The Complexity and Multiplicity of the Specific cAMP Phosphodiesterase Family: PDE4, Open New Adapted Therapeutic Approaches
Abstract
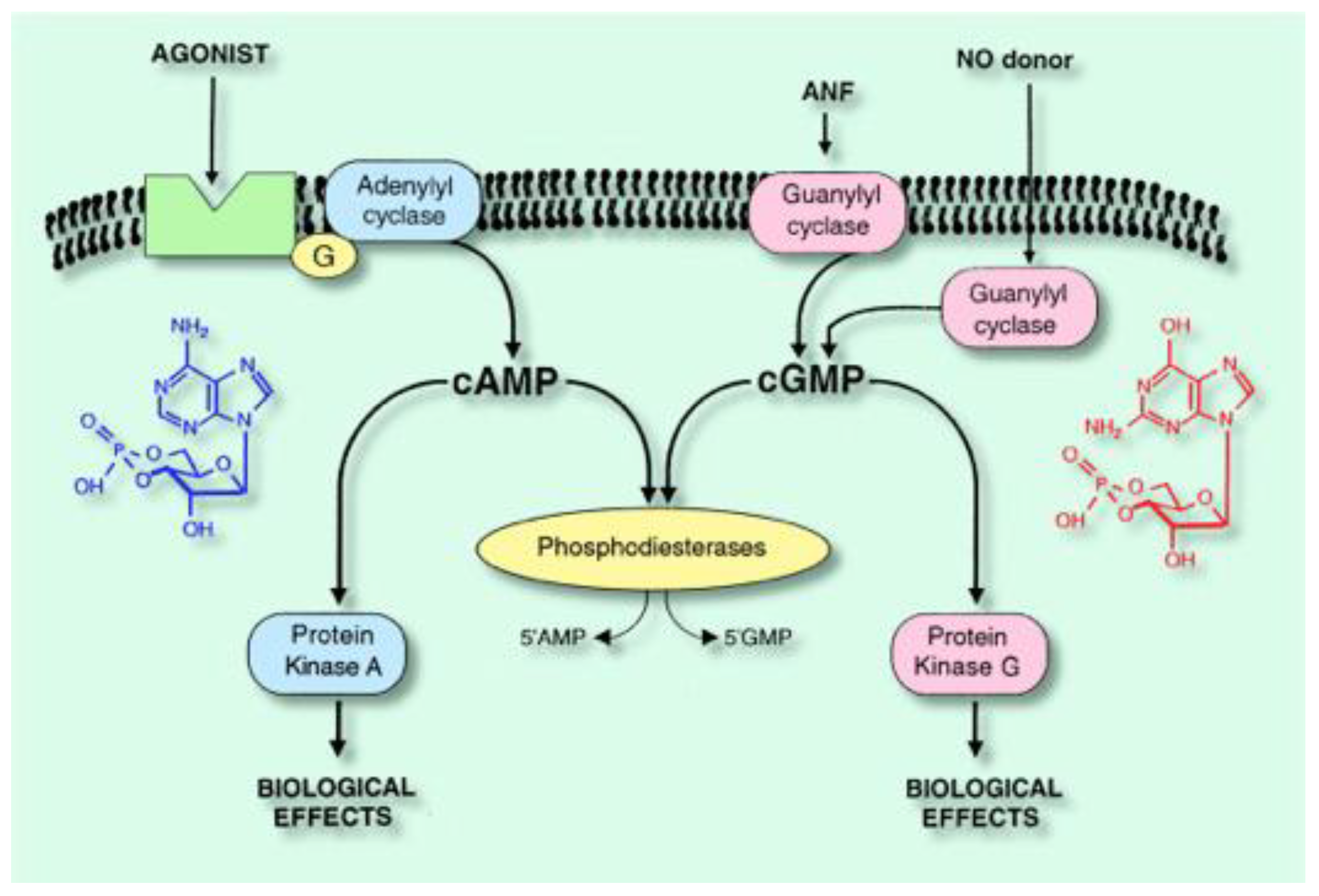
:1. Introduction
- -
- -
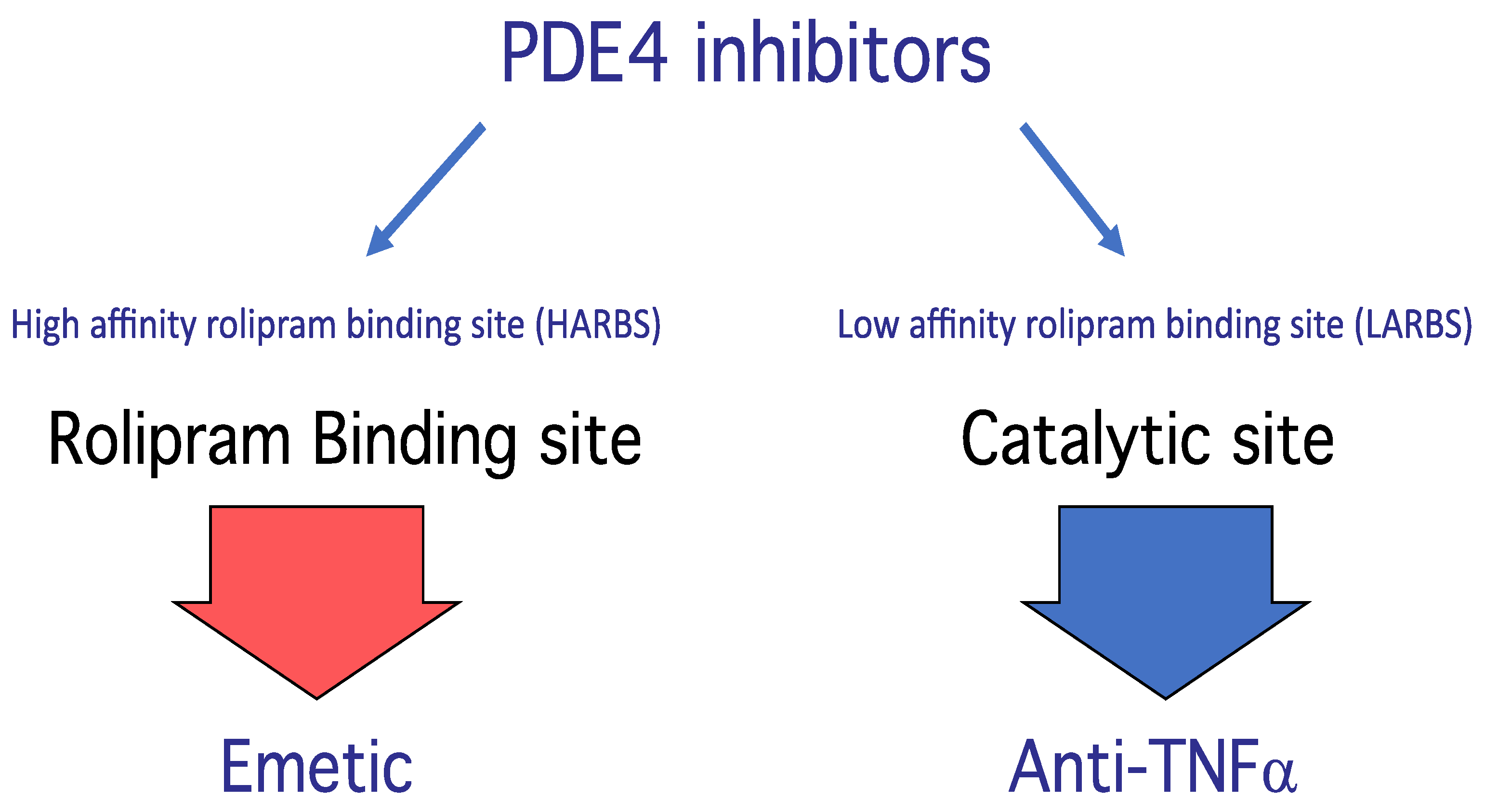
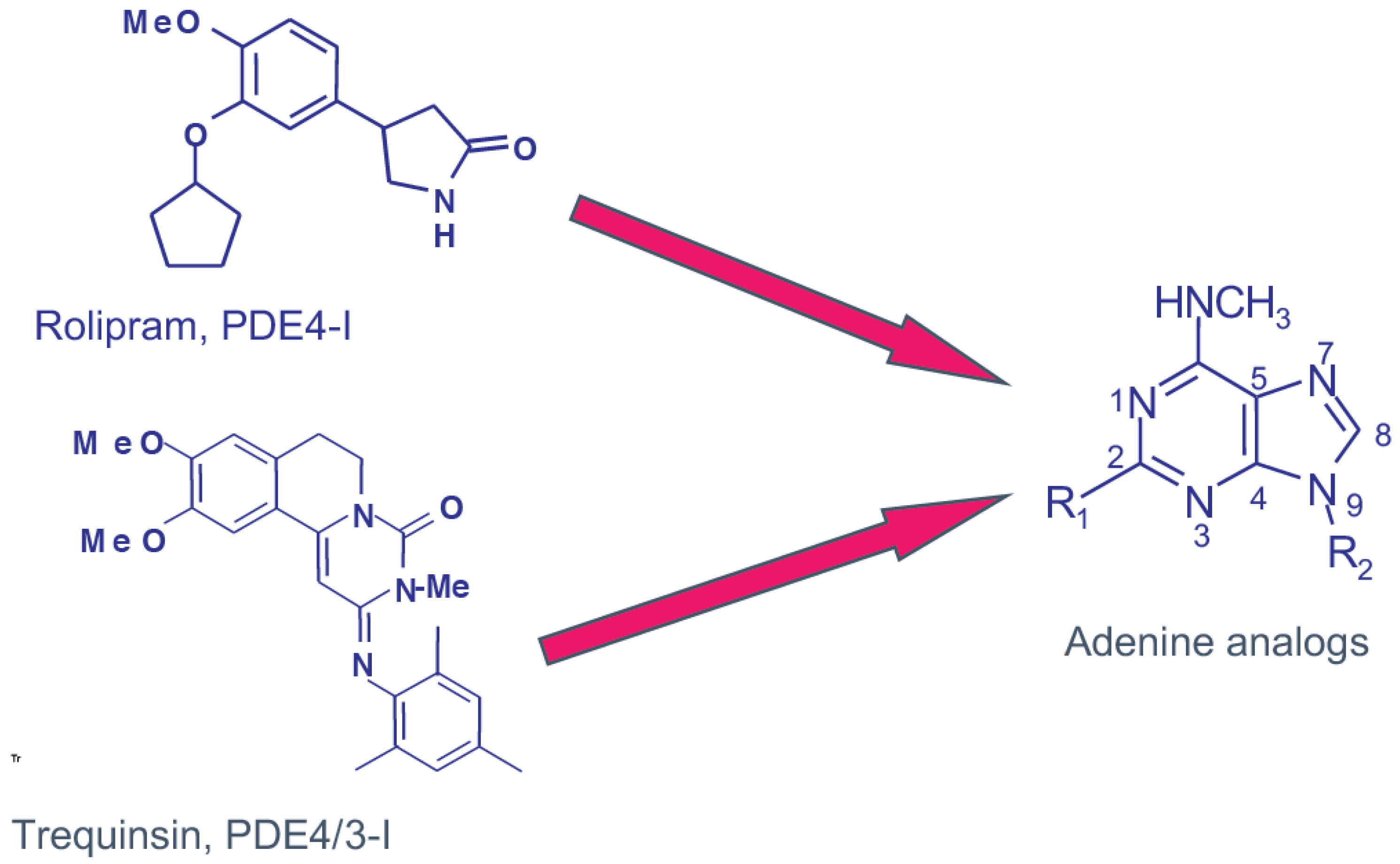
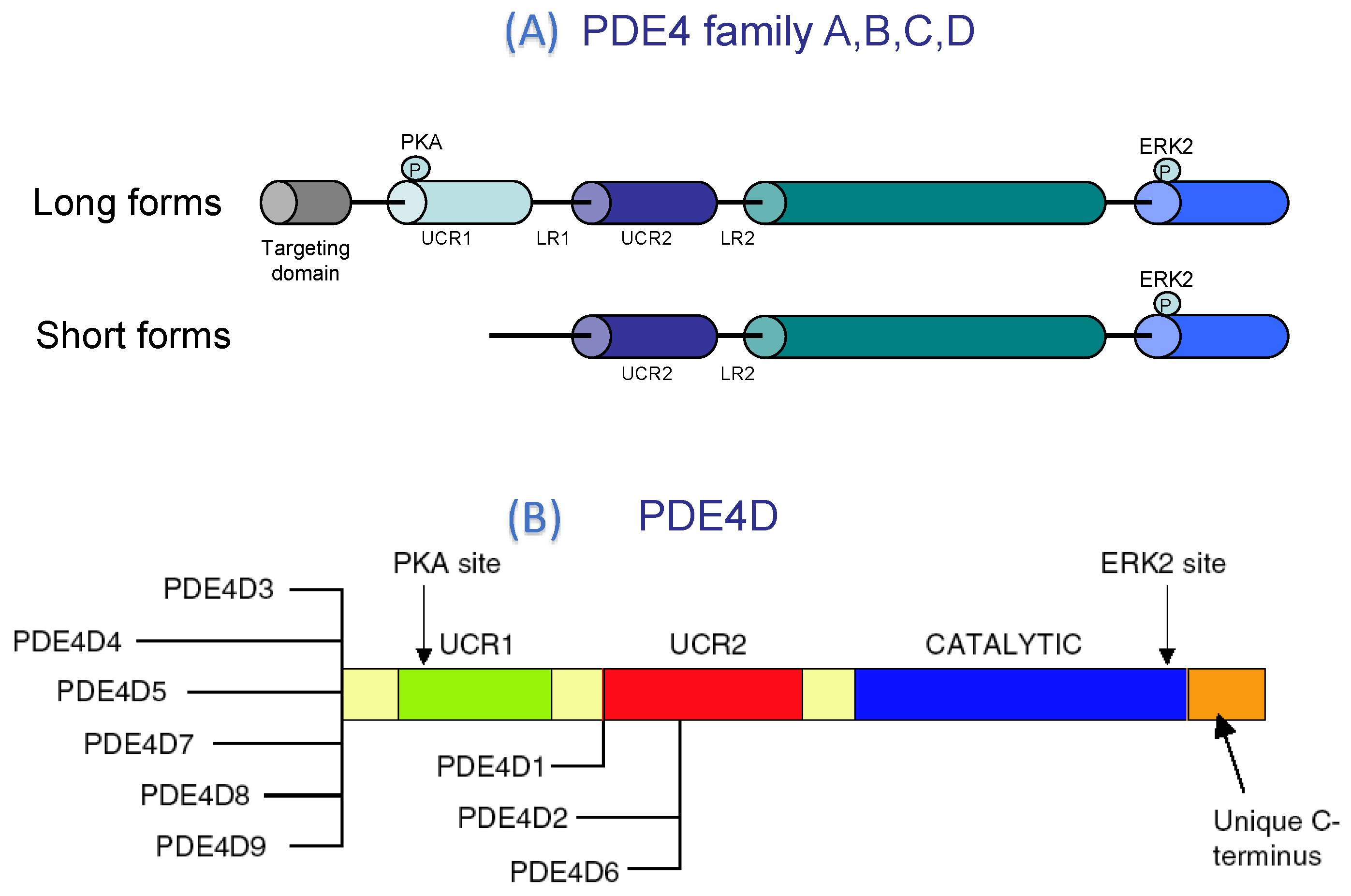
1.1. The PDE4 Family
PDE4 Structure
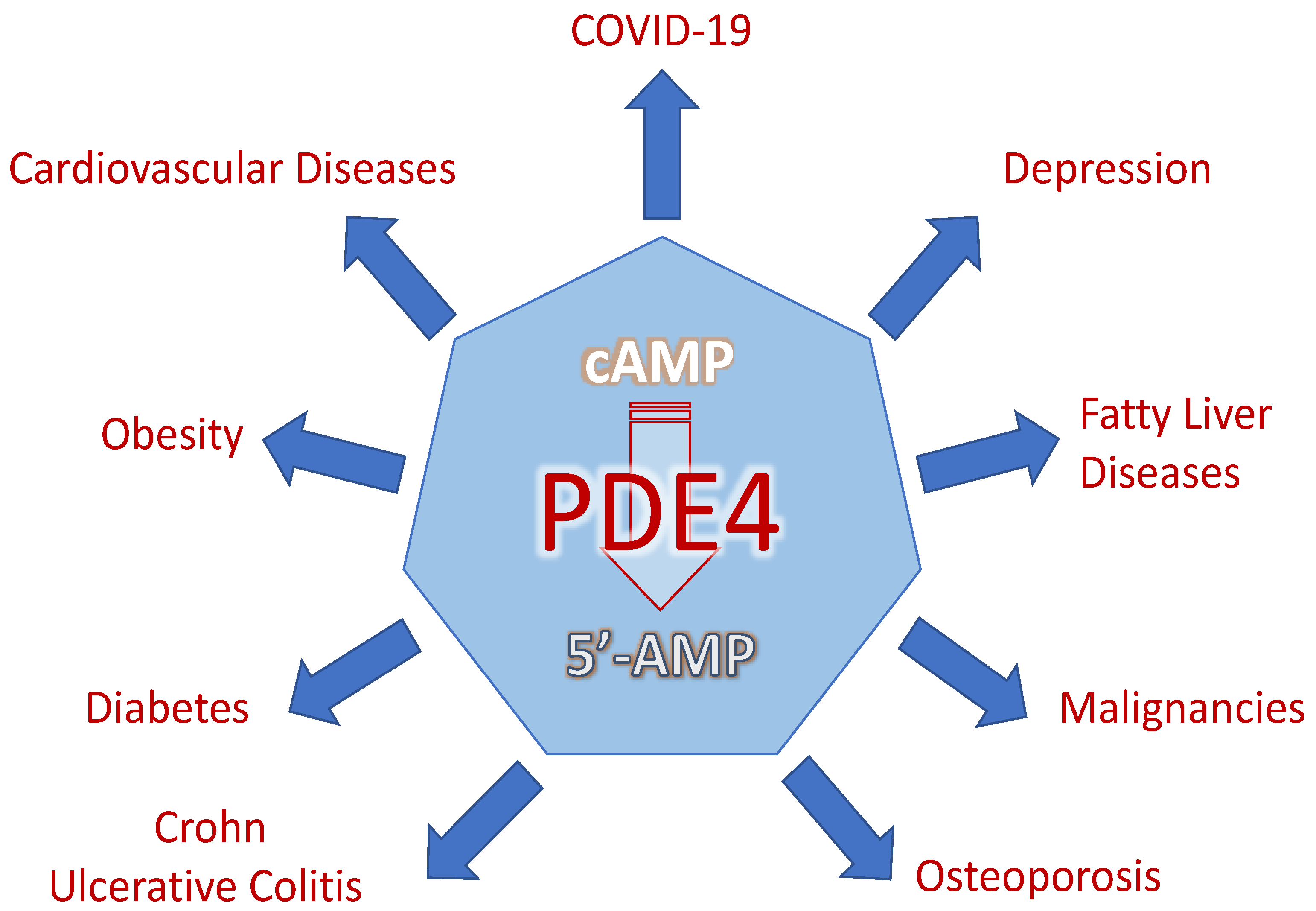
2. PDE4 as Therapeutic Targets
2.1. PDE4 in Cardiovascular Diseases
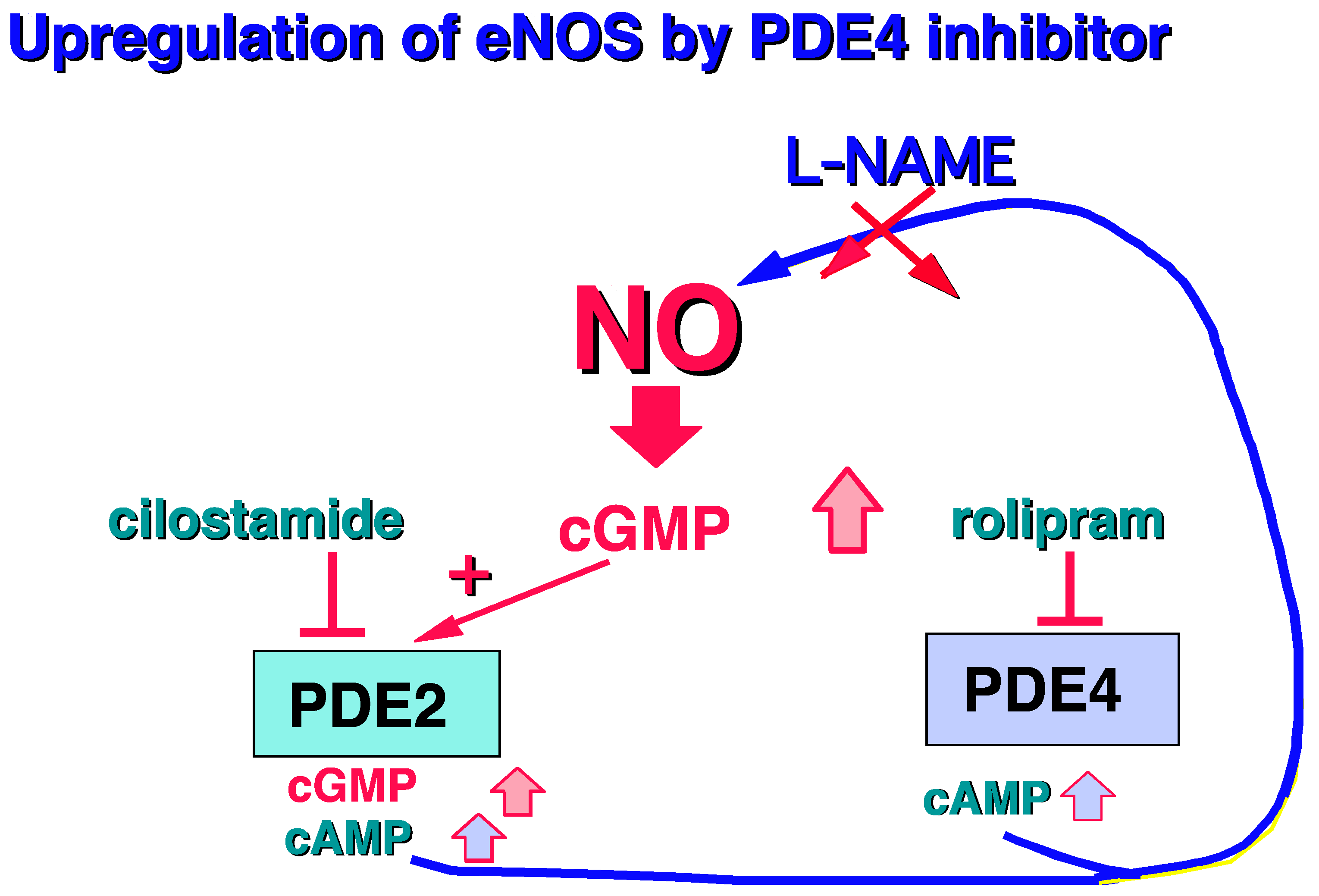
2.1.1. PDE4, and Vessel
2.1.2. PDE4, and Heart
2.2. PDE4 in Obesity
2.3. PDE4 in Diabetes
2.4. PDE4 and Ulcerative Colitis and Crohn’Disease
2.5. PDE4 and Osteoporosis
2.6. PDE4 and Malignancies
2.7. PDE4 and Fatty Liver Disease
2.8. PDE4 and Depression
2.9. PDE4, and COVID-19
3. Conclusions and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lugnier, C. Cyclic nucleotide phosphodiesterase (PDE) superfamily: A new target for the development of specific therapeutic agents. Pharmacol. Ther. 2006, 109, 366–398. [Google Scholar] [CrossRef] [PubMed]
- Mulller, B.; Komas, N.; Keravis, T.; Lugnier, C. Les phosphodiestérases des nucléotides cycliques. Med. Sci. 1993, 12, 1335–1341. [Google Scholar]
- Dessauer, C.W.; Watts, V.J.; Ostrom, R.S.; Conti, M.; Dove, S.; Seifert, R. International Union of Basic and Clinical Pharmacology. CI. Structures and small molecule modulators of mammalian adenylyl cyclases. Pharmacol. Rev. 2017, 69, 93–139. [Google Scholar] [CrossRef]
- Pozdniakova, S.; Ladilov, Y. Functional significance of the adcy10-Dependent intracellular cAMP Compartments. J. Cardiovasc. Dev. Dis. 2018, 5, E29. [Google Scholar] [CrossRef]
- Francis, S.H.; Houslay, M.D.; Conti, M. Phosphodiesterase inhibitors: Factors that influence potency, selectivity, and action. Handb. Exp. Pharmacol. 2011, 204, 47–84. [Google Scholar]
- Keravis, T.; Lugnier, C. Cyclic nucleotide phosphodiesterase (PDE) isozymes as targets of the intracellular signalling network: Benefits of PDE inhibitors in various diseases and perspectives for future therapeutic developments. Br. J. Pharmacol. 2012, 165, 1288–1305. [Google Scholar] [CrossRef]
- Maurice, D.H.; Ke, H.; Ahmad, F.; Wang, Y.; Chung, J.; Manganiello, V.C. Advances in targeting cyclic nucleotide phosphodiesterases. Nat. Rev. Drug. Discov. 2014, 13, 290–314. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Murata, T.; Shimizu, K.; Degerman, E.; Maurice, D.; Manganiello, V. Cyclic nucleotide phosphodiesterases: Important signaling modulators and therapeutic targets. Oral Dis. 2015, 21, e25–e50. [Google Scholar] [CrossRef]
- Lugnier, C.; Meyer, A.; Charloux, A.; Andrès, E.; Geny, B.; Talha, S. The Endocrine function of the heart: Physiology and involvements of natriuretic peptides and cyclic nucleotide phosphodiesterases in heart failure. J. Clin. Med. 2019, 8, 1746. [Google Scholar] [CrossRef]
- Lugnier, C.; Meyer, A.; Talha, S.; Geny, B. Cyclic nucleotide phosphodiesterases: New targets in the metabolic syndrome? Pharmacol. Ther. 2020, 208, 107475. [Google Scholar] [CrossRef]
- Fertig, B.A.; Baillie, G.S. PDE4-Mediated cAMP Signalling. J. Cardiovasc. Dev. Dis. 2018, 5, E8. [Google Scholar] [CrossRef] [PubMed]
- Lugnier, C.; Stierlé, A.; Beretz, A.; Schoeffter, P.; Lebec, A.; Wermuth, C.G.; Cazenave, J.P.; Stoclet, J.C. Tissue and substrate specificity of inhibition by alkoxy-aryl-lactams of platelet and arterial smooth muscle cyclic nucleotide phosphodiesterases relationship to pharmacological activity. Biochem. Biophys. Res. Commun. 1983, 113, 954–959. [Google Scholar] [CrossRef]
- Lugnier, C.; Schoeffter, P.; Le Bec, A.; Strouthou, E.; Stoclet, J.C. Selective inhibition of cyclic nucleotide phosphodiesterases of human, bovine and rat aorta. Biochem. Pharmacol. 1986, 35, 1743–1751. [Google Scholar] [CrossRef]
- Schneider, H.H.; Schmiechen, R.; Brezinski, M.; Seidler, J. Stereospecific binding of the antidepressant rolipram to brain protein structures. Eur. J. Pharmacol. 1986, 127, 105–115. [Google Scholar] [CrossRef]
- Marivet, M.C.; Bourguignon, J.J.; Lugnier, C.; Mann, A.; Stoclet, J.C.; Wermuth, C.G. Inhibition of cyclic adenosine-3′,5′-monophosphate phosphodiesterase from vascular smooth muscle by rolipram analogues. J. Med. Chem. 1989, 32, 1450–1457. [Google Scholar] [CrossRef]
- Dent, G.; Giembycz, M.A.; Rabe, K.F.; Barnes, P.J. Inhibition of eosinophil cyclic nucleotide PDE activity and opsonised zymosan-stimulated respiratory burst by ‘type IV’-selective PDE inhibitors. Br. J. Pharmacol. 1991, 103, 1339–1346. [Google Scholar] [CrossRef]
- Livi, G.P.; Kmetz, P.; McHale, M.M.; Cieslinski, L.B.; Sathe, G.M.; Taylor, D.P.; Davis, R.L.; Torphy, T.J.; Balcarek, J.M. Cloning and expression of cDNA for a human low-Km, rolipram-sensitive cyclic AMP phosphodiesterase. Mol. Cell. Biol. 1990, 10, 2678–2686. [Google Scholar]
- Monaco, L.; Vicini, E.; Conti, M. Structure of two rat genes coding for closely related rolipram-sensitive cAMP phosphodiesterases. Multiple mRNA variants originate from alternative splicing and multiple start sites. J. Biol. Chem. 1994, 269, 347–357. [Google Scholar] [CrossRef]
- Houslay, M.D.; Schafer, P.; Zhang, K.Y. Keynote review: Phosphodiesterase-4 as a therapeutic target. Drug Discov. Today 2005, 10, 1503–1519. [Google Scholar] [CrossRef]
- Houslay, M.D.; Sullivan, M.; Bolger, G.B. The multienzyme PDE4 cyclic adenosine monophosphate-specific phosphodiesterase family: Intracellular targeting, regulation, and selective inhibition by compounds exerting anti-inflammatory and antidepressant actions. Adv. Pharmacol. 1998, 44, 225–342. [Google Scholar]
- Richter, W.; Conti, M. The oligomerization state determines regulatory properties and inhibitor sensitivity of type 4 cAMP-specific phosphodiesterases. J. Biol. Chem. 2004, 279, 30338–30348. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.H.; Turko, I.V.; Corbin, J.D. Cyclic nucleotide phosphodiesterases: Relating structure and function. Prog. Nucleic Acid Res. Mol. Biol. 2001, 65, 1–52. [Google Scholar] [PubMed]
