Abstract
Despite numerous therapies, cancer remains one of the leading causes of death worldwide due to the lack of markers for early detection and response to treatment in many patients. Technological advances in tumor screening and renewed interest in energy metabolism have allowed us to identify new cellular players in order to develop personalized treatments. Among the metabolic actors, the mitochondrial transporter uncoupling protein 2 (UCP2), whose expression is increased in many cancers, has been identified as an interesting target in tumor metabolic reprogramming. Over the past decade, a better understanding of its biochemical and physiological functions has established a role for UCP2 in (1) protecting cells from oxidative stress, (2) regulating tumor progression through changes in glycolytic, oxidative and calcium metabolism, and (3) increasing antitumor immunity in the tumor microenvironment to limit cancer development. With these pleiotropic roles, UCP2 can be considered as a potential tumor biomarker that may be interesting to target positively or negatively, depending on the type, metabolic status and stage of tumors, in combination with conventional chemotherapy or immunotherapy to control tumor development and increase response to treatment. This review provides an overview of the latest published science linking mitochondrial UCP2 activity to the tumor context.
Keywords:
uncoupling protein 2 (UCP2); mitochondria; metabolism; oxidative stress; cancer; therapies 1. Introduction
Cancer is today a major public health issue with a high incidence. With nearly 10 million deaths per year, it was the third leading cause of death worldwide in 2020 [1]. According to the definition of the World Health Organization: “cancer is the rapid multiplication of abnormal cells with unusual growth, which can then invade neighboring parts of the body, and migrate to other organs”. Hanahan and Weinberg have deepened this definition by listing all the common characteristics of cancer cells through periodic updates of several reviews [2,3,4]. After the addition of cellular energetic dysregulations to the “hallmarks of cancer”, the metabolic reprogramming of tumors has gained renewed interest [3]. In the last decade, the study of tumor metabolism, through its mechanisms, signaling pathways and metabolites, has even become a crucial issue for the understanding and treatment of cancer. Otto Warburg’s 1920 hypothesis led for almost a century to the misconception that cancer cells were exclusively dependent on glycolysis to produce adenosine tri phosphate (ATP), and that mitochondria were damaged [5]. Nevertheless, although cancer cells adopt a preferential glycolytic metabolism following the activation of oncogenes, which stabilize under hypoxic conditions, mitochondria are functional and necessary for tumorigenesis [6]. Mitochondria are indeed the production factories of cells. Energy-intensive tumor cells take up oxygen (O2) and nutrients (glucose and essential amino acids) to form building blocks (proteins, lipids and nucleic acids) through mitochondrial biosynthetic pathways to sustain a high proliferation rate and promote resistance to chemotherapy [7,8,9]. However, mitochondria are the main source of reactive oxygen species (ROS) production. The oxidation of glucose by oxidative phosphorylation (OXPHOS) results in the incomplete reduction of O2. Electron leakage at complexes I and III of the electron transport chain (ETC) leads to the release of superoxide (O2−) molecules, which are highly unstable. The superoxide is then successively converted to oxygen peroxide (H2O2) and water (H2O) molecules by antioxidant enzymes to maintain the redox status [10]. However, an uncontrolled increase in ROS is cytotoxic and generates oxidative damage that promotes protein and lipid damage, as well as genomic DNA mutations. This genomic instability can lead to the induction of pro-tumor genes that destabilize cellular integrity [11,12]. To cope with metabolic changes, cells have developed metabolic regulators to control excessive ROS formation. In the past two decades, many studies have highlighted the antioxidant role of uncoupling proteins (UCPs) and more specifically the UCP2 protein. The limitation of ROS production by UCPs reduces cellular stress, which would contribute to improving inflammatory responses in a tumorigenic context [13].
UCPs belong to the family of mitochondrial inner membrane transporters. UCP1 was the first member of the UCP family discovered in 1976 in mammalian brown adipose tissue [14]. Homologs, including UCP2, were then identified [15,16,17]. UCP2 is the uncoupling protein with the highest homology to UCP1 (59%), but its localization and function are distinct [18]. Although its messenger RNA is ubiquitously distributed in all organs, the protein is only detected in certain tissues such as the spleen, pancreas, lung, intestine and white adipose tissue, due to the tight regulation of its translation [19,20]. Even though the highly controversial uncoupling activity has been investigated, a recent biochemical characterization of UCP2 revealed that the protein is involved in the transport of four-carbon (C4) tricarboxylic acid (TCA) metabolites to regulate pyruvate oxidation in mitochondria [21,22]. The role of UCP2 has been implicated in various physiological and pathological conditions. Indeed, through the physiological regulation of ROS [23,24,25], the modulation of metabolism [22,26] and the control of the immune system [27,28], UCP2 has been implicated in the control of autoimmune diseases [29], cardiovascular dysfunction [30], neuronal pathologies [31] and cancers [32,33,34].
Today, although intensive research has led to major advances in the understanding of the mechanisms of tumorigenesis, therapies have evolved little, even with the emergence of immunotherapy. Indeed, immunotherapy and targeted therapies offer only limited success in curing certain patients and in specific cancers [35,36]. Radiotherapy, chemotherapy and surgery remain the first-line treatments despite the development of resistance and significant side effects. However, to improve their effectiveness, the challenge is to combine treatments by delivering, for example, targeted metabolic therapies with conventional treatments or immunotherapies. UCP2, which is a protein involved in metabolic control, could thus represent a therapeutic strategy to fight against the metabolic reprogramming of tumors [37,38].
In this review, we will first characterize and deepen our knowledge of the physiological functions of UCP2. Then, we will understand how this protein, through its multiple roles, is involved in the metabolic and immune reprogramming of tumors. Finally, we will explore whether targeting UCP2 could represent a therapeutic advantage in the fight against cancer and resistance to therapies.
2. Role of UCP2 in the Physiological and Pathophysiological Context
Since the late 1990s, many studies have characterized the involvement of UCP2 in several physiological and pathophysiological processes, but its biological role is not clearly elucidated. Indeed, UCP2, but also its homologue UCP3, have been the subject of much controversy (Figure 1) [13,39]. This first part will allow us to review the biochemical functions attributable to UCP2 in order to better appreciate its involvement in a physiological and pathophysiological context.
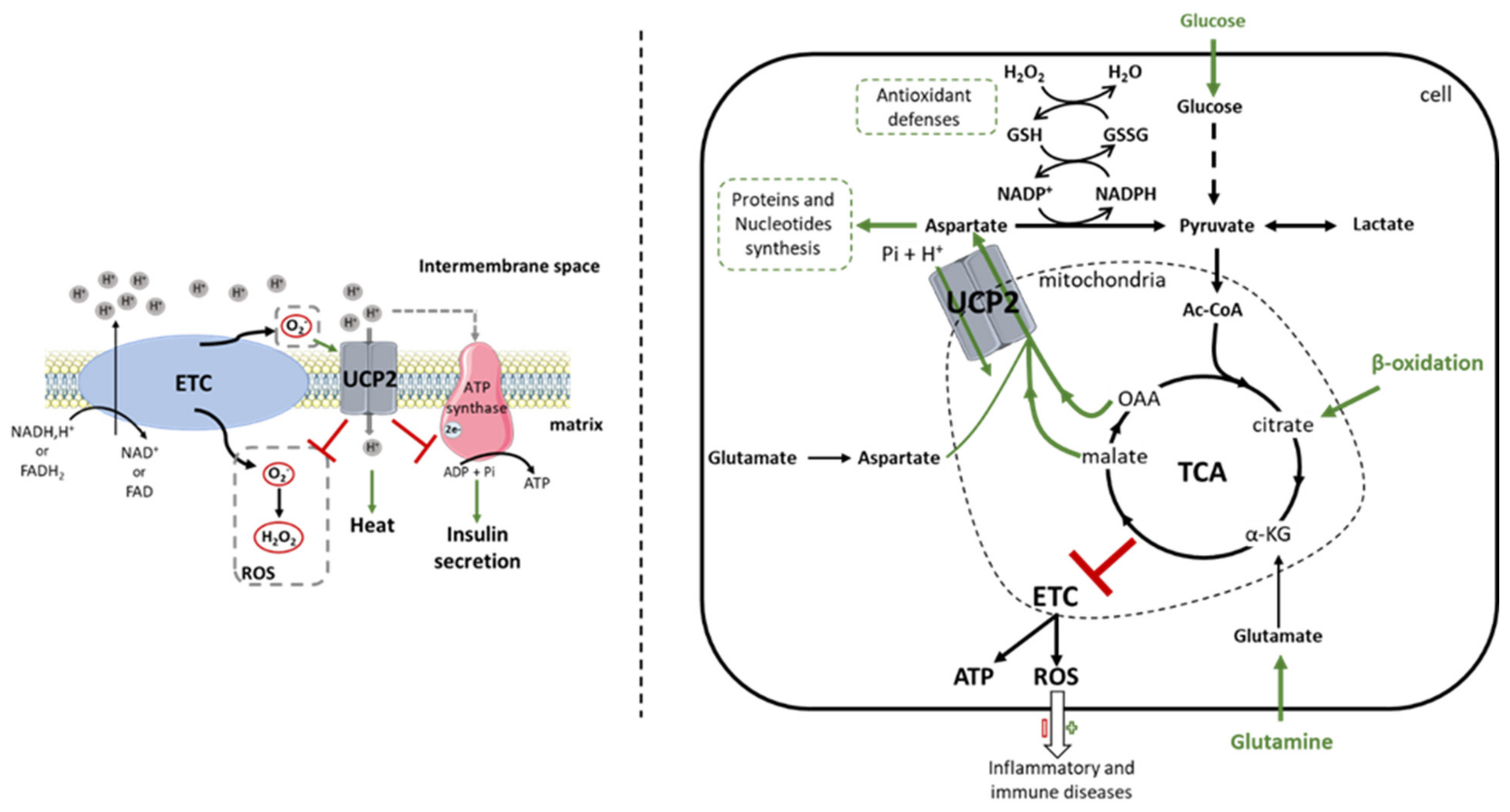
Figure 1.
Comparison between the first function attributed to UCP2 and the new concept of mitochondrial transport highlighted by the work of the Fiermonte team. Since its discovery, the UCP2 protein has been the subject of much controversy regarding its biochemical function. UCP2 was initially identified as an uncoupling protein of the electron transport chain. Thus, the protein captures protons from the intermembrane space and dissipates them into the mitochondrial matrix. Increased thermogenesis, decreased reactive oxygen species (ROS) levels and ATP synthesis, which consequently reduce insulin secretion, are observed due to reduced membrane potential (left). However, the non-reproducibility of these results in different cells and tissues has questioned this function. Since then, work by Vozza et al. [22] in 2014 showed that UCP2 catalyzes the exchange of 4-carbon (C4) metabolites (oxaloacetate, malate, aspartate) from the tricarboxylic acid (TCA) cycle for a phosphate and a proton. UCP2 then regulates pyruvate oxidation in the mitochondrial matrix, which promotes cell proliferation by providing building blocks and increases antioxidant defenses by regulating glutathione oxidation. C4 transport also controls TCA cycle activity to avoid overload by the accumulation of intramitochondrial compounds. The redox pressure of the oxidative phosphorylation is then attenuated, which limits the production of ROS more or less harmful in the fight against pathologies (right). Ac-CoA: Acetyl-CoA; ADP: adenosine diphosphate; ATP: adenosine triphosphate; C4: 4-carbon metabolites; ETC: electron transport chain; FADH2(-FAD): flavine adenine dinucleotide; GSH(-SSG): glutathione; H+: hydrogen ion; H2O: water molecule; H2O2: hydrogen peroxide; NADH(-NAD+): nicotinamide adenine dinucleotide; NADPH(-P+): nicotinamide adenine dinucleotide phosphate; O2-: superoxide; OAA: oxaloacetate; Pi: phosphate ion; ROS: reactive oxygen species; TCA: tricarboxylic acid cycle; UCP2: uncoupling protein 2.
2.1. Biochemical Function
2.1.1. Uncoupling?
The strong homology between UCP1 and UCP2 initially directed research toward similar mitochondrial membrane uncoupling functions (Figure 1). Uncoupling corresponds to the dissociation of ETC oxidative phosphorylation from ATP synthesis; it causes hydrogen ion leakage from the intermembrane space to the mitochondrial matrix to dissipate proton gradient energy. The use of recombinant UCP2 expression systems in yeast and proteoliposomes showed that proton flux decreases the membrane potential in the presence of UCP2 [15,40,41]. The same phenomenon was later described in β-pancreatic cells, thymocytes and isolated kidney mitochondria [24,42,43]. However, these results have been questioned by other studies using mitochondria isolated from Ucp2−/− spleen and kidney [21]. While a thermogenic phenotype has been described as a consequence of uncoupling [44], no dysregulation of energy balance and thermoregulation was observed in Ucp2−/− mice exposed to cold [42,45]. It has been proposed that UCP2 lowers the membrane potential to decrease the ATP/ADP ratio, and thus limit mitochondrial ROS production, during successive oxidation–reduction reactions of the respiratory chain, and negatively regulates insulin secretion [24,42,43]. Indeed, the uncoupling induced by the overexpression of UCP2 in isolated pancreatic islets decreases the electrochemical gradient, which then reduces by 50% the production of ATP by complex V, preventing the exocytosis of insulin vesicles [46]. As for UCP1, these reactions are inhibited by purine nucleotides and activated by free fatty acids, glucose, retinoic acid or ROS [41]. In 2002, Etchay et al. showed that the activation of UCP2 by superoxide increased the proton conductance in a manner dependent on fatty acids, and more particularly on palmitate in isolated mitochondria from the kidney, spleen and pancreas of rats [43]. Other authors have even stated that in the absence of these activators, UCP2 could not perform this uncoupling function [31]. However, these results on the uncoupling activity of UCP2 are now highly controversial. While the characterization of the UCP1 protein showed that two histidine residues (His-145 and His-147) were essential for its protonophore activity, they are absent in UCP2 [47]. These data suggest that if UCP2 is a proton carrier, it uses a different mechanism. Moreover, studies conducted to elucidate the uncoupling role of UCP2 were performed under overexpression conditions in yeast and bacterial systems [15,40,41,48]. However, the artificial overexpression of UCP carriers in these models prevented them from reaching the correct protein conformation [13,49], leading to an uncontrolled artifactual uncoupling of mitochondrial respiration [49,50,51] that is no longer comparable to the physiological level of UCP2 adjusted by tight translation regulation [19]. Furthermore, in the basal state, a comparison of the respiratory rate of mitochondria isolated from spleen and lung of Ucp2+/+ and Ucp2−/− mice showed no change [21]. Additionally, in the latter study, the activation of uncoupling with retinoic acid required micromolar concentrations, and is unlikely to be physiologically relevant [21]. These data are also in agreement with the work of Rial et al., who showed that under standard conditions, the use of purine nucleotides, palmitic acid and retinoic acid had no effect on the respiratory rate of UCP2-overexpressing yeast mitochondria [41]. In contrast, they observed that an increase in pH associated with micromolar concentrations of retinoic acid stimulated respiration through an increase in UCP2 mRNA [41]. However, Ucp2 is translationally regulated and an increase in mRNA expression does not necessarily correlate with protein levels [19]. Unfortunately, much of the data in favor of uncoupling activity have not been replicated. Indeed, studies have described UCP2 activity in renal tissue where UCP2 could never be detected [24,52]. Other studies have used commercial anti-UCP2 antibodies that have been shown to be nonspecific for UCP2 protein detection [42,53,54]. To address the difficulties of the interpretation of western blot, Pecqueur et al. developed in 2001 a sensitive anti-UCP2 polyclonal antibody (UCP2-605 homemade) that has since been approved [19,25]. Since then, other teams have developed antibodies against UCP2 [34]. Thus, the use of commercial antibodies must be carefully validated using Ucp2−/− tissue or UCP2CRISPR/Cas9 cells to ensure the specific detection of UCP2.
