Copper(II) Complexes Containing Natural Flavonoid Pomiferin Show Considerable In Vitro Cytotoxicity and Anti-inflammatory Effects
Abstract
:1. Introduction
2. Results and Discussion
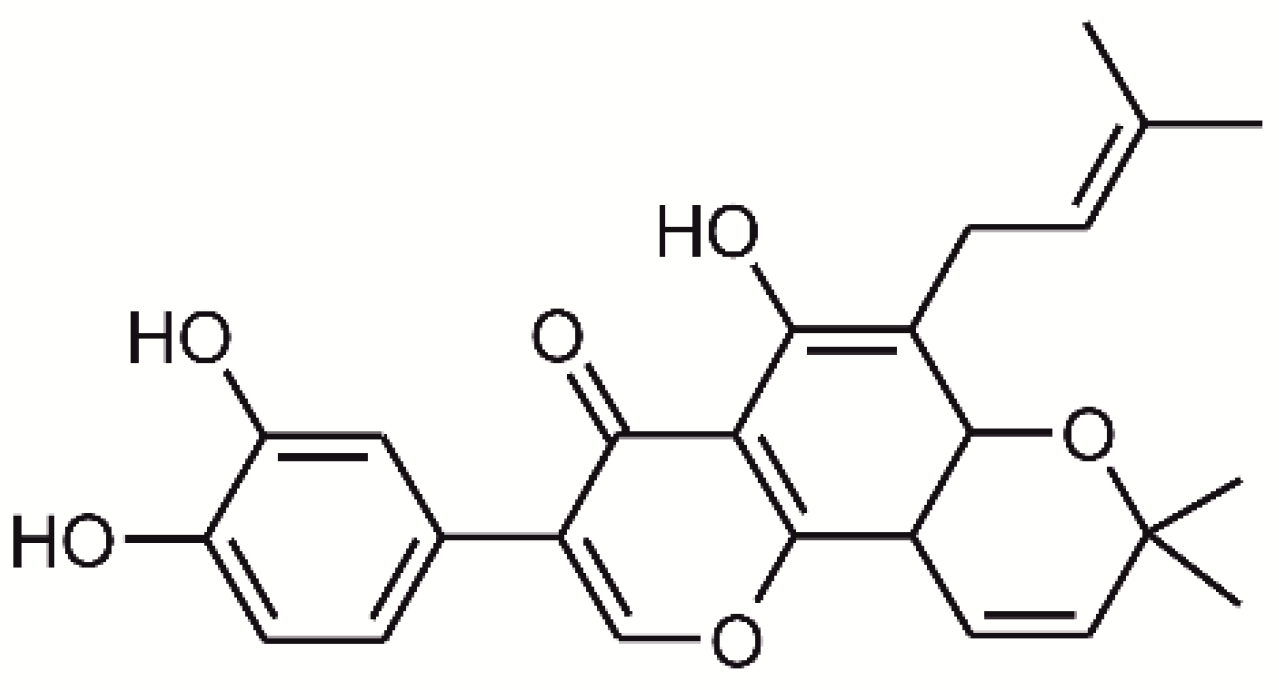
2.1. Synthesis and Characterization
2.2. In Vitro Cytotoxicity of Complexes 1–5
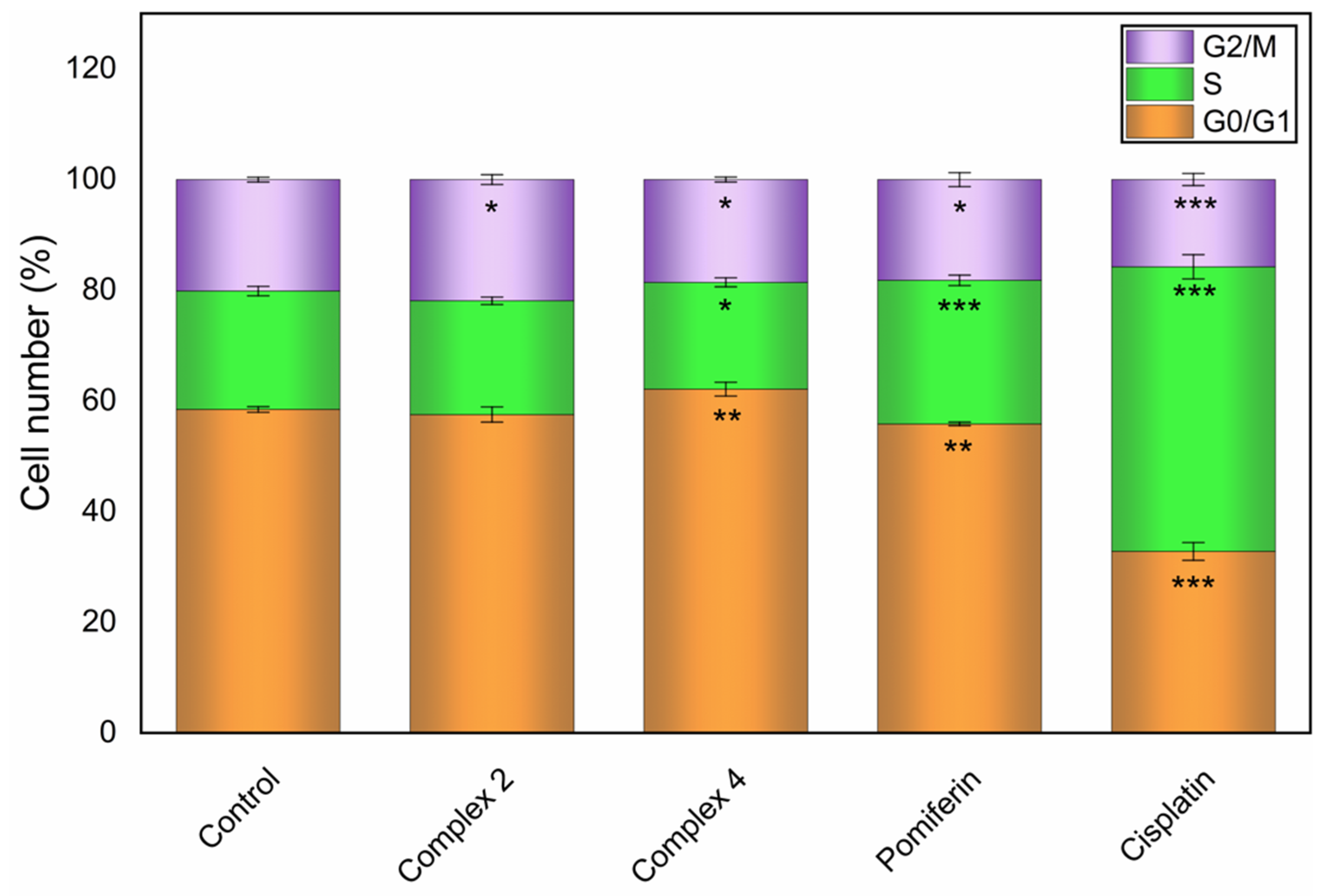
2.3. Cell Cycle Modifications
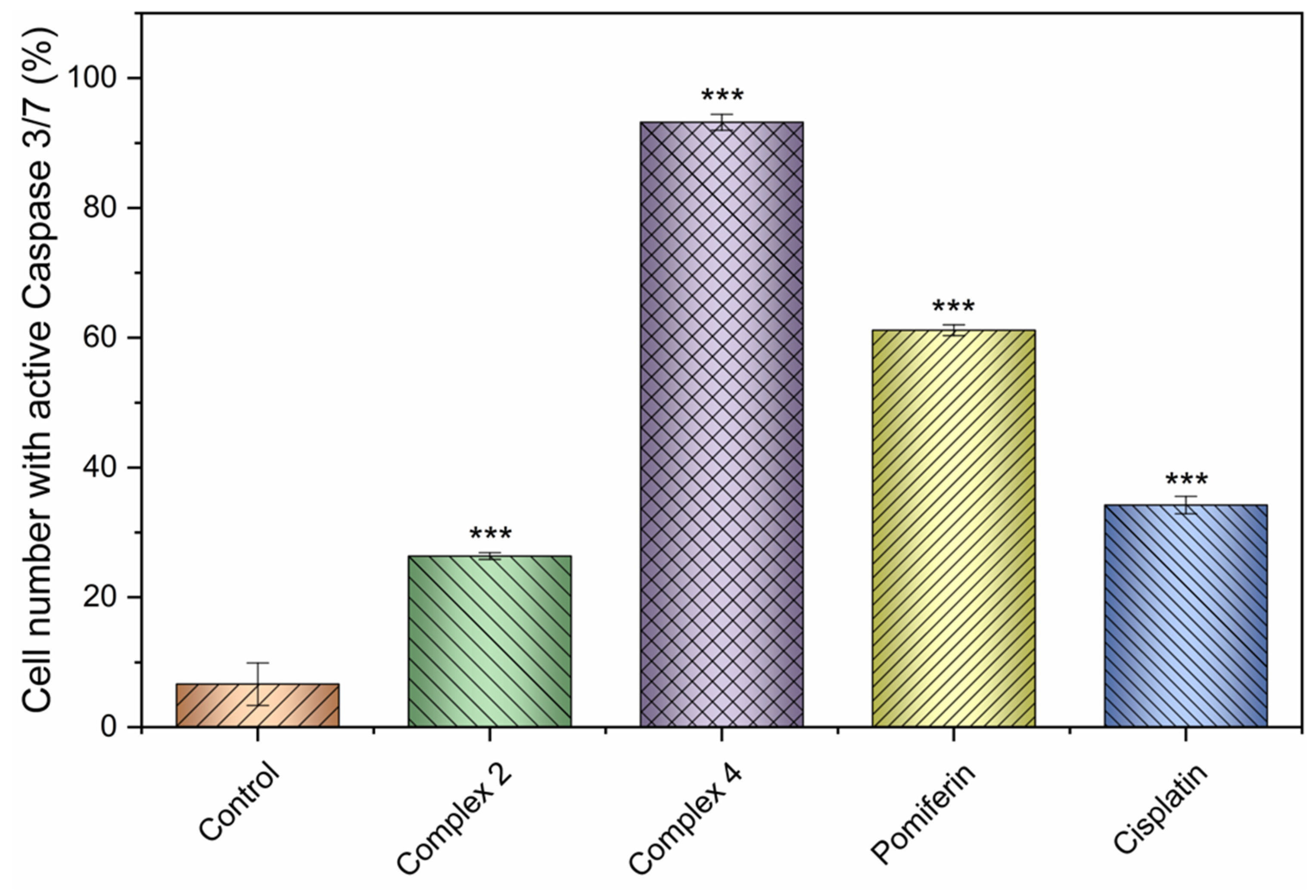
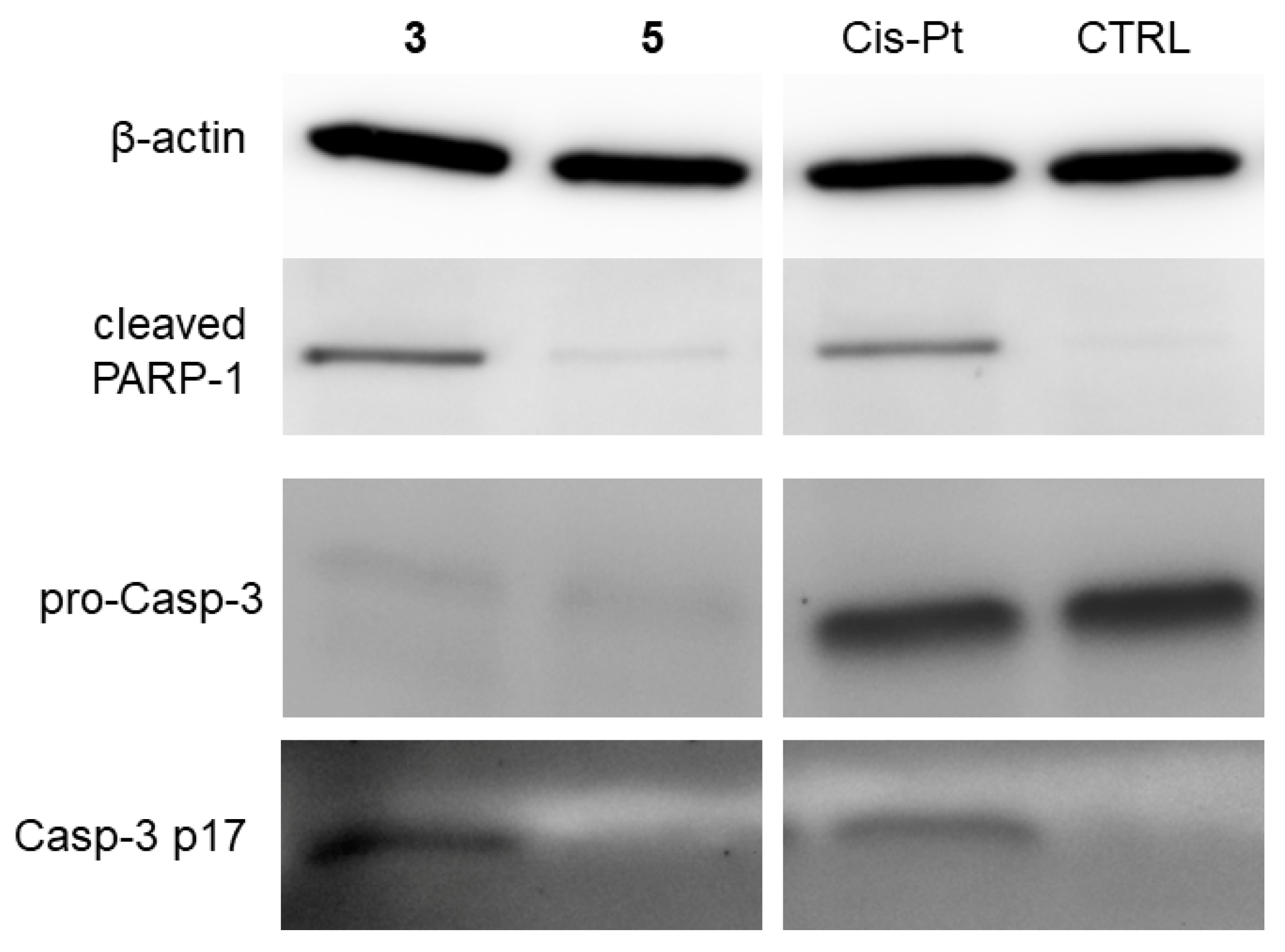
2.4. Induction of Cell Death and Related Processes
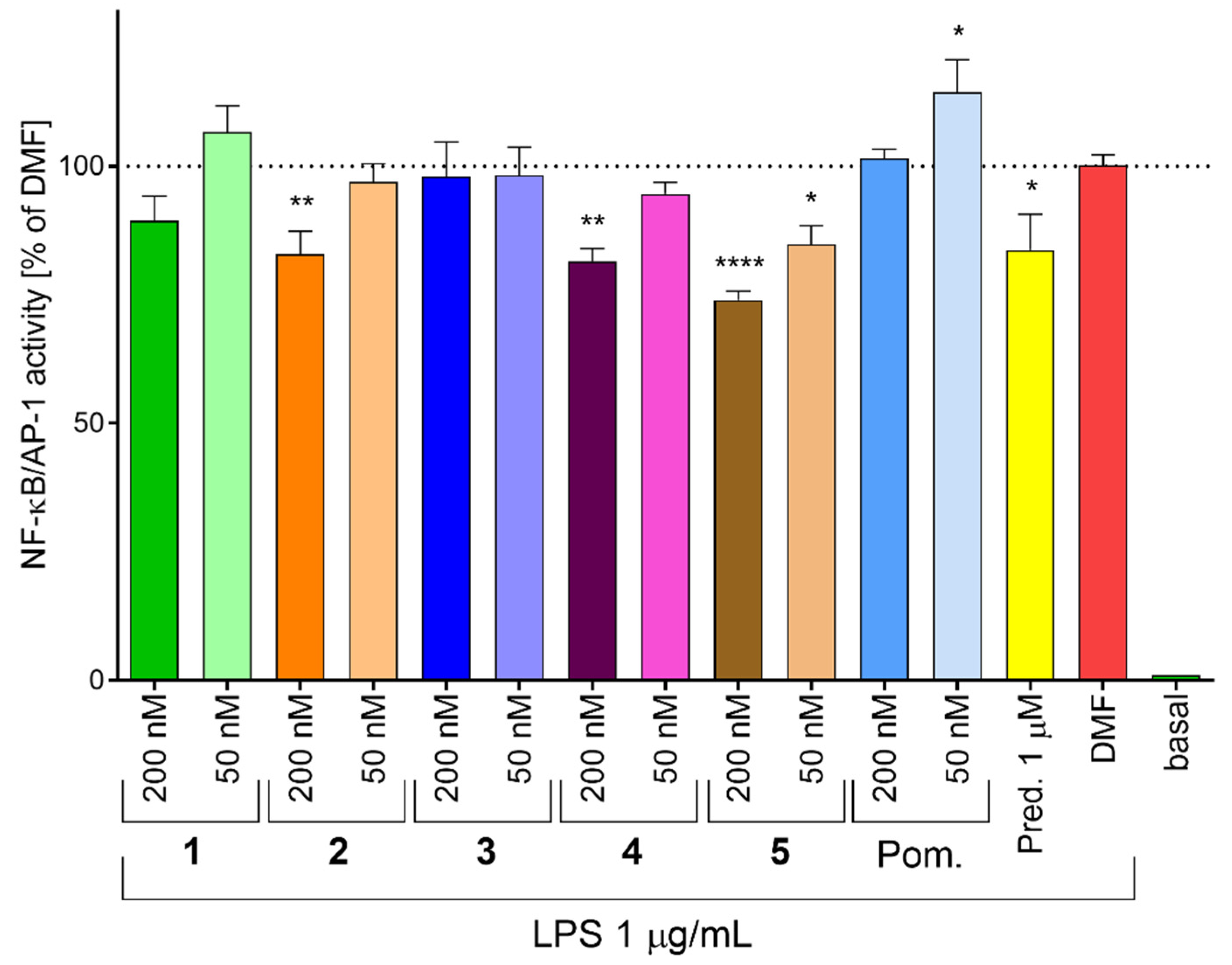
2.5. Inhibition of NF-κB/AP-1 Activity
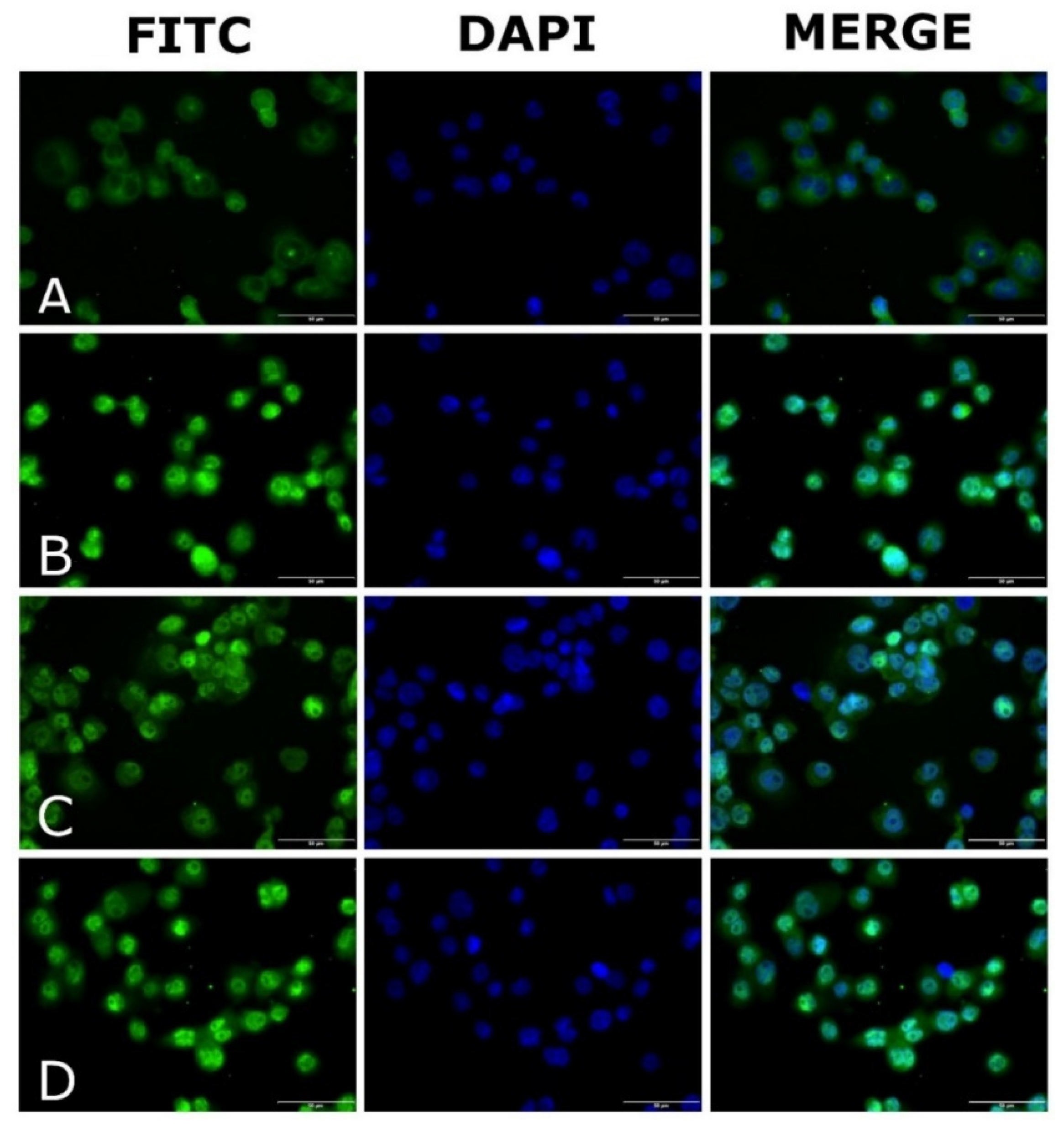
2.6. Evaluation of NF-κB Translocation
2.7. Evaluation of TNF-α Secretion
2.8. Evaluation of Apoptosis and Inflammation-Related Signalling Pathways
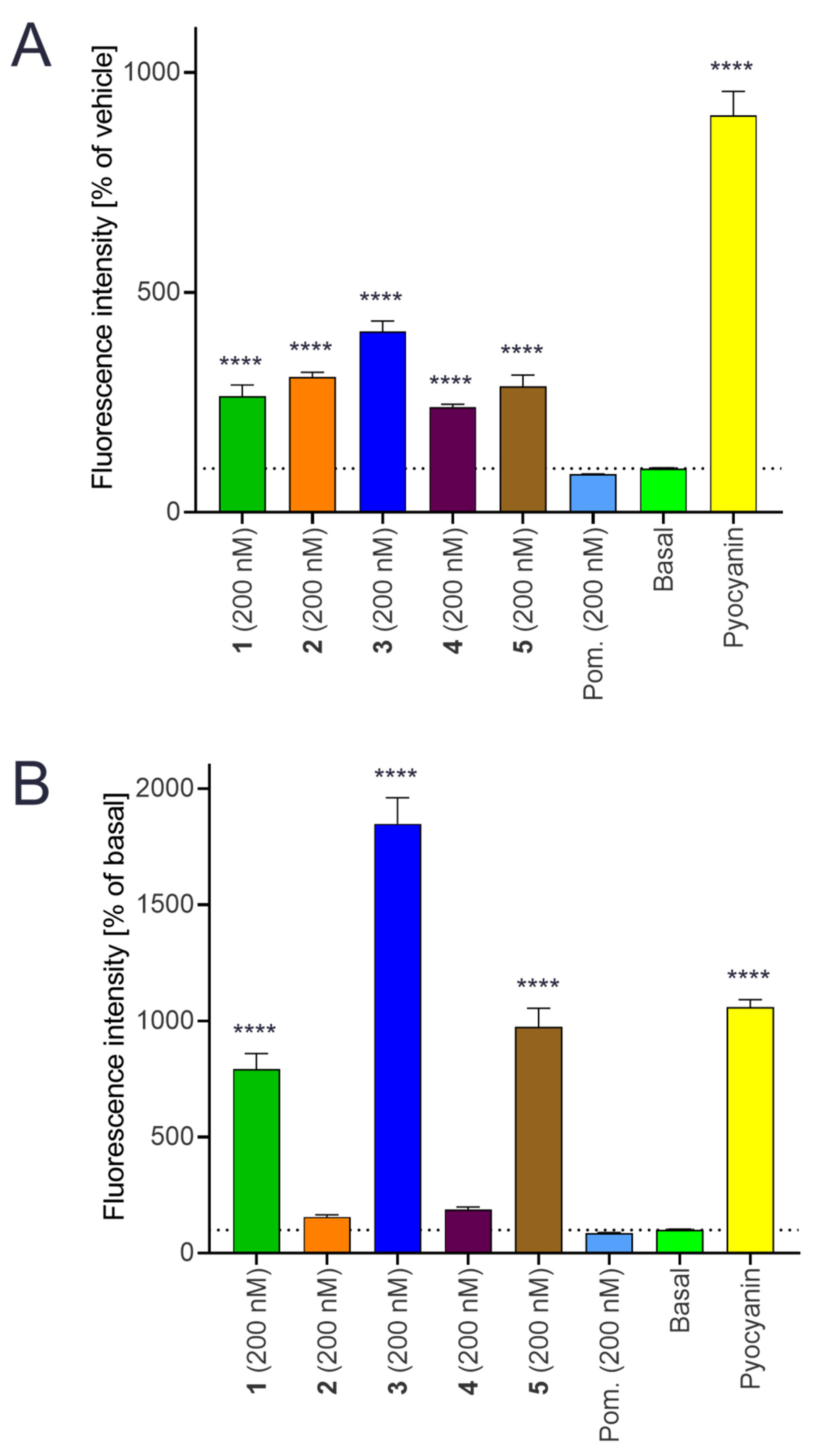
2.9. Intracellular Production of Reactive Oxygen Species (ROS)
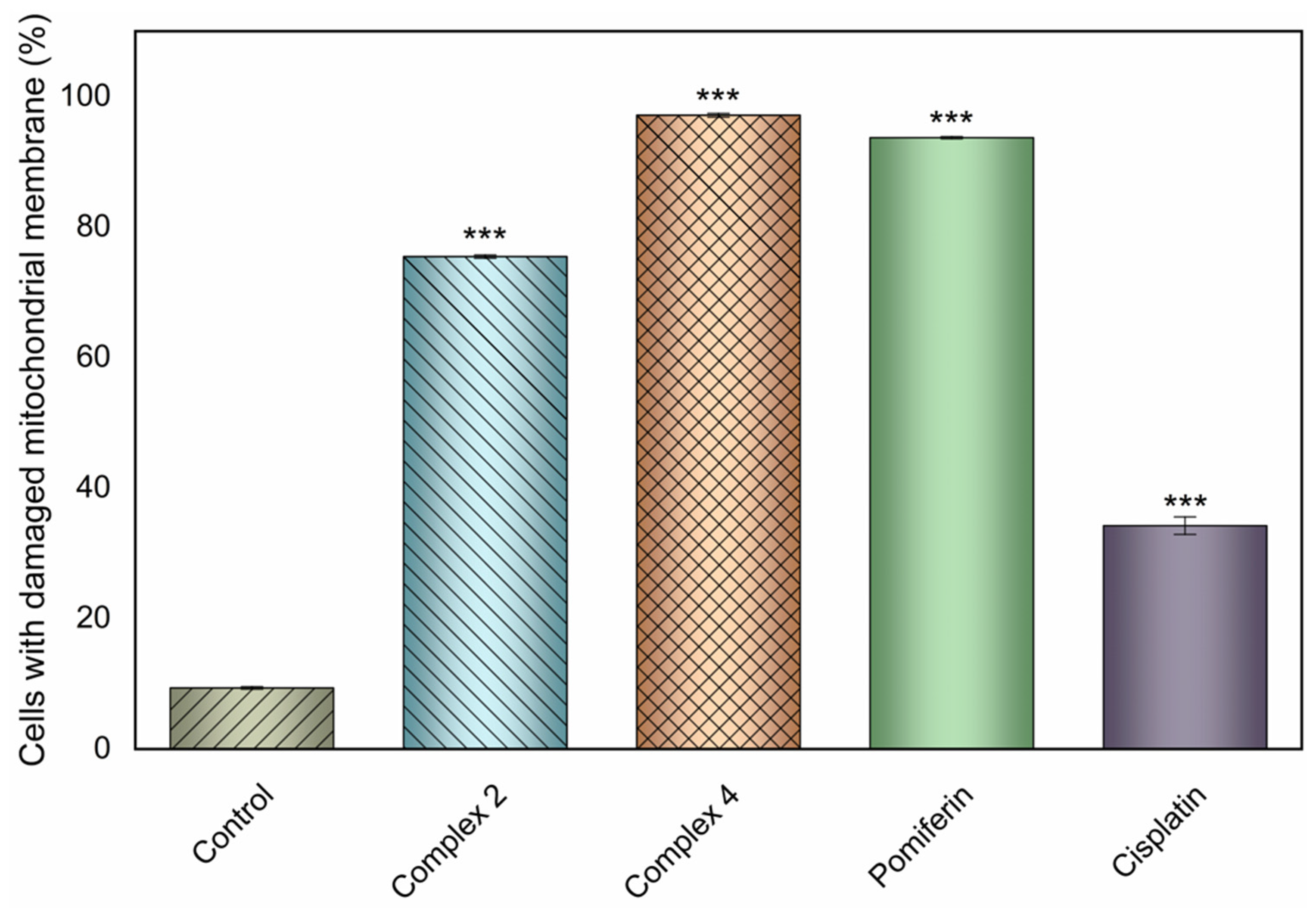
2.10. Mitochondrial Membrane Potential Modifications
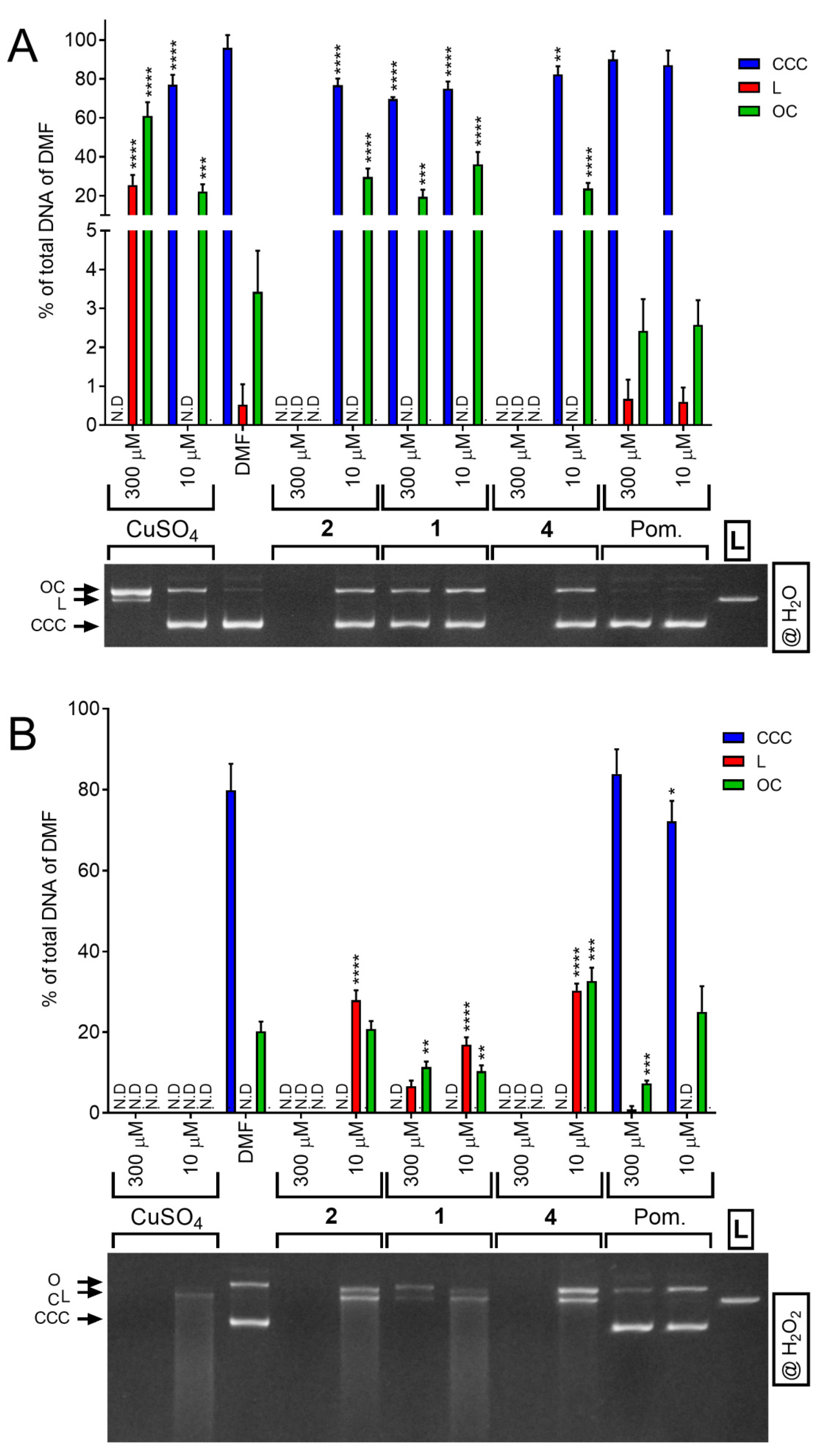
2.11. Nuclease Mimicking Activity
3. Materials and Methods
3.1. General Methods
3.2. Preparation and Characterization of Complexes 1–5
3.3. In Vitro Cytotoxicity
3.4. Cell Cycle Analysis
3.5. Induction of Cell Death and Related Processes
3.6. Detection of the Activation of NF-κB/AP-1
3.7. Immunocytochemical Analysis of NF-κB Nuclear Translocation
3.8. Evaluation of TNF-α Secretion
3.9. Apoptosis and Inflammation-related Signalling Pathways Analysis
3.10. Detection of the Intracellular Production of Reactive Oxygen Species
3.11. Mitochondrial Membrane Potential Modifications
3.12. Nuclease-like Activity Experiments
3.13. Isolation of pUC19 Plasmid DNA for Nuclease Experiments
3.14. Effect of the Antioxidants and Other Inhibitors on the Plasmid DNA Cleavage
3.15. Statistical Evaluations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heffeter, P.; Jungwirth, U.; Jakupec, M.; Hartinger, C.; Galanski, M.; Elbling, L.; Micksche, M.; Keppler, B.; Berger, W. Resistance against novel anticancer metal compounds: Differences and similarities. Drug Resist. Updat. 2008, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, Z. New Trends and Future Developments of Platinum-Based Antitumor Drugs. In Bioinorganic Medicinal Chemistry; Alessio, E., Ed.; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; pp. 97–149. [Google Scholar]