- Baillie, G.S.; MacKenzie, S.J.; McPhee, I.; Houslay, M.D. Sub-family selective actions in the ability of Erk2 MAP kinase to phosphorylate and regulate the activity of PDE4 cyclic AMP-specific phosphodiesterases. Br. J. Pharmacol. 2000, 131, 811–819. [Google Scholar] [CrossRef]
- Bolger, G.B.; Conti, M.; Houslay, M.D. Cellular functions of PDE4 enzymes. In Cyclic Nucleotide Phosphodiesterases in Health and Disease; Beavo, J.A., Francis, S.H., Houslay, M., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 99–129. [Google Scholar]
- Barnette, M.S.; Manning, C.D.; Cieslinski, L.B.; Burman, M.; Christensen, S.B.; Torphy, T.J. The ability of phosphodiesterase IV inhibitors to suppress superoxide production in guinea pig eosinophils is correlated with inhibition of phosphodiesterase IV catalytic activity. J. Pharmacol. Exp. Ther. 1995, 273, 674–679. [Google Scholar] [PubMed]
- Barnette, M.S.; Grous, M.; Cieslinski, L.B.; Burman, M.; Christensen, S.B.; Torphy, T.J. Inhibitors of phosphodiesterase IV (PDE IV) increase acid secretion in rabbit isolated gastric glands: Correlation between function and interaction with a high-affinity rolipram binding site. J. Pharmacol. Exp. Ther. 1995, 273, 1396–1402. [Google Scholar] [PubMed]
- Jacobitz, S.; McLaughlin, M.M.; Livi, G.P.; Burman, M.; Torphy, T.J. Mapping the functional domains of human recombinant phosphodiesterase 4A: Structural requirements for catalytic activity and rolipram binding. Mol. Pharmacol. 1996, 50, 891–899. [Google Scholar]
- Souness, J.E.; Rao, S. Proposal for pharmacologically distinct conformers of PDE4 cyclic AMP phosphodiesterases. Cell Signal. 1997, 9, 227–236. [Google Scholar] [CrossRef]
- Méhats, C.; Tanguy, G.; Paris, B.; Robert, B.; Pernin, N.; Ferré, F.; Leroy, M.J. Pregnancy induces a modulation of the cAMP phosphodiesterase 4-conformers ratio in human myometrium: Consequences for the utero-relaxant effect of PDE4-selective inhibitors. J. Pharmacol. Exp. Ther. 2000, 292, 817–823. [Google Scholar]
- Zhang, H.T.; Zhao, Y.; Huang, Y.; Deng, C.; Hopper, A.T.; De Vivo, M.; Rose, G.M.; O’Donnell, J.M. Antidepressant-like effects of PDE4 inhibitors mediated by the high-affinity rolipram binding state (HARBS) of the phosphodiesterase-4 enzyme (PDE4) in rats. Psychopharmacology 2006, 186, 209–217. [Google Scholar] [CrossRef]
- Hatzelmann, A.; Morcillo, E.J.; Lungarella, G.; Adnot, S.; Sanjar, S.; Beume, R.; Schudt, C.; Tenor, H. The preclinical pharmacology of roflumilast–A selective, oral phosphodiesterase 4 inhibitor in development for chronic obstructive pulmonary disease. Pulm. Pharmacol. Ther. 2010, 23, 235–256. [Google Scholar] [CrossRef]
- Fala, L. Otezla (Apremilast), an oral PDE-4 inhibitor, receives FDA approval for the treatment of patients with active psoriatic arthritis and plaque psoriasis. Am. Health Drug Benefits 2015, 8, 105–110. [Google Scholar] [PubMed]
- Burgin, A.B.; Magnusson, O.T.; Singh, J.; Witte, P.; Staker, B.L.; Bjornsson, J.M.; Thorsteinsdottir, M.; Hrafnsdottir, S.; Hagen, T.; Kiselyov, A.S.; et al. Design of phosphodiesterase 4D (PDE4D) allosteric modulators for enhancing cognition with improved safety. Nat. Biotechnol. 2010, 28, 63–70. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, Y.; Chowdhary, A.; Fox, D., 3rd; Gurney, M.E.; Zhang, H.T.; Auerbach, B.D.; Salvi, R.J.; Yang, M.; Li, G.; et al. Memory enhancing effects of BPN14770, an allosteric inhibitor of phosphodiesterase-4D, in wild-type and humanized mice. Neuropsychopharmacology 2018, 43, 2299–2309. [Google Scholar] [CrossRef] [PubMed]
- Keravis, T.; Lugnier, C. Cyclic nucleotide phosphodiesterases (PDE) and peptide motifs. Curr. Pharm. Des. 2010, 16, 1114–1125. [Google Scholar] [CrossRef] [PubMed]
- Richter, W.; Mika, D.; Blanchard, E.; Day, P.; Conti, M. ß1-adrenergic receptor antagonists signal via PDE4 translocation. EMBO Rep. 2013, 14, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, H.; Ye, M.; Xu, X.T.; Xu, Y.; Yang, W.; Zhang, H.T.; Song, G.; Ke, H. Identification of a PDE4-Specific Pocket for the Design of Selective Inhibitors. Biochemistry 2018, 57, 4518–4525. [Google Scholar] [CrossRef] [PubMed]
- Stoclet, J.C.; Keravis, T.; Komas, N.; Lugnier, C. Cyclic nucleotide phosphodiesterases as therapeutic targets in cardiovascular diseases. Expert Opin. Investig. Drugs 1995, 4, 1081–1100. [Google Scholar]
- Komas, N.; Lugnier, C.; Stoclet, J.C. Endothelium-dependent and independent relaxation of the rat aorta by cyclic nucleotide phosphodiesterase inhibitors. Br. J. Pharmacol. 1991, 104, 495–503. [Google Scholar] [CrossRef]
- Lugnier, C.; Komas, N. Modulation of vascular cyclic nucleotide phosphodiesterases by cyclic GMP: Role in vasodilatation. Eur. Heart J. 1993, 14 (Suppl. 1), 141–148. [Google Scholar]
- Eckly, A.E.; Lugnier, C. Role of phosphodiesterases III and IV in the modulation of vascular cyclic AMP content by the NO/cyclic GMP pathway. Br. J. Pharmacol. 1994, 113, 445–450. [Google Scholar] [CrossRef]
- Keravis, T.; Lugnier, C. Cyclic nucleotide phosphodiesterase (PDE) superfamily and smooth muscle signaling. In New Frontiers in Smooth Muscle Biology and Physiology; Savineau, J.P., Ed.; Transworld Research, Network: Kerala, India, 2007; pp. 269–289. [Google Scholar]
- Lugnier, C.; Schini, V.B. Characterization of cyclic nucleotide phosphodiesterases from cultured bovine aortic endothelial cells. Biochem. Pharmacol. 1990, 39, 75–84. [Google Scholar] [CrossRef]
- Keravis, T.; Komas, N.; Lugnier, C. Cyclic nucleotide hydrolysis in bovine aortic endothelial cells in culture: Differential regulation in cobblestone and spindle phenotypes. J. Vasc. Res. 2000, 37, 235–249. [Google Scholar] [CrossRef] [PubMed]
- Favot, L.; Keravis, T.; Lugnier, C. Modulation of VEGF-induced endothelial cell cycle protein expression through cyclic AMP hydrolysis by PDE2 and PDE4. Thromb. Haemost. 2004, 92, 634–645. [Google Scholar] [CrossRef]
- Keravis, T.; Silva, A.P.; Favot, L.; Lugnier, C. Role of PDEs in vascular health and disease: Endothelial PDEs and angiogenesis. In Cyclic Nucleotide Phosphodiesterases in Health and Disease; Beavo, J.A., Francis, S.H., Houslay, M., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 417–439. [Google Scholar]
