Molecular Regulatory Networks for Improving Nitrogen Use Efficiency in Rice
Abstract
:1. Introduction
2. Genetic Variation in NUE among Various Rice Germplasm
3. Nitrate Relative Genes Play an Important Role in Improving NUE in Rice
4. Ammonium, Amino Acid and Nitrogen Assimilation Relative Genes Apply to NUE
5. Plant Hormones Act on NUE
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Christie, P.; Goulding, K.W.T.; Vitousek, P.M.; Zhang, F.S. Significant acidification in major Chinese croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, H.; Jiang, Z.; Wang, W.; Xu, R.; Wang, Q.; Zhang, Z.; Li, A.; Liang, Y.; Ou, S.; et al. Genomic basis of geographical adaptation to soil nitrogen in rice. Nature 2021, 590, 600–605. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tao, Y.; Tang, D.; Wang, J.; Zhong, J.; Wang, Y.; Yuan, Q.; Yu, X.; Zhang, Y.; Wang, Y.; et al. Identification of QTL Associated with Nitrogen Uptake and Nitrogen Use Efficiency Using High Throughput Genotyped CSSLs in Rice (Oryza sativa L.). Front. Plant Sci. 2017, 8, 1166. [Google Scholar] [CrossRef]
- Tang, J.; Sun, Z.; Chen, Q.; Damaris, R.N.; Lu, B.; Hu, Z. Nitrogen Fertilizer Induced Alterations in The Root Proteome of Two Rice Cultivars. Int. J. Mol. Sci. 2019, 20, 3674. [Google Scholar] [CrossRef] [Green Version]
- Sasaki, T.; Burr, B. International rice genome sequencing project: The effort to completely sequence the rice genome. Curr. Opin. Plant Biol. 2000, 3, 138–142. [Google Scholar] [CrossRef]
- Hu, B.; Wang, W.; Doug, S.J.B.; Tang, J.Y.; Li, H.; Che, R.H.; Zhang, Z.H.; Chai, X.Y.; Wang, H.R.; Wang, Y.Q.; et al. Variation in NRT1.1B contributes to nitrate-use divergence between rice subspecies. Nat. Genet. 2015, 47, 834–838. [Google Scholar] [CrossRef]
- Zhu, J.; Liang, J.; Xu, Z.; Fan, X.; Zhou, Q.; Shen, Q.; Xu, G. Root aeration improves growth and N accumulation in rice seedlings under low nitrogen. AoB Plants 2015, 7, plv131. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Wang, Y.; Chen, G.; Zhang, A.; Yang, S.; Shang, L.; Wang, D.; Ruan, B.; Liu, C.; Jiang, H.; et al. The indica nitrate reductase gene OsNR2 allele enhances rice yield potential and nitrogen use efficiency. Nat. Commun. 2019, 10, 5207. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Chu, C.C. Nitrogen-Use Divergence Between Indica and Japonica Rice: Variation at Nitrate Assimilation. Mol. Plant 2020, 13, 6–7. [Google Scholar] [CrossRef]
- Shan, Y.H.; Wang, Y.L.; Yoshinori, Y. Genotypic difference of nitrogen use efficiency in various types of indica rice. Jiangsu Agric. Res. 2001, 1, 12–15. [Google Scholar]
- Shan, Y.H.; Wang, Y.L.; Pan, X.B. Mapping of QTLs for nitrogen use efficiency and related traits in rice (Oryza sativa L). Acta Agron. Sin. 2005, 4, 721–727. [Google Scholar]
- Li, Y.; Li, M.; Cao, G.; Han, L. Effects of genetic background on expression of QTL for nitrogen efficiency in irrigated rice and upland rice. Sci. Agric. Sin. 2010, 43, 4331–4340. [Google Scholar]
- Lian, X.; Xing, Y.; Yan, H.; Xu, C.; Li, X.; Zhang, Q. QTLs for low nitrogen tolerance at seedling stage identified using a recombinant inbred line population derived from an elite rice hybrid. Appl. Genet. 2005, 112, 85–96. [Google Scholar] [CrossRef]
- Wei, D.; Cui, K.; Ye, G.; Pan, J.; Xiang, J.; Huang, J.; Nie, L. QTL mapping for nitrogen-use efficiency and nitrogen-deficiency tolerance traits in rice. Plant Soil 2012, 359, 281–295. [Google Scholar] [CrossRef]
- Wang, Q.; Nian, J.; Xie, X.; Yu, H.; Zhang, J.; Bai, J.; Dong, G.; Hu, J.; Bai, B.; Chen, L.; et al. Genetic variations in ARE1 mediate grain yield by modulating nitrogen utilization in rice. Nat. Commun. 2018, 9, 735. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Fan, X.; Shen, Q. The relationship between rhizosphere nitrification and nitrogen-use efficiency in rice plants. Plant Cell Env. 2008, 31, 73–85. [Google Scholar] [CrossRef]
- Kirk, G. Plant-mediated processess to acquire nutrients: Nitrogen uptake by rice plants. Plant Soil 2001, 232, 129–134. [Google Scholar] [CrossRef]
- Duan, Y.; Zhang, Y.; Ye, L.; Fan, X.; Xu, G.; Shen, Q. Responses of rice cultivars with different nitrogen use efficiency to partial nitrate nutrition. Ann. Bot. 2007, 99, 1153–1160. [Google Scholar] [CrossRef] [Green Version]
