Abstract
Abscisic acid (ABA) is a key hormone that promotes dormancy during seed development on the mother plant and after seed dispersal participates in the control of dormancy release and germination in response to environmental signals. The modulation of ABA endogenous levels is largely achieved by fine-tuning, in the different seed tissues, hormone synthesis by cleavage of carotenoid precursors and inactivation by 8′-hydroxylation. In this review, we provide an overview of the current knowledge on ABA metabolism in developing and germinating seeds; notably, how environmental signals such as light, temperature and nitrate control seed dormancy through the adjustment of hormone levels. A number of regulatory factors have been recently identified which functional relationships with major transcription factors, such as ABA INSENSITIVE3 (ABI3), ABI4 and ABI5, have an essential role in the control of seed ABA levels. The increasing importance of epigenetic mechanisms in the regulation of ABA metabolism gene expression is also described. In the last section, we give an overview of natural variations of ABA metabolism genes and their effects on seed germination, which could be useful both in future studies to better understand the regulation of ABA metabolism and to identify candidates as breeding materials for improving germination properties.
Keywords:
abscisic acid; biosynthesis; catabolism; dormancy; germination; natural variation; seed; transcription factor 1. Introduction
During seed maturation, abscisic acid (ABA) induces an adaptive trait called primary dormancy that prevents vivipary and, after seed dispersal, delays and spreads germination over time. Exogenous signals, especially temperature variations during seed set, have been described to have a major effect on the modulation of dormancy depth [1]. The dormancy depth determines the timing of germination under seasonally varying conditions at which seedling survival and growth are the most favorable [2]. Seed dormancy is often a combination of a coat-imposed dormancy due to the multiple cell layers surrounding the embryo (testa and endosperm) preventing the radicle protrusion, and embryonic dormancy, when the embryo itself is unable to induce growth [3,4]. After seed shedding, dormancy is released upon imbibition by ABA degradation, which precedes the activation of germination by a second plant hormone, gibberellin (GA). The balance between these two hormones has been shown to integrate light, temperature or nitrate signals and act antagonistically on embryo growth and endosperm weakening [5,6,7,8]. Storage or imbibition of non-dormant seeds in unfavorable conditions for germination can trigger a secondary dormancy. This is a way to prevent seeds from germinating in an untimely fashion while inducing a seasonal cycling of dormancy level in seeds [9,10].
Significant efforts have been made over the last decades to understand the ABA signaling mechanisms controlling dormancy and germination. In seeds as in vegetative tissues, ABA perception involves a multigene family encoding pyrabactin resistance/PYR-like/regulatory components of ABA receptor (PYR/PYL/RCAR), which sequester and inhibit protein phosphatases 2C (PP2C), when ABA is present. PP2C inactivation allows phosphorylation of SNF1-related kinases 2 (SnRK2), which in turn phosphorylate bZIP transcription factors of the ABI5/AREB/ABF family, which bind to ABA response elements (ABRE) in promoter sequences of ABA-inducible genes [11]. The germination phenotypes of PYR/PYL/RCAR, PP2C or SnRK2 multiple mutants suggest that the PYR/PP2C/SnRK2 phosphorylation cascade operates in dormancy regulation [12,13]. Moreover, recent studies reported the interaction of DELAY OF DORMANCY (DOG1), a major regulator of seed dormancy, for which molecular function remains elusive, with a subset of clade A PP2C phosphatases, resulting in their inhibition [14,15,16].
Research on the metabolic pathway of ABA has a long history, and its relationship with germination and dormancy has been broadly explored [17,18,19,20]. Nevertheless, novel ABA metabolism-related genes and factors involved in their regulation have been discovered in recent years. In this review, we first summarize current knowledge about the molecular characterization of genes of the ABA metabolism pathway. How the ABA metabolism-related genes are involved in the induction of dormancy during seed development, as well as in the dormancy release and germination after imbibition is then discussed, with particular emphasis on specific regulatory factors that fine-tune ABA levels, modulate dormancy depth and control germination, in response to major environmental cues. Finally, we outline findings on the natural variations of genes affecting ABA metabolism in seeds and summarize how these variations affect ABA metabolism and seed germination for a given species.
2. An Overview of the ABA Metabolism Pathway
2.1. Carotenoid Interconversion and Cleavage in Plastids
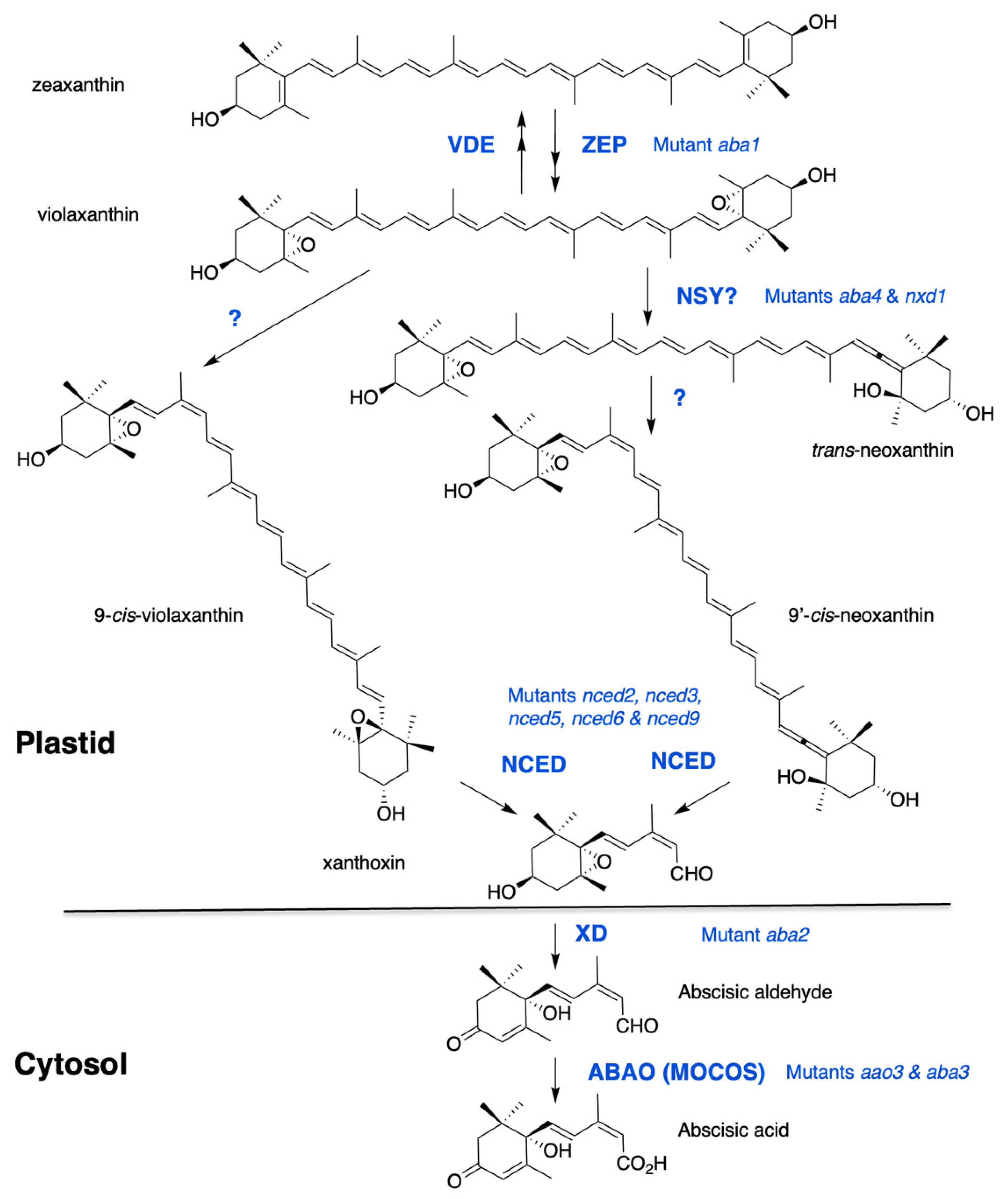
ABA is a sesquiterpenoid that is synthesized in plants via the cleavage of C40 carotenoid precursors. The early steps of ABA biosynthesis take place in plastids and the direct precursors are oxygenated carotenoids, also called xanthophylls. The first carotenoid, cis-phytoene, derives from a C5 precursor, common to all isoprenoids, isopentenyl diphosphate (IPP). Successive desaturation and isomerization reactions lead to the formation of all-trans-lycopene, in which β-cyclization gives rise to β-carotene. Zeaxanthin is produced by the hydroxylation of β-carotene and this first xanthophyll is often chosen as the starting point of the ABA biosynthesis pathway, as shown in Figure 1. Indeed, the first Arabidopsis thaliana mutants identified on the basis of their ABA-deficient phenotypes were affected in zeaxanthin epoxidation; nevertheless, carotenoid cleavage is now considered to be the first committed step in ABA synthesis [21].
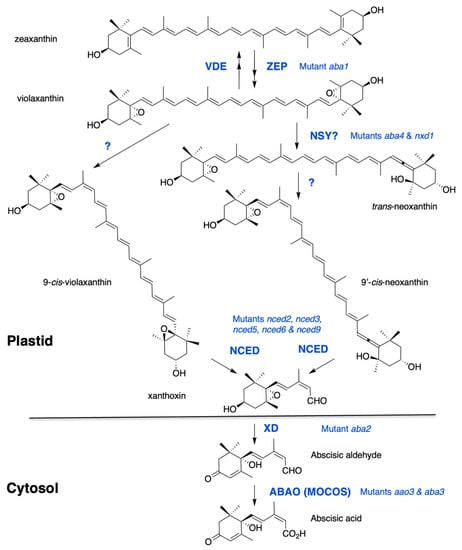
Figure 1.
The ABA biosynthesis pathway from zeaxanthin. Synthesis of violaxanthin is catalyzed by zeaxanthin epoxidase (ZEP). A reverse reaction catalyzed by violaxanthin de-epoxidase (VDE) contributes to energy dissipation in chloroplasts under high light. The formation of cis-isomers of violaxanthin and neoxanthin remains elusive. Two proteins, ABA-DEFICIENT4 and NEOXANTHIN-DEFICIENT1, are necessary for neoxanthin synthase (NSY) activity. Recent evidence suggests that ABA4 is also involved in ABA synthesis from 9-cis-violaxanthin. Cleavage of cis-xanthophylls is catalysed by a family of 9-cis-epoxycarotenoid dioxygenases (NCED). Xanthoxin is then converted by a xanthoxin dehydrogenase (XD) into abscisic aldehyde, which is oxidized into ABA by an abscisic aldehyde oxidase (ABAO). ABAO contains a molydenum cofactor activated by a MoCo sulfurase (MOCOS). Arabidopsis mutants are indicated for each enzymatic step.
Zeaxanthin is converted by zeaxanthin epoxidase (ZEP) to violaxanthin via the intermediate antheraxanthin. Mutants defective in zeaxanthin epoxidation were selected on germination screens and their ABA deficiency resulted in reduced seed dormancy and hypersensitivity to water stress in both Arabidopsis and Nicotiana plumbaginifolia [22,23,24]. ZEP proteins are flavin-dependent monooxygenases that catalyze the introduction of molecular oxygen into zeaxanthin [25,26]. The reverse reactions, from violaxanthin to zeaxanthin, and through antheraxanthin, are catalyzed by violaxanthin epoxidase (VDE). These two proteins share a lipocalin domain involved in lipophilic substrate binding and the so-called xanthophyll cycle has a protective role against excessive light [27]. No correlation between VDE regulation and ABA accumulation has been reported [28].
Violaxanthin is formed as an all-trans-isomer and can be either isomerized into 9-cis-violaxanthin or converted into the all-trans-isomer of neoxanthin (trans-neoxanthin), which is then isomerized into 9′-cis-neoxanthin. Both 9-cis-violaxanthin and 9′-cis-neoxanthin can be cleaved in vitro into the first C15 precursor of ABA, xanthoxin [29]. Despite recent evidence, the enzymatic steps leading to the formation of these last cis-xanthophyll precursors of ABA still remain elusive. Two genes have been identified, ABA-DEFICIENT4 (ABA4) and NEOXANTHIN-DEFICIENT1 (NXD1), whose lack-of-function prevents neoxanthin synthesis. The Arabidopsis aba4 mutant was isolated in a screen for germination resistance to paclobutrazol, which is an inhibitor of GA synthesis [30]. The mutant nxd1 was identified in tomato (Solanum lycopersicon) based on its paler yellow flower color [31]. In both mutants, violaxanthin accumulation was increased compared to wild type and both 9′-cis and trans-neoxanthin isomers were absent, in contrast to 9-cis-violaxanthin, which was still present. Intriguingly, despite their similar carotenoid defects, these mutants showed opposite ABA-related phenotypes. Notably, in Arabidopsis aba4, ABA levels were significantly reduced in both seeds and water-stressed rosettes [30], while in tomato nxd1 no ABA deficiency was observed [31]. A recent genetic analysis confirmed that, in Arabidopsis also, nxd1 alleles were not ABA-deficient and even overproduced ABA compared to wild type, likely due to an increased 9-cis-violaxanthin production in the absence of neoxanthin synthesis. In agreement, nxd1 mutants exhibited increased seed dormancy and water stress tolerance. More importantly, the aba4 mutation was shown to be epistatic to nxd1, suggesting that aba4 prevented ABA production from both 9-cis-violaxanthin and 9′-cis-neoxanthin [32]. Moreover, overexpression of ABA4 in both wild-type and nxd1 backgrounds increased 9-cis-violaxanthin and ABA accumulation [30,32]. Therefore, ABA4 has been postulated to contribute to trans to cis violaxanthin isomerase activity, producing both cis-violaxanthin and neoxanthin precursors. Since NXD1 is a cytosolic protein which likely cannot interact with ABA4 in plastids, it has been further hypothesized that NXD1 possibly produces an unknown factor required for neoxanthin formation, but is not necessary for that of 9-cis-violaxanthin [32]. Further biochemical evidence would be essential to confirm the hypothetical functions of ABA4 and NXD1 and identify putative cofactors.
The first dedicated step to ABA biosynthesis is the cleavage of cis-isomers of violaxanthin and neoxanthin, by a 9-cis epoxycarotenoid dioxygenase (NCED). The first NCED gene, VIVIPAROUS14, was cloned in maize (Zea mays) after the isolation of the viviparous ABA-deficient mutant vp14 [33]. The VP14 protein is a non-heme iron-dependent dioxygenase that cleaves only 9-cis-epoxy-xanthophyll isomers at the 11–12 position [34]. In every plant species examined, NCED genes belong to a multigene family and in Arabidopsis, NCED is encoded by a family of 5 members namely, NCED2, NCED3, NCED5, NCED6 and NCED9 [35] and belongs to a larger family of 9 members, which includes other carotenoid cleavage dioxygenases (CCD1, CCD4, CCD7 and CCD8) having different substrate specificities [36]. Maize VP14 and Arabidopsis NCED3 have been described to exist in two forms—a smaller one, which is soluble in the stroma, and a larger one bound to thylakoid membranes. VP14 and all five NCEDs exhibit similar N-terminal amphipathic helices that are required for attachment to the thylakoid membrane [37,38,39,40]. In contrast to NCED, ZEP and ABA4 are found to be associated with the plastid inner envelope. Furthermore, ABA4 sequence contains predicted transmembrane domains, indicating that it might be an integral protein [30,41]. However, translocation mechanisms of these lipophilic metabolites within plastids remain to be explored. Carotenoid cleavage in plastids produces xanthoxin, which has to move to the cytosol before conversion into ABA, but no transporter has yet been described.
2.2. Cytosolic Steps from Xanthoxin to ABA
Abscisic aldehyde is synthesized from xanthoxin by an enzyme belonging to the family of short-chain dehydrogenases/reductases (SDR). The first mutant aba2-1 was isolated in Arabidopsis, based on its capacity to germinate in the presence of paclobutrazol, an inhibitor of gibberellin biosynthesis [42,43]. It exhibited very severe phenotypes, suggesting the absence of ABA2 functional redundancy with other SDR family members. The ABA2 locus, also named SDR1, encodes a cytosolic NAD-dependent oxidoreductase with xanthoxin dehydrogenase (XD) activity [44,45]. Intragenic complementation of mutant alleles has been observed, suggesting that ABA2 may have a multimeric structure [46,47].
The oxidation of the abscisic aldehyde to the carboxylic acid is the final step in ABA biosynthesis, which is catalyzed by an abscisic aldehyde oxidase. Among four Arabidopsis aldehyde oxidases (AAOs), AAO3 has been shown to be active on abscisic aldehyde [48,49]. In accordance, aao3 alleles exhibited ABA-related phenotypes including the expected reduction in ABA content, water stress tolerance and seed dormancy [50]. AAO3 is a molybdoenzyme that requires a molybdenum cofactor (Moco) for catalytic activity. Mutations in genes for MoCo biosynthesis induce defects in a number of molybdoenzymes inducing pleiotropic phenotypes besides ABA deficiency. Mutants affected in the last step of MoCo biosynthesis have been identified on their ABA-deficient phenotypes and named aba3 in Arabidopsis. ABA3 encodes a Moco sulfurase (MOCOS), which provides the terminal sulfur ligand in Moco [51,52].
