Ortho Isomeric Mn(III) N-Alkyl- and Alkoxyalkylpyridylporphyrins—Enhancers of Hyaluronan Degradation Induced by Ascorbate and Cupric Ions †
Abstract
:1. Introduction
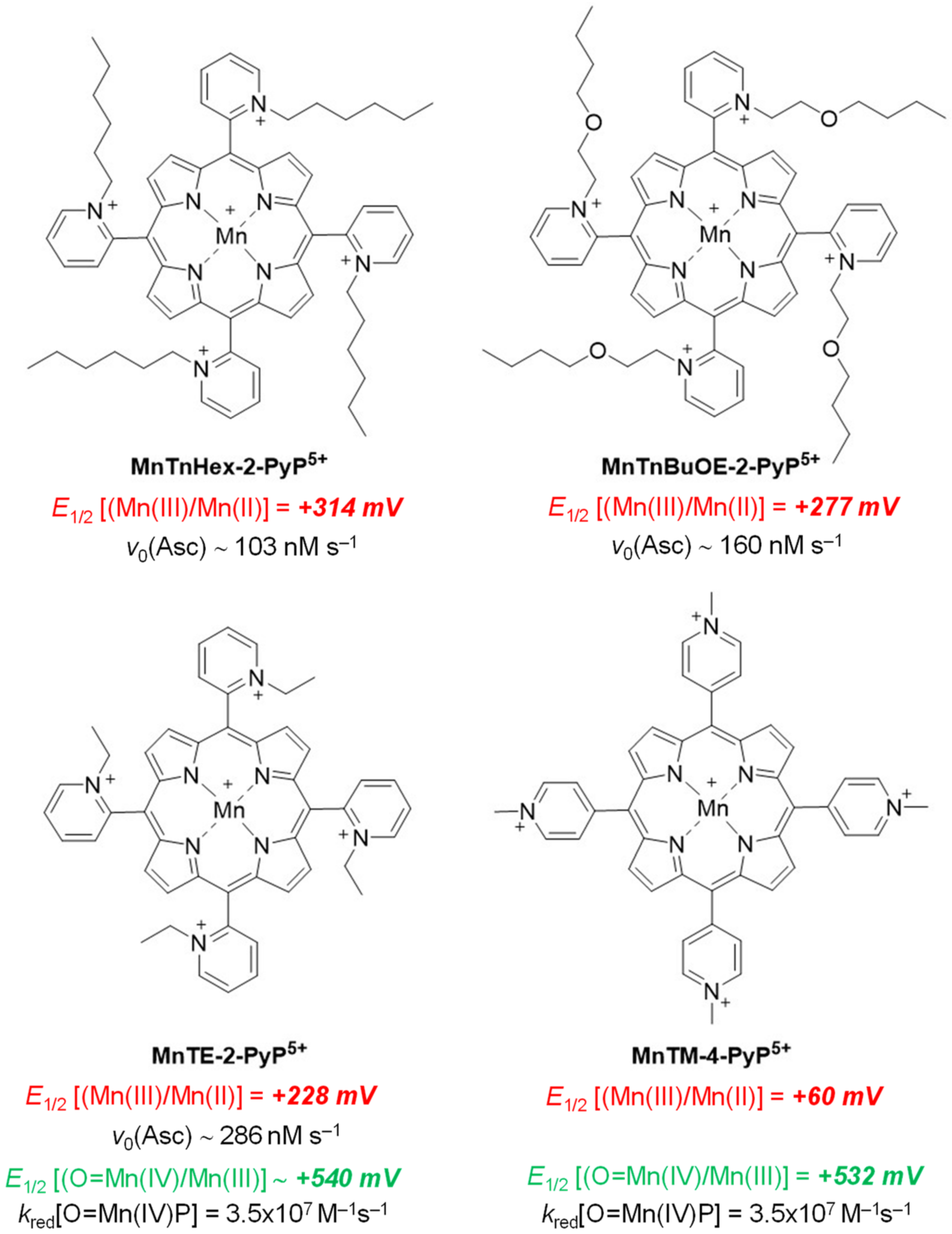
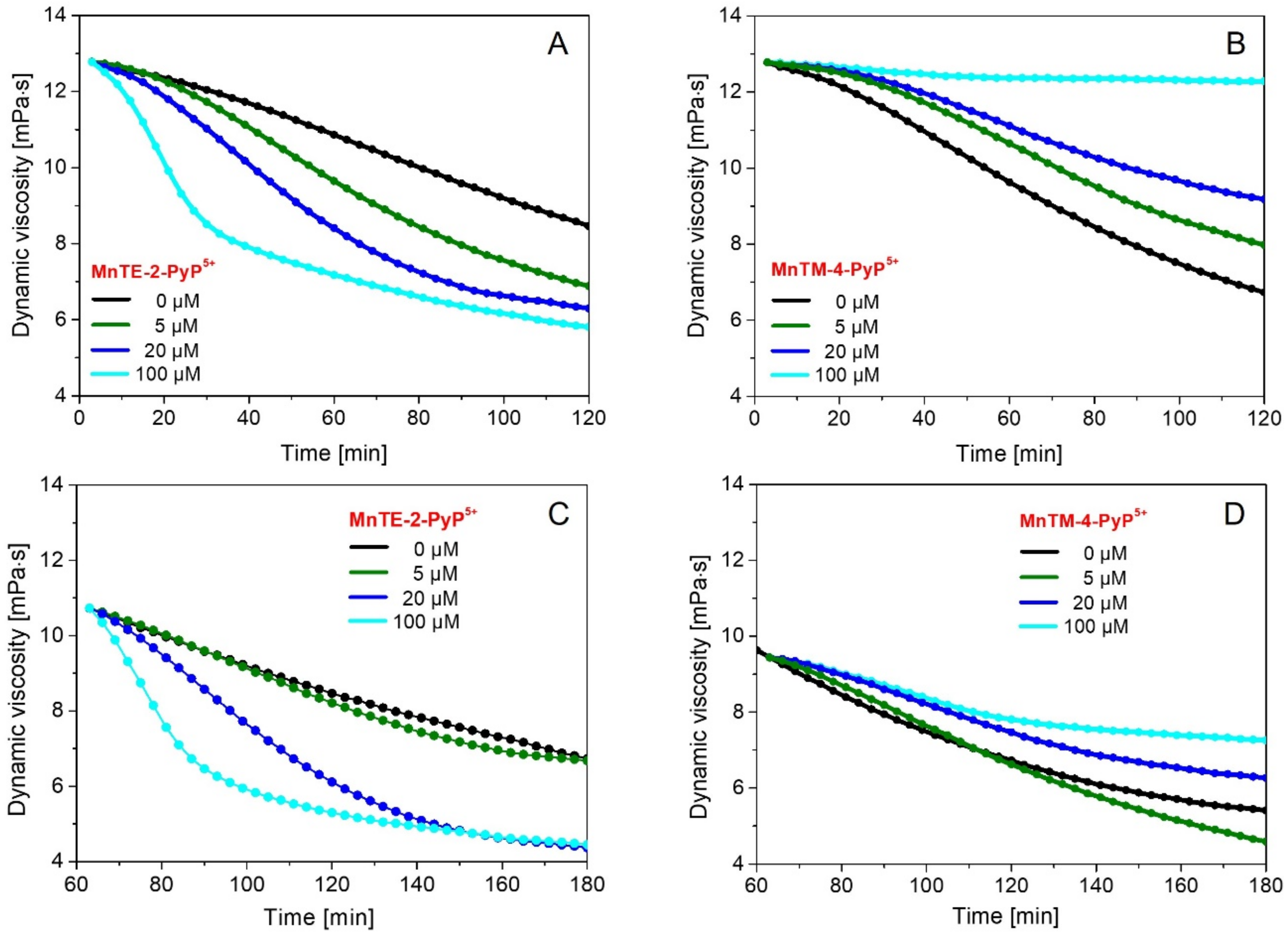
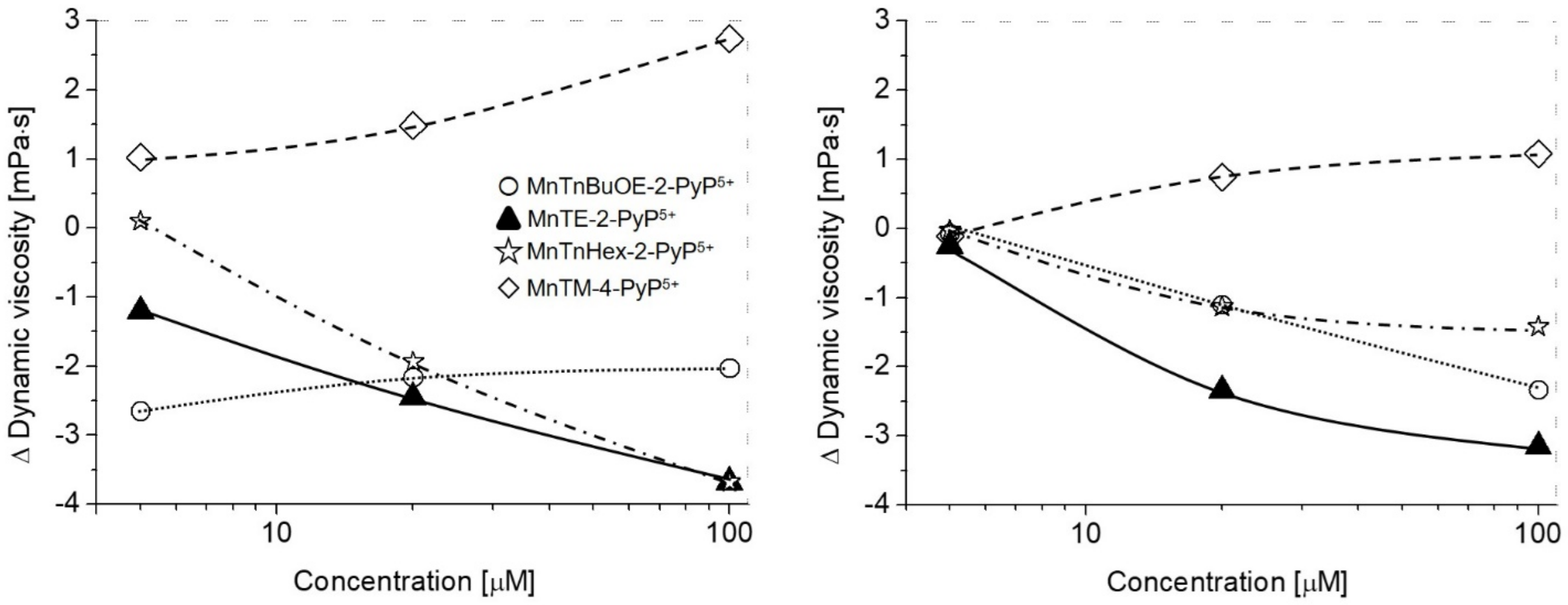
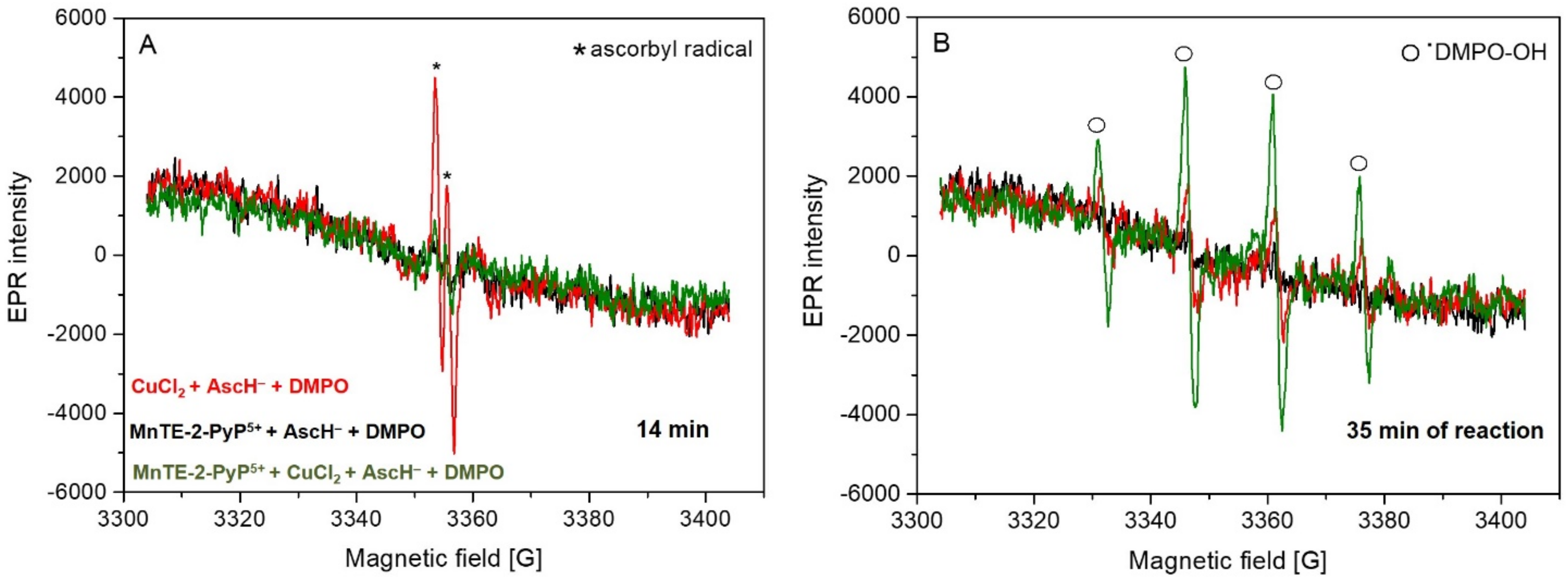
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Stock and Working Solutions
3.3. Studies of Inhibition of Hyaluronan Degradation
- i.
