Sucrose Utilization for Improved Crop Yields: A Review Article
Abstract
:1. Introduction
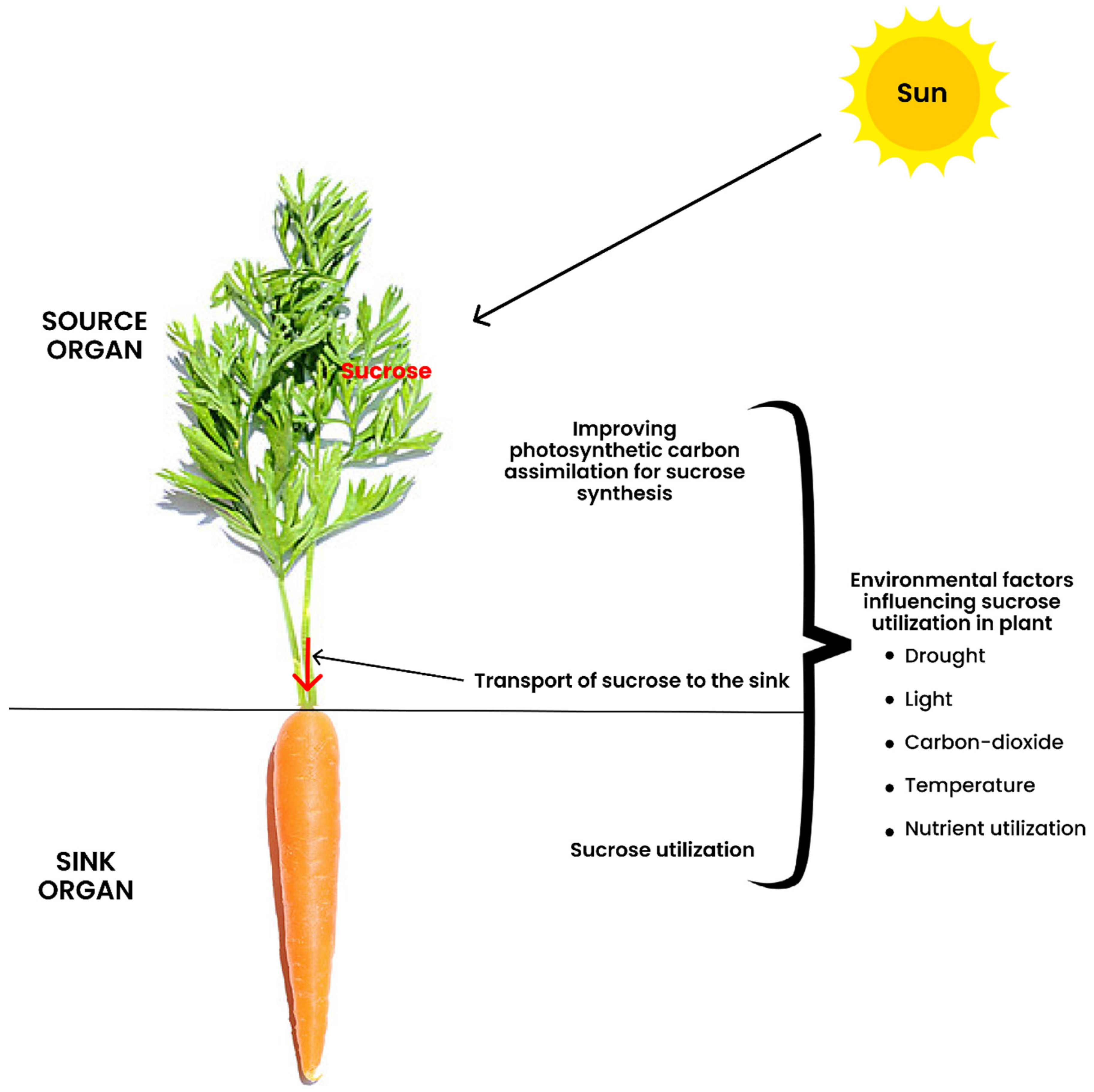
2. The Definition of Terminologies in Source-to-Sink Interaction
3. Improving the Capacity and Efficiency of Photosynthetic Carbon Assimilation
3.1. Impact of Global Warming on the Strategies to Improve Photosynthetic Carbon Assimilation
3.2. Photosynthetic Carbon Assimilation Contributes to Sustainable Development Goals
| Host Species | Strategies | Summary of Findings | References |
|---|---|---|---|
| C3 and C4 plants | Modification of in vitro assay method to measure the variability in carboxylase and decarboxylase activity of C3 and C4 leaf extract. | RuBisCO activation status is lower in mature C4 monocot leaves than in C3 monocots. | [19] |
| Rice | Model analysis conducted on both pot and experiments under various nitrogen rates. | Improved carboxylation rate due to higher RuBisCO content in mutant plants. | [46] |
| Tobacco | Expression of zeaxanthin and violaxanthin in the xanthophyll cycle coupled with an increased amount of the photosystem II subunit. | Greater than 15% increase in plant biomass. | [24] |
| Potato | Overexpression of pyrophosphatase in mesophyll cells. | Enhanced source and sink capacity and a doubling in starch yield of tuber. | [47] |
| Wheat | Overexpression of Brachypodium distachyon sedoheptulose-1,7-biphosphatase. | Increased leaf photosynthesis, biomass, and crop-yield potential. | [40] |
| Tobacco | Overexpression of Arabidopsis sedoheptulose-1,7-bisphosphatase (SBPase). | Improved photosynthetic capacity and crop yield. | [38] |
| Arabidopsis | Independent or synergetic alteration of sedoheptulose 1,7-bisphosphatase (SBPase), glycine decarboxylase H-protein (GDC-H) protein, and fructose 1,6-bisphophate aldolase (FBPA). | Enhanced carboxylation efficiency, vegetative biomass, and maximal seed-yield increase. | [36] |
| Potato | Expression of polyprotein comprising three subunits of Escherichia coli glycolate dehydrogenase (GlcDH). | High carbohydrate levels synthesized in the source leaves were utilized by the sink organ, facilitating a 2.3-fold increase in tuber yield. | [48] |
| Arabidopsis | Expression of a synthetic, light-gated K+ channel BLINK1 in guard cells surrounding stomatal pores. | BLINK1 facilitates a 2.2-fold increase in biomass in fluctuating light without the cost of water use by the plant. | [27] |