- Wehbe, M.; Leung, A.W.Y.; Abrams, M.J.; Orvig, C.; Bally, M.B. A Perspective-can copper complexes be developed as a novel class of therapeutics? Dalton Trans. 2017, 46, 10758–10773. [Google Scholar] [CrossRef]
- Milunović, M.N.M.; Palamarciuc, O.; Sirbu, A.; Shova, S.; Dumitrescu, D.; Dvoranová, D.; Rapta, P.; Petrasheuskaya, T.V.; Enyedy, E.A.; Spengler, G.; et al. Insight into the Anticancer Activity of Copper(II) 5-Methylenetrimethylammonium-Thiosemicarbazonates and Their Interaction with Organic Cation Transporters. Biomolecules 2020, 10, 1213. [Google Scholar] [CrossRef] [PubMed]
- Padnya, P.; Shibaeva, K.; Arsenyev, M.; Baryshnikova, S.; Terenteva, O.; Shiabiev, I.; Khannanov, A.; Boldyrev, A.; Gerasimov, A.; Grishaev, D.; et al. Catechol-Containing Schiff Bases on Thiacalixarene: Synthesis, Copper (II) Recognition, and Formation of Organic-Inorganic Copper-Based Materials. Molecules 2021, 26, 2334. [Google Scholar] [CrossRef]
- Kordestani, N.; Rudbari, H.A.; Fernandes, A.R.; Raposo, L.R.; Luz, A.; Baptista, P.V.; Bruno, G.; Scopelliti, R.; Fateminia, Z.; Micale, N.; et al. Copper(ii) complexes with tridentate halogen-substituted Schiff base ligands: Synthesis, crystal structures and investigating the effect of halogenation, leaving groups and ligand flexibility on antiproliferative activities. Dalton Trans. 2021, 50, 3990–4007. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Xu, P.; Zhi, S.; Zhu, S.; Guo, Y.; Yang, G. Integrated transcriptome and in vitro analysis revealed antiproliferative effects on human gastric cancer cells by a benzimidazole-quinoline copper(II) complex. Process Biochem. 2021, 102, 286–295. [Google Scholar] [CrossRef]
- Karges, J.; Xiong, K.; Blacque, O.; Chao, H.; Gasser, G. Highly cytotoxic copper(II) terpyridine complexes as anticancer drug candidates. Inorg. Chim. Acta 2021, 516, 120137. [Google Scholar] [CrossRef]
- Rodrigues, J.A.O.; de Oliveira Neto, J.G.; da Silva de Barros, A.O.; Ayala, A.P.; Santos-Oliveira, R.; de Menezes, A.S.; de Sousa, F.F. Copper(II):phenanthroline complexes with l-asparagine and l-methionine: Synthesis, crystal structure and in-vitro cytotoxic effects on prostate, breast and melanoma cancer cells. Polyhedron 2020, 191, 114807. [Google Scholar] [CrossRef]
- Teles, R.H.G.; Graminha, A.E.; Rivera-Cruz, C.M.; Nakahata, D.H.; Formiga, A.L.B.; Corbi, P.P.; Figueiredo, M.L.; Cominetti, M.R. Copper transporter 1 affinity as a delivery strategy to improve the cytotoxic profile of rationally designed copper(II) complexes for cancer treatment. Toxicol. In Vitro 2020, 67, 104922. [Google Scholar] [CrossRef]