- Fan, X.; Tang, Z.; Tan, Y.; Zhang, Y.; Luo, B.; Yang, M.; Lian, X.; Shen, Q.; Miller, A.J.; Xu, G. Overexpression of a pH-sensitive nitrate transporter in rice increases crop yields. Proc. Natl. Acad. Sci. USA 2016, 113, 7118–7123. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Hu, B.; Yuan, D.; Liu, Y.; Che, R.; Hu, Y.; Ou, S.; Liu, Y.; Zhang, Z.; Wang, H.; et al. Expression of the nitrate transporter gene OsNRT1.1A/OsNPF6.3 confers high yield and early maturation in rice. Plant Cell 2018, 30, 638–651. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Qu, B.; Li, W.; Zhao, X.; Teng, W.; Ma, W.; Ren, Y.; Li, B.; Li, Z.; Tong, Y. The nitrate-inducible NAC transcription factor TaNAC2-5A controls nitrate response and increases wheat yield. Plant Physiol. 2015, 169, 1991–2005. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Fan, X.; Qian, K.; Zhang, Y.; Song, M.; Liu, Y.; Xu, G.; Fan, X. pOsNAR2.1:OsNAR2.1 expression enhances nitrogen uptake efficiency and grain yield in transgenic rice plants. Plant Biotechnol. J. 2017, 15, 1273–1283. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Liu, X.; Liu, S.; Fan, X.; Zhao, L.; Song, M.; Fan, X.; Xu, G. Co-overexpression of OsNRT2.3a and OsNAR2.1 increased agronomic nitrogen use efficiency in transgenic rice plants. Front. Plant Sci. 2020, 11, 1245. [Google Scholar] [CrossRef]
- Chen, J.; Qi, T.; Hu, Z.; Fan, X.; Zhu, L.; Iqbal, M.F.; Yin, X.; Xu, G.; Fan, X. OsNAR2.1 Positively Regulates Drought Tolerance and Grain Yield Under Drought Stress Conditions in Rice. Front. Plant Sci. 2019, 10, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.; Zhang, Y.; Tan, Y.; Zhang, M.; Zhu, L.; Xu, G.; Fan, X. Agronomic nitrogen-use efficiency of rice can be increased by driving OsNRT2.1 expression with the OsNAR2.1 promoter. Plant Biotechnol. J. 2016, 14, 1705–1715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, H.; Li, B.; Zhi, Y.; Chen, J.; Li, R.; Xia, X.; Xu, G.; Fan, X. Overexpression of the nitrate transporter, OsNRT2.3b, improves rice phosphorus uptake and translocation. Plant Cell Rep. 2017, 36, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Yi, H.; Bao, J.; Gong, J. LeNRT2.3 functions in nitrate acquisition and long-distance transport in tomato. FEBS Lett. 2015, 589, 1072–1079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taulemesse, F.; Le, G.J.; Gouache, D.; Gibon, Y.; Allard, V. Postflowering nitrate uptake in wheat is controlled by N status at flowering, with a putative major role of root nitrate transporter NRT2.1. PLoS ONE 2015, 10, e0120291. [Google Scholar] [CrossRef] [Green Version]
- Yan, M.; Fan, X.; Feng, H.; Miller, A.J.; Shen, Q.; Xu, G. Rice OsNAR2.1 interacts with OsNRT2.1, OsNRT2.2 and OsNRT2.3a nitrate transporters to provide uptake over high and low concentration ranges. Plant Cell Environ. 2011, 34, 1360–1372. [Google Scholar] [CrossRef]
- Feng, H.; Yan, M.; Fan, X.; Li, B.; Shen, Q.; Miller, A.J.; Xu, G. Spatial expression and regulation of rice high-affinity nitrate transporters by nitrogen and carbon status. J. Exp. Bot. 2011, 62, 2319–2332. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Fan, X.; Chen, J.; Qu, H.; Luo, L.; Xu, G. OsNAR2.1 Interaction with OsNIT1 and OsNIT2 Functions in Root-growth Responses to Nitrate and Ammonium. Plant Physiol. 2020, 183, 289–303. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Zheng, Y.; Feng, H.; Qu, H.; Fan, X.; Yamaji, N.; Ma, J.F.; Xu, G. OsNRT2.4 encodes a dual-affinity nitrate transporter and functions in nitrate-regulated root growth and nitrate distribution in rice. J. Exp. Bot. 2018, 69, 1095–1107. [Google Scholar] [CrossRef]
- Liu, X.; Huang, D.; Tao, J.; Miller, A.J.; Fan, X.; Xu, G. Identification and functional assay of the interaction motifs in the partner protein OsNAR2.1 of the two-component system for high-affinity nitrate transport. New Phytol. 2014, 204, 74–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Z.; Fan, X.; Li, Q.; Feng, H.; Miller, A.J.; Shen, Q.; Xu, G. Knockdown of a Rice Stelar Nitrate Transporter Alters Long-Distance Translocation but Not Root Influx. Plant Physiol. 2012, 160, 2052–2063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Chen, A.; Xie, K.; Yang, X.; Luo, Z.; Chen, J.; Zeng, D.; Ren, Y.; Yang, C.; Wang, L.; et al. Functional analysis of the OsNPF4.5 nitrate transporter reveals a conserved mycorrhizal pathway of nitrogen acquisition in plants. Proc. Natl. Acad. Sci. USA 2020, 117, 16649–16659. [Google Scholar] [CrossRef]
- Hu, B.; Jiang, Z.; Wang, W.; Qiu, Y.; Zhang, Z.; Liu, Y.; Li, A.; Gao, X.; Liu, L.; Qian, Y.; et al. Nitrate–NRT1.1B–SPX4 cascade integrates nitrogen and phosphorus signalling networks in plants. Nat. Plants. 2019, 5, 401–413. [Google Scholar] [CrossRef]