2.1.2. Transport of Metabolites
The discovery of other UCPs in plants and fish, organisms not dependent on thermogenesis, further challenged the uncoupling theory and suggested that UCP2 may have other biochemical functions [55,56]. Indeed, UCP2 belongs to the SLC25 family, a group that includes all mitochondrial carriers [57]. The discovery in 2006 of the involvement of UCP3 in the transport of pyruvate was another argument that directed the search for UCP2 activity towards a transport function in mitochondria [58]. It was proposed by Trenker et al., using the overexpression of UCP2 in human endothelial cells, that UCP2 participates in mitochondrial calcium (Ca2+) sequestration [59]. However, after the discovery of mitochondrial calcium uniport (MCU), which was identified as a major contributor to mitochondrial Ca2+ transport, it was suggested that UCP2 might have a modulatory activity. This was confirmed by super-resolution microscopy technology, which showed a specific association between UCP2 and MCU, once the latter was methylated by the protein arginine methyl transferase 1 (PMRT1). This interaction was positively correlated with mitochondrial Ca2+ uptake [60,61,62]. In addition, an interaction has been demonstrated between calcium ions and TCA intermediates, such as citrate and malate [63,64]. Interestingly, Vozza and collaborators demonstrated that UCP2 catalyzes the exchange of C4 metabolites (oxaloacetate, malate, aspartate) for phosphate and hydrogen ions, and therefore regulates pyruvate oxidation in the mitochondrial matrix [22]. Thus, the TCA activity is regulated to avoid overload by the accumulation of intramitochondrial compounds and to reduce the redox pressure, and thus limit ROS production (Figure 1) [22]. These results highlight that the metabolic remodeling (regulation of oxidative stress, insulin secretion, etc.) associated with UCP2 since the early 2000s could be explained by the biochemical transport function of C4 metabolites, and not by an uncoupling activity.
2.2. UCP2 a Metabolic Regulator
Due to the distribution of the UCP2 protein mainly in glycolytic tissues and its role as a mitochondrial carrier, it is now well understood that UCP2 is involved in different physiological and pathological processes related to glucose and lipid metabolism, such as the regulation of food intake, insulin secretion, and immune response [65,66]. Our team has demonstrated that UCP2 acts as a metabolic rewiring regulator to favor fatty acid metabolism over glucose. Indeed, the genetic loss of Ucp2 in murine embryonic fibroblasts (MEFs) was associated with increased proliferation correlated with glycolytic reprogramming at the expense of β-oxidation [26,67]. Indeed, in Ucp2+/+ MEF cells, only 67% of ATP is produced by glucose oxidation, compared to 89% in Ucp2−/− MEF cells [68]. Glycolysis allows the supply of nucleotides through the pentose phosphate pathway to support proliferation. Subsequently, other studies supporting the hypothesis of Pecqueur et al. confirmed that UCP2 is a sensor of glucose to lipid metabolism, especially under energy stress [65]. During caloric restriction or fasting, UCP2 expression is positively correlated with an increased energy response and greater weight loss [69]. Ucp2−/− mice exposed to fasting have a predisposition to hepatic steatosis due to impaired lipid utilization [70]. These mechanisms are also related to the downregulation of insulin synthesis by UCP2 in pancreatic β-cells [71]. UCP2 thus facilitates lipolysis and consequently the resulting β-oxidation [71]. However, insulin release is ineffective after exposure to high glucose concentrations. In this case, UCP2 promotes cell deregulation by inhibiting apoptosis via bcl-2 [72]. Moreover, ghrelin is a hormone that promotes food intake during a fasting state through its activating action on NPY/AgRP neurons. UCP2 mediates the action of ghrelin by activating AMP-activated protein kinase (AMPK), which suppresses acetyl-CoA carboxylase (ACC) enzymatic activity but increases Carnitine palmitoyl transferase I (CPT1) expression to promote energy intake via β-oxidation in these neurons [73]. In contrast, Zhang et al. and Vozza et al. suggested that the loss of UCP2 promotes mitochondrial glucose oxidation by measuring the intramitochondrial accumulation of TCA constituents in stem cells and human hepatocarcinoma cells (HepG2) [22,74]. This accumulation reflects TCA saturation induced by the absence of C4 metabolite efflux. Furthermore, Kukat et al. demonstrated in mice with cardiomyopathy that the absence of the UCP2 protein prevented the oxidation of lipids accumulated in the cells [75]. ATP was then supplied by glycolysis, inducing significant lactic acidosis in disabled mice.
In addition to glucose and fatty acids, cancer and immune cells oxidize glutamine through the glutaminolysis reaction. Glutamine is a major amino acid in plasma and, as a substrate, is successively converted into glutamate and ketoglutarate to integrate the TCA and support oxidative metabolism. In the presence of glutamine, Ucp2−/− macrophages showed altered homeostasis of glutaminolysis-derived metabolites with the accumulation of glutamate, succinate and malate compared to Ucp2+/+ macrophages [76]. Additionally, fatty acids and glutamine have been shown to activate Ucp2 transcription (via Peroxisome Proliferator-activated Receptor (PPAR)) and Ucp2 translation, respectively [77,78,79]. These data reinforce the transporter role of UCP2, which in turn can export the C4 intermediates provided by the oxidation of these two substrates independently of the presence of glucose. The export of C4 metabolites can thus renew the cellular stock of pyruvate and regulate oxidative stress in parallel via glutathione by avoiding a surplus of glucose consumption that would overload the TCA cycle in glycolytic tissues.
In conclusion, these different studies show that UCP2 is an essential regulator of substrate utilization, both at the systemic level via insulin regulation and at the cellular level.
2.3. UCP2 and Oxidative Stress: A Link toward Immunity
The homeostasis of redox status contributes to cellular integrity. It has been recognized that ROSs are involved in many etiologies, such as cancer, aging, neurodegeneration and inflammatory pathologies. Since the first studies on UCP2, its role as a modulator of ROS has been suggested [24,80,81]. Indeed, in 1997, Nègre-Salvayre et al. showed that when UCP2 was inhibited by GDP, mitochondrial H2O2 production was increased [80]. Subsequently, UCP2 overexpression studies in human aortic endothelial cells and neonatal rat cardiomyocytes confirmed these initial results, while emphasizing that the inhibition of ROS production by UCP2 decreased oxidative stress-induced apoptosis [82,83]. At that time, the hypothesis put forward by scientists was that the uncoupling generated by UCP2 jointly lowered mitochondrial membrane potential and ATP synthase activity. The functioning of the ETC was then reduced, which in turn decreased the formation of ROS [24,80,84]. Nowadays, in view of the findings of Vozza et al., it becomes natural to think that since UCP2 controls the oxidation of TCA substrates, the redox pressure of OXPHOS and consequently ROS production is attenuated (Figure 1) [22].
With the identification of the antioxidant role of UCP2, much research has been carried out to investigate its involvement in various pathologies, including those related to immune system activation, as evidenced by the numerous reviews recently published [85,86,87]. Hereafter, we will give a brief and non-exhaustive description of the plurality of oxidative stress responses induced by UCP2 in different pathologies.
2.3.1. Insulin Regulation—Type 2 Diabetes (T2D)—Cardiovascular Diseases
Research on UCP2 quickly highlighted its role in the pathogenesis of diabetes through its regulation of pancreatic β-cell function [24]. In mouse models, UCP2 was shown to be involved in pancreatic development via the ROS-Akt (protein kinase B) pathway by promoting the proliferation of α and β endocrine cells from the embryonic stage [88], and its loss promoted glucose-stimulated insulin secretion (GSIS) compared to wild-type mice [42]. Furthermore, the short-term inhibition of UCP2 activity by Genipin, a pharmacological inhibitor of UCP2 derived from Chinese medicine, improved glycemic response, indicating that UCP2 is a negative regulator of insulin secretion [71]. These combined results may suggest a better response to hyperglycemia. Indeed, Zhang et al. demonstrated a beneficial effect of UCP2 loss on glucose homeostasis in an ob/ob diabetic mouse model [42]. These mice had better GSIS in the first phase after glucose ingestion and better insulin signal transduction in white adipose tissue [42]. However, improved insulin tolerance did not completely restore a healthy phenotype, as no significant changes in body weight, food intake, serum triglycerides, or epididymal fat were found in diabetic Sw/Uni mice treated with an antisense Ucp2 inhibitor [89]. On the contrary, other studies showed that the reduction in Ucp2 expression achieved by transfection of small interfering RNAs in pancreatic islets of ob/ob diabetic mice did not modify plasma insulin levels or blood glucose levels [90], but generated an imbalance of oxidative stress in the pancreatic islets [91]. The loss of UCP2, over several generations of backcrossed mice, in four different strains induced extensive macrophage immune infiltration, which subsequently caused a persistent accumulation of ROS with an imbalance in the reduced glutathione/oxidized glutathione (GSH/GSSG) ratio and an increase in nitrotyrosine levels [92,93]. This excessive accumulation of ROS therefore led to pancreatic α- and β-cells dysfunction, favoring the long-term progression to T2D [94]. Indeed, the lack of insulin feedback on glucagon led to glucose resistance. By analogy, the overexpression of UCP2 in islets conferred protection to pancreatic β-cells against oxidative stress and glucotoxicity [95]. In addition, patients with T2D have an increased risk of developing cardiovascular disease [96]. The genetic deletion of Ucp2 in a mouse model of atherosclerosis accelerated the development of atherosclerotic lesions in the mouse aorta [30]. Cardiovascular dysfunction resulted in endothelial alterations that facilitated monocyte invasion into the intima of Ucp2−/− mice [97]. Moreover, an oxidative burst produced by Ucp2−/−-infiltrating macrophages in atherosclerotic plaques strongly and locally increased ROS, and activated pro-inflammatory cytokine release through overexpression of the pro-inflammatory transcription factor Forkhead Box Protein O1 (FoxO1) [30,98].
2.3.2. Infectious Diseases
UCP2 has also been shown to be involved in antimicrobial defense by increasing ROS production after infection. Shortly after the discovery of Ucp2, Arsenijevic et al. demonstrated that Ucp2−/− mice were resistant to Toxoplasma gondii infection. The loss of the protein stimulates the release of ROS from macrophages, which have a toxoplasmicidal action and thus limit the formation of brain cysts [27]. After listeria infection, the improved survival of Ucp2−/− mice was also observed due to an enhanced phagocytic infiltrate following increased secretion of the MCP1 chemokine for up to 4 days [28]. At the molecular level, isolated Ucp2−/− macrophages stimulated by lipopolysaccharides (LPS) significantly increased ROS production and synergistically potentiated the regulation of the nuclear factor kappa light chain enhancer of activated B cells (NFκB) and Mitogen-activated protein kinases (MAPK) signaling pathways to enhance the inflammatory response compared to wild-type macrophages [23,99]. These in vitro studies were also confirmed after Leishmaniasis infections. Ball et al. showed in 2011 that mice injected with a Ucp2 shRNA into the spleen had a parasite load inversely correlated with ROS production [100]. This result was also confirmed in Ucp2−/− mice [101]. Moreover, in cell lysates from Leishmania-infected Ucp2−/− macrophages, the increased production of pro-inflammatory cytokines (IL1β, IL6, TNFα) was induced by the activation of Erk and p38 MAPK signaling pathways and the inflammasome [100,102]. UCP2 indeed regulates caspase 1 via the NLRP3 inflammasome by stimulating lipid synthesis, whether during localized or systemic infection [103]. Clinical studies have also corroborated these data by showing a positive correlation between UCP2 expression and the severity of sepsis in patients [104].
2.3.3. Autoimmune Diseases
In autoimmune diabetes (type 1 diabetes (T1D)), the role of UCP2 has also been discussed. Indeed, using the streptozotocin (STZ) model with repeated injections of low doses of STZ that induce selective necrosis of pancreatic β-cells, Emre et al. showed in Ucp2−/− mice that the development of the disease was advanced and more severe with increased hyperglycemia compared to Ucp2+/+ mice [29]. This phenotype is accompanied by an increased infiltration of pro-inflammatory lymphocytes and macrophages, as well as the increased production of ROS. Indeed, the promotion of inflammation and oxidative stress accelerated the establishment of T1D by causing the rapid destruction of Ucp2−/− pancreatic β-cells [29]. In contrast, Lee et al. found that the STZ use associated with UCP2 loss essentially alters the function of glucagon-secreting α-cells by increasing ROS [105]. Overall, these results indicate that the loss of UCP2 in autoimmune diabetes can affect both pancreatic β- and α-cells, depending on the study setting and mouse strain (C57BL/6J and C57BL6/129, respectively).
Multiple sclerosis and its corresponding animal model (experimental autoimmune encephalomyelitis (EAE)) is a demyelinating pathology of the central nervous system (CNS). Since no curative treatment has been developed, teams have been interested in studying the impact of the loss of UCP2 on this neurodegenerative disease. Ucp2−/− mice, immunized with a myelin-specific antigen combined with an adjuvant, developed more severe clinical scores compared to wild-type mice [54]. Greater T cell infiltration was found in Ucp2-deficient mice compared with wild-type mice. This accumulation of CD4 and CD8 lymphocytes promoted a strong pro-inflammatory response with the production of high levels of TNFα, IL2 and ROS [54]. Interestingly, the double deficiency of UCP2 and iNOS (inducible nitric oxide synthase) reduced the inflammatory phenotype [106]. More recently, in the spinal cord of wild-type mice, it was shown that the peak of UCP2 expression coincided with the peak of clinical symptoms of EAE occurring 14 days after immunization [107]. These results suggest that UCP2 may be upregulated in the spinal cord to counteract inflammation-induced oxidative stress, and thereby protect the CNS. Accordingly, Alves Guerra et al. showed in 2003 that UCP2 was strongly regulated during the immune response. In the early stages of LPS stimulation, UCP2 expression was inhibited to promote the establishment of the inflammatory response through ROS production. Then, at a later stage, UCP2 was overexpressed to counteract the toxic effect of oxidative stress [25].
In conclusion, all the studies cited above have demonstrated that the mitochondrial transporter UCP2 is involved in energy metabolism and in the regulation of oxidative stress capable of initiating an immune response (Table 1). As both of these features are important aspects of tumorigenesis, we will discuss in the next section how UCP2 might regulate tumor development.

Table 1.
Non exhaustive overview of pathophysiological implications of the mitochondrial protein UCP2.
3. UCP2 and Cancer
In recent years, many studies have shown increased UCP2 protein expression in a large number of human tumors compared to adjacent normal tissues (e.g., head and neck, skin, pancreatic, prostate, colon, gallbladder, breast cancer, etc.) [118,119,120,121]. After screening more than 100 colon adenocarcinomas, Horimoto et al. showed 3- to 4-fold higher levels of Ucp2 mRNA and protein in tumors than in normal tissues [118]. Additionally, UCP2 levels were positively correlated with increased amounts of lipid peroxidation and associated with neoplasia [118]. Naturally, these results lead us to wonder whether increased UCP2 expression is protective or deleterious for tumor development. Thus, in this section, we will attempt to elucidate the role of UCP2 in a tumor context by successively examining its involvement in the oxidative stress, metabolic and immune reprogramming of a tumor.
3.1. UCP2: A Double-Edged Fight against ROS
Oxidative stress plays a complex role in cancer development. UCP2, whose antioxidant role has been detailed previously, has been implicated in tumorigenesis by controlling ROS. The initiation of colon cancer by azoxymethane (AOM) treatment induces the formation of more aberrant crypts in Ucp2−/− mice than in Ucp2+/+ mice [108]. In the absence of UCP2, the increase in lesion number was associated with a lack of protection against oxidative stress and the activation of NFκB-dependent anti-apoptotic genes [108]. Furthermore, Aguilar et al. recently showed an increase in tumor number in Ucp2−/− mice compared to wild-type mice using two models of colorectal carcinogenesis: AOM-DSS (dextran sodium sulfate) and APCmin (Adenomatous polyposis coli) [32]. Loss of UCP2 enhanced colon tumorigenesis by promoting lipid synthesis, which limits the availability of nicotinamide adenine dinucleotide phosphate (NADPH) for glutathione synthesis to buffer oxidative stress [32]. In addition, mutations in the tumor protein 53 (TP53) gene occur during tumorigenesis in over 50% of human cancers, and particularly affect colorectal cancer. The mutant p53 protein loses its antioxidant capacity but acquires new pro-tumor, pro-inflammatory and pro-oxidant biological properties [122]. Indeed, the mutant p53 protein inhibits the Sestrin 1 (SESN1)/AMPK/PPARγ coactivator 1 (PGC1) pathway, which is a transcriptional axis that activates UCP2, and consequently the inhibition of this signaling pathway stimulates O2− production in cancer cell lines. In this context, the loss of UCP2 associated with the gain of function of the p53 mutant confers hyperproliferative anti-apoptotic effects on cancer cells [123].