- Bravo-Gomez, M.E.; Garcia-Ramos, J.C.; Gracia-Mora, I.; Ruiz-Azuara, L. Antiproliferative activity and QSAR study of copper(II) mixed chelate [Cu(N-N)(acetylacetonato)]NO3 and [Cu(N-N)(glycinato)]NO3 complexes, (Casiopeinas). J. Inorg. Biochem. 2009, 103, 299–309. [Google Scholar] [CrossRef]
- Azuara, L.R. Process to Obtain New Mixed Copper Aminoactidate Complexes from Phenylate Phenathrolines to be Used as Anticancerigenic Agents. US5107005A, 21 April 1992. [Google Scholar]
- Galindo-Murillo, R.; Garcia-Ramos, J.C.; Ruiz-Azuara, L.; Cheatham, T.E.; Cortes-Guzman, F. Intercalation processes of copper complexes in DNA. Nucleic. Acids. Res. 2015, 43, 5364–5376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trávníček, Z.; Vančo, J.; Hošek, J.; Buchtík, R.; Dvořák, Z. Cellular responses induced by Cu(II) quinolinonato complexes in human tumor and hepatic cells. Chem. Cent. J. 2012, 6, 160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchtík, R.; Trávníček, Z.; Vančo, J.; Herchel, R.; Dvořák, Z. Synthesis, characterization, DNA interaction and cleavage, and in vitro cytotoxicity of copper(ii) mixed-ligand complexes with 2-phenyl-3-hydroxy-4(1H)- quinolinone. Dalton Trans. 2011, 40, 9404–9412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchtík, R.; Trávníček, Z.; Vančo, J. In vitro cytotoxicity, DNA cleavage and SOD-mimic activity of copper(II) mixed-ligand quinolinonato complexes. J. Inorg. Biochem. 2012, 116, 163–171. [Google Scholar] [CrossRef]
- Křikavová, R.; Vančo, J.; Trávníček, Z.; Buchtík, R.; Dvořák, Z. Copper(II) quinolinonato-7-carboxamido complexes as potent antitumor agents with broad spectra and selective effects. RSC Adv. 2016, 6, 3899–3909. [Google Scholar] [CrossRef] [Green Version]
- Křikavová, R.; Vančo, J.; Trávníček, Z.; Hutyra, J.; Dvořák, Z. Design and characterization of highly in vitro antitumor active ternary copper(II) complexes containing 2′-hydroxychalcone ligands. J. Inorg. Biochem. 2016, 163, 8–17. [Google Scholar] [CrossRef]
- Brezani, V.; Smejkal, K.; Hosek, J.; Tomasova, V. Anti-inflammatory Natural Prenylated Phenolic Compounds—Potential Lead Substances. Curr. Med. Chem. 2018, 25, 1094–1159. [Google Scholar] [CrossRef]