- Kessler, T.; Lugnier, C. Rolipram increases cyclic GMP content in L-arginine-treated cultured bovine aortic endothelial cells. Eur. J. Pharmacol. 1995, 290, 163–167. [Google Scholar] [CrossRef]
- Suttorp, N.; Ehreiser, P.; Hippenstiel, S.; Fuhrmann, M.; Krüll, M.; Tenor, H.; Schudt, C. Hyperpermeability of pulmonary endothelial monolayer: Protective role of phosphodiesterase isoenzymes 3 and 4. Lung 1996, 174, 181–194. [Google Scholar] [PubMed]
- Rampersad, S.N.; Ovens, J.D.; Huston, E.; Umana, M.B.; Wilson, L.S.; Netherton, S.J.; Lynch, M.J.; Baillie, G.S.; Houslay, M.D.; Maurice, D.H. Cyclic AMP phosphodiesterase 4D (PDE4D) tethers EPAC1 in a vascular endothelial cadherin (VE-Cad)-based signaling complex and controls cAMP-mediated vascular permeability. J. Biol. Chem. 2010, 285, 33614–33622. [Google Scholar] [PubMed]
- Hubert, F.; Belacel-Ouari, M.; Manoury, B.; Zhai, K.; Domergue-Dupont, V.; Mateo, P.; Joubert, F.; Fischmeister, R.; Leblais, V. Alteration of vascular reactivity in heart failure: Role of phosphodiesterases 3 and 4. Br. J. Pharmacol. 2014, 171, 5361–5375. [Google Scholar] [CrossRef]
- Varona, S.; Puertas, L.; Galán, M.; Orriols, M.; Cañes, L.; Aguiló, S.; Camacho, M.; Sirvent, M.; Andrés, V.; Martínez-González, J.; et al. Rolipram prevents the formation of abdominal aortic aneurysm (AAA) in Mice: PDE4B as a target in AAA. Antioxidants 2021, 10, 460. [Google Scholar] [CrossRef]
- Wollborn, J.; Siemering, S.; Steiger, C.; Buerkle, H.; Goebel, U.; Schick, M.A. Phosphodiesterase-4 inhibition reduces ECLS-induced vascular permeability and improves microcirculation in a rodent model of extracorporeal resuscitation. Am. J. Physiol. Heart. Circ. Physiol. 2019, 316, H751–H761. [Google Scholar]
- Tsien, R.W. Cyclic AMP and contractile activity in heart. Adv. Cycl. Nucleotide Res. 1977, 8, 363–420. [Google Scholar]
- Harrison, S.A.; Reifsnyder, D.H.; Gallis, B.; Cadd, G.G.; Beavo, J.A. Isolation and characterization of bovine cardiac muscle cGMP- inhibited phosphodiesterase: A receptor for new cardiotonic drugs. Mol. Pharmacol. 1986, 29, 506–514. [Google Scholar] [PubMed]
- Komas, N.; Lugnier, C.; Le Bec, A.; Serradeil-Le Gal, C.; Barthelemy, G.; Stoclet, J.C. Differential sensitivity to cardiotonic drugs of cyclic AMP phosphodiesterases isolated from canine ventricular and sinoatrial-enriched tissues. J. Cardiovasc. Pharmacol. 1989, 14, 213–220. [Google Scholar] [CrossRef]
- Muller, B.; Lugnier, C.; Stoclet, J.C. Implication of cyclic AMP in the positive inotropic effects of cyclic GMP-inhibited cyclic AMP phosphodiesterase inhibitors on guinea pig isolated left atria. J. Cardiovasc. Pharmacol. 1990, 15, 444–451. [Google Scholar] [CrossRef]
- Lanfear, D.E.; Hasan, R.; Gupta, R.C.; Williams, C.; Czerska, B.; Tita, C.; Bazari, R.; Sabbah, H.N. Short term effects of milrinone on biomarkers of necrosis, apoptosis, and inflammation in patients with severe heart failure. J. Transl. Med. 2009, 7, 67. [Google Scholar] [CrossRef]
- Prigent, A.F.; Fougier, S.; Nemoz, G.; Anker, G.; Pacheco, H.; Lugnier, C.; Lebec, A.; Stoclet, J.C. Comparison of cyclic nucleotide phosphodiesterase isoforms from rat heart and bovine aorta. Separation and inhibition by selective reference phosphodiesterase inhibitors. Biochem. Pharmacol. 1988, 37, 3671–3681. [Google Scholar] [CrossRef]
- Stoclet, J.C.; Boulanger-Saunier, C.; Lassègue, B.; Lugnier, C. Cyclic nucleotides and calcium regulation in heart and smooth muscle cells. Ann. N. Y. Acad. Sci. 1988, 522, 106–115. [Google Scholar] [CrossRef]
- Lugnier, C.; Gauthier, C.; Le Bec, A.; Soustre, H. Cyclic nucleotide phosphodiesterases from frog atrial fibers: Isolation and drug sensitivities. Am. J. Physiol. 1992, 262, H654–H660. [Google Scholar] [CrossRef] [PubMed]
- Muller, B.; Stoclet, J.C.; Lugnier, C. Cytosolic and membrane-bound cyclic nucleotide phosphodiesterases from guinea pig cardiac ventricles. Eur. J. Pharmacol. 1992, 225, 263–272. [Google Scholar] [CrossRef]
- Muller, B.; Lugnier, C.; Stoclet, J.C. Involvement of rolipram-sensitive cyclic AMP phosphodiesterase in the regulation of cardiac contraction. Cardiovasc. Pharmacol. 1990, 16, 796–803. [Google Scholar]
- Eschenhagen, T. PDE4 in the human heart–major player or little helper? Br. J. Pharmacol. 2013, 168, 524–527. [Google Scholar] [CrossRef]
- Lugnier, C.; Muller, B.; Le Bec, A.; Beaudry, C.; Rousseau, E. Characterization of indolidan- and rolipram-sensitive cyclic nucleotide phosphodiesterases in canine and human cardiac microsomal fractions. J. Pharmacol. Exp. Ther. 1993, 265, 1142–1151. [Google Scholar] [PubMed]
- Lugnier, C.; Keravis, T.; Le Bec, A.; Pauvert, O.; Proteau, S.; Rousseau, E. Characterization of cyclic nucleotide phosphodiesterase isoforms associated to isolated cardiac nuclei. Biochim. Biophys. Acta 1999, 1472, 431–446. [Google Scholar] [CrossRef]
- Bedioune, I.; Lefebvre, F.; Lechêne, P.; Varin, A.; Domergue, V.; Kapiloff, M.S.; Fischmeister, R.; Vandecasteele, G. PDE4 and mAKAPβ are nodal organizers of β2-ARs nuclear PKA signalling in cardiac myocytes. Cardiovasc. Res. 2018, 114, 1499–1511. [Google Scholar] [CrossRef] [PubMed]
- Verde, I.; Pahlke, G.; Salanova, M.; Zhang, G.; Wang, S.; Coletti, D.; Onuffer, J.; Jin, S.L.; Conti, M. Myomegalin is a novel protein of the Golgi/centrosome that interacts with a cyclic nucleotide phosphodiesterase. J. Biol. Chem. 2001, 276, 11189–11198. [Google Scholar] [CrossRef]
- Lehnart, S.E.; Wehrens, X.H.; Reiken, S.; Warrier, S.; Belevych, A.E.; Harvey, R.D.; Richter, W.; Jin, S.L.; Conti, M.; Marks, A.R. Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell 2005, 123, 25–35, Erratum in Cell 2005, 123, 535–536. [Google Scholar]
- Abi-Gerges, A.; Richter, W.; Lefebvre, F.; Mateo, P.; Varin, A.; Heymes, C.; Samuel, J.L.; Lugnier, C.; Conti, M.; Fischmeister, R.; et al. Decreased expression and activity of cAMP phosphodiesterases in cardiac hypertrophy and its impact on beta-adrenergic cAMP signals. Circ. Res. 2009, 105, 784–792. [Google Scholar]
- Richter, W.; Xie, M.; Scheitrum, C.; Krall, J.; Movsesian, M.A.; Conti, M. Conserved expression and functions of PDE4 in rodent and human heart. Basic Res. Cardiol. 2011, 106, 249–262. [Google Scholar]
