Daily Lifestyle and Cutaneous Malignancies
Abstract
1. Introduction
1.1. Daily Lifestyle-Related Malignancies
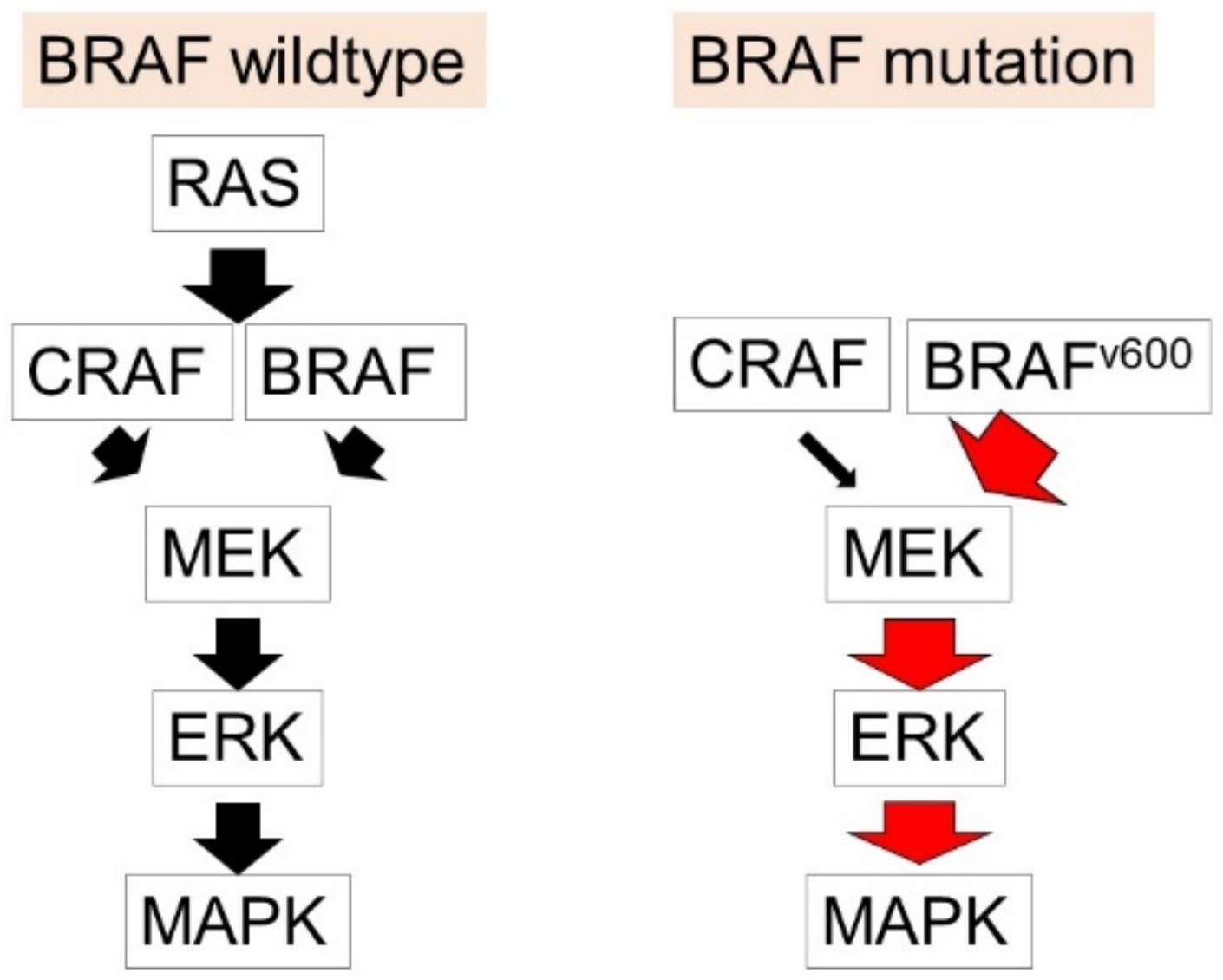
1.2. Malignant Melanoma
1.3. Squamous Cell Carcinoma
1.4. Basal Cell Carcinoma
1.5. Merkel Cell Carcinoma
2. The Daily Lifestyle Associated with the Cutaneous Malignancies
2.1. Circadian Rhythm
2.1.1. Melanoma and Circadian Rhythm
2.1.2. Squamous Cell Carcinoma/Basal Cell Carcinoma and Circadian Rhythm
2.2. Smoking
2.2.1. Melanoma and Smoking
2.2.2. Squamous Cell Carcinoma/Basal Cell Carcinoma and Smoking
2.3. Alcohol
2.3.1. Melanoma and Alcohol
2.3.2. Squamous Cell Carcinoma/Basal Cell Carcinoma and Alcohol
2.4. Dietary Fiber/Vegetables/Fruits
2.4.1. Melanoma and Dietary Fiber/Vegetables/Fruits
2.4.2. Squamous Cell Carcinoma/Basal Cell Carcinoma and Dietary Fiber/Vegetables/Fruits
2.5. Obesity
2.5.1. Melanoma and Obesity
2.5.2. Squamous Cell Carcinoma/Basal Cell Carcinoma and Obesity
2.6. Fatty Acids
2.6.1. Melanoma and Fatty Acids
2.6.2. Squamous Cell Carcinoma/Basal Cell Carcinoma and Fatty Acids
2.7. Coffee/Caffeine
2.7.1. Malignant Melanoma and Coffee/Caffeine
2.7.2. Squamous Cell Carcinoma/Basal Cell Carcinoma and Coffee/Caffeine
2.8. Ultraviolet Light Exposure
2.8.1. Melanoma and Ultraviolet Light
2.8.2. Squamous Cell Carcinoma and Ultraviolet Light
2.8.3. BCC and Ultraviolet Light
2.8.4. Merkel Cell Carcinoma and Ultraviolet Light
3. Recommendations
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dainichi, T.; Kitoh, A.; Otsuka, A.; Nakajima, S.; Nomura, T.; Kaplan, D.H.; Kabashima, K. The epithelial immune microenvironment (EIME) in atopic dermatitis and psoriasis. Nat. Immunol. 2018, 19, 1286–1298. [Google Scholar] [CrossRef]
- Sawada, Y.; Gallo, R.L. Role of Epigenetics in the Regulation of Immune Functions of the Skin. J. Investig. Dermatol. 2020. [Google Scholar] [CrossRef]
- Bernard, J.J.; Gallo, R.L.; Krutmann, J. Photoimmunology: How ultraviolet radiation affects the immune system. Nat. Rev. Immunol. 2019, 19, 688–701. [Google Scholar] [CrossRef]
- Chehade, L.; Jaafar, Z.A.; El Masri, D.; Zmerly, H.; Kreidieh, D.; Tannir, H.; Itani, L.; El Ghoch, M. Lifestyle Modification in Rheumatoid Arthritis: Dietary and Physical Activity Recommendations Based on Evidence. Curr. Rheumatol. Rev. 2019, 15, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Rippe, J.M.; Angelopoulos, T.J. Lifestyle strategies for cardiovascular risk reduction. Curr. Atheroscler. Rep. 2014, 16, 444. [Google Scholar] [CrossRef]
- Sawada, Y.; Saito-Sasaki, N.; Nakamura, M. Omega 3 Fatty Acid and Skin Diseases. Front. Immunol. 2020, 11, 623052. [Google Scholar] [CrossRef] [PubMed]
- Egawa, G.; Kabashima, K. Skin as a peripheral lymphoid organ: Revisiting the concept of skin-associated lymphoid tissues. J. Investig. Dermatol. 2011, 131, 2178–2185. [Google Scholar] [CrossRef] [PubMed]
- Kabashima, K.; Honda, T.; Ginhoux, F.; Egawa, G. The immunological anatomy of the skin. Nat. Rev. Immunol. 2019, 19, 19–30. [Google Scholar] [CrossRef]
- Broggi, G.; Verzì, A.E.; Caltabiano, R.; Micali, G.; Lacarrubba, F. Correlation Between In Vivo Reflectance Confocal Microscopy and Horizontal Histopathology in Skin Cancer: A Review. Front. Oncol. 2021, 11, 653140. [Google Scholar] [CrossRef]
- Broggi, G.; Lacarrubba, F.; Verzì, A.E.; Micali, G.; Caltabiano, R. Confocal microscopy features of patch-stage mycosis fungoides and their correlation with horizontal histopathological sections. A case series. J. Cutan. Pathol. 2019, 46, 163–165. [Google Scholar] [CrossRef]
- Broggi, G.; Verzì, A.E.; Lacarrubba, F.; Caltabiano, R.; Di Natale, A.; Micali, G. Correlation between reflectance confocal microscopy features and horizontal histopathology in cutaneous squamous cell carcinoma in situ: A case series. J. Cutan. Pathol. 2020, 47, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.; Holman, D.M.; Maguire-Eisen, M. Ultraviolet Radiation Exposure and Its Impact on Skin Cancer Risk. Semin. Oncol. Nurs. 2016, 32, 241–254. [Google Scholar] [CrossRef]
- Jenkins, R.W.; Fisher, D.E. Treatment of Advanced Melanoma in 2020 and Beyond. J. Invest. Dermatol. 2020. [Google Scholar] [CrossRef]
- Simiczyjew, A.; Dratkiewicz, E.; Mazurkiewicz, J.; Ziętek, M.; Matkowski, R.; Nowak, D. The Influence of Tumor Microenvironment on Immune Escape of Melanoma. Int. J. Mol. Sci. 2020, 21, 8359. [Google Scholar] [CrossRef]
- Sullivan, R.J.; Fisher, D.E. Understanding the biology of melanoma and therapeutic implications. Hematol. Oncol. Clin. N. Am. 2014, 28, 437–453. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yaman, B.; Akalin, T.; Kandiloğlu, G. Clinicopathological characteristics and mutation profiling in primary cutaneous melanoma. Am. J. Dermatopathol. 2015, 37, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Genomic Classification of Cutaneous Melanoma. Cell 2015, 161, 1681–1696. [CrossRef] [PubMed]
- Hauschild, A.; Grob, J.J.; Demidov, L.V.; Jouary, T.; Gutzmer, R.; Millward, M.; Rutkowski, P.; Blank, C.U.; Miller, W.H., Jr.; Kaempgen, E.; et al. Dabrafenib in BRAF-mutated metastatic melanoma: A multicentre, open-label, phase 3 randomised controlled trial. Lancet 2012, 380, 358–365. [Google Scholar] [CrossRef]
- Ottaviano, M.; Giunta, E.F.; Tortora, M.; Curvietto, M.; Attademo, L.; Bosso, D.; Cardalesi, C.; Rosanova, M.; De Placido, P.; Pietroluongo, E.; et al. BRAF Gene and Melanoma: Back to the Future. Int. J. Mol. Sci. 2021, 22, 3474. [Google Scholar] [CrossRef]
- Peyssonnaux, C.; Eychène, A. The Raf/MEK/ERK pathway: New concepts of activation. Biol. Cell 2001, 93, 53–62. [Google Scholar] [CrossRef]
- Morrison, D.K. MAP kinase pathways. Cold Spring Harb. Perspect. Biol. 2012, 4, a011254. [Google Scholar] [CrossRef]
- Davies, H.; Bignell, G.R.; Cox, C.; Stephens, P.; Edkins, S.; Clegg, S.; Teague, J.; Woffendin, H.; Garnett, M.J.; Bottomley, W.; et al. Mutations of the BRAF gene in human cancer. Nature 2002, 417, 949–954. [Google Scholar] [CrossRef]
