The Current Status of Research on High-Density Lipoproteins (HDL): A Paradigm Shift from HDL Quantity to HDL Quality and HDL Functionality
Abstract
:1. Introduction: What Are HDL Quantities, Qualities, and Functionalities?
2. Change in HDL-C Quantity during One’s Lifetime
2.1. Change in HDL-C Quantity between Gender during One’s Lifetime
2.2. Pubertal Change in HDL-C in Men
2.3. Menopausal Change of HDL-C in Women
2.4. Exercise and Change in HDL
2.5. Nutritional Supplementation and the Change in HDL
2.5.1. Omega-3 Consumption
2.5.2. Consumption of Policosanol from Sugarcane Wax
3. HDL Quality
3.1. HDL Particle Shape, Size, and Antioxidant Ability
3.2. ApoA-I Content in HDL
3.3. ApoA-II Content in HDL
3.4. ApoC-III Content in HDL
3.5. α-Synuclein, β-Amyloid, and Serum Amyloid A in HDL
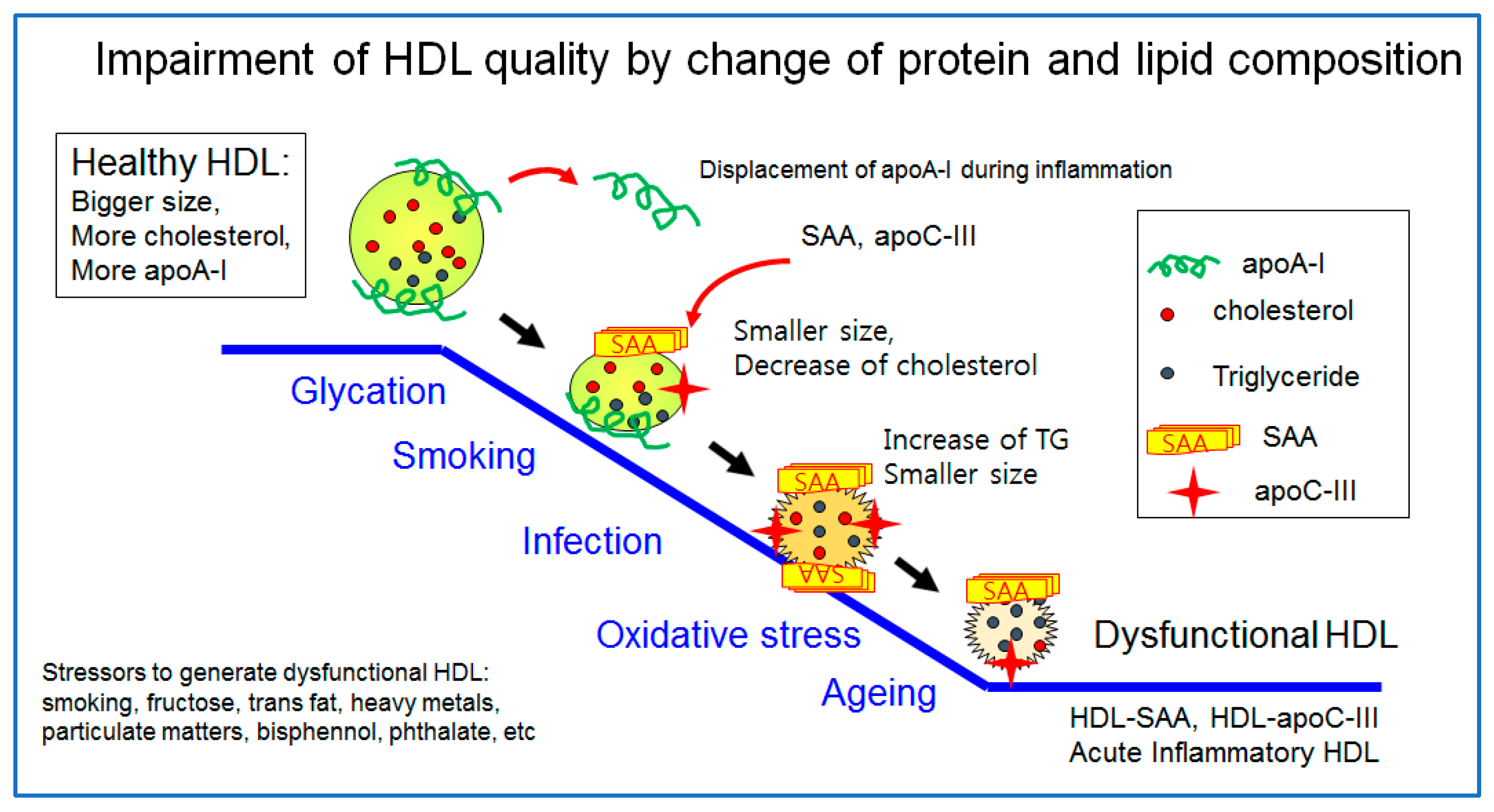
3.6. Lipid Compositions in HDL
3.7. The Best HDL versus the Worst HDL
4. HDL Functionality
4.1. Overview
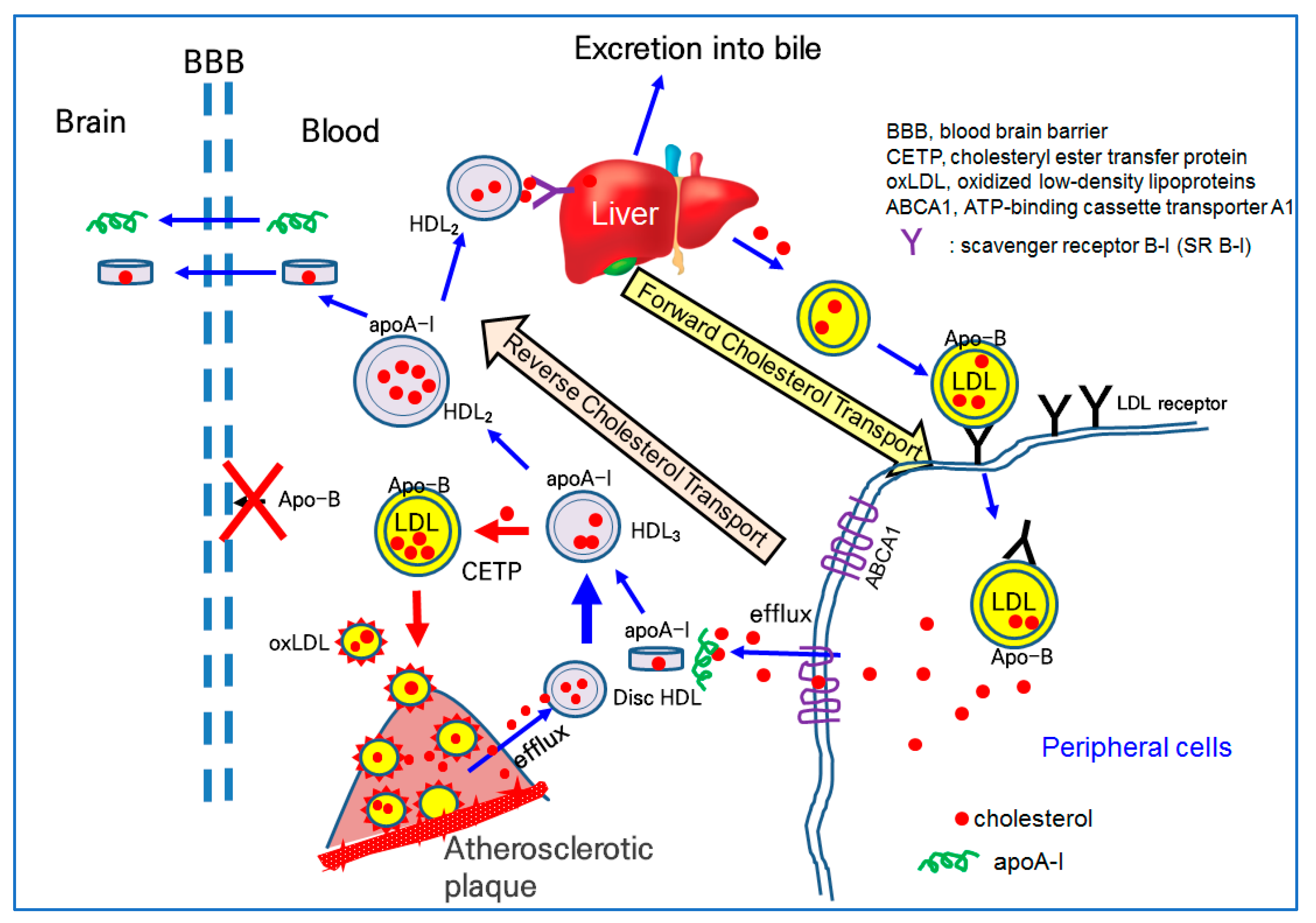
4.2. Cholesterol Efflux and the Treatment of Dyslipidemia via Reverse Cholesterol Transport (RCT)
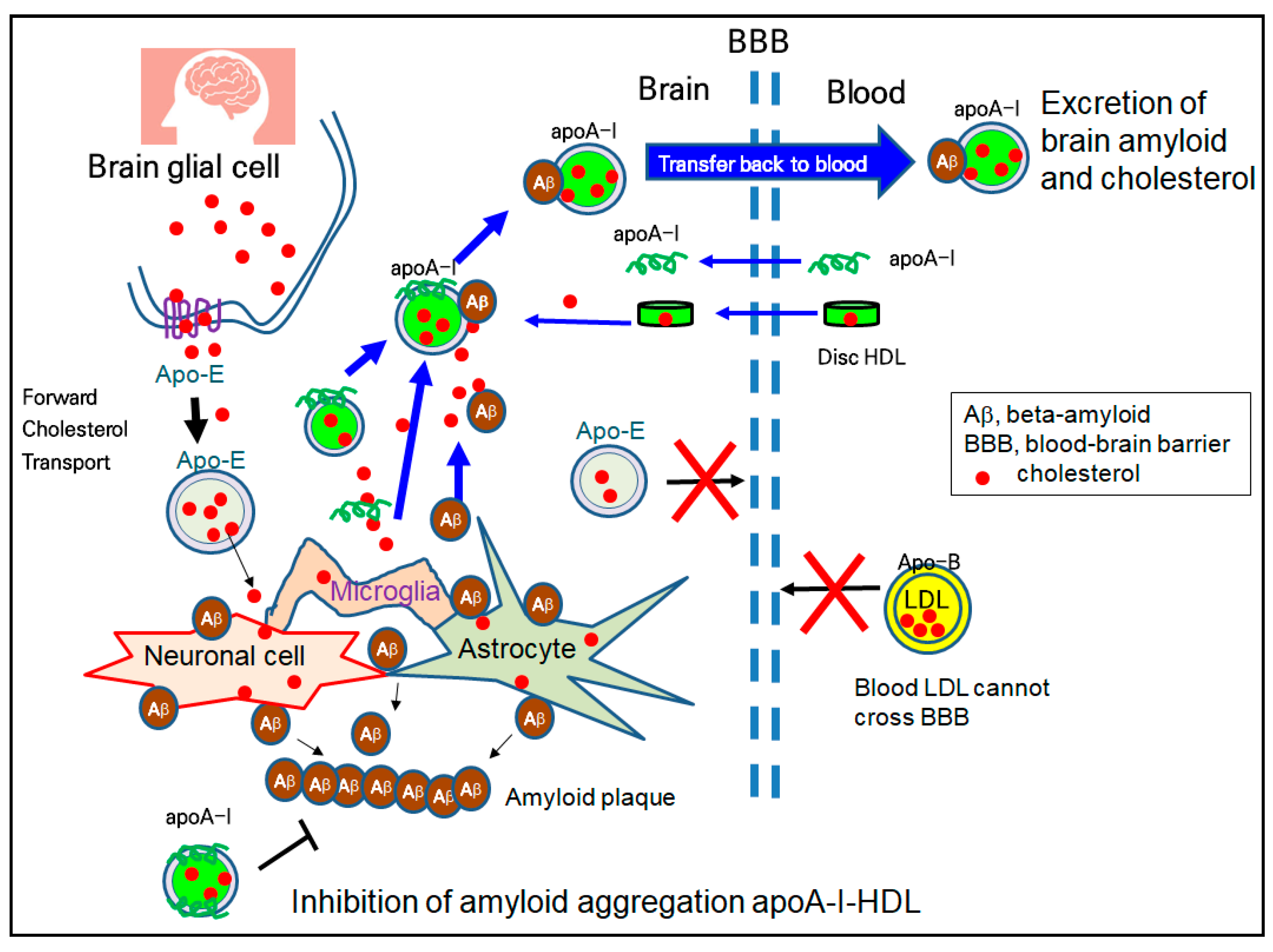
4.3. Treatment of Alzheimer’s Disease via Amyloid Removal
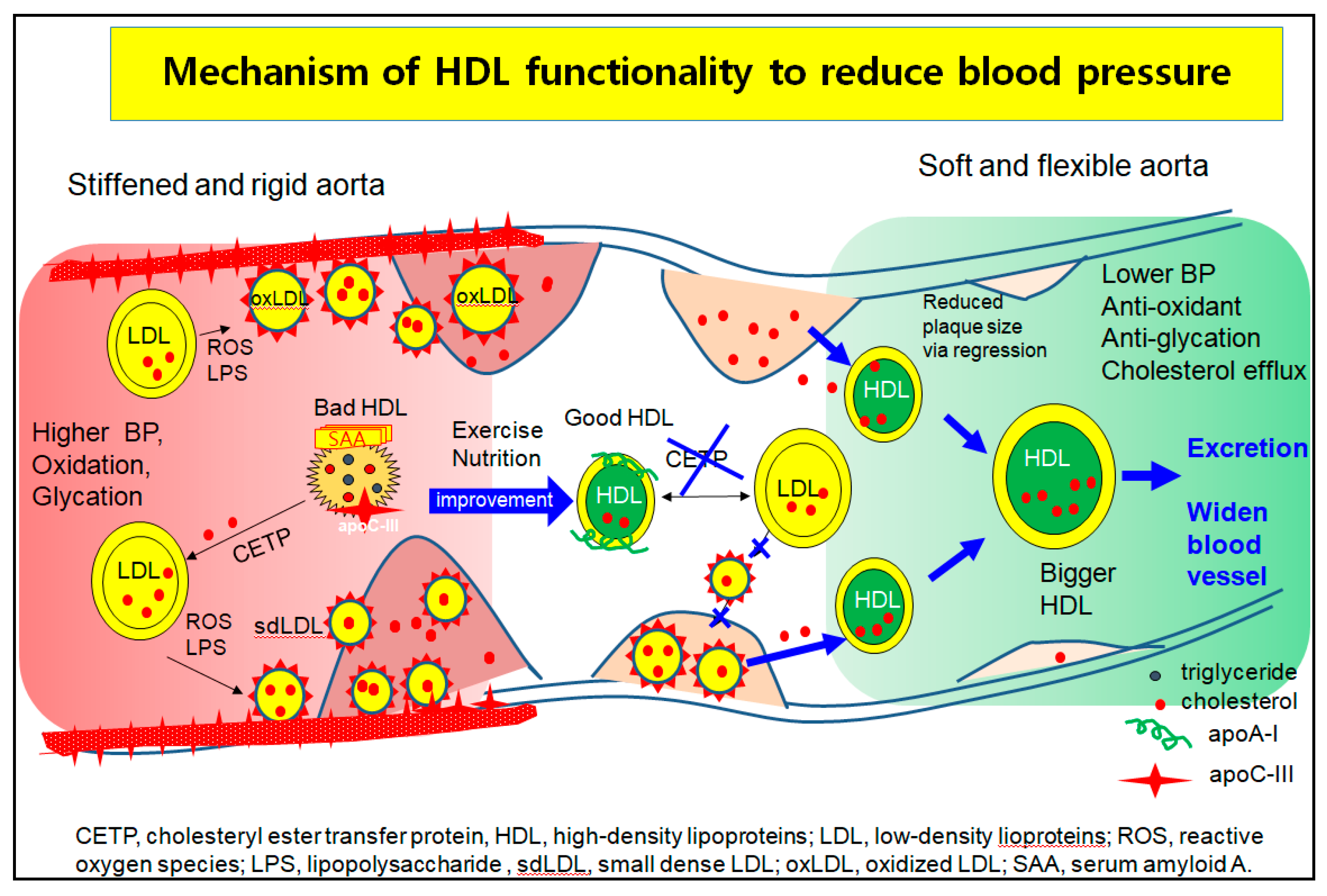
4.4. Treatment of Hypertension via the Regression of Plaque and Reduction in Aortic Stiffness
4.5. Application of HDL
5. Conclusions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cho, K.H. High-Density Lipoproteins as Biomarkers and Therapeutic Tools: Volume 1. Impacts of Lifestyle, Diseases, and Environmental Stressors on HDL, 1st ed.; Springer: New York, NY, USA, 2019. [Google Scholar]
- Cho, K.H. High-Density Lipoproteins as Biomarkers and Therapeutic Tools: Volume 2. Improvement and Enhancement of HDL and Clinical Applications, 1st ed.; Springer: New York, NY, USA, 2019. [Google Scholar]
- Holvoet, P.; De Keyzer, D.; Jacobs, D.R., Jr. Oxidized LDL and the metabolic syndrome. Future Lipidol. 2008, 3, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Khismatullin, D.B. Oxidized low-density lipoprotein contributes to atherogenesis via co-activation of macrophages and mast cells. PLoS ONE 2015, 10, e0123088. [Google Scholar] [CrossRef] [PubMed]
- Hottman, D.A.; Chernick, D.; Cheng, S.; Wang, Z.; Li, L. HDL and cognition in neurodegenerative disorders. Neurobiol. Dis. 2014, 72, 22–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rysz, J.; Gluba-Brzózka, A.; Rysz-Górzyńska, M.; Franczyk, B. The role and function of HDL in patients with chronic kidney disease and the risk of cardiovascular disease. Int. J. Mol. Sci. 2020, 21, 601. [Google Scholar] [CrossRef] [Green Version]
- Masana, L.; Correig, E.; Ibarretxe, D.; Anoro, E.; Arroyo, J.A.; Jericó, C.; Guerrero, C.; Miret, M.; Näf, S.; Pardo, A.; et al. HDL and high triglycerides predict COVID-19 severity. Sci. Rep. 2021, 11, 7217. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Q.; Zhao, X.; Dong, H.; Wu, C.; Wu, F.; Yu, B.; Lv, J.; Zhang, S.; Wu, G.; et al. Low high-density lipoprotein level is correlated with the severity of COVID-19 patients: An observational study. Lipids Health Dis. 2020, 19, 204. [Google Scholar] [CrossRef]
- Feingold, K.R. The bidirectional link between HDL and COVID-19 infections. J. Lipid Res. 2021, 62, 100067. [Google Scholar] [CrossRef]
- Cho, K.H.; Kim, J.R.; Lee, I.C.; Kwon, H.J. Native high-density lipoproteins (HDL) with higher paraoxonase exerts a potent antiviral effect against SARS-CoV-2 (COVID-19), while glycated HDL lost the antiviral activity. Antioxidants 2021, 10, 209. [Google Scholar] [CrossRef]
- Fedder, D.O.; Koro, C.E.; L’Italien, G.J. New national cholesterol education program III guidelines for primary prevention lipid-lowering drug therapy: Projected impact on the size, sex, and age distribution of the treatment-eligible population. Circulation 2002, 105, 152–156. [Google Scholar] [CrossRef] [Green Version]
- Rezapour, M.; Shahesmaeili, A.; Hossinzadeh, A.; Zahedi, R.; Najafipour, H.; Gozashti, M.H. Comparison of lipid ratios to identify metabolic syndrome. Arch. Iran. Med. 2018, 21, 572–577. [Google Scholar]