- Mokni, W.; Keravis, T.; Etienne-Selloum, N.; Walter, A.; Kane, M.O.; Schini-Kerth, V.B.; Lugnier, C. Concerted regulation of cGMP and cAMP phosphodiesterases in early cardiac hypertrophy induced by angiotensin II. PLoS ONE 2010, 5, e14227. [Google Scholar] [CrossRef]
- Beca, S.; Helli, P.B.; Simpson, J.A.; Zhao, D.; Farman, G.P.; Jones, P.; Tian, X.; Wilson, L.S.; Ahmad, F.; Chen, S.R.W.; et al. Phosphodiesterase 4D (PDE4D) regulates baseline sarcoplasmic reticulum Ca2+ release and cardiac contractility, independently of L-type Ca2+ current. Circ. Res. 2011, 109, 1024–1030. [Google Scholar]
- Leroy, J.; Richter, W.; Mika, D.; Castro, L.R.; Abi-Gerges, A.; Xie, M.; Scheitrum, C.; Lefebvre, F.; Schittl, J.; Mateo, P.; et al. Phosphodiesterase 4B in the cardiac L-type Ca2+ channel complex regulates Ca2+ current and protects against ventricular arrhythmias in mice. J. Clin. Investig. 2011, 121, 2651–2661. [Google Scholar] [CrossRef]
- Wang, L.; Burmeister, B.T.; Johnson, K.R.; Baillie, G.S.; Karginov, A.V.; Skidgel, R.A.; O’Bryan, J.P.; Carnegie, G.K. UCR1C is a novel activator of phosphodiesterase 4 (PDE4) long isoforms and attenuates cardiomyocyte hypertrophy. Cell Signal. 2015, 27, 908–922. [Google Scholar] [PubMed]
- Lindner, M.; Mehel, H.; David, A.; Leroy, C.; Burtin, M.; Friedlander, G.; Terzi, F.; Mika, D.; Fischmeister, R.; Prié, D. Fibroblast growth factor 23 decreases PDE4 expression in heart increasing the risk of cardiac arrhythmia; Klotho opposes these effects. Basic Res. Cardiol. 2020, 115, 51. [Google Scholar] [PubMed]
- Levian, C.; Ruiz, E.; Yang, X. The pathogenesis of obesity from a genomic and systems biology perspective. Yale J. Biol. Med. 2014, 87, 113–126. [Google Scholar]
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of obesity among adults and youth: United States, 2015–2016. NCHS Data Briefs 2020, 360, 1–8. [Google Scholar]
- Chung, J.H.; Manganiello, V.; Dyck, J.R. Resveratrol as a calorie restriction mimetic: Therapeutic implications. Trends Cell Biol. 2012, 22, 546–554. [Google Scholar] [CrossRef]
- Omar, B.; Banke, E.; Ekelund, M.; Frederiksen, S.; Degerman, E. Alterations in cyclic nucleotide phosphodiesterase activities in omental and subcutaneous adipose tissues in human obesity. Nutr. Diabetes 2011, 1, e13. [Google Scholar] [CrossRef]
- Kraynik, S.M.; Miyaoka, R.S.; Beavo, J.A. PDE3 and PDE4 isozyme-selective inhibitors are both required for synergistic activation of brown adipose tissue. Mol. Pharmacol. 2013, 83, 1155–1165. [Google Scholar] [CrossRef] [Green Version]
- Jensterle, M.; Salamun, V.; Kocjan, T.; Vrtacnik Bokal, E.; Janez, A. Short term monotherapy with GLP-1 receptor agonist liraglutide or PDE 4 inhibitor roflumilast is superior to metformin in weight loss in obese PCOS women: A pilot randomized study. J. Ovarian Res. 2015, 8, 32. [Google Scholar] [CrossRef]
- Zhang, R.; Maratos-Flier, E.; Flier, J.S. Reduced adiposity and high-fat diet-induced adipose inflammation in mice deficient for phosphodiesterase 4B. Endocrinology 2008, 150, 3076–3082. [Google Scholar]
- Al Amrani, A.; Abdel Karim, M.; Al Zoghaibi, M. PRDM16 Gene Polymorphism is associated with obesity and blood lipids profiles in Saudi population. J. Clin. Med. 2018, 7, E141. [Google Scholar] [CrossRef]
- Muo, I.M.; Park, S.J.; Smith, A.; Springer, A.A.; Allen, M.D.; Hagen, T.J.; Chung, J.H. Compound D159687, a phosphodiesterase 4D inhibitor, induces weight and fat mass loss in aged mice without changing lean mass, physical and cognitive function. Biochem. Biophys. Res. Commun. 2018, 506, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, J.; Xiao, J. Roflumilast suppresses adipogenic differentiation via AMPK mediated pathway. Front. Endocrinol. 2021, 12, 662451. [Google Scholar] [CrossRef] [PubMed]
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Marasco, M.R.; Linnemann, A.K. β-Cell Autophagy in Diabetes Pathogenesis. Endocrinology 2018, 159, 2127–2141. [Google Scholar] [CrossRef]
- Yazdanpanah, S.; Rabiee, M.; Tahriri, M.; Abdolrahim, M.; Rajab, A.; Jazayeri, H.E.; Tayebi, L. Evaluation of glycated albumin (GA) and GA/HbA1c ratio for diagnosis of diabetes and glycemic control: A comprehensive review. Crit. Rev. Clin. Lab. Sci. 2017, 54, 219–232. [Google Scholar] [CrossRef]
- Lugnier, C. Cyclic nucleotide phosphodiesterase families in intracellular signaling and diabetes. Adv. Exp. Med. Biol. 2001, 498, 253–261. [Google Scholar]
- Parker, J.C.; VanVolkenburg, M.A.; Ketchum, R.J.; Brayman, K.L.; Andrews, K.M. Cyclic AMP phosphodiesterases of human and rat islets of Langerhans: Contributions of types III and IV to the modulation of insulin secretion. Biochem. Biophys. Res. Commun. 1995, 217, 916–923. [Google Scholar] [CrossRef]
- Waddleton, D.; Wu, W.; Feng, Y.; Thompson, C.; Wu, M.; Zhou, Y.P.; Howard, A.; Thornberry, N.; Li, J.; Mancini, J.A. Phosphodiesterase 3 and 4 comprise the major cAMP metabolizing enzymes responsible for insulin secretion in INS-1 (832/13) cells and rat islets. Biochem. Pharmacol. 2008, 76, 884–893. [Google Scholar] [CrossRef]
- Tian, G.; Sågetorp, J.; Xu, Y.; Shuai, H.; Degerman, E.; Tengholm, A. Role of phosphodiesterases in the shaping of sub-plasma-membrane cAMP oscillations and pulsatile insulin secretion. J. Cell Sci. 2012, 125, 5084–5095. [Google Scholar] [CrossRef] [Green Version]
- Möllmann, J.; Kahles, F.; Lebherz, C.; Kappel, B.; Baeck, C.; Tacke, F.; Werner, C.; Federici, M.; Marx, N.; Lehrke, M. The PDE4 inhibitor roflumilast reduces weight gain by increasing energy expenditure and leads to improved glucose metabolism. Diabetes Obes. Metab. 2017, 19, 496–508. [Google Scholar] [CrossRef]
- Plock, N.; Vollert, S.; Mayer, M.; Hanauer, G.; Lahu, G. Pharmacokinetic/Pharmacodynamic Modeling of the PDE4 Inhibitor TAK-648 in Type 2 Diabetes: Early Translational Approaches for Human Dose Prediction. Clin. Transl. Sci. 2017, 10, 185–193. [Google Scholar] [PubMed]
- Ookawara, M.; Nio, Y. Phosphodiesterase 4 inhibitors in diabetic nephropathy. Cell Signal. 2022, 90, 110185. [Google Scholar] [CrossRef] [PubMed]