- A volume of 50 µL of CuCl2 solution was added to the HA solution (7.90, 7.85, 7.79 or 7.76 mL), and the mixture, after a stirring of 30 s, was left to stand for 7.5 min at room temperature. Then, the MnP solution (0, 50, 110 or 140 µL) was added to the reaction mixture, followed by stirring again for 30 s. Finally, 50 µL of ascorbic acid solution was added to the solution, and the mixture was stirred for another 30 s. The solution was then immediately transferred into the viscometer Teflon® cup reservoir.
- ii.
- In the second experimental design, a procedure similar to that described in (i) was applied; however, after 7.5 min, the 50 µL of ascorbic acid solution was added and stirred for 1 h. Then 50, 110 or 140 µL of the MnP solution was added, followed by stirring for 30 s. The solution mixture was then immediately transferred into the viscometer Teflon® cup reservoir.Dynamic viscosity of the reaction mixture (8 mL) containing HA (1.75 mg/mL), ascorbate (100 μM), Cu(II) ions (1 μM) and Mn porphyrins (final concentrations of 0, 5, 20, 100 μM) was measured by a Brookfield LVDV−II+PRO digital rotational viscometer (Brookfield Engineering Labs., Middleboro, MA, USA) at 25.0 ± 0.1 °C, 180 rpm at a shear rate of 237.6 s−1 for 2 h. All details of how the degradation of HA can be assessed by dynamic viscosity is described in [31].
3.4. Electron Paramagnetic Resonance (EPR)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Batinic-Haberle, I.; Tovmasyan, A.; Spasojevic, I. Mn porphyrin-based redox-active drugs: Differential effects as cancer therapeutics and protectors of normal tissue against oxidative injury. Antioxid. Redox Signal. 2018, 29, 1691–1724. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Tome, M.E. Thiol regulation by Mn porphyrins, commonly known as SOD mimics. Redox Biol. 2019, 25, 101139. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Spasojevic, I. 25 years of development of Mn porphyrins—From SOD mimics to thiol signaling to clinical trials. The story of our life in USA. J. Porphyr. Phthalocya. 2019, 23, 1326–1335. [Google Scholar] [CrossRef] [Green Version]
