Small RNAs Participate in Plant–Virus Interaction and Their Application in Plant Viral Defense
Abstract
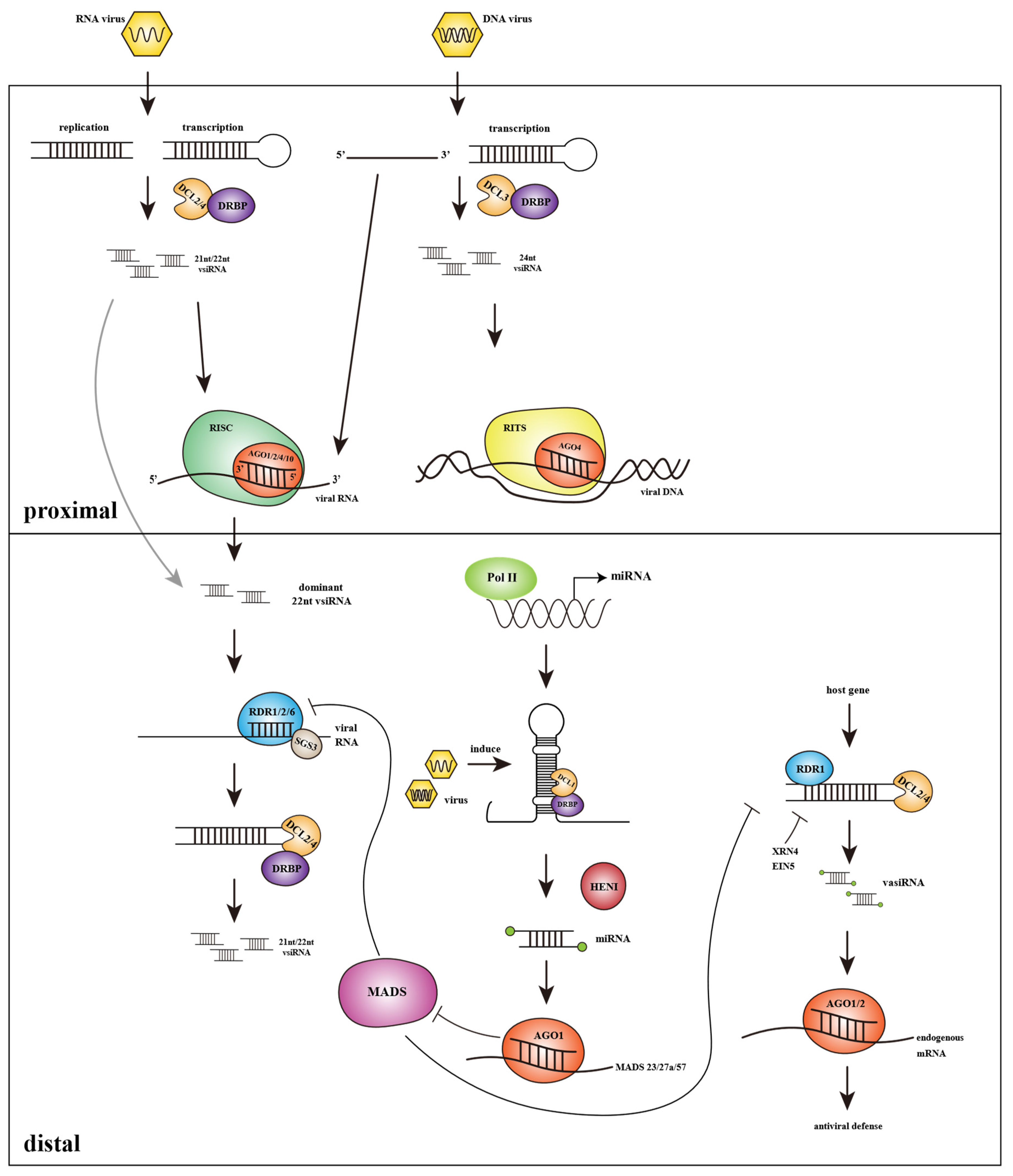
:1. Introduction
2. Small RNAs Biogenesis and Classification
2.1. Overview of Important Components Involved in Small RNAs Biogenesis
2.1.1. Dicer-like (DCL)
2.1.2. RNA-Dependent RNA Polymerases (RDRs)
2.1.3. HUA ENHANCER 1 (HEN1)
2.2. Multiple Ways of Small RNA Biogenesis
2.2.1. The Biogenesis of microRNAs (miRNAs)
2.2.2. The Biogenesis of Small Interfering RNAs (siRNAs)
- Hairpin-siRNAs (hp-siRNAs)
- Natural antisense small interfering RNAs (natsiRNAs)
- Secondary small interfering RNAs (sec-siRNAs)
- Heterochromatic siRNAs (hetsiRNAs)
3. Virus Defense Based on RNAi
3.1. Virus Derived Small Interfering RNAs Based Splicing of Target Viral Transcripts and Systemic Spread of Antiviral Silencing
3.2. Role of Endogenous miRNAs Produced in Host Plants in Virus Defense
3.3. Plant Endogenous siRNAs Can Be Induced by Virus and Participate in Antiviral Silencing
3.4. Confrontation of the Virus to Host Antiviral RNAi
| Panel Point | Interference Effect | VSRs | Virus | Reference |
|---|---|---|---|---|
| Small RNA biogenesis | Long dsRNA binding | P14 | Aureus virus | [104] |
| P38 | Turnip crinkle virus | [105] | ||
| NSs | Tomato spotted wilt virus | [106] | ||
| P22 | Tomato chlorosis virus | [107] | ||
| P21 | Beet yellow virus | [108] | ||
| DCL blocking | P38 | Turnip crinkle virus | [105] | |
| P1 | Rice yellow mottle virus | [109] | ||
| DRB4 inhibition | P6 | Cauliflower Mosaic Virus | [102] | |
| HENI binding | Hc-Pro | Turnip mosaic virus | [110] | |
| RISC assemble and function | AGO blocking | P38 | Turnip crinkle virus | [111] |
| 2b | Cucumber mosaic virus | [112] | ||
| P1 | Sweet potato mild mottle ipomovirus; | [113] | ||
| AGO degradation | P0 | Polerovirus | [114] | |
| P25 | Potato virus X | [115] | ||
| P38 | Carmo virus | [111] | ||
| CP | Nepo virus | [116] | ||
| sRNA binding | P37 | Pelargonium line pattern virus | [117] | |
| P19 | Tomato bushy stunt virus | [118] | ||
| P21 | Clostero virus | [104] | ||
| P15 | Peanut clump virus | [104] | ||
| Hc-Pro | Poty virus | [113] | ||
| 2b | Cucumber mosaic virus | [119] | ||
| NSs | Tomato spotted wilt virus | [106] | ||
| NS3 | Rice Stripe Virus | [120] | ||
| P10 | Grapevine virus | [121] | ||
| P130 | Tomato mosaic virus | [105] | ||
| P122 | Tobacco mosaic virus | [105] | ||
| Rep | Wheat dwarf virus | [122] | ||
| P16 | Cucumber vein yellowing virus | [123] | ||
| PNS10 | Rice Dwarf Phytoreovirus | [124] | ||
| P14 | Aureus virus | [104] | ||
| Systemic antiviral silencing | RDR6 inactivation | βC1 | Tomato yellow leaf curl China virus | [125] |
| P6 | Rice yellow stunt rhabdovirus | [126] | ||
| Hc-Pro | Sugarcane mosaic virus | [127] | ||
| SGS3 blocking | P2 | Rice stripe virus | [128] | |
| V2 | Tomato yellow leaf curl virus | [116] | ||
| VPg | Potato virus A | [129] | ||
| P25 | Plantago asiatica mosaic virus | [130] | ||
| Host miRNA antiviral response | Affect miRNA expression | P19 | Tomato bushy stunt virus | [131] |
| P122 | Tobacco mosaic virus | [132] | ||
| Hc-Pro | Poty virus | [133] | ||
| TGS | Interference with TGS | AC2 | Begono virus | [134] |
| Hc-Pro | Poty virus | [135] | ||
| AL2 | Tomato golden mosaic virus | [136] |
4. The Applications of Small RNAs in Plant Viral Defense
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Calil, I.P.; Fontes, E.P. Plant immunity against viruses: Antiviral immune receptors in focus. Ann. Bot. 2017, 119, 711–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalid, A.; Zhang, Q.; Yasir, M.; Li, F. Small RNA Based Genetic Engineering for Plant Viral Resistance: Application in Crop Protection. Front. Microbiol. 2017, 8, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, R.A.; Naidu, R.A. Global Dimensions of Plant Virus Diseases: Current Status and Future Perspectives. Annu. Rev. Virol. 2019, 6, 387–409. [Google Scholar] [CrossRef] [PubMed]
- Hurley, B.; Subramaniam, R.; Guttman, D.S.; Desveaux, D. Proteomics of effector-triggered immunity (ETI) in plants. Virulence 2014, 5, 752–760. [Google Scholar] [CrossRef] [Green Version]
- BBigeard, J.; Colcombet, J.; Hirt, H. Signaling Mechanisms in Pattern-Triggered Immunity (PTI). Mol. Plant 2015, 8, 521–539. [Google Scholar] [CrossRef] [Green Version]
- Incarbone, M.; Dunoyer, P. RNA silencing and its suppression: Novel insights from in planta analyses. Trends Plant Sci. 2013, 18, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Alcaide-Loridan, C.; Jupin, I. Ubiquitin and plant viruses, let’s play together! Plant Physiol. 2012, 160, 72–82. [Google Scholar] [CrossRef] [Green Version]