However, depending on the stage and type of cancer, UCP2 has a dual regulation mechanism. The hypothesis today is that UCP2 would be repressed in the tumor initiation phases to allow the accumulation of ROS, as described above, while on the contrary the protein would be overexpressed in the late phase to maintain the proliferative capacities of the cells in the tumor microenvironment (TME) that are already under stress conditions [120,124]. This hypothesis is also based on the binary action of ROS. Indeed, when ROS levels are elevated but not uncontrolled, they favor tumor promotion through the acquisition of mutations in oncogenes and the activation of pro-tumor metabolic signals associated with the disruption of the establishment of antioxidant mechanisms. Moreover, a significant increase in oxidative damage will trigger programmed cell death, which relies on caspase activity and cell elimination by phagocytes to limit tumor development. However, tumor cells set up antioxidant activities essential for tumorigenesis to counteract ROS-induced apoptosis [125,126]. Several studies have shown the ROS control induced by UCP2 in established tumor cell lines. Under hypoxic conditions, similar to tumor core conditions, the overexpression of UCP2 in the lung adenocarcinoma cell line A549 showed anti-apoptotic properties. Indeed, the UCP2-induced decrease in ROS accumulation inhibited programmed cell death by blocking cytochrome c release and reducing caspase 9 activity [109]. Correlatively, the transfection of Ucp2 siRNA into A549 and PaCa44 (human pancreatic adenocarcinoma) cell lines inhibited cell growth through a strong increase in ROS production [109,110]. The loss of UCP2 in different human pancreatic adenocarcinoma cell lines further stimulated autophagy through the ROS-dependent nuclear translocation of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) [110]. Autophagy-derived apoptosis was reversed by treatment with the free radical scavenger N-acetylcysteine (NAC) when Ucp2 was inhibited genetically by siRNA and pharmacologically by Genipin [110]. Additionally, Wu et al. demonstrated that the silencing of UCP2 in human glioma cell lines alters the p38 MAPK signaling cascade, which reduces the migration and invasion capabilities of tumor cells [127]. Indeed, p38, widely studied as an antitumorigenic factor in the early stages of the disease, has rather an oncogenic role in advanced disease, since it is involved in the metastatic process when it is no longer blocked by dual-specificity phosphatases (DUSP) [127,128]. Therefore, the loss of UCP2 may favor tumor cells that are less likely to metastasize.
3.2. UCP2 and Tumoral Metabolic Reprogramming
Metabolic dysregulation is one of the factors identified by Hanahan and Weinberg as a common feature of cancer cells [3]. Moreover, we have seen previously that UCP2 plays an important role in controlling cell metabolism by modulating the TCA cycle activity, but what about under conditions of tumorigenesis?
3.2.1. Glycolysis
Glycolysis is the metabolic pathway that converts glucose to pyruvate. In the absence or presence of oxygen, tumors promote the production of lactate from pyruvate [129]. Tumors have an intensely active metabolism to provide energy and building blocks for unregulated cell proliferation. Thus, due to the dysregulated activation of oncogenes that induce the overexpression of glycolysis-stimulating enzymes, cancer cells oxidize abundant amounts of glucose [8]. In this context, Esteves et al. demonstrated that UCP2 overexpression in the murine melanoma cell line B16F10 induces hypoxia-inducible factor-2α (HIF2α)/AMPK axis-dependent metabolic reprogramming [111]. The authors revealed that the reduced level of fumarate observed in UCP2high cells would be the link between AMPK activation and decreased HIF2α expression [111]. Indeed, a decrease in fumarate contributes to stabilize the enzyme pyruvate dehydrogenase (PDH) and inhibits HIF2α, and would therefore be indirectly responsible for the downregulation of the expression of the glycolytic enzymes hexokinase 2 (HK2) and pyruvate kinase M2 (PKM2) [111,130]. In addition, several studies have demonstrated a positive effect of fumarate on AMPK activity [131,132]. Besides this, the decrease in fumarate accumulation also suggests a better regulation of TCA cycle through the export of C4 metabolites by UCP2 [111]. Hence, UCP2 shifts the glycolysis-dependent metabolism to oxidative phosphorylation, which significantly decreases the proliferation of B16F10 cells and thus reduces the cells’ tumorigenic capacity. These results are consistent with a recently published study indicating that saturation of the mitochondrial malate shuttle, which uses a nicotinamide adenine dinucleotide (NADH) cofactor to reduce oxaloacetate to malate, redirects proliferative cellular metabolism to aerobic glycolysis [133]. To go further, metabolomics fluxes using C13-labeled glucose caused an 18% reduction in glucose consumption by the UCP2-overexpressing JB6 P+ murine skin epidermal cell line [134]. In contrast, pyruvate was found to be much more enriched in C13 under aerobic conditions in the same murine cell line. A significant percentage of carbons were rewired to the TCA cycle and not to lactate production, to allow amino acid and nucleic acid synthesis [134]. In addition, palmitate oxidation, as measured by the Seahorse XF apparatus, was higher in JB6 P+ cells transfected with UCP2 compared to control cells [115]. These data demonstrate that the presence of UCP2 induces an increase in β-oxidation in a tumor context as well as in a physiological context, as previously shown by Pecqueur et al. [26,115]. However, although the TCA cycle function is enhanced by UCP2 content, the authors do not show whether this correlates with reduced tumor cell proliferation, migration or invasion.
Nevertheless, other studies have not corroborated these initial results. Yu et al. demonstrated a poor prognosis associated with increased expression of Ucp2 RNA and UCP2 protein in patients with cholangiocarcinoma. Metabolically, the cellular knockdown of UCP2 in the intrahepatic cholangiocarcinoma cell line HuCCT1 and in the extrahepatic cholangiocarcinoma cell line TFK-1 resulted in the attenuation of glycolysis through the activation of AMPK [112]. Phosphorylated AMPK expression, an indicator of its activation, was upregulated in the absence of UCP2 following increased mtROS production and decreased antioxidant activities [111,112]. Indeed, AMPK is an essential sensor for the maintenance of cellular energy homeostasis that is activated by phosphorylation during metabolic stress, such as ROS-mediated oxidative stress [135]. Moreover, activated AMPK reversed the mesenchymal phenotype by inhibiting Akt signaling in cholangiocarcinoma cell lines with low UCP2 expression. Thus, cancer cells lose their ability to migrate and invade healthy tissue [112]. Through its transport activity, UCP2 modified the utilization of substrate by cancer cells. These changes induce Akt-mTOR (mammalian target of rapamycin) signaling, which activates and mediates a glycolytic flux by maintaining an elevated expression of glucose transporter 1 (GLUT1), 6-phosphofructo-2-kinase (PFKB2), and PKM2, which triggers increased lactate release [113,136]. Furthermore, the inhibition of UCP2, either pharmacologically with Genipin or genetically with siRNAs, was sufficient to reverse the metabolic phenotype and reduce proliferation in PaCa44 and JB6 P+ cancer cell lines [113,136].
Although all these results indicate that UCP2 is involved in the glycolytic metabolism of cancer cells, some data are contradictory. These discrepancies can be explained by the fact that the authors used different cell lines that do not necessarily rely on the same energy metabolism. Moreover, the cancer cells used do not express identical basal levels to UCP2.
3.2.2. Glutaminolysis
However, glucose is not the only energy-providing substrate, and glutamine is an important source of nitrogen and carbon for the TCA cycle in tumor cells. Indeed, oncogenes support glutaminolysis to drive mitochondrial metabolism that regulates cellular precursors essential to support increased cell proliferation [137]. The translation of Ucp2 being partly regulated by glutamine, and UCP2 facilitating TCA cycle function, several teams have analyzed the role of the protein in glutamine dependence in oxidative tumors [22,77]. A positive correlation between the expression level of UCP2 and glutamine dependence has been demonstrated in several human and murine tumor cell lines [114,138]. Indeed, when N18TG2 neuroblastoma cells were deprived of glucose, UCP2 was upregulated in response to glutamine consumption, which became the main source of oxidative phosphorylation [138]. However, Sancerni et al. recently demonstrated that the metabolic adaptations of cells to intra- and extracellular glutamine depend on the basal level of UCP2 [114]. Indeed, the loss of UCP2 in HPB-ALL leukemic cells, which highly express UCP2 and are metabolically dependent on glutamine, decreased their oxygen consumption and redirected their metabolism to glycolysis. This cellular metabolic adaptation induced a decrease in cell proliferation. In contrast, Jurkat leukemia cells, which express low levels of UCP2 and are dependent on glycolytic metabolism, were less metabolically affected by Ucp2 knockdown. These results show that Jurkat cells have a more flexible and less oxidation-dependent glutamine metabolism than HPB-ALL cells. However, the loss of UCP2 in Jurkat cells further decreased proliferation by altering the biosynthesis of lipid, protein and nucleotide precursors [114]. In addition, to counteract nutritional stress due to glutamine deficiency, the murine neuroblastoma cell line N18TG2 promoted lactate synthesis; nevertheless, in a manner dependent on activating transcription factor 4 (ATF4), the cells entered quiescent-metabolic and -proliferative phases [138]. Raho et al. also demonstrated that in human pancreatic ductal adenocarcinoma (PDAC), the loss of the UCP2 transporter reduced the mitochondrial glutamine catabolism, leading to increased ROS levels and a shortage of aspartate required for protein and nucleotide biosynthesis [34]. Interestingly, the in vitro and in vivo silencing of UCP2 reduced the rate of cell proliferation only in PDACs mutated for Kirsten Rat Sarcoma Virus (KRAS), which is an oncogene that rewires glutamine consumption for the synthesis of aspartate and oxaloacetate. In parallel, aspartate or GSH supplementation partially or totally rescued the cell growth defect observed in the absence of UCP2 [34,139]. Altogether, these results highlight that UCP2 is essential for glutamine-dependent tumors.
3.2.3. Ca2+ Signaling
Ca2+ signaling is essential for cellular physiological functions, especially in cancer cells, in order to cope with high energy demands and rewire Ca2+ requirements [140]. Upon cellular stress, such as increased oxidative stress reflected by elevated H2O2 levels, receptor tyrosine kinases activate phospholipase Cγ-1 (PLCγ-1), which then cleaves membrane phospholipids, generating two second messengers that stimulate intracellular Ca2+ entry [141]. The overexpression of UCP2 in JB6 P+ cells stimulated with the tumor inducer TPA (12-O-tetradecanoylphorbol 13-acetate) enhanced calcium signaling induced by PLCγ-1 upregulation [115]. Cytoplasmic Ca2+ is then captured by the endoplasmic reticulum (ER) and transferred via mitochondria-associated membranes (MAMs) to mitochondria, stimulating ETC activity and thereby promoting ATP production [140]. Above, we have already pointed out that UCP2 is required for mitochondrial Ca2+ uptake to counteract the inhibitory methylation of the MCU transporter by PRMT1 [61]. Furthermore, it has also been shown that the combined overexpression of UCP2 and PRMT1 improved mitochondrial respiration and cell viability in lung carcinoma cells [142]. In agreement, lung cancer patients with combined Ucp2high and Prmt1high RNA expression had reduced 5-year survival [142]. However, the prolonged binding between mitochondria and MAMs promotes the risk of mitochondrial Ca2+ overload, which, following mitochondrial dysfunction, induces apoptotic cell death. Thus, it has been shown that UCP2 expression is downregulated to avoid overload when the mitochondria–ER interaction is stabilized [116]. Calcium signaling is therefore a finely regulated process that depends on the balance between mitochondria-ER binding and mitochondrial Ca2+ uptake via variations in UCP2 expression to optimally promote tumor development.
Altogether, the studies demonstrated that UCP2 acts differently depending on the metabolic dependence of tumors. In conclusion, the energy metabolism of cancers being very different, it is therefore important to characterize precisely their specific metabolism in order to determine whether a positive or negative targeting of UCP2 will be effective against tumor development.
3.3. UCP2-Activated Antitumor Immunity
Immunometabolism represents a new area of intense research to find new targets to fight cancer, as the infiltration of immune cells into the tumor is indeed influenced by the metabolic characteristics of the TME [143]. UCP2, through its regulation of ROS production, acts on the inflammatory and immune responses of various pathologies (see Section 2.3). In addition, UCP2 modulates the metabolic demands of cancer cells and thus appears to be an interesting candidate to study the immune reprogramming of tumors. Bioinformatics analyses of the Cancer Genome Atlas (TCGA) database of breast cancer and melanoma patients showed a positive correlation between UCP2 expression and antitumor immune infiltration [117,144]. A better prognosis associated with high UCP2 expression also confirmed these correlative findings [117,144]. Based on these observations, Cheng et al. aimed to decipher the role of UCP2 in the immune response of melanoma tumors. First, transcriptomic studies demonstrated that UCP2 induced an CD8+ T cell and conventional dendritic cell (cDC1) infiltration profile [117]. Then, by establishing xenografts with B16-OVA and YUMM1 melanoma cell lines with doxycycline-inducible UCP2 expression, they demonstrated that UCP2 overexpression in these tumor cells induced an immune environment similar to that of melanoma patients. UCP2 facilitated the recruitment of CD8+ T cells through an increased release of the chemokine CXCL10 from cCD1, combined with a decrease in the expression of pro-tumorigenic factors (IL10, M-CSF (macrophage colony-stimulating factor) and VEGF (Vascular Endothelial Growth Factor)) and a normalization of the vasculature that amplifies the antitumor infiltration. Moreover, the induction of UCP2 in combination with immunotherapy (anti-PD1 antibody) resulted in reduced tumor volume and improved survival compared with anti-PD1 antibody alone. These results highlight the potent role of UCP2 in increasing the response to immunotherapy treatment [117]. Based on the results of Esteves et al., the authors hypothesized that increased UCP2 expression in melanoma cells limits the aerobic glycolysis of tumor cells, allowing glucose to be available for consumption by lymphocytes [111,117]. Indeed, when lymphocytes are activated and recruited to the tumor site, they essentially consume glucose to release cytotoxic factors [143]. Other authors have shown that UCP2 improves immune recruitment by acting directly on lymphocytes expressing high levels of UCP2. Indeed, the antigenic stimulation of isolated naive CD4+ and CD8+ T cells induced an increase in UCP2 expression after 24 h, and even more so after restimulation [145]. Furthermore, activated UCP2 inhibits ROS generation to limit ROS-induced apoptosis, and simultaneously regulates glycolytic flux and TCA cycle activity to promote lymphocyte clonal expansion [146].
In conclusion, despite the different functions of UCP2, which may have opposite effects depending on the type and stage of cancer, the authors are in global agreement that the UCP2 protein does not intervene by modulating proton leakage and thus ETC uncoupling. An increase in mitochondrial respiration in the presence of UCP2 independently of the uncoupling function has nevertheless sometimes been described. However, if the effect on metabolism has been well characterized, the effect on tumor immunity remains little studied to date (Figure 2 and Table 1).