- Wan, P.T.; Garnett, M.J.; Roe, S.M.; Lee, S.; Niculescu-Duvaz, D.; Good, V.M.; Jones, C.M.; Marshall, C.J.; Springer, C.J.; Barford, D.; et al. Mechanism of activation of the RAF-ERK signaling pathway by oncogenic mutations of B-RAF. Cell 2004, 116, 855–867. [Google Scholar] [CrossRef]
- Long, G.V.; Menzies, A.M.; Nagrial, A.M.; Haydu, L.E.; Hamilton, A.L.; Mann, G.J.; Hughes, T.M.; Thompson, J.F.; Scolyer, R.A.; Kefford, R.F. Prognostic and clinicopathologic associations of oncogenic BRAF in metastatic melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2011, 29, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Hayward, N.K.; Wilmott, J.S.; Waddell, N.; Johansson, P.A.; Field, M.A.; Nones, K.; Patch, A.M.; Kakavand, H.; Alexandrov, L.B.; Burke, H.; et al. Whole-genome landscapes of major melanoma subtypes. Nature 2017, 545, 175–180. [Google Scholar] [CrossRef]
- Beadling, C.; Jacobson-Dunlop, E.; Hodi, F.S.; Le, C.; Warrick, A.; Patterson, J.; Town, A.; Harlow, A.; Cruz, F., 3rd; Azar, S.; et al. KIT gene mutations and copy number in melanoma subtypes. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2008, 14, 6821–6828. [Google Scholar] [CrossRef]
- Curtin, J.A.; Busam, K.; Pinkel, D.; Bastian, B.C. Somatic activation of KIT in distinct subtypes of melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006, 24, 4340–4346. [Google Scholar] [CrossRef] [PubMed]
- Orlow, I.; Begg, C.B.; Cotignola, J.; Roy, P.; Hummer, A.J.; Clas, B.A.; Mujumdar, U.; Canchola, R.; Armstrong, B.K.; Kricker, A.; et al. CDKN2A germline mutations in individuals with cutaneous malignant melanoma. J. Investig. Dermatol. 2007, 127, 1234–1243. [Google Scholar] [CrossRef]
- Spain, L.; Diem, S.; Larkin, J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat. Rev. 2016, 44, 51–60. [Google Scholar] [CrossRef]
- Delanoy, N.; Michot, J.M.; Comont, T.; Kramkimel, N.; Lazarovici, J.; Dupont, R.; Champiat, S.; Chahine, C.; Robert, C.; Herbaux, C.; et al. Haematological immune-related adverse events induced by anti-PD-1 or anti-PD-L1 immunotherapy: A descriptive observational study. Lancet Haematol. 2019, 6, e48–e57. [Google Scholar] [CrossRef]
- Oda, T.; Sawada, Y.; Okada, E.; Yamaguchi, T.; Ohmori, S.; Haruyama, S.; Yoshioka, M.; Nakamura, M. Hypopituitarism and hypothyroidism following atrioventricular block during nivolumab treatment. J. Dermatol. 2017, 44, e144–e145. [Google Scholar] [CrossRef]
- Zimmer, L.; Livingstone, E.; Hassel, J.C.; Fluck, M.; Eigentler, T.; Loquai, C.; Haferkamp, S.; Gutzmer, R.; Meier, F.; Mohr, P.; et al. Adjuvant nivolumab plus ipilimumab or nivolumab monotherapy versus placebo in patients with resected stage IV melanoma with no evidence of disease (IMMUNED): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 395, 1558–1568. [Google Scholar] [CrossRef]
- Saito, R.; Sawada, Y.; Nakamura, M. Immune Profile Analysis in Peripheral Blood and Tumor in Patients with Malignant Melanoma. Int. J. Mol. Sci. 2021, 22, 1957. [Google Scholar] [CrossRef] [PubMed]
- Mashima, E.; Inoue, A.; Sakuragi, Y.; Yamaguchi, T.; Sasaki, N.; Hara, Y.; Omoto, D.; Ohmori, S.; Haruyama, S.; Sawada, Y.; et al. Nivolumab in the treatment of malignant melanoma: Review of the literature. Oncotargets Ther. 2015, 8, 2045–2051. [Google Scholar]
- Saito, R.; Sawada, Y.; Saito-Sasaki, N.; Yamamoto, K.; Yoshioka, H.; Ohmori, S.; Yoshioka, M.; Okada, E.; Nakamura, M. Profile fluctuation of peripheral blood in advanced melanoma patients treated with nivolumab. J. Dermatol. 2018, 45, 1452–1455. [Google Scholar] [CrossRef] [PubMed]
- Nonomura, Y.; Otsuka, A.; Nakashima, C.; Seidel, J.A.; Kitoh, A.; Dainichi, T.; Nakajima, S.; Sawada, Y.; Matsushita, S.; Aoki, M.; et al. Peripheral blood Th9 cells are a possible pharmacodynamic biomarker of nivolumab treatment efficacy in metastatic melanoma patients. Oncoimmunology 2016, 5, e1248327. [Google Scholar] [CrossRef]
- Lomas, A.; Leonardi-Bee, J.; Bath-Hextall, F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br. J. Dermatol. 2012, 166, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Corchado-Cobos, R.; García-Sancha, N.; González-Sarmiento, R.; Pérez-Losada, J.; Cañueto, J. Cutaneous Squamous Cell Carcinoma: From Biology to Therapy. Int. J. Mol. Sci. 2020, 21, 2956. [Google Scholar] [CrossRef]
- Burton, K.A.; Ashack, K.A.; Khachemoune, A. Cutaneous Squamous Cell Carcinoma: A Review of High-Risk and Metastatic Disease. Am. J. Clin. Dermatol. 2016, 17, 491–508. [Google Scholar] [CrossRef]
- Claveau, J.; Archambault, J.; Ernst, D.S.; Giacomantonio, C.; Limacher, J.J.; Murray, C.; Parent, F.; Zloty, D. Multidisciplinary management of locally advanced and metastatic cutaneous squamous cell carcinoma. Curr. Oncol. 2020, 27, e399–e407. [Google Scholar] [CrossRef]
- Li, G.; Ho, V.C.; Berean, K.; Tron, V.A. Ultraviolet radiation induction of squamous cell carcinomas in p53 transgenic mice. Cancer Res. 1995, 55, 2070–2074. [Google Scholar]
- Becker, J.C.; Zur Hausen, A. Cells of origin in skin cancer. J. Investig. Dermatol. 2014, 134, 2491–2493. [Google Scholar] [CrossRef]
- Sekulic, A.; Migden, M.R.; Oro, A.E.; Dirix, L.; Lewis, K.D.; Hainsworth, J.D.; Solomon, J.A.; Yoo, S.; Arron, S.T.; Friedlander, P.A.; et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N. Engl. J. Med. 2012, 366, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ping, X.L.; Lee, P.K.; Wu, X.L.; Yao, Y.J.; Zhang, M.J.; Silvers, D.N.; Ratner, D.; Malhotra, R.; Peacocke, M.; et al. Role of PTCH and p53 genes in early-onset basal cell carcinoma. Am. J. Pathol. 2001, 158, 381–385. [Google Scholar] [CrossRef]