- Rosa, C.; Kuo, Y.-W.; Yan, Z.; Falk, B.W. RNA Interference Mechanisms and Applications in Plant Pathology. Annu. Rev. Phytopathol. 2018, 56, 581–610. [Google Scholar] [CrossRef]
- Prasad, A.; Sharma, N.; Muthamilarasan, M.; Rana, S.; Prasad, M. Recent advances in small RNA mediated plant-virus interactions. Critical Rev. Biotechnol. 2019, 39, 587–601. [Google Scholar] [CrossRef]
- Muthamilarasan, M.; Prasad, M. Plant innate immunity: An updated insight into defense mechanism. J. Biosci. 2013, 38, 433–449. [Google Scholar] [CrossRef]
- Bonnet, E.; Van de Peer, Y.; Rouzé, P. The small RNA world of plants. New Phytol. 2006, 171, 451–468. [Google Scholar] [CrossRef]
- Ruiz-Ferrer, V.; Voinnet, O. Roles of Plant Small RNAs in Biotic Stress Responses. Annu. Rev. Plant Biol. 2009, 60, 485–510. [Google Scholar] [CrossRef] [Green Version]
- Borges, F.; Martienssen, R.A. The expanding world of small RNAs in plants. Nat. Rev. Mol. Cell Biol. 2015, 16, 727–741. [Google Scholar] [CrossRef] [Green Version]
- Deng, P.; Muhammad, S.; Cao, M.; Wu, L. Biogenesis and regulatory hierarchy of phased small interfering RNAs in plants. Plant Biotechnol. J. 2018, 16, 965–975. [Google Scholar] [CrossRef] [Green Version]
- Arikit, S.; Zhai, J.; Meyers, B. Biogenesis and function of rice small RNAs from non-coding RNA precursors. Curr. Opin. Plant Biol. 2013, 16, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Carthew, R.W.; Sontheimer, E.J. Origins and Mechanisms of miRNAs and siRNAs. Cell 2009, 136, 642–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bologna, N.G.; Voinnet, O. The Diversity, Biogenesis, and Activities of Endogenous Silencing Small RNAs in Arabidopsis. Annu. Rev. Plant Biol. 2014, 65, 473–503. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; You, L.; Li, R.; Fu, D.-Q.; Zhu, B.-Z.; Luo, Y.-B.; Zhu, H.-L. Cloning, identification, and expression analysis of a Dicer-Like gene family from Solanum lycopersicum. Biol. Plant. 2016, 60, 410–418. [Google Scholar] [CrossRef]
- Qin, H.; Chen, F.; Huan, X.; Machida, S.; Song, J.; Yuan, Y.A. Structure of the Arabidopsis thaliana DCL4 DUF283 domain reveals a noncanonical double-stranded RNA-binding fold for protein–protein interaction. RNA 2010, 16, 474–481. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, T.; Zhang, F.; Zhang, Y.; Liang, Y. RNA Interference: A Natural Immune System of Plants to Counteract Biotic Stressors. Cells 2019, 8, 38. [Google Scholar] [CrossRef] [Green Version]
- Barraud, P.; Banerjee, S.; Mohamed, W.I.; Jantsch, M.F.; Allain, F.H.-T. A bimodular nuclear localization signal assembled via an extended double-stranded RNA-binding domain acts as an RNA-sensing signal for transportin 1. Proc. Natl. Acad. Sci. USA 2014, 111, E1852–E1861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hohn, T.; Vazquez, F. RNA silencing pathways of plants: Silencing and its suppression by plant DNA viruses. Biochim. Biophys. Acta (BBA) Bioenerg. 2011, 1809, 588–600. [Google Scholar] [CrossRef] [PubMed]
- Mlotshwa, S.; Schauer, S.E.; Smith, T.H.; Mallory, A.C.; Herr, J., Jr.; Roth, B.; Merchant, D.S.; Ray, A.; Bowman, L.H.; Vance, V.B. Ectopic DICER-LIKE1 expression in P1/HC-Pro Arabidopsis rescues phenotypic anomalies but not defects in microRNA and silencing pathways. Plant Cell 2005, 17, 2873–2885. [Google Scholar] [CrossRef] [Green Version]
- Qi, Y.; Denli, A.M.; Hannon, G.J. Biochemical Specialization within Arabidopsis RNA Silencing Pathways. Mol. Cell 2005, 19, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Slotkin, R.K.; Martienssen, R. Transposable elements and the epigenetic regulation of the genome. Nat. Rev. Genet. 2007, 8, 272–285. [Google Scholar] [CrossRef]
- Matzke, M.A.; Mosher, R.A. RNA-directed DNA methylation: An epigenetic pathway of increasing complexity. Nat. Rev. Genet. 2014, 15, 394–408. [Google Scholar] [CrossRef]
- Bouché, N.; Lauressergues, D.; Gasciolli, V.; Vaucheret, H. An antagonistic function for Arabidopsis DCL2 in development and a new function for DCL4 in generating viral siRNAs. EMBO J. 2006, 25, 3347–3356. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Ruiz, H.; Takeda, A.; Chapman, E.J.; Sullivan, C.M.; Fahlgren, N.; Brempelis, K.J.; Carrington, J.C. Arabidopsis RNA-dependent RNA polymerases and dicer-like proteins in antiviral defense and small interfering RNA biogenesis during Turnip Mosaic Virus infection. Plant Cell 2010, 22, 481–496. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Deng, Z.; Zhang, X.; Wang, H.; Wang, Y.; Liu, X.; Liu, S.; Xu, F.; Li, T.; Fu, D.; et al. Tomato DCL2b is required for the biosynthesis of 22-nt small RNAs, the resulting secondary siRNAs, and the host defense against ToMV. Hortic. Res. 2018, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Brosseau, C.; El Oirdi, M.; Adurogbangba, A.; Ma, X.; Moffett, P. Antiviral Defense Involves AGO4 in an Arabidopsis–Potexvirus Interaction. Mol. Plant-Microbe Interact. 2016, 29, 878–888. [Google Scholar] [CrossRef] [Green Version]