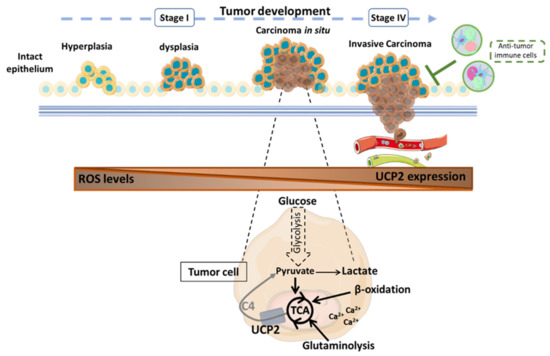
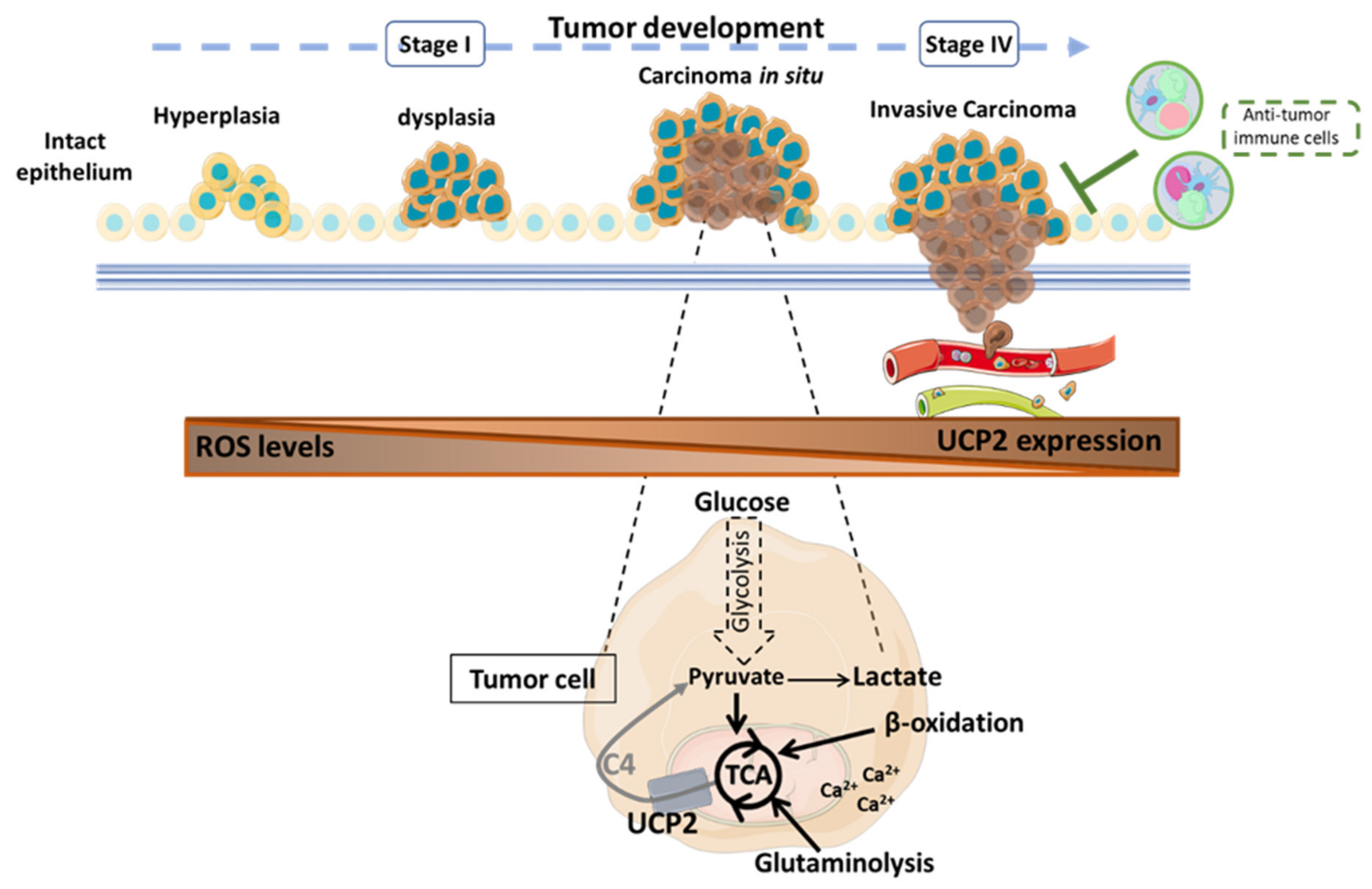
Figure 2.
Role of the mitochondrial carrier UCP2 in tumor progression according to published results [32,111,114,116,117,147]. UCP2 is involved at all stages of tumor development with different consequences depending on the type of cancer. UCP2 acts as an antioxidant to counteract the formation of cellular and mitochondrial reactive oxygen species that could then spread into the tumor microenvironment (TME) to promote the acquisition of new pro-tumor properties of the cells. Through the transport of 4-carbon metabolites of the tricarboxylic acid (TCA) cycle, UCP2 is involved in the regulation of tumor metabolism. When UCP2 is expressed, tumor cells undergo oxidative metabolic reprogramming. Thus, glucose is preferentially converted to pyruvate to support cellular anabolism through TCA cycle activity. In addition, the export of C4 metabolites prevents the overloading of the TCA cycle via the accumulation of intramitochondrial compounds and reduces redox pressure to limit ROS production. Moreover, β-oxidation and glutaminolysis can be promoted to sustain TCA. Calcium dependence also supports the energy requirements of tumor cells. Nevertheless, the presence of UCP2 correlates with a significant antitumor immune infiltration to resist tumor development. C4: 4-carbon metabolites; Ca2+: calcium ion; ROS: reactive oxygen species; TCA: tricarboxylic acid cycle; UCP2: uncoupling protein 2.
3.4. Drug Sensitivity and Therapeutic Improvement
Chemotherapy is a very common strategy used to control tumor growth. Antineoplastic drugs, often given in combination with other treatments, specifically or otherwise target the cell cycle to damage DNA and induce apoptosis in tumor cells. However, the effectiveness of chemotherapy is a major challenge. Indeed, many patients fail to respond due to specific mutations or acquire resistance by adapting antioxidant defense mechanisms and altering the TME [148,149]. Through the roles of UCP2, studies have characterized the involvement of UCP2 in the regulation of tumor cell sensitivity to cancer therapies. In patients with serous ovarian carcinoma, one study showed a negative correlation between UCP2 protein expression and platinum sensitivity. Thus, patients with low UCP2 expression had improved overall survival with platinum-based therapy [150]. However, this work was performed using an AbCam antibody, which was found to be unreliable and non-specific by Aguilar et al. [32]. In vitro, cells overexpressing UCP2 induced chemoresistance by modulating ROS that abrogate p53-induced apoptosis [151]. UCP2 also promoted the expression of the mitochondrial detoxifier superoxide dismutase 2 (SOD2) [121]. The pro-survival activity of UCP2 is also mediated by the NFκB/β-catenin axis. After the treatment of gallbladder cancer cells (G-415) with gentamicin, these transcription factors were activated and, in combination with UCP2, promoted cancer cell survival during chemotherapy [121]. Since gentamycin alone induces Ucp2 gene expression in pancreatic adenocarcinoma cell lines, it has been hypothesized that UCP2 is directly involved in acquired cancer resistance to gentamycin [152]. Conversely, enhanced sensitivity to staurosporine has been reported in several cell lines overexpressing UCP2, reflected in increased pro-apoptotic caspase activities [111]. As pointed out by Wang and colleagues, the deletion of UCP2 under hypoxic conditions increased ROS, but contributed to the stabilization of nuclear factor erythroid 2–related factor 2 (Nrf2), which upregulated the mediate efflux transporter ATP-binding cassette super-family G member 2 (ABCG2), resulting in chemoresistance to cisplatin and docetaxel [153]. Furthermore, these results are consistent with a clinical study that showed poorer response to and survival following cisplatin-based chemotherapy in advanced lung cancer patients with low UCP2 expression [154]. Overall, these studies showed that regulating UCP2 expression, either positively or negatively depending on the type of cancer, represents a therapeutic strategy to be considered to improve responses to chemotherapy (Figure 3).
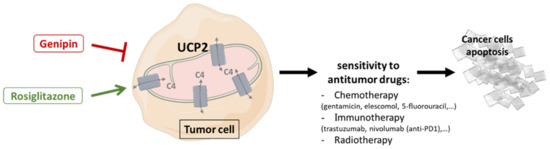
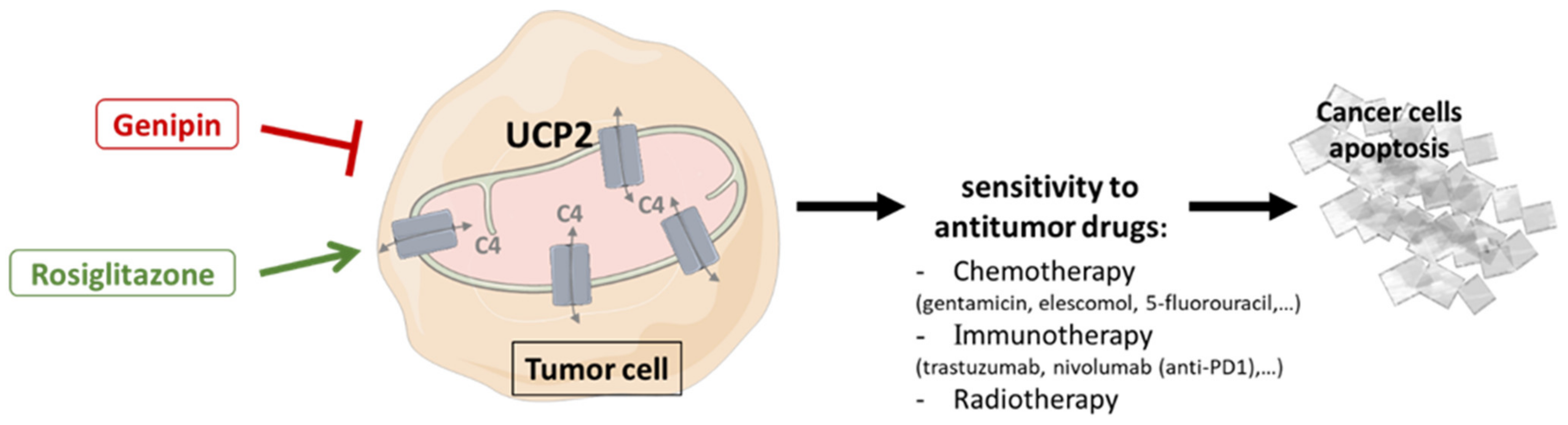
Figure 3.
Therapeutic strategies targeting UCP2 combined with antitumor therapies to promote cancer cell apoptosis. Use of activators (rosiglitazone) or inhibitors (Genipin) of UCP2, depending on tumor-dependent metabolism, leads to changes in the capacities of mitochondria to provide energy and building blocks through the transport of 4-carbon tricarboxylic acid cycle metabolites, and may improve sensitivity to anticancer therapies by increasing tumor cell apoptosis. C4: 4-carbon metabolites; UCP2: uncoupling protein 2.
3.4.1. Genipin
Genipin is a plant chemical extracted from the fruit of Gardenia Jasminoides Ellis. This molecule is widely used in traditional Chinese medicine for its anti-inflammatory, antioxidant, and antipyretic properties. Chemically, Genipin is an excellent cross-linking agent that has been commonly used in industry to produce biomaterials. In 2006, Genipin was also identified as an inhibitor of UCP2; indeed, a specific inhibition of proton transport by the binding of arginine residues on UCP2 was proposed [71]. However, the lack of UCP2-mediated uncoupling highlighted in the studies cited above does not allow us to determine the true mechanism of action of this molecule on UCP2, although the antitumor role of Genipin is well documented [71,155,156,157]. Furthermore, Vozza and his team pointed out in 2014 that Genipin had no effect on UCP2 transport activity. Indeed, this molecule did not catalyze the 33Pi/Pi exchange [22]. In contrast, Genipin, by decreasing UCP2 expression in MCF7 breast cancer lines, inhibited tumor promotion characterized by lower cell proliferation and migration compared to control [158]. The growth, migration and 3D steroid invasion of cholangiocarcinoma cancer lines were also effectively reduced by Genipin in a dose-dependent manner [112]. In vitro, Genipin treatment activated intrinsic apoptotic pathways that enabled cell cycle arrest in the G2/M phase through the inhibitory induction of cyclin-dependent kinase inhibitor 1 (p21) [159,160]. Moreover, the triggering of p38 MAPK signaling inducing the pro-apoptotic activity of caspase 3, which enhances the antitumor effect of Genipin [161], appears to be ROS-dependent [110,113]. Indeed, the inhibition of UCP2 by Genipin induced high levels of total ROS measured by the non-fluorescent diacetylated 2′,7′-dichlorofluorescein (DCFH-DA) probe in the cell medium of pancreatic adenocarcinoma lines triggering cell apoptosis through an autophagic process that was impaired by NAC treatment [110]. In addition, Cho et al. recently demonstrated that Genipin supplementation suppressed the uptake of 18F-FDG (18Fluorine-fluorodeoxyglucose), a glucose analog and radiotracer, in positron emission tomography (PET) scans of breast (T47D and MDA-MB-435) and colon (HCT116 and HT29) cancer cells [162]. The resulting decrease in lactate production and glycolytic flux has been characterized at the molecular level by inhibition of the expression of glycolytic enzymes (GLUT1, PFKFB2, PKM2, Lactate dehydrogenase a (LDHa)…) associated with Akt signaling [113,136]. Finally, the pleiotropic action of Genipin also induced tumor suppression in vivo by depleting tumor-associated macrophages in the TME of hepatocellular carcinoma mouse xenografts [163].
Nevertheless, the real benefit associated with Genipin treatment appears to be the significant improvement in the sensitivity of cancer cells to antitumor treatments. The combined use of cytotoxic molecules, in this case trastuzumab, with Genipin synergized their action and created a similar effect on the inhibition of cell viability to the use of Ucp2 siRNA and trastuzumab on the HER2-positive BT474 cell line [164]. In pancreatic adenocarcinoma cells, the combination treatment of Everolimus, an antitumor agent that inhibits the mTOR pathway, with Genipin synergistically inhibited cancer cell proliferation by increasing the nuclear translocation of GAPDH [147]. In pancreatic, lung and leukemia cancer cells, GAPDH is indeed translocated by increased production of ROS, mainly from the mitochondria [147,165,166]. The resulting amplified triggering of apoptosis strongly reduced the tumor volume of pancreatic adenocarcinoma xenografts in mice without altering their general condition during Everolimus–Genipin treatment [147]. Furthermore, Lee et al. also demonstrated that co-treatment with Genipin and Elescomol, a pro-apoptotic chemotherapy adjuvant, decreased tumor energy metabolism by reducing glycolytic flux [165].
However, although Genipin appears to be a promising molecule for improving response to chemotherapy treatments, the lack of knowledge about its molecular mechanism of action has so far prevented the establishment of clinical trials in cancer.
3.4.2. Rosiglitazone
Since the potent role of UCP2-mediated metabolic reprogramming associated with cancer cell proliferation and immunity has been described above in different studies [32,34,111,114,117], UCP2 activators may represent a promising therapeutic strategy to simultaneous target different energetic pathways essential for tumor growth.
After activation, PPARs, members of the steroid receptor family, are translocated into the nucleus to heterodimerize with the retinoid receptor (RXR) and thus initiate the transcription of target genes such as UCP2 [167]. Among the three PPAR isoforms (PPARα, PPARβ/δ, PPARγ), PPARγ is the most studied to date due to its crucial role in carbohydrate and lipid homeostasis, regulation of apoptosis and tumor progression [168,169]. Numerous studies have demonstrated that PPARγ acts as a tumor suppressor by activating the pro-apoptotic gene TP53 and blocking the cell cycle through the overexpression of p21 in colon, lung, pancreatic, prostate and breast cancer cell lines [170,171,172,173,174]. Furthermore, Kwon and colleagues showed that reduced levels of PPARγ in sporadic colorectal cancers correlated with poor prognosis in patients [175]. The search for synthetic activating molecules led to the identification of thiazolidinediones, including rosiglitazone, as PPARγ agonists [168]. Thus, rosiglitazone has been shown to transcriptionally induce Ucp2 [176]. Recently, increased UCP2 expression following rosiglitazone treatment in melanoma cell lines promoted antitumor immunity and induced the reprogramming of the cytokine profile secreted by tumor-infiltrating T cells [117]. UCP2 induction also significantly improved the response to anti-PD1 immunotherapy, and a greater reduction in tumor volume in melanoma xenografts was reported when anti-PD1 was combined with rosiglitazone [117]. Rosiglitazone also improved radiosensitivity and response to 5-fluorouracil chemotherapy by inhibiting cell growth and suppressing pancreatic and colorectal cancer cells in vitro [177,178].