4. Alterations in Carbohydrate Partitioning When Manipulating Photosynthesis Affect Plant Growth
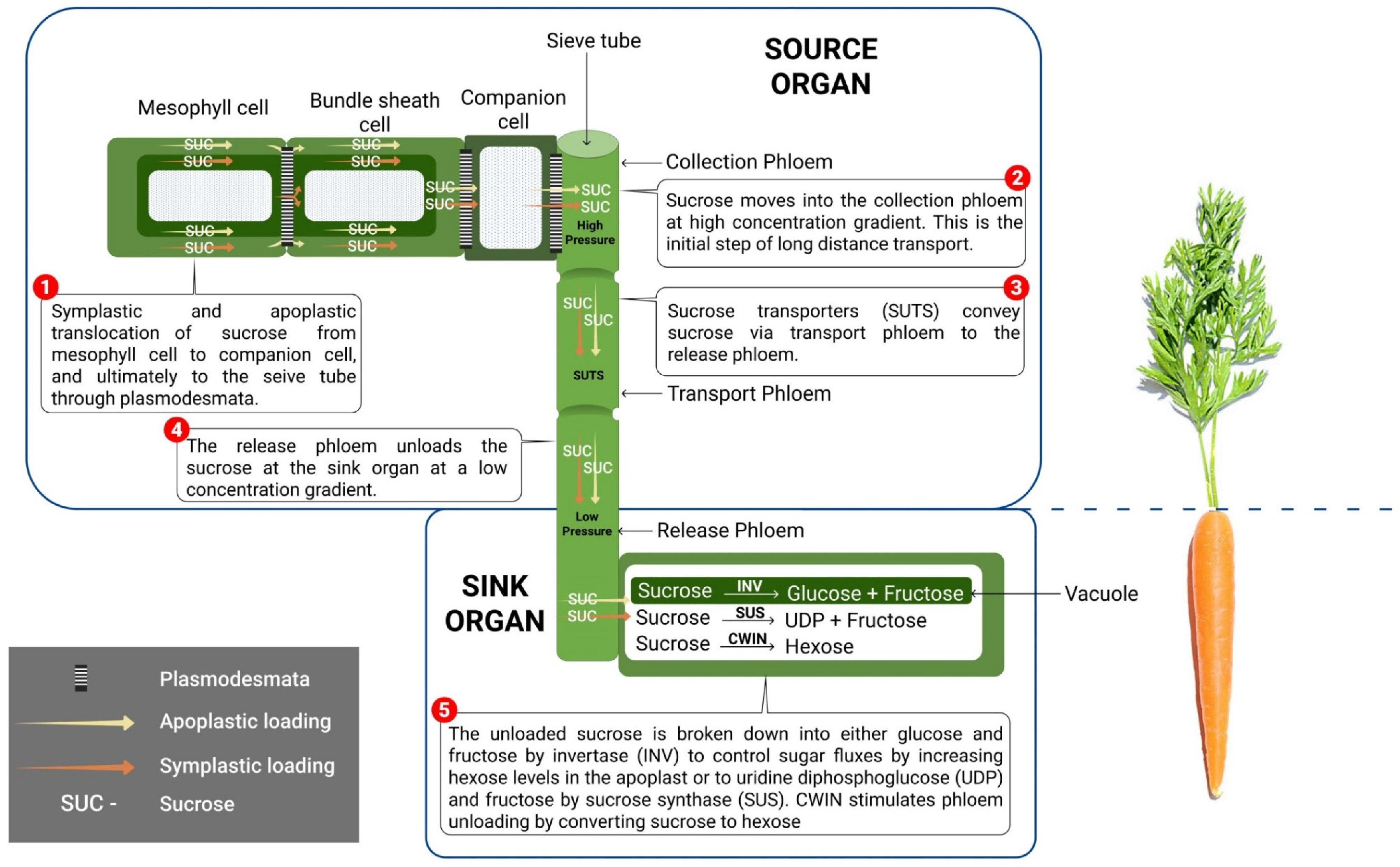
5. Transport of Sucrose to the Sink
6. Sucrose Utilization at the Sink
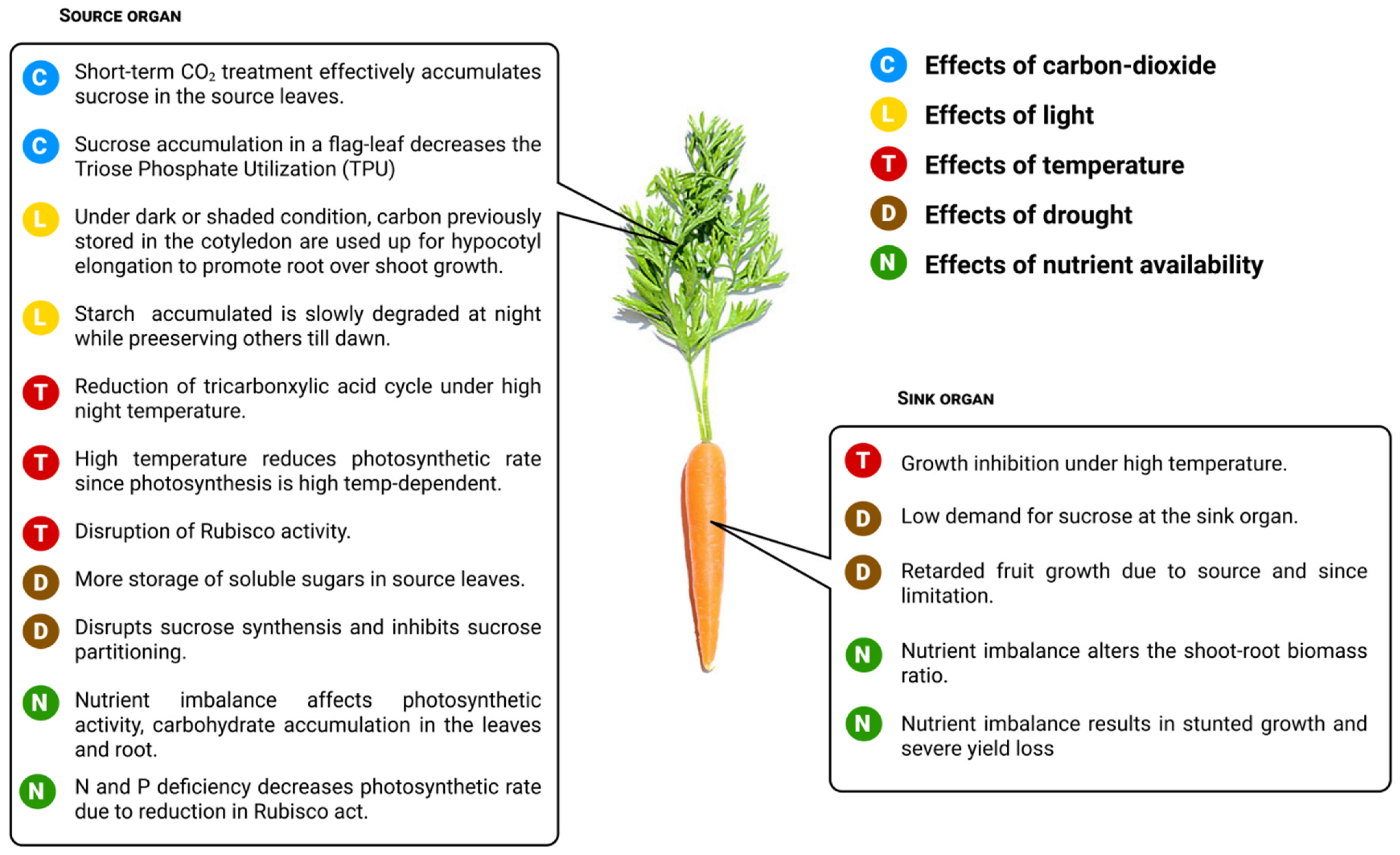
7. Influence of Environmental Factors on Photo-Assimilate Transport
7.1. Effects of Carbon Dioxide
7.2. Effects of Light
7.3. Effects of Temperature
7.4. Effects of Drought
7.5. Effects of Nutrient Availability
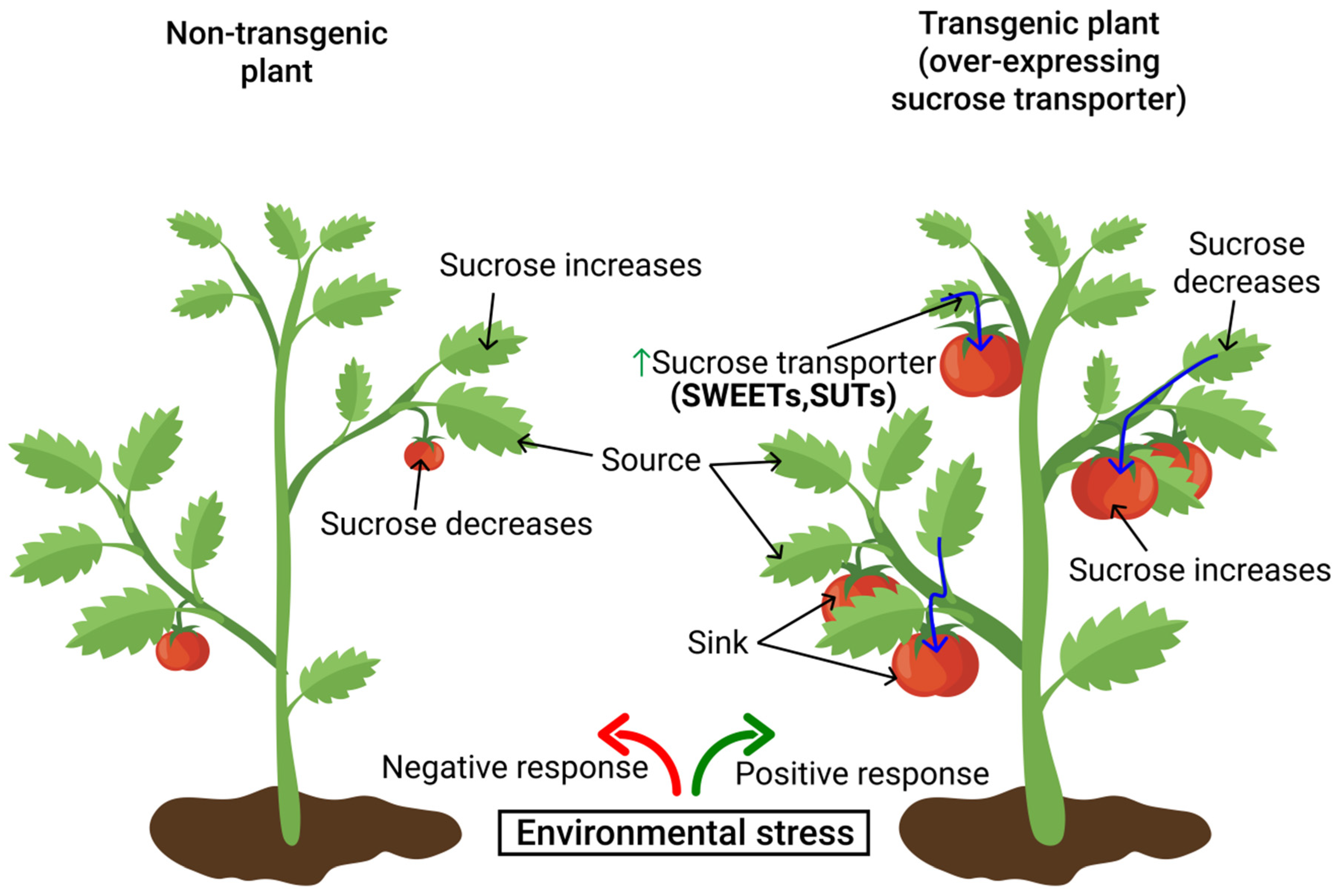
8. Functional Roles of Sugar Transporters in Mitigating Environmental Stress
9. Integrated Approaches to Crop-Yield Improvement
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Taiz, L.; Zeiger, E.; Moller, I.M.; Murphy, A. Plant Physiology and Development, 6th ed.; Oxford University Press: New York, NY, USA, 2018; p. 31. [Google Scholar]
- Osorio, S.; Ruan, Y.L.; Fernie, A.R. An update on source-to-sink carbon partitioning in tomato. Front. Plant Sci. 2014, 5, 516. [Google Scholar] [CrossRef] [Green Version]
- Lemoine, R. Sucrose transporters in plants: Update on function and structure. Biochim. Biophys. Acta (BBA) Biomembr. 2000, 1465, 246–262. [Google Scholar] [CrossRef] [Green Version]
- Kircher, S.; Schopfer, P. Photosynthetic sucrose acts as cotyledon-derived long-distance signal to control root growth during early seedling development in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 11217–11221. [Google Scholar] [CrossRef] [Green Version]
- Mason, M.G.; Ross, J.J.; Babst, B.A.; Wienclaw, B.N.; Beveridge, C.A. Sugar demand, not auxin, is the initial regulator of apical dominance. Proc. Natl. Acad. Sci. USA 2014, 111, 6092–6097. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, T.H.C.; Fernandes-Brum, C.N.; de Souza, C.R.; Dias, F.A.N.; de Almeida-Junior, O.; de Albuquerque Regina, M.; de Oliveira, K.K.P.; dos Reis, G.L.; Oliveira, L.M.; de Paula Fernandes, F. Transcriptome analyses suggest that changes in fungal endophyte lifestyle could be involved in grapevine bud necrosis. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mason, T.; Maskell, E. Studies on the transport of carbohydrates in the cotton plant: II. The factors determining the rate and the direction of movement of sugars. Ann. Bot. 1928, 42, 571–636. [Google Scholar] [CrossRef]
