Abstract
Cadmium (Cd) is one of the most injurious heavy metals, affecting plant growth and development. Melatonin (N-acetyl-5-methoxytryptamine) was discovered in plants in 1995, and it is since known to act as a multifunctional molecule to alleviate abiotic and biotic stresses, especially Cd stress. Endogenously triggered or exogenously applied melatonin re-establishes the redox homeostasis by the improvement of the antioxidant defense system. It can also affect the Cd transportation and sequestration by regulating the transcripts of genes related to the major metal transport system, as well as the increase in glutathione (GSH) and phytochelatins (PCs). Melatonin activates several downstream signals, such as nitric oxide (NO), hydrogen peroxide (H2O2), and salicylic acid (SA), which are required for plant Cd tolerance. Similar to the physiological functions of NO, hydrogen sulfide (H2S) is also involved in the abiotic stress-related processes in plants. Moreover, exogenous melatonin induces H2S generation in plants under salinity or heat stress. However, the involvement of H2S action in melatonin-induced Cd tolerance is still largely unknown. In this review, we summarize the progresses in various physiological and molecular mechanisms regulated by melatonin in plants under Cd stress. The complex interactions between melatonin and H2S in acquisition of Cd stress tolerance are also discussed.
1. Introduction
Heavy metal pollution is the most widespread contamination resulting from anthropogenic activities in the world [1]. It has raised concerns about its various harmful risks to human health via the metal transfer along the food chain [2]. Among the heavy metals, cadmium (Cd) is a toxic element and poses a hazardous impact to living organisms, such as renal tubular dysfunction and bone disease [3]. In plants, Cd disturbs a range of important biochemical, morphological, physiological, and molecular processes, thus resulting in chlorosis and shunted growth [4,5]. Cd stress deceases the chlorophyll content, net photosynthetic rate, stomatal conductance, intracellular CO2 concentration, and transpiration rate [4,5,6]. Cd stress induces the excess accumulation of reactive oxygen species (ROS), mainly due to the imbalance between ROS generation and scavenging [7,8]. Increased concentrations of ROS further induce the lipid peroxidation and oxidative damage, destructing plant membranes, macromolecules, and organelles [7,8]. Additionally, excessive bioaccumulation of Cd in plants inhibits Fe and Zn uptake, and disrupts the uptake and transport of K, Ca, Mg, P, and Mn [9]. In response to Cd stress, plants have evolved the complex biochemical and molecular mechanisms that modulate ROS homeostasis and Cd compartmentation and chelation [7,10,11,12]. Plant hormones (ethylene, salicylic acid (SA), abscisic acid (ABA), jasmonic acid (JA), auxin, brassinosteroids (BRs), and strigolactones (SLs)) and signaling molecules (nitric oxide (NO), carbon monoxide (CO), hydrogen sulfide (H2S), and Ca2+) are involved in plant response to Cd stress [13,14]. Moreover, recent studies have reported that melatonin acts as a master regulator in plant Cd tolerance.
Melatonin (N-acetyl-5-methoxytryptamine) was discovered in plants in 1995, and it is since known to act as a pleiotropic molecule to participate in multiple physiological processes, such as plant growth and development, and protection against abiotic and biotic stresses [15,16]. In recent years, numerous studies have focused on the protective role of melatonin against Cd stress in plants [17]. Application of exogenous melatonin increased photosynthetic pigments, and improved relative water content and stomatal conductance in mallow plants upon Cd stress [18]. Many results showed that melatonin could re-establish redox homeostasis by certain enzymatic and non-enzymatic antioxidant defense systems to alleviate Cd-induced oxidative stress [19,20]. In addition, melatonin decreased Cd accumulation via regulating the transcripts of several heavy metal transporter genes to restrict Cd influx, and promote Cd efflux and chelation [19,21]. Moreover, NO and hydrogen peroxide (H2O2) signaling, microRNAs, heat shock factor HsfA1a and flavonoids may be involved in melatonin-mediated Cd tolerance in plants [19,22,23,24,25].
Apart from NO and H2O2, H2S may also function as a signaling molecule in numerous processes of plants [26,27,28]. It is produced from the degradation of L-cysteine by L-cysteine desulfhydrase (L-CDes), which is encoded by L-cysteine desulfhydrase (LCD), D-cysteine desulfhydrase (DCD), and L-cysteine desulfhydrase1 (DES1) [29,30]. Exogenous application of H2S donors regulated plant growth, and conferred tolerance to salinity, heavy metal, heat, and drought stress among others [27,31,32]. H2S enhanced photosynthesis and antioxidant enzyme activity, and up-regulated the transcripts of PC genes to alleviate Cd stress in tobacco [33]. Moreover, H2S homeostasis and L-cysteine desulfhydrase activity were involved in melatonin-modulated salt stress tolerance in tomato and cucumber seedlings [31,34,35]. Crosstalk of melatonin and H2S in alleviating heat stress was also suggested in wheat [36]. However, the involvement of H2S action in melatonin-mediated abiotic stress tolerance is still largely unknown, especially in Cd stress.
Over the past several years, numerous studies focusing on the role of melatonin in alleviating Cd stress have been steadily increasing in plants. Here, we systematically review and highlight the advanced developments which explore the melatonin-mediated Cd tolerance. For a better understanding of this topic, we also propose and discuss the future studies on the complex interactions between melatonin and H2S during Cd stress.
2. Role of Melatonin in Plant Abiotic Stress Responses
2.1. Melatonin Biosynthesis and Catabolism
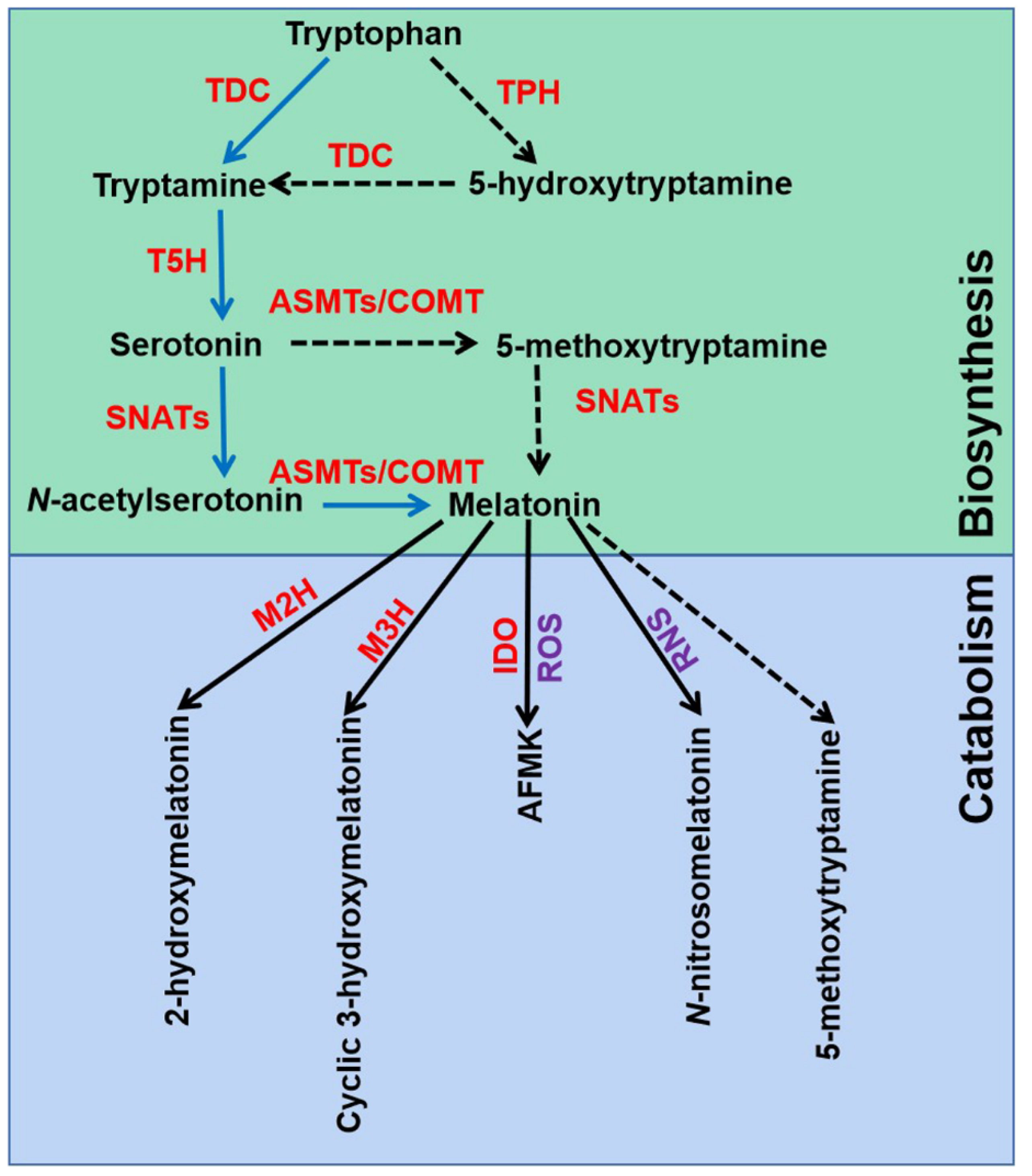
The melatonin metabolic pathway in plants contains two major parts: biosynthesis and catabolism (Figure 1). Melatonin was discovered and confirmed by an isotope tracer study of St. John’s wort (Hypericum perforatum L. cv. Anthos) seedlings [15,37]. It was found that melatonin is synthesized via four continual enzymatic reactions from tryptophan, requiring at least six enzymes: tryptophan decarboxylase (TDC), tryptophan hydroxylase (TPH), tryptamine 5-hydroxylase (T5H), N-acetylserotonin methyltransferase (ASMT), and serotonin N-acetyltransferase (SNAT) [17]. T5H-catalyzed hydroxylation of tryptamine is an important step of melatonin biosynthesis in rice (Oryza sativa) [38]. In animals, serotonin is initially acetylated to form N-acetylserotonin, and then O-methylated to form melatonin (named NM pathway) [39]. It has also been found that serotonin is O-methylated to form 5-methoxytryptamine, and then acetylated to form melatonin (named MN pathway) [39]. Both NM and MN pathways exist in plants [40].
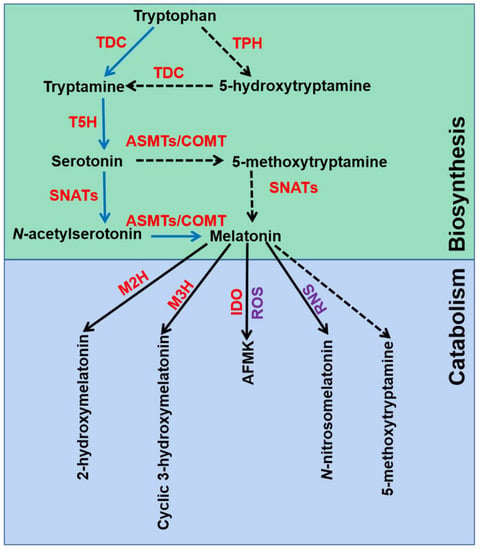
Figure 1.
Melatonin biosynthesis and metabolic pathways in plants. TDC, tryptophan decarboxylase; T5H, tryptamine 5-hydroxylase; TPH, tryptophan hydroxylase; SNATs, serotonin N-acetyltransferases; ASMTs, N-acetylserotonin-O-methyltransferases; COMT, caffeic acid O-methyltransferase; M2H, melatonin 2-hydroxylase; M3H, melatonin 3-hydroxylase; IDO, indoleamine 2,3-dioxygenase; AFMK, N1-acetyl-N2-formyl-5-methoxykynuramine; ROS, reactive oxygen species; RNS, reactive nitrogen species. The green box indicates melatonin biosynthesis pathways, and blue box indicates melatonin metabolic pathways.