- Deleris, A.; Gallego-Bartolome, J.; Bao, J.; Kasschau, K.D.; Carrington, J.C.; Voinnet, O. Hierarchical Action and Inhibition of Plant Dicer-Like Proteins in Antiviral Defense. Science 2006, 313, 68–71. [Google Scholar] [CrossRef]
- Diaz-Pendon, J.; Li, F.; Li, W.-X.; Ding, S.-W. Suppression of Antiviral Silencing by Cucumber Mosaic Virus 2b Protein in Arabidopsis Is Associated with Drastically Reduced Accumulation of Three Classes of Viral Small Interfering RNAs. Plant Cell 2007, 19, 2053–2063. [Google Scholar] [CrossRef] [Green Version]
- Axtell, M.J. Classification and Comparison of Small RNAs from Plants. Annu. Rev. Plant Biol. 2013, 64, 137–159. [Google Scholar] [CrossRef] [Green Version]
- Baulcombe, D.C. RNA silencing in plants. Nature 2004, 431, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Reinhart, B.J.; Bartel, D.P.; Zamore, P.D. A biochemical framework for RNA silencing in plants. Genes Dev. 2003, 17, 49–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zong, J.; Yao, X.; Yin, J.; Zhang, D.; Ma, H. Evolution of the RNA-dependent RNA polymerase (RdRP) genes: Duplications and possible losses before and after the divergence of major eukaryotic groups. Gene 2009, 447, 29–39. [Google Scholar] [CrossRef]
- Willmann, M.R.; Endres, M.W.; Cook, R.T.; Gregory, B.D. The functions of RNA-dependent RNA polymerases in Arabidopsis. Arab. Book Am. Soc. Plant Biol. 2011, 9, e0146. [Google Scholar] [CrossRef] [Green Version]
- Venkataraman, S.; Prasad, B.V.L.S.; Selvarajan, R. RNA Dependent RNA Polymerases: Insights from Structure, Function and Evolution. Viruses 2018, 10, 76. [Google Scholar] [CrossRef] [Green Version]
- Pumplin, N.; Voinnet, O. RNA silencing suppression by plant pathogens: Defence, counter-defence and counter-counter-defence. Nat. Rev. Genet. 2013, 11, 745–760. [Google Scholar] [CrossRef]
- Olmedo-Monfil, V.; Duran-Figueroa, N.; Arteaga-Vazquez, M.A.; Demesa-Arevalo, E.; Autran, D.; Grimanelli, D.; Slotkin, R.K.; Martienssen, R.A.; Vielle-Calzada, J.-P. Control of female gamete formation by a small RNA pathway in Arabidopsis. Nature 2010, 464, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yu, Y.; Zhai, J.; Ramachandran, V.; Dinh, T.T.; Meyers, B.C.; Mo, B.; Chen, X. HESO1, a nucleotidyl transferase in Arabidopsis, uridylates unmethylated miRNAs and siRNAs to trigger their degradation. Curr. Biol. 2012, 22, 689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, G.; Chen, X.; Yu, B. Uridylation of miRNAs by HEN1 SUPPRESSOR1 in Arabidopsis. Curr. Biol. 2012, 22, 695–700. [Google Scholar] [CrossRef] [Green Version]
- Ramachandran, V.; Chen, X. Degradation of microRNAs by a Family of Exoribonucleases in Arabidopsis. Science 2008, 321, 1490–1492. [Google Scholar] [CrossRef] [Green Version]
- Yu, B.; Yang, Z.; Li, J.; Minakhina, S.; Yang, M.; Padgett, R.W.; Steward, R.; Chen, X. Methylation as a Crucial Step in Plant microRNA Biogenesis. Science 2005, 307, 932–935. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Ebright, Y.W.; Yu, B.; Chen, X. HEN1 recognizes 21–24 nt small RNA duplexes and deposits a methyl group onto the 2′ OH of the 3′ terminal nucleotide. Nucleic Acids Res. 2006, 34, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Tkaczuk, K.L.; Obarska, A.; Bujnicki, J.M. Molecular phylogenetics and comparative modeling of HEN1, a methyltransferase involved in plant microRNA biogenesis. BMC Evol. Biol. 2006, 6, 6. [Google Scholar] [CrossRef] [Green Version]
- Chen, X. A marked end. Nat. Struct. Mol. Biol. 2007, 14, 259–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Ji, L.; Huang, Q.; Vassylyev, D.G.; Chen, X.; Ma, J.-B. Structural insights into mechanisms of the small RNA methyltransferase HEN1. Nature 2009, 461, 823–827. [Google Scholar] [CrossRef]
- Kang, C.B.; Hong, Y.; Dhe-Paganon, S.; Yoon, H.S. FKBP family proteins: Immunophilins with versatile biological functions. Neurosignals 2008, 16, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Bevilacqua, P.; Diegelman-Parente, A.; Mathews, M.B. The double-stranded-RNA-binding motif: Interference and much more. Nat. Rev. Mol. Cell Biol. 2004, 5, 1013–1023. [Google Scholar] [CrossRef]
