Hydrogen Sulfide in Plants: Crosstalk with Other Signal Molecules in Response to Abiotic Stresses
Abstract
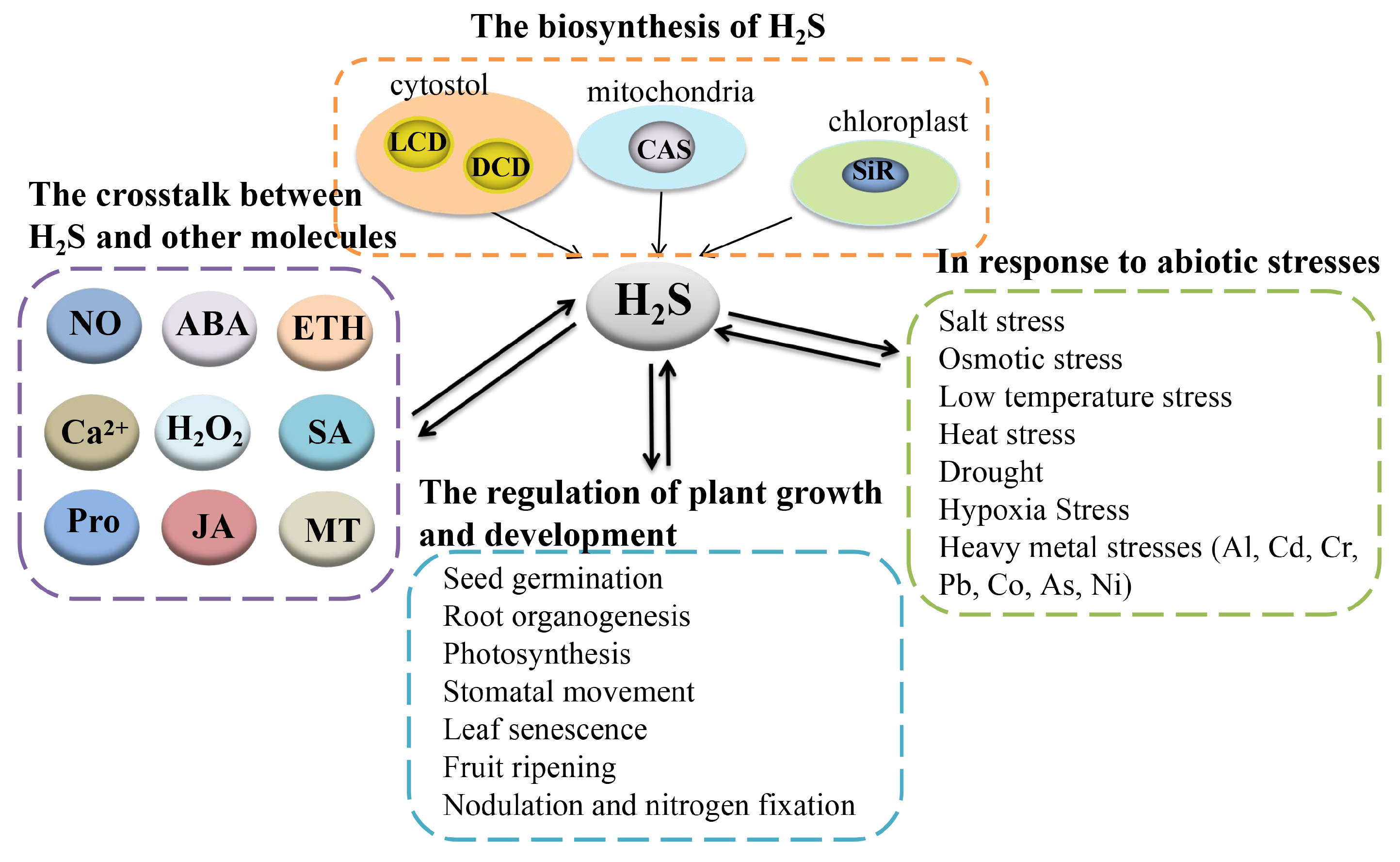
:1. Introduction
2. Crosstalk between H2S and NO in Response to Abiotic Stresses
2.1. Crosstalk between H2S and NO in Response to Heavy Metal Stress
2.2. Crosstalk between H2S and NO in Response to Salt Stress
2.3. Crosstalk between H2S and NO in Response to Other Stresses
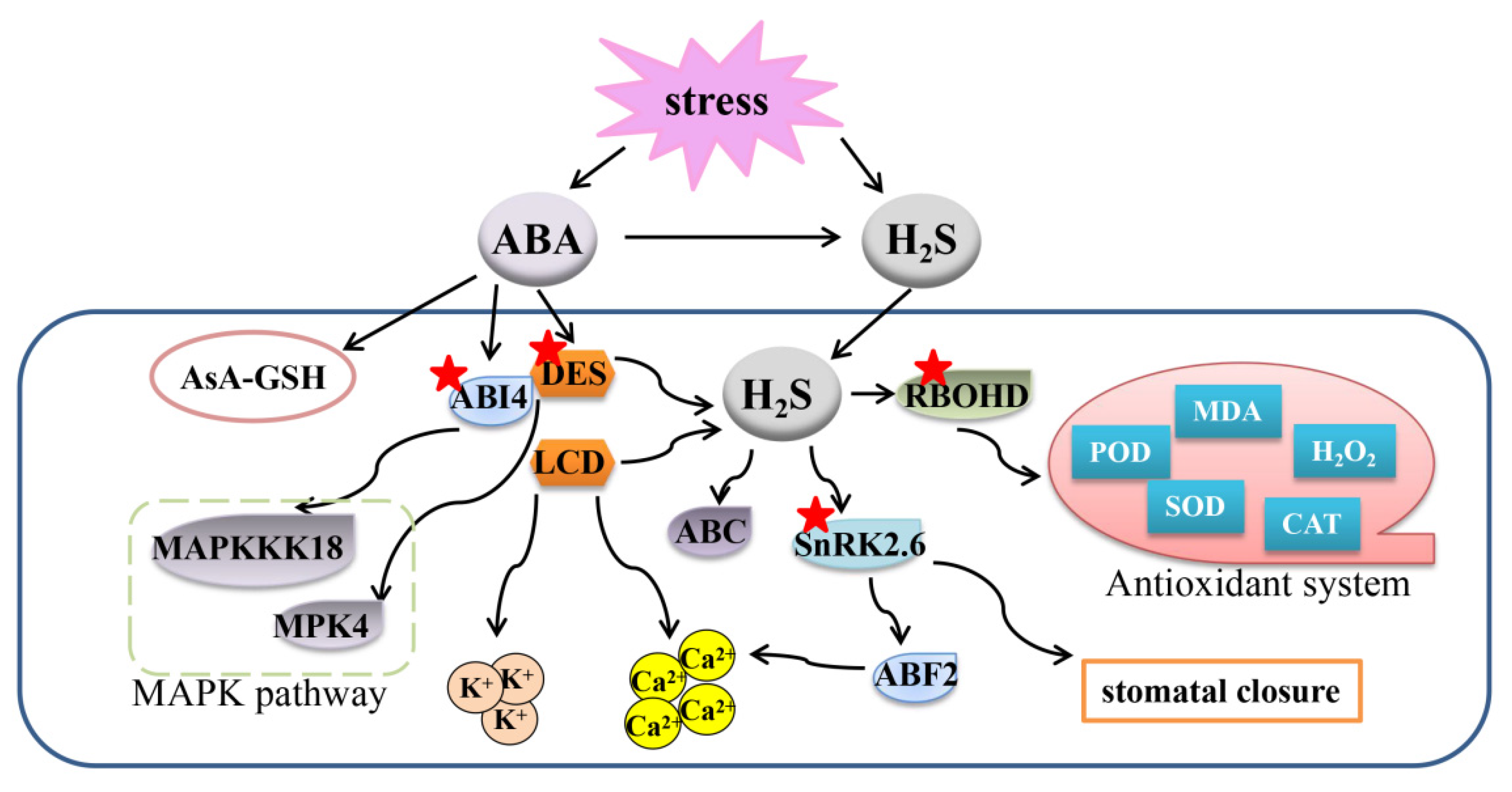
3. Crosstalk between H2S and ABA in Response to Abiotic Stresses
3.1. Crosstalk between H2S and ABA in Response to Abiotic Stresses through Regulating Stomatal Closure
3.2. Crosstalk between DES1/H2S and ABA in Response to Drought Stress through Regulating Protein Persulfidation
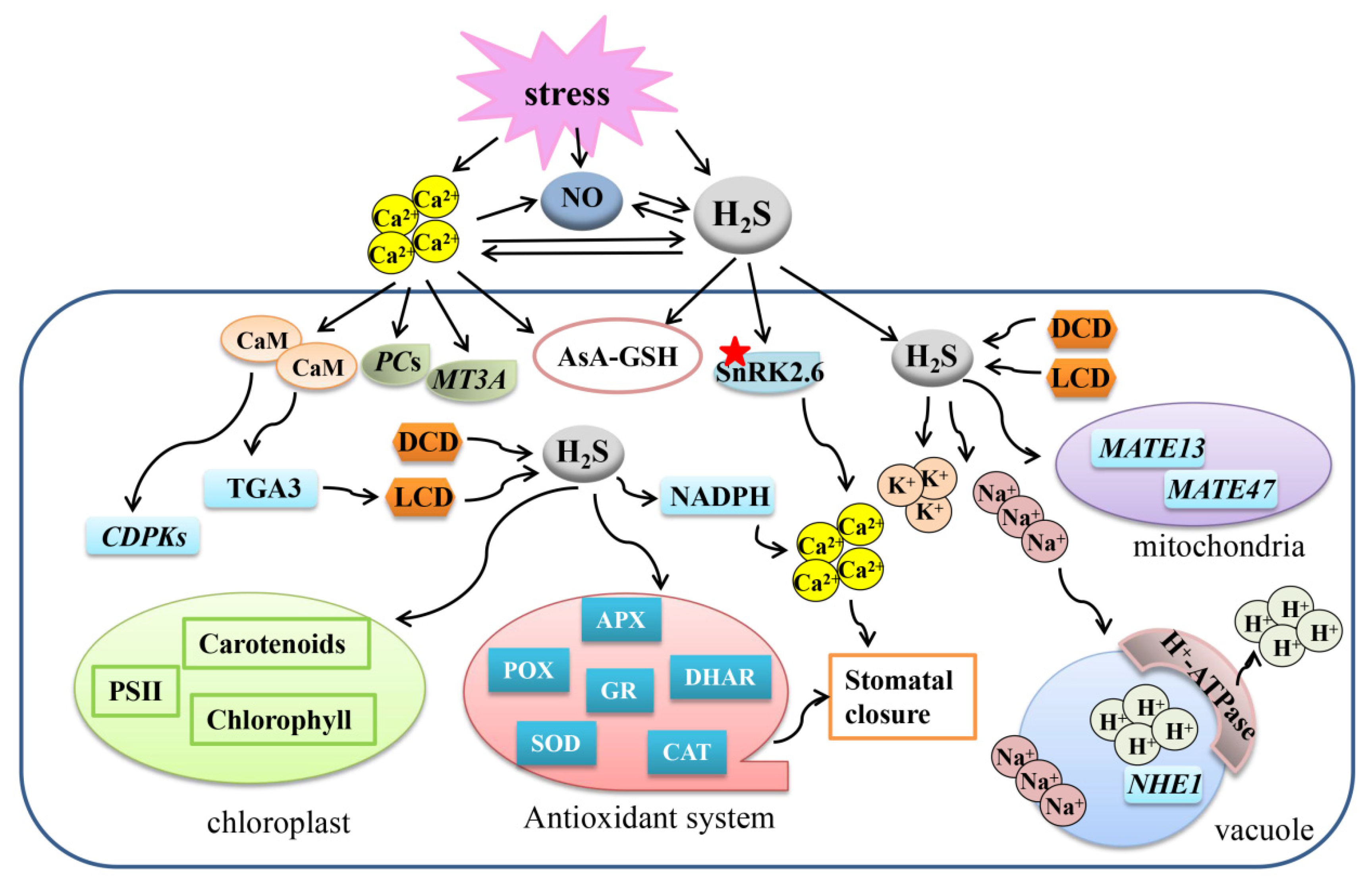
4. Crosstalk between H2S and Ca2+ in Response to Abiotic Stresses
4.1. Crosstalk between H2S and Ca2+ in Response to Heavy Metal Stress
4.2. Crosstalk between H2S and Ca2+ in the Regulation of Stomatal Closure
4.3. Crosstalk between H2S and Ca2+ in Response to Other Stresses
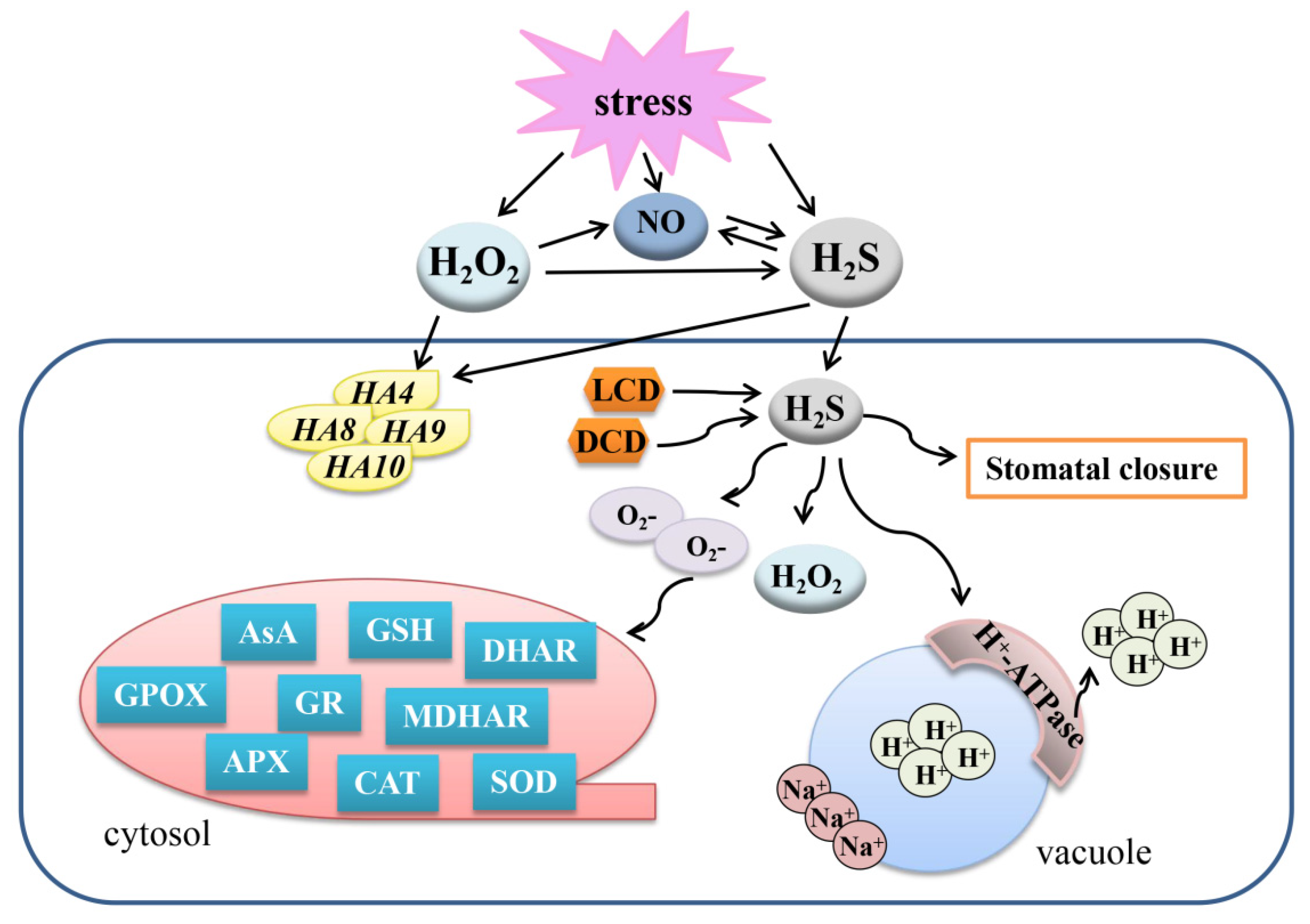
5. Crosstalk between H2S and H2O2 in Response to Abiotic Stresses
5.1. Crosstalk between H2S and H2O2 in Response to Heavy Metal Stress
5.2. Crosstalk between H2S and H2O2 in Response to Salt Stress
5.3. Crosstalk between H2S and H2O2 in Response to Drought Stress
5.4. Crosstalk between H2S and H2O2 in Response to Other Stresses
6. Crosstalk between H2S and Other Signal Molecules in Response to Abiotic Stresses
6.1. Crosstalk between H2S and SA in Response to Abiotic Stresses
6.2. Crosstalk between H2S and ETH in Response to Abiotic Stresses
6.3. Crosstalk between H2S and JA in Response to Abiotic Stresses
6.4. Crosstalk between H2S and Pro in Response to Abiotic Stresses
7. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Kazan, K. Diverse roles of jasmonates and ethylene in abiotic stress tolerance. Trends Plant Sci. 2015, 20, 219–229. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 2015, 756120. [Google Scholar] [CrossRef] [PubMed]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive oxygen species, abiotic stress and stress combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- Devireddy, A.R.; Zandalinas, S.I.; Fichman, Y.; Mittler, R. Integration of reactive oxygen species and hormone signaling during abiotic stress. Plant J. 2021, 105, 459–476. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulos, V.; Christou, A.; Manganaris, G.A. Hydrogen sulfide as a potent regulator of plant responses to abiotic stress factors. Mol. Approaches Plant Abiotic Stress 2013, 353–373. [Google Scholar] [CrossRef]
- Li, W.; Pang, S.; Lu, Z.; Jin, B. Function and mechanism of WRKY transcription factors in abiotic stress responses of plants. Plants 2020, 9, 1515. [Google Scholar] [CrossRef] [PubMed]
- Calderwood, A.; Kopriva, S. Hydrogen sulfide in plants: From dissipation of excess sulfur to signaling molecule. Nitric oxide. 2014, 41, 72–78. [Google Scholar] [CrossRef]
- Wilson, L.G.; Bressan, R.A.; Filner, P. Light-dependent Emission of Hydrogen Sulfide from Plants. Plant Physiol. 1978, 61, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Papenbrock, J.; Riemenschneider, A.; Kamp, A.; Schulz-Vogt, H.N.; Schmidt, A. Characterization of Cysteine-Degrading and H2S-Releasing Enzymes of Higher Plants-From the Field to the Test Tube and Back. Plant Biol. 2007, 9, 582–588. [Google Scholar] [CrossRef]
- Huang, D.; Huo, J.; Liao, W. Hydrogen sulfide: Roles in plant abiotic stress response and crosstalk with other signals. Plant Sci. 2021, 302, 110733. [Google Scholar] [CrossRef]