- Sonnewald, U.; Fernie, A.R. Next-generation strategies for understanding and influencing source–sink relations in crop plants. Curr. Opin. Plant Biol. 2018, 43, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.G.; Zhu, X.G.; Raines, C. Source-sink interaction: A century old concept under the light of modern molecular systems biology. J. Exp. Bot. 2017, 68, 4417–4431. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. What have we learned from 15 years of free-air CO2 enrichment (FACE)? A meta-analytic review of the responses of photosynthesis, canopy properties and plant production to rising CO2. New Phytol. 2005, 165, 351–372. [Google Scholar] [CrossRef]
- Wilson, R.H.; Martin-Avila, E.; Conlan, C.; Whitney, S.M. An improved Escherichia coli screen for Rubisco identifies a protein-protein interface that can enhance CO2-fixation kinetics. J. Biol. Chem. 2018, 293, 18–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgun, V.; Priadkina, G.; Zborovska, O. Dependence of main shoot ear grain yield from stem deposited ability of winter wheat varieties. Ukr. J. Ecol. 2018, 8, 113–118. [Google Scholar]
- Flexas, J. Genetic improvement of leaf photosynthesis and intrinsic water use efficiency in C3 plants: Why so much little success? Plant Sci. 2016, 251, 155–161. [Google Scholar] [CrossRef]
- Andralojc, P.J.; Carmo-Silva, E.; Degen, G.E.; Parry, M.A. Increasing metabolic potential: C-fixation. Essays Biochem. 2018, 62, 109–118. [Google Scholar]
- Sage, R.F. A portrait of the C4 photosynthetic family on the 50th anniversary of its discovery: Species number, evolutionary lineages, and Hall of Fame. J. Exp. Bot. 2017, 68, e11–e28. [Google Scholar] [CrossRef] [Green Version]
- Chowrasia, S.; Mondal, T.K. C4 photosynthesis with Kranz anatomy evolved in the Oryza coarctata Roxb. bioRxiv 2020. [Google Scholar] [CrossRef]
- Ingle, A.P.; Philippini, R.R.; Martiniano, S.; Marcelino, P.R.F.; Gupta, I.; Prasad, S.; da Silva, S.S. Bioresources and their significance: Prospects and obstacles. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–40. [Google Scholar]
- Fernie, A.R.; Bachem, C.W.; Helariutta, Y.; Neuhaus, H.E.; Prat, S.; Ruan, Y.-L.; Stitt, M.; Sweetlove, L.J.; Tegeder, M.; Wahl, V. Synchronization of developmental, molecular and metabolic aspects of source–sink interactions. Nat. Plants 2020, 6, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Sharwood, R.E.; Sonawane, B.V.; Ghannoum, O.; Whitney, S.M. Improved analysis of C4 and C3 photosynthesis via refined in vitro assays of their carbon fixation biochemistry. J. Exp. Bot. 2016, 67, 3137–3148. [Google Scholar] [CrossRef] [Green Version]