- Chen, X.; Liu, J.; Cheng, Y.; Jia, D. HEN1 functions pleiotropically in Arabidopsis development and acts in C function in the flower. Development 2002, 129, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Griffiths-Jones, S.; Saini, H.K.; van Dongen, S.; Enright, A.J. miRBase: Tools for microRNA genomics. Nucleic Acids Res. 2007, 36, D154–D158. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.J.; Zheng, B.; Yu, Y.; Won, S.Y.; Mo, B.; Chen, X. The role of Mediator in small and long noncoding RNA production in Arabidopsis thaliana. EMBO J. 2011, 30, 814–822. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Song, X.; Gu, L.; Li, X.; Cao, S.; Chu, C.; Cui, X.; Chen, X.; Cao, X. NOT2 Proteins Promote Polymerase II–Dependent Transcription and Interact with Multiple MicroRNA Biogenesis Factors in Arabidopsis. Plant Cell 2013, 25, 715–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Z.; Allen, E.; Fahlgren, N.; Calamar, A.; Givan, S.; Carrington, J.C. Expression of Arabidopsis MIRNA Genes. Plant Physiol. 2005, 138, 2145–2154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bielewicz, D.; Kalak, M.; Kalyna, M.; Windels, D.; Barta, A.; Vazquez, F.; Szweykowska-Kulinska, Z.; Jarmolowski, A. Introns of plant pri-miRNAs enhance miRNA biogenesis. EMBO Rep. 2013, 14, 622–628. [Google Scholar] [CrossRef]
- Schwab, R.; Speth, C.; Laubinger, S.; Voinnet, O. Enhanced microRNA accumulation through stemloop-adjacent introns. EMBO Rep. 2013, 14, 615–621. [Google Scholar] [CrossRef]
- Laubinger, S.; Sachsenberg, T.; Zeller, G.; Busch, W.; Lohmann, J.U.; Rätsch, G.; Weigel, D. Dual roles of the nuclear cap-binding complex and SERRATE in pre-mRNA splicing and microRNA processing in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2008, 105, 8795–8800. [Google Scholar] [CrossRef] [Green Version]
- Ben Chaabane, S.; Liu, R.; Chinnusamy, V.; Kwon, Y.; Park, J.-h.; Kim, S.Y.; Zhu, J.-K.; Yang, S.W.; Lee, B.-h. STA1, an Arabidopsis pre-mRNA processing factor 6 homolog, is a new player involved in miRNA biogenesis. Nucleic Acids Res. 2013, 41, 1984–1997. [Google Scholar] [CrossRef] [Green Version]
- Zhan, X.; Wang, B.; Li, H.; Liu, R.; Kalia, R.K.; Zhu, J.-K.; Chinnusamy, V. Arabidopsis proline-rich protein important for development and abiotic stress tolerance is involved in microRNA biogenesis. Proc. Natl. Acad. Sci. USA 2012, 109, 18198–18203. [Google Scholar] [CrossRef] [Green Version]
- Vazquez, F.; Gasciolli, V.; Crété, P.; Vaucheret, H. The nuclear dsRNA binding protein HYL1 is required for microRNA accumulation and plant development, but not posttranscriptional transgene silencing. Curr. Biol. 2004, 14, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Han, M.-H.; Fedoroff, N. The RNA-binding proteins HYL1 and SE promote accurate in vitro processing of pri-miRNA by DCL1. Proc. Natl. Acad. Sci. USA 2008, 105, 9970–9975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, G.; Xie, M.; Dou, Y.; Zhang, S.; Zhang, C.; Yu, B. Regulation of miRNA abundance by RNA binding protein TOUGH in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 12817–12821. [Google Scholar] [CrossRef] [Green Version]
- Yu, B.; Bi, L.; Zheng, B.; Ji, L.; Chevalier, D.; Agarwal, M.; Ramachandran, V.; Li, W.; Lagrange, T.; Walker, J.C. The FHA domain proteins DAWDLE in Arabidopsis and SNIP1 in humans act in small RNA biogenesis. Proc. Natl. Acad. Sci. USA 2008, 105, 10073–10078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machida, S.; Yuan, Y.A. Crystal Structure of Arabidopsis thaliana Dawdle Forkhead-Associated Domain Reveals a Conserved Phospho-Threonine Recognition Cleft for Dicer-Like 1 Binding. Mol. Plant 2013, 6, 1290–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manavella, P.; Hagmann, J.; Ott, F.; Laubinger, S.; Franz, M.; Macek, B.; Weigel, D. Fast-Forward Genetics Identifies Plant CPL Phosphatases as Regulators of miRNA Processing Factor HYL1. Cell 2012, 151, 859–870. [Google Scholar] [CrossRef] [Green Version]
- Park, M.Y.; Wu, G.; Gonzalez-Sulser, A.; Vaucheret, H.; Poethig, R.S. Nuclear processing and export of microRNAs in Arabidopsis. Proc. Natl. Acad. Sci. USA 2005, 102, 3691–3696. [Google Scholar] [CrossRef] [Green Version]
- Eamens, A.L.; Smith, N.A.; Curtin, S.J.; Wang, M.-B.; Waterhouse, P.M. The Arabidopsis thaliana double-stranded RNA binding protein DRB1 directs guide strand selection from microRNA duplexes. RNA 2009, 15, 2219–2235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iki, T.; Yoshikawa, M.; Meshi, T.; Ishikawa, M. Cyclophilin 40 facilitates HSP90-mediated RISC assembly in plants. EMBO J. 2012, 31, 267–278. [Google Scholar] [CrossRef] [Green Version]
- Iki, T.; Yoshikawa, M.; Nishikiori, M.; Jaudal, M.; Matsumoto-Yokoyama, E.; Mitsuhara, I.; Meshi, T.; Ishikawa, M. In Vitro Assembly of Plant RNA-Induced Silencing Complexes Facilitated by Molecular Chaperone HSP90. Mol. Cell 2010, 39, 282–291. [Google Scholar] [CrossRef]
- Devers, E.; Branscheid, A.; May, P.; Krajinski, F. Stars and Symbiosis: MicroRNA- and MicroRNA*-Mediated Transcript Cleavage Involved in Arbuscular Mycorrhizal Symbiosis. Plant Physiol. 2011, 156, 1990–2010. [Google Scholar] [CrossRef] [Green Version]