Melatonin can be degraded by two distinct routes: non-enzymatic and enzymatic transformations [17]. Transgenic tomato (Solanum lycopersicum) plants expressing the gene encoding indoleamine 2,3-dioxygenase (IDO) in rice showed reduced melatonin levels [41]. Thus, the pathway that melatonin converts to N1-acetyl-N2-formyl-5-methoxykynuramine (AFMK) exists in plants. Tan and Reiter speculated that AFMK is the product of melatonin interaction with ROS, which generated during photosynthesis [39]. This might reflect the important role of melatonin in detoxifying ROS accumulation. In addition, melatonin hydroxylation metabolites, 2-hydroxymelatonin (2-OHMel) and cyclic 3-hydroxymelatonin (c3-OHMel), have been identified in plants. Their formation is attributed to melatonin 2-hydroxylase (M2H) and melatonin 3-hydroxylase (M3H), respectively [42,43,44]. Singh et al. suggested that N-nitrosomelatonin (NOmela) likely served as a nitric oxide (NO) carrier that participated in the redox signal transduction [45]. Nevertheless, Mukherjee considered that NOmela served as an intracellular NO reserve in plants was questionable due to its sensitive and unstable characteristics [46]. The processes of NOmela formation and transport are not fully understood and should be thoroughly investigated. In addition, whether 5-methoxytryptamine (5-MT) formed by melatonin deacetylation is of physiological importance remains to be investigated in plants.
2.2. Melatonin Acts as a Master Regulator in Plant Abiotic Stress
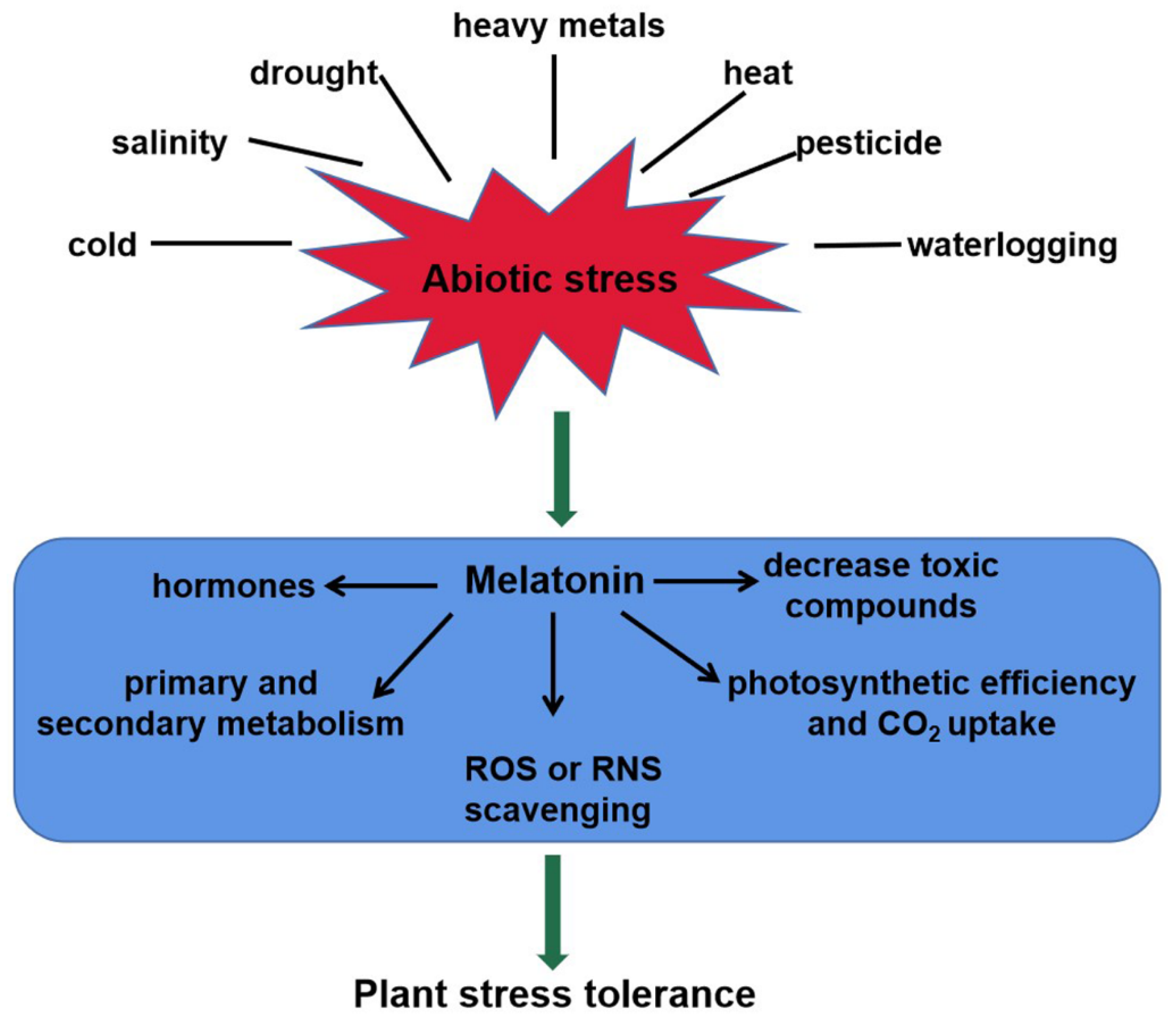
As a master regulator, melatonin plays important roles in plant tolerance to abiotic stresses, such as heavy metals, drought, salinity, cold, heat, waterlogging, and pesticides [19,47,48,49,50,51,52]. This review shows schematically the melatonin-mediated responses to abiotic stresses in plants (Figure 2). Melatonin levels are strongly induced by the above unfavorable conditions. For instance, endogenous melatonin level in Arabidopsis wild-type plants was increased in response to salt stress [47]. Loss-of-function mutation atsnat in the AtSNAT gene showed lower endogenous melatonin content and sensitivity to salinity stress [47]. Cold stress induced melatonin accumulation by upregulating the relative expression of ClASMT in watermelon plants [49]. In tomato seedlings, Cd stress induced COMT1 expression, and thereby improved the accumulation of melatonin [22]. Transcription factor heat shock factor A1a (HsfA1a) bound to the COMT1 gene promoter and activated the transcription of COMT1 gene under Cd stress [22]. However, the post-translational regulation of melatonin biosynthesis genes and modification of related proteins still remains largely unknown and should be elucidated in future.
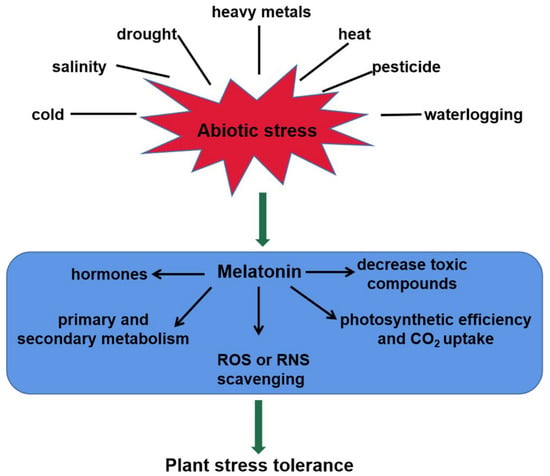
Figure 2.
The roles of melatonin in plant tolerance to abiotic stress. Melatonin content of plants increases significantly in responses to abiotic stresses, such as heavy metals, salinity, drought, heat, cold, waterlogging, and pesticides. It confers plant tolerance via multiple mechanisms, including ROS or RNS scavenging, toxic compounds decrease, photosynthetic efficiency increase, interaction with hormones, and secondary metabolite biosynthesis. ROS, reactive oxygen species; RNS, reactive nitrogen species.
Melatonin confers plant tolerance via multiple mechanisms, including photosynthetic efficiency increase, ROS or RNS scavenging, toxic compounds decrease, interaction with hormones, and secondary metabolite biosynthesis (Figure 2). Melatonin stimulated stomatal conductance and improved photosynthesis, thus enhancing tolerance to water-deficient stress in grape cuttings [53]. Another fact is that the photosynthetic efficiency was maximized by higher rates of CO2 assimilation and stomatal conductance after application of melatonin [54]. Several stresses can induce ROS or RNS accumulation, causing oxidative damage to plants [55]. In this case, melatonin re-establishes the redox balance via activating enzymatic antioxidant defense systems, as well as the ascorbate–glutathione (AsA-GSH) cycle [56]. In plants, the Salt-Overly Sensitive (SOS) pathway mediates ionic homeostasis and contributes to salinity tolerance [57]. This pathway comprises three crucial genes, Salt-Overly Sensitive1 (SOS1), Salt-Overly Sensitive2 (SOS2) and Salt-Overly Sensitive3 (SOS3), which function together to initiate transport of Na+ out of the cell, or activating other transporters, thus leading to the sequestration of Na+ in the vacuole [58]. Melatonin reduced ion toxicity and improved salinity tolerance via the SOS pathway [47]. ABA and H2O2/NO signaling transduction pathways were also modulated for plant tolerance in response to abiotic stress [47,48,56,59]. In addition, melatonin could increase primary and secondary metabolites including amino acids, organic acids and sugars, and thus improving plant cold tolerance [60].
3. Melatonin Improves Cd Tolerance in Plants
It has been found that Cd affects the ecosystem, causing stress and toxicity in plants. Melatonin acts as a key role in protecting plants from Cd stress. Table 1 summarizes that Cd treatment up-regulates the transcripts of melatonin biosynthesis genes, such as TDC, T5H, SNAT, ASMT, and COMT in Arabidopsis thaliana, Oryza sativa L., Solanum lycopersicum, Triticum aestivum L., Nicotiana tabacum L., and Agaricus campestris [59,61,62,63,64,65,66,67]. Therefore, melatonin contents are significantly increased. Notably, four M2H genes, involved in melatonin degradation, were also induced [65]. Byeon et al. suggested that both melatonin degradation and melatonin synthesis occurred in parallel, and 2-hydroxymelatonin of melatonin metabolite also acted as a signaling molecule in plant stress tolerance [65]. As melatonin catabolism is complicated, other pathways and the role of their metabolites should be investigated in plants under Cd stress.

Table 1.
Summary table explaining the effect of Cd on genes related to melatonin metabolic pathway.
Most studies showed that melatonin alleviated Cd-induced seedling growth inhibition, including the biomass (fresh weight and dry weight) and root length [19]. Melatonin improved the photosynthesis rate (Pn), transpiration rate (E), intracellular CO2 concentration and stomatal conductance (Gs) upon Cd stress in Nicotiana tabacum L. [6]. That melatonin enhanced stomatal opening and conductance capacity ultimately favored the photosynthesis in plants. Melatonin also prevented the degradation of the chlorophyll and carotenoid molecules in Chinese cabbage seedlings [68]. Similarly, application of melatonin improved chlorophyll and the maximum quantum efficiency of photosystem II (Fv/Fm) levels of wheat plants [20]. In chloroplasts, superoxide anion (O2·−) in photosystem I (PSI) is generated by two molecules of O2 with two electrons from photosystem II (PSII), and disproportionated to H2O2 catalyzed with superoxide dismutase (SOD) [69]. The better potential in melatonin treated plants under Cd stress can aid in chlorophyll protection, improve photosynthesis, and mediate redox homeostasis from oxidative damage.