- Orazbekov, Y.; Ibrahim, M.A.; Mombekov, S.; Srivedavyasasri, R.; Datkhayev, U.; Makhatov, B.; Chaurasiya, N.D.; Tekwani, B.L.; Ross, S.A. Isolation and Biological Evaluation of Prenylated Flavonoids from Maclura pomifera. Evid-Based Compl Alt 2018, 2018, 1370368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hošek, J.; Šmejkal, K. Flavonoids as Anti-inflammatory Agents. In Compendium of Inflammatory Diseases; Parnham, M.J., Ed.; Springer: Basel, Switzerland, 2016; pp. 482–497. [Google Scholar]
- Selvaraj, S.; Krishnaswamy, S.; Devashya, V.; Sethuraman, S.; Krishnan, U.M. Flavonoid-Metal Ion Complexes: A Novel Class of Therapeutic Agents. Med. Res. Rev. 2014, 34, 677–702. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R.; Yang, R.; Young, J.C. Antioxidant isoflavones in Osage orange, Maclura pomifera (Raf. ) Schneid. J. Agric. Food Chem. 2003, 51, 6445–6451. [Google Scholar] [CrossRef]
- Vesela, D.; Kubinova, R.; Muselik, J.; Zemlicka, M.; Suchy, V. Antioxidative and EROD activities of osajin and pomiferin. Fitoterapia 2004, 75, 209–211. [Google Scholar] [CrossRef]
- Janostikova, E.; Bartosikova, L.; Necas, J.; Jurica, J.; Florian, T.; Bartosik, T.; Klusakova, J.; Suchy, V.; Liskova, M.; Frydrych, M. Effects of pomiferin premedication on the antioxidant status of rats with ischemia-reperfused kidney. Acta. Vet. Brno. 2005, 74, 557–564. [Google Scholar] [CrossRef] [Green Version]
- Necas, J.; Bartosikova, L.; Florian, T.; Klusakova, J.; Suchy, V.; Janostikova, E.; Bartosik, T.; El Naggar, E.B. Protective effects of flavonoid pomiferin on heart ischemia-reperfusion. Acta. Vet. Brno. 2007, 76, 363–370. [Google Scholar] [CrossRef] [Green Version]
- Hosek, J.; Toniolo, A.; Neuwirth, O.; Bolego, C. Prenylated and Geranylated Flavonoids Increase Production of Reactive Oxygen Species in Mouse Macrophages but Inhibit the Inflammatory Response. J. Nat. Prod. 2013, 76, 1586–1591. [Google Scholar] [CrossRef]
- Son, I.H.; Chung, I.M.; Lee, S.I.; Yang, H.D.; Moon, H.I. Pomiferin, histone deacetylase inhibitor isolated from the fruits of Maclura pomifera. Bioorg. Med. Chem. Lett. 2007, 17, 4753–4755. [Google Scholar] [CrossRef] [PubMed]
- Orhan, I.; Senol, F.S.; Kartal, M.; Dvorska, M.; Zemlicka, M.; Smejkal, K.; Mokry, P. Cholinesterase inhibitory effects of the extracts and compounds of Maclura pomifera (Rafin.) Schneider. Food Chem. Toxicol. 2009, 47, 1747–1751. [Google Scholar] [CrossRef]
- Bozkurt, I.; Dilek, E.; Erol, H.S.; Cakir, A.; Hamzaoglu, E.; Koc, M.; Keles, O.N.; Halici, M.B. Investigation on the effects of pomiferin from Maclura pomifera on indomethacin-induced gastric ulcer: An experimental study in rats. Med. Chem. Res. 2017, 26, 2048–2056. [Google Scholar] [CrossRef]