- Weber, A.P.; Bar-Even, A. Update: Improving the efficiency of photosynthetic carbon reactions. Plant Physiol. 2019, 179, 803–812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, S.P.; Marshall-Colon, A.; Zhu, X.-G. Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell 2015, 161, 56–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rae, B.D.; Long, B.M.; Förster, B.; Nguyen, N.D.; Velanis, C.N.; Atkinson, N.; Hee, W.Y.; Mukherjee, B.; Price, G.D.; McCormick, A.J. Progress and challenges of engineering a biophysical CO2-concentrating mechanism into higher plants. J. Exp. Bot. 2017, 68, 3717–3737. [Google Scholar] [CrossRef] [PubMed]
- Orr, D.J.; Pereira, A.M.; da Fonseca Pereira, P.; Pereira-Lima, Í.A.; Zsögön, A.; Araújo, W.L. Engineering photosynthesis: Progress and perspectives. F1000Research 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Kromdijk, J.; Głowacka, K.; Leonelli, L.; Gabilly, S.T.; Iwai, M.; Niyogi, K.K.; Long, S.P. Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 2016, 354, 857–861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betti, M.; Bauwe, H.; Busch, F.A.; Fernie, A.R.; Keech, O.; Levey, M.; Ort, D.R.; Parry, M.A.; Sage, R.; Timm, S. Manipulating photorespiration to increase plant productivity: Recent advances and perspectives for crop improvement. J. Exp. Bot. 2016, 67, 2977–2988. [Google Scholar] [CrossRef] [PubMed]
- Głowacka, K.; Kromdijk, J.; Kucera, K.; Xie, J.; Cavanagh, A.P.; Leonelli, L.; Leakey, A.D.; Ort, D.R.; Niyogi, K.K.; Long, S.P. Photosystem II Subunit S overexpression increases the efficiency of water use in a field-grown crop. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papanatsiou, M.; Petersen, J.; Henderson, L.; Wang, Y.; Christie, J.; Blatt, M. Optogenetic manipulation of stomatal kinetics improves carbon assimilation, water use, and growth. Science 2019, 363, 1456–1459. [Google Scholar] [CrossRef] [Green Version]
- Baslam, M.; Mitsui, T.; Hodges, M.; Priesack, E.; Herritt, M.T.; Aranjuelo, I.; Sanz-Saez, A. Photosynthesis in a Changing Global Climate: Scaling Up and Scaling Down in Crops. Front. Plant Sci. 2020, 11, 882. [Google Scholar] [CrossRef]