- Tang, W.; Ye, J.; Yao, X.; Zhao, P.; Xuan, W.; Tian, Y.; Zhang, Y.; Xu, S.; An, H.; Chen, G.; et al. Genome-wide associated study identifies NAC42-activated nitrate transporter conferring high nitrogen use efficiency in rice. Nat. Commun. 2019, 10, 5279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Ouyang, J.; Wang, Y.Y.; Hu, R.; Xia, K.; Duan, J.; Wang, Y.; Tsay, Y.F.; Zhang, M. Disruption of the rice nitrate transporter OsNPF2.2 hinders root-to-shoot nitrate transport and vascular development. Sci. Rep. 2015, 5, 9635. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Fan, X.; Wei, J.; Feng, H.; Qu, H.; Xie, D.; Miller, A.J.; Xu, G. Rice nitrate transporter OsNPF2.4 functions in low-affinity acquisition and longdistance transport. J. Exp. Bot. 2015, 66, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.T.; Nie, H.P.; Feng, F.; Wang, J.; Lu, K.; Fang, Z.M. Altered expression of OsNPF7.1 and OsNPF7.4 differentially regulates tillering and grain yield in rice. Plant Sci. 2019, 283, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, K.; Nie, H.P.; Zeng, Q.S.; Wu, B.W.; Qian, J.J.; Fang, Z.M. Rice nitrate transporter OsNPF7.2 positively regulates tiller number and grain yield. Rice 2018, 11, 12. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.T.; Bai, G.X.; Wang, J.; Zhu, W.; Zeng, Q.S.; Lu, K.; Sun, S.Y.; Fang, Z.M. Two splicing variants of OsNPF7.7 regulate shoot branching and nitrogen utilization efficiency in rice. Front. Plant Sci. 2018, 9, 300. [Google Scholar] [CrossRef] [Green Version]
- Fang, Z.M.; Bai, G.X.; Huang, W.T.; Wang, Z.X.; Wang, X.L.; Zhang, M.Y. The rice peptide transporter OsNPF7.3 is induced by organic nitrogen, and contributes to nitrogen allocation and grain yield. Front. Plant Sci. 2017, 8, 1338. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Zhang, Z.; Xia, J.; Alfatih, A.; Song, Y.; Huang, Y.; Wan, G.; Sun, L.; Tang, H.; Liu, Y.; et al. Rice NIN-LIKE PROTEIN 4 plays a pivotal role in nitrogen use efficiency. Plant Biotechnol. J. 2021, 19, 448–461. [Google Scholar] [CrossRef]
- Yu, J.; Xuan, W.; Tian, Y.; Fan, L.; Sun, J.; Tang, W.; Chen, G.; Wang, B.; Liu, Y.; Wu, W.; et al. Enhanced OsNLP4-OsNiR cascade confers nitrogen use efficiency by promoting tiller number in rice. Plant Biotechnol. J. 2021, 19, 167–176. [Google Scholar] [CrossRef]
- Fang, Z.M.; Xia, K.F.; Yang, X.; Suter, M.; Meier, S.; Rentsch, D.; Xu, X.L.; Zhang, M.Y. Altered expression of the PTR/NRT1 homologue OsPTR9 affects nitrogen utilization efficiency, growth and grain yield in rice. Plant Biotechnol. J. 2013, 11, 446–458. [Google Scholar] [CrossRef]
- Pellizzaro, A.; Clochard, T.; Planchet, E.; Limami, A.M.; Morere-Le Paven, M.C. Identification and molecular characterization of Medicago truncatula NRT2 and NAR2 families. Physiol. Plant. 2015, 154, 256–269. [Google Scholar] [CrossRef]
- Bagchi, R.; Salehin, M.; Adeyemo, O.S.; Salazar, C.; Shulaev, V.; Sherrier, D.J.; Dickstein, R. Functional assessment of the Medicago truncatula NIP/LATD protein demonstrates that it is a high-affinity nitrate transporter. Plant Physiol. 2012, 160, 906–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, C.; Song, A.; Zhang, X.; Wang, H.; Li, T.; Chen, Y.; Jiang, J.; Chen, F.; Chen, S. Cloning of chrysanthemum high affinity nitrate transporter family (CmNRT2) and characterization of CmNRT2.1. Sci. Rep. 2016, 6, 23462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mounier, E.; Pervent, M.; Ljung, K.; Gojon, A.; Nacry, P. Auxin mediated nitrate signalling by NRT1.1 participates in the adaptive response of Arabidopsis root architecture to the spatial heterogeneity of nitrate availability. Plant Cell Environ. 2014, 37, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Peng, J.S.; He, Y.N.; Zhang, G.B.; Yi, H.Y.; Fu, Y.L.; Gong, J.M. Arabidopsis NRT1.5 mediates the suppression of nitrate starvation induced leaf senescence by modulating foliar potassium level. Mol. Plant. 2016, 9, 461–470. [Google Scholar] [CrossRef] [Green Version]
- Drechsler, N.; Zheng, Y.; Bohner, A.; Nobmann, B.; von Wiren, N.; Kunze, R.; Rausch, C. Nitrate-dependent control of shoot K homeostasis by the nitrate transporter1/peptide transporter family member NPF7.3/ NRT1.5 and the stelar K+ outward rectifier SKOR in Arabidopsis. Plant Physiol. 2015, 169, 2832–2847. [Google Scholar] [PubMed] [Green Version]
- Hsu, P.K.; Tsay, Y.F. Two phloem nitrate transporters, NRT1.11 and NRT1.12, are important for redistributing xylem-borne nitrate to enhance plant growth. Plant Physiol. 2013, 163, 844–856. [Google Scholar] [CrossRef] [Green Version]
- Taochy, C.; Gaillard, I.; Ipotesi, E.; Oomen, R.; Leonhardt, N.; Zimmermann, S.; Boyer, J.C. The Arabidopsis root stele transporter NPF2.3 contributes to nitrate translocation to shoots under salt stress. Plant J. 2015, 83, 466–479. [Google Scholar] [CrossRef]
- Pike, S.; Gao, F.; Kim, M.J.; Kim, S.H.; Schachtman, D.P.; Gassmann, W. Members of the NPF3 transporter subfamily encode pathogen inducible nitrate/nitrite transporters in grapevine and Arabidopsis. Plant Cell Physiol. 2014, 55, 162–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leran, S.; Garg, B.; Boursiac, Y.; Corratg-Faillie, C.; Brachet, C.; Tillard, P.; Gojon, A.; Lacombe, B. AtNPF5.5, a nitrate transporter affecting nitrogen accumulation in Arabidopsis embryo. Sci. Rep. 2015, 5, 7962. [Google Scholar] [CrossRef] [Green Version]