- Yang, R.; Hanwell, H.; Zhang, J.; Tsao, R.; Meckling, K.A. Antiproliferative Activity of Pomiferin in Normal (MCF-10A) and Transformed (MCF-7) Breast Epithelial Cells. J. Agric. Food Chem. 2011, 59, 13328–13336. [Google Scholar] [CrossRef] [PubMed]
- Abourashed, E.A.; Abraha, A.; Khan, S.I.; McCants, T.; Awan, S. Potential of Horse Apple Isoflavones in Targeting Inflammation and Tau Protein Fibrillization. Nat. Prod. Commun. 2015, 10, 1577–1580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chokchaichamnankit, D.; Kongjinda, V.; Khunnawutmanotham, N.; Chimnoi, N.; Pisutcharoenpong, S.; Techasakul, S. Prenylated Flavonoids from the Leaves of Derris malaccensis and their Cytotoxicity. Nat. Prod. Commun. 2011, 6, 1103–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribaudo, G.; Coghi, P.; Zanforlin, E.; Law, B.Y.K.; Wil, Y.Y.J.; Han, Y.; Qiu, A.C.; Qu, Y.Q.; Zagotto, G.; Wong, V.K.W. Semi-synthetic isoflavones as BACE-1 inhibitors against Alzheimer’s disease. Bioorg. Chem. 2019, 87, 474–483. [Google Scholar] [CrossRef]
- Spoerlein, C.; Mahal, K.; Schmidt, H.; Schobert, R. Effects of chrysin, apigenin, genistein and their homoleptic copper(II) complexes on the growth and metastatic potential of cancer cells. J. Inorg. Biochem. 2013, 127, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Draut, H.; Rehm, T.; Begemann, G.; Schobert, R. Antiangiogenic and Toxic Effects of Genistein, Usnic Acid, and Their Copper Complexes in Zebrafish Embryos at Different Developmental Stages. Chem. Biodivers. 2017, 14, e1600302. [Google Scholar] [CrossRef]
- Pouchert, C. Aldrich® Library of Infrared Spectra, 3rd ed.; Aldrich Chemical Co.: Milwaukee, WI, USA, 1981; p. 1850. [Google Scholar]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, 5th ed.; Wiley-Interscience: Hoboken, NJ, USA, 1997; p. 400. [Google Scholar]
- Trávníček, Z.; Vančo, J.; Dvořák, Z. Heteroleptic complexes of copper with osajin or pomiferin and their use for the preparation of drugs for the treatment of cancer. CZ30 8426B6, 2020.
- Trávníček, Z.; Vančo, J.; Dvořák, Z. Heteroleptic complexes of copper with osajin or pomiferin and their utilization for the preparation of drugs for the treatment of tumour diseases. WO2021018324A1, 4 February 2020. [Google Scholar]