3.1. Melatonin Activates Antioxidant Defense Systems in Response to Cd Stress
Cd stress induces ROS overproduction, containing H2O2, O2·−, hydroxyl radical (·OH), and singlet oxygen (1O2) [70]. These could be formed in photosynthetic cells, mitochondrial respiratory electron transport chain, respiratory processes, and nicotinamide-adenine dinucleotide phosphate (NADPH) oxidases in chloroplasts, mitochondria, peroxisomes, and plasma membrane, respectively [71]. Plants have evolved two antioxidant systems to relieve the ROS-triggered damages, including the enzymatic and non-enzymatic defense systems. Enzymatic defense systems including catalase (CAT), ascorbate peroxidase (APX), guaiacol peroxidase (POD), SOD, glutathione peroxidases (GPX), glutathione reductase (GR), dehydroascorbate reductase (DHAR), peroxiredoxins (PRX), thioredoxins (TRX), and glutaredoxins (GRX) are responsible for ROS scavenging [71]. The non-enzymatic systems, including ascorbate, GSH, flavonoid, anthocyanins, sugars, and carotenoids, are also essential for ROS elimination [71,72,73,74].
Melatonin protects plants by enhancing the ROS scavenging efficiency in response to Cd-induced oxidative stress. Application of exogenous melatonin significantly decreased H2O2, malondialdehyde (MDA), and O2·− levels in the tomato leaves/roots under Cd stress [75]. Similar results were also observed in Triticum aestivum L., Nicotiana tabacum L., Brassica napus L., Catharanthus roseus (L.), Malva parviflora, Fragaria x ananassa (Duch.), Agaricus campestris, Carthamus tinctorius L., Oryza sativa L., Raphanus sativus L., Cyphomandra betacea, Malachium aquaticum, and Zea mays [18,20,62,64,68,76,77,78,79,80,81,82,83,84,85,86]. In addition, overexpression of MsSNAT increased endogenous melatonin level, and reduced ROS accumulation in transgenic Arabidopsis plants [19].
Melatonin scavenges the above ROS mainly through two pathways upon Cd stress. Antioxidant enzymes play key roles in melatonin-decreased ROS overproduction, such as APX, CAT, SOD, POD, GPX, GR, DHAR, and monodehydroascorbate reductase (MDHAR). Their functions are confirmed in above plant species. For example, exogenously applied with melatonin counterbalanced the H2O2 and MDA accumulation via enhancing APX, CAT, SOD, and POD activities under Cd stress [77]. Enzymes involved in the ascorbate-glutathione (AsA-GSH) cycle, such as DHAR, MDHAR and GR, were also involved in melatonin-mediated ROS balance in sunflower (Carthamus tinctorius L.) seedlings [80]. In addition, melatonin interacted with ROS by improving antioxidant levels, including GSH, AsA, and dehydroascorbate (DHA) [80]. Other studies reported melatonin also could increase proline, anthocyanins, flavonoid, and sugars contents in response to Cd-induced oxidative stress [18,64,77,79]. These impacts of melatonin on Cd-induced oxidative stress are summarized in Table 2.

Table 2.
Summary table explaining the impacts of melatonin on Cd-induced oxidative stress.
3.2. Melatonin Regulates Cadmium Uptake and Translocation
In general, Cd is taken up by plant roots from soil, then transported to shoots through the xylem and phloem, and eventually accumulated in grains [87]. Several processes regulate Cd accumulation, including Cd apoplastic influx, cell wall adsorption, cytoplasm across the membrane, xylem loading, vacuolar sequestration, and energy-driven transport in plants [88]. Natural resistance-associated macrophage protein (NRAMP) might be involved in several processes, such as uptake, intracellular transport, translocation, and metal detoxification in various plants [89,90]. Moreover, Cd is also transported through Zn, Fe, and Ca transporters, including Zn transporter proteins (ZRT)- and Fe-regulated transporter (IRT)-like protein (ZIP), yellow strip-like (YS1/YSL), and low-affinity calcium (Ca) transporter 1 (LCT1) [91]. ABC transport (PDR8), metal tolerance proteins (MTPs), cation diffusion facilitators (CDFs), and the P18-type metal transporter ATPase (HMAs) take part in Cd homeostasis [92,93,94]. Furthermore, GSH and its derivatives, phytochelatins (PCs), bound with Cd, and then transported Cd to vacuoles by ATP-binding cassette subfamily C proteins (ABCCs) [95,96]. HMA3 and CDF transporter family are also involved in the transfer of Cd–PCs complexes into the vacuole [97,98]. Other high-affinity chelators, including metallothioneins (MTs), organic acids, and amino acids play multiple roles in detoxification of Cd [99].
Recent studies have shown that melatonin regulates Cd homeostasis in plants. Exogenous application of melatonin reduced Cd contents in both roots and leaves of Raphanus sativus L. and Brassica pekinensis (Lour.) Rupr. plants [68,84]. Melatonin significantly decreased Cd contents in the leaves, but not in the roots of Oryza sativa L., Carthamus tinctorius L., and Solanum lycopersicum [61,76,80,81]. However, melatonin increased and decreased Cd contents in roots and shoots of Malva parviflora, respectively [18]. These results suggest that the effect of melatonin on translocation factor (Cd content of shoot/root) are different in the above various plants. Melatonin reduced the transcripts of metal transporter-related genes (iron-regulated transporter1 (OsIRT1), iron-regulated transporter2 (OsIRT2), heavy metal ATPase2 (OsHMA2), heavy metal ATPase3 (OsHMA3), natural resistance-associated macrophage protein1 (OsNramp1), natural resistance-associated macrophage protein5 (OsNramp5), and low-affinity cation transporter1 (OsLCT1) in leaves, but not in the roots of Oryza sativa L. under Cd stress [81]. Expression of YSLs and HMAs were down-regulated by melatonin, thereby reducing the Cd entering the roots of Raphanus sativus L. [84]. In addition, the Metallothionein 1 (RsMT1) gene was involved in melatonin-conferred Cd tolerance in transgenic tobacco [84]. In roots of Brassica pekinensis (Lour.) Rupr. plants, IRT1 transcript was down-regulated significantly by melatonin application [68]. Then, Cd content was reduced in root tissues. These impacts of melatonin on Cd uptake and translocation are summarized in Table 3. Therefore, to characterize the biological roles of these metal transporter genes contributes to understanding the melatonin-mediated Cd homeostasis and detoxification.

Table 3.
Summary table explaining the impacts of melatonin on Cd uptake and translocation.
3.3. Other Regulators Are Involved in Melatonin-Mediated Cd Tolerance
It has been widely reported that NO plays a crucial role in regulating various plant physiological processes [100]. Previous studies found that Cd treatment increased NO production, which promoted Cd accumulation by the IRT1 up-regulation [101,102]. Exogenous melatonin alleviated Cd toxicity by reducing NO accumulation and IRT1 expression in Brassica pekinensis (Lour.) Rupr. [68]. By contrast, melatonin triggered the endogenous NO, and enhanced Cd tolerance via the increase in the activities of antioxidant enzymes in wheat seedlings [20]. Moreover, melatonin can be nitrosated to NOMela by employing four nitrosating entities at the N atom of indole ring [46]. It was suggested that NOMela could release NO. That NO induces S-nitrosation is an important redox-based post-translational modification, which is involved in plant responses to abiotic stress [103,104]. Thus, complex interactions between melatonin and NO in Cd resistance should be further investigated. Another important signaling element, salicylic acid (SA), alleviated Cd toxicity by affecting Cd distribution, the antioxidant defense activities, and photosynthesis [105,106,107]. Amjadi et al. found that there was a possible synergic interaction between melatonin and SA by reducing Cd uptake and modulating the ascorbate–glutathione cycle and glyoxalase system [80].
4. A Possible Role for H2S in Melatonin-Mediated Tolerance against Cd Stress
Acting as a signaling molecule, NO interacts with other molecules (H2O2, CO, and H2S) to mediate plant growth and development, as well as abiotic stress responses [100]. Among the molecules, H2S is also involved in almost all physiological plant processes [27,100]. To date, there is considerable research on the role of NO in melatonin-modulated plant abiotic stress tolerance. However, the functions of H2S have been largely unknown. It will become a research hotspot to contribute to precise analysis of the collaboration between H2S and melatonin, and provide deeper insight into melatonin-mitigated signaling mechanisms.
4.1. H2S Action in Plant Tolerance against Cd Stress
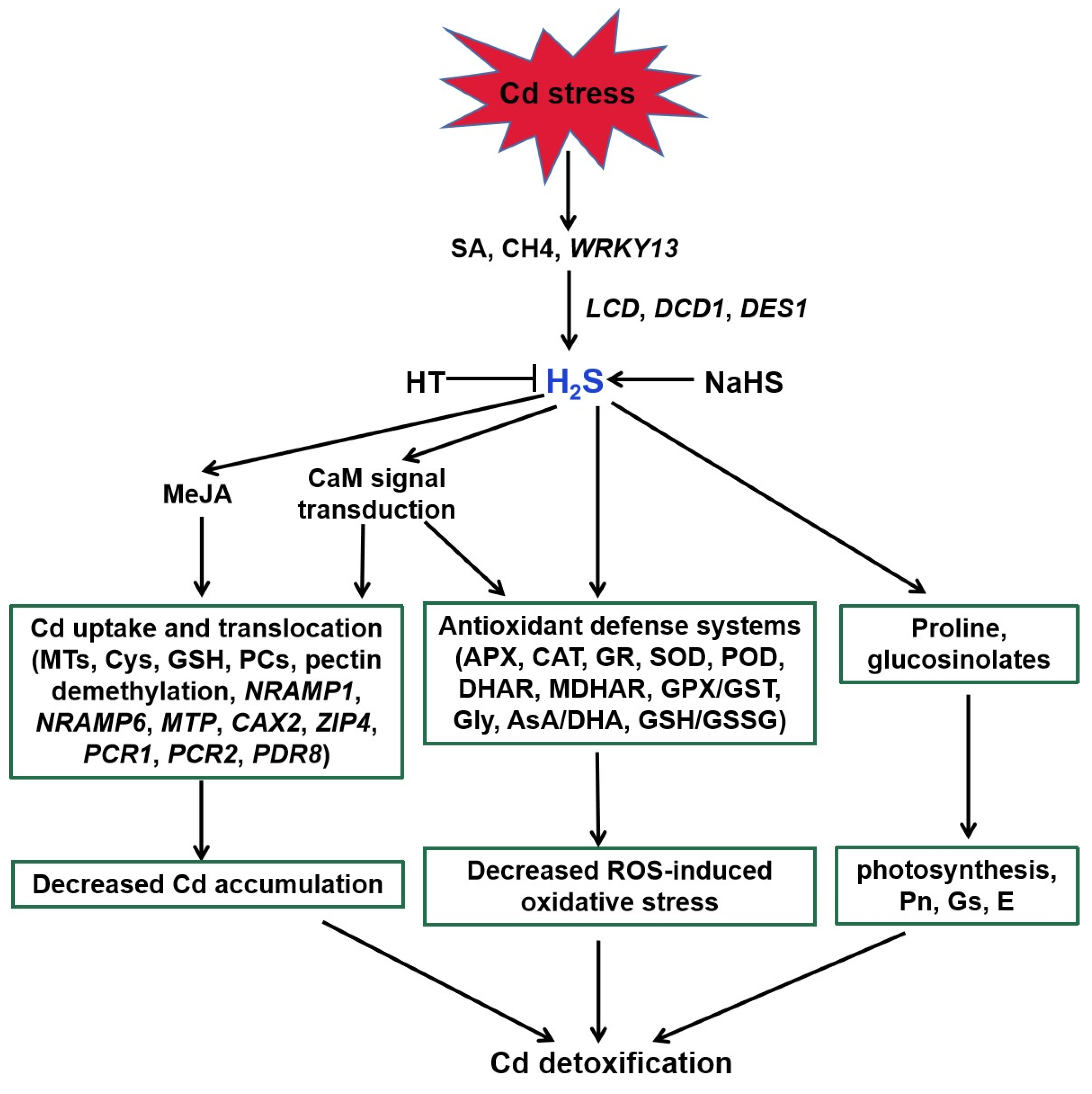
H2S acts as a signaling molecule in modifying various metabolic processes in plants, especially Cd stress (Figure 3, [27]). Endogenous H2S production was induced via expression of LCD, DCD, and DES1 under Cd stress [108,109,110]. SA, methane (CH4), and WRKY DNA-binding protein 13 (WRKY13) transcription factor were suggested to be involved in the above process [30,111,112]. H2S regulated the activities of key enzymes and AsA-GSH cycle involved in ROS homeostasis to alleviate Cd-induced oxidative stress [113,114,115,116,117,118,119,120]. For example, H2S enhanced the activities of antioxidant enzymes, such as POD, CAT, APX, and SOD, and thereby decreased ROS accumulation [120]. Similarly, it also obviously increased AsA and GSH and the redox status (AsA/DHA and GSH/GSSG) levels to improve rice Cd resistance [114,116].