- Takahashi, H.; Kopriva, S.; Giordano, M.; Saito, K.; Hell, R. Sulfur assimilation in photosynthetic organisms: Molecular functions and regulations of transporters and assimilatory enzymes. Annu. Rev. Plant Biol. 2011, 62, 157–184. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, V.; Kapoor, D.; Kumar, S.; Singh, S.; Dhanjal, D.S.; Datta, S.; Samuel, J.; Dey, P.; Wang, S.; et al. Revealing on hydrogen sulfide and nitric oxide signals coordination for plant growth under stress conditions. Physiol. Plant. 2019, 168, 301–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Z.W.; Deng, L.W. Role of H2S Donors in Cancer Biology. Handb. Exp. Pharmacol. 2015, 230, 243–265. [Google Scholar] [CrossRef] [PubMed]
- Akter, F. The role of hydrogen sulfide in burns. Burns 2016, 42, 519–525. [Google Scholar] [CrossRef]
- Tabassum, R.; Jeong, N.; Jung, J. Therapeutic importance of hydrogen sulfide in age-associated neurodegenerative diseases. Neural. Regen. Res. 2020, 15, 653–662. [Google Scholar] [CrossRef]
- Bhatia, M. H2S and inflammation: An overview. Handb. Exp. Pharmacol. 2015, 230, 165–180. [Google Scholar] [CrossRef]
- Mukherjee, S.; Corpas, F.J. Crosstalk among hydrogen sulfide (H2S), nitric oxide (NO) and carbon monoxide (CO) in root-system development and its rhizosphere interactions: A gaseous interactome. Plant Physiol. Bioch. 2020, 155, 800–814. [Google Scholar] [CrossRef]
- Aroca, A.; Gotor, C.; Romero, L.C. Hydrogen Sulfide Signaling in Plants: Emerging Roles of Protein Persulfidation. Front. Plant Sci. 2018, 9, 1369. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhou, H.; Zhou, M.; Ge, Z.; Zhang, F.; Foyer, C.H.; Yuan, X.; Xie, Y. The coordination of guard-cell autonomous ABA synthesis and DES1 function in situ regulates plant water deficit responses. J. Adv. Res. 2020, 27, 191–197. [Google Scholar] [CrossRef]
- Singh, V.P.; Tripathi, D.K.; Fotopoulos, V. Hydrogen sulfide and nitric oxide signal integration and plant development under stressed/non-stressed conditions. Physiol. Plant. 2020, 168, 239–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corpas, F.J.; Barroso, J.B.; González-Gordo, S.; Muñoz-Vargas, M.A.; Palma, J.M. Hydrogen sulfide: A novel component in Arabidopsis peroxisomes which triggers catalase inhibition. J. Integr. Plant Biol. 2019, 61, 871–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Wang, W.H.; Wu, F.H.; He, E.M.; Liu, X.; Shangguan, Z.P.; Zheng, H.L. Hydrogen sulfide enhances salt tolerance through nitric oxide-mediated maintenance of ion homeostasis in barley seedling roots. Sci. Rep. 2015, 5, 12516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xuan, L.; Li, J.; Wang, X.; Wang, C. Crosstalk between hydrogen sulfide and other signal molecules regulates plant growth and development. Int. J. Mol. Sci. 2020, 21, 4593. [Google Scholar] [CrossRef]
- Ahmad, R.; Ali, S.; Rizwan, M.; Dawood, M.; Farid, M.; Hussain, A.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P. Hydrogen sulfide alleviates chromium stress on cauliflower by restricting its uptake and enhancing antioxidative system. Physiol. Plant. 2020, 168, 289–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Cheng, P.; Wang, Y.; Li, Y.; Su, J.; Chen, Z.; Yu, X.; Shen, W. Genetic elucidation of hydrogen signaling in plant osmotic tolerance and stomatal closure via hydrogen sulfide. Free Radic. Bio. Med. 2020, 161, 1–14. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, C.; Wang, N.; Wei, L.; Li, W.; Yao, Y.; Liao, W. Roles of Small-Molecule Compounds in Plant Adventitious Root Development. Biomolecules 2019, 9, 420. [Google Scholar] [CrossRef] [Green Version]
- Corpas, F.J. Nitric oxide and hydrogen sulfide in higher plants under physiological and stress conditions. Antioxidants 2019, 8, 457. [Google Scholar] [CrossRef] [Green Version]