- Murguía-Romero, M.; Jiménez-Flores, J.R.; Sigrist-Flores, S.C.; Espinoza-Camacho, M.A.; Jiménez-Morales, M.; Piña, E.; Méndez-Cruz, A.R.; Villalobos-Molina, R.; Reaven, G.M. Plasma triglyceride/HDL-cholesterol ratio, insulin resistance, and cardiometabolic risk in young adults. J. Lipid Res. 2013, 54, 2795–2799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, K.H.; Park, H.J.; Kim, J.R. Decrease in serum HDL-C level is associated with elevation of blood pressure: Correlation analysis from the Korean National Health and Nutrition Examination Survey 2017. Int. J. Environ. Res. Public Health 2020, 17, 1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemieux, I.; Lamarche, B.; Couillard, C.; Pascot, A.; Cantin, B.; Bergeron, J.; Dagenais, G.R.; Després, J.P. Total cholesterol/HDL cholesterol ratio vs LDL cholesterol/HDL cholesterol ratio as indices of ischemic heart disease risk in men: The Quebec Cardiovascular Study. Arch. Intern. Med. 2001, 161, 2685–2692. [Google Scholar] [CrossRef] [PubMed]
- Reilly, M.P.; Rader, D.J. The metabolic syndrome: More than the sum of its parts? Circulation 2003, 108, 1546–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svensson, T.; Sawada, N.; Mimura, M.; Nozaki, S.; Shikimoto, R.; Tsugane, S. The association between midlife serum high-density lipoprotein and mild cognitive impairment and dementia after 19 years of follow-up. Transl. Psychiatry 2019, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Singh-Manoux, A.; Gimeno, D.; Kivimaki, M.; Brunner, E.; Marmot, M.G. Low HDL cholesterol is a risk factor for deficit and decline in memory in midlife: The Whitehall II study. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1556–1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, K.H.; Park, H.J.; Kim, S.J.; Kim, J.R. Decrease in HDL-C is associated with age and household income in adults from the Korean National Health and Nutrition Examination Survey 2017: Correlation analysis of low HDL-C and poverty. Int. J. Environ. Res. Public Health 2019, 16, 3329. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, A.; Barrett-Connor, E.; Shan, J. Total, LDL, and HDL cholesterol decrease with age in older men and women. The Rancho Bernardo Study. 1984–1994. Circulation 1997, 96, 37–43. [Google Scholar] [CrossRef]
- Shimakawa, T.; Sorlie, P.; Carpenter, M.A.; Dennis, B.; Tell, G.S.; Watson, R.; Williams, O.D. Dietary intake patterns and sociodemographic factors in the atherosclerosis risk in communities study. ARIC Study Investigators. Prev. Med. 1994, 23, 769–780. [Google Scholar] [CrossRef]
- Cho, K.H.; Kim, J.R. Rapid decrease in HDL-C in the puberty period of boys associated with an elevation of blood pressure and dyslipidemia in korean teenagers: An explanation of why and when men have lower HDL-C levels than women. Med. Sci. 2021, 9, 35. [Google Scholar] [CrossRef]
- Kannel, W.B.; Dawber, T.R.; Friedman, G.D.; Glennon, W.E.; McNamara, P.M. Risk factors in coronary heart disease. an evaluation of several serum lipids as predictors of coronary heart disease; the Framingham study. Ann. Intern. Med. 1964, 61, 888–899. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Park, H.A.; Cho, Y.G.; Kang, J.H.; Kim, K.W.; Kang, J.H.; Kim, N.R.; Chung, W.C.; Kim, C.H.; Whang, D.H.; et al. Gender Difference in the Level of HDL Cholesterol in Korean Adults. Korean J. Fam. Med. 2011, 32, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Gorman, B.K.; Read, J. Why men die younger than women. Geriatr. Aging 2007, 10, 179–181. [Google Scholar]
- Fofana, M.; Maboundou, J.C.; Bocquet, J.; Le Goff, D. Transfer of cholesterol between high density lipoproteins and cultured rat Sertoli cells. Biochem. Cell Biol. 1996, 74, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sun, R.; Chen, Q.; Guo, Q.; Wang, J.; Lu, L.; Zhang, Y. Association between HDL-C levels and menopause: A meta-analysis. Hormones 2021, 20, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Collins, P. HDL-C in post-menopausal women: An important therapeutic target. Int. J. Cardiol. 2008, 124, 275–282. [Google Scholar] [CrossRef]
- Matthews, K.A.; Meilahn, E.; Kuller, L.H.; Kelsey, S.F.; Caggiula, A.W.; Wing, R.R. Menopause and risk factors for coronary heart disease. N. Engl. J. Med. 1989, 321, 641–646. [Google Scholar] [CrossRef]
- Pardhe, B.D.; Ghimire, S.; Shakya, J.; Pathak, S.; Shakya, S.; Bhetwal, A.; Khanal, P.R.; Parajuli, N.P. Elevated cardiovascular risks among postmenopausal women: A community based case control study from Nepal. Biochem. Res. Int. 2017, 2017, 3824903. [Google Scholar] [CrossRef]
- Kannel, W.B.; Wilson, P.W. Risk factors that attenuate the female coronary disease advantage. Arch. Intern. Med. 1995, 155, 57–61. [Google Scholar] [CrossRef]