- Schmitt, J.; Haufe, E.; Trautmann, F.; Schulze, H.J.; Elsner, P.; Drexler, H.; Bauer, A.; Letzel, S.; John, S.M.; Fartasch, M.; et al. Is ultraviolet exposure acquired at work the most important risk factor for cutaneous squamous cell carcinoma? Results of the population-based case-control study FB-181. Br. J. Dermatol. 2018, 178, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Ouhtit, A.; Nakazawa, H.; Armstrong, B.K.; Kricker, A.; Tan, E.; Yamasaki, H.; English, D.R. UV-radiation-specific p53 mutation frequency in normal skin as a predictor of risk of basal cell carcinoma. J. Natl. Cancer Inst. 1998, 90, 523–531. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pipas, J.M.; Levine, A.J. Role of T antigen interactions with p53 in tumorigenesis. Semin. Cancer Biol. 2001, 11, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Sastre-Garau, X.; Peter, M.; Avril, M.F.; Laude, H.; Couturier, J.; Rozenberg, F.; Almeida, A.; Boitier, F.; Carlotti, A.; Couturaud, B.; et al. Merkel cell carcinoma of the skin: Pathological and molecular evidence for a causative role of MCV in oncogenesis. J. Pathol. 2009, 218, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Shuda, M.; Feng, H.; Kwun, H.J.; Rosen, S.T.; Gjoerup, O.; Moore, P.S.; Chang, Y. T antigen mutations are a human tumor-specific signature for Merkel cell polyomavirus. Proc. Natl. Acad. Sci. USA 2008, 105, 16272–16277. [Google Scholar] [CrossRef]
- Becker, J.C.; Stang, A.; DeCaprio, J.A.; Cerroni, L.; Lebbé, C.; Veness, M.; Nghiem, P. Merkel cell carcinoma. Nat. Rev. Dis. Primers 2017, 3, 17077. [Google Scholar] [CrossRef]
- Hettwer, S.; Besic Gyenge, E.; Obermayer, B. Influence of cosmetic formulations on the skin’s circadian clock. Int. J. Cosmet. Sci. 2020, 42, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Ando, N.; Nakamura, Y.; Aoki, R.; Ishimaru, K.; Ogawa, H.; Okumura, K.; Shibata, S.; Shimada, S.; Nakao, A. Circadian Gene Clock Regulates Psoriasis-Like Skin Inflammation in Mice. J. Investig. Dermatol. 2015, 135, 3001–3008. [Google Scholar] [CrossRef] [PubMed]
- Schernhammer, E.S.; Razavi, P.; Li, T.Y.; Qureshi, A.A.; Han, J. Rotating night shifts and risk of skin cancer in the nurses’ health study. J. Natl. Cancer Inst. 2011, 103, 602–606. [Google Scholar] [CrossRef]
- Yousef, E.; Mitwally, N.; Noufal, N.; Tahir, M.R. Shift work and risk of skin cancer: A systematic review and meta-analysis. Sci. Rep. 2020, 10, 2012. [Google Scholar] [CrossRef]
- Aiello, I.; Fedele, M.L.M.; Román, F.; Marpegan, L.; Caldart, C.; Chiesa, J.J.; Golombek, D.A.; Finkielstein, C.V.; Paladino, N. Circadian disruption promotes tumor-immune microenvironment remodeling favoring tumor cell proliferation. Sci. Adv. 2020, 6, eaaz4530. [Google Scholar] [CrossRef]
- De Assis, L.V.; Moraes, M.N.; da Silveira Cruz-Machado, S.; Castrucci, A.M. The effect of white light on normal and malignant murine melanocytes: A link between opsins, clock genes, and melanogenesis. Biochim. Biophys. Acta 2016, 1863, 1119–1133. [Google Scholar] [CrossRef] [PubMed]
- De Assis, L.V.M.; Moraes, M.N.; Magalhães-Marques, K.K.; Kinker, G.S.; da Silveira Cruz-Machado, S.; Castrucci, A.M.L. Non-Metastatic Cutaneous Melanoma Induces Chronodisruption in Central and Peripheral Circadian Clocks. Int. J. Mol. Sci. 2018, 19, 1065. [Google Scholar] [CrossRef]
- Lengyel, Z.; Lovig, C.; Kommedal, S.; Keszthelyi, R.; Szekeres, G.; Battyáni, Z.; Csernus, V.; Nagy, A.D. Altered expression patterns of clock gene mRNAs and clock proteins in human skin tumors. Tumour Biol. 2013, 34, 811–819. [Google Scholar] [CrossRef]
- De Assis, L.V.M.; Kinker, G.S.; Moraes, M.N.; Markus, R.P.; Fernandes, P.A.; Castrucci, A.M.L. Expression of the Circadian Clock Gene BMAL1 Positively Correlates with Antitumor Immunity and Patient Survival in Metastatic Melanoma. Front. Oncol. 2018, 8, 185. [Google Scholar] [CrossRef]
- Kiessling, S.; Beaulieu-Laroche, L.; Blum, I.D.; Landgraf, D.; Welsh, D.K.; Storch, K.F.; Labrecque, N.; Cermakian, N. Enhancing circadian clock function in cancer cells inhibits tumor growth. BMC Biol. 2017, 15, 13. [Google Scholar] [CrossRef]
- Hamilton, N.; Diaz-de-Cerio, N.; Whitmore, D. Impaired light detection of the circadian clock in a zebrafish melanoma model. Cell. Cycle 2015, 14, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Li, H.X.; Fu, X.J.; Yang, K.; Chen, D.; Tang, H.; Zhao, Q. The clock gene PER1 suppresses expression of tumor-related genes in human oral squamous cell carcinoma. Oncotarget 2016, 7, 20574–20583. [Google Scholar] [CrossRef]
- Zhao, N.; Yang, K.; Yang, G.; Chen, D.; Tang, H.; Zhao, D.; Zhao, C. Aberrant expression of clock gene period1 and its correlations with the growth, proliferation and metastasis of buccal squamous cell carcinoma. PLoS ONE 2013, 8, e55894. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Yang, Y.; Tang, H.; Yang, K. Loss of the clock gene Per1 promotes oral squamous cell carcinoma progression via the AKT/mTOR pathway. Cancer Sci. 2020, 111, 1542–1554. [Google Scholar] [CrossRef]
- Chen, R.; Yang, K.; Zhao, N.B.; Zhao, D.; Chen, D.; Zhao, C.R.; Tang, H. Abnormal expression of PER1 circadian-clock gene in oral squamous cell carcinoma. Oncotargets Ther. 2012, 5, 403–407. [Google Scholar]
- Hsu, C.M.; Lin, S.F.; Lu, C.T.; Lin, P.M.; Yang, M.Y. Altered expression of circadian clock genes in head and neck squamous cell carcinoma. Tumour Biol. 2012, 33, 149–155. [Google Scholar] [CrossRef]
- Fu, X.J.; Li, H.X.; Yang, K.; Chen, D.; Tang, H. The important tumor suppressor role of PER1 in regulating the cyclin-CDK-CKI network in SCC15 human oral squamous cell carcinoma cells. Oncotargets Ther. 2016, 9, 2237–2245. [Google Scholar]
- Zhao, Q.; Zheng, G.; Yang, K.; Ao, Y.R.; Su, X.L.; Li, Y.; Lv, X.Q. The clock gene PER1 plays an important role in regulating the clock gene network in human oral squamous cell carcinoma cells. Oncotargets 2016, 7, 70290–70302. [Google Scholar] [CrossRef] [PubMed]