However, these potential therapeutic benefits against cancer need to be re-evaluated in humans in terms of dose, because, unfortunately, serious side effects in patients such as fluid retention, heart failure or an increased risk of developing bladder cancer have been described [179]. Although rosiglitazone is still approved by the Food and Drug Administration (FDA), its regulation and use are now very restricted [180]. Moreover, PPAR agonists activate a multitude of target genes, so identifying a specific activator of UCP2 remains a challenge.
4. Conclusions
Cancer can be initiated by an uncontrolled accumulation of ROS, and then cancer cells survive and proliferate until they invade other organs by metabolic and immune reprogramming. Although complex to demonstrate, UCP2 is involved in all these steps with different consequences. Its chemical role has been widely reconsidered in recent years following the questioning of its uncoupling function. In this review, we described that UCP2 acts as a metabolic sensor through its role as a mitochondrial transporter of C4 metabolites of the TCA cycle to regulate and control these processes. Overall, we argue that UCP2 transport-related regulations occur not only in a tumor context, but also in a healthy context. Metabolite modulation then leads to adaptations in cellular and mitochondrial metabolism. Nevertheless, we believe that the metabolic adaptations enabled by UCP2 are more pronounced in a stressful context, such as cancer. Indeed, few effects due to a modification of UCP2 expression can be observed in a model of healthy intestinal epithelium, for example. The latter theoretically has the capacity to maintain its ATP production through the different pathways of energy catabolism. However, when cells are damaged in metabolic diseases, infections or cancers, a loss of UCP2 will have more drastic consequences. Cancer cells or immune cells are indeed dependent on specific metabolic pathways, such as glutamine, which are essential for their activation and expansion. Thus, the absence of UCP2 will lead to a saturation of their TCA cycle, totally unbalancing the metabolism of these cells, and this will decrease in parallel the cellular antioxidant defenses caused by the absence of aspartate transport, and consequently of glutathione synthesis.
In clinical studies, different correlations, negative or positive depending on the type of cancer, have been described between Ucp2 mRNA expression and patient survival. UCP2 is therefore considered today in many studies based on the Oncomine database as a potential cancer biomarker. However, it is crucial to remember that Ucp2 gene expression is not correlated with protein levels due to translational regulation [19]. Moreover, many commercial antibodies targeting UCP2 commonly used by different teams are not effective, and are sometimes even non-specific, because the detected signal is conserved in Ucp2−/− tissues [32]. Thus, caution should be taken in correlating the prognosis of cancer progression with UCP2 protein levels in cancer patients, as has been done in breast, pancreatic and lung cancer with non-specific antibodies [33,124,142]. Nevertheless, based on all the studies cited above, the inhibition or activation of UCP2 according to the metabolic status of the tumor represents a therapeutic opportunity to improve cancer remission. Targeting UCP2 in combination with conventional treatments (chemotherapy, radiotherapy, immunotherapy) could promote a beneficial response in patients. However, we have seen here that the use of Genipin or rosiglitazone is not optimal because their mode of action on UCP2 is not well characterized and not specific. Designing or synthesizing new molecules directly targeting UCP2 should be a priority in the coming years to counter tumor progression.
Author Contributions
Conceptualization, A.L. and M.-C.A.-G.; writing—original draft preparation, A.L. and M.-C.A.-G.; writing—review and editing, A.L. and M.-C.A.-G.; Supervision, M.-C.A.-G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the “Centre National de la Recherche Scientifique” (CNRS), the “Institut National de la Santé et de la Recherche Médicale” (Inserm) and the University Paris-Cité.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Warburg, O.; Wind, F.; Negelein, E. The Metabolism of Tumors in the Body. J. Gen. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef]
- Heiden, M.G.V.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Ahn, C.S.; Metallo, C.M. Mitochondria as Biosynthetic Factories for Cancer Proliferation. Cancer Metab. 2015, 3, 1–10. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. Fundamentals of Cancer Metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef]
- Amoedo, N.D.; Sarlak, S.; Obre, E.; Esteves, P.; Bégueret, H.; Kieffer, Y.; Rousseau, B.; Dupis, A.; Izotte, J.; Bellance, N.; et al. Targeting the Mitochondrial Trifunctional Protein Restrains Tumor Growth in Oxidative Lung Carcinomas. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef]
- Brand, M.D. Mitochondrial Generation of Superoxide and Hydrogen Peroxide as the Source of Mitochondrial Redox Signaling. Free Radic. Biol. Med. 2016, 100, 14–31. [Google Scholar] [CrossRef]
- Chio, I.I.C.; Tuveson, D.A. ROS in Cancer: The Burning Question. Trends Mol. Med. 2017, 23, 411–429. [Google Scholar] [CrossRef]
- Sullivan, L.B.; Chandel, N.S. Mitochondrial Reactive Oxygen Species and Cancer. Cancer Metab. 2014, 2, 1–12. [Google Scholar] [CrossRef]
- Bouillaud, F.; Alves-Guerra, M.-C.; Ricquier, D. UCPs, at the Interface between Bioenergetics and Metabolism. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2016, 1863, 2443–2456. [Google Scholar] [CrossRef]
- Heaton, G.M.; Wagenvoord, R.J.; Kemp, A.; Nicholls, D.G. Brown-Adipose-Tissue Mitochondria: Photoaffinity Labelling of the Regulatory Site of Energy Dissipation. Eur. J. Biochem. 1978, 82, 515–521. [Google Scholar] [CrossRef]
- Fleury, C.; Neverova, M.; Collins, S.; Raimbault, S.; Champigny, O.; Levi-Meyrueis, C.; Bouillaud, F.; Seldin, M.F.; Surwit, R.S.; Ricquier, D.; et al. Uncoupling Protein-2: A Novel Gene Linked to Obesity and Hyperinsulinemia. Nat. Genet. 1997, 15, 269–272. [Google Scholar] [CrossRef]
- Mao, W.; Yu, X.X.; Zhong, A.; Li, W.; Brush, J.; Sherwood, S.W.; Adams, S.H.; Pan, G. UCP4, a Novel Brain-Specific Mitochondrial Protein that Reduces Membrane Potential in Mammalian Cells. FEBS Lett. 1999, 443, 326–330. [Google Scholar] [CrossRef]
- Sanchis, D.; Fleury, C.; Chomiki, N.; Goubern, M.; Huang, Q.; Neverova, M.; Grégoire, F.; Easlick, J.; Raimbault, S.; Lévi-Meyrueis, C.; et al. BMCP1, a Novel Mitochondrial Carrier with High Expression in the Central Nervous System of Humans and Rodents, and Respiration Uncoupling Activity in Recombinant Yeast. J. Biol. Chem. 1998, 273, 34611–34615. [Google Scholar] [CrossRef]
- Enerbäck, S.; Jacobsson, A.; Simpson, E.M.; Guerra, C.; Yamashita, H.; Harper, M.-E.; Kozak, L.P. Mice Lacking Mitochondrial Uncoupling Protein Are Cold-Sensitive but not Obese. Nature 1997, 387, 90–94. [Google Scholar] [CrossRef]
- Pecqueur, C.; Alves-Guerra, M.-C.; Gelly, C.; Lévi-Meyrueis, C.; Couplan, E.; Collins, S.; Ricquier, D.; Bouillaud, F.; Miroux, B. Uncoupling Protein 2, in vivo Distribution, Induction upon Oxidative Stress, and Evidence for Translational Regulation. J. Biol. Chem. 2001, 276, 8705–8712. [Google Scholar] [CrossRef]
- Hurtaud, C.; Gelly, C.; Bouillaud, F.; Lévi-Meyrueis, C. Translation Control of UCP2 Synthesis by the Upstream Open Reading Frame. Cell. Mol. Life Sci. 2006, 63, 1780. [Google Scholar] [CrossRef]
- Couplan, E.; del Mar Gonzalez-Barroso, M.; Alves-Guerra, M.C.; Ricquier, D.; Goubern, M.; Bouillaud, F. No Evidence for a Basal, Retinoic, or Superoxide-Induced Uncoupling Activity of the Uncoupling Protein 2 Present in Spleen or Lung Mitochondria. J. Biol. Chem. 2002, 277, 26268–26275. [Google Scholar] [CrossRef]
- Vozza, A.; Parisi, G.; De Leonardis, F.; Lasorsa, F.M.; Castegna, A.; Amorese, D.; Marmo, R.; Calcagnile, V.M.; Palmieri, L.; Ricquier, D.; et al. UCP2 Transports C4 Metabolites out of Mitochondria, Regulating Glucose and Glutamine Oxidation. Proc. Natl. Acad. Sci. USA 2014, 111, 960–965. [Google Scholar] [CrossRef]
- Bai, Y.; Onuma, H.; Bai, X.; Medvedev, A.V.; Misukonis, M.; Weinberg, J.B.; Cao, W.; Robidoux, J.; Floering, L.M.; Daniel, K.W.; et al. Persistent Nuclear Factor-ΚB Activation in Ucp2-/- Mice Leads to Enhanced Nitric Oxide and Inflammatory Cytokine Production. J. Biol. Chem. 2005, 280, 19062–19069. [Google Scholar] [CrossRef]
- Krauss, S.; Zhang, C.-Y.; Scorrano, L.; Dalgaard, L.T.; St-Pierre, J.; Grey, S.T.; Lowell, B.B. Superoxide-Mediated Activation of Uncoupling Protein 2 Causes Pancreatic β Cell Dysfunction. J. Clin. Investig. 2003, 112, 1831–1842. [Google Scholar] [CrossRef]
- Alves-Guerra, M.-C.; Rousset, S.; Pecqueur, C.; Mallat, Z.; Blanc, J.; Tedgui, A.; Bouillaud, F.; Cassard-Doulcier, A.-M.; Ricquier, D.; Miroux, B. Bone Marrow Transplantation Reveals the in vivo Expression of the Mitochondrial Uncoupling Protein 2 in Immune and Nonimmune Cells during Inflammation. J. Biol. Chem. 2003, 278, 42307–42312. [Google Scholar] [CrossRef]
- Pecqueur, C.; Bui, T.; Gelly, C.; Hauchard, J.; Barbot, C.; Bouillaud, F.; Ricquier, D.; Miroux, B.; Thompson, C.B. Uncoupling Protein-2 Controls Proliferation by Promoting Fatty Acid Oxidation and Limiting Glycolysis-Derived Pyruvate Utilization. FASEB J. 2007, 22, 9–18. [Google Scholar] [CrossRef]
- Arsenijevic, D.; Onuma, H.; Pecqueur, C.; Raimbault, S.; Manning, B.S.; Miroux, B.; Couplan, E.; Alves-Guerra, M.-C.; Goubern, M.; Surwit, R.; et al. Disruption of the Uncoupling Protein-2 Gene in Mice Reveals a Role in Immunity and Reactive Oxygen Species Production. Nat. Genet. 2000, 26, 435–439. [Google Scholar] [CrossRef]
- Rousset, S.; Emre, Y.; Join-Lambert, O.; Hurtaud, C.; Ricquier, D.; Cassard-Doulcier, A.-M. The Uncoupling Protein 2 Modulates the Cytokine Balance in Innate Immunity. Cytokine 2006, 35, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Emre, Y.; Hurtaud, C.; Karaca, M.; Nubel, T.; Zavala, F.; Ricquier, D. Role of Uncoupling Protein UCP2 in Cell-Mediated Immunity: How Macrophage-Mediated Insulitis Is Accelerated in a Model of Autoimmune Diabetes. Proc. Natl. Acad. Sci. USA 2007, 104, 19085–19090. [Google Scholar] [CrossRef]
- Blanc, J.; Alves-Guerra, M.C.; Esposito, B.; Rousset, S.; Gourdy, P.; Ricquier, D.; Tedgui, A.; Miroux, B.; Mallat, Z. Protective Role of Uncoupling Protein 2 in Atherosclerosis. Circulation 2003, 107, 388–390. [Google Scholar] [CrossRef]
- Brand, M.D.; Esteves, T.C. Physiological Functions of the Mitochondrial Uncoupling Proteins UCP2 and UCP3. Cell Metab. 2005, 2, 85–93. [Google Scholar] [CrossRef]
- Aguilar, E.; Esteves, P.; Sancerni, T.; Lenoir, V.; Aparicio, T.; Bouillaud, F.; Dentin, R.; Prip-Buus, C.; Ricquier, D.; Pecqueur, C.; et al. UCP2 Deficiency Increases Colon Tumorigenesis by Promoting Lipid Synthesis and Depleting NADPH for Antioxidant Defenses. Cell Rep. 2019, 28, 2306–2316.e5. [Google Scholar] [CrossRef]
- Pons, D.G.; Nadal-Serrano, M.; Torrens-Mas, M.; Valle, A.; Oliver, J.; Roca, P. UCP2 Inhibition Sensitizes Breast Cancer Cells to Therapeutic Agents by Increasing Oxidative Stress. Free Radic. Biol. Med. 2015, 86, 67–77. [Google Scholar] [CrossRef]
- Raho, S.; Capobianco, L.; Malivindi, R.; Vozza, A.; Piazzolla, C.; De Leonardis, F.; Gorgoglione, R.; Scarcia, P.; Pezzuto, F.; Agrimi, G.; et al. KRAS-Regulated Glutamine Metabolism Requires UCP2-Mediated Aspartate Transport to Support Pancreatic Cancer Growth. Nat. Metab. 2020, 2, 1373–1381. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 377, 1345–1356. [Google Scholar] [CrossRef]
- Bozic, I.; Allen, B.; Nowak, M.A. Dynamics of Targeted Cancer Therapy. Trends Mol. Med. 2012, 18, 311–316. [Google Scholar] [CrossRef][Green Version]
- Wang, K.; Jiang, J.; Lei, Y.; Zhou, S.; Wei, Y.; Huang, C. Targeting Metabolic–Redox Circuits for Cancer Therapy. Trends Biochem. Sci. 2019, 44, 401–414. [Google Scholar] [CrossRef]
- Leary, M.; Heerboth, S.; Lapinska, K.; Sarkar, S. Sensitization of Drug Resistant Cancer Cells: A Matter of Combination Therapy. Cancers 2018, 10, 483. [Google Scholar] [CrossRef]