- Nunes, P.; Correia, I.; Marques, F.; Matos, A.P.; dos Santos, M.M.C.; Azevedo, C.G.; Capelo, J.L.; Santos, H.M.; Gama, S.; Pinheiro, T.; et al. Copper Complexes with 1,10-Phenanthroline Derivatives: Underlying Factors Affecting Their Cytotoxicity. Inorg. Chem. 2020, 59, 9116–9134. [Google Scholar] [CrossRef] [PubMed]
- Perkins, N.D.; Gilmore, T.D. Good cop, bad cop: The different faces of NF-kappa B. Cell Death Differ. 2006, 13, 759–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pahl, H.L. Activators and target genes of Rel/NF-kappa B transcription factors. Oncogene 1999, 18, 6853–6866. [Google Scholar] [CrossRef] [Green Version]
- Karin, M.; Liu, Z.; Zandi, E. AP-1 function and regulation. Curr. Opin. Cell Biol. 1997, 9, 240–246. [Google Scholar] [CrossRef]
- Zelova, H.; Hosek, J. TNF-alpha signalling and inflammation: Interactions between old acquaintances. Inflamm. Res. 2013, 62, 641–651. [Google Scholar] [CrossRef]
- Lelakova, V.; Smejkal, K.; Jakubczyk, K.; Vesely, O.; Landa, P.; Vaclavik, J.; Bobal, P.; Pizova, H.; Temml, V.; Steinacher, T.; et al. Parallel in vitro and in silico investigations into anti-inflammatory effects of non-prenylated stilbenoids. Food Chem. 2019, 285, 431–440. [Google Scholar] [CrossRef]
- Shaulian, E.; Karin, M. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 2002, 4, E131–E136. [Google Scholar] [CrossRef]
- Tummalapalli, K.; Vasavi, C.S.; Munusami, C.P.; Pathak, M.; Balamurali, M.M. Evaluation of DNA/Protein interactions and cytotoxic studies of copper(II) complexes incorporated with N, N donor ligands and terpyridine ligand. Int. J. Biol. Macromol. 2017, 95, 1254–1266. [Google Scholar] [CrossRef] [PubMed]
- Šmejkal, K.; Neuwirth, O.; Treml, J.; Hošek, J. Pro-oxidant Activity of Flavonoids and Their Possible Effects. In Recent Advances in Medicinal Plants; Pathak, M., Govil, J.N., Eds.; Studium Press LLC: Houston, TX, USA, 2016; Volume 40. [Google Scholar]
- Panhwar, Q.K.; Memon, S.; Bhanger, M.I. Synthesis, characterization, spectroscopic and antioxidation studies of Cu(II)-morin complex. J. Mol. Struct. 2010, 967, 47–53. [Google Scholar] [CrossRef]