- Lezhneva, L.; Kiba, T.; Feria-Bourrellier, A.B.; Lafouge, F.; Boutet-Mercey, S.; Zoufan, P.; Sakakibara, H.; Daniel-Vedele, F.; Krapp, A. The Arabidopsis nitrate transporter NRT2.5 plays a role in nitrate acquisition and remobilization in nitrogen-starved plants. Plant J. 2014, 80, 230–241. [Google Scholar] [CrossRef]
- David, L.C.; Dechorgnat, J.; Berquin, P.; Routaboul, J.M.; Debeaujon, I.; Daniel-Vedele, F.; Ferrario-Mery, S. Proanthocyanidin oxidation of Arabidopsis seeds is altered in mutant of the high-affinity nitrate transporter NRT2.7. J. Exp. Bot. 2014, 65, 885–893. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.H.; Wu, J.; Tang, H.; Yuan, Y.; Wang, S.M.; Wang, Y.P.; Zhu, Q.S.; Li, S.G.; Xiang, C.B. Overexpression of Arabidopsis NLP7 improves plant growth under both nitrogen-limiting and -sufficient conditions by enhancing nitrogen and carbon assimilation. Sci. Rep. 2016, 6, 27795. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yao, Q.; Gao, X.; Jiang, C.; Harberd, N.P.; Fu, X. Shoot-to root mobile transcription factor HY5 coordinates plant carbon and nitrogen acquisition. Curr. Biol. 2016, 26, 640–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glass, A.D.; Britto, D.T.; Kaiser, B.N.; Kinghorn, J.R.; Kronzucker, H.J.; Kumar, A.; Vidmar, J.J.; Siddiqi, M.Y.; Rawat, S.; Okamoto, M.; et al. The regulation of nitrate and ammonium transport systems in plants. J. Exp. Bot. 2002, 53, 855–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sonoda, Y.; Ikeda, A.; Saiki, S.; Wirén, N.V.; Yamaya, T.; Yamaguchi, J. Distinct expression and function of three ammonium transporter genes (OsAMT1;1–1;3) in rice. Plant Cell Physiol. 2003, 44, 726–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jahn, T.P.; Møller, A.L.; Zeuthen, T.; Holm, L.M.; Klaerke, D.A.; Mohsin, B.; Kühlbrandt, W.; Schjoerring, J.K. Aquaporin homologues in plants and mammals transport ammonia. FEBS Lett. 2004, 574, 31–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loqué, D.; von Wirén, N. Regulatory levels for the transport of ammonium in plant roots. J. Exp. Bot. 2004, 55, 1293–1305. [Google Scholar] [CrossRef] [Green Version]
- Guo, S.; Kaldenhoff, R.; Uehlein, N.; Sattelmacher, B.; Brueck, H. Relationship between water and nitrogen uptake in nitrate-and ammoniumsupplied Phaseolus vulgaris L. plants. J. Plant Nutr. Soil Sci. 2017, 170, 73–80. [Google Scholar] [CrossRef]
- Bárzana, G.; Aroca, R.; Bienert, G.P.; Chaumont, F.; Ruiz-Lozano, J.M. New insights into the regulation of aquaporins by the arbuscular mycorrhizal symbiosis in maize plants under drought stress and possible implications for plant performance. Mol. Plant Microbe Interact. 2014, 27, 349–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.Y.; Hao, D.L.; Cong, Y.; Jin, M.; Su, Y.H. The rice OsAMT1;1 is a proton-independent feedback regulated ammonium transporter. Plant Cell Rep. 2015, 34, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Ranathunge, K.; El-kereamy, A.; Gidda, S.; Bi, Y.M.; Rothstein, S.J. AMT1;1 transgenic rice plants with enhanced NH+4 permeability show superiorgrowth and higher yield under optimal and suboptimalNH4+ conditions. J. Exp. Bot. 2014, 65, 965–979. [Google Scholar] [CrossRef]
- Li, T.; Liao, K.; Xu, X.; Gao, Y.; Wang, Z.; Zhu, X.; Xuan, Y.; Jia, B. Wheat Ammonium Transporter (AMT) Gene family: Diversity and possible role in host–pathogen interaction with stemrust. Front. Plant Sci. 2017, 8, 1637. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Marmagne, A.; Park, J.; Fabien, C.; Yim, Y.; Kim, S.; Kim, T.; Lim, P.; Masclaux-Daubresse, C.; Nam, H.G. Concurrent activation of OsAMT1;2 and OsGOGAT1 in rice leads to enhanced nitrogen use efficiency under nitrogen limitation. Plant J. 2020, 103, 7–20. [Google Scholar] [CrossRef]
- Bao, A.; Liang, Z.; Zhao, Z.; Cai, H. Overexpressing of OsAMT1-3, a high affinity ammonium transporter gene, modifies rice growth and carbon-nitrogen metabolic status. Int. J. Mol. Sci. 2015, 16, 9037–9063. [Google Scholar] [CrossRef] [Green Version]
- Alfatih, A.; Wu, J.; Zhang, Z.S.; Xia, J.Q.; Jan, S.U.; Yu, L.H.; Xiang, C.B. Rice NIN-LIKE PROTEIN 1 rapidly responds to nitrogen deficiency and improves yield and nitrogen use efficiency. J. Exp. Bot. 2020, 7, 6032–6042. [Google Scholar] [CrossRef] [PubMed]
- Komarova, N.Y.; Thor, K.; Gubler, A.; Meier, S.; Dietrich, D.; Weichert, A.; Grotemeyer, M.S.; Tegeder, M.; Rentsch, D. AtPTR1 and AtPTR5 transport dipeptides in planta. Plant Physiol. 2008, 148, 856–869. [Google Scholar] [CrossRef]
