Beneficial Effects of Vitamin E Supplementation on Endothelial Dysfunction, Inflammation, and Oxidative Stress Biomarkers in Patients Receiving Hemodialysis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources and Search Strategy
2.4. Study Selection and Data Collection
2.5. Data Items
2.6. Risk-of-Bias Assessment
2.7. Data Analysis
3. Results
3.1. Study Selection
3.2. Study Characteristics
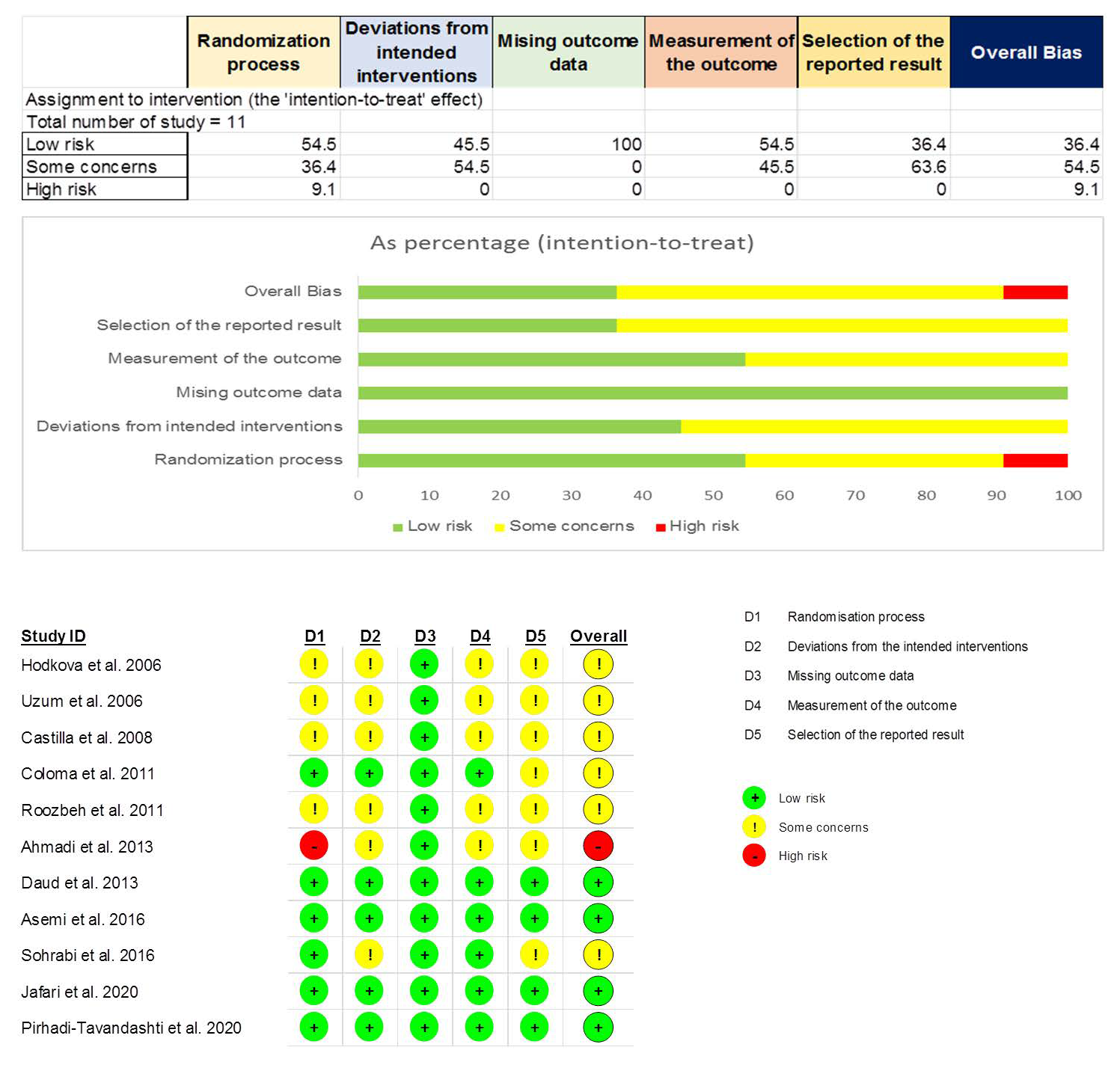
3.3. Risk-of-Bias Assessment
3.4. Meta-Analysis
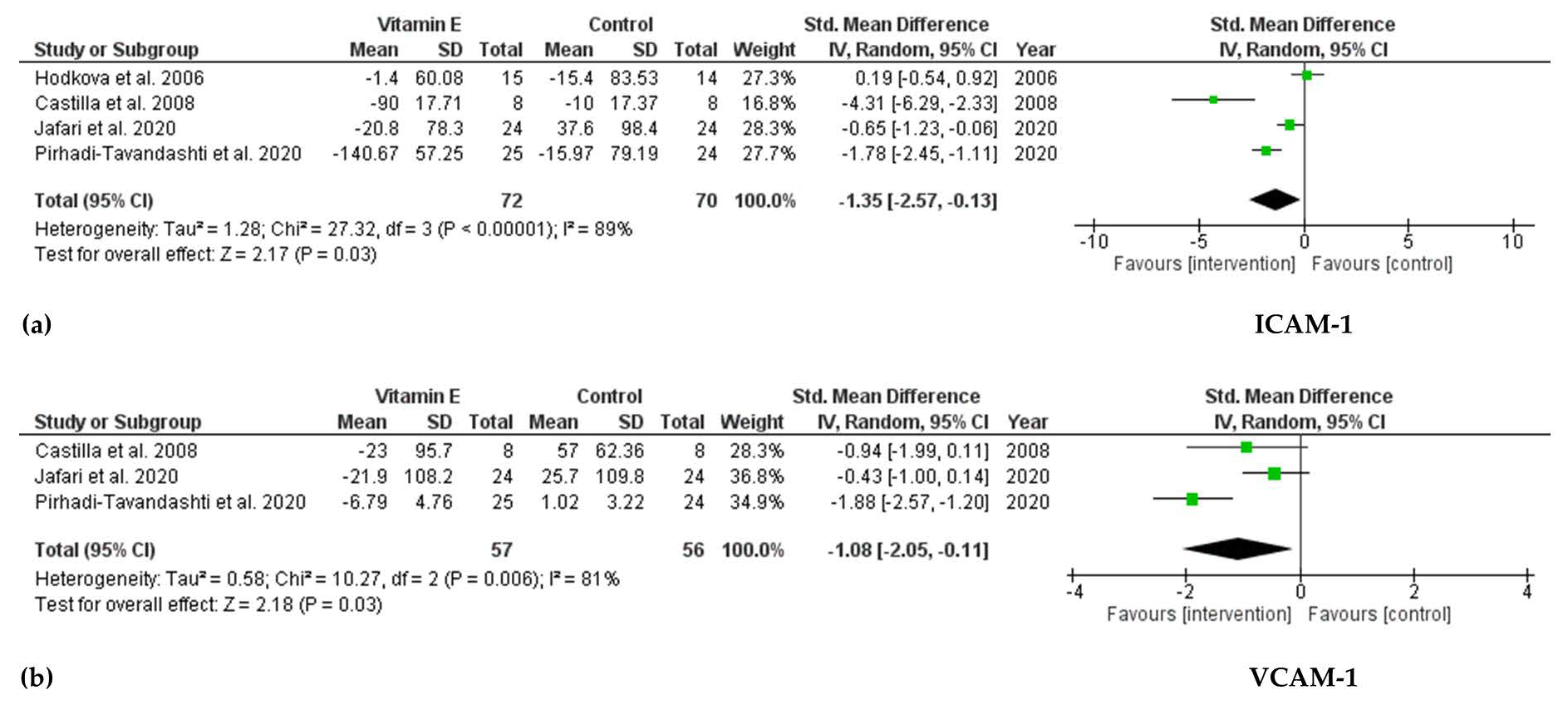
3.4.1. Effect of Vitamin E Supplementation on Biomarkers of Endothelial Dysfunction
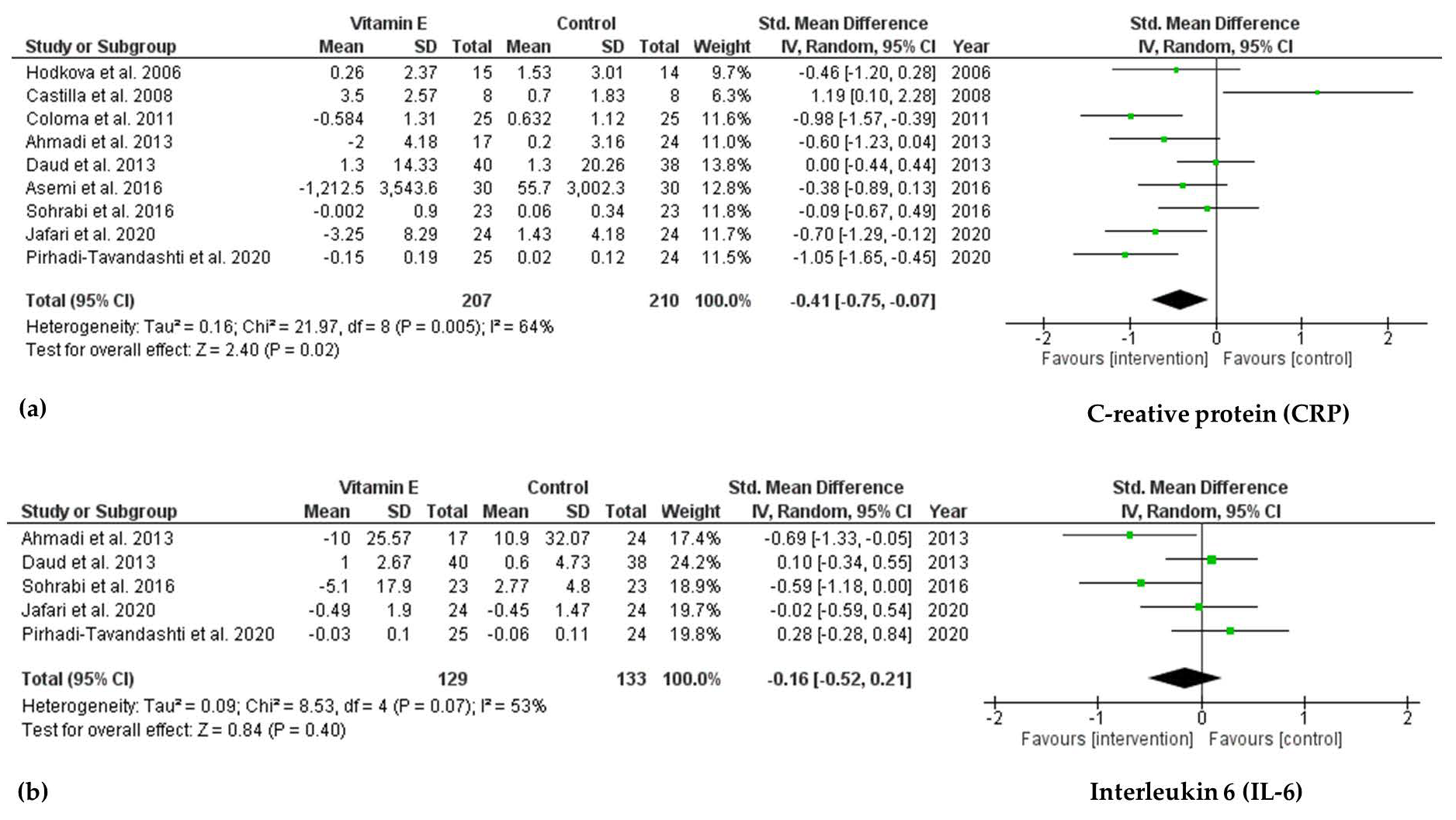
3.4.2. Effect of Vitamin E Supplementation on Biomarkers of Inflammation
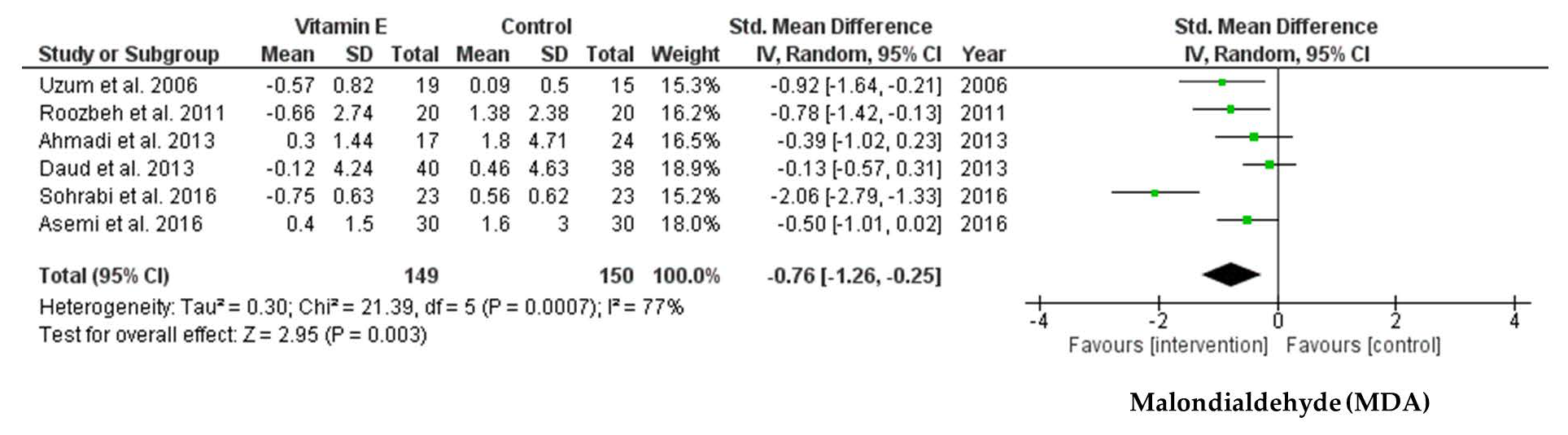
3.4.3. Effect of Vitamin E Supplementation on Biomarkers of Oxidative Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| Database | Detailed Search Strategies | Records Founded |
|---|---|---|
| MEDLINE/PUBMED | (“vitamin e”[MeSH Terms] OR vitamin E[Text Word] OR “tocopherols”[MeSH Terms] OR tocopherol[Text Word] OR “tocotrienols”[MeSH Terms] OR tocotrienol[Text Word]) AND (“renal dialysis”[MeSH Terms] OR hemodialysis[Text Word]) | 463 |
| EMBASE | (“vitamin E” OR “tocopherol” OR “tocotrienol”) AND hemodialysis | 799 |
| Cochrane Library | (“vitamin E” OR “tocopherol” OR “tocotrienol”) AND hemodialysis | 183 |
| Web of Science | (“vitamin E” OR “tocopherol” OR “tocotrienol”) AND hemodialysis | 494 |
References
- Bayés, B.; Pastor, M.C.; Bonal, J.; Juncà, J.; Hernandez, J.M.; Riutort, N.; Foraster, A.; Romero, R. Homocysteine, C-reactive protein, lipid peroxidation and mortality in haemodialysis patients. Nephrol. Dial. Transplant. 2003, 18, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Hong, Y.A.; Ban, T.H.; Kang, C.-Y.; Hwang, S.D.; Choi, S.R.; Lee, H.; Jung, H.-Y.; Kim, K.; Kwon, Y.E.; Kim, S.H.; et al. Trends in epidemiologic characteristics of end-stage renal disease from 2019 Korean Renal Data System (KORDS). Kidney Res. Clin. Pract. 2021, 40, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Stenvinkel, P.; Alvestrand, A. Inflammation in end-stage renal disease: Sources, consequences, and therapy. Semin. Dial. 2002, 15, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Yeun, J.Y.; Levine, R.A.; Mantadilok, V.; Kaysen, G.A. C-Reactive protein predicts all-cause and cardiovascular mortality in hemodialysis patients. Am. J. Kidney Dis. 2000, 35, 469–476. [Google Scholar] [CrossRef]