- Ježek, P.; Holendová, B.; Garlid, K.D.; Jabůrek, M. Mitochondrial Uncoupling Proteins: Subtle Regulators of Cellular Redox Signaling Reviewing Editors: Jerzy Beltowski, Joseph Burgoyne, Gabor Csanyi, Sergey Dikalov, Frank Krause, Anibal Vercesi, and Jeremy Ward. Antioxid. Redox Signal. 2018, 29, 667–714. [Google Scholar] [CrossRef]
- Jabůrek, M.; Vařecha, M.; Gimeno, R.E.; Dembski, M.; Ježek, P.; Zhang, M.; Burn, P.; Tartaglia, L.A.; Garlid, K.D. Transport Function and Regulation of Mitochondrial Uncoupling Proteins 2 and 3 *. J. Biol. Chem. 1999, 274, 26003–26007. [Google Scholar] [CrossRef]
- Rial, E.; González-Barroso, M.; Fleury, C.; Iturrizaga, S.; Sanchis, D.; Jiménez-Jiménez, J.; Ricquier, D.; Goubern, M.; Bouillaud, F. Retinoids Activate Proton Transport by the Uncoupling Proteins UCP1 and UCP2. EMBO J. 1999, 18, 5827–5833. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Baffy, G.; Perret, P.; Krauss, S.; Peroni, O.; Grujic, D.; Hagen, T.; Vidal-Puig, A.J.; Boss, O.; Kim, Y.-B.; et al. Uncoupling Protein-2 Negatively Regulates Insulin Secretion and Is a Major Link between Obesity, β Cell Dysfunction, and Type 2 Diabetes. Cell 2001, 105, 745–755. [Google Scholar] [CrossRef]
- Echtay, K.S.; Roussel, D.; St-Pierre, J.; Jekabsons, M.B.; Cadenas, S.; Stuart, J.A.; Harper, J.A.; Roebuck, S.J.; Morrison, A.; Pickering, S.; et al. Superoxide Activates Mitochondrial Uncoupling Proteins. Nature 2002, 415, 96–99. [Google Scholar] [CrossRef]
- Conti, B.; Sanchez-Alavez, M.; Winsky-Sommerer, R.; Morale, M.C.; Lucero, J.; Brownell, S.; Fabre, V.; Huitron-Resendiz, S.; Henriksen, S.; Zorrilla, E.P.; et al. Transgenic Mice with a Reduced Core Body Temperature Have an Increased Life Span. Science 2006, 314, 825–828. [Google Scholar] [CrossRef]
- Harper, J.A.; Dickinson, K.; Brand, M.D. Mitochondrial Uncoupling as a Target for Drug Development for the Treatment of Obesity. Obes. Rev. 2001, 2, 255–265. [Google Scholar] [CrossRef]
- Chan, C.B.; De Leo, D.; Joseph, J.W.; McQuaid, T.S.; Ha, X.F.; Xu, F.; Tsushima, R.G.; Pennefather, P.S.; Salapatek, A.M.F.; Wheeler, M.B. Increased Uncoupling Protein-2 Levels in β-Cells Are Associated with Impaired Glucose-Stimulated Insulin Secretion: Mechanism of Action. Diabetes 2001, 50, 1302–1310. [Google Scholar] [CrossRef]
- Bienengraeber, M.; Echtay, K.S.; Klingenberg, M. H+ Transport by Uncoupling Protein (UCP-1) Is Dependent on a Histidine Pair, Absent in UCP-2 and UCP-3. Biochemistry 1998, 37, 3–8. [Google Scholar] [CrossRef]
- Gimeno, R.E.; Dembski, M.; Weng, X.; Deng, N.; Shyjan, A.W.; Gimeno, C.J.; Iris, F.; Ellis, S.J.; Woolf, E.A.; Tartaglia, L.A. Cloning and Characterization of an Uncoupling Protein Homolog: A Potential Molecular Mediator of Human Thermogenesis. Diabetes 1997, 46, 900–906. [Google Scholar] [CrossRef]
- Stuart, J.A.; Harper, J.A.; Brindle, K.M.; Jekabsons, M.B.; Brand, M.D. A Mitochondrial Uncoupling Artifact Can Be Caused by Expression of Uncoupling Protein 1 in Yeast. Biochem. J. 2001, 356, 779–789. [Google Scholar] [CrossRef]
- Stuart, J.A.; Harper, J.A.; Brindle, K.M.; Jekabsons, M.B.; Brand, M.D. Physiological Levels of Mammalian Uncoupling Protein 2 Do Not Uncouple Yeast Mitochondria. J. Biol. Chem. 2001, 276, 18633–18639. [Google Scholar] [CrossRef]
- Harper, J.A.; Stuart, J.A.; Jekabsons, M.B.; Roussel, D.; Brindle, K.M.; Dickinson, K.; Jones, R.B.; Brand, M.D. Artifactual Uncoupling by Uncoupling Protein 3 in Yeast Mitochondria at the Concentrations Found in Mouse and Rat Skeletal-Muscle Mitochondria. Biochem. J. 2002, 361, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Echtay, K.S.; Murphy, M.P.; Smith, R.A.J.; Talbot, D.A.; Brand, M.D. Superoxide Activates Mitochondrial Uncoupling Protein 2 from the Matrix Side: Studies using targeted antioxidants. J. Biol. Chem. 2002, 277, 47129–47135. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qian, H.; Huang, X.; Li, J.; Zhang, J.; Zhu, N.; Chen, H.; Zhu, C.; Wang, J.; Zhang, P.; et al. UCP2 Mitigates the Loss of Human Spermatozoa Motility by Promoting MROS Elimination. CPB 2018, 50, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Vogler, S.; Pahnke, J.; Rousset, S.; Ricquier, D.; Moch, H.; Miroux, B.; Ibrahim, S.M. Uncoupling Protein 2 Has Protective Function during Experimental Autoimmune Encephalomyelitis. Am. J. Pathol. 2006, 168, 1570–1575. [Google Scholar] [CrossRef] [PubMed]
- Sweetlove, L.J.; Lytovchenko, A.; Morgan, M.; Nunes-Nesi, A.; Taylor, N.L.; Baxter, C.J.; Eickmeier, I.; Fernie, A.R. Mitochondrial Uncoupling Protein Is Required for Efficient Photosynthesis. Proc. Natl. Acad. Sci. USA 2006, 103, 19587–19592. [Google Scholar] [CrossRef]
- Stuart, J.A.; Harper, J.A.; Brindle, K.M.; Brand, M.D. Uncoupling Protein 2 from Carp and Zebrafish, Ectothermic Vertebrates. Biochim. Biophys. Acta (BBA) Bioenerg. 1999, 1413, 50–54. [Google Scholar] [CrossRef]
- Ruprecht, J.J.; Kunji, E.R.S. The SLC25 Mitochondrial Carrier Family: Structure and Mechanism. Trends Biochem. Sci. 2020, 45, 244–258. [Google Scholar] [CrossRef]
- Mozo, J.; Ferry, G.; Studeny, A.; Pecqueur, C.; Rodriguez, M.; Boutin, J.A.; Bouillaud, F. Expression of UCP3 in CHO Cells Does Not Cause Uncoupling, but Controls Mitochondrial Activity in the Presence of Glucose. Biochem. J. 2006, 393, 431–439. [Google Scholar] [CrossRef]
- Trenker, M.; Malli, R.; Fertschai, I.; Levak-Frank, S.; Graier, W.F. Uncoupling Proteins 2 and 3 Are Fundamental for Mitochondrial Ca2+ Uniport. Nat. Cell Biol. 2007, 9, 445–452. [Google Scholar] [CrossRef]
- Gottschalk, B.; Klec, C.; Leitinger, G.; Bernhart, E.; Rost, R.; Bischof, H.; Madreiter-Sokolowski, C.T.; Radulović, S.; Eroglu, E.; Sattler, W.; et al. MICU1 Controls Cristae Junction and Spatially Anchors Mitochondrial Ca2+ Uniporter Complex. Nat. Commun. 2019, 10, 3732. [Google Scholar] [CrossRef]
- Madreiter-Sokolowski, C.T.; Klec, C.; Parichatikanond, W.; Stryeck, S.; Gottschalk, B.; Pulido, S.; Rost, R.; Eroglu, E.; Hofmann, N.A.; Bondarenko, A.I.; et al. PRMT1-Mediated Methylation of MICU1 Determines the UCP2/3 Dependency of Mitochondrial Ca2+ Uptake in Immortalized Cells. Nat. Commun. 2016, 7, 12897. [Google Scholar] [CrossRef] [PubMed]
- Koshenov, Z.; Oflaz, F.E.; Hirtl, M.; Bachkoenig, O.A.; Rost, R.; Osibow, K.; Gottschalk, B.; Madreiter-Sokolowski, C.T.; Waldeck-Weiermair, M.; Malli, R.; et al. The Contribution of Uncoupling Protein 2 to Mitochondrial Ca2+ Homeostasis in Health and Disease—A Short Revisit. Mitochondrion 2020, 55, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Barati, D.; Walters, J.D.; Pajoum Shariati, S.R.; Moeinzadeh, S.; Jabbari, E. Effect of Organic Acids on Calcium Phosphate Nucleation and Osteogenic Differentiation of Human Mesenchymal Stem Cells on Peptide Functionalized Nanofibers. Langmuir 2015, 31, 5130–5140. [Google Scholar] [CrossRef] [PubMed]
- Reinwald, S.; Weaver, C.M.; Kester, J.J. Chapter 6—The Health Benefits of Calcium Citrate Malate: A Review of the Supporting Science. In Advances in Food and Nutrition Research; Taylor, S.L., Ed.; Academic Press: Cambridge, MA, USA, 2008; Volume 54, pp. 219–346. [Google Scholar]
- Diano, S.; Horvath, T.L. Mitochondrial Uncoupling Protein 2 (UCP2) in Glucose and Lipid Metabolism. Trends Mol. Med. 2012, 18, 52–58. [Google Scholar] [CrossRef]
- Toda, C.; Diano, S. Mitochondrial UCP2 in the Central Regulation of Metabolism. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 757–764. [Google Scholar] [CrossRef]
- Pecqueur, C.; Alves-Guerra, C.; Ricquier, D.; Bouillaud, F. UCP2, a Metabolic Sensor Coupling Glucose Oxidation to Mitochondrial Metabolism? IUBMB Life 2009, 61, 762–767. [Google Scholar] [CrossRef]
- Bouillaud, F. UCP2, Not a Physiologically Relevant Uncoupler but a Glucose Sparing Switch Impacting ROS Production and Glucose Sensing. Biochim. Biophys. Acta 2009, 1787, 377–383. [Google Scholar] [CrossRef]
- Heinitz, S.; Piaggi, P.; Yang, S.; Bonfiglio, S.; Steel, J.; Krakoff, J.; Votruba, S.B. Response of Skeletal Muscle UCP2-Expression during Metabolic Adaptation to Caloric Restriction. Int. J. Obes. 2018, 42, 974–984. [Google Scholar] [CrossRef]
- Sheets, A.R.; Fülöp, P.; Derdák, Z.; Kassai, A.; Sabo, E.; Mark, N.M.; Paragh, G.; Wands, J.R.; Baffy, G. Uncoupling Protein-2 Modulates the Lipid Metabolic Response to Fasting in Mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G1017–G1024. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Parton, L.E.; Ye, C.P.; Krauss, S.; Shen, R.; Lin, C.-T.; Porco, J.A.; Lowell, B.B. Genipin Inhibits UCP2-Mediated Proton Leak and Acutely Reverses Obesity- and High Glucose-Induced β Cell Dysfunction in Isolated Pancreatic Islets. Cell Metab. 2006, 3, 417–427. [Google Scholar] [CrossRef]
- He, Y.; Wang, N.; Shen, Y.; Zheng, Z.; Xu, X. Inhibition of High Glucose-Induced Apoptosis by Uncoupling Protein 2 in Human Umbilical Vein Endothelial Cells. Int. J. Mol. Med. 2014, 33, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Andrews, Z.B.; Liu, Z.-W.; Walllingford, N.; Erion, D.M.; Borok, E.; Friedman, J.M.; Tschöp, M.H.; Shanabrough, M.; Cline, G.; Shulman, G.I.; et al. UCP2 Mediates Ghrelin’s Action on NPY/AgRP Neurons by Lowering Free Radicals. Nature 2008, 454, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Khvorostov, I.; Hong, J.S.; Oktay, Y.; Vergnes, L.; Nuebel, E.; Wahjudi, P.N.; Setoguchi, K.; Wang, G.; Do, A.; et al. UCP2 Regulates Energy Metabolism and Differentiation Potential of Human Pluripotent Stem Cells. EMBO J. 2011, 30, 4860–4873. [Google Scholar] [CrossRef] [PubMed]
- Kukat, A.; Dogan, S.A.; Edgar, D.; Mourier, A.; Jacoby, C.; Maiti, P.; Mauer, J.; Becker, C.; Senft, K.; Wibom, R.; et al. Loss of UCP2 Attenuates Mitochondrial Dysfunction without Altering ROS Production and Uncoupling Activity. PLOS Genet. 2014, 10, e1004385. [Google Scholar] [CrossRef]
- Nübel, T.; Emre, Y.; Rabier, D.; Chadefaux, B.; Ricquier, D.; Bouillaud, F. Modified Glutamine Catabolism in Macrophages of Ucp2 Knock-out Mice. Biochim. Biophys. Acta (BBA) Bioenerg. 2008, 1777, 48–54. [Google Scholar] [CrossRef]
- Hurtaud, C.; Gelly, C.; Chen, Z.; Lévi-Meyrueis, C.; Bouillaud, F. Glutamine Stimulates Translation of Uncoupling Protein 2mRNA. Cell. Mol. Life Sci. 2007, 64, 1853–1860. [Google Scholar] [CrossRef]
- Millet, L.; Vidal, H.; Andreelli, F.; Larrouy, D.; Riou, J.P.; Ricquier, D.; Laville, M.; Langin, D. Increased Uncoupling Protein-2 and -3 MRNA Expression during Fasting in Obese and Lean Humans. J. Clin. Investig. 1997, 100, 2665–2670. [Google Scholar] [CrossRef]
- Schrauwen, P.; Hoppeler, H.; Billeter, R.; Bakker, A.H.; Pendergast, D.R. Fiber Type Dependent Upregulation of Human Skeletal Muscle UCP2 and UCP3 MRNA Expression by High-Fat Diet. Int. J. Obes. Relat. Metab. Disord. 2001, 25, 449–456. [Google Scholar] [CrossRef]
- Nègre-Salvayre, A.; Hirtz, C.; Carrera, G.; Cazenave, R.; Troly, M.; Salvayre, R.; Pénicaud, L.; Casteilla, L. A Role for Uncoupling Protein-2 as a Regulator of Mitochondrial Hydrogen Peroxide Generation. FASEB J. 1997, 11, 809–815. [Google Scholar] [CrossRef]
- Horimoto, M.; Fülöp, P.; Derdák, Z.; Wands, J.R.; Baffy, G. Uncoupling Protein-2 Deficiency Promotes Oxidant Stress and Delays Liver Regeneration in Mice. Hepatology 2004, 39, 386–392. [Google Scholar] [CrossRef]
- Teshima, Y.; Akao, M.; Jones, S.P.; Marbán, E. Uncoupling Protein-2 Overexpression Inhibits Mitochondrial Death Pathway in Cardiomyocytes. Circ. Res. 2003, 93, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-U.; Lee, I.K.; Han, J.; Song, D.-K.; Kim, Y.M.; Song, H.S.; Kim, H.S.; Lee, W.J.; Koh, E.H.; Song, K.-H.; et al. Effects of Recombinant Adenovirus-Mediated Uncoupling Protein 2 Overexpression on Endothelial Function and Apoptosis. Circ. Res. 2005, 96, 1200–1207. [Google Scholar] [CrossRef] [PubMed]
- Mitochondrial uncoupling, ROS generation and cardioprotection. Biochim. Biophys. Acta (BBA) Bioenerg. 2018, 1859, 940–950. [CrossRef]
- Monteiro, B.S.; Freire-Brito, L.; Carrageta, D.F.; Oliveira, P.F.; Alves, M.G. Mitochondrial Uncoupling Proteins (UCPs) as Key Modulators of ROS Homeostasis: A Crosstalk between Diabesity and Male Infertility? Antioxidants 2021, 10, 1746. [Google Scholar] [CrossRef]