- Prather, M.; Flato, G.; Friedlingstein, P.; Jones, C.; Lamarque, J.; Liao, H.; Rasch, P. Annex II: Climate system scenario tables. Clim. Chang. 2013, 2013, 1395–1445. [Google Scholar]
- Rowlands, D.J.; Frame, D.J.; Ackerley, D.; Aina, T.; Booth, B.B.; Christensen, C.; Collins, M.; Faull, N.; Forest, C.E.; Grandey, B.S. Broad range of 2050 warming from an observationally constrained large climate model ensemble. Nat. Geosci. 2012, 5, 256–260. [Google Scholar] [CrossRef] [Green Version]
- Ort, D.R.; Merchant, S.S.; Alric, J.; Barkan, A.; Blankenship, R.E.; Bock, R.; Croce, R.; Hanson, M.R.; Hibberd, J.M.; Long, S.P. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc. Natl. Acad. Sci. USA 2015, 112, 8529–8536. [Google Scholar] [CrossRef] [Green Version]
- Raines, C.A. Transgenic approaches to manipulate the environmental responses of the C3 carbon fixation cycle. Plant Cell Environ. 2006, 29, 331–339. [Google Scholar] [CrossRef]
- Raines, C.A. Increasing photosynthetic carbon assimilation in C3 plants to improve crop yield: Current and future strategies. Plant Physiol. 2011, 155, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Von Caemmerer, S. Biochemical Models of Leaf Photosynthesis; Csiro Publishing: Collingwood, VIC, Australia, 2000. [Google Scholar]
- Long, S.P.; Ainsworth, E.A.; Rogers, A.; Ort, D.R. Rising atmospheric carbon dioxide: Plants FACE the future. Annu. Rev. Plant Biol. 2004, 55, 591–628. [Google Scholar] [CrossRef] [PubMed]
- Simkin, A.J.; Lopez-Calcagno, P.E.; Davey, P.A.; Headland, L.R.; Lawson, T.; Timm, S.; Bauwe, H.; Raines, C.A. Simultaneous stimulation of sedoheptulose 1, 7-bisphosphatase, fructose 1, 6-bisphophate aldolase and the photorespiratory glycine decarboxylase-H protein increases CO2 assimilation, vegetative biomass and seed yield in Arabidopsis. Plant Biotechnol. J. 2017, 15, 805–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simkin, A.J.; McAusland, L.; Headland, L.R.; Lawson, T.; Raines, C.A. Multigene manipulation of photosynthetic carbon assimilation increases CO2 fixation and biomass yield in tobacco. J. Exp. Bot. 2015, 66, 4075–4090. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, S.; Lawson, T.; Fryer, M.; Zakhleniuk, O.V.; Lloyd, J.C.; Raines, C.A. Increased sedoheptulose-1, 7-bisphosphatase activity in transgenic tobacco plants stimulates photosynthesis and growth from an early stage in development. Plant Physiol. 