- Matthan, N.R.; Jalbert, S.M.; Lamon-Fava, S.; Dolnikowski, G.G.; Welty, F.K.; Barrett, H.R.; Schaefer, E.J.; Lichtenstein, A.H. TRL, IDL, and LDL apolipoprotein B-100 and HDL apolipoprotein A-I kinetics as a function of age and menopausal status. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1691–1696. [Google Scholar] [CrossRef]
- Li, Z.; McNamara, J.R.; Fruchart, J.C.; Luc, G.; Bard, J.M.; Ordovas, J.M.; Wilson, P.W.; Schaefer, E.J. Effects of gender and menopausal status on plasma lipoprotein subspecies and particle sizes. J. Lipid Res. 1996, 37, 1886–1896. [Google Scholar] [CrossRef]
- El Khoudary, S.R.; Hutchins, P.M.; Matthews, K.A.; Brooks, M.M.; Orchard, T.J.; Ronsein, G.E.; Heinecke, J.W. Cholesterol efflux capacity and subclasses of HDL particles in healthy women transitioning through menopause. J. Clin. Endocrinol. Metab. 2016, 101, 3419–3428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Khoudary, S.R.; Ceponiene, I.; Samargandy, S.; Stein, J.H.; Li, D.; Tattersall, M.C.; Budoff, M.J. HDL (High-Density Lipoprotein) metrics and atherosclerotic risk in women. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 2236–2244. [Google Scholar] [CrossRef] [PubMed]
- Julian, V.; Bergsten, P.; Forslund, A.; Ahlstrom, H.; Ciba, I.; Dahlbom, M.; Furthner, D.; Gomahr, J.; Kullberg, J.; Maruszczak, K.; et al. Sedentary time has a stronger impact on metabolic health than moderate to vigorous physical activity in adolescents with obesity: A cross-sectional analysis of the Beta-JUDO study. Pediatr. Obes. 2022, e12897. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, P. The effects of different exercise modalities in the treatment of cardiometabolic risk factors in obese adolescents with sedentary behavior-a Systematic review and meta-analysis of randomized controlled trials. Children 2021, 8, 1062. [Google Scholar] [CrossRef] [PubMed]
- Karacabey, K. The effect of exercise on leptin, insulin, cortisol and lipid profiles in obese children. J. Int. Med. Res. 2009, 37, 1472–1478. [Google Scholar] [CrossRef] [Green Version]
- Rowland, T.W.; Mattel, L.; Vanderburgh, P.; Manos, T.; Charkoudian, N. The influence of short term aerobic training on blood lipids in healthy 10–12 year old children. Int. J. Sports Med. 1996, 17, 487–492. [Google Scholar] [CrossRef]
- Prabhakaran, B.; Dowling, E.A.; Branch, J.D.; Swain, D.P.; Leutholtz, B.C. Effect of 14 weeks of resistance training on lipid profile and body fat percentage in premenopausal women. Br. J. Sports Med. 1999, 33, 190–195. [Google Scholar] [CrossRef]
- Zhao, S.; Zhong, J.; Sun, C.; Zhang, J. Effects of aerobic exercise on TC, HDL-C, LDL-C and TG in patients with hyperlipidemia: A protocol of systematic review and meta-analysis. Medicine 2021, 100, e25103. [Google Scholar] [CrossRef]
- LeMura, L.M.; von Duvillard, S.P.; Andreacci, J.; Klebez, J.M.; Chelland, S.A.; Russo, J. Lipid and lipoprotein profiles, cadiovascular fitness, body composition, and diet during and after resistance, aerobic and combination training in young women. Eur. J. Appl. Physiol. 2000, 82, 451–458. [Google Scholar] [CrossRef]
- Liang, M.; Pan, Y.; Zhong, T.; Zeng, Y.; Cheng, A.S.K. Effects of aerobic, resistance, and combined exercise on metabolic syndrome parameters and cardiovascular risk factors: A systematic review and network meta-analysis. Rev. Cardiovasc. Med. 2021, 22, 1523–1533. [Google Scholar] [CrossRef]
- Lee, H.; Park, J.E.; Choi, I.; Cho, K.H. Enhanced functional and structural properties of high-density lipoproteins from runners and wrestlers compared to throwers and lifters. BMB Rep. 2009, 42, 605–610. [Google Scholar] [CrossRef] [Green Version]
- Franceschini, G.; Calabresi, L.; Maderna, P.; Galli, C.; Gianfranceschi, G.; Sirtori, C.R. Omega-3 fatty acids selectively raise high-density lipoprotein 2 levels in healthy volunteers. Metabolism 1991, 40, 1283–1286. [Google Scholar] [CrossRef]
- Kouchaki, E.; Afarini, M.; Abolhassani, J.; Mirhosseini, N.; Bahmani, F.; Masoud, S.A.; Asemi, Z. High-dose omega-3 fatty acid plus vitamin D3 supplementation affects clinical symptoms and metabolic status of patients with multiple sclerosis: A randomized controlled clinical trial. J. Nutr. 2018, 148, 1380–1386. [Google Scholar] [CrossRef] [Green Version]
