Neural and Hormonal Basis of Opposite-Sex Preference by Chemosensory Signals
Abstract
1. Introduction
2. Detection of Sexual Chemicals
3. Sexual Stimuli Attributes
4. Sexually Dimorphic Preference and Organizational Effects of Sex Steroids
5. Hormonal Control for Opposite-Sex Preference
6. Regulation of Opposite-Sex Preference by Integrating the Main and Accessory Olfactory Systems
7. Experience and Sexual Preference
8. Neuropeptidergic Regulation of Opposite-Sex Preference
9. Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Slob, A.; DeKlerk, L.; Brand, T. Homosexual and heterosexual partner preference in ovariectomized female rats: Effects of testosterone, estradiol and mating experience. Physiol. Behav. 1987, 41, 571–576. [Google Scholar] [CrossRef]
- Broekman, M.; De Bruin, M.; Smeenk, J.; Slob, A.K.; Van Der Schoot, P. Partner preference behavior of estrous female rats affected by castration of tethered male incentives. Horm. Behav. 1988, 22, 324–337. [Google Scholar] [CrossRef]
- Merkx, J.; Slob, A.; Bosch, J.V.D.W.T. Preference for an estrous female over a non-estrous female evinced by female rats requires dihydrotestosterone plus estradiol. Horm. Behav. 1989, 23, 466–472. [Google Scholar] [CrossRef]
- Brand, T.; Kroonen, J.; Mos, J.; Slob, A. Adult partner preference and sexual behavior of male rats affected by perinatal endocrine manipulations. Horm. Behav. 1991, 25, 323–341. [Google Scholar] [CrossRef]
- Bakker, J.; Van Ophemert, J.; Eijskoot, F.; Slob, A. A semiautomated test apparatus for studying partner preference behavior in the rat. Physiol. Behav. 1994, 56, 597–601. [Google Scholar] [CrossRef][Green Version]
- Xiao, K.; Kondo, Y.; Sakuma, Y. Sex-specific effects of gonadal steroids on conspecific odor preference in the rat. Horm. Behav. 2004, 46, 356–361. [Google Scholar] [CrossRef]
- Brechbühl, J.; Klaey, M.; Broillet, M.-C. Grueneberg Ganglion Cells Mediate Alarm Pheromone Detection in Mice. Science 2008, 321, 1092–1095. [Google Scholar] [CrossRef] [PubMed]
- Brechbühl, J.; Moine, F.; Klaey, M.; Nenniger-Tosato, M.; Hurni, N.; Sporkert, F.; Giroud, C.; Broillet, M.-C. Mouse alarm pheromone shares structural similarity with predator scents. Proc. Natl. Acad. Sci. USA 2013, 110, 4762–4767. [Google Scholar] [CrossRef] [PubMed]
- Brechbuhl, J.; Klaey, M.; Moine, F.; Bovay, E.; Hurni, N.; Nenniger-Tosato, M.; Broillet, M.C. Morphological and physiological species-dependent characteristics of the rodent Grueneberg ganglion. Front. Neuroanat. 2014, 8, 87. [Google Scholar]
- Stowers, L.; Kuo, T.-H. Mammalian pheromones: Emerging properties and mechanisms of detection. Curr. Opin. Neurobiol. 2015, 34, 103–109. [Google Scholar] [CrossRef]
- Halem, H.A.; Baum, M.J.; Cherry, J.A. Sex Difference and Steroid Modulation of Pheromone-Induced Immediate Early Genes in the Two Zones of the Mouse Accessory Olfactory System. J. Neurosci. 2001, 21, 2474–2480. [Google Scholar] [CrossRef]
- Haga-Yamanaka, S.; Ma, L.; He, J.; Qiu, Q.; Lavis, L.D.; Looger, L.L.; Yu, C.R. Integrated action of pheromone signals in promoting courtship behavior in male mice. eLife 2014, 3, e03025. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.W.; Ackels, T.; Kuo, T.-H.; Cichy, A.; Dey, S.; Hays, C.; Kateri, M.; Logan, D.; Marton, T.F.; Spehr, M.; et al. Murine Pheromone Proteins Constitute a Context-Dependent Combinatorial Code Governing Multiple Social Behaviors. Cell 2014, 157, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Chamero, P.; Marton, T.F.; Logan, D.; Flanagan, K.; Cruz, J.R.; Saghatelian, A.; Cravatt, B.F.; Stowers, L. Identification of protein pheromones that promote aggressive behaviour. Nature 2007, 450, 899–902. [Google Scholar] [CrossRef]
- Roberts, S.A.; Simpson, D.M.; Armstrong, S.D.; Davidson, A.J.; Robertson, D.H.; McLean, L.; Beynon, R.J.; Hurst, J.L. Darcin: A male pheromone that stimulates female memory and sexual attraction to an individual male’s odour. BMC Biol. 2010, 8, 75. [Google Scholar] [CrossRef]
- Demir, E.; Li, K.; Bobrowski-Khoury, N.; Sanders, J.I.; Beynon, R.J.; Hurst, J.L.; Kepecs, A.; Axel, R. The pheromone darcin drives a circuit for innate and reinforced behaviours. Nature 2020, 578, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Haga, S.; Hattori, T.; Sato, T.; Sato, K.; Matsuda, S.; Kobayakawa, R.; Sakano, H.; Yoshihara, Y.; Kikusui, T.; Touhara, K. The male mouse pheromone ESP1 enhances female sexual receptive behaviour through a specific vomeronasal receptor. Nature 2010, 466, 118–122. [Google Scholar] [CrossRef] [PubMed]
- Stopka, P.; Kuntová, B.; Klempt, P.; Havrdová, L.; Černá, M.; Stopková, R. On the saliva proteome of the Eastern European house mouse (Mus musculus musculus) focusing on sexual signalling and immunity. Sci. Rep. 2016, 6, 32481. [Google Scholar] [CrossRef] [PubMed]
- Niimura, Y. Olfactory Receptor Multigene Family in Vertebrates: From the Viewpoint of Evolutionary Genomics. Curr. Genom. 2012, 13, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Liberles, S.D. Trace Amine-associated Receptors Are Olfactory Receptors in Vertebrates. Ann. N. Y. Acad. Sci. 2009, 1170, 168–172. [Google Scholar] [CrossRef]
- Li, Q.; Liberles, S.D. Aversion and Attraction through Olfaction. Curr. Biol. 2015, 25, R120–R129. [Google Scholar] [CrossRef]
- Liberles, S.D. Trace amine-associated receptors: Ligands, neural circuits, and behaviors. Curr. Opin. Neurobiol. 2015, 34, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Harmeier, A.; Meyer, C.A.; Staempfli, A.; Casagrande, F.; Petrinovic, M.M.; Zhang, Y.-P.; Künnecke, B.; Iglesias, A.; Höner, O.P.; Hoener, M.C. How Female Mice Attract Males: A Urinary Volatile Amine Activates a Trace Amine-Associated Receptor That Induces Male Sexual Interest. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Korzan, W.J.; Ferrero, D.M.; Chang, R.B.; Roy, D.S.; Buchi, M.; Lemon, J.K.; Kaur, A.W.; Stowers, L.; Fendt, M.; et al. Synchronous Evolution of an Odor Biosynthesis Pathway and Behavioral Response. Curr. Biol. 2013, 23, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Pacifico, R.; Dewan, A.; Cawley, D.; Guo, C.; Bozza, T. An Olfactory Subsystem that Mediates High-Sensitivity Detection of Volatile Amines. Cell Rep. 2012, 2, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.A.; Tsai, L.; Roy, D.S.; Valenzuela, D.H.; Mosley, C.; Magklara, A.; Lomvardas, S.; Liberles, S.D.; Barnea, G. Neurons expressing trace amine-associated receptors project to discrete glomeruli and constitute an olfactory subsystem. Proc. Natl. Acad. Sci. USA 2012, 109, 13410–13415. [Google Scholar] [CrossRef]
