.2. Radiological Testing
4.1.3. FOB and BAL
4.1.4. Protein Concentration Measurement in BAL
4.1.5. Statistical Analysis—Overview
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fischer, A.; Antoniou, K.M.; Brown, K.K.; Cadranel, J.; Corte, T.J.; du Bois, R.M.; Lee, J.S.; Leslie, K.O.; Lynch, D.A.; Matteson, E.L.; et al. An Official European Respiratory Society/American Thoracic Society Research Statement: Interstitial Pneumonia with Autoimmune Features. Eur. Respir. J. 2015, 46, 976–987. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, K.; Martinez-Gamboa, L.; Meier, S.; Witt, C.; Meisel, C.; Hanitsch, L.G.; Becker, M.O.; Huscher, D.; Burmester, G.R.; Riemekasten, G. Bronchoalveoloar Lavage Fluid Cytokines and Chemokines as Markers and Predictors for the Outcome of Interstitial Lung Disease in Systemic Sclerosis Patients. Arthritis Res. Ther. 2009, 11, R111. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mo, H.; Zhu, M.; Wang, L. Effect of Cyclophosphamide on Cytokines in Patients with Primary Sjögren’s Syndrome-Associated Interstitial Lung Disease in South China. Rheumatol. Int. 2013, 33, 1403–1407. [Google Scholar] [CrossRef] [PubMed]
- Szlubowski, A.; Soja, J.; Grzanka, P.; Tomaszewska, R.; Papla, B.; Kuzdzał, J.; Cmiel, A.; Sładek, K. TGF-Beta1 in Bronchoalveolar Lavage Fluid in Diffuse Parenchymal Lung Diseases and High-Resolution Computed Tomography Score. Pol. Arch. Med. Wewn. 2010, 120, 270–275. [Google Scholar]
- Khalil, N.; O’Connor, R.N.; Unruh, H.W.; Warren, P.W.; Flanders, K.C.; Kemp, A.; Bereznay, O.H.; Greenberg, A.H. Increased Production and Immunohistochemical Localization of Transforming Growth Factor-Beta in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Cell Mol. Biol. 1991, 5, 155–162. [Google Scholar] [CrossRef]
- Merchant, R.K.; Schwartz, D.A.; Helmers, R.A.; Dayton, C.S.; Hunninghake, G.W. Bronchoalveolar Lavage Cellularity: The Distribution in Normal Volunteers. Am. Rev. Respir. Dis. 1992, 146, 448–453. [Google Scholar] [CrossRef]
- Matsushima, K.; Oppenheim, J.J. Interleukin 8 and MCAF: Novel Inflammatory Cytokines Inducible by IL 1 and TNF. Cytokine 1989, 1, 2–13. [Google Scholar] [CrossRef]
- Dobreva, I.; Waeber, G.; James, R.W.; Widmann, C. Interleukin-8 Secretion by Fibroblasts Induced by Low Density Lipoproteins Is P38 MAPK-Dependent and Leads to Cell Spreading and Wound Closure. J. Biol. Chem. 2006, 281, 199–205. [Google Scholar] [CrossRef]
- Feugate, J.; Li, Q.; Wong, L.; Martins-Green, M.; Feugate, J.E.; Li, Q.; Wong, L.; Martins-Green, M. The Cxc Chemokine CCAF Stimulates Differentiation of Fibroblasts into Myofibroblasts and Accelerates Wound Closure. J. Cell Biol. 2002, 156, 161–172. [Google Scholar] [CrossRef]
- Koch, A.; Polverini, P.; Kunkel, S.; Harlow, L.; Di Pietro, L.; Elner, V.; Elner, S.; Strieter, R. Interleukin-8 as a Macrophage-Derived Mediator of Angiogenesis. Science 1992, 258, 1798–1801. [Google Scholar] [CrossRef]
- Kitadai, Y.; Haruma, K.; Sumii, K.; Yamamoto, S.; Ue, T.; Yokozaki, H.; Yasui, W.; Ohmoto, Y.; Kajiyama, G.; Fidler, I.J.; et al. Expression of Interleukin-8 Correlates with Vascularity in Human Gastric Carcinomas. J. Pathol. 1998, 152, 93–100. [Google Scholar]