- Janich, P.; Pascual, G.; Merlos-Suárez, A.; Batlle, E.; Ripperger, J.; Albrecht, U.; Cheng, H.Y.; Obrietan, K.; Di Croce, L.; Benitah, S.A. The circadian molecular clock creates epidermal stem cell heterogeneity. Nature 2011, 480, 209–214. [Google Scholar] [CrossRef]
- Xiong, H.; Yang, Y.; Yang, K.; Zhao, D.; Tang, H.; Ran, X. Loss of the clock gene PER2 is associated with cancer development and altered expression of important tumor-related genes in oral cancer. Int. J. Oncol. 2018, 52, 279–287. [Google Scholar] [CrossRef]
- Liu, H.; Gong, X.; Yang, K. Overexpression of the clock gene Per2 suppresses oral squamous cell carcinoma progression by activating autophagy via the PI3K/AKT/mTOR pathway. J. Cancer 2020, 11, 3655–3666. [Google Scholar] [CrossRef]
- Wang, Q.; Ao, Y.; Yang, K.; Tang, H.; Chen, D. Circadian clock gene Per2 plays an important role in cell proliferation, apoptosis and cell cycle progression in human oral squamous cell carcinoma. Oncol. Rep. 2016, 35, 3387–3394. [Google Scholar] [CrossRef]
- Robb, C.T.; McSorley, H.J.; Lee, J.; Aoki, T.; Yu, C.; Crittenden, S.; Astier, A.; Felton, J.M.; Parkinson, N.; Ayele, A.; et al. Prostaglandin E(2) stimulates adaptive IL-22 production and promotes allergic contact dermatitis. J. Allergy Clin. Immunol. 2018, 141, 152–162. [Google Scholar] [CrossRef]
- Su, X.; Chen, D.; Yang, K.; Zhao, Q.; Zhao, D.; Lv, X.; Ao, Y. The circadian clock gene PER2 plays an important role in tumor suppression through regulating tumor-associated genes in human oral squamous cell carcinoma. Oncol. Rep. 2017, 38, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Cheng, B.; Xie, M.; Chen, Y.; Zhao, J.; Zhou, X.; Chen, L. Circadian Clock Gene Bmal1 Inhibits Tumorigenesis and Increases Paclitaxel Sensitivity in Tongue Squamous Cell Carcinoma. Cancer Res. 2017, 77, 532–544. [Google Scholar] [CrossRef] [PubMed]
- Godlaski, T.M. Holy smoke: Tobacco use among native American tribes in North America. Subst. Use Misuse 2013, 48, 1–8. [Google Scholar] [CrossRef]
- Hackshaw, A.; Morris, J.K.; Boniface, S.; Tang, J.L.; Milenković, D. Low cigarette consumption and risk of coronary heart disease and stroke: Meta-analysis of 141 cohort studies in 55 study reports. BMJ 2018, 360, j5855. [Google Scholar] [CrossRef]
- Tromp, J.; Paniagua, S.M.A.; Lau, E.S.; Allen, N.B.; Blaha, M.J.; Gansevoort, R.T.; Hillege, H.L.; Lee, D.E.; Levy, D.; Ramachandran, V.S.; et al. Age dependent associations of risk factors with heart failure: Pooled population based cohort study. BMJ 2021, 372, n461. [Google Scholar] [CrossRef]
- Parsons, A.; Daley, A.; Begh, R.; Aveyard, P. Influence of smoking cessation after diagnosis of early stage lung cancer on prognosis: Systematic review of observational studies with meta-analysis. BMJ 2010, 340, b5569. [Google Scholar] [CrossRef]
- Sondermeijer, L.; Lamboo, L.G.E.; de Waal, A.C.; Galesloot, T.E.; Kiemeney, L.; van Rossum, M.; Aben, K.H. Cigarette Smoking and the Risk of Cutaneous Melanoma: A Case-Control Study. Dermatology 2020, 236, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Dusingize, J.C.; Olsen, C.M.; Pandeya, N.; Thompson, B.S.; Webb, P.M.; Green, A.C.; Neale, R.E.; Whiteman, D.C. Smoking and Cutaneous Melanoma: Findings from the QSkin Sun and Health Cohort Study. Cancer Epidemiol. Biomark. Prev. 2018, 27, 874–881. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.A.G.; Dobbs, T.D.; Griffiths, R.; Song, J.; Akbari, A.; Whitaker, S.; Watkins, A.; Langan, S.M.; Hutchings, H.A.; Lyons, R.A.; et al. The association of smoking and socioeconomic status on cutaneous melanoma: A population-based, data-linkage, case-control study. Br. J. Dermatol. 2020, 182, 1136–1147. [Google Scholar] [CrossRef] [PubMed]
- Tejera-Vaquerizo, A.; Descalzo-Gallego, M.A.; Traves, V.; Requena, C.; Bolumar, I.; Pla, A.; Nagore, E. No association between smoking and sentinel lymph node metastasis and survival in cutaneous melanoma. J. Eur. Acad. Dermatol. Venereol. 2019, 33, 2283–2290. [Google Scholar] [CrossRef]
- Jones, M.S.; Jones, P.C.; Stern, S.L.; Elashoff, D.; Hoon, D.S.B.; Thompson, J.; Mozzillo, N.; Nieweg, O.E.; Noyes, D.; Hoekstra, H.J.; et al. The Impact of Smoking on Sentinel Node Metastasis of Primary Cutaneous Melanoma. Ann. Surg. Oncol. 2017, 24, 2089–2094. [Google Scholar] [CrossRef] [PubMed]
- De Hertog, S.A.; Wensveen, C.A.; Bastiaens, M.T.; Kielich, C.J.; Berkhout, M.J.; Westendorp, R.G.; Vermeer, B.J.; Bouwes Bavinck, J.N. Relation between smoking and skin cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2001, 19, 231–238. [Google Scholar] [CrossRef]
- Leonardi-Bee, J.; Ellison, T.; Bath-Hextall, F. Lifestyle factors of smoking, BMI and alcohol on the risk of Non-Melanoma Skin Cancer in adults: A systematic review. JBI Libr. Syst. Rev. 2012, 10, 352–398. [Google Scholar] [CrossRef]
- Pirie, K.; Beral, V.; Heath, A.K.; Green, J.; Reeves, G.K.; Peto, R.; McBride, P.; Olsen, C.M.; Green, A.C. Heterogeneous relationships of squamous and basal cell carcinomas of the skin with smoking: The UK Million Women Study and meta-analysis of prospective studies. Br. J. Cancer 2018, 119, 114–120. [Google Scholar] [CrossRef]
- Odenbro, A.; Bellocco, R.; Boffetta, P.; Lindelöf, B.; Adami, J. Tobacco smoking, snuff dipping and the risk of cutaneous squamous cell carcinoma: A nationwide cohort study in Sweden. Br. J. Cancer 2005, 92, 1326–1328. [Google Scholar] [CrossRef]
- McBride, P.; Olsen, C.M.; Green, A.C. Tobacco smoking and cutaneous squamous cell carcinoma: A 16-year longitudinal population-based study. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1778–1783. [Google Scholar] [CrossRef]
- Hughes, M.C.; Olsen, C.M.; Williams, G.M.; Green, A.C. A prospective study of cigarette smoking and basal cell carcinoma. Arch. Dermatol. Res. 2014, 306, 851–856. [Google Scholar] [CrossRef]
- Song, F.; Qureshi, A.A.; Gao, X.; Li, T.; Han, J. Smoking and risk of skin cancer: A prospective analysis and a meta-analysis. Int. J. Epidemiol. 2012, 41, 1694–1705. [Google Scholar] [CrossRef] [PubMed]