- Paungfoo-Lonhienne, C.; Lonhienne, T.G.A.; Rentsch, D.; Robinson, N.; Christie, M.; Webb, R.I. Plants can use protein as a nitrogen source without assistance from other organisms. Proc. Natl. Acad. Sci. USA 2008, 105, 4524–4529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tegeder, M.; Masclaux-Daubresse, C. Source and sink mechanisms of nitrogen transport and use. New Phytol. 2018, 217, 35–53. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.L.; Healey, J.R.; Willett, V.B.; Farrar, J.F.; Hodge, A. Dissolved organic nitrogen uptake by plants—An important N uptake pathway? Soil. Biol. Biochem. 2005, 37, 413–423. [Google Scholar] [CrossRef]
- Moran-Zuloaga, D.; Dippold, M.; Glaser, B.; Kuzyakov, Y. Organic nitrogen uptake by plants: Reevaluation by position-specific labeling of amino acids. Biogeochemistry 2015, 125, 359–374. [Google Scholar] [CrossRef]
- Enggrob, K.L.; Jakobsen, C.M.; Pedersen, I.F.; Rasmussen, J. Newly depolymerized large organic N contributes directly to amino acid uptake in young maize plants. New Phytol. 2019, 224, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Lam, S.K.; Yan, X.; Chen, D. How does recycling of livestock manure in agroecosystems affect crop productivity, reactive nitrogen losses, and soil carbon balance? Environ. Sci. Technol. 2017, 51, 7450–7457. [Google Scholar] [CrossRef]
- Ji, Y.; Huang, W.; Wu, B.; Fang, Z.; Wang, X. The amino acid transporter AAP1 mediates growth and grain yield by regulating neutral amino acid uptake and reallocation in Oryza sativa. J. Exp. Bot. 2020, 71, 4763–4777. [Google Scholar] [CrossRef]
- Lu, K.; Wu, B.; Wang, J.; Zhu, W.; Nie, H.; Qian, J.; Huang, W.; Fang, Z. Blocking amino acid transporter OsAAP3 improves grain yield by promoting outgrowth buds and increasing tiller number in rice. Plant Biotechnol. J. 2018, 16, 1710–1722. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wu, B.; Lu, K.; Wei, Q.; Qian, J.; Chen, Y.; Fang, Z. The amino acid permease 5 (OsAAP5) regulates tiller number and grain yield in rice. Plant Physiol. 2019, 180, 1031–1045. [Google Scholar] [CrossRef]
- Guo, N.; Hu, J.; Yan, M.; Qu, H.; Luo, L.; Tegeder, M.; Xu, G. Oryza sativa Lysine-Histidine-type Transporter 1 functions in root uptake and root-to-shoot allocation of amino acids in rice. Plant J. 2020, 103, 395–411. [Google Scholar] [CrossRef]
- Lam, H.M.; Coschigano, K.T.; Oliveira, I.C.; Melo-Oliveira, R.; Coruzzi, G.M. The molecular-genetics of nitrogen assimilation into amino acids in higher plants. Annu. Rev. Plant Biol. 1996, 47, 569–593. [Google Scholar] [CrossRef] [PubMed]
- Tegeder, M.; Rentsch, D. Uptake and partitioning of amino acids and peptides. Mol. Plant 2010, 3, 997–1011. [Google Scholar] [CrossRef]
- Xu, G.; Fan, X.; Miller, A.J. Plant nitrogen assimilation and use efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Chen, X.; Wu, K.; Fu, X. Nitrogen signaling and use efficiency in plants: What’s new? Curr. Opin. Plant Biol. 2015, 27, 192–198. [Google Scholar] [CrossRef]
- Beevers, L.; Hageman, R.H. Nitrate reduction in higher plants. Annu. Rev. Plant Physiol. 1969, 20, 495–522. [Google Scholar] [CrossRef]
- Djennane, S.; Chauvin, J.E.; Meyer, C. Glasshouse behaviour of eight transgenic potato clones with a modified nitrate reductase expression under two fertilization regimes. J. Exp. Bot. 2002, 53, 1037–1045. [Google Scholar] [CrossRef]
- Chamizo-Ampudia, A.; Sanz-Luque, E.; Llamas, A.; Galvan, A.; Fernandez, E. Nitrate reductase regulates plant nitric oxide homeostasis. Trends Plant Sci. 2017, 22, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Mauceri, A.; Bassolino, L.; Lupini, A.; Badeck, F.; Rizza, F.; Schiavi, M.; Toppino, L.; Abenavoli, M.R.; Rotino, G.L.; Sunseri, F. Genetic variation in eggplant for Nitrogen Use Efficiency under contrasting NO3-supply. J. Integr. Plant Biol. 2020, 62, 487–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaya, T.; Obara, M.; Nakajima, H.; Sasaki, S.; Hayakawa, T.; Sato, T. Genetic manipulation and quantitative-trait loci mapping for nitrogen recycling in rice. J. Exp. Bot. 2002, 53, 917–925. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Zhen, X.; Li, X.; Li, N.; Xu, F. Increased autophagy of rice can increase yield and nitrogen use efficiency (NUE). Front. Plant Sci. 2019, 10, 584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhen, X.; Xu, F.; Zhang, W.; Li, N.; Li, X. Overexpression of rice gene OsATG8b confers tolerance to nitrogen starvation and increases yield and nitrogen use efficiency (NUE) in Arabidopsis. PLoS ONE 2019, 14, e0223011. [Google Scholar] [CrossRef] [Green Version]
- Fan, T.; Yang, W.; Zeng, X.; Xu, X.; Xu, Y.; Fan, X.; Zhang, M. A rice autophagy gene OsATG8b is involved in nitrogen remobilization and control of grain quality. Front. Plant Sci. 2020, 11, 588. [Google Scholar] [CrossRef] [PubMed]
- Zhen, X.; Li, X.; Yu, J.; Xu, F. OsATG8c-mediated increased autophagy regulates the yield and nitrogen use efficiency in rice. Int. J. Mol. Sci. 2019, 20, 4956. [Google Scholar] [CrossRef] [Green Version]