- Palma, J.M.; Mateos, R.M.; López-Jaramillo, J.; Rodríguez-Ruiz, M.; González-Gordo, S.; Lechuga-Sancho, A.M.; Corpas, F.J. Plant catalases as NO and H2S targets. Redox Biol. 2020, 34, 101525. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.P.; Singh, S.; Kumar, J.; Prasad, S.M. Hydrogen sulfide alleviates toxic effects of arsenate in pea seedlings through up-regulation of the ascorbate-glutathione cycle: Possible involvement of nitric oxide. J. Plant Physiol. 2015, 181, 20–29. [Google Scholar] [CrossRef]
- Kharbech, O.; Houmani, H.; Chaoui, A.; Corpas, F.J. Alleviation of Cr(VI)-induced oxidative stress in maize (Zea mays L.) seedlings by NO and H2S donors through differential organ-dependent regulation of ROS and NADPH-recycling metabolisms. J. Plant Physiol. 2017, 219, 71–80. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Shen, W. Roles of hydrogen sulfide and nitric oxide in the alleviation of cadmium-induced oxidative damage in alfalfa seedling roots. Biometals 2012, 25, 617–631. [Google Scholar] [CrossRef]
- Ozfidan-Konakci, C.; Yildiztugay, E.; Elbasan, F.; Kucukoduk, M.; Turkan, I. Hydrogen sulfide (H2S) and nitric oxide (NO) alleviate cobalt toxicity in wheat (Triticum aestivum L.) by modulating photosynthesis, chloroplastic redox and antioxidant capacity. J. Hazard. Mater. 2020, 388, 122061. [Google Scholar] [CrossRef]
- Shi, H.; Ye, T.; Chan, Z. Nitric oxide-activated hydrogen sulfide is essential for cadmium stress response in bermudagrass (Cynodon dactylon (L). Pers.). Plant Physiol. Bioch. 2014, 74, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ji, F.; Zhang, Y.; Hou, J.; Liu, W.; Huang, J.; Liang, W. Interactions between hydrogen sulphide and nitric oxide regulate two soybean citrate transporters during the alleviation of aluminium toxicity. Plant Cell Environ. 2019, 42, 2340–2356. [Google Scholar] [CrossRef]
- Amooaghaie, R.; Zangene-Madar, F.; Enteshari, S. Role of two-sided crosstalk between NO and H2S on improvement of mineral homeostasis and antioxidative defense in Sesamum indicum under lead stress. Ecotox. Environ. Saf. 2017, 139, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Akram, N.A.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Exogenously supplied silicon (Si) improves cadmium tolerance in pepper (Capsicum annuum L.) by up-regulating the synthesis of nitric oxide and hydrogen sulfide. J. Biotechnol. 2020, 316, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Responses of nitric oxide and hydrogen sulfide in regulating oxidative defence system in wheat plants grown under cadmium stress. Physiol. Plant. 2019, 168, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.J.; Batista Fontes, E.P.; Modolo, L.V. Salinity-induced accumulation of endogenous H2S and NO is associated with modulation of the antioxidant and redox defense systems in Nicotiana tabacum L. cv. Havana. Plant Sci. 2017, 256, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Cui, W.; Xu, S.; Shen, W.; Wang, R. Hydrogen sulfide enhances alfalfa (Medicago sativa) tolerance against salinity during seed germination by nitric oxide pathway. Plant Soil 2012, 351, 107–119. [Google Scholar] [CrossRef]
- Da-Silva, C.J.; Mollica, D.C.F.; Vicente, M.H.; Peres, L.E.P.; Modolo, L.V. NO, hydrogen sulfide does not come first during tomato response to high salinity. Nitric Oxide 2018, 76, 164–173. [Google Scholar] [CrossRef]