- Dusingize, J.C.; Olsen, C.M.; Pandeya, N.P.; Subramaniam, P.; Thompson, B.S.; Neale, R.E.; Green, A.C.; Whiteman, D.C. Cigarette Smoking and the Risks of Basal Cell Carcinoma and Squamous Cell Carcinoma. J. Investig. Dermatol. 2017, 137, 1700–1708. [Google Scholar] [CrossRef]
- Arafa, A.; Mostafa, A.; Navarini, A.A.; Dong, J.Y. The association between smoking and risk of skin cancer: A meta-analysis of cohort studies. Cancer Causes Control 2020, 31, 787–794. [Google Scholar] [CrossRef]
- Corona, R.; Dogliotti, E.; D’Errico, M.; Sera, F.; Iavarone, I.; Baliva, G.; Chinni, L.M.; Gobello, T.; Mazzanti, C.; Puddu, P.; et al. Risk factors for basal cell carcinoma in a Mediterranean population: Role of recreational sun exposure early in life. Arch. Dermatol. 2001, 137, 1162–1168. [Google Scholar] [CrossRef]
- Karagas, M.R.; Stukel, T.A.; Greenberg, E.R.; Baron, J.A.; Mott, L.A.; Stern, R.S. Risk of subsequent basal cell carcinoma and squamous cell carcinoma of the skin among patients with prior skin cancer. Skin Cancer Prevention Study Group. JAMA 1992, 267, 3305–3310. [Google Scholar] [CrossRef]
- Freedman, D.M.; Sigurdson, A.; Doody, M.M.; Mabuchi, K.; Linet, M.S. Risk of basal cell carcinoma in relation to alcohol intake and smoking. Cancer Epidemiol. Biomark. Prev. 2003, 12, 1540–1543. [Google Scholar]
- Gon, A.; Minelli, L. Risk factors for basal cell carcinoma in a southern Brazilian population: A case-control study. Int. J. Dermatol. 2011, 50, 1286–1290. [Google Scholar] [CrossRef]
- Leonardi-Bee, J.; Ellison, T.; Bath-Hextall, F. Smoking and the risk of nonmelanoma skin cancer: Systematic review and meta-analysis. Arch. Dermatol. 2012, 148, 939–946. [Google Scholar] [CrossRef] [PubMed]
- Erbagci, Z.; Erkiliç, S. Can smoking and/or occupational UV exposure have any role in the development of the morpheaform basal cell carcinoma? A critical role for peritumoral mast cells. Int. J. Dermatol. 2002, 41, 275–278. [Google Scholar] [CrossRef]
- Bain, C.; Green, A.; Siskind, V.; Alexander, J.; Harvey, P. Diet and melanoma. An exploratory case-control study. Ann. Epidemiol. 1993, 3, 235–238. [Google Scholar]
- Kirkpatrick, C.S.; White, E.; Lee, J.A. Case-control study of malignant melanoma in Washington State. II. Diet, alcohol, and obesity. Am. J. Epidemiol. 1994, 139, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Freedman, D.M.; Sigurdson, A.; Doody, M.M.; Rao, R.S.; Linet, M.S. Risk of melanoma in relation to smoking, alcohol intake, and other factors in a large occupational cohort. Cancer Causes Control 2003, 14, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Millen, A.E.; Tucker, M.A.; Hartge, P.; Halpern, A.; Elder, D.E.; Guerry, D.; Holly, E.A.; Sagebiel, R.W.; Potischman, N. Diet and melanoma in a case-control study. Cancer Epidemiol. Biomark. Prev. 2004, 13, 1042–1051. [Google Scholar]
- Le Marchand, L.; Saltzman, B.S.; Hankin, J.H.; Wilkens, L.R.; Franke, A.A.; Morris, S.J.; Kolonel, L.N. Sun exposure, diet, and melanoma in Hawaii Caucasians. Am. J. Epidemiol. 2006, 164, 232–245. [Google Scholar] [CrossRef] [PubMed]
- Kubo, J.T.; Henderson, M.T.; Desai, M.; Wactawski-Wende, J.; Stefanick, M.L.; Tang, J.Y. Alcohol consumption and risk of melanoma and non-melanoma skin cancer in the Women’s Health Initiative. Cancer Causes Control 2014, 25, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Osterlind, A.; Tucker, M.A.; Stone, B.J.; Jensen, O.M. The Danish case-control study of cutaneous malignant melanoma. IV. No association with nutritional factors, alcohol, smoking or hair dyes. Int. J. Cancer 1988, 42, 825–828. [Google Scholar]
- Osterlind, A. Malignant melanoma in Denmark. Occurrence and risk factors. Acta Oncol. 1990, 29, 833–854. [Google Scholar] [CrossRef]
- Naldi, L.; Gallus, S.; Tavani, A.; Imberti, G.L.; La Vecchia, C. Risk of melanoma and vitamin A, coffee and alcohol: A case-control study from Italy. Eur. J. Cancer Prev. 2004, 13, 503–508. [Google Scholar] [CrossRef]
- Rota, M.; Pasquali, E.; Bellocco, R.; Bagnardi, V.; Scotti, L.; Islami, F.; Negri, E.; Boffetta, P.; Pelucchi, C.; Corrao, G.; et al. Alcohol drinking and cutaneous melanoma risk: A systematic review and dose-risk meta-analysis. Br. J. Dermatol. 2014, 170, 1021–1028. [Google Scholar] [CrossRef]
- Fung, T.T.; Hunter, D.J.; Spiegelman, D.; Colditz, G.A.; Rimm, E.B.; Willett, W.C. Intake of alcohol and alcoholic beverages and the risk of basal cell carcinoma of the skin. Cancer Epidemiol. Biomark. Prev. 2002, 11, 1119–1122. [Google Scholar]
- Husein-Elahmed, H.; Aneiros-Fernandez, J.; Gutierrez-Salmerón, M.T.; Botella-Lopez, M.; Aneiros-Cachaza, J.; Naranjo-Sintes, R. Alcohol intake and risk of aggressive histological basal cell carcinoma: A case-control study. Eur. J. Dermatol. 2012, 22, 525–530. [Google Scholar] [CrossRef]
- Wu, S.; Li, W.Q.; Qureshi, A.A.; Cho, E. Alcohol consumption and risk of cutaneous basal cell carcinoma in women and men: 3 prospective cohort studies. Am. J. Clin. Nutr. 2015, 102, 1158–1166. [Google Scholar] [CrossRef]
- Sahl, W.J.; Glore, S.; Garrison, P.; Oakleaf, K.; Johnson, S.D. Basal cell carcinoma and lifestyle characteristics. Int. J. Dermatol. 1995, 34, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.; Dhana, A.; Okhovat, J.P.; Qureshi, A.; Keum, N.; Cho, E. Alcohol intake and risk of nonmelanoma skin cancer: A systematic review and dose-response meta-analysis. Br. J. Dermatol. 2017, 177, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L. Dietary fiber and body weight. Nutrition 2005, 21, 411–418. [Google Scholar] [CrossRef]