- Jomova, K.; Hudecova, L.; Lauro, P.; Simunkova, M.; Alwasel, S.H.; Alhazza, I.M.; Valko, M. A Switch between Antioxidant and Prooxidant Properties of the Phenolic Compounds Myricetin, Morin, 3′,4′-Dihydroxyflavone, Taxifolin and 4-Hydroxy-Coumarin in the Presence of Copper(II) Ions: A Spectroscopic, Absorption Titration and DNA Damage Study. Molecules 2019, 24, 4335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014, 395, 203–230. [Google Scholar] [CrossRef] [Green Version]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Vančo, J.; Trávníček, Z.; Hošek, J.; Suchý, P. In vitro and in vivo anti-inflammatory active copper(II)-lawsone complexes. PLoS ONE 2017, 12, e0181822. [Google Scholar] [CrossRef] [Green Version]
- Matsuyama, S.; Reed, J.C. Mitochondria-dependent apoptosis and cellular pH regulation. Cell Death Differ. 2000, 7, 1155–1165. [Google Scholar] [CrossRef] [Green Version]
- Kathiresan, S.; Mugesh, S.; Annaraj, J.; Murugan, M. Mixed-ligand copper(II) Schiff base complexes: The vital role of co-ligands in DNA/protein interactions and cytotoxicity. New. J. Chem. 2017, 41, 1267–1283. [Google Scholar] [CrossRef]
- Massoud, S.S.; Perkins, R.S.; Louka, F.R.; Xu, W.; Le Roux, A.; Dutercq, Q.; Fischer, R.C.; Mautner, F.A.; Handa, M.; Hiraoka, Y.; et al. Efficient hydrolytic cleavage of plasmid DNA by chloro-cobalt(II) complexes based on sterically hindered pyridyl tripod tetraamine ligands: Synthesis, crystal structure and DNA cleavage. Dalton. Trans. 2014, 43, 10086–10103. [Google Scholar] [CrossRef] [Green Version]
- Gup, R.; Gokce, C.; Dilek, N. Seven-coordinated cobalt(II) complexes with 2,6-diacetylpyridine bis(4-hydroxybenzoylhydrazone): Synthesis, characterisation, DNA binding and cleavage properties. Supramol. Chem. 2015, 27, 629–641. [Google Scholar] [CrossRef]
- Brezani, V.; Lelakova, V.; Hassan, S.T.S.; Berchova-Bimova, K.; Novy, P.; Kloucek, P.; Marsik, P.; Dall’Acqua, S.; Hosek, J.; Smejkal, K. Anti-Infectivity against Herpes Simplex Virus and Selected Microbes and Anti-Inflammatory Activities of Compounds Isolated from Eucalyptus globulus Labill. Viruses 2018, 10, 360. [Google Scholar] [CrossRef] [Green Version]
- Massoud, S.S.; Ledet, C.C.; Junk, T.; Bosch, S.; Comba, P.; Herchel, R.; Hošek, J.; Trávníček, Z.; Fischer, R.C.; Mautner, F.A. Dinuclear metal(II)-acetato complexes based on bicompartmental 4-chlorophenolate: Syntheses, structures, magnetic properties, DNA interactions and phosphodiester hydrolysis. Dalton Trans. 2016, 45, 12933–12950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bancirova, M. Sodium azide as a specific quencher of singlet oxygen during chemiluminescent detection by luminol and Cypridina luciferin analogues. Luminescence 2011, 26, 685–688. [Google Scholar] [CrossRef] [PubMed]