- Manson, J.E.; Cook, N.R.; Lee, I.M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Albert, C.M.; Gordon, D.; Copeland, T.; et al. Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N. Engl. J. Med. 2019, 380, 23–32. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T., Jr.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular risk reduction with icosapent ethyl for hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Lincoff, A.M.; Garcia, M.; Bash, D.; Ballantyne, C.M.; Barter, P.J.; Davidson, M.H.; Kastelein, J.J.P.; Koenig, W.; McGuire, D.K.; et al. Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: The STRENGTH randomized clinical trial. JAMA 2020, 324, 2268–2280. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Maki, K.C.; Bays, H.E.; Aguilera, F.; Gould, G.; Hegele, R.A.; Moriarty, P.M.; Robinson, J.G.; Shi, P.; Tur, J.F.; et al. Effectiveness of a novel ω-3 krill oil agent in patients with severe hypertriglyceridemia: A randomized clinical trial. JAMA Netw. Open 2022, 5, e2141898. [Google Scholar] [CrossRef]
- Gong, J.; Qin, X.; Yuan, F.; Hu, M.; Chen, G.; Fang, K.; Wang, D.; Jiang, S.; Li, J.; Zhao, Y.; et al. Efficacy and safety of sugarcane policosanol on dyslipidemia: A meta-analysis of randomized controlled trials. Mol. Nutr. Food Res. 2018, 62, 1700280. [Google Scholar] [CrossRef]
- Lim, S.M.; Yoo, J.A.; Lee, E.Y.; Cho, K.H. Enhancement of high-density lipoprotein cholesterol functions by encapsulation of policosanol exerts anti-senescence and tissue regeneration effects via improvement of anti-glycation, anti-apoptosis, and cholesteryl ester transfer inhibition. Rejuvenation Res. 2016, 19, 59–70. [Google Scholar] [CrossRef]
- Lee, E.Y.; Yoo, J.A.; Lim, S.M.; Cho, K.H. Anti-aging and tissue regeneration ability of policosanol along with lipid-lowering effect in hyperlipidemic zebrafish via enhancement of high-density lipoprotein functionality. Rejuvenation Res. 2016, 19, 149–158. [Google Scholar] [CrossRef] [Green Version]
- Potter, L.K.; Sprecher, D.L.; Walker, M.C.; Tobin, F.L. Mechanism of inhibition defines CETP activity: A mathematical model for CETP in vitro. J. Lipid Res. 2009, 50, 2222–2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, D.K.; Li, L.; Porter, T.D. Policosanol inhibits cholesterol synthesis in hepatoma cells by activation of AMP-kinase. J. Pharmacol. Exp. Ther. 2006, 318, 1020–1026. [Google Scholar] [CrossRef] [Green Version]
- Jo, A.L.; Han, J.W.; An, J.I.; Cho, K.H.; Jeoung, N.H. Cuban policosanol prevents the apoptosis and the mitochondrial dysfunction induced by lipopolysaccharide in C2C12 myoblast via activation of Akt and Erk pathways. J. Nutr. Sci. Vitaminol. 2022, 68, 79–86. [Google Scholar]
- McCarty, M.F. Policosanol safely down-regulates HMG-CoA reductase—potential as a component of the Esselstyn regimen. Med. Hypotheses 2002, 59, 268–279. [Google Scholar] [CrossRef]
- Kim, K.M.; Kim, C.H.; Cho, K.H.; Jang, W.G. Policosanol attenuates Pi-induced calcification via AMPK-mediated INSIGs expression in rat VSMCs. Clin. Exp. Pharmacol. Physiol. 2021, 48, 1336–1345. [Google Scholar] [CrossRef]
- Cho, K.H.; Kim, S.J.; Yadav, D.; Kim, J.Y.; Kim, J.R. Consumption of cuban policosanol improves blood pressure and lipid profile via enhancement of HDL functionality in healthy women subjects: Randomized, double-blinded, and placebo-controlled study. Oxid. Med. Cell. Longev. 2018, 2018, 4809525. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Yadav, D.; Park, H.J.; Kim, J.R.; Cho, K.H. Long-term consumption of cuban policosanol lowers central and brachial blood pressure and improves lipid profile with enhancement of lipoprotein properties in healthy korean participants. Front. Physiol. 2018, 9, 412. [Google Scholar] [CrossRef] [Green Version]
- Park, H.J.; Yadav, D.; Jeong, D.J.; Kim, S.J.; Bae, M.A.; Kim, J.R.; Cho, K.H. Short-term consumption of cuban policosanol lowers aortic and peripheral blood pressure and ameliorates serum lipid parameters in healthy korean participants: Randomized, double-blinded, and placebo-controlled study. Int. J. Environ. Res. Public Health. 2019, 16, 809. [Google Scholar] [CrossRef] [Green Version]
- Groenen, A.G.; Bazioti, V.; van Zeventer, I.A.; Chen, L.; Groot, H.E.; Balder, J.W.; Zhernakova, A.; van der Harst, P.; Rimbert, A.; Kuivenhoven, J.A.; et al. Large HDL particles negatively associate with leukocyte counts independent of cholesterol efflux capacity: A cross sectional study in the population-based LifeLines DEEP cohort. Atherosclerosis. 2022, 343, 20–27. [Google Scholar] [CrossRef]