- Rose, D.P. Dietary fiber and breast cancer. Nutr. Cancer 1990, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- De Waure, C.; Quaranta, G.; Gualano, M.R.; Cadeddu, C.; Jovic-Vranes, A.; Djikanovic, B.; La Torre, G.; Ricciardi, W. Systematic review of studies investigating the association between dietary habits and cutaneous malignant melanoma. Public Health 2015, 129, 1099–1113. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Rice, M.S.; Park, M.K.; Chun, O.K.; Melough, M.M.; Nan, H.; Willett, W.C.; Li, W.Q.; Qureshi, A.A.; Cho, E. Intake of Furocoumarins and Risk of Skin Cancer in 2 Prospective US Cohort Studies. J. Nutr. 2020, 150, 1535–1544. [Google Scholar] [CrossRef]
- Shin, J.; Song, I.S.; Pak, J.H.; Jang, S.W. Upregulation of annexin A1 expression by butyrate in human melanoma cells induces invasion by inhibiting E-cadherin expression. Tumour Biol. 2016, 37, 14577–14584. [Google Scholar] [CrossRef] [PubMed]
- Mahamat-Saleh, Y.; Cervenka, I.; Al-Rahmoun, M.; Mancini, F.R.; Severi, G.; Ghiasvand, R.; Veierod, M.B.; Caini, S.; Palli, D.; Botteri, E.; et al. Citrus intake and risk of skin cancer in the European Prospective Investigation into Cancer and Nutrition cohort (EPIC). Eur. J. Epidemiol. 2020, 35, 1057–1067. [Google Scholar] [CrossRef]
- Wu, S.; Cho, E.; Feskanich, D.; Li, W.Q.; Sun, Q.; Han, J.; Qureshi, A.A. Citrus consumption and risk of basal cell carcinoma and squamous cell carcinoma of the skin. Carcinogenesis 2015, 36, 1162–1168. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hakim, I.A.; Harris, R.B.; Ritenbaugh, C. Citrus peel use is associated with reduced risk of squamous cell carcinoma of the skin. Nutr. Cancer 2000, 37, 161–168. [Google Scholar] [CrossRef]
- Lahmann, P.H.; Ibiebele, T.I.; Webb, P.M.; Nagle, C.M.; Whiteman, D.C. A case-control study of glycemic index, glycemic load and dietary fiber intake and risk of adenocarcinomas and squamous cell carcinomas of the esophagus: The Australian Cancer Study. BMC Cancer 2014, 14, 877. [Google Scholar] [CrossRef]
- Skowron, F.; Bérard, F.; Balme, B.; Maucort-Boulch, D. Role of obesity on the thickness of primary cutaneous melanoma. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 262–269. [Google Scholar] [CrossRef]
- Taube, M.; Peltonen, M.; Sjöholm, K.; Anveden, Å.; Andersson-Assarsson, J.C.; Jacobson, P.; Svensson, P.A.; Bergo, M.O.; Carlsson, L.M.S. Association of Bariatric Surgery with Skin Cancer Incidence in Adults with Obesity: A Nonrandomized Controlled Trial. JAMA Dermatol. 2020, 156, 38–43. [Google Scholar] [CrossRef]
- Præstegaard, C.; Kjær, S.K.; Christensen, J.; Tjønneland, A.; Halkjær, J.; Jensen, A. Obesity and risks for malignant melanoma and non-melanoma skin cancer: Results from a large Danish prospective cohort study. J. Investig. Dermatol. 2015, 135, 901–904. [Google Scholar] [CrossRef]
- Pothiawala, S.; Qureshi, A.A.; Li, Y.; Han, J. Obesity and the incidence of skin cancer in US Caucasians. Cancer Causes Control 2012, 23, 717–726. [Google Scholar] [CrossRef] [PubMed]
- De Giorgi, V.; Gori, A.; Savarese, I.; D’Errico, A.; Scarfì, F.; Papi, F.; Maio, V.; Covarelli, P.; Massi, D.; Gandini, S. Role of BMI and hormone therapy in melanoma risk: A case-control study. J. Cancer Res. Clin. Oncol. 2017, 143, 1191–1197. [Google Scholar] [CrossRef]
- Dennis, L.K.; Lowe, J.B.; Lynch, C.F.; Alavanja, M.C. Cutaneous melanoma and obesity in the Agricultural Health Study. Ann. Epidemiol. 2008, 18, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Ueharaguchi, Y.; Honda, T.; Kusuba, N.; Hanakawa, S.; Adachi, A.; Sawada, Y.; Otsuka, A.; Kitoh, A.; Dainichi, T.; Egawa, G.; et al. Thromboxane A(2) facilitates IL-17A production from Vγ4(+) γδ T cells and promotes psoriatic dermatitis in mice. J. Allergy Clin. Immunol. 2018, 142, 680–683.e2. [Google Scholar] [CrossRef]
- Honda, T.; Matsuoka, T.; Ueta, M.; Kabashima, K.; Miyachi, Y.; Narumiya, S. Prostaglandin E(2)-EP(3) signaling suppresses skin inflammation in murine contact hypersensitivity. J. Allergy Clin. Immunol. 2009, 124, 809–818.e2. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Honda, T.; Nakamizo, S.; Nakajima, S.; Nonomura, Y.; Otsuka, A.; Egawa, G.; Yoshimoto, T.; Nakamura, M.; Narumiya, S.; et al. Prostaglandin E(2) (PGE(2))-EP2 signaling negatively regulates murine atopic dermatitis-like skin inflammation by suppressing thymic stromal lymphopoietin expression. J. Allergy Clin. Immunol. 2019, 144, 1265–1273.e9. [Google Scholar] [CrossRef] [PubMed]
- Mahamat-Saleh, Y.; Hughes, M.C.B.; Miura, K.; Malt, M.K.; von Schuckmann, L.; Khosrotehrani, K.; Smithers, B.M.; Green, A.C. Patterns of Omega-3 and Omega-6 Fatty Acid Dietary Intake and Melanoma Thickness at Diagnosis. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1647–1653. [Google Scholar] [CrossRef]
- Saito-Sasaki, N.; Sawada, Y.; Mashima, E.; Yamaguchi, T.; Ohmori, S.; Yoshioka, H.; Haruyama, S.; Okada, E.; Nakamura, M. Maresin-1 suppresses imiquimod-induced skin inflammation by regulating IL-23 receptor expression. Sci. Rep. 2018, 8, 5522. [Google Scholar] [CrossRef]
- Sawada, Y.; Honda, T.; Nakamizo, S.; Otsuka, A.; Ogawa, N.; Kobayashi, Y.; Nakamura, M.; Kabashima, K. Resolvin E1 attenuates murine psoriatic dermatitis. Sci. Rep. 2018, 8, 11873. [Google Scholar] [CrossRef]
- Sawada, Y.; Honda, T.; Hanakawa, S.; Nakamizo, S.; Murata, T.; Ueharaguchi-Tanada, Y.; Ono, S.; Amano, W.; Nakajima, S.; Egawa, G.; et al. Resolvin E1 inhibits dendritic cell migration in the skin and attenuates contact hypersensitivity responses. J. Exp. Med. 2015, 212, 1921–1930. [Google Scholar] [CrossRef]
- Park, M.K.; Li, W.Q.; Qureshi, A.A.; Cho, E. Fat Intake and Risk of Skin Cancer in U.S. Adults. Cancer Epidemiol. Biomark. Prev. 2018, 27, 776–782. [Google Scholar] [CrossRef]
- Noel, S.E.; Stoneham, A.C.; Olsen, C.M.; Rhodes, L.E.; Green, A.C. Consumption of omega-3 fatty acids and the risk of skin cancers: A systematic review and meta-analysis. Int. J. Cancer 2014, 135, 149–156. [Google Scholar] [CrossRef]
- Xia, S.; Lu, Y.; Wang, J.; He, C.; Hong, S.; Serhan, C.N.; Kang, J.X. Melanoma growth is reduced in fat-1 transgenic mice: Impact of omega-6/omega-3 essential fatty acids. Proc. Natl. Acad. Sci. USA 2006, 103, 12499–12504. [Google Scholar] [CrossRef]