2.3. ABA Conjugation and Hydroxylation
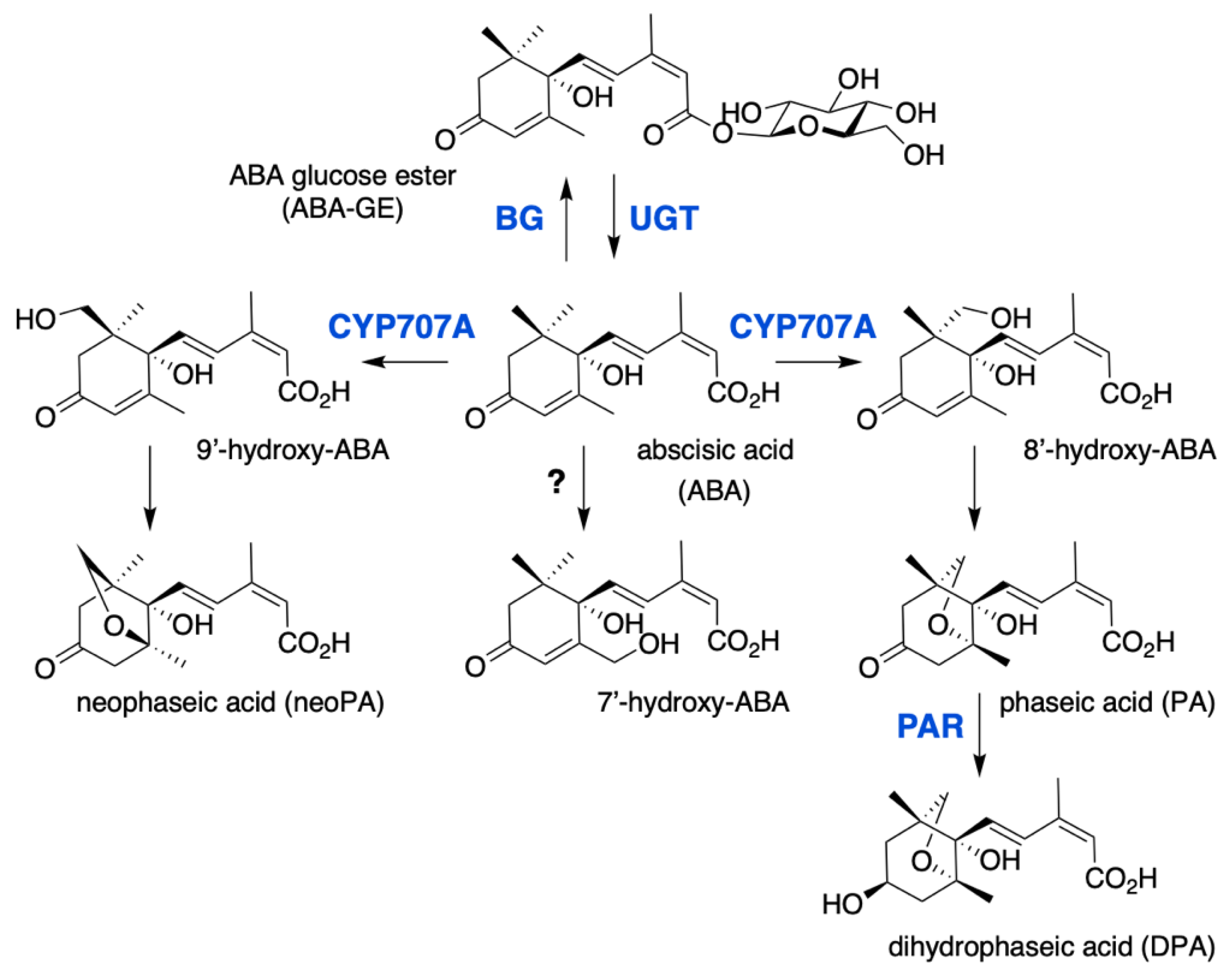
Active hormone levels are modulated by degradation or conjugation (Figure 2). The major catabolic route is the 8′-hydroxylation of ABA by the CYP707A subfamily of P450 monooxygenases [53,54], which are also called ABA 8′-hydroxylases (ABA8′OH or ABA8′ox). In Arabidopsis, this enzyme is encoded by a gene family of four members, CYP707A1 to A4, and multiple mutants were shown to accumulate large ABA amounts and exhibit a very strong seed dormancy [55]. ABA hydroxylation can also occur at the C-7′ and C-9′ position and 9′-hydroxylation is catalyzed by CYP707As as a side reaction, in contrast to C-7′ which hydroxylation mechanism remains unknown. Spontaneous isomerization of 8′- and 9′-hydroxy-ABA gives rise respectively to phaseic acid (PA) and neoPA [56,57]. PA is then converted to dihydrophaseic acid (DPA) by the PA reductase (PAR) which belongs to the family of dihydroflavonol 4-reductase (DFR)-like NAD(P)H-dependent reductases [58]. Moreover, Weng et al. reported that PA was able to activate a subset of ABA-responsive genes and bind to members of the ABA receptor family [58]. Thus, despite less effective than ABA, PA likely has residual biological activity, in contrast to DPA which is inactive.
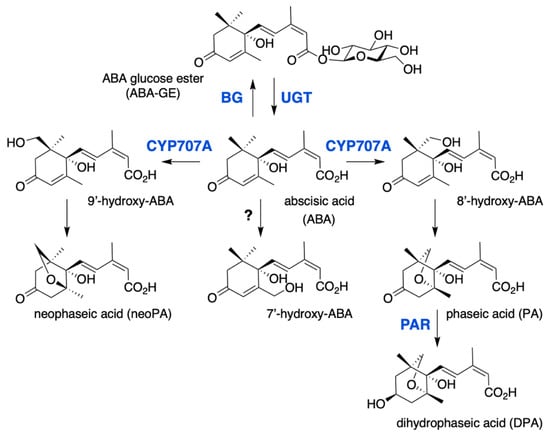
Figure 2.
ABA catabolic pathways. ABA 8′-hydroxylase is encoded by the CYP707A gene family and converts ABA into 8′-hydroxy-ABA, which undergoes a spontaneous isomerization to give phaseic acid (PA). PA reductase (PAR) then converts PA into dihydrophaseic acid (DPA). 7′ and 9′ hydroxylations are minor catabolic routes, 9′-hydroxy-ABA is produced by CYP707A and converted into neoPA, but the enzyme responsible for 7′-hydroxylation remains unknown. ABA is also inactivated to ABA glucose ester (ABA-GE) by UDP-glucosyltransferases (UGT), ABA-GE is then converted to free ABA by β-glucosidases (BG).
The conjugation of ABA with glucose to form the ABA-glucose ester (ABA-GE) is catalyzed by uridine diphosphate glucosyl transferases (UGTs), which belong to the superfamily of glycosyltransferases. Eight Arabidopsis UGTs have been shown to have in vitro activity on ABA, however only UGT71B6 exhibits a selective glucosylation activity towards the natural enantiomer (+)-ABA [59,60]. Gene overexpression or inactivation by either mutation or RNA interference has suggested a biological role for UGT71B6 and its closest homologs, UGT71B7 and UGT71B8, UGT71C5 and UGT75B1 in ABA conjugation in planta [60,61,62,63]. In contrast to hydroxylation that leads to the irreversible degradation of ABA, conjugation to glucose produces a storage form of the hormone that can be converted back to ABA by glucosidases (BGs). In Arabidopsis, two glucosidases BG1 (BGLU18) and BG2 (BGLU33), localized respectively in the endoplasmic reticulum or the vacuole, have been shown to catalyze ABA-GE hydrolysis [64,65].
3. ABA Metabolism and Seed Dormancy Induction
3.1. Control of ABA Levels by Carotenoid Cleavage and ABA Hydroxylation
During seed maturation, ABA positively regulates reserve accumulation, inhibits embryo growth and induces desiccation tolerance and primary dormancy. ABA accumulated in seeds originates from synthesis in both maternal and zygotic tissues and transport from vegetative tissues; however, only ABA produced by zygotic tissues during late maturation stages has a predominant role in dormancy induction [66,67,68,69]. Carotenoid cleavage by NCED and ABA inactivation by CYP707A 8′-hydroxylase have been proven to constitute key regulatory steps for the control of ABA accumulation. Among the five Arabidopsis NCED genes, NCED6 exhibits the highest expression levels in developing seeds and is specifically expressed in the endosperm. NCED9 expression has been detected in testa at early stages and in embryo at later stages. NCED5 is expressed at lower levels, as compared to NCED6 and NCED9, but its tissue specificity is broader in both embryo and endosperm all along seed development. Mutant analysis indicated that expression of these three genes in both embryo and endosperm makes a major contribution to ABA production for dormancy induction [70,71]. Moreover, the comparison of ABA levels in mutants nced2569 and nced259 has suggested that NCED6 activity in endosperm is responsible for most of the xanthoxin production that gives rise to ABA in developing seeds [72]. As in Arabidopsis, ABA levels in barley (Hordeum vulgare) seeds peak at mid-development and ABA abundance is correlated with an increase in HvNCED2 expression [73]. In maize, VP14 transcript has been detected in embryo and its function is required in dormancy induction since gene mutation leads to a viviparous phenotype [74].
ABA catabolism has a great role in the regulation of active hormone levels during seed development. High levels of catabolites, mainly DPA, have been detected in Arabidopsis developing siliques and seeds [55,68,72]. Furthermore, in cyp707a mutants, enhanced dormancy and ABA accumulation has further proven the important contribution of CYP707A1 and CYP707A2, which transcripts are highly accumulated at early and late maturation stages respectively [55]. Maximal accumulation of ABA and DPA is observed at mid-development stages. The higher abundance of DPA relatively to ABA reinforces the importance of inactivation of hormone pools to maintain hormone homeostasis. While ABA production in early stages is essential for embryo development and growth arrest, a too-high seed content at later stages can lead to excessive dormancy depth. Interestingly, a coordinated regulation among catabolic pathways is likely occurring in developing seeds to prevent ABA excess. In cyp707a1a2 double mutants, absence of 8′-hydroxylation can be partially compensated by the production of high amounts of ABA-GE and 7′-OH-ABA [72]. In cereals, ABA catabolism by CYP707A family members has also been described to regulate ABA levels in developing seeds [7]. In barley, contrasting with HvNCED2 which is responsible for ABA accumulation at early and middle stages, HvABA’OH1 has been reported to control ABA levels at later maturation [73]. Furthermore, in wheat (Triticum aestivum), mutations affecting TaABA8′OH1 expression were shown to result in higher ABA content in embryos during seed development, in correlation with a lower germination in the field [75].
3.2. Influence of Maternal Environmental Conditions on Dormancy and Germination
Environmental conditions during seed set modulate dormancy depth and then influence dormancy release and germination after seed dispersal. Temperature, light and soil nitrate conditions experienced by the mother plant during seed development are important signals that regulates dormancy and germination vigour. As reported in a number of species, maternal temperature variations have the greatest effect on dormancy and ABA content in seed progeny. In Arabidopsis, the enhancement of dormancy by cold temperature is correlated with increased ABA and decreased CYP707A2 transcript levels in dry seeds [76]. In wheat, seeds developed under high or low temperatures exhibit either weak or strong dormancy, respectively; however, dormancy depth does not seem to be strictly associated with modifications in ABA content in dry seeds. As reported in a recent study, no differences in ABA contents were observed in dry seeds produced under either low (13 °C) or high (28 °C) temperature. However, the stronger dormancy that was induced by low maternal temperature was associated with higher ABA levels after 24 h imbibition and modulation of the expression of TaNCED1, TaNCED2 and TaCYP707A1 during imbibition [77]. Conversely, in oilseed rape (Brassica napus), seeds produced under elevated temperatures show reduced ABA contents and high pre-harvest sprouting rates [78]. In rice (Oryza sativa), heat stress during seed filling has been described to delay germination even after dormancy release treatment and this delay is correlated with an increased expression of NCED genes and a decreased expression of CYP707A genes in imbibed seeds. Hot temperatures have been also shown to affect methylation of catabolism gene promoters and therefore have been suggested to influence catabolism gene expression, ABA levels and germination rates [79].
High nitrate feeding of Arabidopsis wild-type plants increase endogenous nitrate content in mature dry seeds, which is correlated with the production of less dormant seeds, whereas low nitrate has the opposite effect [18,80,81,82]. In accordance with the reduction of dormancy depth, lower ABA levels are observed in seeds produced under high nitrate conditions. As under cool temperature, the higher expression of the CYP707A2 gene at late maturation stages suggested a possible role for CYP707A2 in controlling seed ABA levels in response to endogenous nitrate [83,84]. Functional genomics analysis of seeds matured under low temperature or low nitrate showed that the similar effects of temperature and nitrate on seed performance, and notably seed dormancy, were reflected by partly overlapping genetic and metabolic networks [85].
Light intensity and spectral quality during seed development can alter dormancy depth, as reviewed by Chahtane et al. [6]. Low light intensity or high ratio of far red (FR) to red (R) have been described to result in deeper dormancy in Arabidopsis seeds. However, light responses may depend on species and culture conditions. Recently, the effect of light quality has been assessed on seeds harvested from soybean (Glycine max) plants grown in the field [86]. The shading of the mother plant on germination of soybean seeds was analyzed in comparison to that of normal light, in two different field culture conditions, either maize-soybean intercropping or soybean monocropping culture. The shade environment, which results in a higher FR/R ratio and a lower photon flux, promoted seed germination and decreased ABA content in both dry and imbibed seeds. In accordance, the expression of the ABA biosynthesis gene ABA2 was decreased while that of the catabolism gene CYP707A1 was increased.
3.3. Regulatory Factors of ABA Metabolism in Developing Seeds
The LAFL proteins, LEAFY COTYLEDON 2 (LEC2), ABSCISIC ACID INSENSTIVE 3 (ABI3), and FUSCA3 (FUS3) B3 domain transcription factors, together with LEC1 and LEC1-LIKE (L1L), homologs of the NF-YB subunit of the CCAAT-binding complex are major regulators of seed development, which control embryo growth arrest, reserve storage and dormancy induction [87]. Mutants in these genes share some common phenotypes, such as a reduced accumulation of storage compounds, desiccation intolerance and precocious germination [88]. Furthermore, LAFL genes have been reported to interact with hormone signaling and synthesis pathways to regulate seed development and germination processes. During seed development, FUS3 promotes ABA accumulation and inhibits GA biosynthesis, thus contributing to dormancy induction [89]. However, while FUS3 directly acts on GA biosynthesis gene expression, its control on ABA pathway may be indirect since no targets have been identified.