- Coller, B.S. Leukocytosis and ischemic vascular disease morbidity and mortality: Is it time to intervene? Arterioscler. Thromb. Vasc. Biol. 2005, 25, 658–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaneko, Y.; Takeuchi, T. Interluekin-6 inhibitors for the treatment of adult-onset Still’s disease. Expert Opin. Biol. Ther. 2022, 32, 12–15. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, E.Y.; Park, J.K.; Song, Y.W.; Kim, J.R.; Cho, K.H. Patients with rheumatoid arthritis show altered lipoprotein profiles with dysfunctional high-density lipoproteins that can exacerbate inflammatory and atherogenic process. PLoS ONE 2016, 11, e0164564. [Google Scholar] [CrossRef]
- Vargas, J.I.; Rivera, K.; Arrese, M.; Benitez, C.; Barrera, F.; Hugo, M.; Arab, J.P.; Pino, K.; Barrera, A.; Lopez-Lastra, M.; et al. LDL particle size and antioxidant HDL function improve after sustained virological response in patients with chronic HCV. Ann. Hepatol. 2022, 27, 100555. [Google Scholar] [CrossRef]
- Ali, E.M.; Shehata, H.H.; Ali-Labib, R.; Esmail Zahra, L.M. Oxidant and antioxidant of arylesterase and paraoxonase as biomarkers in patients with hepatitis C virus. Clin. Biochem. 2009, 42, 1394–1400. [Google Scholar] [CrossRef]
- Li, Y.; Zhai, R.; Li, H.; Mei, X.; Qiu, G. Prognostic value of serum paraoxonase and arylesterase activity in patients with sepsis. J. Int. Med. Res. 2013, 41, 681–687. [Google Scholar] [CrossRef]
- Wei, X.; Zeng, W.; Su, J.; Wan, H.; Yu, X.; Cao, X.; Tan, W.; Wang, H. Hypolipidemia is associated with the severity of COVID-19. J. Clin. Lipidol. 2020, 14, 297–304. [Google Scholar] [CrossRef]
- Agouridis, A.P.; Pagkali, A.; Zintzaras, E.; Rizos, E.C.; Ntzani, E.E. High-density lipoprotein cholesterol: A marker of COVID-19 infection severity? Atheroscler. Plus 2021, 44, 1–9. [Google Scholar] [CrossRef]
- Kluck, G.E.G.; Yoo, J.A.; Sakarya, E.H.; Trigatti, B.L. Good Cholesterol Gone Bad? HDL and COVID-19. Int. J. Mol. Sci. 2021, 22, 10182. [Google Scholar] [CrossRef]
- Hafiane, A.; Gianopoulos, I.; Sorci-Thomas, M.G.; Daskalopoulou, S.S. Current models of apolipoprotein A-I lipidation by adenosine triphosphate binding cassette transporter A1. Curr. Opin. Lipidol. 2022, 33, 139–145. [Google Scholar] [CrossRef]
- Brites, F.; Martin, M.; Guillas, I.; Kontush, A. Antioxidative activity of high-density lipoprotein (HDL): Mechanistic insights into potential clinical benefit. BBA Clin. 2017, 8, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Kontush, A. HDL particle number and size as predictors of cardiovascular disease. Front. Pharmacol. 2015, 6, 218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walldius, G.; de Faire, U.; Alfredsson, L.; Leander, K.; Westerholm, P.; Malmström, H.; Ivert, T.; Hammar, N. Long-term risk of a major cardiovascular event by apoB, apoA-1, and the apoB/apoA-1 ratio-Experience from the Swedish AMORIS cohort: A cohort study. PLoS Med. 2021, 18, e1003853. [Google Scholar] [CrossRef]
- Pownall, H.J.; Morrisett, J.D.; Sparrow, J.T.; Smith, L.C.; Shepherd, J.; Jackson, R.L.; Gotto, A.M., Jr. A review of the unique features of HDL apoproteins. Lipids 1979, 14, 428–434. [Google Scholar] [CrossRef]
- Aleksandrovich, O.V.; Ozerova, I.N.; Olfer’ev, A.M.; Serdyuk, A.P.; Metel’skaya, V.A.; Perova, N.V. Association of serum apolipoprotein A-II concentration with combined hyperlipidemia and impaired glucose tolerance. Bull. Exp. Biol. Med. 2006, 141, 678–681. [Google Scholar] [CrossRef]
- Ribas, V.; Sánchez-Quesada, J.L.; Antón, R.; Camacho, M.; Julve, J.; Escolà-Gil, J.C.; Vila, L.; Ordóñez-Llanos, J.; Blanco-Vaca, F. Human apolipoprotein A-II enrichment displaces paraoxonase from HDL and impairs its antioxidant properties: A new mechanism linking HDL protein composition and antiatherogenic potential. Circ. Res. 2004, 95, 789–797. [Google Scholar] [CrossRef]
- Cho, K.H. Importance of apolipoprotein A-I and A-II composition in HDL and its potential for studying COVID-19 and SARS-CoV-2. Medicines 2021, 8, 38. [Google Scholar] [CrossRef]
- Durbin, D.M.; Jonas, A. The effect of apolipoprotein A-II on the structure and function of apolipoprotein A-I in a homogeneous reconstituted high density lipoprotein particle. J. Biol. Chem. 1997, 272, 31333–31339. [Google Scholar] [CrossRef] [Green Version]
- De Beer, M.C.; Durbin, D.M.; Cai, L.; Mirocha, N.; Jonas, A.; Webb, N.R.; de Beer, F.C.; Van der Westhuyzen, D.R. Apolipoprotein A-II modulates the binding and selective lipid uptake of reconstituted high density lipoprotein by scavenger receptor BI. J. Biol. Chem. 2001, 276, 15832–15839. [Google Scholar] [CrossRef] [Green Version]