- Chichkova, S.; Arellano, J.; Vance, C.P.; Hernández, G. Transgenic tobacco plants that overexpress alfalfa NADH-glutamate synthase have higher carbon and nitrogen content. J. Exp. Bot. 2001, 52, 2079–2087. [Google Scholar] [CrossRef]
- Brauer, E.K.; Rochon, A.; Bi, Y.M.; Bozzo, G.G.; Rothstein, S.J.; Shelp, B.J. Reappraisal of nitrogen use efficiency in rice overexpressing glutamine synthetase1. Physiol. Plant 2011, 141, 361–372. [Google Scholar] [CrossRef]
- Lea, P.J.; Miflin, B.J. “Nitrogen assimilation and its relevance to crop improvement” in Nitrogen Metabolism in Plants in the Post-Genomic Era. Annu. Plant Rev. 2011, 42, 1–40. [Google Scholar]
- Miflin, B.J.; Habash, D.Z. The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. J. Exp. Bot. 2002, 53, 979–987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chardon, F.; Noël, V.; Masclaux-Daubresse, C. Exploring NUE in crops and in Arabidopsis ideotypes to improve yield and seed quality. J. Exp. Bot. 2012, 63, 3401–3412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cren, M.; Hirel, B. Glutamine synthetase in higher plants regulation of gene and protein expression from the organ to the cell. Plant Cell Physiol. 1999, 40, 1187–1193. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, S.; Moreira, E.; Amorim, I.; Santos, C.; Melo, P. Arabidopsis thaliana mutants devoid of chloroplast glutamine synthetase (GS2) have non-lethal phenotype under photorespiratory conditions. Plant Physiol. Biochem. 2019, 144, 365–374. [Google Scholar] [CrossRef]
- Kusano, M.; Tabuchi, M.; Fukushima, A.; Funayama, K.; Diaz, C.; Kobayashi, M.; Saito, K. Metabolomics data reveal a crucial role of cytosolic glutamine synthetase 1;1 in coordinating metabolic balance in rice. Plant J. 2011, 66, 456–466. [Google Scholar] [CrossRef]
- Sun, H.; Huang, Q.; Su, J. Highly effective expression of glutamine synthetase genes GS1 and GS2 in transgenic rice plants increases nitrogen-deficiency tolerance. Zhi Wu Sheng Li Yu Fen Zi Sheng Wu Xue Xue Bao 2005, 31, 492–498. [Google Scholar] [PubMed]
- Migge, A.; Carrayol, E.; Hirel, B.; Becker, T.W. Leaf-specific overexpression of plastidic glutamine synthetase stimulates the growth of transgenic tobacco seedlings. Planta 2000, 210, 252–260. [Google Scholar] [CrossRef]
- Fuentes, S.I.; Allen, D.J.; Ortiz-Lopez, A.; Hernández, G. Over-expression of cytosolic glutamine synthetase increases photosynthesis and growth at low nitrogen concentrations. J. Exp. Bot. 2001, 52, 1071–1081. [Google Scholar] [CrossRef]
- Oliveira, I.C.; Brears, T.; Knight, T.J.; Clark, A.; Coruzzi, G.M. Overexpression of cytosolic glutamine synthetase. Relation to nitrogen, light, and photorespiration. Plant Physiol. 2002, 129, 1170–1180. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Fu, B.; Pan, L.; Chen, L.; Fu, X.; Li, K. Overexpression of Arabidopsis Dof1, GS1 and GS2 enhanced nitrogen assimilation in transgenic tobacco grown under low-nitrogen conditions. Plant Mol. Biol. Rep. 2013, 31, 886–900. [Google Scholar] [CrossRef]
- Zhu, C.; Fan, Q.; Wang, W.; Shen, C.; Meng, X.; Tang, Y.; Mei, B.; Xu, Z.; Song, R. Characterization of a glutamine synthetase gene DvGS2 from Dunaliella viridis and biochemical identification of DvGS2-transgenic Arabidopsis thaliana. Gene 2014, 536, 407–415. [Google Scholar] [CrossRef]
- Urriola, J.; Rathore, K.S. Overexpression of a glutamine synthetase gene affects growth and development in sorghum. Transgenic Res. 2015, 24, 397–407. [Google Scholar] [CrossRef]
- Martin, A.; Lee, J.; Kichey, T.; Gerentes, D.; Zivy, M.; Tatout, C.; Dubois, F.; Balliau, T.; Valot, B.; Davanture, M.; et al. Two cytosolic glutamine synthetase isoforms of maize are specifically involved in the control of grain production. Plant Cell 2006, 18, 3252–3274. [Google Scholar] [CrossRef] [Green Version]
- Habash, D.Z.; Massiah, A.J.; Rong, H.L.; Wallsgrove, R.M.; Leigh, R.A. The role of cytosolic glutamine synthetase in wheat. Ann. App. Biol. 2001, 138, 83–89. [Google Scholar] [CrossRef]
- Hu, M.; Zhao, X.; Liu, Q.; Hong, X.; Zhang, W.; Zhang, Y.; Sun, L.; Li, H.; Tong, Y. Transgenic expression of plastidic glutamine synthetase increases nitrogen uptake and yield in wheat. Plant Biotechnol. J. 2018, 16, 1858–1867. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; de Bang, T.C.; Schjoerring, J.K. Cisgenic overexpression of cytosolic glutamine synthetase improves nitrogen utilization efficiency in barley and prevents grain protein decline under elevated CO2. Plant Biotechnol. J. 2019, 17, 1209–1221. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.; Zhou, Y.; Xiao, J.; Li, X.; Zhang, Q.; Lian, X. Overexpressed glutamine synthetase gene modifies nitrogen metabolism and abiotic stress responses in rice. Plant Cell Rep. 2009, 28, 527–537. [Google Scholar] [CrossRef]