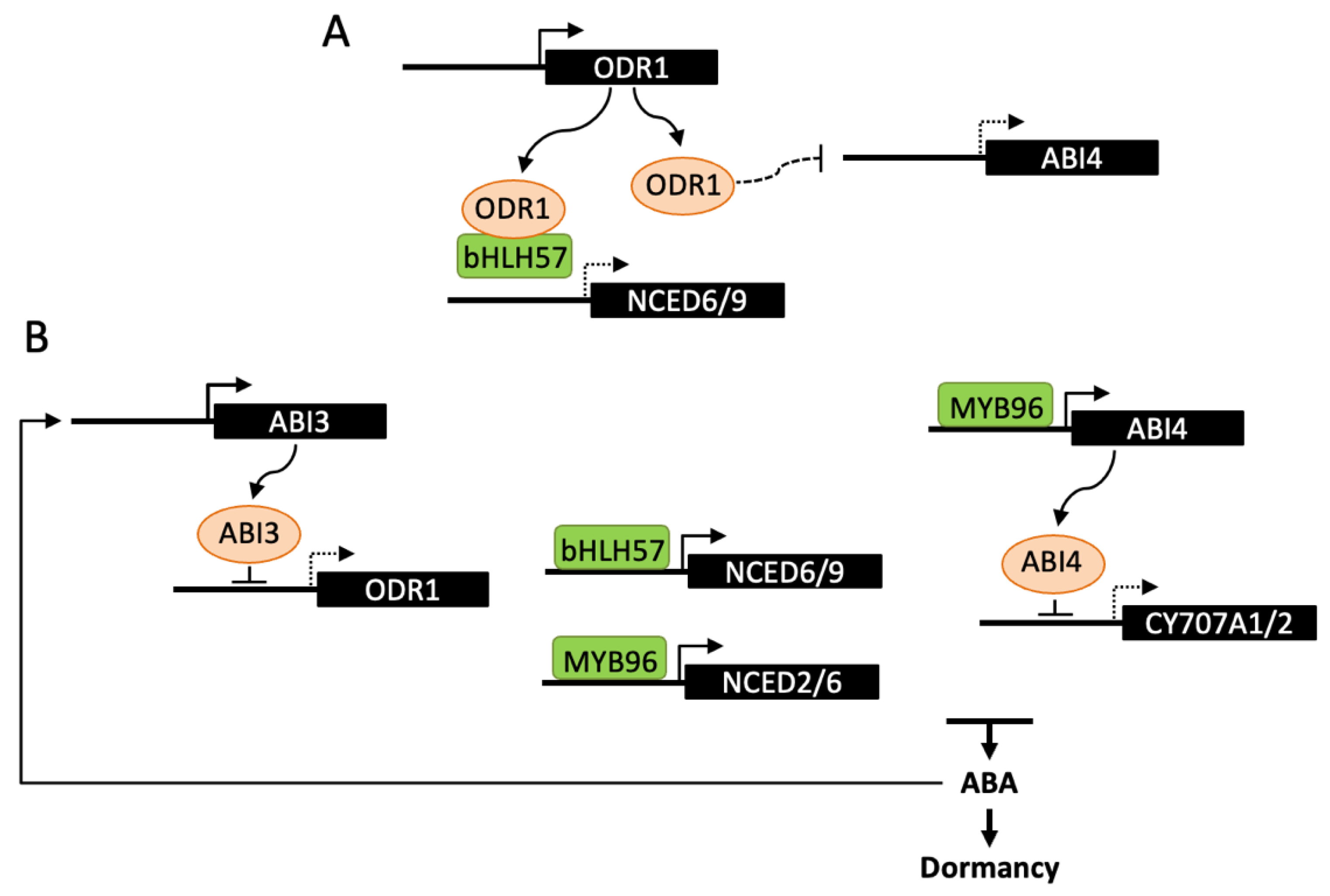
ABI3 is a central transcription factor in the ABA signaling network and, in contrast to other lafl, Arabidopsis abi3 mutants have been identified by their germination insensitivity to ABA [90]. ABI3 has been recently reported to bind to the promoter and repress the expression of REVERSAL of RDO5 1 gene (ODR1) and ODR1 to act as a regulator of ABA biosynthesis [91]. DELAY OF GERMINATION18 (DOG18)/REDUCED DORMANCY 5 (RDO5) was previously identified by both mutant screen and QTL analysis for low dormancy. RDO5 encodes a PP2C pseudophosphatase, that does not belong to the clade A, which includes PP2Cs interacting with ABA receptors [92]. The mutant odr1 was selected in a suppressor screen after rdo5 mutagenesis and odr1 seeds exhibit increased ABA levels and dormancy, in both rdo5 and wild-type backgrounds. ODR1 encodes a homolog of rice Seed dormancy 4 (Sdr4), which is a zinc finger protein identified by QTL cloning [93]. In Arabidopsis, ODR1 was shown to interact with bHLH57, a basic helix-loop-helix transcription factor, which binds to NCED6 and NCED9 promoters and activates gene expression (Figure 3). ODR1 protein itself cannot directly bind to NCED promoters, but its interaction with bHLH57 in a complex negatively impacts on NCED gene transcription and consequently on dormancy induction [91]. In contrast to ODR1, the expression of the rice Sdr4 protein is positively regulated by OsVP1, the rice ortholog of ABI3, and in accordance positively affects dormancy in several rice cultivars [93]. The opposite effects of ODR1 and Sdr4 on seed dormancy may result from their differential regulation by either ABI3 or VP1 and also from differences in expression of Arabidopsis and rice DOG1 homologs, DOG1 being upregulated in odr1 and downregulated in sdr4. The regulation of ABA metabolism by rice Sdr4 still remains to be studied [91]. Together with DOG1, expression of the genes encoding ABI4 and the Drought Response Element binding protein DREB2C is increased in odr1 dry and hydrated seeds, compared to wild-type, however no direct protein interaction with ODR1 has been observed. Since DREB2C and ABI4 transcription factors have been previously shown to interact with NCED6 and NCED9 promoters, ODR1 has been hypothesized to act not only through direct interaction with bHLH57, but also indirectly by downregulating the expression of ABI4 and DREB2C [91,94,95].
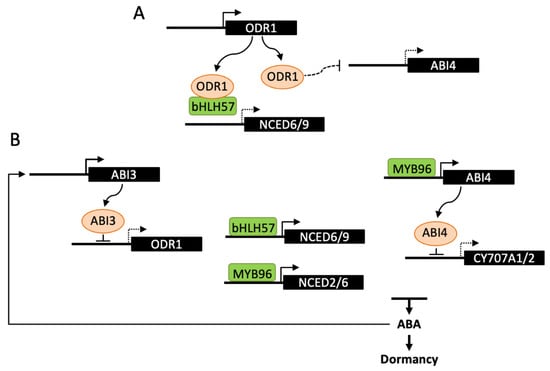
Figure 3.
Transcriptional regulation of ABA metabolism in dormancy induction. The transcription factors ABI4, MYB96 and bHLH57 have been described to directly bind promoters of either NCED or CYP707A genes and regulate ABA levels and dormancy depth. (A) ODR1 interaction with bHLH57 prevents binding to NCED6 and NCED9 promoters and activation of ABA biosynthesis. ODR1 may also regulate ABA levels by decreasing the transcription of ABI4. (B) During seed maturation, ABI3 binding to the ODR1 promoter represses its expression and releases bHLH57 inhibition thus promoting ABA biosynthesis and seed dormancy. This regulation may be amplified by the stimulation of ABI3 by ABA. ABI4 has been shown to bind CYP707A1/2 promoters and inhibit ABA catabolism, leading to higher ABA levels and seed dormancy. MYB96 would have a dual function in the regulation ABA metabolism by directly activating NCED2 and NCED6 and indirectly repressing CYP707A2 through its positive effect on ABI4 expression. Dashed lines indicate either an indirect effect of the signaling factor, or a reduced expression of the downstream gene.
ABI4 and DREB2C belong to the family of APETALA2/ethylene responsive element binding protein (AP2/EREBP)-like transcription factors. In contrast to DREB2C which is one of five members of the clade A2, ABI4 is the single member of the clade A3, suggesting absence of functional redundancy with other family members [96]. As described below (Section 4.3), both have been reported to regulate ABA biosynthesis during seed germination [94,97]. Nevertheless, further evidence suggested an additional role for ABI4 in the regulation of hormone levels during seed development [97]. Like ABI3, ABI4 has been identified based on ABA-insensitive germination of mutants. However, it has been later shown to be involved not only in ABA signaling and germination, but also in a number of other signaling pathways and physiological processes [98]. In abi4 alleles, a mildly reduced dormancy of dry seeds is observed, moreover seeds inside immature green siliques exhibit a viviparous phenotype when siliques are detached and plated on soil or medium. These phenotypes have been correlated with decreased ABA levels and increased CYP707A gene expression in dry seeds. Since ABI4 is able to bind CYP707A1 and A2 promoters, it has been suggested that ABI4 positively regulates dormancy induction by repressing ABA catabolism [97]. The R2R3-type MYB transcription factor MYB96 has also been shown to regulate the establishment of seed dormancy [99]. Seeds of the loss-of function mutant myb96 exhibit a reduced dormancy and viviparous phenotypes are observed after harvesting siliques at long-green stage. Inversely, germination is delayed in activation-tagging myb96-1D seeds. In seedlings, expression of NCED2, NCED5, NCED6 and NCED9 genes is upregulated; however, MYB96 was shown to bind to NCED2 and NCED6 promoters only. MYB96 has also been reported to bind to ABI4 promoter; nevertheless, it has been suggested to stimulate ABA biosynthesis in an ABI4-independent manner [100].
A recent genome-wide association study (GWAS) for seed coat color in soybean (Glycine max) revealed the existence of regulatory mechanisms at the protein level. The G gene that is responsible for seed green color has been shown to encode a CAAX amino-terminal protein protease and control seed dormancy in soybean [101]. G orthologs were selected upon domestication in several other crop species and G gene has a similar function in Arabidopsis. Interestingly, the encoded protein interacts with two ABA biosynthesis enzymes NCED3 and phytoene synthase, and positively affects ABA accumulation. Lower hormone levels and dormancy depth in mutant seeds suggest that G may have an essential contribution in dormancy induction.
Chromatin modification plays an essential role in transcriptional regulation of many physiological processes. In a recent study, Chen et al. reported that histone demethylation by the methyltransferase RELATIVE OF EARLY FLOWERING 6 (REF6) reduces seed dormancy by inducing ABA catabolism in seeds [102]. REF6 catalyzes the lysine (K27) demethylation of histone H3 (H3K27m3), of which methylation is associated with gene silencing. REF6 directly binds to CYP707A1 and A3 genes, but not CYP707A2. In developing siliques, REF6 reduces the level of H3K27 methylation levels in both genes. In accordance, in the ref6 mutant, CYP707A1 and CYP707A3 gene expression is decreased during seed development and also during germination, this lower expression is correlated with enhanced ABA and dormancy levels in freshly-harvested seeds. Another study previously showed that the two Arabidopsis homologs of the human LYSINE SPECIFIC DEMETHYLASE 1, LDL1 and LDL2, which reduce the H3K4 methylation levels, act redundantly in the repression of seed dormancy [103]. LDL1 and LDL2 are highly expressed during early silique development and the increased seed dormancy in ldl1 ldl2 double mutant is associated with an upregulation of ABA2 transcripts at this stage. The increased expression of ABI3 and DOG1 signaling factors, which is observed in mutant seeds at later maturation stages may also contribute to dormancy enhancement. Other chromatin modifications have been described to affect ABA metabolism genes and seed dormancy. The HUB1/RDO4 E3 ubiquitin ligase is involved in histone H2B ubiquitination, which is associated with active gene transcription. HUB1 loss-of-function has been shown to reduce dormancy and be correlated with decreased expression of NCED9 and CYP707A2 in freshly-harvested mutant seeds compared to wild-type [104]. Histone deacetylation mediated by two histone deacetylase-binding factors, SWI-INDEPENDENT3 (SIN3)-LIKE1 (SNL1) and SNL2, has also been reported to influence seed dormancy [105]. Expression of SLN1 and SLN2 genes increase during seed development and these two genes likely have a redundant function in seed dormancy induction, since double mutant seeds exhibits lower dormancy levels than those of simple mutants. Furthermore, the reduced ABA content in mutant seeds correlates with an upregulation of CYP707A1 and CYP707A2 expression and, in accordance, both genes exhibit increased H3K9/18 acetylation levels. SNL1 and SNL2, which also act on ethylene pathway, would regulate seed dormancy and germination by modulating the antagonism between ABA and ethylene.
DNA methylation is an additional epigenetic modification that can repress gene transcription. In tomato, an epimutation that causes an hypermethylation in the promoter of the COLORLESS NON-RIPENING (CNR) gene prevents fruit ripening and coloring. The Cnr mutant possesses a hypermethylated epigenome whose maintenance requires a number of methylation enzymes, including methyltransferase1 (SlMET1). Interestingly, Yao et al. observed a correlation between SlMET1 silencing and seed vivipary in Cnr fruits [106]. A decrease in ABA concentration was also reported together with a reduced accumulation of SlNCED and SlZ-ISO transcripts and a differential methylation of promoter regions, suggesting that an epiregulation of these genes prevents vivipary during fruit development.
4. ABA Metabolism during Dormancy Release and Germination
4.1. Importance of ABA Hydroxylation in the Control of Germination
Dormancy of mature dry seeds is progressively released upon dry storage, also called after-ripening, however, molecular mechanisms involved remain elusive. Limited changes in gene expression between dormant and non-dormant dry seeds have been observed, probably due to the lack of available free water for enzymatic reactions in a fully dry state, while passive and non-enzymatic reaction events such as oxidation and Amadori–Maillard reactions, which occur during after-ripening, have been suggested to be related with dormancy release [6,107]. Moreover, differences in dormancy depth are generally not well correlated with ABA contents in after-ripened seeds compared to dry dormant seeds. In contrast, variations in ABA levels in imbibed seeds appear clearly correlated with seed dormancy. A decrease in ABA levels is observed in both dormant and non-dormant seeds, however dormant seeds maintain higher ABA levels, as observed in Arabidopsis and barley [108,109]. In Arabidopsis, expression of CYP707A2 is rapidly induced upon seed imbibition and plays a major role in the rapid decrease in ABA levels during early seed imbibition [53,55]. Similarly, barley HvCYP707A1 is highly expressed during seed germination and increased expression is followed by a rapid decrease in ABA content [73]. Moreover, compared to after-ripened seeds, both Arabidopsis and barley dormant seeds retain a higher concentration of ABA and, in accordance, exhibit lower CYP707A transcript levels [110]. In parasitic plants, such as Orobanchaeceae, seed germination requires the presence of host-derived strigolactones as signal for germination. The stimulation of germination is correlated with an early upregulation of CYP707A gene expression in seeds upon imbibition in presence of GR24, a strigolactone analog [111,112].
Despite ABA catabolism appears to be a major contribution to regulation of ABA levels and dormancy in imbibed seeds, several lines of evidence suggest that ABA biosynthesis contributes to dormancy maintenance. Indeed, treatment with ABA biosynthesis inhibitors was shown to promote germination of dormant Arabidopsis seeds [109,113]. However, no differential expression of Arabidopsis NCED genes has been observed between dormant and non-dormant imbibed seeds, whereas in barley and Brachypodium distachyon respectively, HvNCED1 and BdNCED1 expression is increased in dormant embryos [110,114].
Beside activation of gene transcription, messenger RNA degradation has been suggested to efficiently act in the fine-tuning of gene expression during seed germination. Two 5′-3′ RNA decay mutants, respectively impaired in the 5′ to 3′ exoribonuclease XRN4 and the component of the decapping complex VARICOSE (VCS) display opposite dormancy and germination phenotypes [115]. In imbibed mutant seeds, germination phenotypes are well correlated with the modifications in the expression of the ABA and GA metabolism genes. In the dormant xrn4 mutant, an increase in NCED6 and NCED9 and a decrease in CYP707A2 transcript abundance are observed, and opposite expression patterns are observed in the nondormant vcs mutant. Moreover, these mutants also display an antagonistic regulation of ABA and GA metabolism genes, suggesting that RNA stability may contribute to the control of ABA/GA balance and dormancy maintenance.
Several studies have highlighted the importance of ABA produced in the endosperm in dormancy maintenance during imbibition [116,117]. A seed coat bedding assay showed that the growth of Arabidopsis dissected embryos is prevented when they are cultured on a layer of dormant seed coats. Indeed, unlike nondormant coats, dormant coats actively produce and release ABA to repress embryo germination [117]. Dissected seed coats are composed of a maternal testa, which in mature seeds is composed of dead dry tissues, and a single layer of endosperm, which is a living tissue that can be a source of ABA for the embryo. Four ATP-binding cassette (ABC) transporters, AtABCG25, AtABCG30, AtABCG31 and AtABCG40 have been reported to mediate ABA transport in imbibed seeds [118]. AtABCG25 and AtABCG31 function in ABA export from the endosperm and AtABCG30 and AtABCG40 in its import into the embryo. Mutant analysis also suggested that these transporters have a role in the hormonal control in seed dormancy and germination. In Arabidopsis, the embryo radicle has been proposed as a decision-making center in dormant seeds, in which ABA and GA metabolism and signaling proteins are expressed in distinct tissues. The decision to germinate or remain dormant, in response to environmental signals, would rely on dynamic regulation of the abundance of both hormones through complex feedback series involving hormone transporters [119].
4.2. Influence of Light, Temperature and Nitrate on Germination
Seeds monitor their environment to germinate under suitable conditions for progeny survival and hormone levels have an essential role in the integration of seed responses to these exogenous signals. Light, temperature and nitrate, together with soil moisture, have been shown to have a prominent influence. Light and temperature responses rely on the antagonistic regulation of the ABA and GA metabolism and signaling pathways, including the opposite regulation of NCED and CYP707A genes. In dicot seeds, white light activates germination by the perception R/FR wavelengths by the phytochrome family of photoreceptors [120]. In Arabidopsis seeds, an R light pulse has been shown to reduce ABA levels and decrease ZEP, NCED6 and AtNCED9 gene expression, whereas expression of CYP707A2 was increased [121]. In lettuce (Lactuca sativa), ABA levels are controlled by the downregulation of LsNCED2 and LsNCED4 expression and upregulation of LsABA8ox4 [122]. In contrast, in numerous monocot species and particularly in cereals, white light inhibits germination and blue light perception by cryptochromes has a similar effect. Crop species, such as barley and wheat, lack the R/FR light response, which has been presumably lost upon domestication [114]. In barley, continuous white light promotes dormancy of freshly-harvested dormant grains, whereas imbibition in the dark breaks dormancy. Germination inhibition by white light is correlated with increased ABA levels and HvNCED1 expression in embryos; however, light or dark had no or little effect on HvNCED2 and HvABA8′OH1 expression. Blue light mimics the effect of white light on ABA and HvNCED1, in contrast to R and FR light that has no effect [123]. Furthermore, a long exposure under blue light at low temperature (10 °C) results in a progressive inability to germinate in the dark, considered as secondary dormancy [124]. In these imbibition conditions, blue-light inhibition was associated with increased ABA levels in embryos and a strong upregulation of both HvNCED1 and HvNCED2.
Together with light, the ability of seeds to sense temperature is ecologically important for the seasonal timing of germination. In crop species, hot temperatures resulting from global warming are regarded as a major threat affecting seed germination. The seeds of many winter annual plants, such as Arabidopsis, germinate in the cool temperatures of autumn, but not in the hot temperatures of summer. In Arabidopsis, high temperature inhibits germination by altering the ABA/GA balance. Increased ABA accumulation results from the activation of ABA biosynthesis genes, including ZEP, NCED2, NCED5 and NCED9 and repression of ABA catabolic genes CYP707A1-3 [125]. Most lettuce varieties exhibit seed thermoinhibition and LsNCED4 has been identified by QTL analysis as the causal gene. When imbibed at 35 °C, seeds from a thermosensitive cultivar accumulate high ABA levels compared to seeds of the thermotolerant Lactuca serriola accession. Furthermore, in thermosensitive accessions, germination inhibition is correlated with an increased LsNCED4 expression in imbibed seeds, which is not observed in thermotolerant accessions [126]. In wheat, germination inhibition at supraoptimal (35 °C) and suboptimal (4 °C) temperatures is better correlated with the expression of TaNCEDs than TaCYP707As, suggesting that ABA biosynthesis has the major role in temperature responses [127].