- Kobayakawa, K.; Kobayakawa, R.; Matsumoto, H.; Oka, Y.; Imai, T.; Ikawa, M.; Okabe, M.; Ikeda, T.; Itohara, S.; Kikusui, T.; et al. Innate versus learned odour processing in the mouse olfactory bulb. Nature 2007, 450, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Omura, M.; Mombaerts, P. Trpc2-Expressing Sensory Neurons in the Main Olfactory Epithelium of the Mouse. Cell Rep. 2014, 8, 583–595. [Google Scholar] [CrossRef]
- Orsulak, P.J.; Gawienowski, A.M. Olfactory Preferences for the Rat Preputial Gland1. Biol. Reprod. 1972, 6, 219–223. [Google Scholar] [CrossRef]
- Bronson, F.H.; Caroom, D. Preputial gland of the male mouse: Attractant function. Reproduction 1971, 25, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.X.; Sun, L.; Zhang, J.H.; Feng, Z.Y. Sex- and gonad-affecting scent compounds and 3 male pheromones in the rat. Chem. Senses 2008, 33, 611–621. [Google Scholar] [CrossRef]
- Logan, D.W.; Marton, T.F.; Stowers, L. Species Specificity in Major Urinary Proteins by Parallel Evolution. PLoS ONE 2008, 3, e3280. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.A.; Prescott, M.C.; Davidson, A.J.; McLean, L.; Beynon, R.J.; Hurst, J.L. Individual odour signatures that mice learn are shaped by involatile major urinary proteins (MUPs). BMC Biol. 2018, 16, 48. [Google Scholar] [CrossRef] [PubMed]
- Mucignat-Caretta, C.; Caretta, A.; Cavaggioni, A. Acceleration of puberty onset in female mice by male urinary proteins. J. Physiol. 1995, 486, 517–522. [Google Scholar] [CrossRef]
- Marchlewska-Koj, A.; Cavaggioni, A.; Mucignat-Caretta, C.; Olejniczak, P. Stimulation of Estrus in Female Mice by Male Urinary Proteins. J. Chem. Ecol. 2000, 26, 2355–2366. [Google Scholar] [CrossRef]
- Pankevich, D.E.; Cherry, J.A.; Baum, M.J. Effect of vomeronasal organ removal from male mice on their preference for and neural Fos responses to female urinary odors. Behav. Neurosci. 2006, 120, 925–936. [Google Scholar] [CrossRef]
- Martínez-Ricós, J.; Agustín-Pavón, C.; Lanuza, E.; Martínez-García, F. Intraspecific Communication Through Chemical Signals in Female Mice: Reinforcing Properties of Involatile Male Sexual Pheromones. Chem. Senses 2006, 32, 139–148. [Google Scholar] [CrossRef]
- Ganfornina, M.D.; Gutiérrez, G.; Bastiani, M. A Phylogenetic Analysis of the Lipocalin Protein Family. Mol. Biol. Evol. 2000, 17, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Timm, D.E.; Baker, L.; Mueller, H.; Zidek, L.; Novotny, M.V. Structural basis of pheromone binding to mouse major urinary protein (MUP-I). Protein Sci. 2001, 10, 997–1004. [Google Scholar] [CrossRef]
- Armstrong, S.D.; Robertson, D.H.L.; Cheetham, S.A.; Hurst, J.L.; Beynon, R.J. Structural and functional differences in isoforms of mouse major urinary proteins: A male-specific protein that preferentially binds a male pheromone. Biochem. J. 2005, 391, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Jemiolo, B.; Alberts, J.; Sochinski-Wiggins, S.; Harvey, S.; Novotny, M. Behavioural and endocrine responses of female mice to synthetic analogues of volatile compounds in male urine. Anim. Behav. 1985, 33, 1114–1118. [Google Scholar] [CrossRef]
- Asaba, A.; Hattori, T.; Mogi, K.; Kikusui, T. Sexual attractiveness of male chemicals and vocalizations in mice. Front. Neurosci. 2014, 8, 231. [Google Scholar] [CrossRef] [PubMed]
- Korzan, W.J.; Freamat, M.; Johnson, A.G.; Cherry, J.A.; Baum, M.J. Either main or accessory olfactory system signaling can mediate the rewarding effects of estrous female chemosignals in sexually naive male mice. Behav. Neurosci. 2013, 127, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, Y.; Si, Y.; Kim, J.-Y.; Chen, Z.-F.; Rao, Y. Molecular regulation of sexual preference revealed by genetic studies of 5-HT in the brains of male mice. Nature 2011, 472, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Pierman, S.; Douhard, Q.; Baum, M.J.; Bakker, J. The vomeronasal organ is required for the expression of lordosis behaviour, but not sex discrimination in female mice. Eur. J. Neurosci. 2006, 23, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Bodo, C.; Rissman, E.F. The Androgen Receptor Is Selectively Involved in Organization of Sexually Dimorphic Social Behaviors in Mice. Endocrinology 2008, 149, 4142–4150. [Google Scholar] [CrossRef] [PubMed]
- Meerts, S.H.; Clark, A.S. Stimulus animal characteristics do not modulate the expression of partner preference by female rats. Physiol. Behav. 2006, 89, 623–626. [Google Scholar] [CrossRef]
- DiBenedictis, B.T.; Ingraham, K.L.; Baum, M.J.; Cherry, J.A. Disruption of urinary odor preference and lordosis behavior in female mice given lesions of the medial amygdala. Physiol. Behav. 2012, 105, 554–559. [Google Scholar] [CrossRef] [PubMed]
- DiBenedictis, B.T.; Olugbemi, A.O.; Baum, M.J.; Cherry, J.A. DREADD-Induced Silencing of the Medial Olfactory Tubercle Disrupts the Preference of Female Mice for Opposite-Sex Chemosignals. Eneuro 2015, 2. [Google Scholar] [CrossRef]
- Bakker, J.; Honda, S.-I.; Harada, N.; Balthazart, J. The Aromatase Knock-Out Mouse Provides New Evidence That Estradiol Is Required during Development in the Female for the Expression of Sociosexual Behaviors in Adulthood. J. Neurosci. 2002, 22, 9104–9112. [Google Scholar] [CrossRef]
- Gubits, R.M.; Lynch, K.R.; Kulkarni, A.B.; Dolan, K.P.; Gresik, E.W.; Hollander, P.; Feigelson, P. Differential regulation of alpha 2u globulin gene expression in liver, lachrymal gland, and salivary gland. J. Biol. Chem. 1984, 259, 12803–12809. [Google Scholar] [CrossRef]