- Branchi, A.; Rovellini, A.; Tomella, C.; Sciariada, L.; Torri, A.; Molgora, M.; Sommariva, D. Association of alcohol consumption with HDL subpopulations defined by apolipoprotein A-I and apolipoprotein A-II content. Eur. J. Clin. Nutr. 1997, 51, 362–365. [Google Scholar] [CrossRef] [Green Version]
- Kido, T.; Kurata, H.; Kondo, K.; Itakura, H.; Okazaki, M.; Urata, T.; Yokoyama, S. Bioinformatic analysis of plasma Apolipoproteins A-I and A-II revealed unique features of A-I/A-II HDL particles in human plasma. Sci. Rep. 2016, 6, 31532. [Google Scholar] [CrossRef] [Green Version]
- Kido, T.; Kondo, K.; Kurata, H.; Fujiwara, Y.; Urata, T.; Itakura, H.; Yokoyama, S. ApoA-I/A-II-HDL positively associates with apoB-lipoproteins as a potential atherogenic indicator. Lipids Health Dis. 2017, 16, 225. [Google Scholar] [CrossRef] [Green Version]
- Shachter, N.S. Apolipoproteins C-I and C-III as important modulators of lipoprotein metabolism. Curr. Opin. Lipidol. 2001, 12, 297–304. [Google Scholar] [CrossRef]
- Luo, M.; Liu, A.; Wang, S.; Wang, T.; Hu, D.; Wu, S.; Peng, D. ApoCIII enrichment in HDL impairs HDL-mediated cholesterol efflux capacity. Sci. Rep. 2017, 7, 2312. [Google Scholar] [CrossRef] [Green Version]
- Park, K.H.; Shin, D.G.; Kim, J.R.; Cho, K.H. Senescence-related truncation and multimerization of apolipoprotein A-I in high-density lipoprotein with an elevated level of advanced glycated end products and cholesteryl ester transfer activity. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 600–610. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.H. Synthesis of reconstituted high density lipoprotein (rHDL) containing apoA-I and apoC-III: The functional role of apoC-III in rHDL. Mol. Cells 2009, 27, 291–297. [Google Scholar] [CrossRef]
- Park, K.H.; Shin, D.G.; Cho, K.H. Dysfunctional lipoproteins from young smokers exacerbate cellular senescence and atherogenesis with smaller particle size and severe oxidation and glycation. Toxicol. Sci. 2014, 140, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Panin, L.E.; Shalbueva, N.I.; Polyakov, L.M. Effects of apolipoproteins C on oxidative phosphorylation in rat liver mitochondria. Bull. Exp. Biol. Med. 2000, 130, 769–771. [Google Scholar] [CrossRef]
- Zewinger, S.; Reiser, J.; Jankowski, V.; Alansary, D.; Hahm, E.; Triem, S.; Klug, M.; Schunk, S.J.; Schmit, D.; Kramann, R.; et al. Apolipoprotein C3 induces inflammation and organ damage by alternative inflammasome activation. Nat. Immunol. 2020, 21, 30–41. [Google Scholar] [CrossRef]
- Cho, K.H.; Park, S.H.; Park, J.E.; Kim, Y.O.; Choi, I.; Kim, J.J.; Kim, J.R. The function, composition, and particle size of high-density lipoprotein were severely impaired in an oliguric phase of hemorrhagic fever with renal syndrome patients. Clin. Biochem. 2008, 41, 56–64. [Google Scholar] [CrossRef]
- Kim, J.; Park, H.H.; Choi, I.; Kim, Y.O.; Cho, K.H. Severely modified lipoprotein properties without a change in cholesteryl ester transfer protein activity in patients with acute renal failure secondary to Hantaan virus infection. BMB Rep. 2010, 43, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Jin, U.; Park, S.J.; Park, S.M. Cholesterol metabolism in the brain and its association with Parkinson’s disease. Exp. Neurobiol. 2019, 28, 554–567. [Google Scholar] [CrossRef] [Green Version]
- Polymeropoulos, M.H.; Lavedan, C.; Leroy, E.; Ide, S.E.; Dehejia, A.; Dutra, A.; Pike, B.; Root, H.; Rubenstein, J.; Boyer, R.; et al. Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science 1997, 276, 2045–2047. [Google Scholar] [CrossRef] [Green Version]
- Hsiao, J.T.; Halliday, G.M.; Kim, W.S. α-Synuclein regulates neuronal cholesterol efflux. Molecules 2017, 22, 1769. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.H. Structural and functional changes of reconstituted high-density lipoprotein (HDL) by incorporation of α-synuclein: A potent antioxidant and anti-glycation activity of α-synuclein and apoA-I in HDL at high molar ratio of α-synuclein. Molecules 2021, 26, 7485. [Google Scholar] [CrossRef]
- Shih, Y.H.; Tsai, K.J.; Lee, C.W.; Shiesh, S.C.; Chen, W.T.; Pai, M.C.; Kuo, Y.M. Apolipoprotein C-III is an amyloid-β-binding protein and an early marker for Alzheimer’s disease. J. Alzheimers Dis. 2014, 41, 855–865. [Google Scholar] [CrossRef]
- Robert, J.; Stukas, S.; Button, E.; Cheng, W.H.; Lee, M.; Fan, J.; Wilkinson, A.; Kulic, I.; Wright, S.D.; Ellington, C.L. Reconstituted high-density lipoproteins acutely reduce soluble brain Aβ levels in symptomatic APP/PS1 mice. Biochim. Biophys. Acta 2016, 1862, 1027–1036. [Google Scholar] [CrossRef]