- Batinic-Haberle, I.; Benov, L.; Spasojevic, I.; Hambright, P.; Crumbliss, A.L.; Fridovich, I. The relationship between redox potentials, proton dissociation constants of pyrrolic nitrogens, and in vitro and in vivo superoxide dismutase activities of manganese(III) and iron(III) cationic and anionic porphyrins. Inorg. Chem. 1999, 38, 4011–4022. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Benov, L.; Spasojevic´, I.; Fridovich, I. The ortho effect makes manganese(III) meso-tetrakis-(N-methylpyridinium-2-yl)porphyrin a powerful and potentially useful superoxide dismutase mimic. J. Biol. Chem. 1998, 273, 24521–24528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrer-Sueta, G.; Batinic-Haberle, I.; Spasojevic, I.; Fridovich, I.; Radi, R. Catalytic Scavenging of Peroxynitrite by Isomeric Mn(III) N-Methylpyridylporphyrins in the Presence of Reductants. Chem. Res. Toxicol. 1999, 12, 442–449. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Reboucas, J.S.; Spasojevic, I. Superoxide dismutase mimics: Chemistry, pharmacology, and therapeutic potential. Antioxid. Redox Signal. 2010, 13, 877–918. [Google Scholar] [CrossRef] [Green Version]
- Weitner, T.; Kos, I.; Mandić, Z.; Batinić-Haberle, I.; Biruš, M. Acid–base and electrochemical properties of manganese meso(ortho- and meta-N-ethylpyridyl)-porphyrins: Voltammetric and chronocoulometric study of protolytic and redox equilibria. Dalton Trans. 2013, 41, 14757–14765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yimcharoen, M.; Kittikunnathum, S.; Suknikorn, C.; Nak-on, W.; Yeethong, P.; Anthony, T.G.; Bunpo, P. Effects of ascorbic acid supplementation on oxidative stress markers in healthy women following a single bout of exercise. J. Int. Soc. Sports Nutr. 2019, 16, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Pehlivan, F.E. Vitamin C: An antioxidant agent. In Vitamin C; IntechOpen: London, UK, 2017; Chapter 2; pp. 23–35. [Google Scholar]