- Yalçınkaya, Y.; Adın-Çınar, S.; Artim-Esen, B.; Kamalı, S.; Pehlivan, Ö.; Öcal, L.; Deniz, G.; Inanç, M. Capillaroscopic Findings and Vascular Biomarkers in Systemic Sclerosis: Association of Low CD40L Levels with Late Scleroderma Pattern. Microvasc. Res. 2016, 108, 17–21. [Google Scholar] [CrossRef]
- Cutolo, M.; Sulli, A.; Pizzorni, C.; Accardo, S. Nailfold Videocapillaroscopy Assessment of Microvascular Damage in Systemic Sclerosis. J. Rheumatol. 2000, 27, 155–160. [Google Scholar] [PubMed]
- Pizzorni, C.; Giampetruzzi, A.R.; Mondino, C.; Facchiano, A.; Abeni, D.; Paolino, S.; Ruaro, B.; Smith, V.; Sulli, A.; Cutolo, M. Nailfold Capillaroscopic Parameters and Skin Telangiectasia Patterns in Patients with Systemic Sclerosis. Microvasc. Res. 2016, 111, 20–24. [Google Scholar] [CrossRef]
- Qazi, B.S.; Tang, K.; Qazi, A. Recent Advances in Underlying Pathologies Provide Insight into Interleukin-8 Expression-Mediated Inflammation and Angiogenesis. Int. J. Inflamm. 2011, 2011, 908468. [Google Scholar] [CrossRef]
- Matsushima, K.; Yang, D.; Oppenheim, J.J. Interleukin-8: An Evolving Chemokine. Cytokine 2022, 153, 155828. [Google Scholar] [CrossRef]
- Bime, C.; Casanova, N.; Oita, R.C.; Ndukum, J.; Lynn, H.; Camp, S.M.; Lussier, Y.; Abraham, I.; Carter, D.; Miller, E.J.; et al. Development of a Biomarker Mortality Risk Model in Acute Respiratory Distress Syndrome. Crit. Care Lond. Engl. 2019, 23, 410. [Google Scholar] [CrossRef]
- Ogushi, F.; Tani, K.; Maniwa, K.; Ichikawa, W.; Tada, H.; Kawano, T.; Sone, S. Interleukin-8 in bronchoalveolar lavage fluid of patients with diffuse panbronchiolitis or idiopathic pulmonary fibrosis. J. Med. Investig. 1997, 44, 53–58. [Google Scholar]
- Carré, P.C.; Mortenson, R.L.; King, T.E.; Noble, P.W.; Sable, C.L.; Riches, D.W. Increased Expression of the Interleukin-8 Gene by Alveolar Macrophages in Idiopathic Pulmonary Fibrosis. A Potential Mechanism for the Recruitment and Activation of Neutrophils in Lung Fibrosis. J. Clin. Investig. 1991, 88, 1802–1810. [Google Scholar] [CrossRef]
- Liang, M.; Jiang, Z.; Huang, Q.; Liu, L.; Xue, Y.; Zhu, X.; Yu, Y.; Wan, W.; Yang, H.; Zou, H. Clinical Association of Chemokine (C-X-C Motif) Ligand 1 (CXCL1) with Interstitial Pneumonia with Autoimmune Features (IPAF). Sci. Rep. 2016, 6, 38949. [Google Scholar] [CrossRef]
- Coker, R.; Laurent, G.; Jeffery, P.; du Bois, R.M.; Black, C.; McAnulty, R. Localisation of Transforming Growth Factor Β1 and Β3 MRNA Transcripts in Normal and Fibrotic Human Lung. Thorax 2001, 56, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Garrison, G.; Huang, S.K.; Okunishi, K.; Scott, J.P.; Kumar Penke, L.R.; Scruggs, A.M.; Peters-Golden, M. Reversal of Myofibroblast Differentiation by Prostaglandin E2. Am. J. Respir. Cell Mol. Biol. 2013, 48, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, H.; Kitamura, H.; Takemura, T.; Ikeda, S.; Sekine, A.; Baba, T.; Iwasawa, T.; Hagiwara, E.; Sato, S.; Ogura, T. Prognostic Factors and Disease Behaviour of Pathologically Proven Fibrotic Non-Specific Interstitial Pneumonia. Respirology 2018, 23, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Hiwatari, N.; Shimura, S.; Yamauchi, K.; Nara, M.; Hida, W.; Shirato, K. Significance of Elevated Procollagen-III-Peptide and Transforming Growth Factor-β Levels of Bronchoalveolar Lavage Fluids from Idiopathic Pulmonary Fibrosis Patients. Tohoku J. Exp. Med. 1997, 181, 285–295. [Google Scholar] [CrossRef][Green Version]