- Barreto, D.V.; Barreto, F.C.; Liabeuf, S.; Temmar, M.; Lemke, H.D.; Tribouilloy, C.; Choukroun, G.; Vanholder, R.; Massy, Z.A. Plasma interleukin-6 is independently associated with mortality in both hemodialysis and pre-dialysis patients with chronic kidney disease. Kidney Int. 2010, 77, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Papayianni, A.; Alexopoulos, E.; Giamalis, P.; Gionanlis, L.; Belechri, A.M.; Koukoudis, P.; Memmos, D. Circulating levels of ICAM-1, VCAM-1, and MCP-1 are increased in haemodialysis patients: Association with inflammation, dyslipidaemia, and vascular events. Nephrol. Dial. Transplant. 2002, 17, 435–441. [Google Scholar] [CrossRef] [Green Version]
- Stenvinkel, P. Inflammatory and atherosclerotic interactions in the depleted uremic patient. Blood Purif. 2001, 19, 53–61. [Google Scholar] [CrossRef]
- Sarnak, M.J.; Levey, A.S. Cardiovascular disease and chronic renal disease: A new paradigm. Am. J. Kidney Dis. 2000, 35, S117–S131. [Google Scholar] [CrossRef]
- Verma, S.; Singh, P.; Khurana, S.; Ganguly, N.K.; Kukreti, R.; Saso, L.; Rana, D.S.; Taneja, V.; Bhargava, V. Implications of oxidative stress in chronic kidney disease: A review on current concepts and therapies. Kidney Res. Clin. Pract. 2021, 40, 183–193. [Google Scholar] [CrossRef]
- Singh, U.; Devaraj, S.; Jialal, I. Vitamin E, oxidative stress, and inflammation. Annu. Rev. Nutr. 2005, 25, 151–174. [Google Scholar] [CrossRef]
- Asbaghi, O.; Sadeghian, M.; Nazarian, B.; Sarreshtedari, M.; Mozaffari-Khosravi, H.; Maleki, V.; Alizadeh, M.; Shokri, A.; Sadeghi, O. The effect of vitamin E supplementation on selected inflammatory biomarkers in adults: A systematic review and meta-analysis of randomized clinical trials. Sci. Rep. 2020, 10, 17234. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.K.; Xiao, L.; Xu, B.; Xu, X.X.; Liu, F.Y.; Sun, L. Effects of vitamin E-coated dialyzer on oxidative stress and inflammation status in hemodialysis patients: A systematic review and meta-analysis. Ren. Fail. 2014, 36, 722–731. [Google Scholar] [CrossRef] [Green Version]