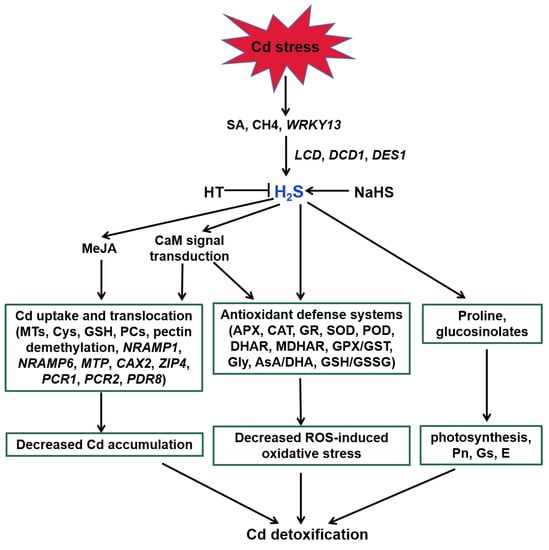
Figure 3.
Function of H2S in plant responses to Cd stress. SA, CH4, and WRKY13 are involved in Cd-induced H2S generation. H2S enhances the antioxidant defense systems to decrease the ROS accumulation, regulates the transcripts of genes related to Cd uptake and translocation to reduce the Cd accumulation, and increases proline and glucosinolates in response to Cd stress in plants. MeJA and Ca participate in the above regulatory pathways. SA, salicylic acid; CH4, methane; HT, hypotaurine; LCD, L-cysteine desulfhydrase; DCD, D-cysteine desulfhydrase; DES1, L-cysteine desulfhydrase 1; MeJA, methyl jasmonate; CaM, calmodulin; NRAMP1, natural resistance-associated macrophage protein1; NRAMP6, natural resistance-associated macrophage protein6; MTP, metal tolerance protein; CAX2, vacuolar cation/proton exchanger2; ZIP4, zinc-iron permease4; PCR1, plant cadmium resistance1; PCR2, plant cadmium resistance2; PDR8, pleiotropic drug resistance8.
Increasing evidence demonstrates that H2S also regulates Cd uptake and translocation in plants [30,117,119,121]. H2S enhanced the expression of genes encoding metallothionein (MTs) and phytochelatin (PCS) in Arabidopsis roots [117]. Therefore, H2S increased the metal chelators synthesis, contributing to Cd detoxification by binding the trace metal. In addition to enhancing the above genes expression, the protective effect of H2S was attributed to a decrease in Cd accumulation associated with the expression of Cd transporter genes, such as PCR1, PCR2, and PDR8 [30]. Exogenous application of NaHS weakened the expression of NRAMP1 and NRAMP6 genes, and intensified the expression of Cd homeostasis-related genes (CAX2 and ZIP4) to enhance Cd tolerance in foxtail millet [122].
A number of studies address that H2S can interact with other signaling molecules, such as SA, proline, MeJA, Ca, and NO during the responses of plants to Cd stress (Figure 3 and Figure 4; [111,122,123]). H2S acted as a downstream molecule of SA-transmitted signals to regulate Cd tolerance in Arabidopsis [111]. The endogenous production of proline and MeJA enhanced by H2S donor NaHS responded significantly to Cd stress in foxtail millet [122,123]. H2S also improved CaM gene expression and controlled the combination of Ca2+ and CaM, which act as signal transducers [33].
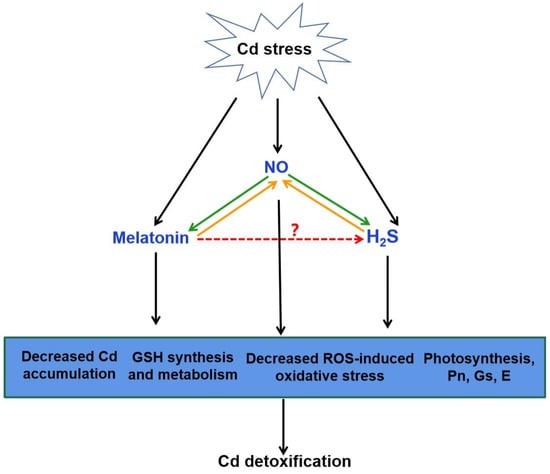
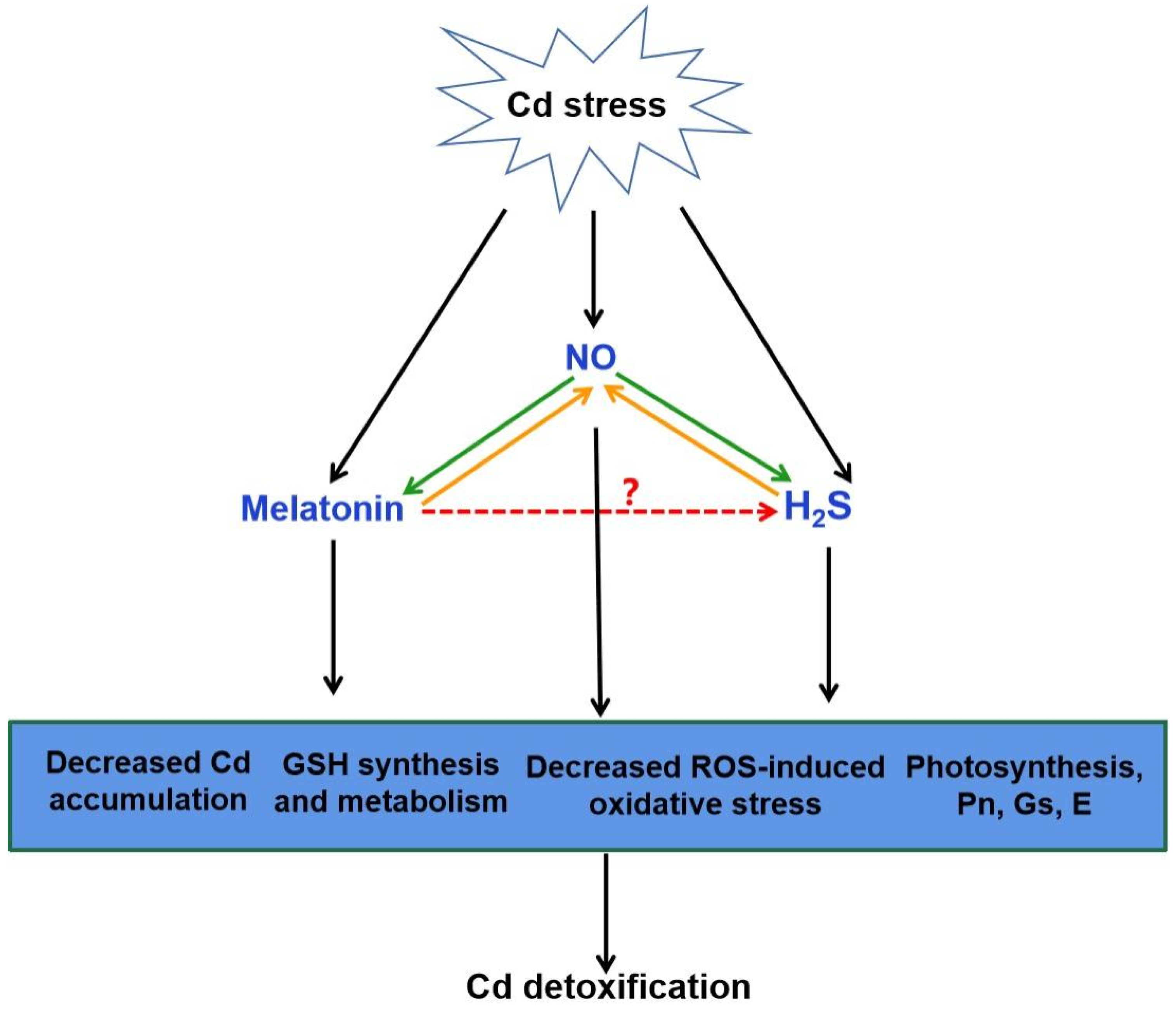
Figure 4.
The possible role of H2S in melatonin-mediated Cd detoxification. NO generation can be induced by Cd stress. Increasing evidence showed that melatonin and H2S act as the downstream of NO in the responses to Cd stress, respectively (green arrow). It is also suggested that NO acts as a downstream of melatonin or H2S to improve Cd tolerance (orange arrow). The combination of melatonin, NO and H2S might be responsible for melatonin-triggered signal transduction in plant Cd tolerance via the decreased Cd accumulation, GSH synthesis and metabolism, decreased ROS-induced oxidative stress and improved photosynthesis. Red arrow, yet largely unknown. Cd, cadmium; NO, nitric oxide; H2S, hydrogen sulfide; GSH, glutathione; ROS, reactive oxygen species; Pn, photosynthesis rate; Gs, stomatal conductance; E, transpiration.
There exists a complicated and synergistic relationship between H2S and NO in response to Cd stress in plants (Figure 4; [115,118,124,125]). Exogenous NO and H2S application increased the Cd tolerance in plants [115,124,126]. Subsequent pharmacological experiments proved that H2S donor NaHS triggered NO production, which might act as a signal for alleviation of Cd-induced oxidative damage in alfalfa seedling roots [124]. Nevertheless, H2S production activated by NO is essential in Cd stress response of bermudagrass [115]. As a second messenger, Ca acted both upstream and downstream of NO signal, and crosstalk of Ca and NO regulated the cysteine and H2S to mitigate Cd toxicity in Vigna radiata [126]. Moreover, application of sodium nitroprusside (SNP), the donor of NO, increased H2S generation, and thus enhanced Cd stress tolerance in wheat [118]. However, this protective effect was reversed by hypotaurine (HT), the scavenger of H2S [118]. These results suggested that H2S and NO can function in a coordinated way under certain signaling cascades in plants under Cd stress.
4.2. Crosstalk of Melatonin and H2S in Plants
The interaction between melatonin and H2S plays a beneficial role in abiotic stress response [32]. Exogenous melatonin regulated the endogenous H2S homeostasis by modulating the L-DES activity in salt-stressed tomato cotyledons [31]. Moreover, an endogenous H2S-dependent pathway was involved in melatonin-mediated salt stress tolerance in tomato seedling roots [34]. Synergistic effects of melatonin and H2S regulated K+/Na+ homeostasis, and reduced excessive accumulation of ROS by enhancing the activity of antioxidant enzymes. Inhibition of H2S by HT reversed the melatonin-modulated heat tolerance by inhibiting photosynthesis, carbohydrate metabolism, and the activity of antioxidant enzymes in wheat [36]. Recent investigation has revealed that melatonin-induced pepper tolerance to iron deficiency and salt stress was dependent on H2S and NO [118]. It was further confirmed that H2S and NO jointly participated in melatonin-mitigated salt tolerance in cucumber [35]. Thus, these results postulate that H2S might act as a downstream signaling molecule of melatonin. Combined with the roles of H2S and melatonin in alleviating Cd stress, it is easy to speculate that H2S might be involved in melatonin-mediated Cd tolerance in plants (Figure 4).