- Kaya, C.; Higgs, D.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Integrative roles of nitric oxide and hydrogen sulfide in melatonin-induced tolerance of pepper (Capsicum annuum L.) plants to iron deficiency and salt stress alone or in combination. Physiol. Plant. 2020, 168, 256–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Yang, S.; Long, W.; Yang, G.; Shen, Z. Hydrogen sulphide may be a novel downstream signal molecule in nitric oxide-induced heat tolerance of maize (Zea mays L.) seedlings. Plant Cell Environ. 2013, 36, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Peng, R.; Bian, Z.; Zhou, L.; Cheng, W.; Hai, N.; Yang, C.; Yang, T.; Wang, X.; Wang, C. Hydrogen sulfide enhances nitric oxide-induced tolerance of hypoxia in maize (Zea mays L.). Plant Cell Rep. 2016, 35, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Hou, Z.; Liu, G.; Hou, L.; Liu, X. Hydrogen sulfide may function downstream of nitric oxide in ethylene-induced stomatal closure in Vicia faba L. J. Integr. Agric. 2012, 11, 1644–1653. [Google Scholar] [CrossRef]
- Mishra, V.; Singh, P.; Tripathi, D.K.; Corpas, F.J.; Singh, V.P. Nitric oxide and hydrogen sulfide: An indispensable combination for plant functioning. Trends Plant Sci. 2021, 1360–1385. [Google Scholar] [CrossRef]
- Khan, M.N.; Mobin, M.; Abbas, Z.K.; Siddiqui, M.H. Nitric oxide-induced synthesis of hydrogen sulfide alleviates osmotic stress in wheat seedlings through sustaining antioxidant enzymes, osmolyte accumulation and cysteine homeostasis. Nitric Oxide 2017, 68, 91–102. [Google Scholar] [CrossRef]
- Lisjak, M.; Srivastava, N.; Teklic, T.; Civale, L.; Lewandowski, K.; Wilson, I.; Wood, M.E.; Whiteman, M.; Hancock, J.T. A novel hydrogen sulfide donor causes stomatal opening and reduces nitric oxide accumulation. Plant Physiol. Bioch. 2010, 48, 931–935. [Google Scholar] [CrossRef]
- Antoniou, C.; Xenofontos, R.; Chatzimichail, G.; Christou, A.; Kashfi, K.; Fotopoulos, V. Exploring the potential of nitric oxide and hydrogen sulfide (NOSH)-releasing synthetic compounds as novel priming agents against drought stress in Medicago sativa plants. Biomolecules 2020, 10, 120. [Google Scholar] [CrossRef] [Green Version]
- Urhonen, T.; Lie, A.; Aamodt, G. Associations between long commutes and subjective health complaints among railway workers in Norway. Prev. Med. Rep. 2016, 4, 490–495. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Duan, Y.; Hua, D.; Fan, G.; Wang, L.; Liu, Y.; Chen, Z.; Han, L.; Qu, L.; Gong, Z. DEXH box RNA helicase-mediated mitochondrial reactive oxygen species production in Arabidopsis mediates crosstalk between abscisic acid and auxin signaling. Plant Cell 2012, 24, 1815–1833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Xue, S. Interplay between hydrogen sulfide and other signaling molecules in the regulation of guard cell signaling and abiotic/biotic stress response. Plant Commun. 2021, 2, 100179. [Google Scholar] [CrossRef] [PubMed]
- García-Mata, C.; Lamattina, L. Hydrogen sulphide, a novel gasotransmitter involved in guard cell signalling. New Phytol. 2010, 188, 977–984. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, Y.; Zhai, F.; Zhang, J.; Zhang, F.; Yuan, X.; Xie, Y. Hydrogen sulfide promotes rice drought tolerance via reestablishing redox homeostasis and activation of ABA biosynthesis and signaling. Plant Physiol. Bioch. 2020, 155, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Xue, S.; Luo, Y.; Tian, B.; Fang, H.; Li, H.; Pei, Y. Hydrogen sulfide interacting with abscisic acid in stomatal regulation responses to drought stress in Arabidopsis. Plant Physiol. Bioch. 2013, 62, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Ding, H.; Wang, C.; Qin, H.; Han, Q.; Hou, J.; Lu, H.; Xie, Y.; Guo, T. Alleviation of drought stress by hydrogen sulfide is partially related to the abscisic acid signaling pathway in wheat. PLoS ONE 2016, 11, e163082. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Jin, Z.; Zhang, L.; Liu, X.; Yang, G.; Pei, Y. H2S is involved in ABA-mediated stomatal movement through MPK4 to alleviate drought stress in Arabidopsis thaliana. Plant Soil 2019, 435, 295–307. [Google Scholar] [CrossRef]