- Bergin, P.; Leggett, A.; Cardwell, C.R.; Woodside, J.V.; Thakkinstian, A.; Maxwell, A.P.; McKay, G.J. The effects of vitamin E supplementation on malondialdehyde as a biomarker of oxidative stress in haemodialysis patients: A systematic review and meta-analysis. BMC Nephrol. 2021, 22, 126. [Google Scholar] [CrossRef]
- Jafari, T.; Fallah, A.A.; Reyhanian, A.; Sarmast, E. Effects of pomegranate peel extract and vitamin E on the inflammatory status and endothelial function in hemodialysis patients: A randomized controlled clinical trial. Food Funct. 2020, 11, 7987–7993. [Google Scholar] [CrossRef]
- Pirhadi-Tavandashti, N.; Imani, H.; Ebrahimpour-Koujan, S.; Samavat, S.; Hakemi, M.S. The effect of vitamin E supplementation on biomarkers of endothelial function and inflammation among hemodialysis patients: A double-blinded randomized clinical trial. Complement. Ther. Med. 2020, 49, 102357. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M. Cochrane Handbook for Systematic Reviews of Interventions; Version 6.2 (updated February 2021); Cochrane Collaboration: Melbourne, Australia, 2021. [Google Scholar]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.; Thompson, S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002, 21, 1539–1558. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Hodkova, M.; Dusilova-Sulkova, S.; Kalousova, M.; Soukupova, J.; Zima, T.; Mikova, D.; Malbohan, I.M.; Bartunkova, J. Influence of oral vitamin E therapy on micro-inflammation and cardiovascular disease markers in chronic hemodialysis patients. Ren. Fail. 2006, 28, 395–399. [Google Scholar] [CrossRef]
- Uzum, A.; Toprak, O.; Gumustas, M.K.; Ciftci, S.; Sen, S. Effect of vitamin E therapy on oxidative stress and erythrocyte osmotic fragility in patients on peritoneal dialysis and hemodialysis. J. Nephrol. 2006, 19, 739–745. [Google Scholar]
- Castilla, P.; Dávalos, A.; Teruel, J.L.; Cerrato, F.; Fernández-Lucas, M.; Merino, J.L.; Sánchez-Martín, C.C.; Ortuño, J.; Lasunción, M.A. Comparative effects of dietary supplementation with red grape juice and vitamin E on production of superoxide by circulating neutrophil NADPH oxidase in hemodialysis patients. Am. J. Clin. Nutr. 2008, 87, 1053–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coloma, R.S.; Jocson, V.R.A. Effects of vitamin E on a biomarker of inflammation and precursors of atherogenesis in chronic hemodialysis patients. Philipp. J. Intern. Med. 2011, 49, 206–215. [Google Scholar]