As mentioned above in Section 3, GSH plays a critical role in plant Cd tolerance. It is synthesized from glutamate, cysteine and glycine by γ-glutamyl cysteine synthetase (γ-ECS, encoded by GSH1/ECS gene) and glutathione synthetase (GS, encoded by GSH2/GS gene) [127]. The catalysis of GSH1 is the rate-limiting step of GSH biosynthesis [128]. Cd stress induced the transcripts of GSH1 and GSH2 in Arabidopsis, as well as ECS and GS in Medicago sativa [114,129,130,131]. It was suggested that H2S could be quickly incorporated into cysteine and subsequently into GSH [132]. Application of NaHS re-established (h)GSH homeostasis by further strengthening the up-regulation of ECS and GS genes [114]. Similar results were also found in strawberry and cucumber plants [133,134]. Interestingly, exogenous melatonin also increased the GSH content by inducing the transcript levels of SlGSH1 in tomato [75]. Hence, there might be a certain connection between H2S and melatonin in regulating the GSH homeostasis at the transcriptional regulatory pathway. This will provide an interesting direction for further research on the complex interactions between melatonin and H2S in improving Cd tolerance in plants.
5. Conclusions and Future Prospects
Recent studies have strongly indicated that melatonin, a multifunctional molecule, regulates Cd tolerance in plants. To further promote related research in plant Cd tolerance, this review summarizes the regulatory roles and mechanisms of melatonin in response to Cd stress. Melatonin reduces Cd damage mainly through re-establishing the redox homeostasis and decreasing Cd accumulation, but its underlying mechanisms remain to be determined. Intriguingly, melatonin is suggested to be a phytohormone due to the identification of the putative receptor CAND2/PMTR1 [135], although there is still a debate on whether it is a bona fide receptor for melatonin [136]. More importantly, more receptor gene(s) should be characterized, which will be critical for precisely understanding the signal transduction pathway of melatonin in plants in response to Cd stress.
Currently, as a signal molecule, the role of NO has been revealed in melatonin-mediated Cd tolerance, likewise H2S plays a key messenger in plant resistance to Cd stress. That the effects of H2S have been less explored has prevented precise analysis of the collaboration of H2S and melatonin. Recently, we presented the underlying mechanisms of H2S action and its multifaceted roles in plant stress responses [137]. Hence, it would be interesting to fully evaluate the effects of H2S-based signaling on regulating melatonin-induced Cd tolerance. For directions of future research, biochemical and genetic characterization of H2S-producing proteins and persulfidation signaling is needed and will shed more light on the integration of H2S and melatonin signaling during Cd stress.
5.1. Pharmacological, Genetic and ‘Omics’ Approach to Understand the Crosstalk of H2S–Melatonin during Cd Stress
Various pharmacological, enzyme activity, and gene expression investigations revealed the crosstalk of H2S–melatonin in response to salt and heat stress in tomato and cucumber [34,35,36]. Exogenous melatonin induced the H2S generation by activating the L-cysteine desulfhydrase (L-CDes) activity, which was encoded by LCD, DCD, and DES1 [31,35]. Then, the interaction of H2S and melatonin enhanced the antioxidant defense, and regulated carbohydrate metabolism and ion homeostasis [34,35,36,118]. Similar pharmacological experiments with an effective concentration range of 1–200 μM in exogenous melatonin, 10–100 μM in 4-Chloro-DL-phenylalanine (p-CPA, melatonin synthesis inhibitor), 10–100 μM in hypotaurine (HT, H2S inhibitor), and 10–100 μM in NaHS (H2S donor) could be used to investigate the crosstalk of H2S–melatonin during Cd stress. Furthermore, genetic modifications with altering melatonin and H2S levels, such as snat, comt, lcd, dcd, and des1 mutants, should be used to explore their possible roles.
H2S plays a critical signal mediator in plants in response to Cd stress [111,112,115]. However, there is still an urgent need to elucidate the interactions of H2S with other signaling molecules in melatonin-mediated Cd tolerance. With the advent of transcriptomic and proteomic analysis, scientists shall reveal the intrinsic regulatory mechanisms of melatonin and H2S interaction on the regulation of various biological processes. For example, the expression of genes and proteins related to GSH synthesis and metabolism and redox homoeostasis, as well as the hormone biosynthesis pathways, might be used to establish a model system to decipher their signaling interaction.
5.2. The Potential Role of Persulfidation Driven by H2S in Melatonin-Mediated Cd Tolerance
Recently, it was found that H2S-mediated post-translational modification (PTM, persulfidation) of protein cysteine residues (RSSH) is an important mechanism in plants to adapt to external environments [27,32]. Protein persulfidation cause various changes in structures, activities, as well as the subcellular localizations of the candidate proteins [138,139]. These proteins are mainly involved in plant growth and development, abiotic stress responses, and carbon/nitrogen metabolism [138]. For example, H2S production regulated the persulfidation of NADPH oxidase RBOHD at Cys825 and Cys890, leading to improving the ability to produce H2O2 signal [140]. It also led to the persulfidation of ABSCISIC ACID INSENSITIVE 4 (ABI4) at Cys250, and persulfidation of SnRK2.6, contributing to reveal the function of H2S in the complex signal-transduction system [141,142,143]. By contrast, the residue Cys32 of APX could be persulfidated, thereby enhancing its activity [144]. Therefore, the persulfidation might become a promising direction to investigate the roles of H2S in melatonin-mediated Cd tolerance in plants. To conclude, the progresses in the various physiological and molecular mechanisms regulated by melatonin are not enough, and future studies along with the above lines should be used to unveil the regulatory mechanism of melatonin and H2S signaling pathways in plant Cd tolerance.
Author Contributions
Writing—original draft preparation, Q.G., C.W., and Q.X.; writing—review and editing, Z.C. and Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was mainly supported by the Anhui Provincial Natural Science Foundation (2008085MC101), the Natural Science Foundation of China (31300225), Talent Research Fund Project of Hefei University (No. 18-19RC13), and “Borrowing transfer to supplement” Foundation of Hefei (J2019D04).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Weissmannová, H.D.; Pavlovský, J. Indices of soil contamination by heavy metals-methodology of calculation for pollution assessment. Environ. Monit. Assess. 2017, 189, 616. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S. Safer food through plant science: Reducing toxic element accumulation in crops. J. Exp. Bot. 2019, 70, 5537–5557. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S.; Aarts, M.G.; Thomine, S.; Verbruggen, N. Plant science: The key to preventing slow cadmium poisoning. Trends Plant Sci. 2013, 18, 92–99. [Google Scholar] [CrossRef]
- DalCorso, G.; Manara, A.; Furini, A. An overview of heavy metal challenge in plants: From roots to shoots. Metallomics 2013, 5, 1117–1132. [Google Scholar] [CrossRef]
- Ismael, M.A.; Elyamine, A.M.; Moussa, M.G.; Cai, M.; Zhao, X.H.; Hu, C.X. Cadmium in plants: Uptake, toxicity, and its interactions with selenium fertilizers. Metallomics 2019, 11, 255–277. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Duan, S.; Zhou, Z.; Chen, S.; Wang, D. Foliar spraying of melatonin confers cadmium tolerance in Nicotiana tabacum L. Ecotoxicol. Environ. Saf. 2019, 170, 68–76. [Google Scholar] [CrossRef]
- Sharma, S.S.; Dietz, K.J. The relationship between metal toxicity and cellular redox imbalance. Trends Plant Sci. 2009, 14, 43–50. [Google Scholar] [CrossRef]
- Pérez-Chaca, M.V.; Rodríguez-Serrano, M.; Molina, A.S.; Pedranzani, H.E.; Zirulnik, F.; Sandalio, L.M.; Romero-Puertas, M.C. Cadmium induces two waves of reactive oxygen species in Glycine max (L.) roots. Plant Cell Environ. 2014, 37, 1672–1687. [Google Scholar] [CrossRef]
- Khaliq, M.A.; James, B.; Chen, Y.H.; Saqib, H.; Li, H.H.; Jayasuriya, P.; Guo, W. Uptake, translocation, and accumulation of Cd and its interaction with mineral nutrients (Fe, Zn, Ni, Ca, Mg) in upland rice. Chemosphere 2019, 215, 916–924. [Google Scholar] [CrossRef]
- Noctor, G.; Mhamdi, A.; Chaouch, S.; Han, Y.; Neukermans, J.; Marquez-Garcia, B.; Foyer, C.H. Glutathione in plants: An integrated overview. Plant Cell Environ. 2021, 35, 454–484. [Google Scholar] [CrossRef]
- Cobbett, C.; Goldsbrough, P. Phytochelatins and metallothioneins: Roles in heavy metal detoxification and homeostasis. Rev. Plant Biol. 2002, 53, 159–182. [Google Scholar] [CrossRef] [Green Version]
- Rizwan, M.; Ali, S.; Adrees, M.; Rizvi, H.; Zia-ur-Rehman, M.; Hannan, F.; Qayyum, M.F.; Hafeez, F.; Ok, Y.S. Cadmium stress in rice: Toxic effects, tolerance mechanisms, and management: A critical review. Environ. Sci. Pollut. Res. Int. 2016, 23, 17859–17879. [Google Scholar] [CrossRef]
- Thao, N.P.; Khan, M.I.R.; Thu, N.B.A.; Hoang, X.L.T.; Asgher, M.; Khan, N.A.; Tran, L.S.P. Role of ethylene and its cross talk with other signaling molecules in plant responses to heavy metal stress. Plant Physiol. 2015, 169, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Rabia, A.; Faiza, M.; Ghulam, K.; Tooba, I.; Maryam, K. Plant signaling molecules and cadmium stress tolerance. Cadmium Toler. Plants 2019, 367–399. [Google Scholar] [CrossRef]
- Hattori, A.; Migitaka, H.; Iigo, M.; Itoh, M.; Yamamoto, K.; Ohtani-Kaneko, R.; Hara, M.; Suzuki, T.; Reiter, R.J. Identification of melatonin in plants and its effects on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem. Mol. Biol. Int. 1995, 35, 627–634. [Google Scholar]
- Arnao, M.B.; Hernandez-Ruiz, J. Melatonin: A New Plant Hormone and/or a Plant Master Regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2021, 63, 126–145. [Google Scholar] [CrossRef]