2005, 138, 451–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, F.; Wang, M.; Zhang, S.; Ai, X. Changes in SBPase activity influence photosynthetic capacity, growth, and tolerance to chilling stress in transgenic tomato plants. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- Driever, S.M.; Simkin, A.J.; Alotaibi, S.; Fisk, S.J.; Madgwick, P.J.; Sparks, C.A.; Jones, H.D.; Lawson, T.; Parry, M.A.; Raines, C.A. Increased SBPase activity improves photosynthesis and grain yield in wheat grown in greenhouse conditions. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köhler, I.H.; Ruiz-Vera, U.M.; VanLoocke, A.; Thomey, M.L.; Clemente, T.; Long, S.P.; Ort, D.R.; Bernacchi, C.J. Expression of cyanobacterial FBP/SBPase in soybean prevents yield depression under future climate conditions. J. Exp. Bot. 2017, 68, 715–726. [Google Scholar] [CrossRef] [Green Version]
- Fuso Nerini, F.; Sovacool, B.; Hughes, N.; Cozzi, L.; Cosgrave, E.; Howells, M.; Tavoni, M.; Tomei, J.; Zerriffi, H.; Milligan, B. Connecting climate action with other Sustainable Development Goals. Nat. Sustain. 2019, 2, 674–680. [Google Scholar] [CrossRef]
- Simkin, A.J.; López-Calcagno, P.E.; Raines, C.A. Feeding the world: Improving photosynthetic efficiency for sustainable crop production. J. Exp. Bot. 2019, 70, 1119–1140. [Google Scholar] [CrossRef] [Green Version]
- The Lancet HIV. The syndemic threat of food insecurity and HIV. Lancet HIV 2020, 7. [Google Scholar] [CrossRef] [Green Version]
- Taylor, S.F.; Roberts, M.J.; Milligan, B.; Ncwadi, R. Measurement and implications of marine food security in the Western Indian Ocean: An impending crisis? Food Secur. 2019, 11, 1395–1415. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.; Zhou, Z.; Li, Z.; Chen, Y.; Wang, Z.; Zhang, H. Rice (Oryza sativa L.) with reduced chlorophyll content exhibit higher photosynthetic rate and efficiency, improved canopy light distribution, and greater yields than normally pigmented plants. Field Crop. Res. 2017, 200, 58–70. [Google Scholar] [CrossRef]
- Jonik, C.; Sonnewald, U.; Hajirezaei, M.R.; Flügge, U.I.; Ludewig, F. Simultaneous boosting of source and sink capacities doubles tuber starch yield of potato plants. Plant Biotechnol. J. 2012, 10, 1088–1098. [Google Scholar] [CrossRef]