- Roozbeh, J.; Shahriyari, B.; Akmali, M.; Vessal, G.; Pakfetrat, M.; Jalali, G.A.R.; Afshariani, R.; Hasheminasab, M.; Ghahramani, N. Comparative effects of silymarin and vitamin E supplementation on oxidative stress markers, and hemoglobin levels among patients on hemodialysis. Ren. Fail. 2011, 33, 118–123. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, A.; Mazooji, N.; Roozbeh, J.; Mazloom, Z.; Hasanzade, J. Effect of alpha-lipoic acid and vitamin E supplementation on oxidative stress, inflammation, and malnutrition in hemodialysis Patients. Iran. J. Kidney Dis. 2013, 7, 461–467. [Google Scholar]
- Daud, Z.A.M.; Tubie, B.; Sheyman, M.; Osia, R.; Adams, J.; Tubie, S.; Khosla, P. Vitamin E tocotrienol supplementation improves lipid profiles in chronic hemodialysis patients. Vasc. Health Risk Manag. 2013, 9, 747–761. [Google Scholar] [CrossRef] [Green Version]
- Asemi, Z.; Soleimani, A.; Shakeri, H.; Mazroii, N.; Esmaillzadeh, A. Effects of omega-3 fatty acid plus alpha-tocopherol supplementation on malnutrition–inflammation score, biomarkers of inflammation and oxidative stress in chronic hemodialysis patients. Int. Urol. Nephrol. 2016, 48, 1887–1895. [Google Scholar] [CrossRef]
- Sohrabi, Z.; Eftekhari, M.H.; Eskandari, M.H.; Rezaianzadeh, A.; Sagheb, M.M. Intradialytic Oral Protein Supplementation and Nutritional and Inflammation Outcomes in Hemodialysis: A Randomized Controlled Trial. Am. J. Kidney Dis. 2016, 68, 122–130. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K. Recent advances in understanding the malnutrition-inflammation-cachexia syndrome in chronic kidney disease patients: What is next? Semin. Dial. 2005, 18, 365–369. [Google Scholar] [CrossRef] [Green Version]
- Del Vecchio, L.; Locatelli, F.; Carini, M. What we know about oxidative stress in patients with chronic kidney disease on dialysis--clinical effects, potential treatment, and prevention. Semin. Dial. 2011, 24, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Ribera, L.; Corredor, Z.; Silva, I.; Díaz, J.M.; Ballarín, J.; Marcos, R.; Pastor, S.; Coll, E. Vitamin E-coated dialysis membranes reduce the levels of oxidative genetic damage in hemodialysis patients. Mutat. Res. 2017, 815, 16–21. [Google Scholar] [CrossRef]