- Tousi, S.; Zoufan, P.; Ghahfarrokhie, A.R. Alleviation of cadmium-induced phytotoxicity and growth improvement by exogenous melatonin pretreatment in mallow (Malva parviflora) plants. Ecotoxicol. Environ. Saf. 2020, 206, 111403. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Chen, Z.; Yu, X.; Cui, W.; Pan, J.; Zhao, G.; Xu, S.; Wang, R.; Shen, W. Melatonin confers plant tolerance against cadmium stress via the decrease of cadmium accumulation and reestablishment of microRNA-mediated redox homeostasis. Plant Sci. 2017, 261, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Kaya, C.; Okant, M.; Ugurlar, F.; Alyemeni, M.N.; Ashraf, M.; Ahmad, P. Melatonin-mediated nitric oxide improves tolerance to cadmium toxicity by reducing oxidative stress in wheat plants. Chemosphere 2019, 225, 627–638. [Google Scholar] [CrossRef]
- He, J.; Zhuang, X.; Zhou, J.; Sun, L.; Wan, H.; Li, H.; Lyu, D. Exogenous melatonin alleviates cadmium uptake and toxicity in apple rootstocks. Tree Physiol. 2020, 40, 746–761. [Google Scholar] [CrossRef]
- Cai, S.Y.; Zhang, Y.; Xu, Y.P.; Qi, Z.Y.; Li, M.Q.; Ahammed, G.J.; Xia, X.; Shi, K.; Zhou, Y.; Reiter, R.; et al. HsfA1a upregulates melatonin biosynthesis to confer cadmium tolerance in tomato plants. J. Pineal Res. 2017, 62, e12387. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Choi, G.H.; Back, K. Cadmium-induced melatonin synthesis in rice requires light, hydrogen peroxide, and nitric oxide: Key regulatory roles for tryptophan decarboxylase and caffeic acid O-methyltransferase. J. Pineal Res. 2017, 63, e12441. [Google Scholar] [CrossRef] [PubMed]
- Kyungjin, L.; Jin, H.O.; Reiter, R.J.; Kyoungwhan, B. Flavonoids inhibit both rice and sheep serotonin N-acetyltransferases and reduce melatonin levels in plants. J. Pineal Res. 2018, 65, e12512. [Google Scholar] [CrossRef]
- Lu, R.; Liu, Z.; Shao, Y.; Sun, F.; Zhang, Y.; Cui, J.; Zhou, T. Melatonin is responsible for rice resistance to rice stripe virus infection through a nitric oxide-dependent pathway. Virol. J. 2019, 16, 141. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Chen, Y.; Zhai, F.; Zhang, J.; Zhang, F.; Yuan, X.; Xie, Y. Hydrogen sulfide promotes rice drought tolerance via reestablishing redox homeostasis and activation of ABA biosynthesis and signaling. Plant Physiol. Biochem. 2020, 155, 213–220. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, M.; Zhou, H.; Zhao, D.; Gotor, C.; Romero, L.C.; Shen, J.; Ge, Z.; Zhang, Z.; Shen, W.; et al. Hydrogen sulfide, a signaling molecule in plant stress responses. J. Integr. Plant Biol. 2021, 63, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhou, H.; Shen, J.; Zhang, Z.; Xie, Y. H2S action in plant life cycle. Plant Growth Regul. 2021, 94, 1–9. [Google Scholar] [CrossRef]
- Zhou, H.; Guan, W.; Zhou, M.; Shen, J.; Liu, X.; Wu, D.; Yin, X.; Xie, Y. Cloning and characterization of a gene encoding true D-cysteine desulfhydrase from Oryza sativa. Plant Mol. Biol. Rep. 2020, 38, 95–113. [Google Scholar] [CrossRef]
- Zhang, Q.; Cai, W.; Ji, T.T.; Ye, L.; Lu, Y.T.; Yuan, T.T. WRKY13 enhances cadmium tolerance by promoting D-cysteine desulfhydrase and hydrogen sulfide production. Plant Physiol. 2020, 183, 345–357. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bhatla, S.C. Exogenous melatonin modulates endogenous H2S homeostasis and L-cysteine desulfhydrase activity in salt-stressed tomato (Solanum lycopersicum L. var. cherry) seedling cotyledons. J. Plant Growth Regul. 2020, 4, 1–13. [Google Scholar] [CrossRef]
- Huang, D.; Huo, J.; Liao, W. Hydrogen sulfide: Roles in plant abiotic stress response and crosstalk with other signals. Plant Sci. 2021, 302, 110733. [Google Scholar] [CrossRef]
- Wang, H.R.; Che, Y.H.; Wang, Z.H.; Zhang, B.N.; Ao, H. The multiple effects of hydrogen sulfide on cadmium toxicity in tobacco may be interacted with CaM signal transduction. J. Hazard. Mater. 2021, 403, 123651. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.; Khan, M.; Mukherjee, S.; Basahi, R.; Alamri, S.; Al-Amri, A.; Alsubaie, Q.; Ali, H.; Al-Munqedhi, B.; Almohisen, I. Exogenous melatonin-mediated regulation of K+/Na+ transport, H+-ATPase activity and enzymatic antioxidative defence operate through endogenous hydrogen sulphide signalling in NaCl-stressed tomato seedling roots. Plant Biol. 2021, 23, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ma, C.; Kang, X.; Zhang, L.; Wang, J.; Zheng, S.; Zhang, T. Hydrogen sulfide and nitric oxide are involved in melatonin-induced salt tolerance in cucumber. Plant Physiol. Biochem. 2021, 167, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Fatma, M.; Gautam, H.; Umar, S.; Khan, N.A. The crosstalk of melatonin and hydrogen sulfide determines photosynthetic performance by regulation of carbohydrate metabolism in wheat under heat stress. Plants 2021, 10, 1778. [Google Scholar] [CrossRef]
- Murch, S.J.; KrishnaRaj, S.; Saxena, P.K. Tryptophan is a precursor for melatonin and serotonin biosynthesis in in vitro regenerated St. John’s wort (Hypericum perforatum L. cv. Anthos) plants. Plant Cell Rep. 2000, 19, 698–704. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.; Kim, Y.S.; Back, K. Tryptamine 5-hydroxylase-deficient Sekiguchi rice induces synthesis of 5-hydroxytryptophan and N-acetyltryptamine but decreases melatonin biosynthesis during senescence process of detached leaves. J. Pineal Res. 2012, 52, 211–216. [Google Scholar] [CrossRef]
- Tan, D.X.; Reiter, R.J. An evolutionary view of melatonin synthesis and metabolism related to its biological functions in plants. J. Exp. Bot. 2020, 71, 4677–4689. [Google Scholar] [CrossRef]
- Ye, T.; Yin, X.; Yu, L.; Zheng, S.J.; Cai, W.J.; Wu, Y.; Feng, Y.Q. Metabolic analysis of the melatonin biosynthesis pathway using chemical labeling coupled with liquid chromatography-mass spectrometry. J. Pineal Res. 2019, 66, e12531. [Google Scholar] [CrossRef] [Green Version]
- Okazaki, M.; Higuchi, K.; Aouini, A.; Ezura, H. Lowering intercellular melatonin levels by transgenic analysis of indoleamine 2,3-dioxygenase from rice in tomato plants. J. Pineal Res. 2010, 49, 239–247. [Google Scholar] [CrossRef]
- Byeon, Y.; Back, K. Molecular cloning of melatonin 2-hydroxylase responsible for 2-hydroxymelatonin production in rice (Oryza sativa). J. Pineal Res. 2015, 58, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Back, K. 2-Hydroxymelatonin promotes the resistance of rice plant to multiple simultaneous abiotic stresses (combined cold and drought). J. Pineal Res. 2016, 61, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Zawadzka, A.; Czarnocki, Z.; Reiter, R.J.; Back, K. Molecular cloning of melatonin 3-hydroxylase and its production of cyclic 3-hydroxymelatonin in rice (Oryza sativa). J. Pineal Res. 2016, 61, 470–478. [Google Scholar] [CrossRef]
- Singh, N.; Kaur, H.; Yadav, S.; Bhatla, S.C. Does N-nitrosomelatonin compete with S-nitrosothiols as a long distance nitric oxide carrier in plants? Biochem. Anal. Biochem. 2016, 5, 262. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S. Insights into nitric oxide-melatonin crosstalk and N-nitrosomelatonin functioning in plants. J. Exp. Bot. 2019, 70, 6035–6047. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, Y.; Gu, Q.; Zhao, G.; Zhang, Y.; Cui, W.; Xu, S.; Wang, R.; Shen, W. The AtrbohF-dependent regulation of ROS signaling is required for melatonin-induced salinity tolerance in Arabidopsis. Free Radical. Biol. Med. 2017, 108, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Shazad, R.; Bilal, S.; Imran, Q.M.; Lee, I.J. Exogenous melatonin mediates the regulation of endogenous nitric oxide in Glycine max L. to reduce effects of drought stress. Environ. Exp. Bot. 2021, 188, 104511. [Google Scholar] [CrossRef]
- Li, H.; Guo, Y.; Lan, Z.; Xu, K.; Chang, J.; Ahammed, G.J.; Ma, J.; Wei, C.; Zhang, X. Methyl jasmonate mediates melatonin-induced cold tolerance of grafted watermelon plants. Hortic. Res. 2021, 8, 57. [Google Scholar] [CrossRef]
- Xia, H.; Zhou, Y.; Deng, H.; Lin, L.; Deng, Q.; Wang, J.; Lv, X.; Zhang, X.; Liang, D. Melatonin improves heat tolerance in Actinidia deliciosa via carotenoid biosynthesis and heat shock proteins expression. Physiol Plant. 2021, 172, 1582–1593. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhou, J.; Tan, D.X.; Wang, N.; Wang, L.; Shan, D.; Kong, J. Melatonin improves waterlogging tolerance of Malus baccata (Linn.) Borkh. seedlings by maintaining aerobic respiration, photosynthesis and ROS migration. Front. Plant Sci. 2017, 8, 483. [Google Scholar] [CrossRef] [Green Version]
- Pang, Y.W.; Jiang, X.L.; Wang, Y.C.; Wang, Y.Y.; Hao, H.S.; Zhao, S.J.; Du, W.H.; Zhao, X.M.; Wang, L.; Zhu, H.B. Melatonin protects against paraquat-induced damage during in vitro maturation of bovine oocytes. J. Pineal Res. 2019, 66, e12532. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.F.; Xu, T.F.; Wang, Z.Z.; Fang, Y.L.; Xi, Z.M.; Zhang, Z.W. The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: Antioxidant metabolites, leaf anatomy, and chloroplast morphology. J. Pineal Res. 2014, 57, 200–212. [Google Scholar] [CrossRef]
- Wang, P.; Yin, L.; Liang, D.; Li, C.; Ma, F.; Yue, Z. Delayed senescence of apple leaves by exogenous melatonin treatment: Toward regulating the ascorbate-glutathione cycle. J. Pineal Res. 2012, 53, 11–20. [Google Scholar] [CrossRef] [PubMed]
- DalCorso, G.; Farinati, S.; Maistri, S.; Furini, A. How plants cope with cadmium: Staking all on metabolism and gene expression. J. Integr. Plant Biol. 2008, 50, 1268–1280. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin against environmental plant stressors: A review. Curr. Protein Pept. Sci. 2021, 21, 413–429. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Zelm, E.V.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.Y.; Back, K. Melatonin is required for H2O2- and NO-mediated defense signaling through MAPKKK3 and OXI1 in Arabidopsis thaliana. J. Pineal Res. 2017, 62, e12379. [Google Scholar] [CrossRef]
- Shi, H.; Jiang, C.; Ye, T.; Tan, D.X.; Reiter, R.J.; Zhang, H.; Liu, R.; Chan, Z. Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass [Cynodon dactylon (L). Pers.] by exogenous melatonin. J. Exp. Bot. 2015, 66, 681–694. [Google Scholar] [CrossRef] [Green Version]