- Shahan, K.; Denaro, M.; Gilmartin, M.; Shi, Y.; Derman, E. Expression of six mouse major urinary protein genes in the mammary, parotid, sublingual, submaxillary, and lachrymal glands and in the liver. Mol. Cell. Biol. 1987, 7, 1947–1954. [Google Scholar] [CrossRef] [PubMed]
- Dhungel, S.; Rai, D.; Terada, M.; Orikasa, C.; Nishimori, K.; Sakuma, Y.; Kondo, Y. Oxytocin is indispensable for conspecific-odor preference and controls the initiation of female, but not male, sexual behavior in mice. Neurosci. Res. 2019, 148, 34–41. [Google Scholar] [CrossRef]
- Achiraman, S.; Ponmanickam, P.; Ganesh, D.S.; Archunan, G. Detection of estrus by male mice: Synergistic role of olfactory–vomeronasal system. Neurosci. Lett. 2010, 477, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, M.C.; Nagata, K.; Sagoshi, S.; Ogawa, S. Estrogen and oxytocin involvement in social preference in male mice: A study using a novel long-term social preference paradigm with aromatase, estrogen receptor-α and estrogen receptor-β, oxytocin, and oxytocin receptor knockout male mice. Integr. Zoöl. 2018, 13, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Kumagai, R.; Kondo, Y. Why does castrated male odor attract sexually active male rats?–Attractivity induced by hypothalamus-pituitary-gonad axis block. Physiol. Behav. 2021, 230, 113288. [Google Scholar] [CrossRef]
- Xiao, K.; Chiba, A.; Sakuma, Y.; Kondo, Y. Transient reversal of olfactory preference following castration in male rats: Implication for estrogen receptor involvement. Physiol. Behav. 2015, 152, 161–167. [Google Scholar] [CrossRef]
- Luo, M.; Fee, M.S.; Katz, L.C. Encoding Pheromonal Signals in the Accessory Olfactory Bulb of Behaving Mice. Science 2003, 299, 1196–1201. [Google Scholar] [CrossRef] [PubMed]
- Hammen, G.F.; Turaga, D.; Holy, T.E.; Meeks, J.P. Functional organization of glomerular maps in the mouse accessory olfactory bulb. Nat. Neurosci. 2014, 17, 953–961. [Google Scholar] [CrossRef] [PubMed]
- Bakker, J.; Honda, S.; Harada, N.; Balthazart, J. Restoration of male sexual behavior by adult exogenous estrogens in male aromatase knockout mice. Horm. Behav. 2004, 46, 1–10. [Google Scholar] [CrossRef]
- Vega Matuszczyk, J.; Larsson, K. Role of androgen, estrogen and sexual experience on the female rat’s partner preference. Physiol Behav 1991, 50, 139–142. [Google Scholar] [CrossRef]
- Brand, T.; Slob, A. Neonatal organization of adult partner preference behavior in male rats. Physiol. Behav. 1991, 49, 107–111. [Google Scholar] [CrossRef]
- Matuszczyk, J.V.; Larsson, K. Sexual Preference and Feminine and Masculine Sexual Behavior of Male Rats Prenatally Exposed to Antiandrogen or Antiestrogen. Horm. Behav. 1995, 29, 191–206. [Google Scholar] [CrossRef]
- Bakker, J.; Brand, T.; van Ophemert, J.; Slob, A.K. Hormonal regulation of adult partner preference behavior in neonatally ATD-treated male rats. Behav. Neurosci. 1993, 107, 480–487. [Google Scholar] [CrossRef]
- Houtsmuller, E.; Brand, T.; de Jonge, F.; Joosten, R.; van de Poll, N.; Slob, A. SDN-POA volume, sexual behavior, and partner preference of male rats affected by perinatal treatment with ATD. Physiol. Behav. 1994, 56, 535–541. [Google Scholar] [CrossRef]
- Cruz, C.D.; Pereira, O.C.M. Prenatal testosterone supplementation alters puberty onset, aggressive behavior, and partner preference in adult male rats. J. Physiol. Sci. 2012, 62, 123–131. [Google Scholar] [CrossRef]
- Henley, C.; Nunez, A.; Clemens, L. Exogenous androgen during development alters adult partner preference and mating behavior in gonadally intact male rats. Horm. Behav. 2010, 57, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Henley, C.; Nunez, A.; Clemens, L. Estrogen treatment during development alters adult partner preference and reproductive behavior in female laboratory rats. Horm. Behav. 2009, 55, 68–75. [Google Scholar] [CrossRef] [PubMed]
- De Jonge, F.H.; Muntjewerff, J.W.; Louwerse, A.L.; Van De Poll, N.E. Sexual behavior and sexual orientation of the female rat after hormonal treatment during various stages of development. Horm. Behav. 1988, 22, 100–115. [Google Scholar] [CrossRef]
- Wersinger, S.R.; Rissman, E.F. Oestrogen receptor alpha is essential for female-directed chemo-investigatory behaviour but is not required for the pheromone-induced luteinizing hormone surge in male mice. J. Neuroendocr. 2000, 12, 103–110. [Google Scholar] [CrossRef]
- Bodo, C.; Rissman, E.F. New roles for estrogen receptor beta in behavior and neuroendocrinology. Front. Neuroendocrinol. 2006, 27, 217–232. [Google Scholar] [CrossRef]
- Bakker, J.; Honda, S.; Harada, N.; Balthazart, J. Sexual Partner Preference Requires a Functional Aromatase (Cyp19) Gene in Male Mice. Horm. Behav. 2002, 42, 158–171. [Google Scholar] [CrossRef] [PubMed]
- Bodo, C.; Rissman, E.F. Androgen receptor is essential for sexual differentiation of responses to olfactory cues in mice. Eur. J. Neurosci. 2007, 25, 2182–2190. [Google Scholar] [CrossRef] [PubMed]
- Raskin, K.; De Gendt, K.; Duittoz, A.; Liere, P.; Verhoeven, G.; Tronche, F.; Mhaouty-Kodja, S. Conditional Inactivation of Androgen Receptor Gene in the Nervous System: Effects on Male Behavioral and Neuroendocrine Responses. J. Neurosci. 2009, 29, 4461–4470. [Google Scholar] [CrossRef]
- Brock, O.; Douhard, Q.; Baum, M.J.; Bakker, J. Reduced Prepubertal Expression of Progesterone Receptor in the Hypothalamus of Female Aromatase Knockout Mice. Endocrinology 2010, 151, 1814–1821. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brock, O.; Baum, M.J.; Bakker, J. The Development of Female Sexual Behavior Requires Prepubertal Estradiol. J. Neurosci. 2011, 31, 5574–5578. [Google Scholar] [CrossRef]
- Mazzucco, C.A.; Walker, H.A.; Pawluski, J.; Lieblich, S.E.; Galea, L.A. ERα, but not ERβ, mediates the expression of sexual behavior in the female rat. Behav. Brain Res. 2008, 191, 111–117. [Google Scholar] [CrossRef]
- Ogawa, S.; Lubahn, D.B.; Korach, K.; Pfaff, D.W. Behavioral effects of estrogen receptor gene disruption in male mice. Proc. Natl. Acad. Sci. USA 1997, 94, 1476–1481. [Google Scholar] [CrossRef] [PubMed]