- Muo, I.M.; MacDonald, S.D.; Madan, R.; Park, S.J.; Gharib, A.M.; Martinez, P.E.; Walter, M.F.; Yang, S.B.; Rodante, J.A.; Courville, A.B.; et al. Early effects of roflumilast on insulin sensitivity in adults with prediabetes and overweight/obesity involve age-associated fat mass loss–results of an exploratory study. Diabetes Metab. Syndr. Obes. 2019, 12, 743–759. [Google Scholar] [CrossRef] [PubMed]
- Gajendran, M.; Loganathan, P.; Catinella, A.P.; Jana, G.; Hashash, J.G. A comprehensive review and update on Crohn’s disease. Dis. Mon. 2018, 64, 20–57. [Google Scholar] [CrossRef]
- Reimund, J.-M.; Wittersheim, C.; Dumont, S.; Muller, C.D.; Kenney, J.S.; Baumann, R.; Poindron, P.; Duclos, B. Increased production of tumour necrosis factor-α, interleukin-1β, and interleukin-6 by morphologically normal intestinal biopsies from patients with Crohn’s disease. Gut 1996, 39, 684–689. [Google Scholar] [CrossRef]
- Reimund, J.M.; Dumont, S.; Muller, C.D.; Kenney, J.S.; Kedinger, M.; Baumann, R.; Poindron, P.; Duclos, B. In vitro effects of oxpentifylline on inflammatory cytokine release in patients with inflammatory bowel disease. Gut 1997, 40, 475–480. [Google Scholar] [CrossRef]
- Tetsi, L.; Charles, A.L.; Paradis, S.; Lejay, A.; Talha, S.; Geny, B.; Lugnier, C. Effects of cyclic nucleotide phosphodiesterases (PDEs) on mitochondrial skeletal muscle functions. Cell. Mol. Life Sci. 2017, 74, 1883–1893. [Google Scholar] [CrossRef]
- Arondel, Y.; Keravis, T.; Le Bec, A.; Baumann, R.; Duclos, B.; Reimund, J.M.; Lugnier, C. First characterisation of cyclic nucleotide phosphodiesterase isoforms in normal human mucosa and inflamed mucosa from Crohn’s disease patients. Gastroenterology 1999, 116, G3716. [Google Scholar]
- Banan, A.; Fitzpatrick, L.; Zhang, Y.; Keshavarzian, A. OPC-compounds prevent oxidant-induced carbonylation and depolymerization of the F-actin cytoskeleton and intestinal barrier hyperpermeability. Free Radic. Biol. Med. 2001, 30, 287–298. [Google Scholar]
- O’Mahony, S. Tetomilast. IDrugs 2005, 8, 502–507. [Google Scholar]
- Hartmann, G.; Bidlingmaier, C.; Siegmund, B.; Albrich, S.; Schulze, J.; Tschoep, K.; Eigler, A.; Lehr, H.A.; Endres, S. Specific type IV phosphodiesterase inhibitor rolipram mitigates experimental colitis in mice. J. Pharmacol. Exp. Ther. 2000, 292, 22–30. [Google Scholar] [PubMed]
- Videla, S.; Vilaseca, J.; Medina, C.; Mourelle, M.; Guarner, F.; Salas, A.; Malagelada, J.R. Selective inhibition of phosphodiesterase-4 ameliorates chronic colitis and prevents intestinal fibrosis. J. Pharmacol. Exp. Ther. 2006, 316, 940–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spadaccini, M.; D’Alessio, S.; Peyrin-Biroulet, L.; Danese, S. PDE4 Inhibition and Inflammatory Bowel Disease: A novel therapeutic avenue. Int. J. Mol. Sci. 2017, 18, E1276. [Google Scholar] [CrossRef]
- Picchianti-Diamanti, A.; Spinelli, F.R.; Rosado, M.M.; Conti, F.; Laganà, B. Inhibition of Phosphodiesterase-4 in Psoriatic Arthritis and Inflammatory Bowel Diseases. Int. J. Mol. Sci. 2021, 22, 2638. [Google Scholar] [CrossRef]
- Oh, K.; Oh, E.-H.; Noh, S.M.; Park, S.H.; Kim, N.; Hwang, S.W.; Park, S.H.; Yang, D.-H.; Byeon, J.-S.; Myung, S.-J.; et al. Combined endoscopic and radiologic healing is associated with a better prognosis than endoscopic healing only in patients with Crohn’s disease receiving anti-TNF therapy. Clin. Transl. Gastroenterol. 2022, 13, e00442. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.C. Advances in osteoporosis from 1970 to 2018. Menopause 2018, 25, 1403–1417. [Google Scholar] [CrossRef]
- Barrios-Moyano, A.; De la Peña-García, C. Prevalencia de osteoporosis y osteopenia en pacientes laboralmente activos. Acta Ortop. Mex. 2018, 32, 131–133. [Google Scholar]
- Zamani, M.; Zamani, V.; Heidari, B.; Parsian, H.; Esmaeilnejad-Ganji, S.M. Prevalence of osteoporosis with the World Health Organization diagnostic criteria in the Eastern Mediterranean Region: A systematic review and meta-analysis. Arch. Osteoporos. 2018, 13, 129. [Google Scholar] [CrossRef]
- Lugnier, C.; Stoclet, J.C.; Wilke, R. Phosphodiesterase isoenzymes in different tissues and the selective inhibition by denbufylline. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1989, 339, R106. [Google Scholar]
- Nicholson, C.D.; Jackman, S.A.; Wilke, R. The ability of denbufylline to inhibit cyclic nucleotide phosphodiesterase and its affinity for adenosine receptors and the adenosine re-uptake site. Br. J. Pharmacol. 1989, 97, 889–897. [Google Scholar] [CrossRef]
- Miyamoto, K.; Waki, Y.; Horita, T.; Kasugai, S.; Ohya, K. Reduction of bone loss by denbufylline, an inhibitor of phosphodiesterase 4. Biochem. Pharmacol. 1997, 54, 613–617. [Google Scholar] [CrossRef]
- Waki, Y.; Horita, T.; Miyamoto, K.; Ohya, K.; Kasugai, S. Effects of XT-44, a phosphodiesterase 4 inhibitor, in osteoblastgenesis and osteoclastgenesis in culture and its therapeutic effects in rat osteopenia models. Jpn. J. Pharmacol. 1999, 79, 477–483. [Google Scholar] [PubMed]
- Kinoshita, T.; Kobayashi, S.; Ebara, S.; Yoshimura, Y.; Horiuchi, H.; Tsutsumimoto, T.; Wakabayashi, S.; Takaoka, K. Phosphodiesterase inhibitors, pentoxifylline and rolipram, increase bone mass mainly by promoting bone formation in normal mice. Bone 2000, 27, 811–817. [Google Scholar] [CrossRef]
- Boudjema, N. Inhibiteurs de phosphodiestrase type 4: Intérêt potentiel dans le traitement de l’ostéoporose. Master’s Thesis, Faculty of Pharmacy, Strasbourg, France, 15 March 2002. [Google Scholar]
- Yao, W.; Tian, X.Y.; Chen, J.; Setterberg, R.B.; Lundy, M.W.; Chmielzwski, P.; Froman, C.A.; Jee, W.S. Rolipram, a phosphodiesterase 4 inhibitor, prevented cancellous and cortical bone loss by inhibiting endosteal bone resorption and maintaining the elevated periosteal bone formation in adult ovariectomized rats. J. Musculoskelet. Neuronal Interact. 2007, 7, 119–130. [Google Scholar]
- Ahlström, M.; Pekkinen, M.; Huttunen, M.; Lamberg-Allardt, C. Cyclic nucleotide phosphodiesterases (PDEs) in human osteoblastic cells; the effect of PDE inhibition on cAMP accumulation. Cell. Mol. Biol. Lett. 2005, 10, 305–319. [Google Scholar]