As previously mentioned, when Arabidopsis mother plants are fed with high nitrate, endogenous nitrate in developing seeds activates ABA catabolism by CYP707A2, resulting in reduced ABA levels and low dormancy, whereas low nitrate nutrition has the opposite effect [80,81,83]. Upon imbibition, exogenously applied nitrate also alleviates seed dormancy and stimulates germination by reducing ABA levels [82]. In Arabidopsis and the hedge mustard Sisymbrium officinale, low concentrations of nitrate (0.1 mM) have been shown to promote seed germination independently of its assimilation, suggesting that nitrate itself acts as a signal. In particular, nitrate is able to stimulate germination of the Arabidopsis nitrate reductase-deficient mutant nia1nia2 mutant [80]. In both species, nitrate has been reported to reduce ABA levels by upregulating CYP707A2 expression and, in accordance, nitrate fails to decrease the ABA content in the Arabidopsis cyp707a2 mutants [80,83,128]. Altogether, nitrate negatively regulates both the induction of primary dormancy during seed development and its maintenance during germination, by activating ABA catabolism at both developmental stages.
4.3. Regulatory Factors of ABA Metabolism in Imbibed Seeds
Germination is regulated by the antagonistic ABA/GA balance and ABA catabolism upon imbibition precedes GA synthesis and activation of germination. Several transcription factors have been shown to oppositely regulate ABA and GA metabolism pathways. Among them, ABI4 has been described above to act as a positive regulator of dormancy induction during seed development and a repressor of ABA catabolism through its binding to the CYP707A1 and CYP707A2 promoters. During germination, ABI4 regulates both ABA synthesis and catabolism. In abi4 imbibed seeds, a downregulation of NCED genes and an upregulation of CYP707A genes is observed, CYP707A2 transcripts being the most differentially accumulated [97]. At seedling stage, ABI4 has been shown to activate NCED6 and GA2ox7, a GA-inactivating gene, by direct binding to promoters. Moreover, ABA and GA are able to antagonistically modify ABI4 expression and stability, suggesting the existence of regulatory loops [95]. However, the occurrence of such regulatory processes in germinating seeds remains to be confirmed. Interestingly, the ABA-responsive transcription factor MYB96, highly expressed in embryo, regulates seed germination by controlling the expression of ABI4 in Arabidopsis [129]. In rice, the transcription factor OsAP2-39 which, like ABI4, belongs to the APETALA2-domain family has been shown to be involved in the regulation of the ABA/GA balance and to bind to OsNCED1 promoter. Furthermore, OsAP2-39 overexpression upregulates OsNCED1 transcript and ABA levels in leaves; however, again evidence for a regulatory role in germinating seeds is still lacking [130].
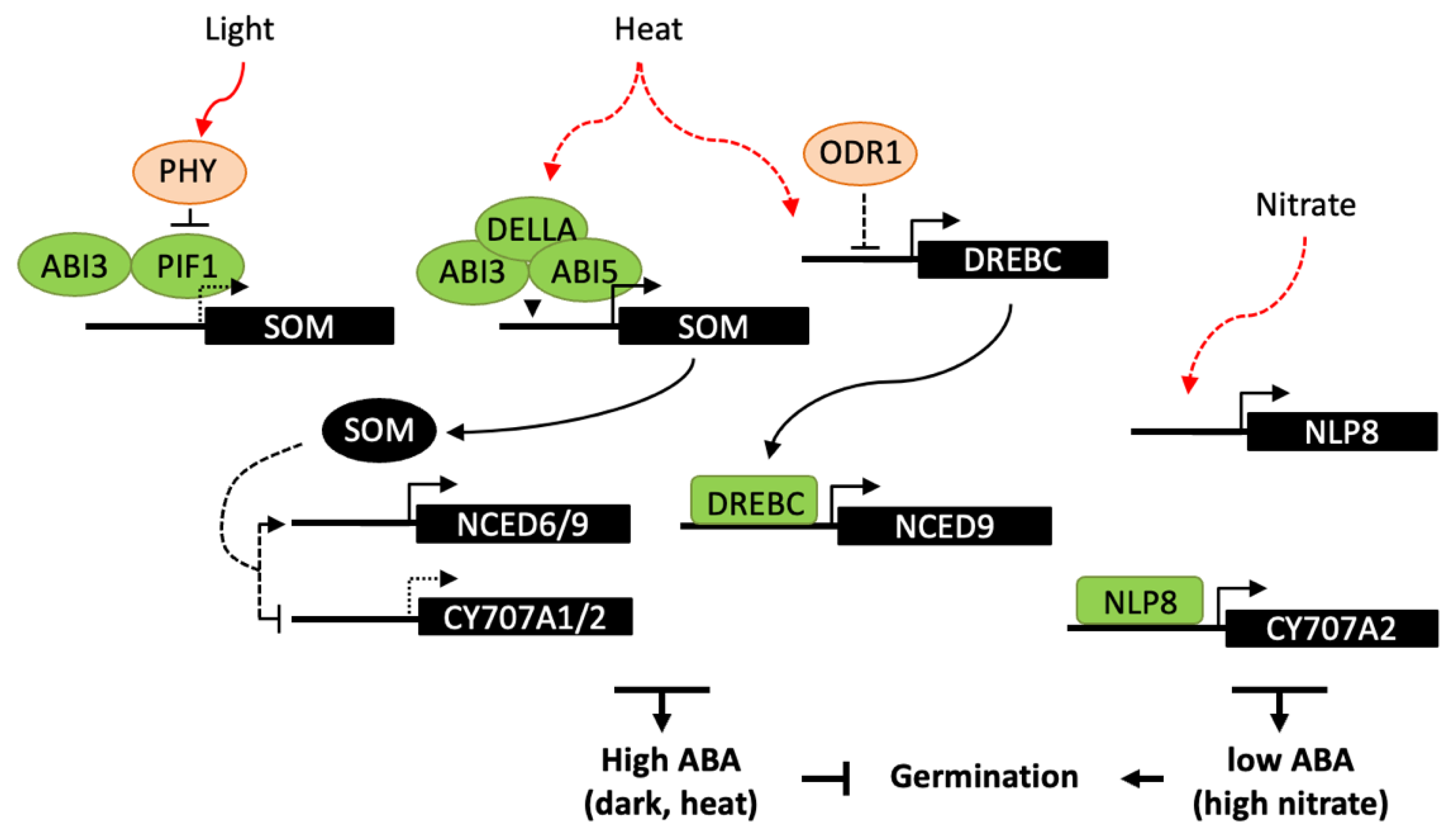
A number of transcriptional regulators have been shown to be involved in germination responses to light, including ABA and GA signaling factors [120,131]. In Arabidopsis, phytochrome B (PHYB) is the main photoreceptor in germination activation by R light and reversion by FR light, whereas PHYA promotes germination responses in continuous FR light and at very low fluence rates. Light-activated phytochromes interact with the basic helix–loop–helix (bHLH) transcription factor PHYTOCHROME-INTERACTING FACTOR 1 (PIF1) and promote its degradation by the 26S proteasome (Figure 4). PIF1 directly regulates the expression of several downstream genes, including the C3H-type zinc finger protein SOMNUS (SOM), which contributes to the inhibition of seed germination through the modulation the GA and ABA metabolism genes, including the activation of the ABA biosynthesis genes, ABA1, NCED6 and NCED9 and the inhibition of the catabolic gene CYP707A2 [132]. Like PIF1, ABI3 is a positive regulator of SOM expression by direct binding to RY motifs in its promoter and, at the protein level, ABI3 and PIF1 interact to collaboratively activate SOM expression in imbibed seeds [133]. In maturing seeds, ABI3 has also been shown to upregulate SOM expression but independently of PIF1, suggesting that other transcription factors may be involved at this stage. Light responses in Arabidopsis seeds have been shown to be spatially-controlled [134]. FR light inactivates PHYB in the endosperm whereas it activates PHYA in the embryo. PIF1 stabilization in the endosperm results in the downstream repression of GA synthesis genes and activation of NCED6 and NCED9; ABA released from the endosperm would then counteract PHYA signaling in the embryo and repress germination.
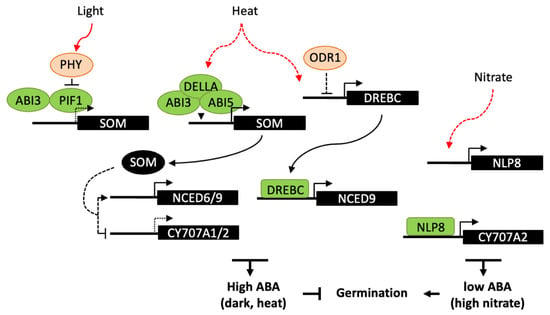
Figure 4.
Transcriptional regulation of ABA metabolism in germination responses to light, high temperature and nitrate. Light-activated phytochromes interact with PIF1 and promote its degradation. In the dark, PIF1 interacts with ABI3 and both bind to the promoter of SOM gene, which indirectly regulates ABA metabolism genes, resulting in an increase in ABA levels and inhibition of germination. Similarly, SOM is also indirectly involved in germination thermoinhibition, ABI3 and ABI5 form a complex with DELLA proteins and bind to the SOM promoter. In response to heat, DREBC has been shown to directly binds to NCED9 promoter and upregulates ABA accumulation. Moreover DREBC expression would be indirectly subject to a negative regulation by ODR1, which role in temperature response has not been investigated. Nitrate promotes germination through NLP8 binding to CYP707A2 promoter and activation of its expression, thus reducing ABA levels upon imbibition. Dashed lines indicate either an indirect effect of the signaling factor, or a reduced expression of the downstream gene.
Downstream factors in the light signaling network controlling seed germination remain to identify, in particular the direct regulators of ABA biosynthesis or catabolism genes. Recently, ABI4 has been reported to promote PHYA-dependent seed germination [135]. A 48-h FR light treatment results in the activation of PHYA and deactivation of PHYB. Under these conditions, abi4 mutant seeds germinated at lower rates and expression of NCED6 and NCED9 genes was upregulated. Therefore, PHYA has been suggested to promote ABI4 expression, through the action of PIF1, then ABI4 would in turn repress ABA biosynthesis and activate germination. The repressive function of ABI4 on ABA biosynthesis under FR light contrasts with the conclusions of the other study detailed above [97], which described ABI4 as a repressor of germination and activator of ABA biosynthesis. Since observations have been made in very different experimental conditions, understanding of ABI4 regulatory functions would require further study.
The control of plant growth by light and temperature has been shown to share a large set of common regulators, including phytochromes and PIF proteins [136]. However, in seeds, molecular mechanisms involved in temperature sensing to activate or suppress germination remain elusive. In Arabidopsis seeds, the SOM gene appears to integrate both light and high-temperature signals. ABI3 interacts with either PIF1 or the bZIP transcription factor ABI5, in the dark or at high temperature respectively, resulting in repression of germination. At high temperature, both ABI3 and ABI5 form a complex with the GA-signalling proteins DELLA and bind to the SOM promoter to activate its expression [137]. Moreover, SOM may participate to a positive feedback loop with ABI3, ABI5, and DELLAs, through the activation ABA biosynthesis and inhibition of GA biosynthesis. Besides thermoinhibition, high temperature can inhibit germination by the induction of secondary dormancy, which does not allow seeds to resume germination for a while even after a return to favorable temperatures [138]. PHYD has been shown to modulate secondary dormancy and could act by controlling PIF1 degradation, which in turn may impact on expression on ABA and GA metabolism genes and favor ABA accumulation [139]. High temperature has also been reported to enhance the expression of FUS3 [140]. FUS3 overexpression results in delayed seed germination at high temperature, while fus3 mutants are more tolerant than wild type. FUS3 activation by heat stress is correlated with the antagonistic regulation of ABA and GA metabolism and signaling genes, in particular with an upregulation of NCED5 and NCED9, however the exact role of FUS3 is still unknown and might be indirect. A more direct regulatory function has been reported for the transcription factor DREBC, since it is able to bind NCED9 promoter and its constitutive overexpression delays germination at supraoptimal temperature (33 °C) and increase seed ABA content [94].
In Arabidopsis, the nitrate transporter NRT1.1/CHL1 has been shown to have a dual function, acting as both a nitrate sensor and transporter, and to induce primary nitrate response independent of its function in nitrate transport [82]. Downstream of the nitrate sensor, NIN-like proteins (NLP) constitute a family of transcription factors that are involved in nitrate responses. In seeds, NLP8 is the most abundantly expressed NLP and, in good correlation, nitrate fails to promote germination of nlp8 seeds in response to nitrate. NLP8 directly binds to the nitrate response element (NRE) in the CYP707A2 promoter region and induces gene expression, suggesting a key function for NLP8 in nitrate signaling for the control of germination. Furthermore, since nrt1.1/chl1 mutant exhibits a much milder germination insensitivity to nitrate than nlp8, NRT1.1/CHL1 may have a minor role in seed germination and other nitrate sensors may be involved upstream of NLP8 [141]. A number of other regulators upstream of ABI and SOMNUS have been described [5], which could have a downstream effect on ABA accumulation and gene expression. Furthermore, ODR1 which functions downstream of ABI3 in seed dormancy regulation [91] may possibly have a role in germination responses to exogenous signals.
5. Natural Variations of Genes Affecting ABA Metabolism in Seeds
5.1. Relationship between ABA Levels and Germination in Natural Lines
Natural inbred lines or strains of plants have been generated from wild ancestral plants under both natural and human selection. They exhibit a large variety of phenotypes in terms of shape, growth, or physiological response to the environment, and are valuable resources for crop improvement, as well as being successfully employed to identify the genes and the allelic diversity that are causative for the phenotypic differences [142,143,144,145]. In Arabidopsis, more than 1000 natural inbred lines have been collected from a range of ecologically diverse habitats [146,147] and were often called “ecotypes”. However, as it does not conform strictly to its ecological definition, they are now referred to as “accessions”, which has been often used to assign a unique identifier for a plant genotype of a species collected at a specific location [148,149].
It has been reported that levels of endogenous ABA in imbibed seeds differ between the natural lines or accessions of different plant species, being associated with germination phenotypes. For instance, in Arabidopsis seeds, ABA content decreased at 6 h after the onset of imbibition; however, the reduction was less in the deeply-dormant accession from Cape Verde Islands (Cvi) than in the weakly-dormant accession Columbia (Col) [150]. In wheat, freshly-harvested seeds from varieties Gifu and OS38 both maintained their dormancy when imbibed at 20 °C, with no significant changes in the endogenous amount of ABA. After imbibition at low temperature (15 °C), Gifu seeds became less dormant and germinated but OS38 did not, in good correlation with ABA level decrease in Gifu and increase in OS38 [151]. In lettuce, the accession Salinas showed an inability to germinate at a high temperature, while the accession UC96US23 was thermoinhibition-resistant; moreover, the ABA levels in imbibed seeds in the former accession were almost 5-fold greater than those in the latter accession [152]. The link between seed ABA levels and germination ability in natural lines suggests that natural variations in ABA metabolism genes may affect seed germination phenotypes. Again, the focus remains on genes involved in the ABA metabolism pathway, downstream of zeaxanthin. Thus, we summarize here the natural variations in both biosynthesis and catabolism genes and highlight their effect on ABA levels in seeds and germination-related phenotypes (Table 1), including also carotenoid content and seed color that often affect ABA biosynthesis and seed germination [153,154,155].
5.2. Natural Variations of ABA Metabolism Genes Associated with Germination-Related Phenotypes
Natural variations in ZEP genes have been reported in GWAS for carotenoid content rather than ABA levels in seeds. In Arabidopsis, GWAS for the ratio of violaxanthin to antheraxanthin (V/A) in mature dry seeds, followed by haplotype analysis, identified three haploblocks with contrasting haplotypes located near the ZEP genetic region that significantly affected the V/A ratio. The most significant haplotype is HB4a, which is located at the 3′ intergenic region of ZEP. This was corroborated by a quantitative trait locus (QTL) analysis with two parental accessions having contrasting HB4a haplotypes, Col-0 and Blanes-1 (Bla-1), which showed that the ZEP gene is within the QTL region significantly associated with the V/A trait. ZEP mRNAs were induced during mid-seed development, and the haplotype HB4a was shown to be correlated with modifications in transcript level and V/A content between accessions [156]. Similarly, single nucleotide polymorphisms (SNPs) within and proximal to ZEP1 gene in maize were detected by GWAS for kernel carotenoid composition and kernel color, respectively [157,158]. SNPs in ZEP1 associated with zeaxanthin levels in grain were also identified by GWAS in sorghum (Sorghum bicolor) [159]. A possible effect of natural variations in maize ZEP on ABA levels was reported by QTL analyses for kernel desiccation and ABA content during seed maturation. A colocation of ZEP1 gene was found with both QTLs; however, the ZEP1 mRNA levels in parental lines were not clearly correlated with the ABA contents [160]. Natural variation of ABA4 and NXD1, of which mutations in Arabidopsis and tomato affect synthesis of neoxanthin and violaxanthin isomers and germination in seeds has also not been reported so far.