- Imwalle, D.B.; Scordalakes, E.M.; Rissman, E.F. Estrogen Receptor α Influences Socially Motivated Behaviors. Horm. Behav. 2002, 42, 484–491. [Google Scholar] [CrossRef]
- Honda, S.; Harada, N.; Ito, S.; Takagi, Y.; Maeda, S. Disruption of sexual behavior in male aromatase-deficient mice lacking exons 1 and 2 of the cyp19 gene. Biochem. Biophys. Res. Commun. 1998, 252, 445–449. [Google Scholar] [CrossRef]
- Kondo, Y.; Tomihara, K.; Sakuma, Y. Sensory requirements for noncontact penile erection in the rat. Behav. Neurosci. 1999, 113, 1062–1070. [Google Scholar] [CrossRef]
- Bialy, M.; Nikolaev-Diak, A.; Kalata, U.; Nikolaev, E. Blockade of androgen receptor in the medial amygdala inhibits noncontact erections in male rats. Physiol. Behav. 2011, 103, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Bialy, M.; Sachs, B.D. Androgen Implants in Medial Amygdala Briefly Maintain Noncontact Erection in Castrated Male Rats. Horm. Behav. 2002, 42, 345–355. [Google Scholar] [CrossRef]
- Manzo, J.; Cruz, M.; Hernandez, M.E.; Pacheco, P.; Sachs, B. Regulation of Noncontact Erection in Rats by Gonadal Steroids. Horm. Behav. 1999, 35, 264–270. [Google Scholar] [CrossRef]
- Seo, S.I.; Kim, S.W.; Paick, J.S. The effects of androgen on penile reflex, erectile response to electrical stimulation and penile NOS activity in the rat. Asian J. Androl. 1999, 1, 169–174. [Google Scholar]
- Muroi, Y.; Ishii, T.; Komori, S.; Nishimura, M. A competitive effect of androgen signaling on male mouse attraction to volatile female mouse odors. Physiol. Behav. 2006, 87, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Schellino, R.; Trova, S.; Cimino, I.; Farinetti, A.; Jongbloets, B.C.; Pasterkamp, R.J.; Panzica, G.; Giacobini, P.; De Marchis, S.; Peretto, P. Opposite-sex attraction in male mice requires testosterone-dependent regulation of adult olfactory bulb neurogenesis. Sci. Rep. 2016, 6, 36063. [Google Scholar] [CrossRef]
- Eliasson, M.; Meyerson, B.J. Sexual preference in female rats during estrous cycle, pregnancy and lactation. Physiol. Behav. 1975, 14, 705–710. [Google Scholar] [CrossRef]
- Clark, A.S.; Kelton, M.C.; Guarraci, F.A.; Clyons, E.Q. Hormonal status and test condition, but not sexual experience, modulate partner preference in female rats. Horm. Behav. 2004, 45, 314–323. [Google Scholar] [CrossRef]
- Monchobogani, J.; Lanuza, E.; Lorente, M.; Martinez-Garcia, F. Attraction to male pheromones and sexual behaviour show different regulatory mechanisms in female mice. Physiol. Behav. 2004, 81, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Lukas, M.; Neumann, I.D. Social preference and maternal defeat-induced social avoidance in virgin female rats: Sex differences in involvement of brain oxytocin and vasopressin. J. Neurosci. Methods 2014, 234, 101–107. [Google Scholar] [CrossRef]
- Yao, S.; Bergan, J.; Lanjuin, A.; Dulac, C. Oxytocin signaling in the medial amygdala is required for sex discrimination of social cues. eLife 2017, 6, e31373. [Google Scholar] [CrossRef] [PubMed]
- Eidson, L.N.; Maras, P.M.; Epperson, E.; Petrulis, A. Female hamster preference for odors is not regulated by circulating gonadal hormones. Physiol. Behav. 2007, 91, 134–141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chamero, P.; Leinders-Zufall, T.; Zufall, F. From genes to social communication: Molecular sensing by the vomeronasal organ. Trends Neurosci. 2012, 35, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, C.J.; Lepri, J.J. Consequences of removing the vomeronasal organ. J. Steroid Biochem. Mol. Biol. 1991, 39, 661–669. [Google Scholar] [CrossRef]
- Powers, J.; Winans, S. Vomeronasal organ: Critical role in mediating sexual behavior of the male hamster. Science 1975, 187, 961–963. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.R. Copulatory Behavior of Male Rats Paired with Natural Proestrous and Hormone-treated Ovariectomized Females. Exp. Anim. 1987, 36, 91–93. [Google Scholar] [CrossRef]
- Kondo, Y.; Sudo, T.; Tomihara, K.; Sakuma, Y. Activation of accessory olfactory bulb neurons during copulatory behavior after deprivation of vomeronasal inputs in male rats. Brain Res. 2003, 962, 232–236. [Google Scholar] [CrossRef]
- Dhungel, S.; Masaoka, M.; Rai, D.; Kondo, Y.; Sakuma, Y. Both olfactory epithelial and vomeronasal inputs are essential for activation of the medial amygdala and preoptic neurons of male rats. Neuroscience 2011, 199, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Pankevich, D.E.; Baum, M.J.; Cherry, J.A. Olfactory Sex Discrimination Persists, Whereas the Preference for Urinary Odorants from Estrous Females Disappears in Male Mice after Vomeronasal Organ Removal. J. Neurosci. 2004, 24, 9451–9457. [Google Scholar] [CrossRef] [PubMed]
- Cross, S.K.J.; Martin, Y.H.; Salia, S.; Gamba, I.; Major, C.A.; Hassan, S.; Parsons, K.A.; Swift-Gallant, A. Puberty is a Critical Period for Vomeronasal Organ Mediation of Socio-sexual Behavior in Mice. Front. Behav. Neurosci. 2021, 14. [Google Scholar] [CrossRef]
- Stowers, L.; Holy, T.E.; Meister, M.; Dulac, C.; Koentges, G. Loss of Sex Discrimination and Male-Male Aggression in Mice Deficient for TRP2. Science 2002, 295, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Beny, Y.; Kimchi, T. Conditioned odor aversion induces social anxiety towards females in wild-type and TrpC2 knockout male mice. Genes Brain Behav. 2016, 15, 722–732. [Google Scholar] [CrossRef]
- Beny-Shefer, Y.; Zilkha, N.; Lavi-Avnon, Y.; Bezalel, N.; Rogachev, I.; Brandis, A.; Dayan, M.; Kimchi, T. Nucleus Accumbens Dopamine Signaling Regulates Sexual Preference for Females in Male Mice. Cell Rep. 2017, 21, 3079–3088. [Google Scholar] [CrossRef]
- Kimchi, T.; Xu, J.; Dulac, C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature 2007, 448, 1009–1014. [Google Scholar] [CrossRef]
- Martel, K.L.; Baum, M.J. Adult Testosterone Treatment But Not Surgical Disruption of Vomeronasal Function Augments Male-Typical Sexual Behavior in Female Mice. J. Neurosci. 2009, 29, 7658–7666. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.A. Trpc2. Handb. Exp. Pharmacol. 2014, 222, 53–65. [Google Scholar]