- Ahlström, M.; Pekkinen, M.; Huttunen, M.; Lamberg-Allardt, C. Dexamethasone down-regulates cAMP-phosphodiesterase in human osteosarcoma cells. Biochem. Pharmacol. 2005, 69, 267–275. [Google Scholar] [CrossRef]
- Chen, L.; Zheng, T.; Park, H.; Noh, A.L.; Lee, J.M.; Lee, D.S.; Yim, M. PDE4 inhibitor suppresses PGE2-induced osteoclast formation via COX-2-mediated p27(KIP1) expression in RAW264.7 cells. Pharmazie 2011, 66, 201–206. [Google Scholar]
- Stern, A.R.; Yao, X.; Wang, Y.; Berhe, A.; Dallas, M.; Johnson, M.L.; Yao, W.; Kimmel, D.B.; Lane, N.E. Effect of osteoporosis treatment agents on the cortical bone osteocyte microenvironment in adult estrogen-deficient, osteopenic rats. Bone Rep. 2018, 8, 115–124. [Google Scholar] [CrossRef]
- Porwal, K.; Pal, S.; Bhagwati, S.; Siddiqi, M.I.; Chattopadhyay, N. Therapeutic potential of phosphodiesterase inhibitors in the treatment of osteoporosis: Scopes for therapeutic repurposing and discovery of new oral osteoanabolic drugs. Eur. J. Pharmacol. 2021, 899, 174015. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Kim, D.H.; Lerner, A. Type 4 cyclic adenosine monophosphate phosphodiesterase as a therapeutic target in chronic lymphocytic leukemia. Blood 1998, 92, 2484–2494. [Google Scholar] [CrossRef] [PubMed]
- Marko, D.; Romanakis, K.; Zankl, H.; Fürstenberger, G.; Steinbauer, B.; Eisenbrand, G. Induction of apoptosis by an inhibitor of cAMP-specific PDE in malignant murine carcinoma cells overexpressing PDE activity in comparison to their nonmalignant counterparts. Cell. Biochem. Biophys. 1998, 28, 75–101. [Google Scholar] [CrossRef] [PubMed]
- Narita, M.; Murata, T.; Shimizu, K.; Sugiyama, T.; Nakagawa, T.; Manganiello, V.C.; Tagawa, T. Phosphodiesterase 4 in osteoblastic osteosarcoma cells as a potential target for growth inhibition. Anticancer. Drugs 2003, 14, 377–381. [Google Scholar] [CrossRef]
- Favot, L.; Keravis, T.; Holl, V.; Le Bec, A.; Lugnier, C. VEGF-induced HUVEC migration and proliferation are decreased by PDE2 and PDE4 inhibitors. Thromb. Haemost. 2003, 90, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Abusnina, A.; Keravis, T.; Zhou, Q.; Justiniano, H.; Lobstein, A.; Lugnier, C. Tumour growth inhibition and anti-angiogenic effects using curcumin correspond to combined PDE2 and PDE4 inhibition. Thromb. Haemost. 2015, 113, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Suhasini, A.N.; Wang, L.; Holder, K.N.; Lin, A.P.; Bhatnagar, H.; Kim, S.W.; Moritz, A.W.; Aguiar, R.C.T. A phosphodiesterase 4B-dependent interplay between tumor cells and the microenvironment regulates angiogenesis in B-cell lymphoma. Leukemia 2016, 30, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Parrella, E.; Gianni, M.; Cecconi, V.; Nigro, E.; Barzago, M.M.; Rambaldi, A.; Rochette-Egly, C.; Terao, M.; Garattini, E. Phosphodiesterase IV inhibition by piclamilast potentiates the cytodifferentiating action of retinoids in myeloid leukemia cells. Cross-talk between the cAMP and the retinoic acid signaling pathways. J. Biol. Chem. 2004, 279, 42026–42040. [Google Scholar] [CrossRef] [Green Version]
- Keravis, T.; Justiniano, H.; Guillemin, M.C.; de Thé, H.; Rochette-Egly, C.; Lugnier, C. cAMP-PDE activity, PDE4 activity and PDE4D protein expression are increased in RA-resistant NB4-R2 cells. In Proceedings of the EMBO Retinoids 2011: Mechanisms, Biology and pathology of Signaling by Retinoic acid and Retinoic acid Receptors, Strasbourg, France, 22–25 September 2011.Poster. [Google Scholar]
- Pleiman, J.K.; Irving, A.A.; Wang, Z.; Toraason, E.; Clipson, L.; Dove, W.F.; Dustin, A.; Deming, D.A.; Newton, M.A. The conserved protective cyclic AMP-phosphodiesterase function PDE4B is expressed in the adenoma and adjacent normal colonic epithelium of mammals and silenced in colorectal cancer. PLoS Genet. 2018, 14, e1007611. [Google Scholar] [CrossRef]
- Warrington, N.M.; Gianino, S.M.; Jackson, E.; Goldhoff, P.; Garbow, J.R.; Piwnica-Worms, D.; Gutmann, D.H.; Rubin, J.B. Cyclic AMP suppression is sufficient to induce gliomagenesis in a mouse model of Neurofibromatosis-1. Cancer Res. 2010, 70, 5717–5727. [Google Scholar] [CrossRef]
- Gong, S.; Chen, Y.; Meng. F.; Zhang. Y.; Wu, H.; Wu, F. Roflumilast restores cAMP/PKA/CREB signaling axis for FtMt-mediated tumor inhibition of ovarian cancer. Oncotarget 2017, 8, 112341–112353. [Google Scholar] [CrossRef]
- Henderson, D.J.P.; Houslay, M.D.; Bangma, C.H.; Hoffmann, R. Creating a potential diagnostic for prostate cancer risk stratification (InformMDx™) by translating novel scientific discoveries concerning cAMP degrading phosphodiesterase-4D7 (PDE4D7). Clin. Sci. 2019, 133, 269–286. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.U.; Nam, J.; Cha, M.D.; Kim, S.W. Inhibition of phosphodiesterase 4D decreases the malignant properties of DLD-1 colorectal cancer cells by repressing the AKT/mTOR/Myc signaling pathway. Oncol. Lett. 2019, 17, 3589–3598. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Ai, G.; Wang, D.; Chen, R.; Guo, D.; Yao, Y.; Wang, K.; Liang, G.; Qi, F.; Liu, W.; et al. PDE4 and Epac1 Synergistically Promote Rectal Carcinoma via the cAMP Pathway. Anal. Cell. Pathol. 2019, 2019, 7145198. [Google Scholar] [CrossRef]
- Guo, C.H.; Bai, L.; Wu, H.H.; Yang, J.; Cai, G.H.; Wang, X.; Wu, S.X.; Ma, W. The analgesic effect of rolipram is associated with the inhibition of the activation of the spinal astrocytic JNK/CCL2 pathway in bone cancer pain. Int. J. Mol. Med. 2016, 38, 1433–1442. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Gong, J.; Jin, Y.; Zhou, Y.; Tong, R.; Wei, X.; Bai, L.; Shi, J. Inhibitors of phosphodiesterase as cancer therapeutics. Eur. J. Med. Chem. 2018, 150, 742–756. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.P.; Martires, K.; Wu, J.J. The risk of melanoma and hematologic cancers in patients with psoriasis. J. Am. Acad. Dermatol. 2017, 76, 639–647. [Google Scholar] [PubMed]