- Valachova, K.; Topolska, D.; Mendichi, R.; Collins, M.N.; Sasinková, V.; Soltes, L. Hydrogen peroxide generation by the Weissberger biogenic oxidative system during hyaluronan degradation. Carbohydr. Polym. 2016, 148, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Ind. J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valachova, K.; Hrabarova, E.; Priesolova, E.; Nagy, M.; Banasova, M.; Juranek, I.; Soltes, L. Free-radical degradation of high-molecular-weight hyaluronan induced by ascorbate plus cupric ions. Testing of bucillamine and its SA981-metabolite as antioxidants. J. Pharm. Biomed. Anal. 2011, 56, 664–670. [Google Scholar] [CrossRef]
- Buffone, A.; Weaver, V.M. Don’t sugarcoat it: How glycocalyx composition influences cancer progression. J. Cell Biol. 2020, 219, 1–14. [Google Scholar] [CrossRef]
- Lahir, Y.H. Understanding the basic role of glycocalyx during cancer. J. Rad. Cancer Res. 2016, 17, 79–84. [Google Scholar] [CrossRef] [Green Version]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, M.J.; King, M.R. Physical Biology in Cancer. 3. The role of cell glycocalyx in vascular transport of circulating tumor cells. Am. J. Physiol. Cell Physiol. 2014, 306, 89–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soltes, L.; Stankovska, M.; Brezova, V.; Schiller, J.; Arnhold, J.; Kogan, G.; Gemeiner, P. Hyaluronan degradation by copper(II) chloride and ascorbate: Rotational viscometric, EPR spin-trapping, and MALDI–TOF mass spectrometric investigations. Carbohydr. Res. 2006, 341, 2826–2834. [Google Scholar] [CrossRef]
- Tovmasyan, A.; Sampaio, R.S.; Boss, M.K.; Bueno-Janice, J.C.; Bader, B.H.; Thomas, M.; Reboucas, J.S.; Orr, M.; Chandler, J.D.; Young-Mi Go, J.M.; et al. Anticancer therapeutic potential of Mn porphyrin/ascorbate system. Free Radic. Biol. Med. 2015, 89, 1231–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valachova, K.; Rapta, P.; Batinic-Haberle, I.; Soltes, L. Manganese porphyrins as pro-oxidants in high-molar-mass hyaluronan oxidative degradation-possible implications in anticancer effects. In Green Chemistry and Green Engineering. Processing Technologies, Properties and Applications; Kulkarni, S., Rawat, N.K., Haghi, A.K., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2021; Chapter 12; pp. 223–241. [Google Scholar]
- Lokeshwar, V.B.; Mirza, S.; Jordan, A. Targeting hyaluronic acid family for cancer chemoprevention and therapy. Adv. Cancer Res. 2014, 123, 35–65. [Google Scholar]