- Hua, D.; Wang, C.; He, J.; Liao, H.; Duan, Y.; Zhu, Z.; Guo, Y.; Chen, Z.; Gong, Z. A plasma membrane receptor kinase, GHR1, mediates abscisic acid- and hydrogen peroxide-regulated stomatal movement in Arabidopsis. Plant Cell 2012, 24, 2546–2561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Wan, R.; Shi, Y.; Xue, S. Hydrogen sulfide activates S-type anion channel via OST1 and Ca2+ Modules. Mol. Plant 2016, 9, 489–491. [Google Scholar] [CrossRef] [Green Version]
- Golldack, D.; Lüking, I.; Yang, O. Plant tolerance to drought and salinity: Stress regulating transcription factors and their functional significance in the cellular transcriptional network. Plant Cell Rep. 2011, 30, 1383–1391. [Google Scholar] [CrossRef]
- Shan, C.; Zhang, S.; Zhou, Y. Hydrogen sulfide is involved in the regulation of ascorbate-glutathione cycle by exogenous ABA in wheat seedling leaves under osmotic stress. Cereal Res. Commun. 2017, 45, 411–420. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhou, M.; Ge, Z.; Shen, J.; Zhou, C.; Gotor, C.; Romero, L.C.; Duan, X.; Liu, X.; Wu, D.; et al. Abscisic acid-triggered guard cell L-cysteine desulfhydrase function and in situ hydrogen sulfide production contributes to heme oxygenase-modulated stomatal closure. Plant Cell Environ. 2020, 43, 624–636. [Google Scholar] [CrossRef] [PubMed]
- Scuffi, D.; álvarez, C.; Laspina, N.; Gotor, C.; Lamattina, L.; García-Mata, C. Hydrogen sulfide generated by L-cysteine desulfhydrase acts upstream of nitric oxide to modulate abscisic acid-dependent stomatal closure. Plant Physiol. 2014, 166, 2065–2076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Jia, H.; Wang, X.; Shi, C.; Wang, X.; Ma, P.; Wang, J.; Ren, M.; Li, J. Hydrogen sulfide positively regulates abscisic acid signaling through persulfidation of SnRK2.6. Guard Cells 2020, 13, 732–744. [Google Scholar] [CrossRef]
- Hou, Z.; Wang, L.; Liu, J.; Hou, L.; Liu, X. Hydrogen sulfide regulates ethylene-induced stomatal closure in Arabidopsis thaliana. J. Integr. Plant Biol. 2013, 55, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Böhmer, M.; Hu, H.; Nishimura, N.; Schroeder, J.I. Guard cell signal transduction network: Advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu. Rev. Plant Biol. 2010, 61, 561–591. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Zhang, J.; Zhou, M.; Zhou, H.; Cui, B.; Gotor, C.; Romero, L.C.; Fu, L.; Yang, J.; Foyer, C.H.; et al. Persulfidation-based modification of cysteine desulfhydrase and the NADPH oxidase RBOHD controls guard cell abscisic acid signaling. Plant Cell 2020, 32, 1000–1017. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, J.; Shen, J.; Zhou, H.; Zhao, D.; Gotor, C.; Romero, L.C.; Fu, L.; Li, Z.; Yang, J.; et al. Hydrogen sulfide-linked persulfidation of ABI4 controls ABA responses through the transactivation of MAPKKK18. Arabidopsis 2021, 14, 921–936. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, J.; Shen, J.; Zhou, M.; Yuan, X.; Xie, Y. Redox-based protein persulfidation in guard cell ABA signaling. Plant Signal. Behav. 2020, 15, 1741987. [Google Scholar] [CrossRef]
- Peiter, E. The plant vacuole: Emitter and receiver of calcium signals. Cell Calcium 2011, 50, 120–128. [Google Scholar] [CrossRef]
- Fang, H.; Jing, T.; Liu, Z.; Zhang, L.; Jin, Z.; Pei, Y. Hydrogen sulfide interacts with calcium signaling to enhance the chromium tolerance in Setaria italica. Cell Calcium 2014, 56, 472–481. [Google Scholar] [CrossRef]