- Digiovine, B.; Lynch, J.; Martinez, F.; Whyte, R.; Iannettoni, M.; Hariharan, K.; Arenberg, D.; Burdick, M.; Glass, M.; Morris, S.; et al. The Presence of Pro-Fibrotic Cytokines Correlate with Outcome in Patients with Idiopathic Pulmonary Fibrosis. Chest 1996, 110, 37S. [Google Scholar]
- Broekelmann, T.J.; Limper, A.H.; Colby, T.V.; McDonald, J.A. Transforming Growth Factor Beta 1 Is Present at Sites of Extracellular Matrix Gene Expression in Human Pulmonary Fibrosis. Proc. Natl. Acad. Sci. USA 1991, 88, 6642–6646. [Google Scholar] [CrossRef]
- Meloni, F.; Caporali, R.; Marone Bianco, A.; Paschetto, E.; Morosini, M.; Fietta, A.M.; Patrizio, V.; Bobbio-Pallavicini, F.; Pozzi, E.; Montecucco, C. BAL Cytokine Profile in Different Interstitial Lung Diseases: A Focus on Systemic Sclerosis. Sarcoidosis Vasc. Diffus. Lung Dis. Off. J. WASOG 2004, 21, 111–118. [Google Scholar]
- Graham, B.L.; Steenbruggen, I.; Miller, M.R.; Barjaktarevic, I.Z.; Cooper, B.G.; Hall, G.L.; Hallstrand, T.S.; Kaminsky, D.A.; McCarthy, K.; McCormack, M.C.; et al. Standardization of Spirometry 2019 Update. An Official American Thoracic Society and European Respiratory Society Technical Statement. Am. J. Respir. Crit. Care Med. 2019, 200, e70–e88. [Google Scholar] [CrossRef]
- Stocks, J.; Godfrey, S.; Beardsmore, C.; Bar-Yishay, E.; Castile, R. Plethysmographic Measurements of Lung Volume and Airway Resistance. Eur. Respir. J. 2001, 17, 302–312. [Google Scholar] [CrossRef]
- Graham, B.L.; Brusasco, V.; Burgos, F.; Cooper, B.G.; Jensen, R.; Kendrick, A.; MacIntyre, N.R.; Thompson, B.R.; Wanger, J. 2017 ERS/ATS Standards for Single-Breath Carbon Monoxide Uptake in the Lung. Eur. Respir. J. 2017, 49, 1600016. [Google Scholar] [CrossRef]
- Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Available online: https://www.atsjournals.org/doi/epdf/10.1164/rccm.201807-1255ST (accessed on 13 September 2022).
- van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.; Riemekasten, G.; Carreira, P.; et al. Classification Criteria for Systemic Sclerosis: An ACR-EULAR Collaborative Initiative. Arthritis Rheum. 2013, 65, 2737–2747. [Google Scholar] [CrossRef] [PubMed]
- Rider, L.G.; Ruperto, N.; Pistorio, A.; Erman, B.; Bayat, N.; Lachenbruch, P.A.; Rockette, H.; Feldman, B.M.; Huber, A.M.; Hansen, P.; et al. 2016 ACR-EULAR Adult Dermatomyositis and Polymyositis and Juvenile Dermatomyositis Response Criteria-Methodological Aspects. Rheumatol. Oxf. Engl. 2017, 56, 1884–1893. [Google Scholar] [CrossRef] [PubMed]
- Aringer, M. EULAR/ACR Classification Criteria for SLE. Semin. Arthritis Rheum. 2019, 49, S14–S17. [Google Scholar] [CrossRef] [PubMed]
- Shiboski, C.H.; Shiboski, S.C.; Seror, R.; Criswell, L.A.; Labetoulle, M.; Lietman, T.M.; Rasmussen, A.; Scofield, H.; Vitali, C.; Bowman, S.J.; et al. 2016 ACR-EULAR Classification Criteria for Primary Sjögren’s Syndrome: A Consensus and Data-Driven Methodology Involving Three International Patient Cohorts. Arthritis Rheumatol. 2017, 69, 35–45. [Google Scholar] [CrossRef] [PubMed]
- ATS Statement | Guidelines for the Six-Minute Walk Test | American Journal of Respiratory and Critical Care Medicine. Available online: https://www.atsjournals.org/doi/full/10.1164/ajrccm.166.1.at1102 (accessed on 27 September 2022).