- Cho, K.H. Structural and Functional Impairments of Reconstituted High-Density Lipoprotein by Incorporation of Recombinant β-Amyloid42. Molecules 2021, 26, 4317. [Google Scholar] [CrossRef]
- Zuliani, G.; Cavalieri, M.; Galvani, M.; Volpato, S.; Cherubini, A.; Bandinelli, S.; Corsi, A.M.; Lauretani, F.; Guralnik, J.M.; Fellin, R.; et al. Relationship between low levels of high-density lipoprotein cholesterol and dementia in the elderly. The InChianti study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2010, 65, 559–564. [Google Scholar] [CrossRef] [Green Version]
- Shridas, P.; Patrick, A.C.; Tannock, L.R. Role of Serum Amyloid A in Abdominal Aortic Aneurysm and Related Cardiovascular Diseases. Biomolecules 2021, 11, 1883. [Google Scholar] [CrossRef]
- Malle, E.; De Beer, F.C. Human serum amyloid A (SAA) protein: A prominent acute-phase reactant for clinical practice. Eur. J. Clin. Investig. 1996, 26, 427–435. [Google Scholar] [CrossRef]
- Benditt, E.P.; Eriksen, N. Amyloid protein SAA is associated with high density lipoprotein from human serum. Proc. Natl. Acad. Sci. USA 1977, 74, 4025–4028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Ye, R.D. Serum amyloid A1: Structure, function and gene polymorphism. Gene 2016, 583, 48–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reisinger, A.C.; Schuller, M.; Sourij, H.; Stadler, J.T.; Hackl, G.; Eller, P.; Marsche, G. Impact of sepsis on high-Density lipoprotein metabolism. Front. Cell. Dev. Biol. 2022, 9, 795460. [Google Scholar] [CrossRef]
- Webb, N.R. High-Density Lipoproteins and Serum Amyloid A (SAA). Curr. Atheroscler. Rep. 2021, 23, 7. [Google Scholar] [CrossRef]
- Tohidi, M.; Hatami, M.; Hadaegh, F.; Azizi, F. Triglycerides and triglycerides to high-density lipoprotein cholesterol ratio are strong predictors of incident hypertension in Middle Eastern women. J. Hum. Hypertens. 2012, 26, 525–532. [Google Scholar] [CrossRef] [Green Version]
- Justin, B.N.; Turek, M.; Hakim, A.M. Heart disease as a risk factor for dementia. Clin. Epidemiol. 2013, 5, 135–145. [Google Scholar] [CrossRef] [Green Version]
- Deckers, K.; Schievink, S.H.J.; Rodriquez, M.M.F.; van Oostenbrugge, R.J.; van Boxtel, M.P.J.; Verhey, F.R.J.; Köhler, S. Coronary heart disease and risk for cognitive impairment or dementia: Systematic review and meta-analysis. PLoS ONE 2017, 12, e0184244. [Google Scholar] [CrossRef]
- Craft, S. The role of metabolic disorders in Alzheimer disease and vascular dementia. Arch. Neurol. 2009, 66, 300–305. [Google Scholar] [CrossRef] [Green Version]
- Rohrer, L.; Hersberger, M.; von Eckardstein, A. High density lipoproteins in the intersection of diabetes mellitus, inflammation and cardiovascular disease. Curr. Opin. Lipidol. 2004, 15, 269–278. [Google Scholar] [CrossRef]
- Kosmas, C.E.; Martinez, I.; Sourlas, A.; Bouza, K.V.; Campos, F.N.; Torres, V.; Montan, P.D.; Guzman, E. High-density lipoprotein (HDL) functionality and its relevance to atherosclerotic cardiovascular disease. Drugs Context 2018, 7, 212525. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, W. Molecular links between Alzheimer’s disease and diabetes mellitus. Neuroscience 2013, 250, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Chou, P.S.; Wu, M.N.; Yang, C.C.; Shen, C.T.; Yang, Y.H. Effect of advanced glycation end products on the progression of Alzheimer’s disease. J. Alzheimers Dis. 2019, 72, 191–197. [Google Scholar] [CrossRef]
- Liu, D.; Ji, L.; Zhang, D.; Tong, X.; Pan, B.; Liu, P.; Zhang, Y.; Huang, Y.; Su, J.; Willard, B.; et al. Nonenzymatic glycation of high-density lipoprotein impairs its anti-inflammatory effects in innate immunity. Diabetes Metab. Res. Rev. 2012, 28, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ramie, J.J.; Barber, J.L.; Sarzynski, M.A. Effects of exercise on HDL functionality. Curr. Opin. Lipidol. 2019, 30, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Cuchel, M.; Rader, D.J. Macrophage reverse cholesterol transport: Key to the regression of atherosclerosis? Circulation 2006, 113, 2548–2555. [Google Scholar] [CrossRef]
- Yancey, P.G.; Bortnick, A.E.; Kellner-Weibel, G.; de la Llera-Moya, M.; Phillips, M.C.; Rothblat, G.H. Importance of different pathways of cellular cholesterol efflux. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 712–719. [Google Scholar] [CrossRef]