- Dunoyer, P.; Himber, C.; Ruiz-Ferrer, V.; Alioua, A.; Voinnet, O. Intra- and intercellular RNA interference in Arabidopsis thaliana requires components of the microRNA and heterochromatic silencing pathways. Nat. Genet. 2007, 39, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Kasschau, K.D.; Fahlgren, N.; Chapman, E.J.; Sullivan, C.M.; Cumbie, J.S.; Givan, S.A.; Carrington, J.C. Genome-wide profiling and analysis of Arabidopsis siRNAs. PLoS Biol. 2007, 5, e57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.-J.; Gaasterland, T.; Chua, N.-H. Genome-wide prediction and identification of cis-natural antisense transcripts in Arabidopsis thaliana. Genome Biol. 2005, 6, R30. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Lii, Y.; Wu, Z.; Polishko, A.; Zhang, H.; Chinnusamy, V.; Lonardi, S.; Zhu, J.-K.; Liu, R.; Jin, H. Mechanisms of Small RNA Generation from Cis-NATs in Response to Environmental and Developmental Cues. Mol. Plant 2013, 6, 704–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshikawa, M.; Peragine, A.; Park, M.Y.; Poethig, R.S. A pathway for the biogenesis of trans-acting siRNAs in Arabidopsis. Genes Dev. 2005, 19, 2164–2175. [Google Scholar] [CrossRef] [Green Version]
- Allen, E.; Xie, Z.; Gustafson, A.M.; Carrington, J.C. microRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 2005, 121, 207–221. [Google Scholar] [CrossRef] [Green Version]
- Montgomery, T.A.; Howell, M.D.; Cuperus, J.T.; Li, D.; Hansen, J.E.; Alexander, A.L.; Chapman, E.J.; Fahlgren, N.; Allen, E.; Carrington, J.C. Specificity of ARGONAUTE7-miR390 interaction and dual functionality in TAS3 trans-acting siRNA formation. Cell 2008, 133, 128–141. [Google Scholar] [CrossRef] [Green Version]
- Fei, Q.; Xia, R.; Meyers, B.C. Phased, secondary, small interfering RNAs in posttranscriptional regulatory networks. Plant Cell 2013, 25, 2400–2415. [Google Scholar] [CrossRef] [Green Version]
- Ronemus, M.; Vaughn, M.W.; Martienssen, R.A. MicroRNA-targeted and small interfering RNA–mediated mRNA degradation is regulated by Argonaute, Dicer, and RNA-dependent RNA polymerase in Arabidopsis. Plant Cell 2006, 18, 1559–1574. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, M.; Iki, T.; Tsutsui, Y.; Miyashita, K.; Poethig, R.S.; Habu, Y.; Ishikawa, M. 3′ fragment of miR173-programmed RISC-cleaved RNA is protected from degradation in a complex with RISC and SGS3. Proc. Natl. Acad. Sci. USA 2013, 110, 4117–4122. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Medina, C.; Atkins, C.A.; Mann, A.J.; Jordan, M.E.; Smith, P.M. Macromolecular composition of phloem exudate from white lupin (Lupinus albus L.). BMC Plant Biol. 2011, 11, 36. [Google Scholar] [CrossRef] [Green Version]
- Uslu, V.V.; Wassenegger, M. Critical view on RNA silencing-mediated virus resistance using exogenously applied RNA. Curr. Opin. Virol. 2020, 42, 18–24. [Google Scholar] [CrossRef]
- Sharma, N.; Sahu, P.P.; Puranik, S.; Prasad, M. Recent Advances in Plant–Virus Interaction with Emphasis on Small Interfering RNAs (siRNAs). Mol. Biotechnol. 2012, 55, 63–77. [Google Scholar] [CrossRef] [PubMed]
- De Ronde, D.; Butterbach, P.; Kormelink, R. Dominant resistance against plant viruses. Front. Plant Sci. 2014, 5, 307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brosseau, C.; Moffett, P. Functional and Genetic Analysis Identify a Role for Arabidopsis ARGONAUTE5 in Antiviral RNA Silencing. Plant Cell 2015, 27, 1742–1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Li, Y. Dissection of RNAi-based antiviral immunity in plants. Curr. Opin. Virol. 2018, 32, 88–99. [Google Scholar] [CrossRef]
- Vazquez, F.; Hohn, T. Biogenesis and Biological Activity of Secondary siRNAs in Plants. Scientifica 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Zhang, X.; Lai, T.; Zhang, P.; Zhang, X.; Yuan, C.; Jin, Z.; Li, H.; Yu, Z.; Qin, C.; Tör, M.; et al. Mini review: Revisiting mobile RNA silencing in plants. Plant Sci. 2018, 278, 113–117. [Google Scholar] [CrossRef]
- Parent, J.-S.; Bouteiller, N.; Elmayan, T.; Vaucheret, H. Respective contributions of Arabidopsis DCL2 and DCL4 to RNA silencing. Plant J. 2014, 81, 223–232. [Google Scholar] [CrossRef]
- Dalakouras, A.; Wassenegger, M.; Dadami, E.; Ganopoulos, I.; Pappas, M.L.; Papadopoulou, K. Genetically modified organism-free RNA interference: Exogenous application of RNA molecules in plants. Plant Physiol. 2020, 182, 38–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melnyk, C.W.; Molnar, A.; Bassett, A.; Baulcombe, D. Mobile 24 nt Small RNAs Direct Transcriptional Gene Silencing in the Root Meristems of Arabidopsis thaliana. Curr. Biol. 2011, 21, 1678–1683. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.-B.; Wu, Q.; Ito, T.; Cillo, F.; Li, W.-X.; Chen, X.; Yu, J.-L.; Ding, S.-W. RNAi-mediated viral immunity requires amplification of virus-derived siRNAs in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2010, 107, 484–489. [Google Scholar] [CrossRef] [Green Version]