- Li, M.Q.; Hasan, M.K.; Li, C.X.; Ahammed, G.J.; Xia, X.J.; Shi, K.; Zhou, Y.; Reiter, R.; Yu, J.; Xu, M.; et al. Melatonin mediates selenium-induced tolerance to cadmium stress in tomato plants. J. Pineal Res. 2016, 61, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wang, Q.; Shah, F.A.; Liu, W.; Wang, D.; Huang, S.; Fu, S.; Wu, L. Exogenous melatonin confers cadmium tolerance by counterbalancing the hydrogen peroxide homeostasis in wheat seedlings. Molecules 2018, 23, 799. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Yao, Z.; Zhang, R.; Mou, Z.; Yin, H.; Xu, T.; Zhao, D.; Chen, S. Genome-wide identification and expression profile of the SNAT gene family in tobacco (Nicotiana tabacum). Front. Genet. 2020, 11, 591984. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, Y.; Qian, J.; Si, W.; Tan, Q.; Xu, J.; Zhao, Y. Melatonin enhances the cadmium tolerance of mushrooms through antioxidant-related metabolites and enzymes. Food Chem. 2020, 330, 127263. [Google Scholar] [CrossRef]
- Byeon, Y.; Lee, H.Y.; Hwang, O.J.; Lee, H.J.; Back, K. Coordinated regulation of melatonin synthesis and degradation genes in rice leaves in response to cadmium treatment. J. Pineal Res. 2015, 58, 470–478. [Google Scholar] [CrossRef]
- Byeon, Y.; Lee, H.J.; Lee, H.Y.; Back, K. Cloning and functional characterization of the Arabidopsis N-acetylserotonin O-methyltransferase responsible for melatonin synthesis. J. Pineal Res. 2016, 60, 65–73. [Google Scholar] [CrossRef]
- Byeon, Y.; Lee, H.Y.; Back, K. Cloning and characterization of the serotoninn-acetyltransferase-2 gene (SNAT2) in rice (Oryza sativa). J. Pineal Res. 2016, 61, 198–207. [Google Scholar] [CrossRef]
- Wang, T.; Song, J.; Liu, Z.; Liu, Z.; Cui, J. Melatonin alleviates cadmium toxicity by reducing nitric oxide accumulation and IRT1 expression in Chinese cabbage seedlings. Environ. Sci. Pollut. Res. Int. 2021, 28, 15394–15405. [Google Scholar] [CrossRef]
- Asada, K. THE WATER-WATER CYCLE IN CHLOROPLASTS: Scavenging of active oxygens and dissipation of excess photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef]
- Considine, M.J.; Foyer, C.H. Oxygen and reactive oxygen species-dependent regulation of plant growth and development. Plant Physiol. 2020, 186, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Kohli, S.K.; Khanna, K.; Bhardwaj, R.; Abd_Allah, E.F.; Ahmad, P.; Corpas, F.J. Assessment of Subcellular ROS and NO Metabolism in Higher Plants: Multifunctional Signaling Molecules. Antioxidants 2019, 8, 641. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.P.; Dong, X.J.; Ma, H.H. Molecular mechanism for cadmium-induced anthocyanin accumulation in Azolla imbricata. Chemosphere 2012, 87, 319–325. [Google Scholar] [CrossRef]
- Alzahrani, Y.; Rady, M.M. Compared to antioxidants and polyamines, the role of maize grain-derived organic biostimulants in improving cadmium tolerance in wheat plants. Ecotoxicol. Environ. Saf. 2019, 182, 109378. [Google Scholar] [CrossRef]
- Kanu, A.S.; Ashraf, U.; Mo, Z.; Sabir, S.; Tang, X. Calcium amendment improved the performance of fragrant rice and reduced metal uptake under cadmium toxicity. Environ. Sci. Pollut. Res. 2019, 26, 24748–24757. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.K.; Ahammed, G.J.; Yin, L.; Shi, K.; Xia, X.; Zhou, Y.; Yu, J.; Zhou, J. Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicum L. Front. Plant Sci. 2015, 6, 601. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.K.; Ahammed, G.J.; Sun, S.; Li, M.; Yin, H.; Zhou, J. Melatonin inhibits cadmium translocation and enhances plant tolerance by regulating sulfur uptake and assimilation in Solanum lycopersicum L. J. Agric. Food Chem. 2019, 67, 10563–10576. [Google Scholar] [CrossRef]
- Sami, A.; Shah, F.A.; Abdullah, M.; Zhou, X.Y.; Zhou, K.J. Melatonin mitigates cadmium and aluminium toxicity through modulation of antioxidant potential in Brassica napus L. Plant Biol. 2020, 22, 679–690. [Google Scholar] [CrossRef]
- Nabaei, M.; Amooaghaie, R. Melatonin and nitric oxide enhance cadmium tolerance and phytoremediation efficiency in Catharanthus roseus (L.) G. Don. Environ. Sci. Pollut. Res. Int. 2020, 27, 6981–6994. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Y.; Zhang, J.; Gong, X.; Wang, Y. Exogenous melatonin improves physiological characteristics and promotes growth of strawberry seedlings under cadmium stress. Hortic. Plant J. 2021, 7, 13–22. [Google Scholar] [CrossRef]
- Amjadi, Z.; Namdjoyan, S.; Soorki, A.A. Exogenous melatonin and salicylic acid alleviates cadmium toxicity in safflower (Carthamus tinctorius L.) seedlings. Ecotoxicology 2021, 30, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Dai, S.; Wang, B.; Xie, Z.; Li, J.; Wang, L.; Li, S.; Tan, Y.; Tian, B.; Shu, Q.; et al. Gold nanoparticles synthesized using melatonin suppress cadmium uptake and alleviate its toxicity in rice. Environ. Sci. Nano 2021, 8, 1042–1056. [Google Scholar] [CrossRef]
- Ma, L.; Huang, Z.; Li, S.; Ashraf, U.; Yang, W.; Liu, H.; Xu, D.; Li, W.; Mo, Z. Melatonin and nitrogen applications modulate early growth and related physio-biochemical attributes in Maize under Cd stress. J. Soil Sci. Plant Nutr. 2021, 21, 978–990. [Google Scholar] [CrossRef]
- Bao, Q.; Bao, W.; Li, Y.; Zhang, S.; Lian, F.; Huang, Y. Silicon combined with foliar melatonin for reducing the absorption and translocation of Cd and As by Oryza sativa L. in two contaminated soils. J. Environ. Manag. 2021, 287, 112343. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Zhang, F.; Tang, M.; Wang, Y.; Dong, J.; Ying, J.; Chen, Y.; Hu, B.; Li, C.; Liu, L. Melatonin confers cadmium tolerance by modulating critical heavy metal chelators and transporters in radish plants. J. Pineal Res. 2020, 69, e12659. [Google Scholar] [CrossRef]
- Tang, Y.; Lin, L.; Xie, Y.; Liu, J.; Sun, G.; Li, H.; Liao, M.; Wang, Z.; Liang, D.; Xia, H.; et al. Melatonin affects the growth and cadmium accumulation of Malachium aquaticum and Galinsoga parviflora. Int. J. Phytoremediation 2018, 20, 295–300. [Google Scholar] [CrossRef]
- Lin, L.; Li, J.; Chen, F.; Liao, M.; Tang, Y.; Liang, D.; Xia, H.; Lai, Y.; Wang, X.; Chen, C.; et al. Effects of melatonin on the growth and cadmium characteristics of Cyphomandra betacea seedlings. Environ. Monit. Assess. 2018, 190, 119. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Shu, F.; Fujiwara, T.; Yoneyama, T.; Hayashi, H. Quantitative estimation of the contribution of the phloem in cadmium transport to grains in rice plants (Oryza sativa L.). Soil Sci. Plant Nutr. 2010, 53, 72–77. [Google Scholar] [CrossRef] [Green Version]
- Raza, A.; Habib, M.; Kakavand, S.N.; Zahid, Z.; Zahra, N.; Sharif, R.; Hasanuzzaman, M. Phytoremediation of cadmium: Physiological, biochemical, and molecular mechanisms. Biology 2020, 9, 177. [Google Scholar] [CrossRef]
- Nevo, Y.; Nelson, N. The NRAMP family of metal-ion transporters. Biochim. Biophys. Acta Mol. Cell Res. 2006, 1763, 609–620. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Yamaji, N.; Yamane, M.; Kashino-Fujii, M.; Sato, K.; Ma, J.F. The HvNramp5 transporter mediates uptake of cadmium and manganese, but not iron. Plant Physiol. 2016, 172, 1899–1910. [Google Scholar] [CrossRef] [Green Version]
- Uraguchi, S.; Kamiya, T.; Sakamoto, T.; Kasai, K.; Sato, Y.; Nagamura, Y.; Yoshida, A.; Kyozuka, J.; Ishikawa, S.; Fujiwara, T. Low-affinity cation transporter (OsLCT1) regulates cadmium transport into rice grains. Proc. Natl. Acad. Sci. USA 2011, 108, 20959–20964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.Y.; Bovet, L.; Maeshima, M.; Martinoia, E.; Lee, Y. The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. Plant J. 2007, 50, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Migocka, M.; Kosieradzka, A.; Papierniak, A.; Maciaszczyk-Dziubinska, E.; Posyniak, E.; Garbiec, A.; Filleur, S. Two metal-tolerance proteins, MTP1 and MTP4, are involved in Zn homeostasis and Cd sequestration in cucumber cells. J. Exp. Bot. 2015, 66, 1001–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lekeux, G.; Crowet, J.-M.; Nouet, C.; Joris, M.; Jadoul, A.; Bosman, B.; Carnol, M.; Motte, P.; Lins, L.; Galleni, M. Homology modeling and in vivo functional characterization of the zinc permeation pathway in a heavy metal P-type ATPase. J. Exp. Bot. 2019, 70, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Song, W.Y.; Ko, D.; Eom, Y.; Hansen, T.H.; Schiller, M.; Lee, T.G.; Martinoia, E.; Lee, Y. The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J. 2012, 69, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, P.; Zanella, L.; De Paolis, A.; Di Litta, D.; Cecchetti, V.; Falasca, G.; Barbieri, M.; Altamura, M.M.; Costantino, P.; Cardarelli, M. Cadmium-inducible expression of the ABC-type transporter AtABCC3 increases phytochelatin-mediated cadmium tolerance in Arabidopsis. J. Exp. Bot. 2015, 66, 3815–3829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Zhao, H.; Wu, L.; Liu, A.; Zhao, F.J.; Xu, W. Heavy metal ATPase 3 (HMA3) confers cadmium hypertolerance on the cadmium/zinc hyperaccumulator Sedum plumbizincicola. New Phytol. 2017, 215, 687–698. [Google Scholar] [CrossRef] [Green Version]
- Krämer, U.; Talke, I.N.; Hanikenne, M. Transition metal transport. FEBS Lett. 2007, 581, 2263–2272. [Google Scholar] [CrossRef]
- Rahman, M.F.; Ghosal, A.; Alam, M.F.; Kabir, A.H. Remediation of cadmium toxicity in field peas (Pisum sativum L.) through exogenous silicon. Ecotoxicol. Environ. Saf. 2017, 135, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.; Singh, P.; Tripathi, D.K.; Corpas, F.J.; Singh, V.P. Nitric oxide and hydrogen sulfide: An indispensable combination for plant functioning. Trends Plant Sci. 2021, 17, S1360–S1385. [Google Scholar] [CrossRef]
- Besson-Bard, A.; Gravot, A.; Richaud, P.; Auroy, P.; Duc, C.; Gaymard, F.; Taconnat, L.; Renou, J.P.; Pugin, A.; Wendehenne, D. Nitric oxide contributes to cadmium toxicity in Arabidopsis by promoting cadmium accumulation in roots and by up-regulating genes related to iron uptake. Plant Physiol. 2009, 149, 1302–1315. [Google Scholar] [CrossRef] [Green Version]
- Han, B.; Yang, Z.; Xie, Y.; Nie, L.; Jin, C.; Shen, W. Arabidopsis HY1 confers cadmium tolerance by decreasing nitric oxide production and improving iron homeostasis. Mol. Plant 2014, 7, 388–403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sehrawat, A.; Deswal, R. S-Nitrosylation in Abiotic Stress in Plants and Nitric Oxide Interaction with Plant Hormones; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 399–411. [Google Scholar]