- Kirmizis, D.; Papagianni, A.; Belechri, A.-M.; Memmos, D. Effects of vitamin E-coated membrane dialyser on markers of oxidative stress and inflammation in patients on chronic haemodialysis. Nephrol. Dial. Transplant. 2010, 26, 2296–2301. [Google Scholar] [CrossRef] [Green Version]
- D’Arrigo, G.; Baggetta, R.; Tripepi, G.; Galli, F.; Bolignano, D. Effects of Vitamin E-Coated versus Conventional Membranes in Chronic Hemodialysis Patients: A Systematic Review and Meta-Analysis. Blood Purif. 2017, 43, 101–122. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, Q.; Hou, H.; Liu, Z.; Wang, L.; Rasekhmagham, R.; Kord-Varkaneh, H.; Santos, H.O.; Yao, G. The effects of pomegranate supplementation on biomarkers of inflammation and endothelial dysfunction: A meta-analysis and systematic review. Complement. Ther. Med. 2020, 49, 102358. [Google Scholar] [CrossRef] [PubMed]
- Stam, F.; van Guldener, C.; Schalkwijk, C.G.; ter Wee, P.M.; Donker, A.J.; Stehouwer, C.D. Impaired renal function is associated with markers of endothelial dysfunction and increased inflammatory activity. Nephrol. Dial. Transplant. 2003, 18, 892–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blankenberg, S.; Rupprecht, H.J.; Bickel, C.; Peetz, D.; Hafner, G.; Tiret, L.; Meyer, J. Circulating cell adhesion molecules and death in patients with coronary artery disease. Circulation 2001, 104, 1336–1342. [Google Scholar] [CrossRef] [Green Version]
- Tedgui, A. The role of inflammation in atherothrombosis: Implications for clinical practice. Vasc. Med. 2005, 10, 45–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habas, K.; Shang, L. Alterations in intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) in human endothelial cells. Tissue Cell 2018, 54, 139–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shuvaev, V.V.; Muzykantov, V.R. Targeted modulation of reactive oxygen species in the vascular endothelium. J. Control. Release 2011, 153, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Korevaar, J.C.; van Manen, J.G.; Dekker, F.W.; de Waart, D.R.; Boeschoten, E.W.; Krediet, R.T. Effect of an increase in C-reactive protein level during a hemodialysis session on mortality. J. Am. Soc. Nephrol. 2004, 15, 2916–2922. [Google Scholar] [CrossRef] [Green Version]