- Keller, M.; Douhard, Q.; Baum, M.J.; Bakker, J. Sexual Experience Does Not Compensate for the Disruptive Effects of Zinc Sulfate—Lesioning of the Main Olfactory Epithelium on Sexual Behavior in Male Mice. Chem. Senses 2006, 31, 753–762. [Google Scholar] [CrossRef]
- Keller, M.; Douhard, Q.; Baum, M.J.; Bakker, J. Destruction of the Main Olfactory Epithelium Reduces Female Sexual Behavior and Olfactory Investigation in Female Mice. Chem. Senses 2006, 31, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, T.; Hattori, T.; Asaba, A.; Inoue, N.; Kanomata, N.; Kikusui, T.; Kobayakawa, R.; Kobayakawa, K. Genetic dissection of pheromone processing reveals main olfactory system-mediated social behaviors in mice. Proc. Natl. Acad. Sci. USA 2015, 112, E311–E320. [Google Scholar] [CrossRef]
- Choi, J.-M.; Kim, S.-S.; Choi, C.-I.; Cha, H.-L.; Oh, H.-H.; Ghil, S.; Lee, Y.-D.; Birnbaumer, L.; Suh-Kim, H. Development of the main olfactory system and main olfactory epithelium-dependent male mating behavior are altered in Go-deficient mice. Proc. Natl. Acad. Sci. USA 2016, 113, 10974–10979. [Google Scholar] [CrossRef] [PubMed]
- Shimomi, Y.; Kondo, Y. Blunt olfaction in sexually sluggish male rats. Exp. Anim. Jpn. Assoc. Lab. Anim. Sci. 2020, 69, 441–447. [Google Scholar] [CrossRef]
- Portillo, W.; Paredes, R.G. Sexual incentive motivation, olfactory preference, and activation of the vomeronasal projection pathway by sexually relevant cues in non-copulating and naive male rats. Horm. Behav. 2004, 46, 330–340. [Google Scholar] [CrossRef]
- McCarthy, E.A.; Kunkhyen, T.; Korzan, W.J.; Naik, A.; Maqsudlu, A.; Cherry, J.A.; Baum, M.J. A comparison of the effects of male pheromone priming and optogenetic inhibition of accessory olfactory bulb forebrain inputs on the sexual behavior of estrous female mice. Horm. Behav. 2017, 89, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Jakupovic, J.; Kang, N.; Baum, M.J. Effect of bilateral accessory olfactory bulb lesions on volatile urinary odor discrimination and investigation as well as mating behavior in male mice. Physiol. Behav. 2008, 93, 467–473. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martel, K.L.; Baum, M.J. Sexually dimorphic activation of the accessory, but not the main, olfactory bulb in mice by urinary volatiles. Eur. J. Neurosci. 2007, 26, 463–475. [Google Scholar] [CrossRef]
- Vargas-Barroso, V.; Ordaz-Sanchez, B.; Pena-Ortega, F.; Larriva-Sahd, J.A. Electrophysiological Evidence for a Direct Link between the Main and Accessory Olfactory Bulbs in the Adult Rat. Front. Neurosci. 2015, 9, 518. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Barroso, V.; Peña-Ortega, F.; Larriva-Sahd, J.A. Olfaction and Pheromones: Uncanonical Sensory Influences and Bulbar Interactions. Front. Neuroanat. 2017, 11. [Google Scholar] [CrossRef] [PubMed]
- Kunkhyen, T.; McCarthy, E.A.; Korzan, W.J.; Doctor, D.; Han, X.; Baum, M.J.; Cherry, J.A. Optogenetic Activation of Accessory Olfactory Bulb Input to the Forebrain Differentially Modulates Investigation of Opposite versus Same-Sex Urinary Chemosignals and Stimulates Mating in Male Mice. Eneuro 2017, 4. [Google Scholar] [CrossRef] [PubMed]
- Kang, N.; Baum, M.J.; Cherry, J.A. A direct main olfactory bulb projection to the ‘vomeronasal’ amygdala in female mice selectively responds to volatile pheromones from males. Eur. J. Neurosci. 2009, 29, 624–634. [Google Scholar] [CrossRef] [PubMed]
- Martel, K.L.; Baum, M.J. A centrifugal pathway to the mouse accessory olfactory bulb from the medial amygdala conveys gender-specific volatile pheromonal signals. Eur. J. Neurosci. 2009, 29, 368–376. [Google Scholar] [CrossRef]
- Stowers, L.; Marton, T.F. What Is a Pheromone? Mammalian Pheromones Reconsidered. Neuron 2005, 46, 699–702. [Google Scholar] [CrossRef]
- Lin, D.Y.; Zhang, S.-Z.; Block, E.; Katz, L.C. Encoding social signals in the mouse main olfactory bulb. Nature 2005, 434, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Inokuchi, K.; Imamura, F.; Takeuchi, H.; Kim, R.; Okuno, H.; Nishizumi, H.; Bito, H.; Kikusui, T.; Sakano, H. Nrp2 is sufficient to instruct circuit formation of mitral-cells to mediate odour-induced attractive social responses. Nat. Commun. 2017, 8, 15977. [Google Scholar] [CrossRef]
- McCarthy, E.A.; Maqsudlu, A.; Bass, M.; Georghiou, S.; Cherry, J.A.; Baum, M.J. DREADD-induced silencing of the medial amygdala reduces the preference for male pheromones and the expression of lordosis in estrous female mice. Eur. J. Neurosci. 2017, 46, 2035–2046. [Google Scholar] [CrossRef] [PubMed]
- Billing, A.; Correia, M.H.; Kelly, D.A.; Li, G.-L.; Bergan, J.F. Synaptic Connections of Aromatase Circuits in the Medial Amygdala Are Sex Specific. Eneuro 2020, 7. [Google Scholar] [CrossRef] [PubMed]
- Bergan, J.F.; Ben-Shaul, Y.; Dulac, C. Sex-specific processing of social cues in the medial amygdala. eLife 2014, 3, e02743. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.M.; Marcotulli, D.; Shen, A.; Zweifel, L.S. Divergent medial amygdala projections regulate approach–avoidance conflict behavior. Nat. Neurosci. 2019, 22, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Iurilli, G.; Datta, S.R. Population Coding in an Innately Relevant Olfactory Area. Neuron 2017, 93, 1180–1197. [Google Scholar] [CrossRef]
- Root, C.M.; Denny, C.A.; Hen, R.; Axel, R. The participation of cortical amygdala in innate, odour-driven behaviour. Nature 2014, 515, 269–273. [Google Scholar] [CrossRef]
- Pardo-Bellver, C.; Martínez-Bellver, S.; Martínez-García, F.; Lanuza, E.; Teruel-Martí, V. Synchronized Activity in The Main and Accessory Olfactory Bulbs and Vomeronasal Amygdala Elicited by Chemical Signals in Freely Behaving Mice. Sci. Rep. 2017, 7, 1–16. [Google Scholar] [CrossRef]