- Hirschenson, J.; Melgar-Bermudez, E.; Mailloux, R.J. The Uncoupling Proteins: A Systematic Review on the Mechanism Used in the Prevention of Oxidative Stress. Antioxidants 2022, 11, 322. [Google Scholar] [CrossRef] [PubMed]
- Hass, D.T.; Barnstable, C.J. Uncoupling Proteins in the Mitochondrial Defense against Oxidative Stress. Prog. Retin. Eye Res. 2021, 83, 100941. [Google Scholar] [CrossRef]
- Broche, B.; Fradj, S.B.; Aguilar, E.; Sancerni, T.; Bénard, M.; Makaci, F.; Berthault, C.; Scharfmann, R.; Alves-Guerra, M.-C.; Duvillié, B. Mitochondrial Protein UCP2 Controls Pancreas Development. Diabetes 2018, 67, 78–84. [Google Scholar] [CrossRef]
- DeSouza, C.T.; Araújo, E.P.; Stoppiglia, L.F.; Pauli, J.R.; Ropelle, E.; Rocco, S.A.; Marin, R.M.; Franchini, K.G.; Carvalheira, J.B.; Saad, M.J.; et al. Inhibition of UCP2 Expression Reverses Diet-Induced Diabetes Mellitus by Effects on both Insulin Secretion and Action. FASEB J. 2007, 21, 1153–1163. [Google Scholar] [CrossRef]
- Saleh, M.C.; Wheeler, M.B.; Chan, C.B. Endogenous Islet Uncoupling Protein-2 Expression and Loss of Glucose Homeostasis in Ob/Ob Mice. J. Endocrinol. 2006, 190, 659–667. [Google Scholar] [CrossRef]
- Produit-Zengaffinen, N.; Davis-Lameloise, N.; Perreten, H.; Bécard, D.; Gjinovci, A.; Keller, P.A.; Wollheim, C.B.; Herrera, P.; Muzzin, P.; Assimacopoulos-Jeannet, F. Increasing Uncoupling Protein-2 in Pancreatic Beta Cells Does Not Alter Glucose-Induced Insulin Secretion but Decreases Production of Reactive Oxygen Species. Diabetologia 2007, 50, 84–93. [Google Scholar] [CrossRef]
- Pi, J.; Bai, Y.; Daniel, K.W.; Liu, D.; Lyght, O.; Edelstein, D.; Brownlee, M.; Corkey, B.E.; Collins, S. Persistent Oxidative Stress Due to Absence of Uncoupling Protein 2 Associated with Impaired Pancreatic β-Cell Function. Endocrinology 2009, 150, 3040–3048. [Google Scholar] [CrossRef] [PubMed]
- Pi, J.; Collins, S. Reactive Oxygen Species and Uncoupling Protein 2 in Pancreatic β-Cell Function. Diabetes Obes. Metab. 2010, 12, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Robson-Doucette, C.A.; Sultan, S.; Allister, E.M.; Wikstrom, J.D.; Koshkin, V.; Bhattacharjee, A.; Prentice, K.J.; Sereda, S.B.; Shirihai, O.S.; Wheeler, M.B. β-Cell Uncoupling Protein 2 Regulates Reactive Oxygen Species Production, Which Influences Both Insulin and Glucagon Secretion. Diabetes 2011, 60, 2710–2719. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Karaca, M.; Maechler, P. Upregulation of UCP2 in Beta-Cells Confers Partial Protection against Both Oxidative Stress and Glucotoxicity. Redox Biol. 2017, 13, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Martín-Timón, I.; Sevillano-Collantes, C.; Segura-Galindo, A.; del Cañizo-Gómez, F.J. Type 2 Diabetes and Cardiovascular Disease: Have all Risk Factors the Same Strength? World J. Diabetes 2014, 5, 444–470. [Google Scholar] [CrossRef]
- Moukdar, F.; Robidoux, J.; Lyght, O.; Pi, J.; Daniel, K.W.; Collins, S. Reduced Antioxidant Capacity and Diet-Induced Atherosclerosis in Uncoupling Protein-2-Deficient Mice S⃞. J. Lipid Res. 2009, 50, 59–70. [Google Scholar] [CrossRef]
- Luo, J.-Y.; Cheng, C.K.; He, L.; Pu, Y.; Zhang, Y.; Lin, X.; Xu, A.; Lau, C.W.; Tian, X.Y.; Ma, R.C.W.; et al. Endothelial UCP2 Is a Mechanosensitive Suppressor of Atherosclerosis. Circ. Res. 2022, 131, 424–441. [Google Scholar] [CrossRef]
- Emre, Y.; Hurtaud, C.; Nübel, T.; Criscuolo, F.; Ricquier, D.; Cassard-Doulcier, A.-M. Mitochondria Contribute to LPS-Induced MAPK Activation via Uncoupling Protein UCP2 in Macrophages. Biochem. J. 2007, 402, 271–278. [Google Scholar] [CrossRef]
- Ball, W.B.; Kar, S.; Mukherjee, M.; Chande, A.G.; Mukhopadhyaya, R.; Das, P.K. Uncoupling Protein 2 Negatively Regulates Mitochondrial Reactive Oxygen Species Generation and Induces Phosphatase-Mediated Anti-Inflammatory Response in Experimental Visceral Leishmaniasis. J. Immunol. 2011, 187, 1322–1332. [Google Scholar] [CrossRef]
- Carrión, J.; Abengozar, M.A.; Fernández-Reyes, M.; Sánchez-Martín, C.; Rial, E.; Domínguez-Bernal, G.; González-Barroso, M.M. UCP2 Deficiency Helps to Restrict the Pathogenesis of Experimental Cutaneous and Visceral Leishmaniosis in Mice. PLOS Negl. Trop. Dis. 2013, 7, e2077. [Google Scholar] [CrossRef]
- Gupta, A.K.; Ghosh, K.; Palit, S.; Barua, J.; Das, P.K.; Ukil, A. Leishmania Donovani Inhibits Inflammasome-Dependent Macrophage Activation by Exploiting the Negative Regulatory Proteins A20 and UCP2. FASEB J. 2017, 31, 5087–5101. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-S.; Lee, S.; Park, M.-A.; Siempos, I.I.; Haslip, M.; Lee, P.J.; Yun, M.; Kim, C.K.; Howrylak, J.; Ryter, S.W.; et al. UCP2-Induced Fatty Acid Synthase Promotes NLRP3 Inflammasome Activation during Sepsis. J. Clin. Investig. 2015, 125, 665–680. [Google Scholar] [CrossRef]
- Jiang, Z.-M.; Yang, Q.-H.; Zhu, C.-Q. UCP2 in Early Diagnosis and Prognosis of Sepsis. Eur. Rev. Med. Pharm. Sci. 2017, 21, 549–553. [Google Scholar]
- Lee, S.C.; Robson-Doucette, C.A.; Wheeler, M.B. Uncoupling Protein 2 Regulates Reactive Oxygen Species Formation in Islets and Influences Susceptibility to Diabetogenic Action of Streptozotocin. J. Endocrinol. 2009, 203, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Aheng, C.; Ly, N.; Kelly, M.; Ibrahim, S.; Ricquier, D.; Alves-Guerra, M.-C.; Miroux, B. Deletion of UCP2 in iNOS Deficient Mice Reduces the Severity of the Disease during Experimental Autoimmune Encephalomyelitis. PLoS ONE 2011, 6, e22841. [Google Scholar] [CrossRef]
- Smorodchenko, A.; Schneider, S.; Rupprecht, A.; Hilse, K.; Sasgary, S.; Zeitz, U.; Erben, R.G.; Pohl, E.E. UCP2 Up-Regulation within the Course of Autoimmune Encephalomyelitis Correlates with T-Lymphocyte Activation. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 1002–1012. [Google Scholar] [CrossRef]
- Derdák, Z.; Fülöp, P.; Sabo, E.; Tavares, R.; Berthiaume, E.P.; Resnick, M.B.; Paragh, G.; Wands, J.R.; Baffy, G. Enhanced Colon Tumor Induction in Uncoupling Protein-2 Deficient Mice Is Associated with NF-KappaB Activation and Oxidative Stress. Carcinogenesis 2006, 27, 956–961. [Google Scholar] [CrossRef]
- Deng, S.; Yang, Y.; Han, Y.; Li, X.; Wang, X.; Li, X.; Zhang, Z.; Wang, Y. UCP2 Inhibits ROS-Mediated Apoptosis in A549 under Hypoxic Conditions. PLoS ONE 2012, 7, e30714. [Google Scholar] [CrossRef]
- Dando, I.; Fiorini, C.; Pozza, E.D.; Padroni, C.; Costanzo, C.; Palmieri, M.; Donadelli, M. UCP2 Inhibition Triggers ROS-Dependent Nuclear Translocation of GAPDH and Autophagic Cell Death in Pancreatic Adenocarcinoma Cells. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2013, 1833, 672–679. [Google Scholar] [CrossRef]
- Esteves, P.; Pecqueur, C.; Ransy, C.; Esnous, C.; Lenoir, V.; Bouillaud, F.; Bulteau, A.-L.; Lombès, A.; Prip-Buus, C.; Ricquier, D.; et al. Mitochondrial Retrograde Signaling Mediated by UCP2 Inhibits Cancer Cell Proliferation and Tumorigenesis. Cancer Res. 2014, 74, 3971–3982. [Google Scholar] [CrossRef]
- Yu, J.; Shi, L.; Shen, X.; Zhao, Y. UCP2 Regulates Cholangiocarcinoma Cell Plasticity via Mitochondria-to-AMPK Signals. Biochem. Pharmacol. 2019, 166, 174–184. [Google Scholar] [CrossRef]
- Brandi, J.; Cecconi, D.; Cordani, M.; Torrens-Mas, M.; Pacchiana, R.; Dalla Pozza, E.; Butera, G.; Manfredi, M.; Marengo, E.; Oliver, J.; et al. The Antioxidant Uncoupling Protein 2 Stimulates HnRNPA2/B1, GLUT1 and PKM2 Expression and Sensitizes Pancreas Cancer Cells to Glycolysis Inhibition. Free Radic. Biol. Med. 2016, 101, 305–316. [Google Scholar] [CrossRef]
- Sancerni, T. UCP2 Silencing Restrains Leukemia Cell Proliferation through Glutamine Metabolic Remodeling. Front. Immunol. 2022, 13, 960226. [Google Scholar] [CrossRef]
- Sreedhar, A.; Lefort, J.; Petruska, P.; Gu, X.; Shi, R.; Miriyala, S.; Panchatcharam, M.; Zhao, Y. UCP2 Upregulation Promotes PLCγ-1 Signaling during Skin Cell Transformation. Mol. Carcinog. 2017, 56, 2290–2300. [Google Scholar] [CrossRef]
- Madreiter-Sokolowski, C.T.; Gottschalk, B.; Sokolowski, A.A.; Malli, R.; Graier, W.F. Dynamic Control of Mitochondrial Ca2+ Levels as a Survival Strategy of Cancer Cells. Front. Cell Dev. Biol. 2021, 9, 614668. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.-C.; Tsui, Y.-C.; Ragusa, S.; Koelzer, V.H.; Mina, M.; Franco, F.; Läubli, H.; Tschumi, B.; Speiser, D.; Romero, P.; et al. Uncoupling Protein 2 Reprograms the Tumor Microenvironment to Support the Anti-Tumor Immune Cycle. Nat. Immunol. 2019, 20, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Horimoto, M.; Resnick, M.B.; Konkin, T.A.; Routhier, J.; Wands, J.R.; Baffy, G. Expression of Uncoupling Protein-2 in Human Colon Cancer. Clin. Cancer Res. 2004, 10, 6203–6207. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Nichols, K.; Nathan, C.-A.; Zhao, Y. Mitochondrial uncoupling protein 2 is up-regulated in human head and neck, skin, pancreatic, and prostate tumors. Cancer Biomark. 2013, 13, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Sayeed, A.; Meng, Z.; Luciani, G.; Chen, L.-C.; Bennington, J.L.; Dairkee, S.H. Negative Regulation of UCP2 by TGFβ Signaling Characterizes Low and Intermediate-Grade Primary Breast Cancer. Cell Death Dis. 2010, 1, e53. [Google Scholar] [CrossRef]
- Yu, J.; Shi, L.; Lin, W.; Lu, B.; Zhao, Y. UCP2 Promotes Proliferation and Chemoresistance through Regulating the NF-ΚB/β-Catenin Axis and Mitochondrial ROS in Gallbladder Cancer. Biochem. Pharmacol. 2020, 172, 113745. [Google Scholar] [CrossRef] [PubMed]
- Cordani, M.; Butera, G.; Pacchiana, R.; Masetto, F.; Mullappilly, N.; Riganti, C.; Donadelli, M. Mutant P53-Associated Molecular Mechanisms of ROS Regulation in Cancer Cells. Biomolecules 2020, 10, 361. [Google Scholar] [CrossRef] [PubMed]
- Cordani, M.; Butera, G.; Dando, I.; Torrens-Mas, M.; Butturini, E.; Pacchiana, R.; Oppici, E.; Cavallini, C.; Gasperini, S.; Tamassia, N.; et al. Mutant P53 Blocks SESN1/AMPK/PGC-1α/UCP2 Axis Increasing Mitochondrial O2−· Production in Cancer Cells. Br. J. Cancer 2018, 119, 994–1008. [Google Scholar] [CrossRef] [PubMed]
- Donadelli, M.; Dando, I.; Pozza, E.D.; Palmieri, M. Mitochondrial Uncoupling Protein 2 and Pancreatic Cancer: A New Potential Target Therapy. World J. Gastroenterol. 2015, 21, 3232–3238. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in Cancer Therapy: The Bright Side of the Moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Cheung, E.C.; Vousden, K.H. The Role of ROS in Tumour Development and Progression. Nat. Rev. Cancer 2022, 22, 280–297. [Google Scholar] [CrossRef]
- Wu, S.; Luo, C.; Hameed, N.U.F.; Wang, Y.; Zhuang, D. UCP2 Silencing in Glioblastoma Reduces Cell Proliferation and Invasiveness by Inhibiting P38 MAPK Pathway. Exp. Cell Res. 2020, 394, 112110. [Google Scholar] [CrossRef]
- Martínez-Limón, A.; Joaquin, M.; Caballero, M.; Posas, F.; de Nadal, E. The P38 Pathway: From Biology to Cancer Therapy. Int. J. Mol. Sci. 2020, 21, 1913. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Chandel, N.S. We Need to Talk about the Warburg Effect. Nat. Metab. 2020, 2, 127–129. [Google Scholar] [CrossRef]
- Esteves, P.; Pecqueur, C.; Alves-Guerra, M.-C. UCP2 Induces Metabolic Reprogramming to Inhibit Proliferation of Cancer Cells. Mol. Cell. Oncol. 2015, 2, e975024. [Google Scholar] [CrossRef]
- Klaus, A.; Polge, C.; Zorman, S.; Auchli, Y.; Brunisholz, R.; Schlattner, U. A Two-Dimensional Screen for AMPK Substrates Identifies Tumor Suppressor Fumarate Hydratase as a Preferential AMPKα2 Substrate. J. Proteom. 2012, 75, 3304–3313. [Google Scholar] [CrossRef]
- Tong, W.-H.; Sourbier, C.; Kovtunovych, G.; Jeong, S.Y.; Vira, M.; Ghosh, M.; Romero, V.V.; Sougrat, R.; Vaulont, S.; Viollet, B.; et al. The Glycolytic Shift in Fumarate-Hydratase-Deficient Kidney Cancer Lowers AMPK Levels, Increases Anabolic Propensities and Lowers Cellular Iron Levels. Cancer Cell 2011, 20, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Stancliffe, E.; Fowle-Grider, R.; Wang, R.; Wang, C.; Schwaiger-Haber, M.; Shriver, L.P.; Patti, G.J. Saturation of the Mitochondrial NADH Shuttles Drives Aerobic Glycolysis in Proliferating Cells. Mol. Cell 2022, 82, 3270–3283. [Google Scholar] [CrossRef] [PubMed]
- Sreedhar, A.; Cassell, T.; Smith, P.; Lu, D.; Nam, H.W.; Lane, A.N.; Zhao, Y. UCP2 Overexpression Redirects Glucose into Anabolic Metabolic Pathways. Proteomics 2019, 19, 1800353. [Google Scholar] [CrossRef] [PubMed]