- Bao, A.; Zhao, Z.; Ding, G.; Shi, L.; Xu, F.; Cai, H. Accumulated expression level of cytosolic glutamine synthetase 1 gene (OsGS1;1 or OsGS1;2) alter plant development and the carbon-nitrogen metabolic status in rice. PLoS ONE 2014, 9, e95581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomsen, H.C.; Eriksson, D.; Møller, I.S.; Schjoerring, J.K. Cytosolic glutamine synthetase: A target for improvement of crop nitrogen use efficiency? Trends Plant Sci. 2014, 19, 656–663. [Google Scholar] [CrossRef]
- Garnett, T.; Plett, D.; Heuer, S.; Okamoto, M. Genetic approaches to enhancing nitrogen-use efficiency (NUE) in cereals: Challenges and future directions. Funct. Plant Biol. 2015, 42, 921–941. [Google Scholar] [CrossRef] [PubMed]
- Sweetlove, L.J.; Nielsen, J.; Fernie, A.R. Engineering central metabolism–a grand challenge for plant biologists. Plant J. 2017, 90, 749–763. [Google Scholar] [CrossRef] [Green Version]
- Luo, L.; Wang, H.X.; Liu, X.H.; Hu, J.Q.; Zhu, X.L.; Pan, S.; Qin, R.Y.; Wang, Y.F.; Zhao, P.P.; Fan, X.R.; et al. Strigolactones affect the translocation of nitrogen in rice. Plant Sci. 2018, 270, 190–197. [Google Scholar] [CrossRef]
- Benjamins, R.; Scheres, B. Auxin: The Looping Star in Plant Development. Annu. Rev. Plant Biol. 2008, 59, 443–465. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Zhu, L.M.; Shen, C.B.; Ji, Z.; Zhang, H.P.; Zhang, T.; Li, Y.; Yu, J.P.; Yang, N.; He, Y.B.; et al. Natural allelic variation in a modulator of auxin homeostasis improves grain yield and nitrogen use efficiency in rice. Plant Cell 2021, 5, 566–580. [Google Scholar] [CrossRef]
- Hou, M.; Luo, F.; Wu, D.; Zhang, X.; Lou, M.; Shen, D.; Yan, M.; Mao, C.; Fan, X.; Xu, G.; et al. OsPIN9, an auxin efflux carrier, is required for the regulation of rice tiller bud outgrowth by ammonium. New Phytol. 2021, 229, 935–949. [Google Scholar] [CrossRef]
- Itoh, H.; Ueguchi-Tanaka, M.; Sato, Y.; Matsuoka, A.M. The gibberellin signaling pathway is regulated by the appearance and disappearance of SLENDER RICE1 in nuclei. Plant Cell 2002, 14, 57–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asano, K.; Yamasaki, M.; Takuno, S.; Miura, K.; Katagiri, S.; Ito, T.; Doi, K.; Wu, J.; Ebana, K.; Matsumoto, T.; et al. Artificial selection for a green revolution gene during japonica rice domestication. Proc. Natl. Acad. Sci. USA 2011, 108, 11034–11039. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Tian, Y.; Wu, K.; Ye, Y.; Yu, J.; Zhang, J.; Liu, Q.; Hu, M.; Li, H.; Tong, Y.; et al. Modulating plant growth-metabolism coordination for sustainable agriculture. Nature 2018, 560, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Hedden, P. The genes of the Green Revolution. Trends Genet. 2003, 19, 5–9. [Google Scholar] [CrossRef]
- Wu, K.; Wang, S.S.; Song, W.Z.; Zhang, J.Q.; Wang, Y.; Liu, Q.; Yu, J.P.; Ye, Y.F.; Li, S.; Chen, J.F.; et al. Enhanced sustainable green revolution yield via nitrogen-responsive chromatin modulation in rice. Science 2020, 367, eaaz2046. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.Y.; Ding, C.H.; Baerson, S.R.; Lian, F.Z.; Lin, X.H.; Zhang, L.Q.; Wu, C.F.; Hwang, S.Y.; Zeng, R.S.; Song, Y.Y. The roles of jasmonate signalling in nitrogen uptake and allocation in rice (Oryza sativa L.). Plant Cell Environ. 2019, 42, 659–672. [Google Scholar] [CrossRef]
- Wang, C.G.; Wang, G.K.; Gao, Y.; Lu, G.H.; Habben, J.E.; Mao, G.F.; Chen, G.W.; Wang, J.T.; Yang, F.; Zhao, X.Q.; et al. A cytokinin-activation enzyme-like gene improves grain yield under various field conditions in rice. Plant Mol. Biol. 2020, 102, 373–388. [Google Scholar] [CrossRef]
- Xu, G.H.; Takahashi, H. Improving nitrogen use efficiency: From cells to plant systems. J. Exp. Bot. 2020, 71, 4359–4364. [Google Scholar] [CrossRef]
- Qin, P.; Lu, H.; Du, H.; Wang, H.; Chen, W.; Chen, Z.; He, Q.; Ou, S.; Zhang, H.; Li, X.; et al. Pangenome analysis of 33 genetically diverse rice accessions reveals hidden genomic variations. Cell 2021, 184, 3542–3558.e16. [Google Scholar] [CrossRef]
- Fan, X.R.; Naz, M.; Fan, X.R.; Xuan, W.; Miller, A.J.; Xu, G.H. Plant nitrate transporters: From gene function to application. J. Exp. Bot. 2020, 68, 2463–2475. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, M.R.; Longo, C.; Lupini, A.; Miller, A.J.; Araniti, F.; Mercati, F.; Princi, M.P.; Sunseri, F. Phenotyping two tomato genotypes with different nitrogen use efficiency. Plant Physiol. Biochem. 2016, 107, 21–32. [Google Scholar] [CrossRef]
- Naz, M.; Luo, B.; Guo, X.; Li, B.; Chen, J.; Fan, X. Overexpression of Nitrate Transporter OsNRT2.1 Enhances Nitrate-Dependent Root Elongation. Genes 2019, 10, 290. [Google Scholar] [CrossRef] [Green Version]
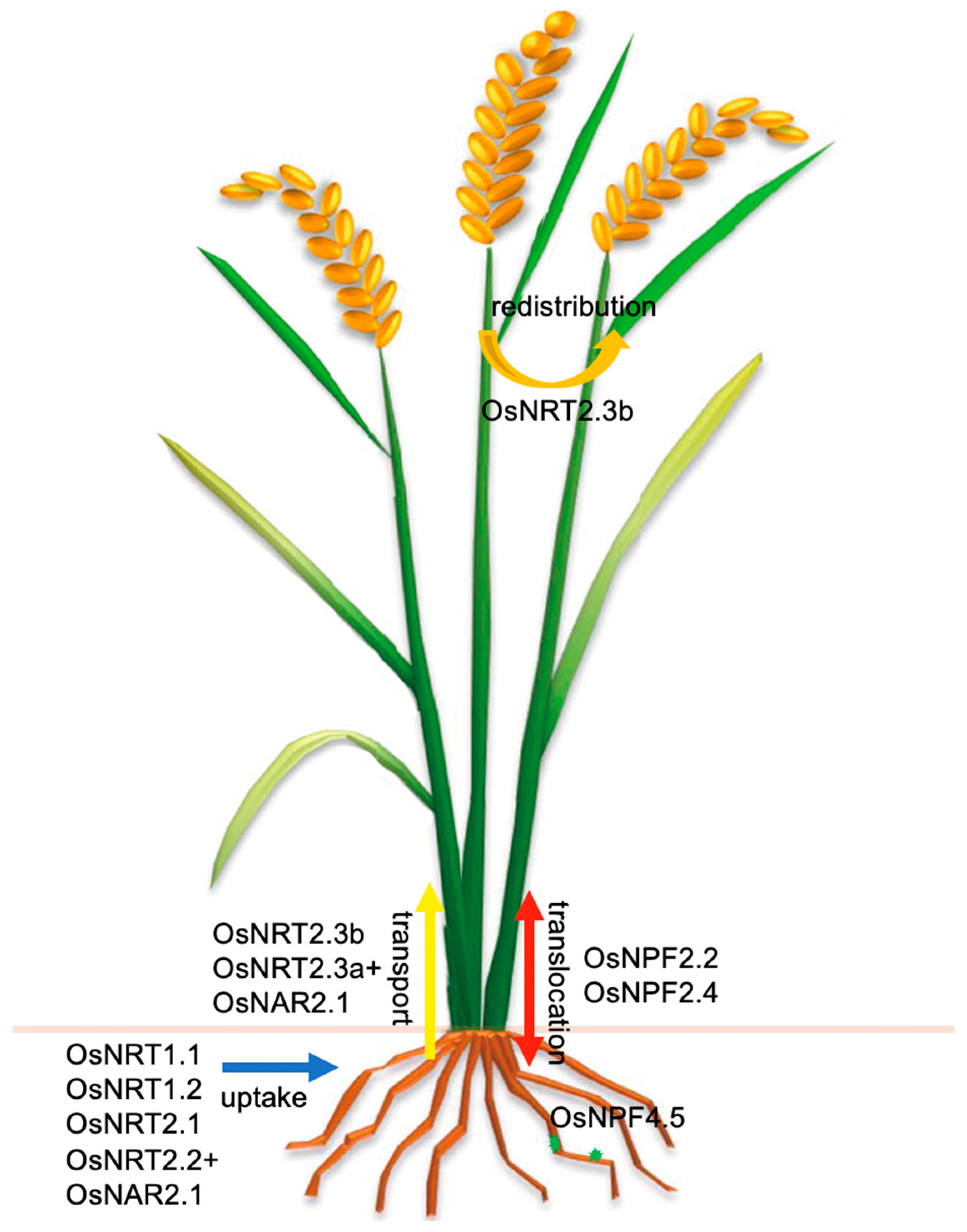
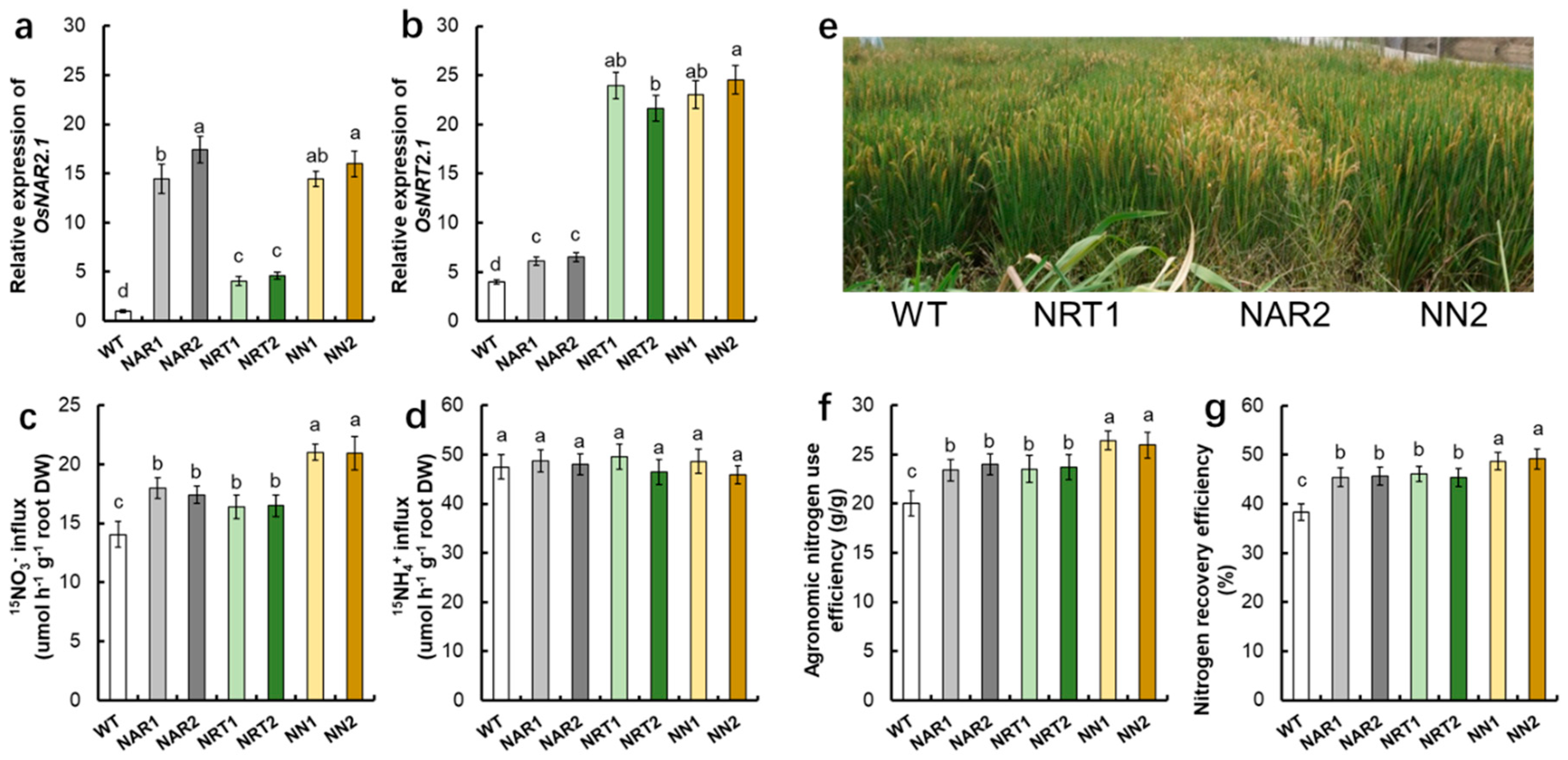
| Gene | Accession NO. | Species | Characteristic | Nnitrogen Behavior | Reference |
|---|---|---|---|---|---|