In terms of the effects of natural variations in NCED genes on seed germination, resistance to thermoinhibition in lettuce seeds has been studied in detail. As mentioned above, seeds of the UC96US23 accession have a lower ABA endogenous content than Salinas seeds and exhibit a thermoinhibition-resistant phenotype [152]. QTL analysis for the alleviation of thermoinhibition, with near-isogenic lines (NILs) derived from the two accessions, identified LsNCED4 as a most promising candidate for a responsive gene [161]. Transgenic expression of Salinas LsNCED4 driven by its native promoter in UC96US23 seeds resulted in thermoinhibition, with increased levels of endogenous ABA compared to wild-type UC96US23 seeds. LsNCED4 coding regions from both UC96US23 and Salinas could rescue the thermotolerant phenotype of Arabidopsis nced6-1 nced9-1 double mutant, indicating that both LsNCED4 alleles of UC96US23 and Salinas encode functional NCED proteins. Nineteen SNPs on the promoter regions of LsNCED4 were, however, conserved within thermotolerant or thermosensitive accession groups and the mRNAs expression patterns were associated with the two different groups. After imbibition at high temperature, LsNCED4 transcripts declined to low levels in thermotolerant accessions, whereas levels were maintained in thermosensitive accessions. Variations in promoter sequences have been suggested to induce changes in binding specificities for trans-acting factors controlling LsNCED4 expression [126]. In rice, nced3 mutants germinated earlier than wild type [162]. In the cultivar PA64s mature embryos, NCED3 expression was 3.9-fold higher than in the cultivar 9311, which was consistent with stronger dormancy and remarkably higher ABA levels in PA64s dry seeds, as compared to 9311. A comparison of NCED3 sequences in both cultivars inferred SNPs and insertions/deletions (Indels) within the gene. A low similarity between 9311 and PA64 was found in regulatory sequences (promoter and UTRs), as well as potential amino acid changes in the coding regions [163], suggesting that these variations in rice NCED3 may be involved in regulation of dormancy. Recently, it has been reported that natural variation of NCED3 in Arabidopsis affects the accumulation of ABA in seedlings under low water potential stress. The ABA accumulation in the accession Shahdara (Sha) was less than that in the accession Landsberg erecta (Ler). A QTL analysis for the ABA levels with recombinant inbred lines (RILs) derived from the two accessions revealed the major-effect QTL for the trait, which included NCED3 as a candidate gene. Complementation experiments indicated that Sha has a reduced-function NCED3 allele and has four amino acid substitutions within AtNCED3 compared with Ler, although how these amino acid substitutions could alter AtNCED3 activity remain to be elucidated [164]. Interestingly, the QTL for ABA accumulation including NCED3 colocalized with previously detected QTLs for speed of seed germination (faster germination of Sha than Ler) and ability to germinate on salt- or ABA- containing media (higher germination rate in Sha than Ler) [165], implying that NCED3 natural variation may be involved in these differences through the regulation of ABA levels. Aiming to know ABA-mediated anthocyanin biosynthesis and pericarp coloration, natural variation of rice NCED2 has been recently analyzed [166]. No clear link was observed between the pericarp color and a single nucleotide substitution from C to T in the NCED2 gene that causes a valine to isoleucine change in the protein. The same substitution to isoleucine in NCED2 was however, dominant in many upland rice varieties and these rice varieties exhibited a higher level of ABA in leaves and were more tolerant to drought [167]. Altogether, future research is still needed concerning the relationship between endogenous ABA levels, seed phenotypes and the natural variations of NCEDs.
For steps downstream of xanthoxin synthesis, little information is available on the natural variation of these biosynthesis genes that directly affect seed ABA content. Nevertheless, in wheat grain, yellow pigment content and yellow index (color intensity) were significantly associated with SNPs in the coding sequences of ABA2 and AO3 [168]. Moreover, AO3 mRNAs were more abundant in seeds of the cultivar Ciccio than in those of the cultivar Svevo that have a higher carotenoid content, indicating that the allele present in Ciccio is associated with a more active carotenoid degradation, although no polymorphisms were observed in the promoter regions between the two cultivars [169]. In soybean, nine possible candidate genes were identified by GWAS for seed germination under salt stress, in which a candidate gene is homologous to ABA3 and located near the SNP marker [170].
Among genes in ABA catabolic pathways, natural variations in CYP707A/ABA8′OH, may have a link to ABA amount and germination ability in seeds. For instance, mRNA expression of the gene CYP707A5 in rice was 3.5-fold lower in the cultivar PA64s than in the cultivar 9311, and coincided with levels of dormancy and ABA in the two cultivars. SNPs and Indels in regulatory and coding regions of CYP707A5 may induce these differences between the two cultivars [163]. In wheat, ABA8′OH-2 has also been mapped near to a major QTL for seed dormancy. Five amino acid residue substitutions in ABA8′OH-2 were inferred by comparing allele sequences between parental accessions for the QTL. One of the substitutions occurs in a highly conserved amino acid residue in CYP707A family, implying that the substitution may impact on the enzymatic activity of ABA8′OH-2 [171].

Table 1.
Natural variations of ABA biosynthesis and catabolic genes that affect germination-related phenotypes.
Table 1.
Natural variations of ABA biosynthesis and catabolic genes that affect germination-related phenotypes.
| Gene | Species | Types of Genetic Variation | Involvement in ABA Metabolism and Phenotype | Ref. |
|---|---|---|---|---|
| ZEP | Arabidopsis | Intergenic SNPs/QTL region | Affecting mRNA levels of ZEP and ratio of violaxanthin to antheraxanthin | [156] |
| ZEP1 | Maize | SNPs in coding region | Associated with carotenoid composition | [157] |
| Intergenic SNPs | Associated with kernel color | [158] | ||
| QTL regions | Colocating with QTLs for kernel desiccation and ABA content | [160] | ||
| ZEP | Sorghum | SNPs inside the gene | Associated with zeaxanthin levels | [159] |
| NCED4 | Lettuce | SNPs in promoter | Affecting mRNA levels of NCED4, ABA levels and thermoinhibition | [126] |
| NCED3 | Rice | Low similarity of regulatory region SNPs and Indels in coding region | Higher expression at mRNA level in variety seeds with higher ABA level and stronger dormancy | [163] |
| NCED2 | Nonsynonymous SNPs | Affecting ABA levels in leaves and tolerance to drought but not clearly associated with pericarp color | [166] [167] | |
| NCED3 | Arabidopsis | QTL region/Nonsynonymous substitutions | Affecting ABA accumulation in seedling under low water potential stress | [164] |
| QTL region | Colocating with QTLs for germination speed and ability on media containing salt or ABA | [165] | ||
| ABA2 AO3 | Wheat | SNPs in coding region | Associated with yellow pigment content and yellow index of grain | [168] |
| AO3 | No clear polymorphisms in promoter | Highly expressed at the mRNA level in variety seeds with lower carotenoid content | [169] | |
| ABA3 | Soybean | Intergenic SNPs | Associated with seed germination under salt stress | [170] |
| CYP707A5 | Rice | SNPs and Indels in regulatory and coding region | Lower expression at mRNA level in variety seeds with higher ABA level and stronger dormancy | [163] |
| ABA8′OH-2 | Wheat | QTL region/Nonsynonymous substitutions | Colocating with QTL for seed dormancy | [171] |
5.3. Perspectives for Understanding Natural Variations That Underlie ABA Metabolism in Seeds
Although several studies have reported altered ABA levels or germination in seeds of natural variants, our current knowledge regarding the causative natural variations of genes for ABA metabolism remains fragmentary. Furthermore, to date, no genetic screen specifically for ABA levels in seeds has been performed, although such an approach could be interesting to develop. Aiming to understand drought-induced ABA accumulation in natural accessions, GWAS for ABA levels in seedlings under low water potential was performed in Arabidopsis [172]. The genetic regions containing clusters of ABA-associated SNPs were validated by reverse genetic analyses and six effectors of ABA accumulation such as plasma membrane protein and RING-U box protein were identified. Interestingly, none of these six genes were previously reported to affect ABA or abiotic stress response. Further insight into the natural variations affecting ABA metabolism in seeds may be gained by a similar strategy combining GWAS for seed ABA levels and reverse genetics.
GWAS have succeeded to expand the catalog of variants underlying human diseases, however, as many as 90% of the variants fall within non-coding regions having unknown functional importance [173]. Similarly, many of natural variations in genes summarized in this review are in intergenic or regulatory regions rather than in the coding regions implying that, in seeds of natural variant lines, their function is often regulated at the transcript level rather than enzyme activity. Recently, the natural variations in regulatory regions that control gene expression response to drought stress in maize were revealed by GWAS focusing on transcriptome in leaves. Ninety-seven genes were prioritized in relation to their association with drought tolerance due to their variation in expression and, among the candidates, Zm00001d051554 (abh2) encoding ABA 8′-hydroxylase was verified to play a negative role in the drought tolerance [174]. Therefore, transcriptome-wide association study would also be a promising approach to understand natural variations of genes related to ABA metabolism in seeds.
Natural variations of ABA metabolism genes may play an important role in adapting plants to various environments within a species. Nonetheless, it should be noted that the identified genetic variations do not always explain the local adaptation within the species because in a typical approach, a subset of genotyped lines is grown together under conditions defined the “common garden”, without considering the complex natural environment in which each line inhabits [175,176]. Furthermore, differences in endogenous levels of ABA cannot always explain germination variety between natural lines, and other processes such as ABA perception and signal transduction are also important for the regulation for variety in seed dormancy within a species. For instance, during seed development in 14 accessions of rice, pre-harvest sprouting (PHS)-susceptible accessions maintained similar or even higher ABA levels compared with PHS-resistant accessions [177]. In addition, a number of genes identified in QTLs functioning in cereal PHS are involved in ABA signaling rather than metabolism, although many causal genes for this trait remain to be identified [178,179].
6. Conclusions
In recent years, our understanding of the regulation of ABA metabolism in seeds has been greatly improved and most enzymes of the ABA biosynthesis and catabolism pathways have been identified. Nevertheless, several key steps still remain obscure, such as the synthesis of the last carotenoid precursors of ABA or translocation mechanisms of precursors within the chloroplast and from chloroplast to cytosol. Facets of the molecular factors and mechanisms that regulate ABA synthesis and degradation are also gradually being resolved. Interestingly, several novel regulators have been identified as directly acting on ABA metabolism genes in seeds. Some others have been characterized in vegetative tissues, of which function could be assessed in seeds. Regarding the factors that control the tissue localization of ABA, several ABA transporters have been identified but only a small number has been functionally characterized in seeds [16]. ABA movement within seed tissues, notably between endosperm and embryo, and from mother plant tissues to seeds would be particularly important to decipher for a better understanding of the role of these distinct ABA pools in signaling pathways that control dormancy induction and germination, in response to environmental conditions such as light, temperature and nitrate. It would also require the development of efficient technologies to precisely assess local ABA concentrations in seed tissues. Besides strategies such as engineering ABA receptors or developing ABA receptor agonists [180], natural variants implicated in ABA metabolism or signaling could be valuable materials for improvement of important traits for crop cultivation such as stress tolerance and germination characteristics. Since, in general, mutations in key genes of plant hormone metabolism/signaling affect overall plant growth, the discovery of natural variations that fine-tune ABA action in a specific spatiotemporal manner during vegetative or reproductive development and in response to stress will be important in the future. Identification of the variations that directly affect the expression of ABA metabolism-related genes may be prioritized for the analysis.
Author Contributions
N.S. and A.M.-P. wrote the article together. All authors have read and agreed to the published version of the manuscript.
Funding
N.S. has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No [846387]. This work was financed by ANR-MAPKSEED (ANR-18-CE20-0019) and ANR-KABAGRAPE (ANR-20-CE20-011). The IJPB also benefits from the support of Saclay Plant Sciences-SPS (ANR-17-EUR-0007).
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- Penfield, S.; MacGregor, D.R. Effects of environmental variation during seed production on seed dormancy and germination. J. Exp. Bot. 2017, 68, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Burghardt, L.T.; Edwards, B.R.; Donohue, K. Multiple paths to similar germination behavior in Arabidopsis thaliana. New Phytol. 2016, 209, 1301–1312. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Leubner-Metzger, G. Seed dormancy and the control of germination. New Phytol. 2006, 171, 501–523. [Google Scholar] [CrossRef]
- Yan, D.; Duermeyer, L.; Leoveanu, C.; Nambara, E. The functions of endosperm during seed germination. Plant Cell Physiol. 2014, 55, 1521–1533. [Google Scholar] [CrossRef] [PubMed]
- Carrera-Castano, G.; Calleja-Cabrera, J.; Pernas, M.; Gomez, L.; Onate-Sanchez, L. An updated overview on the regulation of seed germination. Plants 2020, 9, 703. [Google Scholar] [CrossRef] [PubMed]
- Chahtane, H.; Kim, W.; Lopez-Molina, L. Primary seed dormancy: A temporally multilayered riddle waiting to be unlocked. J. Exp. Bot. 2017, 68, 857–869. [Google Scholar] [CrossRef]