- Nölke, G.; Houdelet, M.; Kreuzaler, F.; Peterhänsel, C.; Schillberg, S. The expression of a recombinant glycolate dehydrogenase polyprotein in potato (Solanum tuberosum) plastids strongly enhances photosynthesis and tuber yield. Plant Biotechnol. J. 2014, 12, 734–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ainsworth, E.A.; Bush, D.R. Carbohydrate export from the leaf: A highly regulated process and target to enhance photosynthesis and productivity. Plant Physiol. 2011, 155, 64–69. [Google Scholar] [CrossRef] [Green Version]
- Oszvald, M.; Primavesi, L.F.; Griffiths, C.A.; Cohn, J.; Basu, S.S.; Nuccio, M.L.; Paul, M.J. Trehalose 6-phosphate regulates photosynthesis and assimilate partitioning in reproductive tissue. Plant Physiol. 2018, 176, 2623–2638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Turgeon, R. Downregulating the sucrose transporter VpSUT1 in Verbascum phoeniceum does not inhibit phloem loading. Proc. Natl. Acad. Sci. USA 2009, 106, 18849–18854. [Google Scholar] [CrossRef] [Green Version]
- Stitt, M.; Lunn, J.; Usadel, B. Arabidopsis and primary photosynthetic metabolism-more than the icing on the cake. Plant J. 2010, 61, 1067–1091. [Google Scholar] [CrossRef] [PubMed]
- Kaschuk, G.; Hungria, M.; Leffelaar, P.; Giller, K.; Kuyper, T. Differences in photosynthetic behaviour and leaf senescence of soybean (Glycine max [L.] Merrill) dependent on N2 fixation or nitrate supply. Plant Biol. 2010, 12, 60–69. [Google Scholar] [CrossRef]
- Petridis, A.; van der Kaay, J.; Sungurtas, J.; Verrall, S.R.; McCallum, S.; Graham, J.; Hancock, R.D. Photosynthetic plasticity allows blueberry (Vaccinium corymbosum L.) plants to compensate for yield loss under conditions of high sink demand. Environ. Exp. Bot. 2020, 174. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Long, S.P. 30 years of free-air carbon dioxide enrichment (FACE): What have we learned about future crop productivity and its potential for adaptation? Glob. Chang. Biol. 2021, 27, 27–49. [Google Scholar] [CrossRef]
- Arp, W. Effects of source-sink relations on photosynthetic acclimation to elevated CO2. Plant Cell Environ. 1991, 14, 869–875. [Google Scholar] [CrossRef]
- Leakey, A.D.; Ainsworth, E.A.; Bernacchi, C.J.; Rogers, A.; Long, S.P.; Ort, D.R. Elevated CO2 effects on plant carbon, nitrogen, and water relations: Six important lessons from FACE. J. Exp. Bot. 2009, 60, 2859–2876. [Google Scholar] [CrossRef]