- Raja, P.; Wolf, J.N.; Bisaro, D.M. RNA silencing directed against geminiviruses: Post-transcriptional and epigenetic components. Biochim. Biophys. Acta (BBA) Gene Regul. Mech. 2010, 1799, 337–351. [Google Scholar] [CrossRef]
- De Felippes, F.F.; Wang, J.-W.; Weigel, D. MIGS: miRNA-induced gene silencing. Plant J. 2011, 70, 541–547. [Google Scholar] [CrossRef]
- Zhai, J.; Jeong, D.-H.; De Paoli, E.; Park, S.; Rosen, B.D.; Li, Y.; González, A.J.; Yan, Z.; Kitto, S.L.; Grusak, M.A.; et al. MicroRNAs as master regulators of the plant NB-LRR defense gene family via the production of phased, trans-acting siRNAs. Genes Dev. 2011, 25, 2540–2553. [Google Scholar] [CrossRef] [Green Version]
- Boccara, M.; Sarazin, A.; Thiébeauld, O.; Jay, F.; Voinnet, O.; Navarro, L.; Colot, V. The Arabidopsis miR472-RDR6 Silencing Pathway Modulates PAMP- and Effector-Triggered Immunity through the Post-transcriptional Control of Disease Resistance Genes. PLoS Pathog. 2014, 10, e1003883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Lu, Y.-G.; Shi, Y.; Wu, L.; Xu, Y.-J.; Huang, F.; Guo, X.-Y.; Zhang, Y.; Fan, J.; Zhao, J.-Q.; et al. Multiple Rice MicroRNAs Are Involved in Immunity against the Blast Fungus Magnaporthe oryzae. Plant Physiol. 2013, 164, 1077–1092. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Jiao, X.; Kong, X.; Hamera, S.; Wu, Y.; Chen, X.; Fang, R.; Yan, Y. A Signaling Cascade from miR444 to RDR1 in Rice Antiviral RNA Silencing Pathway. Plant Physiol. 2016, 170, 2365–2377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lian, S.; Cho, W.K.; Kim, S.-M.; Choi, H.; Kim, K.-H. Time-Course Small RNA Profiling Reveals Rice miRNAs and Their Target Genes in Response to Rice Stripe Virus Infection. PLoS ONE 2016, 11, e0162319. [Google Scholar] [CrossRef]
- Cao, M.; Du, P.; Wang, X.-B.; Yu, Y.-Q.; Qiu, Y.-H.; Li, W.; Gal-On, A.; Zhou, C.; Li, Y.; Ding, S.-W. Virus infection triggers widespread silencing of host genes by a distinct class of endogenous siRNAs in Arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 14613–14618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laird, J.; McInally, C.; Carr, C.; Doddiah, S.; Yates, G.; Chrysanthou, E.; Khattab, A.; Love, A.J.; Geri, C.; Sadanandom, A. Identification of the domains of cauliflower mosaic virus protein P6 responsible for suppression of RNA silencing and salicylic acid signalling. J. Gen. Virol. 2013, 94, 2777. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Senthil-Kumar, M.; Ryu, C.-M.; Kang, L.; Mysore, K.S. Phytosterols Play a Key Role in Plant Innate Immunity against Bacterial Pathogens by Regulating Nutrient Efflux into the Apoplast. Plant Physiol. 2012, 158, 1789–1802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merai, Z.; Kerényi, Z.; Kertész, S.; Magna, M.; Lakatos, L.; Silhavy, D. Double-Stranded RNA Binding May Be a General Plant RNA Viral Strategy To Suppress RNA Silencing. J. Virol. 2006, 80, 5747–5756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgyán, J.; Havelda, Z. Viral suppressors of RNA silencing. Trends Plant Sci. 2011, 16, 265–272. [Google Scholar] [CrossRef] [PubMed]
- De Ronde, D.; Pasquier, A.; Ying, S.; Butterbach, P.; Lohuis, D.; Kormelink, R. Analysis of T omato spotted wilt virus NSs protein indicates the importance of the N-terminal domain for avirulence and RNA silencing suppression. Mol. Plant Pathol. 2014, 15, 185–195. [Google Scholar] [CrossRef]
- Landeo-Ríos, Y.; Navas-Castillo, J.; Moriones, E.; Cañizares, M.C. The p22 RNA silencing suppressor of the crinivirus Tomato chlorosis virus is dispensable for local viral replication but important for counteracting an antiviral RDR6-mediated response during systemic infection. Viruses 2016, 8, 182. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.Y.; Park, J.M. Cross-Talk in Viral Defense Signaling in Plants. Front. Microbiol. 2016, 7, 2068. [Google Scholar] [CrossRef] [Green Version]
- Lacombe, S.; Bangratz, M.; Vignols, F.; Brugidou, C. The rice yellow mottle virus P1 protein exhibits dual functions to suppress and activate gene silencing. Plant J. 2010, 61, 371–382. [Google Scholar] [CrossRef] [PubMed]
- Sanobar, N.; Lin, P.-C.; Pan, Z.-J.; Fang, R.-Y.; Tjita, V.; Chen, F.-F.; Wang, H.-C.; Tsai, H.-L.; Wu, S.-H.; Shen, T.-L. Investigating the viral suppressor HC-pro inhibiting small rna methylation through functional comparison of HEN1 in angiosperm and bryophyte. Viruses 2021, 13, 1837. [Google Scholar] [CrossRef]