- Maras, P.; Petrulis, A. The posteromedial cortical amygdala regulates copulatory behavior, but not sexual odor preference, in the male Syrian hamster (Mesocricetus auratus). Neuroscience 2008, 156, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Maras, P.M.; Petrulis, A. The anterior medial amygdala transmits sexual odor information to the posterior medial amygdala and related forebrain nuclei. Eur. J. Neurosci. 2010, 32, 469–482. [Google Scholar] [CrossRef] [PubMed]
- Maras, P.M.; Petrulis, A. Lesions that functionally disconnect the anterior and posterodorsal sub-regions of the medial amygdala eliminate opposite-sex odor preference in male Syrian hamsters (Mesocricetus auratus). Neuroscience 2010, 165, 1052–1062. [Google Scholar] [CrossRef][Green Version]
- Vaz, R.P.; Cardoso, A.; Sá, S.; Pereira, P.; Madeira, M.D. The integrity of the nucleus of the lateral olfactory tract is essential for the normal functioning of the olfactory system. Brain Struct. Funct. 2017, 222, 3615–3637. [Google Scholar] [CrossRef]
- Kondo, Y.; Arai, Y. Functional association between the medial amygdala and the medial preoptic area in regulation of mating behavior in the male rat. Physiol. Behav. 1995, 57, 69–73. [Google Scholar] [CrossRef]
- Kondo, Y.; Yamanouchi, K. The possible involvement of the nonstrial pathway of the amygdala in neural control of sexual behavior in male rats. Brain Res. Bull. 1995, 38, 37–40. [Google Scholar] [CrossRef]
- Been, L.E.; Petrulis, A. Dissociated functional pathways for appetitive and consummatory reproductive behaviors in male Syrian hamsters. Horm. Behav. 2012, 61, 204–211. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Been, L.E.; Petrulis, A. Lesions of the posterior bed nucleus of the stria terminalis eliminate opposite-sex odor preference and delay copulation in male Syrian hamsters: Role of odor volatility and sexual experience. Eur. J. Neurosci. 2010, 32, 483–493. [Google Scholar] [CrossRef]
- Dhungel, S.; Urakawa, S.; Kondo, Y.; Sakuma, Y. Olfactory preference in the male rat depends on multiple chemosensory inputs converging on the preoptic area. Horm. Behav. 2011, 59, 193–199. [Google Scholar] [CrossRef]
- Hurtazo, H.A.; Paredes, R.G. Olfactory preference and Fos expression in the accessory olfactory system of male rats with bilateral lesions of the medial preoptic area/anterior hypothalamus. Neuroscience 2005, 135, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Hurtazo, H.; Paredes, R.; Ågmo, A. Inactivation of the medial preoptic area/anterior hypothalamus by lidocaine reduces male sexual behavior and sexual incentive motivation in male rats. Neuroscience 2008, 152, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Guarraci, F.A.; Clark, A.S. Ibotenic acid lesions of the medial preoptic area disrupt the expression of partner preference in sexually receptive female rats. Brain Res. 2006, 1076, 163–170. [Google Scholar] [CrossRef]
- Martinez, L.A.; Petrulis, A. The medial preoptic area is necessary for sexual odor preference, but not sexual solicitation, in female Syrian hamsters. Horm. Behav. 2013, 63, 606–614. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McHenry, J.A.; Otis, J.M.; Rossi, M.A.; Robinson, J.E.; Kosyk, O.; Miller, N.W.; McElligott, Z.A.; Budygin, E.A.; Rubinow, D.R.; Stuber, G.D. Hormonal gain control of a medial preoptic area social reward circuit. Nat. Neurosci. 2017, 20, 449–458. [Google Scholar] [CrossRef]
- Sánchez-Catalán, M.J.; Orrico, A.; Hipólito, L.; Zornoza, T.; Polache, A.; Lanuza, E.; Martínez-García, F.; Granero, L.; Agustín-Pavón, C. Glutamate and Opioid Antagonists Modulate Dopamine Levels Evoked by Innately Attractive Male Chemosignals in the Nucleus Accumbens of Female Rats. Front. Neuroanat. 2017, 11, 8. [Google Scholar] [CrossRef]
- Wesson, D.W.; Wilson, D. Sniffing out the contributions of the olfactory tubercle to the sense of smell: Hedonics, sensory integration, and more? Neurosci. Biobehav. Rev. 2011, 35, 655–668. [Google Scholar] [CrossRef]
- Pardo-Bellver, C.; Cádiz-Moretti, B.; Novejarque, A.; Martínez-García, F.; Lanuza, E. Differential efferent projections of the anterior, posteroventral, and posterodorsal subdivisions of the medial amygdala in mice. Front. Neuroanat. 2012, 6, 33. [Google Scholar] [CrossRef] [PubMed]
- DiBenedictis, B.T.; Helfand, A.I.; Baum, M.J.; Cherry, J.A. A quantitative comparison of the efferent projections of the anterior and posterior subdivisions of the medial amygdala in female mice. Brain Res. 2014, 1543, 101–108. [Google Scholar] [CrossRef]
- Agustín-Pavón, C.; Martinez-Garcia, F.; Lanuza, E. Focal lesions within the ventral striato-pallidum abolish attraction for male chemosignals in female mice. Behav. Brain Res. 2014, 259, 292–296. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Y.; Rao, Y. Serotonin signaling in the brain of adult female mice is required for sexual preference. Proc. Natl. Acad. Sci. USA 2013, 110, 9968–9973. [Google Scholar] [CrossRef] [PubMed]
- Angoa-Pérez, M.; Herrera-Mundo, N.; Kane, M.J.; Sykes, C.E.; Anneken, J.H.; Francescutti, D.M.; Kuhn, D.M. Brain Serotonin Signaling Does Not Determine Sexual Preference in Male Mice. PLoS ONE 2015, 10, e0118603. [Google Scholar] [CrossRef]
- Sachs, B.D. Erection evoked in male rats by airborne scent from estrous females. Physiol. Behav. 1997, 62, 921–924. [Google Scholar] [CrossRef]
- Lydell, K.; Doty, R.L. Male rat of odor preferences for female urine as a function of sexual experience, urine age, and urine source. Horm. Behav. 1972, 3, 205–212. [Google Scholar] [CrossRef]
- Olvera-Hernández, S.; Hernández, A.; Reyes, R.; Fernández-Guasti, A. Establishment of partner preference in male rats: Effect of prenatal letrozole and sexual experience. Horm. Behav. 2019, 109, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Pankevich, D.E.; Cherry, J.A.; Baum, M.J. Accessory olfactory neural Fos responses to a conditioned environment are blocked in male mice by vomeronasal organ removal. Physiol. Behav. 2006, 87, 781–788. [Google Scholar] [CrossRef][Green Version]
- Ballard, C.L.; Wood, R.I. Partner preference in male hamsters: Steroids, sexual experience and chemosensory cues. Physiol. Behav. 2007, 91, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Maras, P.M.; Petrulis, A. Olfactory experience and the development of odor preference and vaginal marking in female Syrian hamsters. Physiol. Behav. 2008, 94, 545–551. [Google Scholar] [CrossRef]