- Tuan, P.A.; Kumar, R.; Rehal, P.K.; Toora, P.K.; Ayele, B.T. Molecular mechanisms underlying abscisic acid/gibberellin balance in the control of seed dormancy and germination in cereals. Front. Plant Sci. 2018, 9, 668. [Google Scholar] [CrossRef]
- Liu, X.; Hou, X. Regulation of ABA and GA in metabolism and signaling. Front. Plant Sci. 2018, 9, 251. [Google Scholar] [CrossRef]
- Cadman, C.S.C.; Toorop, P.E.; Hilhorst, H.W.M.; Finch-Savage, W.E. Gene expression profiles of Arabidopsis Cvi seeds during dormancy cycling indicate a common underlying dormancy control mechanism. Plant J. 2006, 46, 805–822. [Google Scholar] [CrossRef] [PubMed]
- Footitt, S.; Douterelo-Soler, I.; Clay, H.; Finch-Savage, W.E. Dormancy cycling in Arabidopsis seeds is controlled by seasonally distinct hormone-signaling pathways. Proc. Natl. Acad. Sci. USA 2011, 108, 20236–20241. [Google Scholar] [CrossRef] [PubMed]
- Cutler, S.R.; Rodriguez, P.L.; Finkelstein, R.R.; Abrams, S.R. Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 2010, 61, 651–679. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Zhu, J.K. Arabidopsis mutant deficient in 3 abscisic acid-activated protein kinases reveals critical roles in growth, reproduction, and stress. Proc. Natl. Acad. Sci. USA 2009, 106, 8380–8385. [Google Scholar] [CrossRef]
- Nakashima, K.; Fujita, Y.; Kanamori, N.; Katagiri, T.; Umezawa, T.; Kidokoro, S.; Maruyama, K.; Yoshida, T.; Ishiyama, K.; Kobayashi, M.; et al. Three Arabidopsis SnRK2 protein kinases, SRK2D/SnRK2.2, SRK2E/SnRK2.6/OST1 and SRK2I/SnRK2.3, involved in ABA signaling are essential for the control of seed development and dormancy. Plant Cell Physiol. 2009, 50, 1345–1363. [Google Scholar] [CrossRef]
- Née, G.; Kramer, K.; Nakabayashi, K.; Yuan, B.; Xiang, Y.; Miatton, E.; Finkemeier, I.; Soppe, W.J.J. DELAY OF GERMINATION1 requires PP2C phosphatases of the ABA signalling pathway to control seed dormancy. Nat. Commun. 2017, 8, 72. [Google Scholar] [CrossRef]
- Nishimura, N.; Tsuchiya, W.; Moresco, J.J.; Hayashi, Y.; Satoh, K.; Kaiwa, N.; Irisa, T.; Kinoshita, T.; Schroeder, J.I.; Yates, I.J.R.; et al. Control of seed dormancy and germination by DOG1-AHG1 PP2C phosphatase complex via binding to heme. Nat. Commun. 2018, 9, 2132. [Google Scholar] [CrossRef]
- Carrillo-Barral, N.; Rodríguez-Gacio, M.C.; Matilla, A.J. Delay of germination-1 (DOG1): A key to understanding seed dormancy. Plants 2020, 9, 480. [Google Scholar] [CrossRef]
- Nambara, E.; Okamoto, M.; Tatematsu, K.; Yano, R.; Seo, M.; Kamiya, Y. Abscisic acid and the control of seed dormancy and germination. Seed Sci. Res. 2010, 20, 55–67. [Google Scholar] [CrossRef]
- Matilla, A.J.; Carrillo-Barral, N.; Rodriguez-Gacio, M.d.C. An update on the role of NCED and CYP707A ABA metabolism genes in seed dormancy induction and the response to after-ripening and nitrate. J. Plant Growth Regul. 2015, 34, 274–293. [Google Scholar] [CrossRef]
- Seo, M.; Marion-Poll, A. Abscisic acid metabolism and transport. In Abscisic Acid in Plants; Seo, M., Marion-Poll, A., Eds.; Advances in Botanical Research; Academic Press: London, UK, 2019; Volume 92, pp. 1–49. [Google Scholar]
- Chen, K.; Li, G.J.; Bressan, R.A.; Song, C.P.; Zhu, J.K.; Zha, Y. Abscisic acid dynamics, signaling, and functions in plants. J. Integr. Plant Biol. 2020, 62, 25–64. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.H.; Qin, X.; Zeevaart, J.A.D. Elucidation of the indirect pathway of abscisic acid biosynthesis by mutants, genes, and enzymes. Plant Physiol. 2003, 131, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Koornneef, M.; Jorna, M.L.; Brinkhorst-van der Swan, D.L.C.; Karssen, C.M. The isolation of abscisic acid (ABA) deficient mutants by selection of induced revertants in non-germinating gibberellin sensitive lines of Arabidopsis thaliana (L.) Heynh. Theor. Appl. Genet. 1982, 61, 385–393. [Google Scholar] [CrossRef]
- Rock, C.D.; Zeevaart, J.A.D. The aba mutant of Arabidopsis thaliana is impaired in epoxy-carotenoid biosynthesis. Proc. Natl. Acad. Sci. USA 1991, 88, 7496–7499. [Google Scholar] [CrossRef] [PubMed]
- Marin, E.; Nussaume, L.; Quesada, A.; Gonneau, M.; Sotta, B.; Hugueney, P.; Frey, A.; Marion-Poll, A. Molecular identification of zeaxanthin epoxidase of Nicotiana plumbaginifolia, a gene involved in abscisic acid biosynthesis and corresponding to the ABA locus of Arabidopsis thaliana. EMBO J. 1996, 15, 2331–2342. [Google Scholar] [CrossRef]
- Buch, K.; Stransky, H.; Hager, A. FAD is a further essential cofactor of the NAD(P)H and O2-dependent zeaxanthin-epoxidase. FEBS Lett. 1995, 376, 45–48. [Google Scholar] [CrossRef]
- Bouvier, F.; d’Harlingue, A.; Hugueney, P.; Marin, E.; Marion-Poll, A.; Camara, B. Xanthophyll biosynthesis. Cloning, expression, functional reconstitution, and regulation of b-cyclohexenyl carotenoid epoxidase from pepper (Capsicum annuum). J. Biol. Chem. 1996, 271, 28861–28867. [Google Scholar] [CrossRef] [PubMed]
- Bugos, R.C.; Hieber, A.D.; Yamamoto, H.Y. Xanthophyll cycle enzymes are members of the lipocalin family, the first identified from plants. J. Biol. Chem. 1998, 273, 15321–15324. [Google Scholar] [CrossRef] [PubMed]
- North, H.M.; Frey, A.; Boutin, J.P.; Sotta, B.; Marion-Poll, A. Analysis of xanthophyll cycle gene expression during the adaptation of Arabidopsis to excess light and drought stress: Changes in RNA steady state levels do not contribute to short-term responses. Plant Sci. 2005, 169, 115–124. [Google Scholar] [CrossRef]
- Schwartz, S.H.; Tan, B.C.; McCarty, D.R.; Welch, W.; Zeevaart, J.A.D. Substrate specificity and kinetics for VP14, a carotenoid cleavage dioxy- genase in the ABA biosynthetic pathway. Biochim. Biophys. Acta 2003, 1619, 9–14. [Google Scholar] [CrossRef]
- North, H.; De Almeida, A.; Boutin, J.P.; Frey, A.; To, A.; Botran, L.; Sotta, B.; Marion-Poll, A. The Arabidopsis ABA-deficient mutant aba4 demonstrates that the major route for stress-induced ABA accumulation is via neoxanthin isomers. Plant J. 2007, 50, 810–824. [Google Scholar] [CrossRef]
- Neuman, H.; Galpaz, N.; Cunningham, F.X.; Zamir, D.; Hirschberg, J. The tomato mutation nxd1 reveals a gene necessary for neoxanthin biosynthesis and demonstrates that violaxanthin is a sufficient precursor for abscisic acid biosynthesis. Plant J. 2014, 78, 80–93. [Google Scholar] [CrossRef]
- Perreau, F.; Frey, A.; Effroy-Cuzzi, D.; Savane, P.; Berger, A.; Gissot, L.; Marion-Poll, A. ABA4 has an essential function in both cis-violaxanthin and cis-neoxanthin synthesis. Plant Physiol. 2020, 184, 1303–1316. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.H.; Tan, B.C.; Gage, D.A.; Zeevaart, J.A.; McCarty, D.R. Specific oxidative cleavage of carotenoids by VP14 of maize. Science 1997, 276, 1872–1874. [Google Scholar] [CrossRef]
- Schwartz, S.H.; Qin, X.; Zeevaart, J.A.D. Characterization of a novel carotenoid cleavage dioxygenase from plants. J. Biol. Chem. 2001, 276, 25208–25211. [Google Scholar] [CrossRef] [PubMed]
- Iuchi, S.; Kobayashi, M.; Taji, T.; Naramoto, M.; Seki, M.; Kato, T.; Tabata, S.; Kakubari, Y.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Regulation of drought tolerance by gene manipulation of 9-cis-epoxycarotenoid dioxygenase, a key enzyme in abscisic acid biosynthesis in Arabidopsis. Plant J. 2001, 27, 325–333. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A global perspective on carotenoids: Metabolism, biotechnology, and benefits for nutrition and health. Progr. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef]
- Tan, B.C.; Cline, K.; McCarty, D.R. Localization and targeting of the VP14 epoxy-carotenoid dioxygenase to chloroplast membranes. Plant J. 2001, 27, 373–382. [Google Scholar] [CrossRef]
- Tan, B.C.; Joseph, L.M.; Deng, W.T.; Liu, L.; Li, Q.B.; Cline, K.; McCarty, D.R. Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J. 2003, 35, 44–56. [Google Scholar] [CrossRef]
- Endo, A.; Sawada, Y.; Takahashi, H.; Okamoto, M.; Ikegami, K.; Koiwai, H.; Seo, M.; Toyomasu, T.; Mitsuhashi, W.; Shinozaki, K.; et al. Drought induction of Arabidopsis 9-cis-epoxycarotenoid dioxygenase occurs in vascular parenchyma cells. Plant Physiol. 2008, 147, 1984–1993. [Google Scholar] [CrossRef]
- Messing, S.A.J.; Gabelli, S.B.; Echeverria, I.; Vogel, J.T.; Guan, J.C.; Tan, B.C.; Klee, H.J.; McCarty, D.R.; Amzel, L.M. Structural insights into maize Viviparous14, a key enzyme in the biosynthesis of the phytohormone abscisic acid. Plant Cell 2010, 22, 2970–2980. [Google Scholar] [CrossRef]
- Bouchnak, I.; Brugière, S.; Moyet, L.; Le Gall, S.; Salvi, D.; Kuntz, M.; Tardif, M.; Rolland, N. Unraveling hidden components of the chloroplast envelope proteome: Opportunities and limits of better MS sensitivity. Mol. Cell Proteom. 2019, 18, 1285–1306. [Google Scholar] [CrossRef] [PubMed]
- Leon-Kloosterziel, K.M.; Alvarez Gil, M.; Ruijs, G.J.; Jacobsen, S.E.; Olszewski, N.E.; Schwartz, S.H.; Zeevaart, J.A.D.; Koornneef, M. Isolation and characterization of abscisic acid-deficient Arabidopsis mutants at two new loci. Plant J. 1996, 10, 655–661. [Google Scholar] [CrossRef]
- Schwartz, S.H.; Leon-Kloosterziel, K.M.; Koornneef, M.; Zeevaart, J.A.D. Biochemical characterization of the aba2 and aba3 mutants in Arabidopsis thaliana. Plant Physiol. 1997, 114, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Guzman, M.; Apostolova, N.; Belles, J.M.; Barrero, J.M.; Piqueras, P.; Ponce, M.R.; Micol, J.L.; Serrano, R.; Rodriguez, P.L. The short-chain alcohol dehydrogenase ABA2 catalyzes the conversion of xanthoxin to abscisic aldehyde. Plant Cell 2002, 14, 1833–1846. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.H.; Endo, A.; Zhou, L.; Penney, J.; Chen, H.C.; Arroyo, A.; Leon, P.; Nambara, E.; Asami, T.; Seo, M.; et al. Unique short-chain dehydrogenase/reductase in Arabidopsis glucose signaling and abscisic acid biosynthesis and functions. Plant Cell 2002, 14, 2723–2743. [Google Scholar] [CrossRef]
- Rook, F.; Corke, F.; Card, R.; Munz, G.; Smith, C.; Bevan, M.W. Impaired sucrose-induction mutants reveal the modulation of sugar-induced starch biosynthetic gene expression by abscisic acid signalling. Plant J. 2001, 26, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Merlot, S.; Mustilli, A.C.; Genty, B.; North, H.; Lefebvre, V.; Sotta, B.; Vavasseur, A.; Giraudat, J. Use of infrared thermal imaging to isolate Arabidopsis mutants defective in stomatal regulation. Plant J. 2002, 30, 601–609. [Google Scholar] [CrossRef]
- Seo, M.; Peeters, A.J.; Koiwai, H.; Oritani, T.; Marion-Poll, A.; Zeevaart, J.A.D.; Koornneef, M.; Kamiya, Y.; Koshiba, T. The Arabidopsis aldehyde oxidase 3 (AAO3) gene product catalyzes the final step in abscisic acid biosynthesis in leaves. Proc. Natl. Acad. Sci. USA 2000, 97, 12908–12913. [Google Scholar] [CrossRef]
- Seo, M.; Aoki, H.; Koiwai, H.; Kamiya, Y.; Nambara, E.; Koshiba, T. Comparative studies on the Arabidopsis aldehyde oxidase (AAO) gene family revealed a major role of AAO3 in ABA biosynthesis in seeds. Plant Cell Physiol. 2004, 45, 1694–1703. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Guzman, M.; Abia, D.; Salinas, J.; Serrano, R.; Rodriguez, P.L. Two new alleles of the abscisic aldehyde oxidase 3 gene reveal its role in abscisic acid biosynthesis in seeds. Plant Physiol. 2004, 135, 325–333. [Google Scholar] [CrossRef]
- Bittner, F.; Oreb, M.; Mendel, R.R. ABA3 is a molybdenum cofactor sulfurase required for activation of aldehyde oxidase and xanthine dehydrogenase in Arabidopsis thaliana. J. Biol. Chem. 2001, 276, 40381–40384. [Google Scholar] [CrossRef]
- Xiong, L.M.; Ishitani, M.; Lee, H.; Zhu, J.K. The Arabidopsis LOS5/ABA3 locus encodes a molybdenum cofactor sulfurase and modulates cold stress- and osmotic stress-responsive gene expression. Plant Cell 2001, 13, 2063–2083. [Google Scholar] [PubMed]
- Kushiro, T.; Okamoto, M.; Nakabayashi, K.; Yamagishi, K.; Kitamura, S.; Asami, T.; Hirai, N.; Koshiba, T.; Kamiya, Y.; Nambara, E. The Arabidopsis cytochrome P450 CYP707A encodes ABA 8′–hydroxylases: Key enzymes in ABA catabolism. EMBO J. 2004, 23, 1647–1656. [Google Scholar] [CrossRef]
- Saito, S.; Hirai, N.; Matsumoto, C.; Ohigashi, H.; Ohta, D.; Sakata, K.; Mizutani, M. Arabidopsis CYP707As encode (+)-abscisic acid 8’-hydroxylase, a key enzyme in the oxidative catabolism of abscisic acid. Plant Physiol. 2004, 134, 1439–1449. [Google Scholar] [CrossRef]
- Okamoto, M.; Kuwahara, A.; Seo, M.; Kushiro, T.; Asami, T.; Hirai, N.; Kamiya, Y.; Koshiba, T.; Nambara, E. CYP707A1 and CYP707A2, which encode abscisic acid 8′-hydroxylases, are indispensable for proper control of seed dormancy and germination in Arabidopsis. Plant Physiol. 2006, 141, 97–107. [Google Scholar] [CrossRef]
- Zhou, R.; Cutler, A.J.; Ambrose, S.J.; Galka, M.M.; Nelson, K.M.; Squires, T.M.; Loewen, M.K.; Jadhav, A.S.; Ross, A.R.S.; Taylor, D.C.; et al. A new abscisic acid catabolic pathway. Plant Physiol. 2004, 134, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, M.; Kushiro, T.; Jikumaru, Y.; Abrams, S.R.; Kamiya, Y.; Seki, M.; Nambara, E. ABA 9′-hydroxylation is catalysed by CYP707A in Arabidopsis. Phytochemistry 2011, 72, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.K.; Ye, M.; Li, B.; Noel, J.P. Co-evolution of hormone metabolism and signaling networks expands plant adaptive plasticity. Cell 2016, 166, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.K.; Doucet, C.J.; Hou, B.; Jackson, R.G.; Abrams, S.R.; Bowles, D.J. Resolution of (+)-abscisic acid using an Arabidopsis glycosyltransferase. Tetrahedron Asymmetry 2005, 16, 143–147. [Google Scholar] [CrossRef]
- Priest, D.M.; Ambrose, S.J.; Vaistij, F.E.; Elias, L.; Higgins, G.S.; Ross, A.R.; Abrams, S.R.; Bowles, D.J. Use of the glucosyltransferase UGT71B6 to disturb abscisic acid homeostasis in Arabidopsis thaliana. Plant J. 2006, 46, 492–502. [Google Scholar] [CrossRef]
- Dong, T.; Xu, Z.Y.; Park, Y.; Kim, D.H.; Lee, Y.; Hwang, I. Abscisic acid uridine diphosphate glucosyltransferases play a crucial role in abscisic acid homeostasis in Arabidopsis. Plant Physiol. 2014, 165, 277–289. [Google Scholar] [CrossRef]
- Liu, Z.; Yan, J.P.; Li, D.K.; Luo, Q.; Yan, Q.; Liu, Z.B.; Ye, L.M.; Wang, J.M.; Li, X.F.; Yang, Y. UDP-Glucosyltransferase71C5, a major glucosyltransferase, mediates abscisic acid homeostasis in Arabidopsis. Plant Physiol. 2015, 167, 1659–1670. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.T.; Liu, F.F.; Xiao, D.W.; Jiang, X.Y.; Li, P.; Zhao, S.M.; Hou, B.K.; Li, Y.J. The Arabidopsis UDP-glycosyltransferase75B1, conjugates abscisic acid and affects plant response to abiotic stresses. Plant Mol. Biol. 2020, 102, 389–401. [Google Scholar] [CrossRef]
- Lee, K.H.; Piao, H.L.; Kim, H.Y.; Choi, S.M.; Jiang, F.; Hartung, W.; Hwang, I.; Kwak, J.M.; Lee, I.J.; Hwang, I. Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid. Cell 2006, 126, 1109–1120. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.Y.; Lee, K.H.; Dong, T.; Jeong, J.C.; Jin, J.B.; Kanno, Y.; Kim, D.H.; Kim, S.Y.; Seo, M.; Bressan, R.A.; et al. A vacuolar beta-glucosidase homolog that possesses glucose-conjugated abscisic acid hydrolyzing activity plays an important role in osmotic stress responses in Arabidopsis. Plant Cell 2012, 24, 2184–2199. [Google Scholar] [CrossRef] [PubMed]