- Gupta, K.J.; Hancock, J.T.; Petrivalsky, M.; Kolbert, Z.; Lindermayr, C.; Durner, J.; Barroso, J.B.; Palma, J.M.; Brouquisse, R.; Wendehenne, D.; et al. Recommendations on terminology and experimental best practice associated with plant nitric oxide research. New Phytol. 2020, 225, 1828–1834. [Google Scholar] [CrossRef] [Green Version]
- Metwally, A.; Finkemeier, I.; Georgi, M.; Dietz, K.J. Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol. 2003, 132, 272–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krantev, A.; Yordanova, R.; Janda, T.; Szalai, G.; Popova, L. Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. J. Plant Physiol. 2008, 165, 920–931. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Liang, Y.C.; Zhu, Y.G.; Zhao, F.J. Role of salicylic acid in alleviating oxidative damage in rice roots (Oryza sativa) subjected to cadmium stress. Environ. Pollut. 2007, 147, 743–749. [Google Scholar] [CrossRef]
- Zhang, L.; Pei, Y.; Wang, H.; Jin, Z.; Liu, Z.; Qiao, Z.; Fang, H.; Zhang, Y. Hydrogen sulfide alleviates cadmium-induced cell death through restraining ROS accumulation in roots of Brassica rapa L. ssp. pekinensis. Oxidative Med. Cell. Longev. 2015, 2015, 804603. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Li, H.; Huang, S.; Wang, C.; Sun, W.J.; Mo, H.Z.; Shi, Z.Q.; Chen, J. Eugenol confers cadmium tolerance via intensifying endogenous hydrogen sulfide signaling in Brassica rapa. J. Agric. Food Chem. 2018, 66, 9914–9922. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, M.J.; Ge, Z.L.; Shen, J.; Zhou, C.; Gotor, C.; Romero, L.C.; Duan, X.L.; Liu, X.; Wu, D.L.; et al. Abscisic acid-triggered guard cell L-cysteine desulfhydrase function and in situ hydrogen sulfide production contributes to heme oxygenase-modulated stomatal closure. Plant Cell Environ. 2020, 43, 624–636. [Google Scholar] [CrossRef]
- Qiao, Z.; Tao, J.; Liu, Z.; Zhang, L.; Jin, Z.; Liu, D.; Pei, Y. H2S acting as a downstream signaling molecule of SA regulates Cd tolerance in Arabidopsis. Plant Soil 2015, 393, 137–146. [Google Scholar] [CrossRef]
- Yang, X.; Kong, L.; Wang, Y.; Su, J.; Shen, W. Methane control of cadmium tolerance in alfalfa roots requires hydrogen sulfide. Environ. Pollut. 2021, 284, 117123. [Google Scholar] [CrossRef]
- Sun, J.; Wang, R.; Zhang, X.; Yu, Y.; Zhao, R.; Li, Z.; Chen, S. Hydrogen sulfide alleviates cadmium toxicity through regulations of cadmium transport across the plasma and vacuolar membranes in Populus euphratica cells. Plant Physiol. Biochem. 2013, 65, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Chen, H.; Zhu, K.; Jin, Q.; Xie, Y.; Cui, J.; Xia, Y.; Zhang, J.; Shen, W. Cadmium-induced hydrogen sulphide synthesis is involved in cadmium tolerance in Medicago sativa by reestablishment of reduced (homo) glutathione and reactive oxygen species homeostases. PLoS ONE 2014, 9, e109669. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Ye, T.; Chan, Z. Nitric oxide-activated hydrogen sulfide is essential for cadmium stress response in bermudagrass (Cynodon dactylon (L). pers.). Plant Physiol. Biochem. 2014, 74, 99–107. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Rahman, A.; Ansary, M.; Watanabe, A.; Fujita, M.; Tran, L.P. Hydrogen sulfide modulates cadmium-induced physiological and biochemical responses to alleviate cadmium toxicity in rice. Sci. Rep. 2015, 5, 14078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, H.; Wang, X.; Dou, Y.; Liu, D.; Si, W.; Fang, H.; Zhao, C.; Chen, S.; Xi, J.; Li, J. Hydrogen sulfide-cysteine cycle system enhances cadmium tolerance through alleviating cadmium-induced oxidative stress and ion toxicity in Arabidopsis roots. Sci. Rep. 2016, 6, 39702. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Responses of nitric oxide and hydrogen sulfide in regulating oxidative defence system in wheat plants grown under cadmium stress. Physiol. Plantarum 2020, 168, 345–360. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Xu, J.; Bo, T.; Wang, W. Comparative transcriptome analysis uncovers roles of hydrogen sulfide for alleviating cadmium toxicity in Tetrahymena thermophila. BMC Genom. 2021, 22, 21. [Google Scholar] [CrossRef]
- Li, G.; Shah, A.A.; Khan, W.U.; Yasin, N.A.; Ahmad, A.; Abbas, M.; Ali, A.; Safdar, N. Hydrogen sulfide mitigates cadmium induced toxicity in Brassica rapa by modulating physiochemical attributes, osmolyte metabolism and antioxidative machinery. Chemosphere 2021, 263, 127999. [Google Scholar] [CrossRef]
- Jia, H.; Wang, X.; Shi, C.; Guo, J.; Ma, P.; Ren, X.; Wei, T.; Liu, H.; Li, J. Hydrogen sulfide decreases Cd translocation from root to shoot through increasing Cd accumulation in cell wall and decreasing Cd2+ influx in Isatis indigotica. Plant Physiol. Biochem. 2020, 155, 605–612. [Google Scholar] [CrossRef]
- Tian, B.; Zhang, Y.; Jin, Z.; Liu, Z.; Pei, Y. Role of hydrogen sulfide in the methyl jasmonate response to cadmium stress in foxtail millet. Front. Biosci. 2017, 22, 530–538. [Google Scholar] [CrossRef] [Green Version]
- Tian, B.; Qiao, Z.; Zhang, L.; Li, H.; Pei, Y. Hydrogen sulfide and proline cooperate to alleviate cadmium stress in foxtail millet seedlings. Plant Physiol. Biochem. 2016, 109, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Y.; Shen, W. Roles of hydrogen sulfide and nitric oxide in the alleviation of cadmium-induced oxidative damage in alfalfa seedling roots. Biometals 2012, 25, 617–631. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Siddiqui, M.H.; AlSolami, M.A.; Alamri, S.; Hu, Y.; Ali, H.M.; Al-Amri, A.A.; Alsubaie, Q.D.; Al-Munqedhi, B.M.A.; Al-Ghamdi, A. Crosstalk of hydrogen sulfide and nitric oxide requires calcium to mitigate impaired photosynthesis under cadmium stress by activating defense mechanisms in Vigna radiata. Plant Physiol. Biochem. 2020, 156, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Ju, W.; Yang, C.; Jin, X.; Liu, D.; Li, M.; Yu, J.; Zhao, W.; Zhang, C. Exogenous application of signaling molecules to enhance the resistance of legume-rhizobium symbiosis in Pb/Cd-contaminated soils. Environ. Pollut. 2020, 265, 114744. [Google Scholar] [CrossRef]
- Graham, N.; Arisi, A.M.; Lise, J.; Kunert, K.J.; Heinz, R.; Foyer, C.H. Glutathione: Biosynthesis, metabolism and relationship to stress tolerance explored in transformed plants. J. Exp. Bot. 1998, 49, 623–647. [Google Scholar] [CrossRef]
- Jozefczak, M.; Remans, T.; Vangronsveld, J.; Cuypers, A. Glutathione is a key player in metal-induced oxidative stress defenses. Int. J. Mol. Sci. 2012, 13, 3145–3175. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Yang, L.; Yan, X.; Liu, Y.; Wang, R.; Fan, T.; Ren, Y.; Tang, X.; Xiao, F.; Liu, Y.; et al. Zinc-finger Transcription Factor ZAT6 positively regulates cadmium tolerance through the glutathione-dependent pathway in Arabidopsis. Plant Physiol. 2016, 171, 707–719. [Google Scholar] [CrossRef]
- Shen, J.; Su, Y.; Zhou, C.; Zhang, F.; Yuan, X. A putative rice L-cysteine desulfhydrase encodes a true L-cysteine synthase that regulates plant cadmium tolerance. Plant Growth Regul. 2019, 89, 217–226. [Google Scholar] [CrossRef]
- Gu, Q.; Chen, Z.; Cui, W.; Zhang, Y.; Hu, H.; Yu, X.; Wang, Q.; Shen, W. Methane alleviates alfalfa cadmium toxicity via decreasing cadmium accumulation and reestablishing glutathione homeostasis. Ecotoxicol. Environ. Saf. 2018, 147, 861–871. [Google Scholar] [CrossRef]
- Kok, L.; Bosma, W.; Maas, F.M.; Kuiper, P. The effect of short-term H2S fumigation on water-soluble sulphydryl and glutathione levels in spinach. Plant Cell Environ. 1985, 8, 189–194. [Google Scholar] [CrossRef]
- Anastasis, C.; Manganaris, G.A.; Ioannis, P.; Vasileios, F. Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways. J. Exp. Bot. 2013, 7, 1953–1966. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, X.; Cai, B.; Pan, D.; Ai, X. Physiological response and transcription profiling analysis reveal the role of glutathione in H2S-induced chilling stress tolerance of cucumber seedlings. Plant Sci. 2020, 291, 110363. [Google Scholar] [CrossRef]
- Wei, J.; Li, D.X.; Zhang, J.R.; Shan, C.; Rengel, Z.; Song, Z.B.; Chen, Q. Phytomelatonin receptor PMTR1-mediated signaling regulates stomatal closure in Arabidopsis thaliana. J. Pineal Res. 2018, 65, e12500. [Google Scholar] [CrossRef]
- Lee, H.Y.; Back, K. The phytomelatonin receptor (PMRT1) Arabidopsis Cand2 is not a bona fide G protein-coupled melatonin receptor. Melatonin Res. 2020, 3, 177–186. [Google Scholar] [CrossRef]
- Chen, T.; Tian, M.; Han, Y. Hydrogen sulfide: A multi-tasking signal molecule in the regulation of oxidative stress responses. J. Exp. Bot. 2020, 71, 2862–2869. [Google Scholar] [CrossRef]
- Aroca, A.; Benito, J.M.; Gotor, C.; Romero, L.C. Persulfidation proteome reveals the regulation of protein function by hydrogen sulfide in diverse biological processes in Arabidopsis. J. Exp. Bot. 2017, 68, 4915–4927. [Google Scholar] [CrossRef] [Green Version]
- Filipovic, M.R.; Zivanovic, J.; Alvarez, B.; Banerjee, R. Chemical biology of H2S signaling through persulfidation. Chem. Rev. 2018, 118, 1253–1337. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, J.; Zhou, M.; Zhou, H.; Cui, B.; Gotor, C.; Romero, L.C.; Fu, L.; Yang, J.; Foyer, C.H.; et al. Persulfidation-based modification of cysteine desulfhydrase and the NADPH oxidase RBOHD controls guard cell abscisic acid signaling. Plant Cell 2020, 32, 1000–1017. [Google Scholar] [CrossRef]
- Zhou, M.; Zhang, J.; Shen, J.; Zhou, H.; Zhao, D.; Gotor, C.; Romero, L.C.; Fu, L.; Li, Z.; Yang, J.; et al. Hydrogen sulfide-linked persulfidation of ABI4 controls ABA responses through the transactivation of MAPKKK18 in Arabidopsis. Mol. Plant 2021, 14, 921–936. [Google Scholar] [CrossRef]
- Chen, S.S.; Jia, H.L.; Wang, X.F.; Shi, C.; Wang, X.; Ma, P.; Wang, J.; Wang, M.J.; Li, J. Hydrogen sulfide positively regulates abscisic acid signaling through persulfidation of SnRK2.6 in guard cells. Mol. Plant 2020, 13, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, H.; Xie, Y. SnRK2.6 phosphorylation/persulfidation: Where ABA and H2S signaling meet. Trends Plant Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Aroca, Á.; Serna, A.; Gotor, C.; Romero, L.C. S-sulfhydration: A cysteine posttranslational modification in plant systems. Plant Physiol. 2015, 168, 334–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).