- Albino, A.P.; Juan, G.; Traganos, F.; Reinhart, L.; Connolly, J.; Rose, D.P.; Darzynkiewicz, Z. Cell cycle arrest and apoptosis of melanoma cells by docosahexaenoic acid: Association with decreased pRb phosphorylation. Cancer Res. 2000, 60, 4139–4145. [Google Scholar]
- Serini, S.; Zinzi, A.; Ottes Vasconcelos, R.; Fasano, E.; Riillo, M.G.; Celleno, L.; Trombino, S.; Cassano, R.; Calviello, G. Role of β-catenin signaling in the anti-invasive effect of the omega-3 fatty acid DHA in human melanoma cells. J. Dermatol. Sci. 2016, 84, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Denkins, Y.; Kempf, D.; Ferniz, M.; Nileshwar, S.; Marchetti, D. Role of omega-3 polyunsaturated fatty acids on cyclooxygenase-2 metabolism in brain-metastatic melanoma. J. Lipid. Res. 2005, 46, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Yu, X.W.; Zhu, P.; Zhang, Y.M.; Zhang, X.H.; Wang, F.; Zhang, J.J.; Yan, W.; Xi, Y.; Wan, J.B.; et al. Endogenously synthesized n-3 fatty acids in fat-1 transgenic mice prevent melanoma progression by increasing E-cadherin expression and inhibiting β-catenin signaling. Mol. Med. Rep. 2016, 14, 3476–3484. [Google Scholar] [CrossRef] [PubMed]
- Ottes Vasconcelos, R.; Serini, S.; de Souza Votto, A.P.; Santos Trindade, G.; Fanali, C.; Sgambato, A.; Calviello, G. Combination of ω-3 fatty acids and cisplatin as a potential alternative strategy for personalized therapy of metastatic melanoma: An in-vitro study. Melanoma Res. 2019, 29, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chen, C.Y.; Arita, M.; Kim, K.; Li, X.; Zhang, H.; Kang, J.X. An omega-3 polyunsaturated fatty acid derivative, 18-HEPE, protects against CXCR4-associated melanoma metastasis. Carcinogenesis 2018, 39, 1380–1388. [Google Scholar] [CrossRef]
- Bachi, A.L.; Kim, F.J.; Nonogaki, S.; Carneiro, C.R.; Lopes, J.D.; Jasiulionis, M.G.; Correa, M. Leukotriene B4 creates a favorable microenvironment for murine melanoma growth. Mol. Cancer Res. 2009, 7, 1417–1424. [Google Scholar] [CrossRef]
- Sulciner, M.L.; Serhan, C.N.; Gilligan, M.M.; Mudge, D.K.; Chang, J.; Gartung, A.; Lehner, K.A.; Bielenberg, D.R.; Schmidt, B.; Dalli, J.; et al. Resolvins suppress tumor growth and enhance cancer therapy. J. Exp. Med. 2018, 215, 115–140. [Google Scholar] [CrossRef]
- Miura, K.; Way, M.; Jiyad, Z.; Marquart, L.; Plasmeijer, E.I.; Campbell, S.; Isbel, N.; Fawcett, J.; Ferguson, L.E.; Davis, M.; et al. Omega-3 fatty acid intake and decreased risk of skin cancer in organ transplant recipients. Eur. J. Nutr. 2020, 9, 1–9. [Google Scholar] [CrossRef]
- Wallingford, S.C.; van As, J.A.; Hughes, M.C.; Ibiebele, T.I.; Green, A.C.; van der Pols, J.C. Intake of omega-3 and omega-6 fatty acids and risk of basal and squamous cell carcinomas of the skin: A longitudinal community-based study in Australian adults. Nutr. Cancer 2012, 64, 982–990. [Google Scholar] [CrossRef]
- Wallingford, S.C.; Hughes, M.C.; Green, A.C.; van der Pols, J.C. Plasma omega-3 and omega-6 concentrations and risk of cutaneous basal and squamous cell carcinomas in Australian adults. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1900–1905. [Google Scholar] [CrossRef]
- Rehman, K.; Mohd Amin, M.C.; Yuen, N.P.; Zulfakar, M.H. Immunomodulatory Effectiveness of Fish Oil and omega-3 Fatty Acids in Human Non-melanoma Skin Carcinoma Cells. J. Oleo Sci. 2016, 65, 217–224. [Google Scholar] [CrossRef]
- Ye, Y.; Scheff, N.N.; Bernabé, D.; Salvo, E.; Ono, K.; Liu, C.; Veeramachaneni, R.; Viet, C.T.; Viet, D.T.; Dolan, J.C.; et al. Anti-cancer and analgesic effects of resolvin D2 in oral squamous cell carcinoma. Neuropharmacology 2018, 139, 182–193. [Google Scholar] [CrossRef]
- Daneschvar, H.L.; Smetana, G.W.; Brindamour, L.; Bain, P.A.; Mukamal, K.J. Impact of Coffee Consumption on Physiological Markers of Cardiovascular Risk: A Systematic Review. Am. J. Med. 2020. [Google Scholar] [CrossRef]
- Park, S.Y.; Freedman, N.D.; Haiman, C.A.; Le Marchand, L.; Wilkens, L.R.; Setiawan, V.W. Prospective Study of Coffee Consumption and Cancer Incidence in Non-White Populations. Cancer Epidemiol Biomark. Prev. 2018, 27, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Micek, A.; Godos, J.; Lafranconi, A.; Marranzano, M.; Pajak, A. Caffeinated and decaffeinated coffee consumption and melanoma risk: A dose-response meta-analysis of prospective cohort studies. Int. J. Food Sci. Nutr. 2018, 69, 417–426. [Google Scholar] [CrossRef]
- Loftfield, E.; Freedman, N.D.; Graubard, B.I.; Hollenbeck, A.R.; Shebl, F.M.; Mayne, S.T.; Sinha, R. Coffee drinking and cutaneous melanoma risk in the NIH-AARP diet and health study. J. Natl. Cancer Inst. 2015, 107. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, X.; Zhang, D. Coffee consumption and the risk of cutaneous melanoma: A meta-analysis. Eur. J. Nutr. 2016, 55, 1317–1329. [Google Scholar] [CrossRef]
- Liu, J.; Shen, B.; Shi, M.; Cai, J. Higher Caffeinated Coffee Intake Is Associated with Reduced Malignant Melanoma Risk: A Meta-Analysis Study. PLoS ONE 2016, 11, e0147056. [Google Scholar] [CrossRef] [PubMed]
- Caini, S.; Masala, G.; Saieva, C.; Kvaskoff, M.; Savoye, I.; Sacerdote, C.; Hemmingsson, O.; Hammer Bech, B.; Overvad, K.; Tjønneland, A.; et al. Coffee, tea and melanoma risk: Findings from the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer 2017, 140, 2246–2255. [Google Scholar] [CrossRef]
- Miura, K.; Hughes, M.C.; Green, A.C.; van der Pols, J.C. Caffeine intake and risk of basal cell and squamous cell carcinomas of the skin in an 11-year prospective study. Eur. J. Nutr. 2014, 53, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Song, F.; Qureshi, A.A.; Han, J. Increased caffeine intake is associated with reduced risk of basal cell carcinoma of the skin. Cancer Res. 2012, 72, 3282–3289. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, L.M.; Cartmel, B.; Molinaro, A.M.; Leffell, D.J.; Bale, A.E.; Mayne, S.T. Tea, coffee, and caffeine and early-onset basal cell carcinoma in a case-control study. Eur. J. Cancer Prev. 2014, 23, 296–302. [Google Scholar] [CrossRef]