- Fang, H.; Liu, Z.; Long, Y.; Liang, Y.; Jin, Z.; Zhang, L.; Liu, D.; Li, H.; Zhai, J.; Pei, Y. The Ca2/calmodulin2-binding transcription factor TGA3 elevates LCD expression and H2S production to bolster Cr6+ tolerance in Arabidopsis. Plant J. 2017, 91, 1038–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, H.; Li, Y.; He, L. The central role of hydrogen sulfide in plant responses to toxic metal stress. Ecotox. Environ. Saf. 2018, 157, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Valivand, M.; Amooaghaie, R.; Ahadi, A. Seed priming with H2S and Ca2+ trigger signal memory that induces cross-adaptation against nickel stress in zucchini seedlings. Plant Physiol. Biochem. 2019, 143, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Shi, C.; Ding, X.; Liu, C.; Li, J. Hydrogen sulfide induces Ca2+ signal in guard cells by regulating reactive oxygen species accumulation. Plant Signal. Behav. 2020, 15, 1805228. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gong, M.; Xie, H.; Yang, L.; Li, J. Hydrogen sulfide donor sodium hydrosulfide-induced heat tolerance in tobacco (Nicotiana tabacum L) suspension cultured cells and involvement of Ca2+ and calmodulin. Plant Sci. 2012, 185, 185–189. [Google Scholar] [CrossRef]
- Kolupaev, Y.E.; Firsova, E.N.; Yastreb, T.O.; Lugovaya, A.A. The participation of calcium ions and reactive oxygen species in the induction of antioxidant enzymes and heat resistance in plant cells by hydrogen sulfide donor. Appl. Biochem. Microbiol. 2017, 53, 573–579. [Google Scholar] [CrossRef]
- Khan, M.N.; Siddiqui, M.H.; Mukherjee, S.; Alamri, S.; Al-Amri, A.A.; Alsubaie, Q.D.; Al-Munqedhi, B.; Ali, H.M. Calcium-hydrogen sulfide crosstalk during K+-deficient NaCl stress operates through regulation of Na+/H+ antiport and antioxidative defense system in mung bean roots. Plant Physiol. Biochem. 2021, 159, 211–225. [Google Scholar] [CrossRef] [PubMed]
- Waszczak, C.; Carmody, M.; Kangasjarvi, J. Reactive oxygen species in plant signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef] [Green Version]
- Černý, M.; Habánová, H.; Berka, M.; Luklová, M.; Brzobohatý, B. Hydrogen peroxide: Its role in plant biology and crosstalk with signalling networks. Int. J. Mol. Sci. 2018, 19, 2812. [Google Scholar] [CrossRef] [Green Version]
- Lv, W.; Yang, L.; Xu, C.; Shi, Z.; Shao, J.; Xian, M.; Chen, J. Cadmium disrupts the balance between hydrogen peroxide and superoxide radical by regulating endogenous hydrogen sulfide in the root tip of Brassica rapa. Front. Plant Sci. 2017, 8, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kabała, K.; Zboińska, M.; Głowiak, D.; Reda, M.; Jakubowska, D.; Janicka, M. Interaction between the signaling molecules hydrogen sulfide and hydrogen peroxide and their role in vacuolar H+-ATPase regulation in cadmium-stressed cucumber roots. Physiol. Plant. 2019, 166, 688–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janicka, M.; Reda, M.; Czyżewska, K.; Kabała, K. Involvement of signalling molecules NO, H2O2 and H2S in modification of plasma membrane proton pump in cucumber roots subjected to salt or low temperature stress. Funct. Plant Biol. 2018, 45, 428–439. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, Y.; He, X.; Yong, B.; Peng, Y.; Zhang, X.; Ma, X.; Yan, Y.; Huang, L.; Nie, G. The hydrogen sulfide, a downstream signaling molecule of hydrogen peroxide and nitric oxide, involves spermidine-regulated transcription factors and antioxidant defense in white clover in response to dehydration. Environ. Exp. Bot. 2019, 161, 255–264. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, X.; Wang, X.; Wu, K.; Li, P.; Chang, N.; Wang, J.; Wang, F.; Li, J.; Bi, Y. Glucose-6-phosphate dehydrogenase and alternative oxidase are involved in the cross tolerance of highland barley to salt stress and UV-B radiation. J. Plant Physiol. 2015, 181, 83–95. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.; Zhao, Y.; Zhang, X.; Zhang, S.; Bo, L.; Wang, Y.; Ding, Y.; An, L. Putrescine protects hulless barley from damage due to UV-B stress via H2S- and H2O2-mediated signaling pathways. Plant Cell Rep. 2016, 35, 1155–1168. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Luo, L.; Sun, Y. Signal crosstalk between nitric oxide and hydrogen sulfide may be involved in hydrogen peroxide-induced thermotolerance in maize seedlings. Russ. J. Plant Physiol. 2015, 62, 507–514. [Google Scholar] [CrossRef]