- Karssen, C.M.; Brinkhorstvanderswan, D.L.C.; Breekland, A.E.; Koornneef, M. Induction of dormancy during seed development by endogenous abscisic acid: Studies on abscisic acid deficient genotypes of Arabidopsis thaliana (L.) Heynh. Planta 1983, 157, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Frey, A.; Godin, B.; Bonnet, M.; Sotta, B.; Marion-Poll, A. Maternal synthesis of abscisic acid controls seed development and yield in Nicotiana plumbaginifolia. Planta 2004, 218, 958–964. [Google Scholar] [CrossRef]
- Kanno, Y.; Jikumaru, Y.; Hanada, A.; Nambara, E.; Abrams, S.R.; Kamiya, Y.; Seo, M. Comprehensive hormone profiling in developing Arabidopsis seeds: Examination of the site of ABA biosynthesis, ABA transport and hormone interactions. Plant Cell Physiol. 2010, 51, 1988–2001. [Google Scholar] [CrossRef]
- Seo, M.; Kanno, Y.; Frey, A.; North, H.M.; Marion-Poll, A. Dissection of Arabidopsis NCED9 promoter regulatory regions reveals a role for ABA synthesized in embryos in the regulation of GA-dependent seed germination. Plant Sci. 2016, 246, 91–97. [Google Scholar] [CrossRef]
- Lefebvre, V.; North, H.; Frey, A.; Sotta, B.; Seo, M.; Okamoto, M.; Nambara, E.; Marion-Poll, A. Functional analysis of Arabidopsis NCED6 and NCED9 genes indicates that ABA synthesized in the endosperm is involved in the induction of seed dormancy. Plant J. 2006, 45, 309–319. [Google Scholar] [CrossRef]
- Frey, A.; Effroy, D.; Lefebvre, V.; Seo, M.; Perreau, F.; Berger, A.; Sechet, J.; To, A.; North, H.M.; Marion-Poll, A. Epoxycarotenoid cleavage by NCED5 fine-tunes ABA accumulation and affects seed dormancy and drought tolerance with other NCED family members. Plant J. 2012, 70, 501–512. [Google Scholar] [CrossRef]
- Chauffour, F.; Bailly, M.; Perreau, F.; Cueff, G.; Suzuki, H.; Collet, B.; Frey, A.; Clément, G.; Soubigou-Taconnat, L.; Balliau, T.; et al. Multi-omics analysis reveals sequential roles for ABA during Arabidopsis seed maturation. Plant Physiol. 2019, 180, 1198–2018. [Google Scholar] [CrossRef] [PubMed]
- Chono, M.; Honda, I.; Shinoda, S.; Kushiro, T.; Kamiya, Y.; Nambara, E.; Kawakami, N.; Kaneko, S.; Watanabe, Y. Field studies on the regulation of abscisic acid content and germinability during grain development of barley: Molecular and chemical analysis of pre-harvest sprouting. J. Exp. Bot. 2006, 57, 2421–2434. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.C.; Schwartz, S.H.; Zeevaart, J.A.D.; McCarty, D.R. Genetic control of abscisic acid biosynthesis in maize. Proc. Natl. Acad. Sci. USA 1997, 94, 12235–122240. [Google Scholar] [CrossRef]
- Chono, M.; Matsunaka, H.; Seki, M.; Fujita, M.; Kiribuchi-Otobe, C.; Oda, S.; Kojima, H.; Kobayashi, D.; Kawakami, N. Isolation of a wheat (Triticum aestivum L.) mutant in ABA 8′-hydroxylase gene: Effect of reduced ABA catabolism on germination inhibition under field condition. Breed. Sci. 2013, 63, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Kendall, S.L.; Hellwege, A.; Marriot, P.; Whalley, C.; Graham, I.A.; Penfield, S. Induction of dormancy in Arabidopsis summer annuals requires parallel regulation of DOG1 and hormone metabolism by low temperature and CBF transcription factors. Plant Cell 2011, 23, 2568–2580. [Google Scholar] [CrossRef]
- Tuan, P.A.; Nguyen, T.N.; Jordan, M.C.; Ayele, B.T. A shift in abscisic acid/gibberellin balance underlies retention of dormancy induced by seed development temperature. Plant Cell Environ. 2020. [Google Scholar] [CrossRef]
- Brunel-Muguet, S.; D’Hooghe, P.; Bataillé, M.-P.; Larré, C.; Kim, T.-H.; Trouverie, J.; Avice, J.-C.; Etienne, P.; Dürr, C. Heat stress during seed filling interferes with sulfur restriction on grain composition and seed germination in oilseed rape (Brassica napus L.). Front. Plant Sci. 2015, 6, 213. [Google Scholar] [CrossRef]
- Suriyasak, C.; Oyama, Y.; Ishida, T.; Mashiguchi, K.; Yamaguchi, S.; Hamaoka, N.; Iwaya-Inoue, M.; Ishibashi, Y. Mechanism of delayed seed germination caused by high temperature during grain filling in rice (Oryza sativa L.). Sci. Rep. 2020, 10, 17378. [Google Scholar] [CrossRef]
- Alboresi, A.; Gestin, C.; Leydecker, M.T.; Bedu, M.; Meyer, C.; Truong, H.N. Nitrate, a signal relieving seed dormancy in Arabidopsis. Plant Cell Environ. 2005, 28, 500–512. [Google Scholar] [CrossRef]
- He, H.; de Souza Vidigal, D.; Snoek, L.B.; Schnabel, S.; Nijveen, H.; Hilhorst, H.; Bentsink, L. Interaction between parental environment and genotype affects plant and seed performance in Arabidopsis. J. Exp. Bot. 2014, 65, 6603–6615. [Google Scholar] [CrossRef] [PubMed]
- Duermeyer, L.; Khodapanahi, E.; Yan, D.; Krapp, A.; Rothstein, S.J.; Nambara, E. Regulation of seed dormancy and germination by nitrate. Seed Sci. Res. 2018, 28, 150–157. [Google Scholar] [CrossRef]
- Matakiadis, T.; Alboresi, A.; Jikumaru, Y.; Tatematsu, K.; Pichon, O.; Renou, J.P.; Kamiya, Y.; Nambara, E.; Truong, H.N. The Arabidopsis abscisic acid catabolic gene CYP707A2 plays a key role in nitrate control of seed dormancy. Plant Physiol. 2009, 149, 949–960. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. The control of seed dormancy and germination by temperature, light and nitrate. Bot. Rev. 2020, 86, 39–75. [Google Scholar] [CrossRef]
- He, H.; Willems, L.; Batushansky, A.; Fait, A.; Hanson, J.; Nijveen, H.; Hilhorst, H.W.M.; Bentsink, L. Effects of parental temperature and nitrate on seed performance are reflected by partly overlapping genetic and metabolic pathways. Plant Cell Physiol. 2016, 57, 473–487. [Google Scholar] [CrossRef]
- Chen, F.; Zhou, W.; Yin, H.; Luo, X.; Chen, W.; Liu, X.; Wang, X.; Meng, Y.; Feng, L.; Qin, Y.; et al. Shading of the mother plant during seed development promotes subsequent seed germination in soybean. J. Exp. Bot. 2020, 71, 2072–2084. [Google Scholar] [CrossRef]
- Carbonero, P.; Iglesias-Fernandez, R.; Vicente-Carbajosa, J. The AFL subfamily of B3 transcription factors: Evolution and function in angiosperms seeds. J. Exp. Bot. 2017, 68, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Lepiniec, L.; Devic, M.; Roscoe, T.; Bouyer, D.; Zhou, D.X.; Boulard, C.; Baud, S.; Dubreucq, B. Molecular and epigenetic regulations and functions of the LAFL transcriptional regulators that control seed development. Plant Reprod. 2018, 31, 291–307. [Google Scholar] [CrossRef]
- Gazzarrini, S.; Tsuchiya, Y.; Lumba, S.; Okamoto, M.; McCourt, P. The transcription factor FUSCA3 controls developmental timing in Arabidopsis through the action of the hormones gibberellins and abscisic acid. Dev. Cell 2004, 7, 373–385. [Google Scholar] [CrossRef] [PubMed]
- Koornneef, M.; Reuling, G.; Karssen, C.M. The isolation and characterization of abscisic acid-insensitive mutants of Arabidopsis thaliana. Physiol. Plant. 1984, 61, 377–383. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, H.; Ding, L.; Soppe, W.J.J.; Xiang, Y. REVERSAL OF RDO5 1, a homolog of rice Seed dormancy4, interacts with bHLH57 and controls ABA biosynthesis and seed dormancy in Arabidopsis. Plant Cell 2020, 32, 1933–1948. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Nakabayashi, K.; Ding, J.; He, F.; Bentsink, L.; Soppe, W.J.J. Reduced Dormancy5 encodes a protein phosphatase 2C that is required for seed dormancy in Arabidopsis. Plant Cell 2014, 26, 4362–4375. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, K.; Takeuchi, Y.; Ebana, K.; Miyao, A.; Hirochika, H.; Hara, N.; Ishiyama, K.; Kobayashi, M.; Ban, Y.; Hattori, T.; et al. Molecular cloning of Sdr4, a regulator involved in seed dormancy and domestication of rice. Proc. Natl. Acad. Sci. USA 2010, 107, 5792–5797. [Google Scholar] [CrossRef]
- Je, J.; Chen, H.; Song, C.; Lim, C.O. Arabidopsis DREB2C modulates ABA biosynthesis during germination. Biochem. Biophys. Res. Commun. 2014, 452, 91–98. [Google Scholar] [CrossRef]
- Shu, K.; Chen, Q.; Wu, Y.; Liu, X.D.; Zhang, H.; Wang, P.; Li, Y.; Wang, S.; Tang, S.; Liu, C.; et al. ABI4 mediates antagonistic effects of abscisic acid and gibberellins at transcript and protein levels. Plant J. 2016, 85, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Dietz, K.J.; Vogel, M.O.; Viehhauser, A. AP2/EREBP transcription factors are part of gene regulatory networks and integrate metabolic, hormonal and environmental signals in stress acclimation and retrograde signalling. Protoplasma 2010, 245, 3–14. [Google Scholar] [CrossRef]
- Shu, K.; Zhang, H.; Wang, S.; Chen, M.; Wu, Y.; Tang, S.; Liu, C.; Feng, Y.; Cao, X.; Xie, Q. ABI4 regulates primary seed dormancy by regulating the biogenesis of abscisic acid and gibberellins in Arabidopsis. PLoS Genet. 2013, 9, e1003577. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, U.; Luo, X.; Zhou, W.; Shu, K. Multifaceted signaling networks mediated by Abscisic Acid Insensitive 4. Plant Commun. 2020, 1, 100040. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.G.; Lee, K.; Seo, P.J. The Arabidopsis MYB96 transcription factor plays a role in seed dormancy. Plant Mol. Biol. 2015, 87, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Seo, P.J. Coordination of seed dormancy and germination processes by MYB96. Plant Signal. Behav. 2015, 10, e1056423. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, W.; Fang, C.; Xu, F.; Liu, Y.; Wang, Z.; Yang, R.; Zhang, M.; Liu, S.; Lu, S.; et al. Parallel selection on a dormancy gene during domestication of crops from multiple families. Nature Genet. 2018, 50, 1435–1441. [Google Scholar] [CrossRef]
- Chen, H.; Tong, J.; Fu, W.; Liang, Z.; Ruan, J.; Yu, Y.; Song, X.; Yuan, L.; Xiao, L.; Liu, J.; et al. The H3K27me3 demethylase RELATIVE OF EARLY FLOWERING6 suppresses seed dormancy by inducing abscisic acid catabolism. Plant Physiol. 2020, 184, 1969–1978. [Google Scholar] [CrossRef]
- Zhao, M.; Yang, S.; Liu, X.; Wu, K. Arabidopsis histone demethylases LDL1 and LDL2 control primary seed dormancy by regulating DELAY OF GERMINATION 1 and ABA signaling-related genes. Front. Plant Sci. 2015, 6, 159. [Google Scholar] [CrossRef]
- Liu, Y.; Koornneef, M.; Soppe, W.J.J. The absence of histone h2b monoubiquitination in the arabidopsis hub1 (rdo4) mutant reveals a role for chromatin remodeling in seed dormancy. Plant Cell 2007, 19, 433–444. [Google Scholar] [CrossRef]
- Wang, Z.; Cao, H.; Sun, Y.; Li, X.; Chen, F.; Carles, A.; Li, Y.; Ding, M.; Zhang, C.; Deng, X.; et al. Arabidopsis paired amphipathic helix proteins SLN1 and SLN2 redundantly regulate primary seed dormancy via abscisic acid–ethylene antagonism mediated by histone deacetylation. Plant Cell 2013, 25, 149–166. [Google Scholar] [CrossRef]
- Yao, M.; Chen, W.; Kong, J.; Zhang, X.; Shi, N.; Zhong, S.; Ma, P.; Gallusci, P.; Jackson, S.; Liu, Y.; et al. METHYLTRANSFERASE1 and ripening modulate vivipary during tomato fruit development. Plant Physiol. 2020, 183, 1883–1897. [Google Scholar] [CrossRef] [PubMed]
- Sano, N.; Rajjou, L.; North, H.M. Lost in Translation: Physiological roles of stored mRNAs in seed germination. Plants 2020, 9, 347. [Google Scholar] [CrossRef]
- Jacobsen, J.V.; Pearce, D.W.; Poole, A.T.; Pharis, R.P.; Mander, L.N. Abscisic acid, phaseic acid and gibberellin contents associated with dormancy and germination in barley. Physiol. Plant. 2002, 115, 428–441. [Google Scholar] [CrossRef]
- Ali-Rachedi, S.; Bouinot, D.; Wagner, M.H.; Bonnet, M.; Sotta, B.; Grappin, P.; Jullien, M. Changes in endogenous abscisic acid levels during dormancy release and maintenance of mature seeds: Studies with the Cape Verde Islands ecotype, the dormant model of Arabidopsis thaliana. Planta 2004, 219, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Millar, A.A.; Jacobsen, J.V.; Ross, J.J.; Helliwell, C.A.; Poole, A.T.; Scofield, G.; Reid, J.B.; Gubler, F. Seed dormancy and ABA metabolism in Arabidopsis and barley: The role of ABA 8′-hydroxylase. Plant J. 2006, 45, 942–954. [Google Scholar] [CrossRef] [PubMed]
- Lechat, M.M.; Pouvreau, J.B.; Péron, T.; Gauthier, M.; Montiel, G.; Véronési, C.; Todoroki, Y.; Le Bizec, B.; Monteau, F.; Macherel, D.; et al. PrCYP707A1, an ABA catabolic gene, is a key component of Phelipanche ramosa seed germination in response to the strigolactone analogue GR24. J. Exp. Bot. 2012, 63, 5311–5322. [Google Scholar] [CrossRef] [PubMed]
- Brun, G.; Thoiron, S.; Braem, L.; Pouvreau, J.B.; Montiel, G.; Lechat, M.-M.; Simier, P.; Gevaert, K.; Goormachtig, S.; Delavault, P. CYP707As are effectors of karrikin and strigolactone signalling pathways in Arabidopsis thaliana and parasitic plants. Plant Cell Environ. 2019, 42, 2612–2626. [Google Scholar] [CrossRef]
- Debeaujon, I.; Koornneef, M. Gibberellin requirement for Arabidopsis seed germination is determined both by testa characteristics and embryonic abscisic acid. Plant Physiol. 2000, 122, 415–424. [Google Scholar] [CrossRef]
- Barrero, J.M.; Jacobsen, J.V.; Talbot, M.J.; White, R.G.; Swain, S.M.; Garvin, D.F.; Gubler, F. Grain dormancy and light quality effects on germination in the model grass Brachypodium distachyon. New Phytol. 2012, 193, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Basbouss-Serhal, I.; Pateyron, S.; Cochet, F.; Leymarie, J.; Bailly, C. 5′ to 3′ mRNA decay contributes to the regulation of Arabidopsis seed germination by dormancy. Plant Physiol. 2017, 173, 1709. [Google Scholar] [CrossRef] [PubMed]
- Bethke, P.C.; Libourel, I.G.L.; Aoyama, N.; Chung, Y.Y.; Still, D.W.; Jones, R.L. The Arabidopsis aleurone layer responds to nitric oxide, gibberellin, and abscisic acid and is sufficient and necessary for seed dormancy. Plant Physiol. 2007, 143, 1173–1188. [Google Scholar] [CrossRef]
- Lee, K.P.; Piskurewicz, U.; Tureckova, V.; Strnad, M.; Lopez-Molina, L. A seed coat bedding assay shows that RGL2-dependent release of abscisic acid by the endosperm controls embryo growth in Arabidopsis dormant seeds. Proc. Natl. Acad. Sci. USA 2010, 107, 19108–19113. [Google Scholar] [CrossRef]
- Kang, J.; Yim, S.; Choi, H.; Kim, A.; Lee, K.P.; Lopez-Molina, L.; Martinoia, E.; Lee, Y. Abscisic acid transporters cooperate to control seed germination. Nat. Commun. 2015, 6, 8113. [Google Scholar] [CrossRef]
- Topham, A.T.; Taylor, R.E.; Yan, D.; Nambara, E.; Johnston, I.G.; Bassel, G.W. Temperature variability is integrated by a spatially embedded decision-making center to break dormancy in Arabidopsis seeds. Proc. Natl. Acad. Sci. USA 2017, 114, 6629–6634. [Google Scholar] [CrossRef]
- Yang, L.; Liu, S.; Lin, R. The role of light in regulating seed dormancy and germination. J. Integr. Plant Biol. 2020, 62, 1310–1326. [Google Scholar] [CrossRef]
- Seo, M.; Hanada, A.; Kuwahara, A.; Endo, A.; Okamoto, M.; Yamauchi, Y.; North, H.; Marion-Poll, A.; Sun, T.P.; Koshiba, T.; et al. Regulation of hormone metabolism in Arabidopsis seeds: Phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. Plant J. 2006, 48, 354–366. [Google Scholar] [CrossRef]
- Sawada, Y.; Aoki, M.; Nakaminami, K.; Mitsuhashi, W.; Tatematsu, K.; Kushiro, T.; Koshiba, T.; Kamiya, Y.; Inoue, Y.; Nambara, E.; et al. Phytochrome- and gibberellin-mediated regulation of abscisic acid metabolism during germination of photoblastic lettuce seeds. Plant Physiol. 2008, 146, 1386–1396. [Google Scholar] [CrossRef]