- Barter, P.J.; Brewer, H.B., Jr.; Chapman, M.J.; Hennekens, C.H.; Rader, D.J.; Tall, A.R. Cholesteryl ester transfer protein: A novel target for raising HDL and inhibiting atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 160–167. [Google Scholar] [CrossRef]
- Koudinov, A.R.; Berezov, T.T.; Kumar, A.; Koudinova, N.V. Alzheimer’s amyloid b interaction with normal human plasma high density lipoprotein: Association with apolipoprotein and lipids. Clin. Chim. Acta 1998, 270, 75–84. [Google Scholar] [CrossRef]
- Paula-Lima, A.C.; Tricerri, M.A.; Brito-Moreira, J.; Bomfim, T.R.; Oliveira, F.F.; Magdesian, M.H.; Grinberg, L.T.; Panizzutti, R.; Ferreira, S.T. Human apolipoprotein A-I binds amyloid-beta and prevents Aβ-induced neurotoxicity. Int. J. Biochem. Cell. Biol. 2009, 41, 1361–1370. [Google Scholar] [CrossRef]
- Merched, A.; Xia, Y.; Visvikis, S.; Serot, J.M.; Siest, G. Decreased high-density lipoprotein cholesterol and serum apolipoprotein AI concentrations are highly correlated with the severity of Alzheimer’s disease. Neurobiol. Aging 2000, 21, 27–30. [Google Scholar] [CrossRef]
- Lv, P.; Zhao, M.; Liu, Y.; Jin, H.; Cui, W.; Fan, C.; Teng, Y.; Zheng, L.; Huang, Y. Apolipoprotein C-III in the high-density lipoprotein proteome of cerebral lacunar infarction patients impairs its anti-inflammatory function. Int. J. Mol. Med. 2018, 41, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, A.; Barreto, G.E.; Lombardi, G.; Pirro, M.; Sahebkar, A. Emerging roles for high-density lipoproteins in neurodegenerative disorders. BioFactors 2019, 45, 725–739. [Google Scholar] [CrossRef] [PubMed]
- Dietschy, J.M.; Turley, S.D. Cholesterol metabolism in the brain. Curr. Opin. Lipidol. 2001, 12, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, C.A.; Gatz, M.; Prince, J.A.; Berg, S.; Pedersen, N.L. Serum lipid levels and cognitive change in late life. J. Am. Geriatr Soc. 2010, 58, 501–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nelson, R.H. Hyperlipidemia as a risk factor for cardiovascular disease. Prim. Care 2013, 40, 195–211. [Google Scholar] [CrossRef] [Green Version]
- Mcgrowder, D.; Riley, C.; Morrison, E.Y.S.A.; Gordon, L. The role of high-density lipoproteins in reducing the risk of vascular diseases, neurogenerative disorders, and cancer. Cholesterol 2011, 2011, 496925. [Google Scholar] [CrossRef]
- Reitz, C.; Tang, M.X.; Schupf, N.; Manly, J.J.; Mayeux, R.; Luchsinger, J.A. Association of higher levels of high-density lipoprotein cholesterol in elderly individuals and lower risk of late-onset Alzheimer disease. Arch. Neurol. 2010, 67, 1491–1497. [Google Scholar] [CrossRef] [Green Version]
- Halperin, R.O.; Sesso, H.D.; Ma, J.; Buring, J.E.; Stampfer, M.J.; Gaziano, J.M. Dyslipidemia and the risk of incident hypertension in men. Hypertension 2006, 47, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Rohatgi, A.; Khera, A.; Berry, J.D.; Givens, E.G.; Ayers, C.R.; Wedin, K.E.; Neeland, I.J.; Yuhanna, I.S.; Rader, D.R.; de Lemos, J.A.; et al. HDL cholesterol efflux capacity and incident cardiovascular events. N. Engl. J. Med. 2014, 371, 2383–2393. [Google Scholar] [CrossRef] [Green Version]
- Grundy, S.M. Metabolic syndrome update. Trends Cardiovasc. Med. 2016, 26, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.Y.; Rainey, W.E.; Bollag, W.B. Very low-density lipoprotein (VLDL)-induced signals mediating aldosterone production. J. Endocrinol. 2017, 232, 115–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansurudeen, I.; Pietzsch, J.; Graessler, J.; Ehrhart-Bornstein, M.; Saha, S.; Bornstein, S.R.; Kopprasch, S. Modulation of adrenal aldosterone release by oxidative modification of low-density lipoprotein. Am. J. Hypertens. 2010, 23, 1061–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, K.-H. The Current Status of Research on High-Density Lipoproteins (HDL): A Paradigm Shift from HDL Quantity to HDL Quality and HDL Functionality. Int. J. Mol. Sci. 2022, 23, 3967. https://doi.org/10.3390/ijms23073967
Cho K-H. The Current Status of Research on High-Density Lipoproteins (HDL): A Paradigm Shift from HDL Quantity to HDL Quality and HDL Functionality. International Journal of Molecular Sciences. 2022; 23(7):3967. https://doi.org/10.3390/ijms23073967
Chicago/Turabian StyleCho, Kyung-Hyun. 2022. "The Current Status of Research on High-Density Lipoproteins (HDL): A Paradigm Shift from HDL Quantity to HDL Quality and HDL Functionality" International Journal of Molecular Sciences 23, no. 7: 3967. https://doi.org/10.3390/ijms23073967