- Rusu, C.C.; Racasan, S.; Kacso, I.M.; Moldovan, D.; Potra, A.; Patiu, I.M.; Vladutiu, D.; Caprioara, M.G. Malondialdehyde can predict survival in hemodialysis patients. Clujul. Med. 2016, 89, 250–256. [Google Scholar] [CrossRef] [Green Version]
- Sung, C.C.; Hsu, Y.C.; Chen, C.C.; Lin, Y.F.; Wu, C.C. Oxidative stress and nucleic acid oxidation in patients with chronic kidney disease. Oxid. Med. Cell Longev. 2013, 2013, 301982. [Google Scholar] [CrossRef] [Green Version]
- Rusu, A.E. Vitamin E in Hemodialysis Patients. In Vitamin E in Health and Disease; Morales-Gonzalez, J.A., Ed.; InTechOpen: London, UK, 2018. [Google Scholar]
- Kosmadakis, G.; Da Costa Correia, E.; Carceles, O.; Somda, F.; Aguilera, D. Vitamins in dialysis: Who, when and how much? Ren. Fail. 2014, 36, 638–650. [Google Scholar] [CrossRef]
- Galli, F.; Buoncristiani, U.; Conte, C.; Aisa, C.; Floridi, A. Vitamin E in uremia and dialysis patients. Ann. N. Y. Acad. Sci. 2004, 1031, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Glynn, R.J.; Ridker, P.M.; Goldhaber, S.Z.; Zee, R.Y.; Buring, J.E. Effects of random allocation to vitamin E supplementation on the occurrence of venous thromboembolism: Report from the Women’s Health Study. Circulation 2007, 116, 1497–1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freedman, J.E.; Farhat, J.H.; Loscalzo, J.; Keaney, J.F., Jr. alpha-tocopherol inhibits aggregation of human platelets by a protein kinase C-dependent mechanism. Circulation 1996, 94, 2434–2440. [Google Scholar] [CrossRef]
- Rota, S.; McWilliam, N.A.; Baglin, T.P.; Byrne, C.D. Atherogenic lipoproteins support assembly of the prothrombinase complex and thrombin generation: Modulation by oxidation and vitamin E. Blood 1998, 91, 508–515. [Google Scholar] [CrossRef] [Green Version]
- Pecoits-Filho, R.; Bárány, P.; Lindholm, B.; Heimbürger, O.; Stenvinkel, P. Interleukin-6 is an independent predictor of mortality in patients starting dialysis treatment. Nephrol. Dial. Transplant. 2002, 17, 1684–1688. [Google Scholar] [CrossRef]