- Batinic-Haberle, I.; Spasojevic, I. Complex chemistry and biology of redox-active compounds, commonly known as SOD mimics, affect their therapeutic effects. Antioxid. Redox Signal. 2014, 20, 2323–2325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, Q.; Weaver, M.R.; Kosmacek, E.A.; O’Connor, B.P.; Harmacek, L.; Venkataraman, S.; Oberley-Deegan, R.E. MnTE-2-PyP reduces prostate cancer growth and metastasis by suppressing p300 activity and p300/HIF-1/CREB binding to the promoter region of the PAI-1 gene. Free Radic. Biol. Med. 2016, 94, 185–194. [Google Scholar] [CrossRef] [Green Version]
- Flórido, A.; Saraiva, N.; Cerqueira, S.; Almeida, N.; Parsons, M.; Batinic-Haberle, I.; Miranda, J.P.; Costa, J.G.; Carrara, G.; Castro, M.; et al. The manganese(III) porphyrin MnTnHex-2-PyP5+ modulates intracellular ROS and breast cancer cell migration: Impact on doxorubicin-treated cell. Redox Biol. 2019, 20, 367–378. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Tovmasyan, A.; Huang, Z.; Duan, W.; Du, L.; Siamakpour-Reihani, S.; Cao, Z.; Sheng, H.; Spasojevic, I.; Secord, A.A. H2O2–driven anticancer activity of Mn porphyrins and the underlying molecular pathways. Oxid. Med. Cell. Longev. 2021, 2021, 23. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013, 13, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dvoranová, D.; Barbieriková, Z.; Brezová, V. Radical Intermediates in Photoinduced Reactions on TiO2 (An EPR Spin Trapping Study). Molecules 2014, 19, 17279–17304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spasojevic, I.; Batinic-Haberle, I.; Fridovich, I. Nitrosylation of manganese(II) tetrakis(N-ethylpyridinium-2-yl)porphyrin. Nitric Oxide 2000, 4, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Bonnesœur, S.; Morin-Grognet, S.; Thoumire, O.; Le Cerf, D.; Boyer, O.; Vannier, J.-P.; Labat, B. Hyaluronan-based hydrogels as versatile tumor-like models: Tunable ECM and stiffness with genipin-crosslinking. J. Biomed. Mat. Res. 2020, 108A, 1256–1268. [Google Scholar] [CrossRef]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic acid and its biomedical applications: A review. Eng. Regen. 2020, 1, 102–113. [Google Scholar] [CrossRef]