- Janda, M.; Ruelland, E. Magical mystery tour: Salicylic acid signalling. Environ. Exp. Bot. 2015, 114, 117–128. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, M.; Zhou, H.; Zhao, D.; Gotor, C.; Romero, L.C.; Shen, J.; Ge, Z.; Zhang, Z.; Shen, W.; et al. Hydrogen sulfide, a signaling molecule in plant stress responses. J. Integr. Plant Biol. 2021, 63, 146–160. [Google Scholar] [CrossRef]
- Li, Z. Synergistic effect of antioxidant system and osmolyte in hydrogen sulfide and salicylic acid crosstalk-induced heat tolerance in maize (Zea mays L.) seedlings. Plant Signal. Behav. 2015, 10, e1051278. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Xie, L.; Li, X. Hydrogen sulfide acts as a downstream signal molecule in salicylic acid-induced heat tolerance in maize (Zea mays L.) seedlings. J. Plant Physiol. 2015, 177, 121–127. [Google Scholar] [CrossRef]
- Qiao, Z.; Jing, T.; Liu, Z.; Zhang, L.; Jin, Z.; Liu, D.; Pei, Y. H2S acting as a downstream signaling molecule of SA regulates Cd tolerance in Arabidopsis. Plant Soil. 2015, 393, 137–146. [Google Scholar] [CrossRef]
- Kaya, C. Salicylic acid-induced hydrogen sulphide improves lead stress tolerance in pepper plants by upraising the ascorbate-glutathione cycle. Physiol. Plant. 2020, 173, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Chen, S.; Liu, D.; Liesche, J.; Shi, C.; Wang, J.; Ren, M.; Wang, X.; Yang, J.; Shi, W.; et al. Ethylene-induced hydrogen sulfide negatively regulates ethylene biosynthesis by persulfidation of ACO in tomato under osmotic stress. Front. Plant Sci. 2018, 9, 1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, Y.; Wu, X.; Sun, M.; Peng, F. Hydrogen sulfide alleviates waterlogging-induced damage in peach seedlings via enhancing antioxidative system and inhibiting ethylene synthesis. Front Plant Sci. 2020, 11, 696. [Google Scholar] [CrossRef] [PubMed]
- Pauwels, L.; Barbero, G.F.; Geerinck, J.; Tilleman, S.; Grunewald, W.; Pérez, A.C.; Chico, J.M.; Bossche, R.V.; Sewell, J.; Gil, E.; et al. NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature 2010, 464, 788–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, T.; Song, S.; Ren, Q.; Wu, D.; Huang, H.; Chen, Y.; Fan, M.; Peng, W.; Ren, C.; Xie, D. The Jasmonate-ZIM-domain proteins interact with the WD-Repeat/bHLH/MYB complexes to regulate Jasmonate-mediated anthocyanin accumulation and trichome initiation in Arabidopsis thaliana. Plant Cell 2011, 23, 1795–1814. [Google Scholar] [CrossRef] [Green Version]
- Qi, T.; Huang, H.; Song, S.; Xie, D. Regulation of Jasmonate-mediated stamen development and seed production by a bHLH-MYB complex in Arabidopsis. Plant Cell 2015, 27, 1620–1633. [Google Scholar] [CrossRef] [Green Version]
- Qi, T.; Wang, J.; Huang, H.; Liu, B.; Gao, H.; Liu, Y.; Song, S.; Xie, D. Regulation of Jasmonate-Induced Leaf Senescence by Antagonism between bHLH Subgroup IIIe and IIId Factors in Arabidopsis. Plant Cell 2015, 27, 1634–1649. [Google Scholar] [CrossRef] [Green Version]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Hu, Y.; Zhang, G.; Jiang, Y.; Chen, X.; Yu, D. Jasmonate negatively regulates stomatal development in Arabidopsis cotyledons. Plant Physiol. 2018, 176, 2871–2885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, G.; Zhou, L.; Wang, Y.; Zhang, G.; Chen, X. Hydrogen sulfide acts downstream of jasmonic acid to inhibit stomatal development in Arabidopsis. Planta 2020, 251, 42. [Google Scholar] [CrossRef]