- Upritchard, J.E.; Sutherland, W.H.; Mann, J.I. Effect of supplementation with tomato juice, vitamin E, and vitamin C on LDL oxidation and products of inflammatory activity in type 2 diabetes. Diabetes Care 2000, 23, 733–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pecoits-Filho, R.; Heimbürger, O.; Bárány, P.; Suliman, M.; Fehrman-Ekholm, I.; Lindholm, B.; Stenvinkel, P. Associations between circulating inflammatory markers and residual renal function in CRF patients. Am. J. Kidney Dis. 2003, 41, 1212–1218. [Google Scholar] [CrossRef]

| Author, Year | Country | Type of Study | Population (M/F) | Mean Age (Years) | Mean BMI (kg/m2) | Hemodialysis | Intervention Group (n) | Control Group (n) | Outcome | Duration (Weeks) |
|---|---|---|---|---|---|---|---|---|---|---|
| Hodkova et al. 2006 [22] | Czech Republic | randomized controlled trial | 29 (10/19) | 62 | NA | HD thrice weekly, 4 h/session, Kt/V >1.2 | alpha-tocopherol 400 mg = 888 IU of vitamin E daily (n = 15) | No intervention (n = 14) | CRP, ICAM-1, E-selectin, PAPP-A | 5 |
| Uzum et al. 2006 [23] | Turkey | prospective, randomized placebo-controlled trial | 34 (18/16) | 46 | 23 | HD thrice weekly, 4 h/day | 300 mg vitamin E daily (n = 19) | Placebo (n = 15) | MDA, SOD | 20 |
| Castilla et al. 2008 [24] | Spain | randomized controlled trial | 32 (16/16) | range (33–79) | HD thrice weekly for 3.5–4.5 h/session | 800 IU/day α-tocopherol (n = 8) | No intervention (n = 8) | CRP, ICAM-1, VCAM-1, MCP-1 | 2 | |
| Coloma et al. 2011 [25] | Philippines | Prospective randomized double-blind placebo-controlled clinical trial | 50 (36/14) | 60 | 22 | HD 4 h/session, 2 sessions/week, Kt/V: 1.61 ± 0.4066 (intervention); 1.7 ± 0.4594 (placebo) | vitamin E (400 IU) daily (n = 25) | Placebo (n = 25) | CRP | 8 |
| Roozbeh et al. 2011 [26] | Iran | randomized controlled trial | 40 (27/13) | 44 | NA | HD thrice weekly | vitamin E 400 IU daily (n = 20) | No intervention (n = 20) | MDA, GPX | 3 |
| Ahmadi et al. 2013 [27] | Iran | randomized placebo-controlled trial | 41 (20/21) | 47 | 25.5 | at least 2 times weekly for at least 1 year | vitamin E (400 IU) daily (n = 17) | Placebo (n = 24) | CRP, IL-6, MDA | 8 |