- Azevedo, J.; Garcia, D.; Pontier, D.; Ohnesorge, S.; Yu, A.; Garcia, S.; Braun, L.; Bergdoll, M.; Hakimi, M.A.; Lagrange, T. Argonaute quenching and global changes in Dicer homeostasis caused by a pathogen-encoded GW repeat protein. Genes Dev. 2010, 24, 904–915. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Yuan, Y.-R.; Pei, Y.; Lin, S.-S.; Tuschl, T.; Patel, D.J.; Chua, N.-H. Cucumber mosaic virus-encoded 2b suppressor inhibits Arabidopsis Argonaute1 cleavage activity to counter plant defense. Genes Dev. 2006, 20, 3255–3268. [Google Scholar] [CrossRef] [Green Version]
- Li, M.-L.; Weng, K.-F.; Shih, S.-R.; Brewer, G. The evolving world of small RNAs from RNA viruses. Wiley Interdiscip. Rev. RNA 2016, 7, 575–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendelman, A.; Kravchik, M.; Stav, R.; Zik, M.; Lugassi, N.; Arazi, T. The developmental outcomes of P0-mediated ARGONAUTE destabilization in tomato. Planta 2013, 237, 363–377. [Google Scholar] [CrossRef] [PubMed]
- Chiu, M.H.; Chen, I.H.; Baulcombe, D.C.; Tsai, C.H. The silencing suppressor P25 of Potato virus X interacts with Argonaute1 and mediates its degradation through the proteasome pathway. Mol. Plant Pathol. 2010, 11, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Sinha, K.V.; Sopory, S.K.; Sanan-Mishra, N. Influence of virus–host interactions on plant response to abiotic stress. Plant Cell Rep. 2021, 40, 2225–2245. [Google Scholar] [CrossRef]
- Pérez-Cañamás, M.; Hernández, C. Key Importance of Small RNA Binding for the Activity of a Glycine-Tryptophan (GW) Motif-containing Viral Suppressor of RNA Silencing. J. Biol. Chem. 2015, 290, 3106–3120. [Google Scholar] [CrossRef] [Green Version]
- Vargason, J.M.; Szittya, G.; Burgyán, J.; Hall, T.M. Size Selective Recognition of siRNA by an RNA Silencing Suppressor. Cell 2003, 115, 799–811. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Yang, J.; Lin, C.; Yuan, Y.A. Structural basis for RNA-silencing suppression by Tomato aspermy virus protein 2b. EMBO Rep. 2008, 9, 754–760. [Google Scholar] [CrossRef] [Green Version]
- Shen, M.; Xu, Y.; Jia, R.; Zhou, X.; Ye, K. Size-Independent and Noncooperative Recognition of dsRNA by the Rice Stripe Virus RNA Silencing Suppressor NS3. J. Mol. Biol. 2010, 404, 665–679. [Google Scholar] [CrossRef]
- Zhou, Z.S.; Dell’Orco, M.; Saldarelli, P.; Turturo, C.; Minafra, A.; Martelli, G.P. Identification of an RNA-silencing suppressor in the genome of Grapevine virus A. J. Gen. Virol. 2006, 87, 2387–2395. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dang, M.; Hou, H.; Mei, Y.; Qian, Y.; Zhou, X. Identification of an RNA silencing suppressor encoded by a mastrevirus. J. Gen. Virol. 2014, 95, 2082–2088. [Google Scholar] [CrossRef]
- Valli, A.; Oliveros, J.C.; Molnar, A.; Baulcombe, D.; García, J.A. The specific binding to 21-nt double-stranded RNAs is crucial for the anti-silencing activity of Cucumber vein yellowing virus P1b and perturbs endogenous small RNA populations. RNA 2011, 17, 1148–1158. [Google Scholar] [CrossRef] [Green Version]
- Ren, B.; Guo, Y.; Gao, F.; Zhou, P.; Wu, F.; Meng, Z.; Wei, C.; Li, Y. Multiple Functions of Rice Dwarf Phytoreovirus Pns10 in Suppressing Systemic RNA Silencing. J. Virol. 2010, 84, 12914–12923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Huang, C.; Li, Z.; Zhou, X. Suppression of RNA silencing by a plant DNA virus satellite requires a host calmodulin-like protein to repress RDR6 expression. PLoS Pathog. 2014, 10, e1003921. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Song, X.; Xie, C.; Huo, Y.; Zhang, F.; Chen, X.; Geng, Y.; Fang, R. Rice yellow stunt rhabdovirus protein 6 suppresses systemic RNA silencing by blocking RDR6-mediated secondary siRNA synthesis. Mol. Plant-Microbe Interact. 2013, 26, 927–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Du, P.; Lu, L.; Xiao, Q.; Wang, W.; Cao, X.; Ren, B.; Wei, C.; Li, Y. Contrasting effects of HC-Pro and 2b viral suppressors from Sugarcane mosaic virus and Tomato aspermy cucumovirus on the accumulation of siRNAs. Virology 2008, 374, 351–360. [Google Scholar] [CrossRef] [Green Version]
- Du, Z.; Xiao, D.; Wu, J.; Jia, D.; Yuan, Z.; Liu, Y.; Hu, L.; Han, Z.; Wei, T.; Lin, Q.; et al. p2 of Rice stripe virus (RSV) interacts with OsSGS3 and is a silencing suppressor. Mol. Plant Pathol. 2011, 12, 808–814. [Google Scholar] [CrossRef]
- Rajamäki, M.-L.; Streng, J.; Valkonen, J.P. Silencing suppressor protein VPg of a potyvirus interacts with the plant silencing-related protein SGS3. Mol. Plant-Microbe Interact. 2014, 27, 1199–1210. [Google Scholar] [CrossRef] [Green Version]