- Been, L.E.; Petrulis, A. The role of the medial preoptic area in appetitive and consummatory reproductive behaviors depends on sexual experience and odor volatility in male Syrian hamsters. Neuroscience 2010, 170, 1120–1132. [Google Scholar] [CrossRef]
- Van Der Linden, C.; Jakob, S.; Gupta, P.; Dulac, C.; Santoro, S.W. Sex separation induces differences in the olfactory sensory receptor repertoires of male and female mice. Nat. Commun. 2018, 9, 1–15. [Google Scholar] [CrossRef]
- Dillon, T.S.; Fox, L.C.; Han, C.; Linster, C. 17beta-estradiol enhances memory duration in the main olfactory bulb in CD-1 mice. Behav Neurosci 2013, 127, 923–931. [Google Scholar] [CrossRef]
- Shea, S.D.; Katz, L.C.; Mooney, R. Noradrenergic Induction of Odor-Specific Neural Habituation and Olfactory Memories. J. Neurosci. 2008, 28, 10711–10719. [Google Scholar] [CrossRef]
- Unda, N.M.; Eportillo, W.; Corona, R.; Paredes, R.G. Sexual Stimulation Increases the Survival of New Cells in the Accessory Olfactory Bulb of the Male Rat. Front. Neurosci. 2016, 10, 65. [Google Scholar] [CrossRef]
- Swaney, W.T.; Curley, J.P.; Champagne, F.A.; Keverne, E.B. The paternally expressed gene Peg3 regulates sexual experience-dependent preferences for estrous odors. Behav. Neurosci. 2008, 122, 963–973. [Google Scholar] [CrossRef]
- Li, Y.; Mathis, A.; Grewe, B.; Osterhout, J.A.; Ahanonu, B.; Schnitzer, M.J.; Murthy, V.; Dulac, C. Neuronal Representation of Social Information in the Medial Amygdala of Awake Behaving Mice. Cell 2017, 171, 1176–1190. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, J.M.; Brann, J.H.; Gil, M.; Hull, E.M. Sexual experience increases nitric oxide synthase in the medial preoptic area of male rats. Behav. Neurosci. 2006, 120, 1389–1394. [Google Scholar] [CrossRef]
- Swaney, W.; Dubose, B.N.; Curley, J.P.; Champagne, F.A. Sexual experience affects reproductive behavior and preoptic androgen receptors in male mice. Horm. Behav. 2012, 61, 472–478. [Google Scholar] [CrossRef] [PubMed]
- Maejima, S.; Abe, Y.; Yamaguchi, S.; Musatov, S.; Ogawa, S.; Kondo, Y.; Tsukahara, S. VGF in the Medial Preoptic Nucleus Increases Sexual Activity Following Sexual Arousal Induction in Male Rats. Endocrinology 2018, 159, 3993–4005. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.E.; Brameld, J.M.; Jethwa, P.H. Neuroendocrine Role for VGF. Front. Endocrinol. 2015, 6, 3. [Google Scholar] [CrossRef]
- Succu, S.; Mascia, M.S.; Melis, T.; Sanna, F.; Melis, M.R.; Possenti, R.; Argiolas, A. Pro-VGF-derived peptides induce penile erection in male rats: Involvement of paraventricular nitric oxide. Neuropharmacology 2005, 49, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.R.; Meerts, S.H.; Sisk, C.L. Male Syrian hamsters demonstrate a conditioned place preference for sexual behavior and female chemosensory stimuli. Horm. Behav. 2010, 58, 410–414. [Google Scholar] [CrossRef][Green Version]
- Gadziola, M.A.; Tylicki, K.A.; Christian, D.L.; Wesson, D.W. The Olfactory Tubercle Encodes Odor Valence in Behaving Mice. J. Neurosci. 2015, 35, 4515–4527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, Q.; Wen, P.; Zhang, J.; Rao, X.; Zhou, Z.; Zhang, H.; He, X.; Li, J.; Zhou, Z.; et al. Activation of the dopaminergic pathway from VTA to the medial olfactory tubercle generates odor-preference and reward. eLife 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Lopez, H.H.; Ettenberg, A. Exposure to female rats produces differences in c-fos induction between sexually-naive and experienced male rats. Brain Res. 2002, 947, 57–66. [Google Scholar] [CrossRef]
- Hosokawa, N.; Chiba, A. Effects of sexual experience on conspecific odor preference and estrous odor-induced activation of the vomeronasal projection pathway and the nucleus accumbens in male rats. Brain Res. 2005, 1066, 101–108. [Google Scholar] [CrossRef]
- Fujiwara, M.; Chiba, A. Sexual odor preference and dopamine release in the nucleus accumbens by estrous olfactory cues in sexually naïve and experienced male rats. Physiol. Behav. 2018, 185, 95–102. [Google Scholar] [CrossRef]
- Moncho-Bogani, J.; Martinez-Garcia, F.; Novejarque, A.; Lanuza, E. Attraction to sexual pheromones and associated odorants in female mice involves activation of the reward system and basolateral amygdala. Eur. J. Neurosci. 2005, 21, 2186–2198. [Google Scholar] [CrossRef]
- Moncho-Bogani, J.; Lanuza, E.; Hernandez, A.; Novejarque, A.; Martinez-Garcia, F. Attractive properties of sexual pheromones in mice: Innate or learned? Physiol. Behav. 2002, 77, 167–176. [Google Scholar] [CrossRef]
- Ramm, S.; Cheetham, S.A.; Hurst, J.L. Encoding choosiness: Female attraction requires prior physical contact with individual male scents in mice. Proc. R. Soc. B Biol. Sci. 2008, 275, 1727–1735. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, E.A.; Naik, A.S.; Coyne, A.F.; Cherry, J.A.; Baum, M.J. Effect of Ovarian Hormones and Mating Experience on the Preference of Female Mice to Investigate Male Urinary Pheromones. Chem. Senses 2017, 43, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, N.; Chiba, A. Effects of sexual experience on conspecific odor preference and male odor-induced activation of the vomeronasal projection pathway and the nucleus accumbens in female rats. Brain Res. 2007, 1175, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Meerts, S.H.; Park, S.; Sekhawat, R. Sexual experience modulates partner preference and mPOA nitric oxide synthase in female rats. Behav. Neurosci. 2016, 130, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Piergies, A.M.; Hicks, M.E., Jr.; Schwartz, J.P.; Meerts, S.H. Sexually experienced, but not naïve, female rats show a conditioned object preference (COP) for mating after a single training trial. Physiol. Behav. 2019, 198, 42–47. [Google Scholar] [CrossRef]