- Chan, E.K.L.; von Mühlen, C.A.; Fritzler, M.J.; Damoiseaux, J.; Infantino, M.; Klotz, W.; Satoh, M.; Musset, L.; García-De La Torre, I.; Carballo, O.G.; et al. The International Consensus on ANA Patterns (ICAP) in 2021—The 6th Workshop and Current Perspectives. J. Appl. Lab. Med. 2022, 7, 322–330. [Google Scholar] [CrossRef]
- Technical Recommendations and Guidelines for Bronchoalveolar Lavage (BAL). Report of the European Society of Pneumology Task Group. Eur. Respir. J. 1989, 2, 561–585. [Google Scholar] [CrossRef]
- Chciałowski, A.; Chorostowska-Wynimko, J.; Fal, A.; Pawłowicz, R.; Domagała-Kulawik, J. Wskazówki Polskiego Towarzystwa Chorób Płuc dotyczące metod pozyskiwania, opracowywania oraz oceny płynu z płukania oskrzelowo-pęcherzykowego (BAL) [Recommendation of the Polish Respiratory Society for bronchoalveolar lavage (BAL) sampling, processing and analysis methods]. Pneumonol. Alergol. Pol. 2011, 79, 75–89. [Google Scholar]
| IPAF | CTD-ILD | IIP | p | |||||
|---|---|---|---|---|---|---|---|---|
| DEMOGRAPHICS | Age (years) | 68.78 ± 7.89 | 65.80 ± 8.98 | 69.13 ± 12.21 | 0.43 | |||
| Sex (% female) | 88.9 | 80.0 | 31.3 | 0.004 | ||||
| Height (cm) | 160.83 ± 7.08 | 164.80 ± 6.80 | 168.75 ± 8.95 | 0.06 | ||||
| Weight (kg) | 75.67 ± 11.56 | 72.60 ± 13.82 | 80.53 ± 14.52 | 0.51 | ||||
| History of tobacco smoke exposure (%) | 55.6 | 78.6 | 62.5 | 0.47 | ||||
| FUNCTIONAL PARAMETERS | Me | IQR | Me | IQR | Me | IQR | ||
| FEV1 (l) | 1.90 | 0.75 | 1.84 | 0.68 | 2.33 | 1.13 | 0.23 | |
| FEV1 (%) | 83 | 0.15 | 0.77 | 0.29 | 0.85 | 0.25 | 0.53 | |
| FVC (l) | 2.74 | 1.50 | 2.45 | 1.60 | 2.84 | 2.08 | 0.54 | |
| FVC (%) | 74.00 | 30.00 | 73.00 | 44.00 | 79.00 | 28.00 | 0.54 | |
| FEV1/FVC | 79.55 | 11.91 | 79.20 | 10.36 | 82.40 | 21.16 | 0.467 | |
| TLC (l) | 3.92 | 1.32 | 4.89 | 1.86 | 4.55 | 2.41 | 0.28 | |
| TLC (%) | 77.00 | 20.50 | 87.00 | 32.00 | 82.50 | 27.50 | 0.43 | |
| DLCO (mmol/min/kPa) | 4.62 | 2.24 | 4.69 | 2.17 | 4.76 | 3.93 | 0.77 | |
| DLCO (%N) | 64.00 | 24.50 | 73.00 | 43.00 | 61.50 | 48.50 | 0.443 | |
| 6MWT (m) | 287.00 | 95.00 | 289.00 | 230.00 | 323.00 | 286.25 | 0.99 | |
| IPAF (n = 9) | CTD-ILDs (n = 15) | IIPs (n = 16) | p | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Me | IQR | Me | IQR | Me | IQR | ||||
| BAL TCC (×106) N: 18.1 ± 0.9 (non-smokers); 59.8 ± 7.3 (smokers) [5] | 22.3 | 12.4 | 25.05 | 15.35 | 27.05 | 6.59 | 0.8 | ||