- Hsien Lai, S.; Zervoudakis, G.; Chou, J.; Gurney, M.E.; Quesnelle, K.M. PDE4 subtypes in cancer. Oncogene 2020, 39, 3791–3802. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Vonghia, L.; Van Herck, M.A.; Weyler, J.; Francque, S. Targeting myeloid-derived cells: New frontiers in the treatment of non-alcoholic and alcoholic liver disease. Front. Immunol. 2019, 10, 563. [Google Scholar] [CrossRef]
- Gobejishvili, L.; Barve, S.; Breitkopf-Heinlein, K.; Li, Y.; Zhang, J.; Avila, D.V.; Dooley, S.; McClain, C.J. Rolipram attenuates bile duct ligation-induced liver injury in rats: A potential pathogenic role of PDE4. J. Pharmacol. Exp. Pathol. 2013, 347, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Ratziu, V.; Bedossa, P.; Francque, S.M.; Larrey, D.; Aithal, G.P.; Serfaty, L.; Voiculescu, M.; Preotescu, L.; Nevens, F.; De Lédinghen, V.; et al. Lack of efficacy of an inhibitor of PDE4 in phase 1 and 2 trials of patients with nonalcoholic steatohepatitis. Clin. Gastroenterol. Hepatol. 2014, 12, 1724–1730.e5. [Google Scholar] [CrossRef] [PubMed]
- Avila, D.V.; Barker, D.F.; Zhang, J.; McClain, C.J.; Barve, S.; Gobejishvili, L. Dysregulation of hepatic cAMP levels via altered Pde4b expression plays a critical role in alcohol-induced steatosis. J. Pathol. 2016, 240, 96–107. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, W.E.; Wahlang, B.; Wang, Y.; Zhang, J.; Vadhanam, M.V.; Joshi-Barve, S.; Bauer, P.; Cannon, R.; Ahmadi, A.R.; Sun, Z.; et al. Phosphodiesterase 4 inhibition as a therapeutic target for alcoholic liver disease: From bedside to bench. Hepatology 2019, 70, 1958–1971. [Google Scholar] [CrossRef]
- Hu, W.; Lu, T.; Chen, A.; Huang, Y.; Hansen, R.; Chandler, L.J.; Zhang, H.-T. Inhibition of phosphodiesterase-4 decreases ethanol intake in mice. Psychopharmacology 2011, 218, 331–339. [Google Scholar] [CrossRef]
- Tao, X.; He, H.; Peng, J.; Xu, R.; Fu, J.; Hu, Y.; Li, L.; Yang, X.; Feng, X.; Zhang, C.; et al. Overexpression of PDE4D in mouse liver is sufficient to trigger NAFLD and hypertension in a CD36-TGF-beta1 pathway: Therapeutic role of roflumilast. Pharmacol. Res. 2022, 175, 106004. [Google Scholar] [CrossRef]
- Gobejishvili, L.; Rodriguez, W.E.; Bauer, P.; Wang, Y.; Soni, C.; Lydic, T.; Barve, S.; McClain, C.; Maldonado, C. Novel liposomal rolipram formulation for clinical application to reduce emesis. Drug Des. Devel. Ther. 2022, 16, 1301–1309. [Google Scholar] [CrossRef]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Wachtel, H. Potential antidepressant activity of rolipram and other selective cyclic adenosine 3′,5′-monophosphate phosphodiesterase inhibitors. Neuropharmacology 1983, 22, 267–272. [Google Scholar] [CrossRef]
- O’Donnell, J.M. Antidepressant-like effects of rolipram and other inhibitors of cyclic adenosine monophosphate phosphodiesterase on behavior maintained by differential reinforcement of low response rate. J. Pharmacol. Exp. Ther. 1993, 264, 1168–1178. [Google Scholar]
- Barad, M.; Bourtchouladze, R.; Winder, D.G.; Golan, H.; Kandel, E. Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc. Natl. Acad. Sci. USA 1998, 95, e15020–e15025. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.T.; Huang, Y.; Jin, S.L.; Frith, S.A.; Suvarna, N.; Conti, M.; O’Donnell, J.M. Antidepressant-like profile and reduced sensitivity to rolipram in mice deficient in the PDE4D phosphodiesterase enzyme. Neuropsychopharmacology 2002, 27, 587–595. [Google Scholar] [PubMed]
- Menniti, F.S.; Faraci, W.S.; Schmidt, C.J. Phosphodiesterases in the CNS: Targets for drug development. Nat. Rev. Drug Discov. 2006, 5, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Rutten, K.; Wallace, T.L.; Works, M.; Prickaerts, J.; Blokland, A.; Novak, T.J.; Santarelli, L.; Misner, D. L Enhanced long-term depression and impaired reversal learning in phosphodiesterase 4B- knockout (PDE4B−/−) mice. Neuropharmacology 2011, 61, 138–147. [Google Scholar]
- Omar, F.; Jane, E.; Findlay, J.E.; Carfray, G.; Allcock, R.W.; Jiang, Z.; Moore, C.; Muir, A.L.; Lannoy, M.; Fertig, B.A.; et al. Small-molecule allosteric activators of PDE4 long form cyclic AMP phosphodiesterases. Proc. Natl. Acad. Sci. USA 2019, 116, 13320–13329. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.L.; van Groen, T.; Kadish, I.; Smoot, L.H.M.; Bolger, G.B. Altered phosphorylation, electrophysiology, and behavior on attenuation of PDE4B action in hippocampus. BMC Neurosci. 2017, 18, 77. [Google Scholar] [CrossRef]
- Wang, Z.Z.; Yang, W.X.; Zhang, Y.; Zhao, N.; Zhang, Y.Z.; Liu, Y.Q.; Xu, Y.; Wilson, S.P.; O’Donnell, J.M.; Zhang, H.T.; et al. Phosphodiesterase-4D knock-down in the prefrontal cortex alleviates chronic unpredictable stress-induced depressive-like behaviors and memory deficits in mice. Sci. Rep. 2015, 5, 11332. [Google Scholar] [CrossRef]
- Vanmierlo, T.; Creemers, P.; Akkerman, S.; van Duinen, M.; Sambeth, A.; De Vry, J.; Uz, T.; Blokland, A.; Prickaerts, J. The PDE4 inhibitor roflumilast improves memory in rodents at non-emetic doses. Behav. Brain. Res. 2016, 303, 26–33. [Google Scholar] [CrossRef]
- Bolger, G.B. The PDE4 cAMP-specific phosphodiesterases: Targets for drugs with antidepressant and memory-enhancing action. Adv. Neurobiol. 2017, 17, 63–102. [Google Scholar]
- Tibbo, A.J.; Tejeda, G.S.; Baillie, G.S. Understanding PDE4′s function in Alzheimer’s disease; a target for novel therapeutic approaches. Biochem. Soc. Trans. 2019, 47, 1557–1565. [Google Scholar]
- Wang, H.; Zhang, M.; Xie, Q.; Yu, J.; Qi, Y.; Yue, Q. Identification of diagnostic markers for major depressive disorder by cross-validation of data from whole blood samples. PeerJ 2019, 7, e7171. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, F.F.; Xu, Y.; Fu, H.R.; Wang, X.D.; Wang, L.; Chen, W.; Xu, X.Y.; Gao, Y.F.; Zhang, J.G.; et al. The Phosphodiesterase-4 Inhibitor Roflumilast, a Potential Treatment for the Comorbidity of Memory Loss and Depression in Alzheimer’s Disease: A Preclinical Study in APP/PS1 Transgenic Mice. Int. J. Neuropsychopharmacol. 2020, 23, 700–711. [Google Scholar]
- Blokland, A.; Heckman, P.; Vanmierlo, T.; Schreiber, R.; Paes, D.; Prickaerts, J. Phosphodiesterase type 4 inhibition in CNS diseases. TIPS 2019, 40, 971–985. [Google Scholar] [CrossRef]