- Repine, J.E.; Eaton, J.W.; Anders, M.W.; Hoidal, J.R.; Fox, R.B. Generation of Hydroxyl Radical by Enzymes, Chemicals, and Human Phagocytes Invitro - Detection with the Anti-Inflammatory Agent, Dimethyl-Sulfoxide. J. Clin. Investig. 1979, 64, 1642–1651. [Google Scholar] [CrossRef] [Green Version]





| Comp. | MCF-7 | HOS | A549 | PC-3 | A2780 | A2780R | Caco-2 | THP-1 * | Hep |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 23.8 ± 0.2 | 21.5 ± 1.4 | 20.8 ± 1.5 | 22.1 ± 1.9 | 12.1 ± 1.5 | 5.6 ± 0.3 | 13.7 ± 7.4 | 4.0 ± 1.1 | >100 |
| 2 | 29.6 ± 2.0 | 21.1 ± 1.6 | 20.9 ± 0.9 | >50 | 5.4 ± 2.4 | 5.3 ± 1.1 | 5.1 ± 1.6 | 7.9 ± 1.1 | >100 |
| 3 | 5.9 ± 0.6 | 3.5 ± 0.1 | 3.5 ± 0.2 | 13.0 ± 1.4 | 5.8 ± 2.2 | 2.9 ± 1.2 | 4.2 ± 1.9 | >10 | >100 |
| 4 | 16.0 ± 1.1 | 2.6 ± 0.5 | 8.7 ± 0.8 | 13.0 ± 1.1 | 12.8 ± 3.1 | 6.6 ± 2.3 | 22.9 ± 12.9 | 3.3 ± 1.2 | >100 |
| 5 | 3.0 ± 0.5 | 2.4 ± 0.9 | 3.1 ± 0.2 | 3.3 ± 0.1 | 2.5 ± 0.9 | 2.2 ± 0.5 | 2.6 ± 1.6 | 3.1 ± 1.1 | >100 |
| pomiferin | 22.0 ± 0.1 | 20.7 ± 0.5 | 21.2 ± 1.4 | 18.5 ± 0.7 | 8.4 ± 2.0 | 4.3 ± 1.3 | 19.8 ± 2.4 | >10 | >50 |
| cisplatin | 30.8 ± 1.8 | 30.0 ± 5.9 | >80 | >80 | 26.3 ± 2.1 | >80 | >80 | n.t. | >80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vančo, J.; Trávníček, Z.; Hošek, J.; Malina, T.; Dvořák, Z. Copper(II) Complexes Containing Natural Flavonoid Pomiferin Show Considerable In Vitro Cytotoxicity and Anti-inflammatory Effects. Int. J. Mol. Sci. 2021, 22, 7626. https://doi.org/10.3390/ijms22147626
Vančo J, Trávníček Z, Hošek J, Malina T, Dvořák Z. Copper(II) Complexes Containing Natural Flavonoid Pomiferin Show Considerable In Vitro Cytotoxicity and Anti-inflammatory Effects. International Journal of Molecular Sciences. 2021; 22(14):7626. https://doi.org/10.3390/ijms22147626
Chicago/Turabian StyleVančo, Ján, Zdeněk Trávníček, Jan Hošek, Tomáš Malina, and Zdeněk Dvořák. 2021. "Copper(II) Complexes Containing Natural Flavonoid Pomiferin Show Considerable In Vitro Cytotoxicity and Anti-inflammatory Effects" International Journal of Molecular Sciences 22, no. 14: 7626. https://doi.org/10.3390/ijms22147626
APA StyleVančo, J., Trávníček, Z., Hošek, J., Malina, T., & Dvořák, Z. (2021). Copper(II) Complexes Containing Natural Flavonoid Pomiferin Show Considerable In Vitro Cytotoxicity and Anti-inflammatory Effects. International Journal of Molecular Sciences, 22(14), 7626. https://doi.org/10.3390/ijms22147626