| BAL N (%) | 9.70 | 1.60 | 4.35 | 7.90 | 3.05 | 8.68 | 0.49 | 0.78 | <0.01 |
| N: ≤3% | |||||||||
| [6] | |||||||||
| BAL M (%) N: >85% [6] | 66.30 | 23.5 | 67.30 | 24.90 | 82.95 | 26.48 | 3.23 | 0.19 | 0.03 |
| BAL L (%) | 12.80 | 18.00 | 22.90 | 22.48 | 7.75 | 14.00 | 5.28 | 0.07 | 0.09 |
| N: 10–15% | |||||||||
| [6] | |||||||||
| BAL E (%) N: ≤1% [6] | 0.90 | 3.85 | 0.45 | 2.35 | 0.85 | 1.40 | 0.95 | 0.62 | <0.01 |
| Rheumatological Symptoms | IPAF | CTD-ILDs | IIPs | |||
|---|---|---|---|---|---|---|
| % | % | % | χ2 | p | V | |
| Fever/subfebrile states | 11.1 | 6.7 | 12.5 | 0.61 | 1.000 | 0.09 |
| Peripheral lymphadenopathy/hepato- and splenomegaly/ | 11.1 | 6.7 | 6.3 | 0.71 | 1.000 | 0.07 |
| Raynaud phenomenon | 44.4 | 13.3 | 0 | 7.79 | 0.008 | 0.47 |
| Arthritis/articular edema | 55.6 | 60.0 | 0 | 14.41 | <0.001 | 0.60 |
| Articular pains | 100.0 | 93.3 | 68.8 | 4.59 | 0.12 | 0.38 |
| Muscle weakness | 0 | 28.6 | 12.5 | 3.06 | 0.16 | 0.30 |
| Sclerodactyly (proximal to elbows and knees) | 0 | 20.0 | 0 | 3.83 | 0.06 | 0.37 |
| Teleangiectasias | 11.1 | 46.7 | 6.3 | 7.20 | 0.02 | 0.45 |
| Sicca syndrome | 0 | 20.0 | 0 | 3.83 | 0.06 | 0.37 |
| Erythema (heliotrope rash/butterfly rash/DLE type symptoms/SCLE symptoms) | 0 | 6.7 | 0 | 1.72 | 0.60 | 0.21 |
| Morning joint stiffness > 1 h | 77.8 | 53.3 | 0 | 17.43 | <0.001 | 0.66 |
| History of pleural effusion | 11.1 | 6.7 | 0 | 1.94 | 0.51 | 0.20 |
| Digital edema | 22.2 | 33.3 | 0 | 6.51 | 0.03 | 0.39 |
| RF | ||||||
| Negative | 66.7 | 10 | 14 | 2.35 | 0.338 | 0.24 |
| Positive | 33.3 | 5 | 2 | |||
| Anti-CCP | ||||||
| Negative | 66.7 | 9 | 15 | 5.36 | 0.060 | 0.36 |
| Positive | 33.3 | 6 | 1 | |||
| ANA | ||||||
| Negative | 11.1 | 5 | 13 | 13.25 | 0.001 | 0.58 |
| Positive | 88.9 | 10 | 3 |
| IL—8 | TGF—β1 | SPD | SPA | CXCL1 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Eta/rs | p | Eta/rs | p | Eta/rs | p | Eta/rs | p | Eta/rs | p | |
| FVC (1 −≥ 50% pred. v.; 0 −< 50% pred. v.) | 0.09 | 0.59 | 0.13 | 0.43 | 0.33 | 0.04 | 0.24 | 0.13 | 0.14 | 0.40 |
| DLCO (1 −≥ 60% pred.v.; 0 < 60% pred. v) | 0.05 | 0.77 | −0.09 | 0.60 | −0.06 | 0.74 | 0.12 | 0.45 | −0.36 | 0.02 |
| FVC (l) | 0.08 | 0.62 | −0.14 | 0.39 | −0.17 | 0.31 | −0.38 | 0.02 | 0.15 | 0.38 |
| BAL TCC (×106) | 0.46 | 0.003 | 0.07 | 0.69 | 0.08 | 0.61 | 0.31 | 0.06 | 0.52 | 0.001 |
| BAL N (%) | −0.15 | 0.36 | 0.03 | 0.86 | −0.16 | 0.33 | −0.30 | 0.06 | 0.10 | 0.56 |