- He, H.; He, L. Regulation of gaseous signaling molecules on proline metabolism in plants. Plant Cell Rep. 2018, 37, 387–392. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, Y.; Li, C.; Huang, D.; Zhang, J.; Wang, C.; Li, W.; Wang, N.; Deng, Y.; Liao, W. Research progress on the functions of gasotransmitters in plant responses to abiotic stresses. Plants 2019, 8, 605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, B.; Qiao, Z.; Zhang, L.; Li, H.; Pei, Y. Hydrogen sulfide and proline cooperate to alleviate cadmium stress in foxtail millet seedlings. Plant Physiol. Bioch. 2016, 109, 293–299. [Google Scholar] [CrossRef] [PubMed]

| Crosstalk between H2S and other Molecules | Stresses | Plant Species | Tissue | Regulated Genes | References |
|---|---|---|---|---|---|
| H2S and NO | salt stress | Medicago sativa | seeds | APX-1, APX-2, and Cu/Zn-SOD | [39] |
| Hordeum vulgare L. | seedlings | HvHA, HvVHA-β, HvSOS1, HvVNHX2, HvAKT1 and HvHAK4 | [22] | ||
| Solanum lycopersicum | seedlings | SlL-DES, SlCAS and SlCS | [40] | ||
| drought | M. sativa L. | leaves | GST17, Cu/ZnSOD, FeSOD, NR, cAPX, PIP | [48] | |
| hypoxia stress | Zea mays L. | seedlings | P4H, ADH, CRT1, GS, CYP51 and ME | [43] | |
| cadmium stress | M. sativa L. | seedlings | Cu/Zn–SOD, APX and POD | [31] | |
| cobalt stress | Triticum aestivum L. | seedlings | RbcL | [32] | |
| aluminum stress | Glycine max L. | seedlings | MATE13, MATE47, MATE58, MATE74, MATE79, MATE84, and MATE87 | [34] | |
| H2S and ABA | drought | Oryza sativa L. | seedlings | NCED2, NCED3, NCED5, AREB1, AREB8, bZIP23 and LEA3 | [54] |
| Arabidopsis | seedlings | TPC1, GORK, SKOR, KCO1, MYP5, ACA9, ACA11, CAX1, SLAC1, AKT1A, KT2, KC1 and KAT1 | [55] | ||
| T. aestivum L. | leaves and roots | TaZEP, TaNCED, TaAAO and TaSDR | [56] | ||
| Arabidopsis thaliana | - | MAPKs | [57] | ||
| chromium stress | A. thaliana | seedlings | LCD | [72] | |
| nickel stress | Cucurbita pepo L. | seedlings | CDPK and PCS1 | [74] | |
| H2S and Ca2+ | chromium stress | Setaria italica | seedlings | MT3A, PCS, CaM, CBL and CDPK | [71] |
| H2S-H2O2 | cadmium stress | Brassica rapa. | seedlings | Br_UPB1A, Br_UPB1B↑; Bra035235, Bra033551, Bra006423, ra023639 | [89] |
| cadmium stress | Cucumis sativus L. | roots | CsVHA-A, CsVHA-B, CsVHA-a1, CsVHA-a2, CsVHA-a3, CsVHA-c1, CsVHA-c2 and CsVHA-c3 | [82] | |
| H2S, NO and H2O2 | salt or low temperature | C. sativus L. | roots | CsHA1, CsHA2, CsH4, CsH8, CsH9 and CsHA10 | [83] |
| dehydration | Trifolium repens | seedlings | bZIP37, bZIP107, DREB2, DREB4 and WRKY108715 | [84] | |
| H2S and ETH | osmotic stress | S. lycopersicum | seedlings | LeACO1 and LeACO2 | [94] |
| H2S and Pro | cadmium stress | Foxtail millet | seedlings | PDH and P5CR | [105] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Deng, Y.; Liu, Z.; Liao, W. Hydrogen Sulfide in Plants: Crosstalk with Other Signal Molecules in Response to Abiotic Stresses. Int. J. Mol. Sci. 2021, 22, 12068. https://doi.org/10.3390/ijms222112068
Wang C, Deng Y, Liu Z, Liao W. Hydrogen Sulfide in Plants: Crosstalk with Other Signal Molecules in Response to Abiotic Stresses. International Journal of Molecular Sciences. 2021; 22(21):12068. https://doi.org/10.3390/ijms222112068
Chicago/Turabian StyleWang, Chunlei, Yuzheng Deng, Zesheng Liu, and Weibiao Liao. 2021. "Hydrogen Sulfide in Plants: Crosstalk with Other Signal Molecules in Response to Abiotic Stresses" International Journal of Molecular Sciences 22, no. 21: 12068. https://doi.org/10.3390/ijms222112068