- Lugnier, C.; Al-Kuraishy, H.M.; Rousseau, E. PDE4 inhibition as a therapeutic strategy for improvement of pulmonary dysfunctions in Covid-19 and cigarette smoking. Biochem. Pharmacol. 2021, 185, 114431. [Google Scholar] [PubMed]
- Angel, J.B.; Saget, B.M.; Walsh, S.P.; Greten, T.F.; Dinarello, C.A.; Skolnik, P.R.; Endres, S. Rolipram, a specific type IV phosphodiesterase inhibitor, is a potent inhibitor of HIV-1 replication. AIDS 1995, 9, 1137–1144. [Google Scholar] [PubMed]
- Navarro, J.; Punzon, C.; Jimenez, J.L.; Fernandez-Cruz, E.; Pizarro, A.; Fresno, M.; Muñoz-Fernández, M.A. Inhibition of phosphodiesterase type IV suppresses human immunodeficiency virus type 1 replication and cytokine production in primary T cells: Involvement of NF-κB and NFAT. J. Virol. 1998, 72, 4712–4720. [Google Scholar] [PubMed]
- Sun, Y.; Li, L.; Lau, F.; Beavo, J.A.; Clark, E.A. Infection of CD4+ memory T cells by HIV-1 requires expression of phosphodiesterase 4. J. Immunol. 2000, 165, 1755–1761. [Google Scholar] [CrossRef]
- Mata, M.; Martinez, I.; Melero, J.A.; Tenor, H.; Cortijo, J. Roflumilast inhibits respiratory syncytial virus infection in human differentiated bronchial epithelial cells. PLoS ONE 2013, 8, e69670. [Google Scholar] [CrossRef]
- Van Ly, D.; De Pedro, M.; James, P.; Morgan, L.; Black, J.L.; Burgess, J.K.; Oliver, B.G. Inhibition of phosphodiesterase 4 modulates cytokine induction from toll like receptor activated, but not rhinovirus infected, primary human airway smooth muscle. Respir. Res. 2013, 14, 127. [Google Scholar]
- Li, G.; Nunoya, J.I.; Cheng, L.; Reszka-Blanco, N.; Tsao, L.C.; Jeffrey, J.; Su, L. Regulatory T Cells Contribute to HIV-1 Reservoir persistence in CD4+ T cells through cyclic adenosine monophosphate–dependent mechanisms in humanized mice in vivo. J. Inf. Dis. 2017, 216, 1579–1591. [Google Scholar]
- Kuba, K.; Imai, Y.; Rao, S.; Gao, H.; Feng Guo, F.; Guan, B.; Huan, Y.; Yang, P.; Zhang, Y.; Deng, W.; et al. A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS Coronavirus-Induced lung injury. Nat. Med. 2005, 11, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Dalamaga, M.; Karampela, I.; Mantzoros, C.S. Commentary: Phosphodiesterase 4 inhibitors as potential adjunct treatment targeting the cytokine storm in COVID-19. Metabolism 2020, 109, 154282. [Google Scholar] [CrossRef] [PubMed]
- Chemboli, R.; Kapavarapu, R.; Deepti, K.; Prasad, K.R.S.; Reddy, A.G.; Kumar, A.V.D.N.; Rao, M.V.B.; Pal, M. Pyrrolo[2,3-b]quinoxalines in attenuating cytokine storm in COVID-19: Their sonochemical synthesis and in silico/in vitro assessment. J. Mol. Struct. 2021, 1230, 129868. [Google Scholar] [CrossRef] [PubMed]
- Bridgewood, C.; Damiani, G.; Sharif, K.; Watad, A.; Bragazzi, N.L.; Quartuccio, L.; Savic, S.; McGonagle, D. Rationale for evaluating PDE4 inhibition for mitigating against severe inflammation in COVID-19 pneumonia and beyond. Isr. Med. Assoc. J. 2020, 22, 335–339. [Google Scholar] [PubMed]
- Sugin Lal Jabaris, S.; Ranju, V. Scope of adjuvant therapy using roflumilast, a PDE-4 inhibitor against COVID-19. Pulm. Pharmacol. Ther. 2021, 66, 101978. [Google Scholar]
- Nguyen, H.O.; Schioppa, T.; Tiberio, L.; Facchinetti, F.; Villetti, G.; Civelli, M.; Del Prete, A.; Sozio, F.; Gaudenzi, C.; Passari, M.; et al. The PDE4 Inhibitor Tanimilast Blunts Proinflammatory Dendritic Cell Activation by SARS-CoV-2 ssRNAs. Front. Immunol. 2022, 12, 797390. [Google Scholar] [CrossRef]
- Geller, S.; Xu, H.; Lebwohl, M.; Nardone, B.; Lacouture, M.E.; Kheterpal, M. Malignancy risk and recurrence with psoriasis and its treatments: A concise update. Am. J. Clin. Dermatol. 2018, 19, 363–375. [Google Scholar] [CrossRef]
- Xu, B.; Qin, Y.; Li, D.; Cai, N.; Wu, J.; Jiang, L.; Jie, L.; Zhou, Z.; Xu, J.; Wang, H. Inhibition of PDE4 protects neurons against oxygen-glucose deprivation-induced endoplasmic reticulum stress through activation of the Nrf-2/HO-1 pathway. Redox Biol. 2020, 28, 101342. [Google Scholar] [CrossRef]
- Lugnier, C. PDE inhibitors: A new approach to treat metabolic syndrome? Curr. Opin. Pharmacol. 2011, 11, 698–706. [Google Scholar] [CrossRef]
- Klussmann, E. Protein–protein interactions of PDE4 family members–Functions, interactions and therapeutic value. Cell Signal. 2016, 28, 713–718. [Google Scholar] [CrossRef]
- Houslay, K.F.; Christian, F.; MacLeod, R.; Adams, D.R.; Houslay, M.D.; Baillie, G.S. Identification of a multifunctional docking site on the catalytic unit of phosphodiesterase-4 (PDE4) that is utilized by multiple interaction partners. Biochem. J. 2017, 474, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Baillie, G.S.; Tejeda, G.S.; Kelly, M.P. Therapeutic targeting of 3′,5′-cyclic nucleotide phosphodiesterases: Inhibition and beyond. Nat. Drug Discov. 2019, 18, 770–796. [Google Scholar] [CrossRef] [PubMed]
- Viña, D.; Seoane, N.; Vasquez, E.C.; Campos-Toimil, M. cAMP compartmentalization in cerebrovascular endothelial cells: New therapeutic opportunities in Alzheimer’s disease. Cells 2021, 10, 1951. [Google Scholar] [CrossRef] [PubMed]
- Schick, M.A.; Schlegel, N. Clinical implication of phosphodiesterase-4-inhibition. Int. J. Mol. Sci. 2022, 23, 1209. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lugnier, C. The Complexity and Multiplicity of the Specific cAMP Phosphodiesterase Family: PDE4, Open New Adapted Therapeutic Approaches. Int. J. Mol. Sci. 2022, 23, 10616. https://doi.org/10.3390/ijms231810616
Lugnier C. The Complexity and Multiplicity of the Specific cAMP Phosphodiesterase Family: PDE4, Open New Adapted Therapeutic Approaches. International Journal of Molecular Sciences. 2022; 23(18):10616. https://doi.org/10.3390/ijms231810616
Chicago/Turabian StyleLugnier, Claire. 2022. "The Complexity and Multiplicity of the Specific cAMP Phosphodiesterase Family: PDE4, Open New Adapted Therapeutic Approaches" International Journal of Molecular Sciences 23, no. 18: 10616. https://doi.org/10.3390/ijms231810616
APA StyleLugnier, C. (2022). The Complexity and Multiplicity of the Specific cAMP Phosphodiesterase Family: PDE4, Open New Adapted Therapeutic Approaches. International Journal of Molecular Sciences, 23(18), 10616. https://doi.org/10.3390/ijms231810616