- Kudwa, A.E.; López, F.J.; McGivern, R.F.; Handa, R.J. A Selective Androgen Receptor Modulator Enhances Male-Directed Sexual Preference, Proceptive Behavior, and Lordosis Behavior in Sexually Experienced, But Not Sexually Naive, Female Rats. Endocrinology 2010, 151, 2659–2668. [Google Scholar] [CrossRef] [PubMed]
- Mitre, M.; Marlin, B.J.; Schiavo, J.K.; Morina, E.; Norden, S.E.; Hackett, T.A.; Aoki, C.J.; Chao, M.V.; Froemke, R.C. A Distributed Network for Social Cognition Enriched for Oxytocin Receptors. J. Neurosci. 2016, 36, 2517–2535. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Rodriguez, A.; Mani, S.K.; Handa, R.J. Oxytocin and Estrogen Receptor beta in the Brain: An Overview. Front. Endocrinol. 2015, 6, 160. [Google Scholar] [CrossRef]
- Vaccari, C.; Lolait, S.J.; Ostrowski, N.L. Comparative Distribution of Vasopressin V1b and Oxytocin Receptor Messenger Ribonucleic Acids in Brain1. Endocrinology 1998, 139, 5015–5033. [Google Scholar] [CrossRef] [PubMed]
- Wacker, D.; Ludwig, M. The role of vasopressin in olfactory and visual processing. Cell Tissue Res. 2018, 375, 201–215. [Google Scholar] [CrossRef]
- Ferguson, J.N.; Young, L.J.; Hearn, E.F.; Matzuk, M.M.; Insel, T.R.; Winslow, J.T. Social amnesia in mice lacking the oxytocin gene. Nat. Genet. 2000, 25, 284–288. [Google Scholar] [CrossRef]
- Ferguson, J.N.; Aldag, J.M.; Insel, T.R.; Young, L.J. Oxytocin in the Medial Amygdala is Essential for Social Recognition in the Mouse. J. Neurosci. 2001, 21, 8278–8285. [Google Scholar] [CrossRef]
- Oettl, L.-L.; Ravi, N.; Schneider, M.; Scheller, M.F.; Schneider, P.; Mitre, M.; da Silva Gouveia, M.; Froemke, R.; Chao, M.; Young, W.S.; et al. Oxytocin Enhances Social Recognition by Modulating Cortical Control of Early Olfactory Processing. Neuron 2016, 90, 609–621. [Google Scholar] [CrossRef]
- Wersinger, S.R.; Ginns, E.I.; O’Carroll, A.M.; Lolait, S.J.; Young, W.S., 3rd. Vasopressin V1b receptor knockout reduces aggressive behavior in male mice. Mol. Psychiatry 2002, 7, 975–984. [Google Scholar] [CrossRef] [PubMed]
- De la Zerda, S.H.; Netser, S.; Magalnik, H.; Wagner, S. Impaired sex preference, but not social and social novelty preferences, following systemic blockade of oxytocin receptors in adult male mice. Psychoneuroendocrinology 2020, 116, 104676. [Google Scholar] [CrossRef]
- Blitzer, D.; Wells, T.; Hawley, W. Administration of an oxytocin receptor antagonist attenuates sexual motivation in male rats. Horm. Behav. 2017, 94, 33–39. [Google Scholar] [CrossRef]
- Kent, K.; Arientyl, V.; Khachatryan, M.M.; Wood, R.I. Oxytocin Induces a Conditioned Social Preference in Female Mice. J. Neuroendocr. 2013, 25, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Rio, R.T.-D.; Silvaran, M.B.T.; Díaz-Estrada, V.X.; Herrera-Covarrubias, D.; Corona-Morales, A.A.; Pfaus, J.G.; Coria-Avila, G.A. Conditioned same-sex partner preference in male rats is facilitated by oxytocin and dopamine: Effect on sexually dimorphic brain nuclei. Behav. Brain Res. 2015, 283, 69–77. [Google Scholar] [CrossRef]
- Shimizu, K.; Nakamura, K.; Yokosuka, M.; Kondo, Y. Modulation of male mouse sociosexual and anxiety-like behaviors by vasopressin receptors. Physiol. Behav. 2018, 197, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Swaab, D.; Slob, A.; Houtsmuller, E.; Brand, T.; Zhou, J. Increased number of vasopressin neurons in the suprachiasmatic nucleus (SCN) of ‘bisexual’ adult male rats following perinatal treatment with the aromatase blocker ATD. Dev. Brain Res. 1995, 85, 273–279. [Google Scholar] [CrossRef]
- Wersinger, S.R.; Kelliher, K.R.; Zufall, F.; Lolait, S.J.; O’Carroll, A.M.; Young, W.S., 3rd. Social motivation is reduced in vasopressin 1b receptor null mice despite normal performance in an olfactory discrimination task. Horm. Behav. 2004, 46, 638–645. [Google Scholar] [CrossRef]
- DiBenedictis, B.T.; Cheung, H.K.; Nussbaum, E.R.; Veenema, A. Involvement of ventral pallidal vasopressin in the sex-specific regulation of sociosexual motivation in rats. Psychoneuroendocrinology 2020, 111, 104462. [Google Scholar] [CrossRef]
- Bakker, J.; Pierman, S.; Gonzales-Martinez, D. Effects of aromatase mutation (ArKO) on the sexual differentiation of kisspeptin neuronal numbers and their activation by same versus opposite sex urinary pheromones. Horm. Behav. 2010, 57, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Hellier, V.; Brock, O.; Candlish, M.; Desroziers, E.; Aoki, M.; Mayer, C.; Piet, R.; Herbison, A.; Colledge, W.H.; Prevot, V.; et al. Female sexual behavior in mice is controlled by kisspeptin neurons. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Adekunbi, D.; Li, X.F.; Lass, G.; Shetty, K.; Adegoke, O.A.; Yeo, S.H.; Colledge, W.H.; Lightman, S.L.; O’Byrne, K.T. Kisspeptin neurones in the posterodorsal medial amygdala modulate sexual partner preference and anxiety in male mice. J. Neuroendocr. 2018, 30, e12572. [Google Scholar] [CrossRef] [PubMed]
- Gresham, R.; Li, S.; Adekunbi, D.; Hu, M.; Li, X.F.; O’Byrne, K.T. Kisspeptin in the medial amygdala and sexual behavior in male rats. Neurosci. Lett. 2016, 627, 13–17. [Google Scholar] [CrossRef] [PubMed]
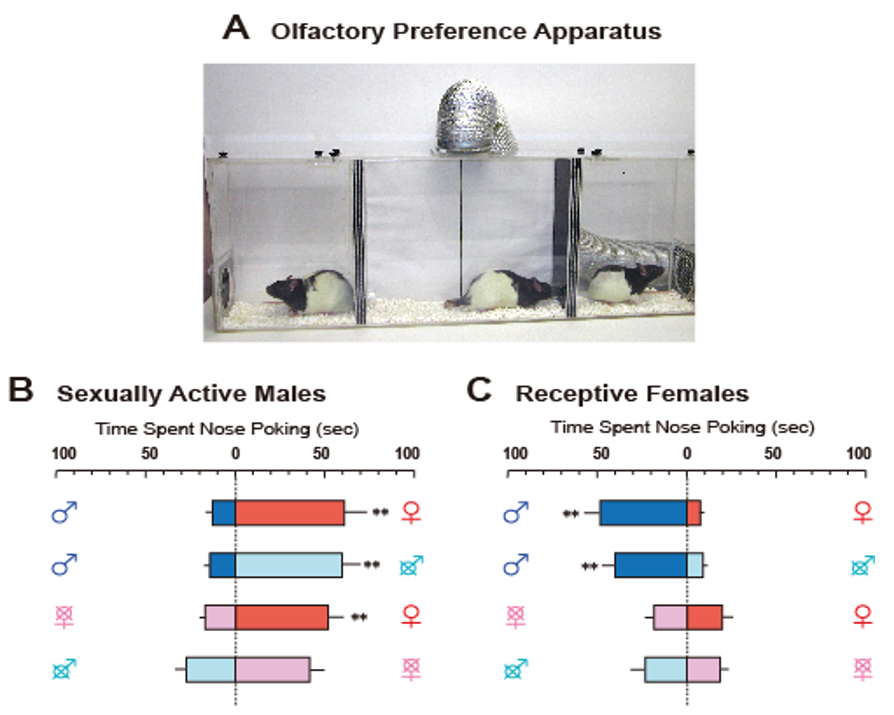
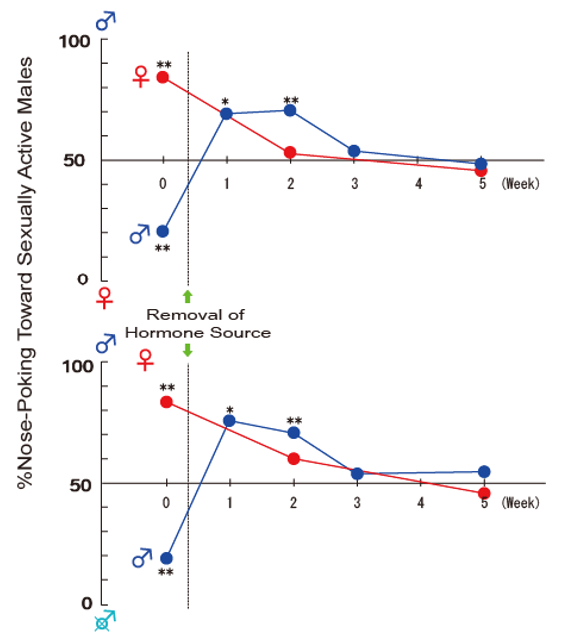
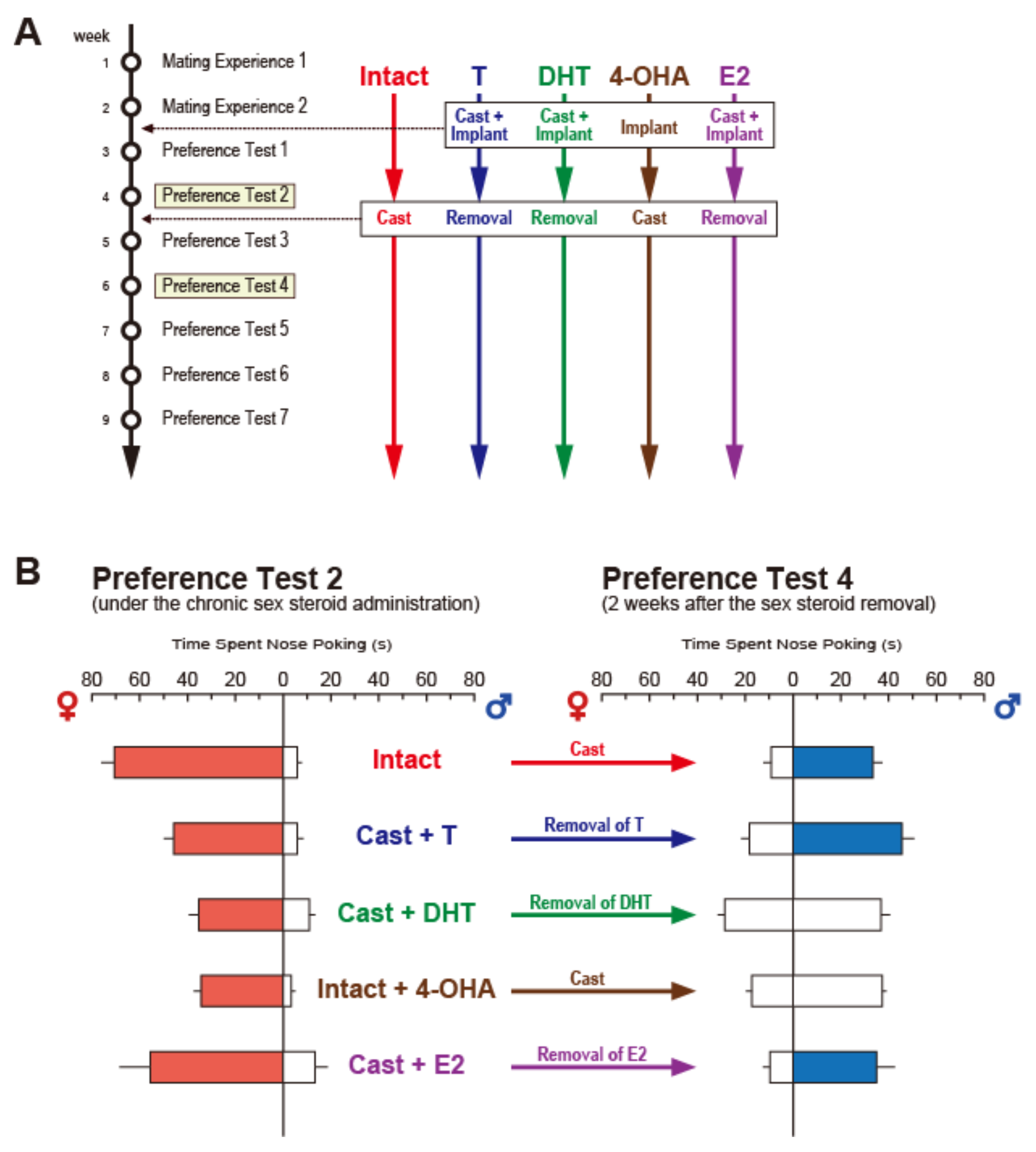


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kondo, Y.; Hayashi, H. Neural and Hormonal Basis of Opposite-Sex Preference by Chemosensory Signals. Int. J. Mol. Sci. 2021, 22, 8311. https://doi.org/10.3390/ijms22158311
Kondo Y, Hayashi H. Neural and Hormonal Basis of Opposite-Sex Preference by Chemosensory Signals. International Journal of Molecular Sciences. 2021; 22(15):8311. https://doi.org/10.3390/ijms22158311
Chicago/Turabian StyleKondo, Yasuhiko, and Himeka Hayashi. 2021. "Neural and Hormonal Basis of Opposite-Sex Preference by Chemosensory Signals" International Journal of Molecular Sciences 22, no. 15: 8311. https://doi.org/10.3390/ijms22158311
APA StyleKondo, Y., & Hayashi, H. (2021). Neural and Hormonal Basis of Opposite-Sex Preference by Chemosensory Signals. International Journal of Molecular Sciences, 22(15), 8311. https://doi.org/10.3390/ijms22158311