| Daud et al. 2013 [28] | USA | randomized, double-blind, place bo-controlled, parallel trial | 81 (43/38) | 58.5 | 29.5 | Kt/V: 1.45 ± 0.20 (intervention); 1.48 ± 0.26 (placebo) | vitamin E tocotrienol-rich fraction (TRF) (180 mg tocotrienols, 40 mg tocopherols) daily (n = 40) | Placebo (n = 38) | CRP, IL-6, MDA | 16 |
| Asemi et al. 2016 [29] | Iran | randomized double-blind placebo-controlled clinical trial | 60 (40/20) | 60 | NA | NA | 400 IU/day alpha-tocopherol (n = 30) | Placebo (n = 30) | CRP, MDA, TAC, GSH | 12 |
| Sohrabi et al. 2016 [30] | Iran | randomized, controlled, nonblinded, parallel trial | 46 (23/23) | 57 | 22.5 | NA | vitamin E (600 IU) 3 times a week (n = 23) | No intervention (n = 23) | CRP, IL-6, MDA | 8 |
| Jafari et al. 2020 [14] | Iran | randomized, double-blind, placebo-controlled clinical trial | 48 (23/25) | 54 | 23 | HD thrice weekly Kt/V: 1.41 ± 0.71 (intervention); 1.60 ± 0.10 (placebo) | vitamin E soft gel (400 IU) daily (n = 24) | Placebo (n = 24) | CRP, IL-6, TNF-α, ICAM-1, VCAM-1, P-selectin | 8 |
| Pirhadi-Tavandashti et al. 2020 [15] | Iran | randomized, double-blinded, and placebo-controlled clinical trial | 49 (15/34) | 45 | 26 | NA | 600 IU alpha-tocopherol soft gel (n = 25) | Placebo (n = 24) | CRP, ICAM-1, VCAM-1, IL-6 | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.T.U.; Yeom, J.-h.; Kim, W. Beneficial Effects of Vitamin E Supplementation on Endothelial Dysfunction, Inflammation, and Oxidative Stress Biomarkers in Patients Receiving Hemodialysis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Mol. Sci. 2021, 22, 11923. https://doi.org/10.3390/ijms222111923
Nguyen TTU, Yeom J-h, Kim W. Beneficial Effects of Vitamin E Supplementation on Endothelial Dysfunction, Inflammation, and Oxidative Stress Biomarkers in Patients Receiving Hemodialysis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. International Journal of Molecular Sciences. 2021; 22(21):11923. https://doi.org/10.3390/ijms222111923
Chicago/Turabian StyleNguyen, Thi Thuy Uyen, Ji-hyun Yeom, and Won Kim. 2021. "Beneficial Effects of Vitamin E Supplementation on Endothelial Dysfunction, Inflammation, and Oxidative Stress Biomarkers in Patients Receiving Hemodialysis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" International Journal of Molecular Sciences 22, no. 21: 11923. https://doi.org/10.3390/ijms222111923