| BAL M (%) | −0.01 | 0.93 | −0.12 | 0.47 | 0.07 | 0.68 | 0.07 | 0.70 | 0.00 | 0.99 |
| BAL L (%) | 0.05 | 0.75 | 0.15 | 0.35 | 0.11 | 0.50 | 0.06 | 0.74 | 0.00 | 0.99 |
| BAL E (%) | 0.05 | 0.79 | 0.04 | 0.83 | −0.18 | 0.27 | −0.12 | 0.48 | 0.09 | 0.59 |
| IL-8_mean | TGF-β1_mean | SPD_mean | SPA_mean | CXCL1_mean | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Eta/rs | p | Eta/rs | p | Eta/rs | p | Eta/rs | p | Eta/rs | p | |
| RF (1—positive; 0—negative) | 0.12 | 0.46 | 0.11 | 0.52 | 0.08 | 0.65 | 0.14 | 0.39 | 0.03 | 0.87 |
| anti-CCP (1—positive; 0—negative) | 0.12 | 0.46 | 0.17 | 0.30 | 0.21 | 0.19 | 0.24 | 0.14 | −0.16 | 0.35 |
| ANA (1—positive; 0—negative) | 0.10 | 0.56 | −0.35 | 0.03 | −0.12 | 0.46 | −0.06 | 0.72 | −0.08 | 0.65 |
| 95.0% CI for B | |||||||
|---|---|---|---|---|---|---|---|
| B | SE | Beta | T | P | LL | UL | |
| (Constant) | 21.90 | 7.88 | 2.78 | 0.009 | 5.83 | 37.98 | |
| IL-8_mean | 0.04 | 0.02 | 0.32 | 2.18 | 0.04 | 0.00 | 0.07 |
| TGFβ1_mean | 0.00 | 0.00 | −0.10 | −0.69 | 0.49 | 0.00 | 0.00 |
| SPD_mean | −0.01 | 0.02 | −0.05 | −0.30 | 0.77 | −0.05 | 0.04 |
| SPA_mean | 0.00 | 0.00 | 0.17 | 1.09 | 0.28 | 0.00 | 0.01 |
| CXCL1_mean | 0.00 | 0.00 | 0.45 | 3.08 | 0.004 | 0.00 | 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rzepka-Wrona, P.; Skoczyński, S.; Barczyk, A. Are There Differences in Inflammatory and Fibrotic Pathways between IPAF, CTD-ILDs, and IIPs? A Single-Center Pilot Study. Int. J. Mol. Sci. 2022, 23, 15205. https://doi.org/10.3390/ijms232315205
Rzepka-Wrona P, Skoczyński S, Barczyk A. Are There Differences in Inflammatory and Fibrotic Pathways between IPAF, CTD-ILDs, and IIPs? A Single-Center Pilot Study. International Journal of Molecular Sciences. 2022; 23(23):15205. https://doi.org/10.3390/ijms232315205
Chicago/Turabian StyleRzepka-Wrona, Patrycja, Szymon Skoczyński, and Adam Barczyk. 2022. "Are There Differences in Inflammatory and Fibrotic Pathways between IPAF, CTD-ILDs, and IIPs? A Single-Center Pilot Study" International Journal of Molecular Sciences 23, no. 23: 15205. https://doi.org/10.3390/ijms232315205
APA StyleRzepka-Wrona, P., Skoczyński, S., & Barczyk, A. (2022). Are There Differences in Inflammatory and Fibrotic Pathways between IPAF, CTD-ILDs, and IIPs? A Single-Center Pilot Study. International Journal of Molecular Sciences, 23(23), 15205. https://doi.org/10.3390/ijms232315205