| OsTCP19 | Os06g12230 | Rice | Responding to nitrate, negatively regulate rice tillering | High tilling response to nitrogen | [2] |
| OsNRT2.3b | AK072215 | Rice | Enhancing pH homeostasis, grain yield and NUE | Uptake | [19] |
| OsNRT2.3a | AK109776 | Rice | Root-to-shoot NO3− transport | Transport | [34] |
| OsNAR2.1 | NC_029257.1 | Rice | Interaction with OsNRT2.1/2.2/2.3a | Promote uptake and transport after interaction | [29,33] |
| OsNRT1.1B | Os10g40600 | Rice | Improving NUE in Indica rice | Uptake and transport | [6] |
| OsNPF2.2 | Os12g44100 | Rice | Unloading NO3− from the xylem and effects on root-to-shoot NO3−transport | Transport | [38] |
| OsNPF2.4 | AK099321.1 | Rice | Long-distance NO3− transport and regulating NO3− and K+ shuttle | Uptake and transport | [39] |
| OsNRT2.4 | Os01g36720 | Rice | Dual-affinity nitrate transporter, nitrate-regulated root growth | Uptake | [32] |
| OsNRT1.1A | Os08g05910 | Rice | Regulating N utilization and flowering | Uptake | [20] |
| OsNPF4.5 | Os01g54515 | Rice | Low-affinity NO3− transport activity involved in symbiotic N uptake | Uptake | [35] |
| OsNPF6.1 | Os01g01360 | Rice | OsNPF6.1HapB enhances nitrate uptake and confers high NUE by increasing yield under low nitrogen supply | Uptake | [15] |
| OsNPF7.1 | Os07g41250 | Rice | regulates tillering and grain yield | Uptake | [40] |
| OsNPF7.4 | Os04g50940 | Rice | regulates tillering and grain yield | Uptake and transport | [40] |
| OsNPF7.2 | Os02g47090 | Rice | positively regulates tiller number and grain yield | Uptake and transport | [41] |
| OsNPF7.7 | Os10g42870 | Rice | regulate shoot branching and nitrogen utilization efficiency | Uptake | [42] |
| OsNPF7.3 | Os04g50950 | Rice | contributes to nitrogen allocation and grain yield | Translocation | [43] |
| OsPTR9 | Os06g49250 | Rice | nitrogen utilization efficiency and grain yield | Uptake | [46] |
| TaNAC2-5A | AY625683 | Wheat | Positive control of NO3− transporter expression and improving NO3− accumulation and plant growth | Regualating TaNRT2.5 Enhance nitrate uptake | [21] |
| TaNRT2.1 | AF332214 | Wheat | NO3− uptake at post-flowering stage | Uptake | [28] |
| MtNRT2.1 | Medtr4g104730 | M. truncatula | Nitrate transport with HATS activity | Uptake | [47] |
| MtNRT2.3 | Medtr4g057865 | M. truncatula | Controlling post-inoculation processes in nodule functioning | Transport | [47] |
| MtNIP/LATD | GQ401665 | M. truncatula | Controlling nodulation and root architecture | Uptake | [48] |
| LeNRT2.3 | AY038800 | Tomato | NO3− uptake and long-distance transport | Uptake and transport | [27] |
| CmNRT2.1 | KT203959.1 | Chrysanthemum | Enhancing N uptake | Uptake | [49] |
| AtNRT1.1 | AT1G12110 | Arabidopsis | Directing root growth in sensing external NO3− concentration | Uptake | [50] |
| AtNPF7.3/AtNRT1.5 | AT1G32450 | Arabidopsis | Low NO3− dependent K+ translocation from root to shoot | Transport | [51,52] |
| AtNRT1.11 | AT1G52190 | Arabidopsis | Phloem-specific NO3− transporter redistributing xylem-borne NO3− to enhance plant growth | Transport | [53] |
| AtNRT1.12 | AT3G16180 | Arabidopsis | Phloem-specific NO3− transporter redistributing xylem-borne NO3− to enhance plant growth | Transport | [53] |
| AtNPF2.3 | AT3G45680 | Arabidopsis | NO3− excretion transporter and contribution to NO3− translocation to the shoot | Transport | [54] |
| AtNPF3.1 | AT1G68570 | Arabidopsis | Encoding pathogen-inducible NO3−/NO2− transporters | Uptake | [55] |
| AtNPF5.5 | AT2G38100 | Arabidopsis | Affecting N accumulation in Arabidopsis embryo | Uptake | [56] |
| AtNRT2.5 | AT1G12940 | Arabidopsis | Loading NO3− into phloem of N-starved adult plants | Uptake andtransport | [57] |
| AtNRT2.7 | AT5G14570 | Arabidopsis | Seed-specific NO3− transporter for accumulation/oxidation of proanthocyanidins | Transport | [58] |
| AtNLP7 | AT4G24020 | Arabidopsis | Enhancing plant growth in N-sufficient conditions | Sensing and assimilation | [59] |
| AtHY5 | AT3G17609 | Arabidopsis | Binding NRT2.1 promoter | Activating NRT2.1 expression and nitrate uptake | [60] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, M.; Yu, M.; Li, Z.; Ai, Z.; Chen, J. Molecular Regulatory Networks for Improving Nitrogen Use Efficiency in Rice. Int. J. Mol. Sci. 2021, 22, 9040. https://doi.org/10.3390/ijms22169040
Hou M, Yu M, Li Z, Ai Z, Chen J. Molecular Regulatory Networks for Improving Nitrogen Use Efficiency in Rice. International Journal of Molecular Sciences. 2021; 22(16):9040. https://doi.org/10.3390/ijms22169040
Chicago/Turabian StyleHou, Mengmeng, Ming Yu, Zhiqiang Li, Zhiyuan Ai, and Jingguang Chen. 2021. "Molecular Regulatory Networks for Improving Nitrogen Use Efficiency in Rice" International Journal of Molecular Sciences 22, no. 16: 9040. https://doi.org/10.3390/ijms22169040