- Chuang, H.-C.; Chou, C.-C.; Kulp, S.K.; Chen, C.-S. AMPK as a Potential Anticancer Target—Friend or Foe? Curr. Pharm. Des. 2014, 20, 2607–2618. [Google Scholar] [CrossRef]
- Sreedhar, A.; Petruska, P.; Miriyala, S.; Panchatcharam, M.; Zhao, Y. UCP2 Overexpression Enhanced Glycolysis via Activation of PFKFB2 during Skin Cell Transformation. Oncotarget 2017, 8, 95504–95515. [Google Scholar] [CrossRef]
- Wise, D.R.; DeBerardinis, R.J.; Mancuso, A.; Sayed, N.; Zhang, X.-Y.; Pfeiffer, H.K.; Nissim, I.; Daikhin, E.; Yudkoff, M.; McMahon, S.B.; et al. Myc Regulates a Transcriptional Program That Stimulates Mitochondrial Glutaminolysis and Leads to Glutamine Addiction. Proc. Natl. Acad. Sci. USA 2008, 105, 18782–18787. [Google Scholar] [CrossRef]
- Rupprecht, A. Glutamine regulates mitochondrial uncoupling protein 2 to promote glutaminolysis in neuroblastoma cells. Biochim. Biophys. Acta (BBA) Bioenerg. 2019, 1860, 391–401. [Google Scholar] [CrossRef]
- Gorgoglione, R.; Impedovo, V.; Riley, C.L.; Fratantonio, D.; Tiziani, S.; Palmieri, L.; Dolce, V.; Fiermonte, G. Glutamine-Derived Aspartate Biosynthesis in Cancer Cells: Role of Mitochondrial Transporters and New Therapeutic Perspectives. Cancers 2022, 14, 245. [Google Scholar] [CrossRef]
- Roderick, H.L.; Cook, S.J. Ca2+ Signalling Checkpoints in Cancer: Remodelling Ca2+ for Cancer Cell Proliferation and Survival. Nat. Rev. Cancer 2008, 8, 361–375. [Google Scholar] [CrossRef]
- Patterson, R.L.; van Rossum, D.B.; Nikolaidis, N.; Gill, D.L.; Snyder, S.H. Phospholipase C-γ: Diverse Roles in Receptor-Mediated Calcium Signaling. Trends Biochem. Sci. 2005, 30, 688–697. [Google Scholar] [CrossRef]
- Madreiter-Sokolowski, C.T.; Győrffy, B.; Klec, C.; Sokolowski, A.A.; Rost, R.; Waldeck-Weiermair, M.; Malli, R.; Graier, W.F. UCP2 and PRMT1 Are Key Prognostic Markers for Lung Carcinoma Patients. Oncotarget 2017, 8, 80278–80285. [Google Scholar] [CrossRef] [PubMed]
- Luby, A.; Alves-Guerra, M.-C. Targeting Metabolism to Control Immune Responses in Cancer and Improve Checkpoint Blockade Immunotherapy. Cancers 2021, 13, 5912. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Shi, M.; Wu, Q.; Wei, W.; Sun, S.; Zhu, S. Identification of UCP1 and UCP2 as Potential Prognostic Markers in Breast Cancer: A Study Based on Immunohistochemical Analysis and Bioinformatics. Front. Cell Dev. Biol. 2022, 10, 891731. [Google Scholar] [CrossRef]
- Rupprecht, A.; Bräuer, A.U.; Smorodchenko, A.; Goyn, J.; Hilse, K.E.; Shabalina, I.G.; Infante-Duarte, C.; Pohl, E.E. Quantification of Uncoupling Protein 2 Reveals Its Main Expression in Immune Cells and Selective Up-Regulation during T-Cell Proliferation. PLoS ONE 2012, 7, e41406. [Google Scholar] [CrossRef]
- Chaudhuri, L.; Srivastava, R.K.; Kos, F.; Shrikant, P.A. Uncoupling Protein 2 Regulates Metabolic Reprogramming and Fate of Antigen-Stimulated CD8+ T Cells. Cancer Immunol. Immunother. 2016, 65, 869–874. [Google Scholar] [CrossRef]
- Dando, I.; Pacchiana, R.; Pozza, E.D.; Cataldo, I.; Bruno, S.; Conti, P.; Cordani, M.; Grimaldi, A.; Butera, G.; Caraglia, M.; et al. UCP2 Inhibition Induces ROS/Akt/MTOR Axis: Role of GAPDH Nuclear Translocation in Genipin/Everolimus Anticancer Synergism. Free Radic. Biol. Med. 2017, 113, 176–189. [Google Scholar] [CrossRef]
- Katzung, B.G. (Ed.) Basic & Clinical Pharmacology, 14th ed.; A Lange Medical Book; McGraw-Hill Education: New York, NY, USA, 2018; ISBN 978-1-259-64115-2. [Google Scholar]
- Kachalaki, S.; Ebrahimi, M.; Mohamed Khosroshahi, L.; Mohammadinejad, S.; Baradaran, B. Cancer Chemoresistance; Biochemical and Molecular Aspects: A Brief Overview. Eur. J. Pharm. Sci. 2016, 89, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, M.; Fukuda, T.; Shimomura, M.; Inoue, Y.; Wada, T.; Tasaka, R.; Yasui, T.; Sumi, T. Expression of UCP2 Is Associated with Sensitivity to Platinum-Based Chemotherapy for Ovarian Serous Carcinoma. Oncol. Lett. 2018, 15, 9923–9928. [Google Scholar] [CrossRef]
- Derdak, Z.; Mark, N.M.; Beldi, G.; Robson, S.C.; Wands, J.R.; Baffy, G. The Mitochondrial Uncoupling Protein-2 Promotes Chemoresistance in Cancer Cells. Cancer Res. 2008, 68, 2813–2819. [Google Scholar] [CrossRef] [PubMed]
- Dalla Pozza, E.; Fiorini, C.; Dando, I.; Menegazzi, M.; Sgarbossa, A.; Costanzo, C.; Palmieri, M.; Donadelli, M. Role of Mitochondrial Uncoupling Protein 2 in Cancer Cell Resistance to Gemcitabine. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2012, 1823, 1856–1863. [Google Scholar] [CrossRef]
- Wang, M.; Li, G.; Yang, Z.; Wang, L.; Zhang, L.; Wang, T.; Zhang, Y.; Zhang, S.; Han, Y.; Jia, L. Uncoupling Protein 2 Downregulation by Hypoxia through Repression of Peroxisome Proliferator-Activated Receptor γ Promotes Chemoresistance of Non-Small Cell Lung Cancer. Oncotarget 2016, 8, 8083–8094. [Google Scholar] [CrossRef] [PubMed]
- Su, W.-P.; Lo, Y.-C.; Yan, J.-J.; Liao, I.-C.; Tsai, P.-J.; Wang, H.-C.; Yeh, H.-H.; Lin, C.-C.; Chen, H.H.W.; Lai, W.-W.; et al. Mitochondrial uncoupling protein 2 regulates the effects of paclitaxel on Stat3 activation and cellular survival in lung cancer cells. Carcinogenesis 2012, 33, 2065–2075. [Google Scholar] [CrossRef] [PubMed]
- Kreiter, J.; Rupprecht, A.; Zimmermann, L.; Moschinger, M.; Rokitskaya, T.I.; Antonenko, Y.N.; Gille, L.; Fedorova, M.; Pohl, E.E. Molecular Mechanisms Responsible for Pharmacological Effects of Genipin on Mitochondrial Proteins. Biophys. J. 2019, 117, 1845–1857. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, M.K.; Shen, H.; Tang, F.R.; Arfuso, F.; Rajesh, M.; Wang, L.; Kumar, A.P.; Bian, J.; Goh, B.C.; Bishayee, A.; et al. Potential Role of Genipin in Cancer Therapy. Pharmacol. Res. 2018, 133, 195–200. [Google Scholar] [CrossRef]
- Cho, Y.S. Genipin, an Inhibitor of UCP2 as a Promising New Anticancer Agent: A Review of the Literature. Int. J. Mol. Sci. 2022, 23, 5637. [Google Scholar] [CrossRef]
- Ayyasamy, V.; Owens, K.M.; Desouki, M.M.; Liang, P.; Bakin, A.; Thangaraj, K.; Buchsbaum, D.J.; LoBuglio, A.F.; Singh, K.K. Cellular Model of Warburg Effect Identifies Tumor Promoting Function of UCP2 in Breast Cancer and Its Suppression by Genipin. PLoS ONE 2011, 6, e24792. [Google Scholar] [CrossRef]
- Ko, H.; Kim, J.M.; Kim, S.-J.; Shim, S.H.; Ha, C.H.; Chang, H.I. Induction of Apoptosis by Genipin Inhibits Cell Proliferation in AGS Human Gastric Cancer Cells via Egr1/P21 Signaling Pathway. Bioorg. Med. Chem. Lett. 2015, 25, 4191–4196. [Google Scholar] [CrossRef]
- Feng, Q.; Cao, H.; Xu, W.; Li, X.; Ren, Y.; Du, L. Apoptosis Induced by Genipin in Human Leukemia K562 Cells: Involvement of c-Jun N-Terminal Kinase in G2/M Arrest. Acta Pharm. Sin. 2011, 32, 519–527. [Google Scholar] [CrossRef]
- Wang, N.; Zhu, M.; Tsao, S.-W.; Man, K.; Zhang, Z.; Feng, Y. Up-Regulation of TIMP-1 by Genipin Inhibits MMP-2 Activities and Suppresses the Metastatic Potential of Human Hepatocellular Carcinoma. PLoS ONE 2012, 7, e46318. [Google Scholar] [CrossRef]
- Cho, Y.S.; Lee, J.H.; Jung, K.-H.; Park, J.-W.; Moon, S.H.; Choe, Y.S.; Lee, K.-H. Molecular Mechanism of 18F-FDG Uptake Reduction Induced by Genipin in T47D Cancer Cell and Role of Uncoupling Protein-2 in Cancer Cell Glucose Metabolism. Nucl. Med. Biol. 2016, 43, 587–592. [Google Scholar] [CrossRef]
- Tan, H.-Y.; Wang, N.; Tsao, S.-W.; Che, C.-M.; Yuen, M.-F.; Feng, Y. Inhibition by Natural Compound Genipin on Tumour Associated Macrophages Reduces Growth of Hepatocellular Carcinoma. Oncotarget 2016, 7, 43792–43804. [Google Scholar] [CrossRef]
- Hua, J.; Zhang, Z.; Zhang, L.; Sun, Y.; Yuan, Y. UCP-2 Inhibitor Enhanced the Efficacy of Trastuzumab against HER2 Positive Breast Cancer Cells. Cancer Chemother. Pharm. 2021, 88, 633–642. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, Y.S.; Jung, K.-H.; Park, J.W.; Lee, K.-H. Genipin Enhances the Antitumor Effect of Elesclomol in A549 Lung Cancer Cells by Blocking Uncoupling Protein-2 and Stimulating Reactive Oxygen Species Production. Oncol. Lett. 2020, 20, 374. [Google Scholar] [CrossRef] [PubMed]
- Mailloux, R.J.; Adjeitey, C.N.-K.; Harper, M.-E. Genipin-Induced Inhibition of Uncoupling Protein-2 Sensitizes Drug-Resistant Cancer Cells to Cytotoxic Agents. PLoS ONE 2010, 5, e13289. [Google Scholar] [CrossRef] [PubMed]
- Bugge, A.; Siersbæk, M.; Madsen, M.S.; Göndör, A.; Rougier, C.; Mandrup, S. A Novel Intronic Peroxisome Proliferator-Activated Receptor γ Enhancer in the Uncoupling Protein (UCP) 3 Gene as a Regulator of Both UCP2 and -3 Expression in Adipocytes. J. Biol. Chem. 2010, 285, 17310–17317. [Google Scholar] [CrossRef]
- Hernandez-Quiles, M.; Broekema, M.F.; Kalkhoven, E. PPARgamma in Metabolism, Immunity, and Cancer: Unified and Diverse Mechanisms of Action. Front. Endocrinol. 2021, 12, 624112. [Google Scholar] [CrossRef] [PubMed]
- Laganà, A.S.; Vitale, S.G.; Nigro, A.; Sofo, V.; Salmeri, F.M.; Rossetti, P.; Rapisarda, A.M.C.; La Vignera, S.; Condorelli, R.A.; Rizzo, G.; et al. Pleiotropic Actions of Peroxisome Proliferator-Activated Receptors (PPARs) in Dysregulated Metabolic Homeostasis, Inflammation and Cancer: Current Evidence and Future Perspectives. Int. J. Mol. Sci. 2016, 17, 999. [Google Scholar] [CrossRef] [PubMed]
- Sarraf, P.; Mueller, E.; Jones, D.; King, F.J.; DeAngelo, D.J.; Partridge, J.B.; Holden, S.A.; Chen, L.B.; Singer, S.; Fletcher, C.; et al. Differentiation and Reversal of Malignant Changes in Colon Cancer through PPARγ. Nat. Med. 1998, 4, 1046–1052. [Google Scholar] [CrossRef] [PubMed]
- Bonofiglio, D.; Cione, E.; Qi, H.; Pingitore, A.; Perri, M.; Catalano, S.; Vizza, D.; Panno, M.L.; Genchi, G.; Fuqua, S.A.W.; et al. Combined Low Doses of PPARγ and RXR Ligands Trigger an Intrinsic Apoptotic Pathway in Human Breast Cancer Cells. Am. J. Pathol. 2009, 175, 1270–1280. [Google Scholar] [CrossRef]
- Motomura, W.; Okumura, T.; Takahashi, N.; Obara, T.; Kohgo, Y. Activation of Peroxisome Proliferator-Activated Receptor Gamma by Troglitazone Inhibits Cell Growth through the Increase of P27KiP1 in Human. Pancreatic Carcinoma Cells. Cancer Res. 2000, 60, 5558–5564. [Google Scholar]
- Bren-Mattison, Y.; Van Putten, V.; Chan, D.; Winn, R.; Geraci, M.W.; Nemenoff, R.A. Peroxisome Proliferator-Activated Receptor-γ (PPARγ) Inhibits Tumorigenesis by Reversing the Undifferentiated Phenotype of Metastatic Non-Small-Cell Lung Cancer Cells (NSCLC). Oncogene 2005, 24, 1412–1422. [Google Scholar] [CrossRef]
- Kubota, T.; Koshizuka, K.; Williamson, E.A.; Asou, H.; Said, J.W.; Holden, S.; Miyoshi, I.; Koeffler, H.P. Ligand for Peroxisome Proliferator-Activated Receptor Gamma (Troglitazone) Has Potent Antitumor Effect against Human Prostate Cancer Both in vitro and in vivo. Cancer Res. 1998, 58, 3344–3352. [Google Scholar] [PubMed]
- Kwon, K.A.; Yun, J.; Oh, S.Y.; Seo, B.-G.; Lee, S.; Lee, J.-H.; Kim, S.-H.; Choi, H.J.; Roh, M.S.; Kim, H.-J. Clinical Significance of Peroxisome Proliferator-Activated Receptor γ and TRAP220 in Patients with Operable Colorectal Cancer. Cancer Res. Treat. 2016, 48, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Villarroya, F.; Iglesias, R.; Giralt, M. PPARs in the Control of Uncoupling Proteins Gene Expression. PPAR Res. 2007, 2007, 74364. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shen, W.; Li, X.; Feng, Y.; Qian, K.; Wang, G.; Gao, Y.; Xu, X.; Zhang, S.; Yue, L.; et al. The PPARγ agonist rosiglitazone enhances the radiosensitivity of human pancreatic cancer cells. Drug Design, Development and Therapy. DDDT 2020, 14, 3099–3110. [Google Scholar] [CrossRef]
- Lau, M.-F.; Chua, K.-H.; Sabaratnam, V.; Kuppusamy, U.R. Rosiglitazone Enhances the Apoptotic Effect of 5-Fluorouracil in Colorectal Cancer Cells with High-Glucose-Induced Glutathione. Sci. Prog. 2020, 103, 0036850419886448. [Google Scholar] [CrossRef]
- Wang, S.; Dougherty, E.J.; Danner, R.L. PPARγ Signaling and Emerging Opportunities for Improved Therapeutics. Pharmacol. Res. 2016, 111, 76–85. [Google Scholar] [CrossRef]
- Center for Drug Evaluation and FDA Drug Safety Communication: Avandia (Rosiglitazone) Labels Now Contain Updated Information about Cardiovascular Risks and Use in Certain Patients. FDA. 2019. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-avandia-rosiglitazone-labels-now-contain-updated-information-about (accessed on 30 November 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).