- Soltes, L.; Valachova, K.; Mendichi, R.; Kogan, G.; Arnhold, J.; Gemeiner, P. Solution properties of high-molar-mass hyaluronans: The biopolymer degradation by ascorbate. Carbohydr. Res. 2007, 342, 1071–1077. [Google Scholar] [CrossRef]
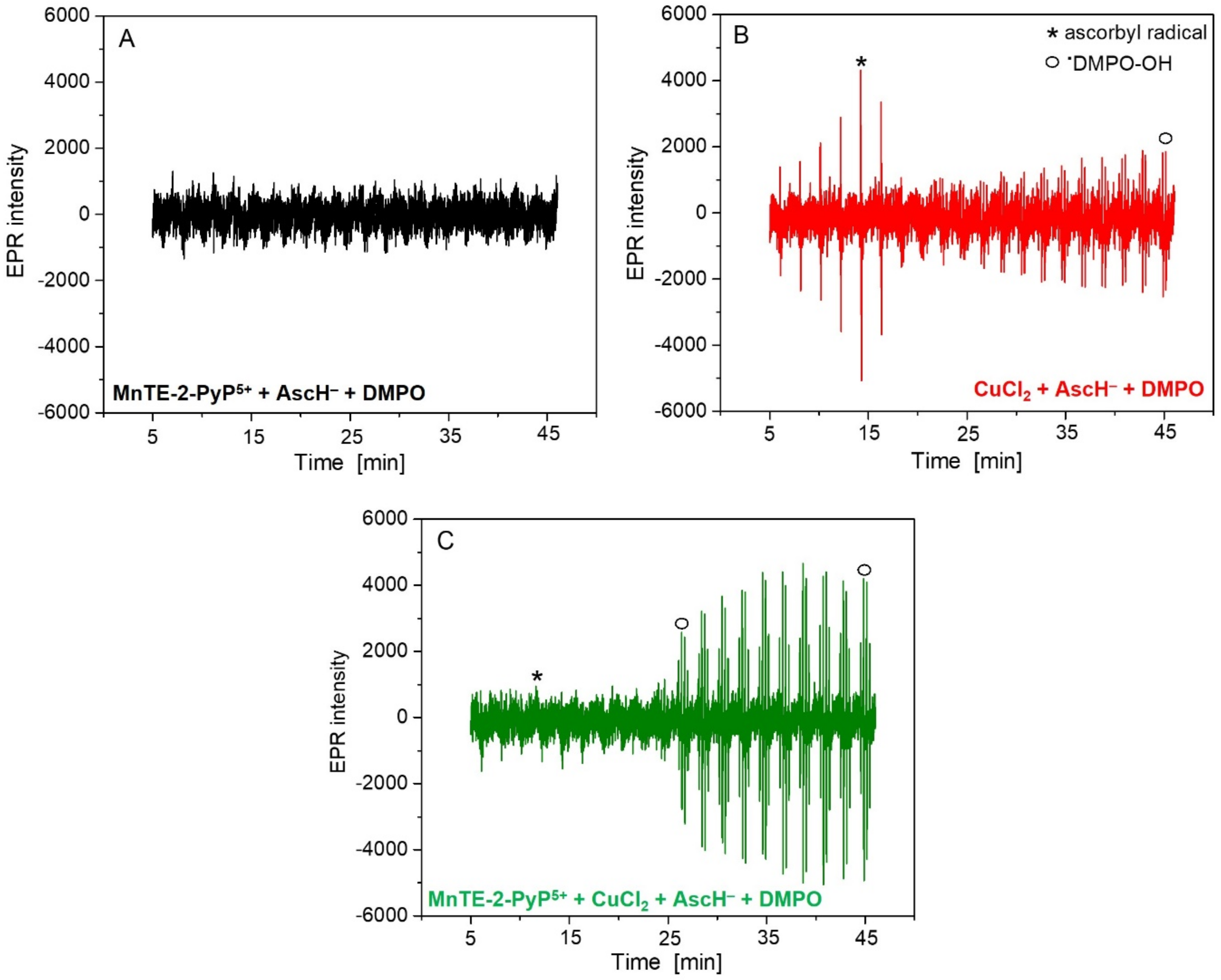

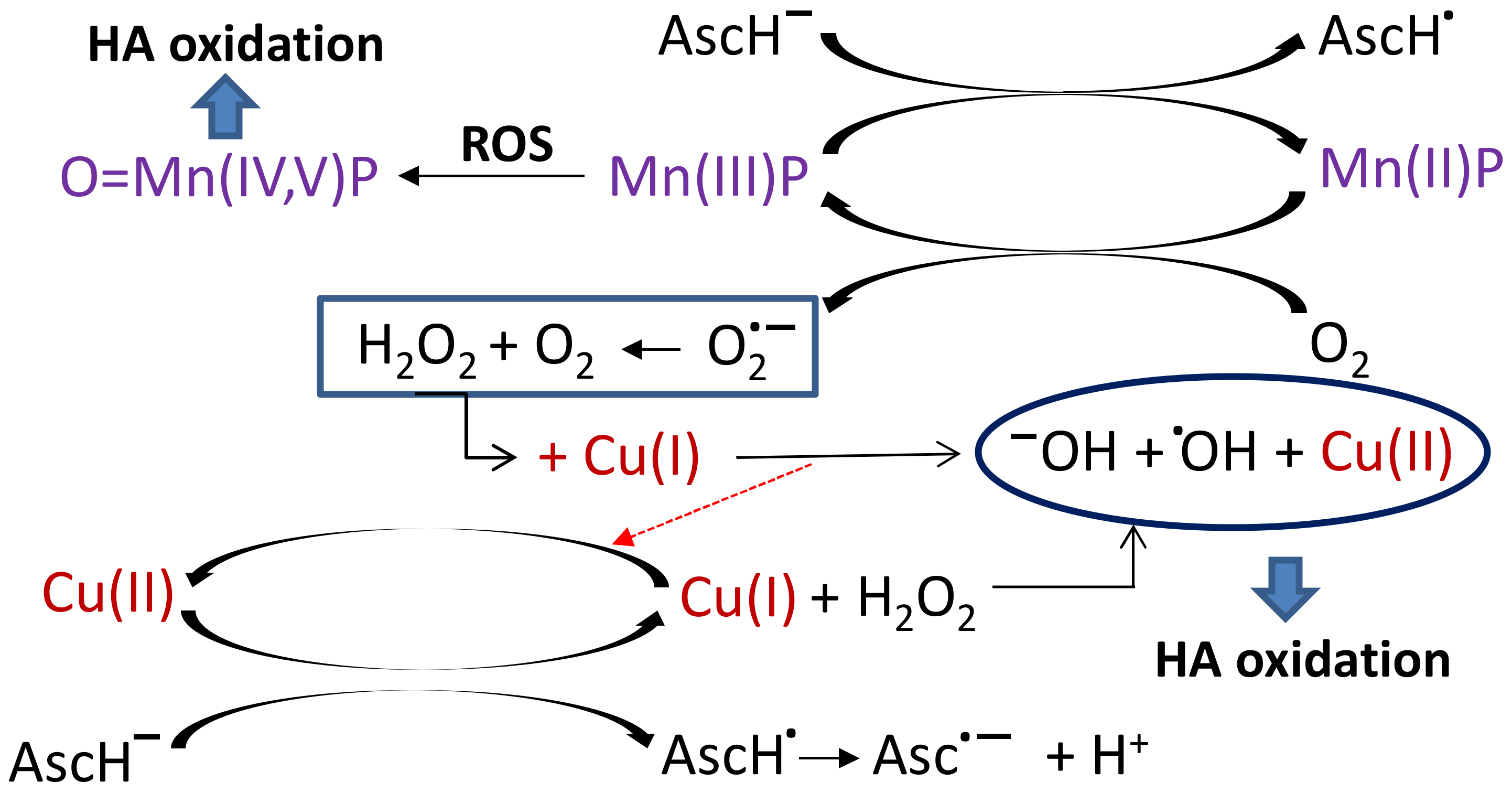

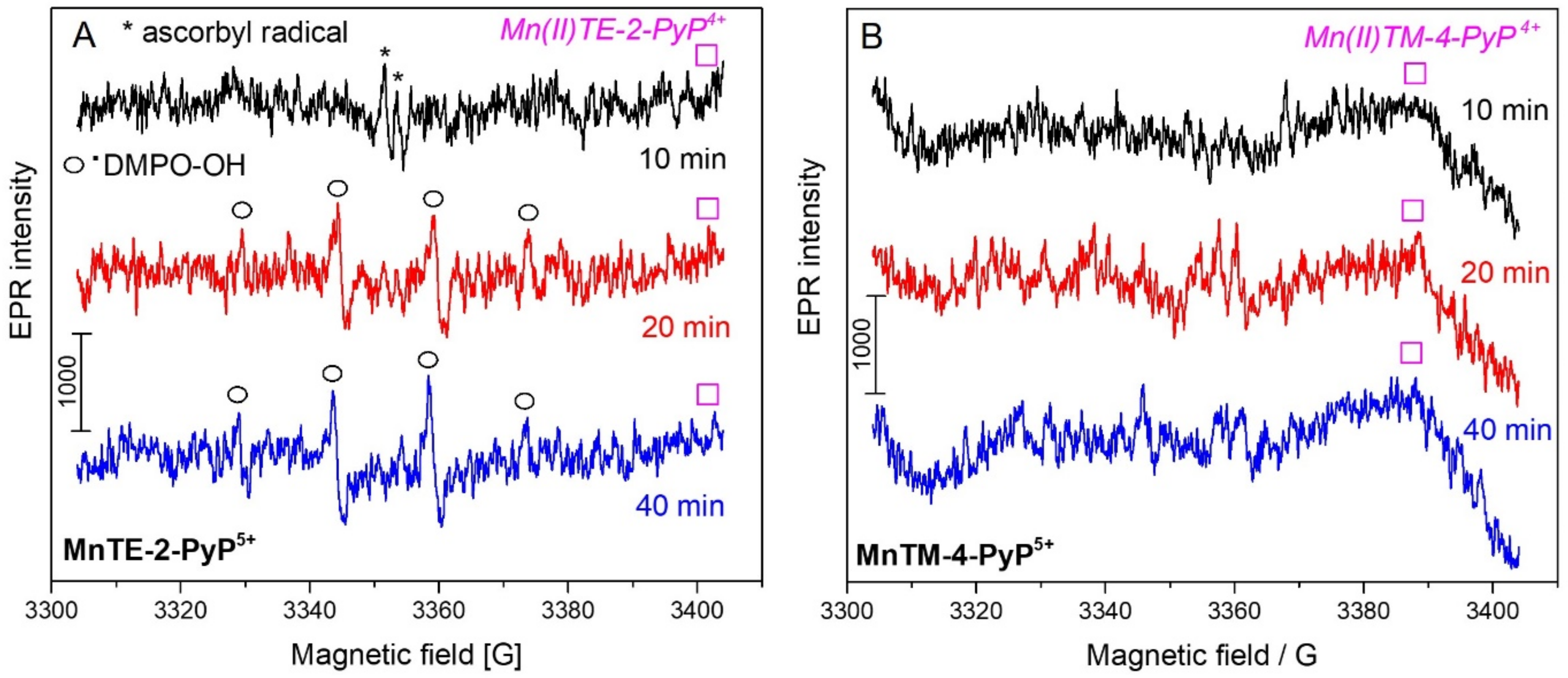
| Mn Complex | 0 µM | 5 µM | 20 µM | 100 µM |
|---|---|---|---|---|
| Initial rates −(dη/dt)t=0/mPa·s·min−1 | ||||
| MnTE-2-PyP5+ | 0.0209 | 0.0244 | 0.0434 | 0.0954 |
| MnTM-4-PyP5+ | 0.0311 | 0.0163 | 0.0085 | 0.0074 |
| Rates at t = 60 min, −(dη/dt)t=60/mPa·s·min−1 | ||||
| MnTE-2-PyP5+ | 0.0354 | 0.0354 | 0.0597 | 0.1282 |
| MnTM-4-PyP5+ | 0.0597 | 0.0401 | 0.0190 | 0.0190 |
| Rates at t = 20 min −(dη/dt)t=20/mPa·s·min−1 | ||||
|---|---|---|---|---|
| MnTE-2-PyP5+ | 0 µM | 5 µM | 20 µM | 100 µM |
| 0 µM Cu(II) | 0 | 0.006 | 0.017 | 0.115 |
| 1 µM Cu(II) | 0.020 | 0.045 | 0.075 | 0.215 |
| Initial rates at t ~ 0 –(dη/dt)t=0/mPa·s·min−1 | ||||
| MnTE-2-PyP5+ | 0 µM | 5 µM | 20 µM | 100 µM |
| 0 µM Cu(II) | 0 | 0.006 | 0.0331 | 0.0779 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valachová, K.; Rapta, P.; Moura, N.M.M.; Batinic-Haberle, I.; Šoltés, L. Ortho Isomeric Mn(III) N-Alkyl- and Alkoxyalkylpyridylporphyrins—Enhancers of Hyaluronan Degradation Induced by Ascorbate and Cupric Ions. Int. J. Mol. Sci. 2021, 22, 8608. https://doi.org/10.3390/ijms22168608
Valachová K, Rapta P, Moura NMM, Batinic-Haberle I, Šoltés L. Ortho Isomeric Mn(III) N-Alkyl- and Alkoxyalkylpyridylporphyrins—Enhancers of Hyaluronan Degradation Induced by Ascorbate and Cupric Ions. International Journal of Molecular Sciences. 2021; 22(16):8608. https://doi.org/10.3390/ijms22168608
Chicago/Turabian StyleValachová, Katarína, Peter Rapta, Nuno M. M. Moura, Ines Batinic-Haberle, and Ladislav Šoltés. 2021. "Ortho Isomeric Mn(III) N-Alkyl- and Alkoxyalkylpyridylporphyrins—Enhancers of Hyaluronan Degradation Induced by Ascorbate and Cupric Ions" International Journal of Molecular Sciences 22, no. 16: 8608. https://doi.org/10.3390/ijms22168608