- Aguilar, E.; Almendral, D.; Allende, L.; Pacheco, R.; Chung, B.N.; Canto, T.; Tenllado, F. The P25 Protein of Potato Virus X (PVX) Is the Main Pathogenicity Determinant Responsible for Systemic Necrosis in PVX-Associated Synergisms. J. Virol. 2014, 89, 2090–2103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Várallyay, É.; Válóczi, A.; Ágyi, Á.; Burgyán, J.; Havelda, Z. Plant virus-mediated induction of miR168 is associated with repression of ARGONAUTE1 accumulation. EMBO J. 2010, 29, 3507–3519. [Google Scholar] [CrossRef] [Green Version]
- Csorba, T.; Bovi, A.; Dalmay, T.; Burgyán, J. The p122 subunit of Tobacco Mosaic Virus replicase is a potent silencing suppressor and compromises both small interfering RNA-and microRNA-mediated pathways. J. Virol. 2007, 81, 11768–11780. [Google Scholar] [CrossRef] [Green Version]
- Pruss, G.J.; Lawrence, C.B.; Bass, T.; Li, Q.; Bowman, L.H.; Vance, V. The potyviral suppressor of RNA silencing confers enhanced resistance to multiple pathogens. Virology 2004, 320, 107–120. [Google Scholar] [CrossRef] [Green Version]
- Guerrero, J.; Regedanz, E.; Lu, L.; Ruan, J.; Bisaro, D.M.; Sunter, G. Manipulation of the plant host by the Geminivirus AC2/C2 protein, a central player in the infection cycle. Front. Plant Sci. 2020, 11, 591. [Google Scholar] [CrossRef]
- Yang, L.; Xu, Y.; Liu, Y.; Meng, D.; Jin, T.; Zhou, X. HC-Pro viral suppressor from tobacco vein banding mosaic virus interferes with DNA methylation and activates the salicylic acid pathway. Virology 2016, 497, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Sanan-Mishra, N.; Jailani, A.A.K.; Mandal, B.; Mukherjee, S.K. Secondary siRNAs in Plants: Biosynthesis, Various Functions, and Applications in Virology. Front. Plant Sci. 2021, 12, 610283. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Malinina, L.; Patel, D.J. Recognition of small interfering RNA by a viral suppressor of RNA silencing. Nature 2003, 426, 874–878. [Google Scholar] [CrossRef] [Green Version]
- Brigneti, G.; Voinnet, O.; Li, W.X.; Ji, L.H.; Ding, S.W.; Baulcombe, D.C. Retracted: Viral pathogenicity determinants are suppressors of transgene silencing in Nicotiana benthamiana. EMBO J. 1998, 17, 6739–6746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goto, K.; Kobori, T.; Kosaka, Y.; Natsuaki, T.; Masuta, C. Characterization of Silencing Suppressor 2b of Cucumber Mosaic Virus Based on Examination of its Small RNA-Binding Abilities. Plant Cell Physiol. 2007, 48, 1050–1060. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Hernández, A.M.; Gosalvez, B.; Sempere, R.N.; Burgos, L.; Aranda, M.A.; Truniger, V. Melon RNA interference (RNAi) lines silenced for Cm-eIF4E show broad virus resistance. Mol. Plant Pathol. 2012, 13, 755–763. [Google Scholar] [CrossRef]
- Niu, Q.-W.; Lin, S.-S.; Reyes, J.L.; Chen, K.-C.; Wu, H.-W.; Yeh, S.-D.; Chua, N.-H. Expression of artificial microRNAs in transgenic Arabidopsis thaliana confers virus resistance. Nat. Biotechnol. 2006, 24, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.; Biedenkopf, D.; Furch, A.; Weber, L.; Rossbach, O.; Abdellatef, E.; Linicus, L.; Johannsmeier, J.; Jelonek, L.; Goesmann, A.; et al. An RNAi-Based Control of Fusarium graminearum Infections Through Spraying of Long dsRNAs Involves a Plant Passage and Is Controlled by the Fungal Silencing Machinery. PLoS Pathog. 2016, 12, e1005901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, H.; Zhang, J.; Zhang, C.; Gong, P.; Ziaf, K.; Xiao, F.; Ye, Z. Expression of artificial microRNAs in tomato confers efficient and stable virus resistance in a cell-autonomous manner. Transgenic Res. 2011, 20, 569–581. [Google Scholar] [CrossRef]
- Jacobs, T.B.; Lawler, N.J.; Lafayette, P.R.; Vodkin, L.O.; Parrott, W.A. Simple gene silencing using the trans-acting siRNA pathway. Plant Biotechnol. J. 2015, 14, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Betti, F.; Ladera-Carmona, M.J.; Weits, D.A.; Ferri, G.; Iacopino, S.; Novi, G.; Svezia, B.; Kunkowska, A.B.; Santaniello, A.; Piaggesi, A.; et al. Exogenous miRNAs induce post-transcriptional gene silencing in plants. Nat. Plants 2021, 7, 1379–1388. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Z.; Ma, L.; Zhang, P.; Zhu, H. Small RNAs Participate in Plant–Virus Interaction and Their Application in Plant Viral Defense. Int. J. Mol. Sci. 2022, 23, 696. https://doi.org/10.3390/ijms23020696
Deng Z, Ma L, Zhang P, Zhu H. Small RNAs Participate in Plant–Virus Interaction and Their Application in Plant Viral Defense. International Journal of Molecular Sciences. 2022; 23(2):696. https://doi.org/10.3390/ijms23020696
Chicago/Turabian StyleDeng, Zhiqi, Liqun Ma, Peiyu Zhang, and Hongliang Zhu. 2022. "Small RNAs Participate in Plant–Virus Interaction and Their Application in Plant Viral Defense" International Journal of Molecular Sciences 23, no. 2: 696. https://doi.org/10.3390/ijms23020696