- Caini, S.; Cattaruzza, M.S.; Bendinelli, B.; Tosti, G.; Masala, G.; Gnagnarella, P.; Assedi, M.; Stanganelli, I.; Palli, D.; Gandini, S. Coffee, tea and caffeine intake and the risk of non-melanoma skin cancer: A review of the literature and meta-analysis. Eur. J. Nutr. 2017, 56, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Laikova, K.V.; Oberemok, V.V.; Krasnodubets, A.M.; Gal’chinsky, N.V.; Useinov, R.Z.; Novikov, I.A.; Temirova, Z.Z.; Gorlov, M.V.; Shved, N.A.; Kumeiko, V.V.; et al. Advances in the Understanding of Skin Cancer: Ultraviolet Radiation, Mutations, and Antisense Oligonucleotides as Anticancer Drugs. Molecules 2019, 24, 1516. [Google Scholar] [CrossRef]
- Arnold, M.; de Vries, E.; Whiteman, D.C.; Jemal, A.; Bray, F.; Parkin, D.M.; Soerjomataram, I. Global burden of cutaneous melanoma attributable to ultraviolet radiation in 2012. Int. J. Cancer 2018, 143, 1305–1314. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, N.; Yin, C.; Zhu, B.; Li, X. Ultraviolet Radiation and Melanomagenesis: From Mechanism to Immunotherapy. Front. Oncol. 2020, 10, 951. [Google Scholar] [CrossRef]
- Moshinsky, D.J.; Wogan, G.N. UV-induced mutagenesis of human p53 in a vector replicated in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 1997, 94, 2266–2271. [Google Scholar] [CrossRef]
- Viros, A.; Sanchez-Laorden, B.; Pedersen, M.; Furney, S.J.; Rae, J.; Hogan, K.; Ejiama, S.; Girotti, M.R.; Cook, M.; Dhomen, N.; et al. Ultraviolet radiation accelerates BRAF-driven melanomagenesis by targeting TP53. Nature 2014, 511, 478–482. [Google Scholar] [CrossRef]
- Menzies, A.M.; Haydu, L.E.; Visintin, L.; Carlino, M.S.; Howle, J.R.; Thompson, J.F.; Kefford, R.F.; Scolyer, R.A.; Long, G.V. Distinguishing clinicopathologic features of patients with V600E and V600K BRAF-mutant metastatic melanoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2012, 18, 3242–3249. [Google Scholar] [CrossRef] [PubMed]
- Ratushny, V.; Gober, M.D.; Hick, R.; Ridky, T.W.; Seykora, J.T. From keratinocyte to cancer: The pathogenesis and modeling of cutaneous squamous cell carcinoma. J. Clin. Investig. 2012, 122, 464–472. [Google Scholar] [CrossRef]
- Brash, D.E.; Rudolph, J.A.; Simon, J.A.; Lin, A.; McKenna, G.J.; Baden, H.P.; Halperin, A.J.; Pontén, J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc. Natl. Acad. Sci. USA 1991, 88, 10124–10128. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Park, H.J.; Baek, S.C.; Byun, D.G.; Houh, D. Mutations of the p53 and PTCH gene in basal cell carcinomas: UV mutation signature and strand bias. J. Dermatol. Sci. 2002, 29, 1–9. [Google Scholar] [CrossRef]
- Lesiak, A.; Sobolewska-Sztychny, D.; Bednarski, I.A.; Wódz, K.; Sobjanek, M.; Woźniacka, A.; Narbutt, J. Alternative activation of hedgehog pathway induced by ultraviolet B radiation: Preliminary study. Clin. Exp. Dermatol. 2018, 43, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Mogha, A.; Fautrel, A.; Mouchet, N.; Guo, N.; Corre, S.; Adamski, H.; Watier, E.; Misery, L.; Galibert, M.D. Merkel cell polyomavirus small T antigen mRNA level is increased following in vivo UV-radiation. PLoS ONE 2010, 5, e11423. [Google Scholar] [CrossRef] [PubMed]
- Girschik, J.; Thorn, K.; Beer, T.W.; Heenan, P.J.; Fritschi, L. Merkel cell carcinoma in Western Australia: A population-based study of incidence and survival. Br. J. Dermatol. 2011, 165, 1051–1057. [Google Scholar] [CrossRef]
- Wong, S.Q.; Waldeck, K.; Vergara, I.A.; Schröder, J.; Madore, J.; Wilmott, J.S.; Colebatch, A.J.; De Paoli-Iseppi, R.; Li, J.; Lupat, R.; et al. UV-Associated Mutations Underlie the Etiology of MCV-Negative Merkel Cell Carcinomas. Cancer Res. 2015, 75, 5228–5234. [Google Scholar] [CrossRef]

| Melanoma | Squamous Cell Carcinoma | Basal Cell Carcinoma | Merkel Cell Carcinoma | |
|---|---|---|---|---|
| Circadian rhythm disruption | Risk up [54] | No association [54] | Risk down [54] | |
| Smoking | Risk down [80,81,82] | Risk up [85,86,87] No association [88,89]. | Risk down [90,91,92,93] No association [85,86,94,95,96,97,98]. | |
| Alcohol | Risk up [109] | Risk up [114] | Risk up [114] | |
| Dietary fiber/vegetables/fruits | Vitamin A: Risk down [108,116,117] Furocoumarins: No association [118] | Citrus: Risk down [120] No association [118,121,122] | Furocoumarins: Risk up [118,120,121] | |
| Obesity | Risk up [124,128,129] No association [125,126,127] | Risk up [126] Risk down [127] | Risk down [126,127] | |
| Fatty acids | omega-3 fatty acid: Risk down [138] omega-6 fatty acid: Risk up [137] | Omega-3 fatty acid: No association [138] Omega-6 fatty acid: Risk up [149] | Omega-3 fatty acid: No association [138] Omega-6 fatty acid: Risk up [137] | |
| Coffee/caffeine | Risk down [154,155,156] | Risk down [160,161,162] | ||
| Ultraviolet light | Risk up [165,166] | Risk up [45,170] | Risk up [45,46] | Risk up [165,176] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawada, Y.; Nakamura, M. Daily Lifestyle and Cutaneous Malignancies. Int. J. Mol. Sci. 2021, 22, 5227. https://doi.org/10.3390/ijms22105227
Sawada Y, Nakamura M. Daily Lifestyle and Cutaneous Malignancies. International Journal of Molecular Sciences. 2021; 22(10):5227. https://doi.org/10.3390/ijms22105227
Chicago/Turabian StyleSawada, Yu, and Motonobu Nakamura. 2021. "Daily Lifestyle and Cutaneous Malignancies" International Journal of Molecular Sciences 22, no. 10: 5227. https://doi.org/10.3390/ijms22105227
APA StyleSawada, Y., & Nakamura, M. (2021). Daily Lifestyle and Cutaneous Malignancies. International Journal of Molecular Sciences, 22(10), 5227. https://doi.org/10.3390/ijms22105227