- Gubler, F.; Hughes, T.; Waterhouse, P.; Jacobsen, J. Regulation of dormancy in barley by blue light and after-ripening: Effects on abscisic acid and gibberellin metabolism. Plant Physiol. 2008, 147, 886–896. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.H.; Sechet, J.; Bailly, C.; Leymarie, J.; Corbineau, F. Inhibition of germination of dormant barley (Hordeum vulgare L.) grains by blue light as related to oxygen and hormonal regulation. Plant Cell Environ. 2014, 37, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Toh, S.; Imamura, A.; Watanabe, A.; Nakabayashi, K.; Okamoto, M.; Jikumaru, Y.; Hanada, A.; Aso, Y.; Ishiyama, K.; Tamura, N.; et al. High temperature-induced abscisic acid biosynthesis and its role in the inhibition of gibberellin action in Arabidopsis seeds. Plant Physiol. 2008, 146, 1368–1385. [Google Scholar] [CrossRef]
- Huo, H.; Dahal, P.; Kunusoth, K.; McCallum, C.M.; Bradford, K.J. Expression of 9-cis-EPOXYCAROTENOID DIOXYGENASE4 is essential for thermoinhibition of lettuce seed germination but not for seed development or stress tolerance. Plant Cell 2013, 25, 884–900. [Google Scholar] [CrossRef]
- Izydorczyk, C.; Nguyen, T.N.; Jo, S.H.; Son, S.H.; Tuan, P.A.; Ayele, B.T. Spatiotemporal modulation of abscisic acid and gibberellin metabolism and signalling mediates the effects of suboptimal and supraoptimal temperatures on seed germination in wheat (Triticum aestivum L.). Plant Cell Environ. 2018, 41, 1022–1037. [Google Scholar] [CrossRef]
- Carrillo-Barral, N.; Matilla, A.J.; Rodriguez-Gacio, M.C.; Iglesias-Fernandez, R. Nitrate affects sensu-stricto germination of after-ripened Sisymbrium officinale seeds by modifying expression of SoNCED5, SoCYP707A2 and SoGA3ox2 genes. Plant Sci. 2014, 217–218, 99–108. [Google Scholar] [CrossRef]
- Lee, K.; Lee, H.G.; Yoon, S.; Kim, H.U.; Seo, P.J. The Arabidopsis MYB96 transcription factor is a positive regulator of ABSCISIC ACID-INSENSITIVE4 in the control of seed germination. Plant Physiol. 2015, 168, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Yaish, M.W.; El-Kereamy, A.; Zhu, T.; Beatty, P.H.; Good, A.G.; Bi, Y.M.; Rothstein, S.J. The APETALA-2-like transcription factor OsAP2-39 controls key interactions between abscisic acid and gibberellin in rice. PLoS Genet. 2010, 6, e1001098. [Google Scholar] [CrossRef]
- De Wit, M.; Galvão, V.C.; Fankhauser, C. Light-mediated hormonal regulation of plant growth and development. Annu. Rev. Plant Biol. 2016, 67, 513–537. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Yamaguchi, S.; Lim, S.; Oh, E.; Park, J.; Hanada, A.; Kamiya, Y.; Choi, G. SOMNUS, a CCCH-type zinc finger protein in Arabidopsis, negatively regulates light-dependent seed germination downstream of PIL5. Plant Cell 2008, 20, 1260–1277. [Google Scholar] [CrossRef]
- Park, J.; Lee, N.; Kim, W.; Lim, S.; Cho, G. ABI3 and PIL5 collaboratively activate the expression of SOMNUS by directly binding to its promoter in imbibed Arabidopsis seeds. Plant Cell 2011, 23, 1404–1415. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.P.; Piskurewicz, U.; Tureckova, V.; Carat, S.; Chappuis, R.; Strnad, M.; Fankhauser, C.; Lopez-Molina, L. Spatially and genetically distinct control of seed germination by phytochromes A and B. Genes Dev. 2012, 26, 1984–1996. [Google Scholar] [CrossRef] [PubMed]
- Barros-Galvao, T.; Dave, A.; Gilday, A.D.; Harvey, D.; Vaistij, F.E.; Graham, I.A. ABA INSENSITIVE4 promotes rather than represses PHYA-dependent seed germination in Arabidopsis thaliana. New Phytol. 2020, 226, 953–956. [Google Scholar] [CrossRef] [PubMed]
- Vu, L.D.; Xu, X.; Gevaert, K.; De Smet, I. Developmental plasticity at high temperature. Plant Physiol. 2019, 181, 399–411. [Google Scholar] [CrossRef]
- Lim, S.; Park, J.; Lee, N.; Jeong, J.; Toh, S.; Watanabe, A.; Kim, J.; Kang, H.; Kim, D.H.; Kawakami, N.; et al. ABA-insensitive3, ABA-insensitive5, and DELLAs interact to activate the expression of SOMNUS and other high-temperature-inducible genes in imbibed seeds in Arabidopsis. Plant Cell 2013, 25, 4863–4878. [Google Scholar] [CrossRef]
- Buijs, G. A perspective on secondary seed dormancy in Arabidopsis thaliana. Plants 2020, 9, 749. [Google Scholar] [CrossRef]
- Martel, C.; Blair, L.K.; Donohue, K. PHYD prevents secondary dormancy establishment of seeds exposed to high temperature and is associated with lower PIL5 accumulation. J. Exp. Bot. 2018, 69, 3157–3169. [Google Scholar] [CrossRef]
- Chiu, R.S.; Nahal, H.; Provart, N.J.; Gazzarrini, S. The role of the Arabidopsis FUSCA3 transcription factor during inhibition of seed germination at high temperature. BMC Plant Biol. 2012, 12, 15. [Google Scholar] [CrossRef]
- Yan, D.; Easwaran, V.; Chau, V.; Okamoto, M.; Ierullo, M.; Kimura, M.; Endo, A.; Yano, R.; Pasha, A.; Gong, Y.; et al. NIN-like protein 8 is a master regulator of nitrate-promoted seed germination in Arabidopsis. Nat. Commun. 2016, 7, 13179. [Google Scholar] [CrossRef]
- Huang, X.; Han, B. Natural variations and genome-wide association studies in crop plants. Annu. Rev. Plant Biol. 2014, 65, 531–551. [Google Scholar] [CrossRef]
- Ogura, T.; Busch, W. From phenotypes to causal sequences: Using genome wide association studies to dissect the sequence basis for variation of plant development. Curr. Opin. Plant Biol. 2015, 23, 98–108. [Google Scholar] [CrossRef]
- Bazakos, C.; Hanemian, M.; Trontin, C.; Jiménez-Gómez, J.M.; Loudet, O. New strategies and tools in quantitative genetics: How to go from the phenotype to the genotype. Annu. Rev. Plant Biol. 2017, 68, 435–455. [Google Scholar] [CrossRef]
- Varshney, R.K.; Sinha, P.; Singh, V.K.; Kumar, A.; Zhang, Q.; Bennetzen, J.L. 5Gs for crop genetic improvement. Curr. Opin. Plant Biol. 2020, 56, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Weigel, D.; Mott, R. The 1001 genomes project for Arabidopsis thaliana. Genome Biol. 2009, 10, 107. [Google Scholar] [CrossRef]
- The 1001 Genomes Consortium. 1,135 genomes reveal the global pattern of polymorphism in Arabidopsis thaliana. Cell 2016, 166, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Blanco, C.; Koornneef, M. Naturally occurring variation in Arabidopsis: An underexploited resource for plant genetics. Trends Plant Sci. 2000, 5, 22–29. [Google Scholar] [CrossRef]
- Weigel, D. Natural variation in Arabidopsis: From molecular genetics to ecological genomics. Plant Physiol. 2012, 158, 2–22. [Google Scholar] [CrossRef] [PubMed]
- Preston, J.; Tatematsu, K.; Kanno, Y.; Hobo, T.; Kimura, M.; Jikumaru, Y.; Yano, R.; Kamiya, Y.; Nambara, E. Temporal expression patterns of hormone metabolism genes during imbibition of Arabidopsis thaliana seeds: A comparative study on dormant and non-dormant accessions. Plant Cell Physiol. 2009, 50, 1786–1800. [Google Scholar] [CrossRef]
- Kashiwakura, Y.; Kobayashi, D.; Jikumaru, Y.; Takebayashi, Y.; Nambara, E.; Seo, M.; Kamiya, Y.; Kushiro, T.; Kawakami, N. Highly sprouting-tolerant wheat grain exhibits extreme dormancy and cold imbibition-resistant accumulation of abscisic acid. Plant Cell Physiol. 2016, 57, 715–732. [Google Scholar] [CrossRef]
- Argyris, J.; Dahal, P.; Hayashi, E.; Still, D.W.; Bradford, K.J. Genetic variation for lettuce seed thermoinhibition is associated with temperature-sensitive expression of abscisic acid, gibberellin, and ethylene biosynthesis, metabolism, and response genes. Plant Physiol. 2008, 148, 926–947. [Google Scholar] [CrossRef]
- Howitt, C.A.; Pogson, B.J. Carotenoid accumulation and function in seeds and non-green tissues. Plant Cell Environ. 2006, 29, 435–445. [Google Scholar] [CrossRef]
- Fang, J.; Chai, C.; Qian, Q.; Li, C.; Tang, J.; Sun, L.; Huang, Z.; Guo, X.; Sun, C.; Liu, M.; et al. Mutations of genes in synthesis of the carotenoid precursors of ABA lead to pre-harvest sprouting and photo-oxidation in rice. Plant J. 2008, 54, 177–189. [Google Scholar] [CrossRef]
- Stanley, L.; Yuan, Y.W. Transcriptional regulation of carotenoid biosynthesis in plants: So many regulators, so little consensus. Front. Plant Sci. 2019, 10, 1017. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Jorge, S.; Mehrshahi, P.; Magallanes-Lundback, M.; Lipka, A.E.; Angelovici, R.; Gore, M.A.; DellaPenna, D. ZEAXANTHIN EPOXIDASE activity potentiates carotenoid degradation in maturing seed. Plant Physiol. 2016, 171, 1837–1851. [Google Scholar] [CrossRef] [PubMed]
- Owens, B.F.; Lipka, A.E.; Magallanes-Lundback, M.; Tiede, T.; Diepenbrock, C.H.; Kandianis, C.B.; Kim, E.; Cepela, J.; Mateos-Hernandez, M.; Buell, C.R.; et al. foundation for provitamin a biofortification of maize: Genome-wide association and genomic prediction models of carotenoid levels. Genetics 2014, 198, 1699–1716. [Google Scholar] [CrossRef]
- Owens, B.F.; Mathew, D.; Diepenbrock, C.H.; Tiede, T.; Wu, D.; Mateos-Hernandez, M.; Gore, M.A.; Rocheford, T. Genome-wide association study and pathway-level analysis of kernel color in maize. G3 2019, 9, 1945–1955. [Google Scholar] [CrossRef]
- Cruet-Burgos, C.; Cox, S.; loerger, B.P.; Perumal, R.; Hu, Z.; Herald, T.J.; Bean, S.R.; Rhodes, D.H. Advancing provitamin a biofortification in sorghum: Genome-wide association studies of grain carotenoids in global germplasm. Plant Genome 2020, 13, e20013. [Google Scholar] [CrossRef]
- Capelle, V.; Remoué, C.; Moreau, L.; Reyss, A.; Mahé, A.; Massonneau, A.; Falque, M.; Charcosset, A.; Thévenot, C.; Rogowsky, P.; et al. QTLs and candidate genes for desiccation and abscisic acid content in maize kernels. BMC Plant Biol. 2010, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Argyris, J.; Truco, M.J.; Ochoa, O.; McHale, L.; Dahal, P.; Van Deynze, A.; Michelmore, R.W.; Bradford, K.J. A gene encoding an abscisic acid biosynthetic enzyme (LsNCED4) collocates with the high temperature germination locus Htg6.1 in lettuce (Lactuca sp.). Theor. Appl. Genet. 2011, 122, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Guo, Y.; Liu, Y.; Zhang, F.; Wang, Z.; Wang, H.; Wang, F.; Li, D.; Mao, D.; Luan, S.; et al. 9-cis-epoxycarotenoid dioxygenase 3 regulates plant growth and enhances multi-abiotic stress tolerance in rice. Front. Plant Sci. 2018, 9, 162. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, H.; Wu, G.; Sun, J.; Hao, L.; Ge, X.; Yu, J.; Wang, W. Sequence variation and expression analysis of seed dormancy- and germination-associated ABA- and GA-related genes in rice cultivars. Front. Plant Sci. 2011, 2, 17. [Google Scholar] [CrossRef]
- Kalladan, R.; Lasky, J.R.; Sharma, S.; Kumar, M.N.; Juenger, T.E.; Des Marais, D.L.; Verslues, P.E. Natural variation in 9-cis-epoxycartenoid dioxygenase 3 and ABA accumulation. Plant Physiol. 2019, 179, 1620–1631. [Google Scholar] [CrossRef] [PubMed]
- Clerkx, E.J.; El-Lithy, M.E.; Vierling, E.; Ruys, G.J.; Blankestijn-De Vries, H.; Groot, S.P.; Vreugdenhil, D.; Koornneef, M. Analysis of natural allelic variation of Arabidopsis seed germination and seed longevity traits between the accessions Landsberg erecta and Shakdara, using a new recombinant inbred line population. Plant Physiol. 2004, 135, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Amer Hamzah, M.; Mohd Kasim, N.A.; Shamsuddin, A.; Mustafa, N.; Mohamad Rusli, N.I.; Teh, C.Y.; Ho, C.L. Nucleotide variations of 9-cis-epoxycarotenoid dioxygenase 2 (NCED2) and pericarp coloration genes (Rc and Rd) from upland rice varieties. 3 Biotech 2020, 10, 105. [Google Scholar] [CrossRef] [PubMed]
- Lyu, J.; Zhang, S.; Dong, Y.; He, W.; Zhang, J.; Deng, X.; Zhang, Y.; Li, X.; Li, B.; Huang, W.; et al. Analysis of elite variety tag SNPs reveals an important allele in upland rice. Nat. Commun. 2013, 4, 2138. [Google Scholar] [CrossRef] [PubMed]
- Colasuonno, P.; Lozito, M.L.; Marcotuli, I.; Nigro, D.; Giancaspro, A.; Mangini, G.; De Vita, P.; Mastrangelo, A.M.; Pecchioni, N.; Houston, K.; et al. The carotenoid biosynthetic and catabolic genes in wheat and their association with yellow pigments. BMC Genom. 2017, 18, 122. [Google Scholar] [CrossRef]
- Colasuonno, P.; Marcotuli, I.; Lozito, M.L.; Simeone, R.; Blanco, A.; Gadaleta, A. Characterization of aldehyde oxidase (AO) genes involved in the accumulation of carotenoid pigments in wheat grain. Front. Plant Sci. 2017, 8, 863. [Google Scholar] [CrossRef]
- Kan, G.; Zhang, W.; Yang, W.; Ma, D.; Zhang, D.; Hao, D.; Hu, Z.; Yu, D. Association mapping of soybean seed germination under salt stress. Mol. Genet. Genom. 2015, 290, 2147–2162. [Google Scholar] [CrossRef]
- Nakamura, S.; Chono, M.; Abe, F.; Miura, H. Mapping a diploid wheat abscisic acid 8′-hydroxylase homologue in the seed dormancy QTL region on chromosome 5Am. Euphytica 2010, 171, 111–120. [Google Scholar] [CrossRef]
- Kalladan, R.; Lasky, J.R.; Chang, T.Z.; Sharma, S.; Juenger, T.E.; Verslues, P.E. Natural variation identifies genes affecting drought-induced abscisic acid accumulation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2017, 114, 11536–11541. [Google Scholar] [CrossRef]
- Farh, K.K.; Marson, A.; Zhu, J.; Kleinewietfeld, M.; Housley, W.J.; Beik, S.; Shoresh, N.; Whitton, H.; Ryan, R.J.; Shishkin, A.A.; et al. Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature 2015, 518, 337–343. [Google Scholar] [CrossRef]
- Liu, S.; Li, C.; Wang, H.; Wang, S.; Yang, S.; Liu, X.; Yan, J.; Li, B.; Beatty, M.; Zastrow-Hayes, G.; et al. Mapping regulatory variants controlling gene expression in drought response and tolerance in maize. Genome Biol. 2020, 21, 163. [Google Scholar] [CrossRef]
- De Villemereuil, P.; Gaggiotti, O.E.; Mouterde, M.; Till-Bottraud, I. Common garden experiments in the genomic era: New perspectives and opportunities. Heredity 2016, 116, 249–254. [Google Scholar] [CrossRef]
- Ferrero-Serrano, Á.; Assmann, S.M. Phenotypic and genome-wide association with the local environment of Arabidopsis. Nat. Ecol. Evol. 2019, 3, 274–285. [Google Scholar] [CrossRef]
- Lee, G.A.; Jeon, Y.A.; Lee, H.S.; Hyun, D.Y.; Lee, J.R.; Lee, M.C.; Lee, S.Y.; Ma, K.H.; Koh, H.J. Variation in pre-harvest sprouting resistance, seed germination and changes in abscisic acid levels during grain development in diverse rice genetic resources. Plant Genet. Resour. 2016, 16, 18–27. [Google Scholar] [CrossRef]
- Ali, A.; Cao, J.; Jiang, H.; Chang, C.; Zhang, H.P.; Sheikh, S.W.; Shah, L.; Ma, C. Unraveling molecular and genetic studies of wheat (Triticum aestivum L.) Resistance against factors causing pre-harvest sprouting. Agronomy 2019, 9, 117. [Google Scholar] [CrossRef]
- Tai, L.; Wang, H.J.; Xu, X.J.; Sun, W.H.; Ju, L.; Liu, W.T.; Li, W.Q.; Sun, J.; Chen, K.M. Cereal pre-harvest sprouting: A global agricultural disaster regulated by complex genetic and biochemical mechanisms. J. Exp. Bot. 2021, in press. [Google Scholar]
- Dejonghe, W.; Cutler, S.R. Abscisic acid as a gateway for the crops of tomorrow. In Abscisic Acid in Plants; Seo, M., Marion-Poll, A., Eds.; Advances in Botanical Research; Academic Press: London, UK, 2019; Volume 92, pp. 341–370. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).