- Parvin, S.; Uddin, S.; Tausz-Posch, S.; Armstrong, R.; Tausz, M. Carbon sink strength of nodules but not other organs modulates photosynthesis of faba bean (Vicia faba) grown under elevated [CO2] and different water supply. New Phytol. 2020, 227, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Davey, P.; Olcer, H.; Zakhleniuk, O.; Bernacchi, C.; Calfapietra, C.; Long, S.; Raines, C. Can fast-growing plantation trees escape biochemical down-regulation of photosynthesis when grown throughout their complete production cycle in the open air under elevated carbon dioxide? Plant Cell Environ. 2006, 29, 1235–1244. [Google Scholar] [CrossRef]
- Sulpice, R.; Flis, A.; Ivakov, A.A.; Apelt, F.; Krohn, N.; Encke, B.; Abel, C.; Feil, R.; Lunn, J.E.; Stitt, M. Arabidopsis coordinates the diurnal regulation of carbon allocation and growth across a wide range of photoperiods. Mol. Plant 2014, 7, 137–155. [Google Scholar] [CrossRef] [PubMed]
- Kolling, K.; Thalmann, M.; Muller, A.; Jenny, C.; Zeeman, S.C. Carbon partitioning in Arabidopsis thaliana is a dynamic process controlled by the plants metabolic status and its circadian clock. Plant Cell Environ. 2015, 38, 1965–1979. [Google Scholar] [CrossRef] [Green Version]
- Weraduwage, S.M.; Chen, J.; Anozie, F.C.; Morales, A.; Weise, S.E.; Sharkey, T.D. The relationship between leaf area growth and biomass accumulation in Arabidopsis thaliana. Front. Plant Sci. 2015, 6, 167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulpice, R.; Pyl, E.-T.; Ishihara, H.; Trenkamp, S.; Steinfath, M.; Witucka-Wall, H.; Gibon, Y.; Usadel, B.; Poree, F.; Piques, M.C. Starch as a major integrator in the regulation of plant growth. Proc. Natl. Acad. Sci. USA 2009, 106, 10348–10353. [Google Scholar] [CrossRef] [Green Version]
- Lemoine, R.; La Camera, S.; Atanassova, R.; Dédaldéchamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.-L.; Laloi, M.; Coutos-Thévenot, P.; Maurousset, L. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 2013, 4, 272. [Google Scholar] [CrossRef] [Green Version]
- Minchin, P.; Lacointe, A. New understanding on phloem physiology and possible consequences for modelling long-distance carbon transport. New Phytol. 2005, 166, 771–779. [Google Scholar] [CrossRef]
- Rennie, E.A.; Turgeon, R. A comprehensive picture of phloem loading strategies. Proc. Natl. Acad. Sci. USA 2009, 106, 14162–14167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Bel, A.J. The phloem, a miracle of ingenuity. Plant Cell Environ. 2003, 26, 125–149. [Google Scholar] [CrossRef]
- Braun, D.M.; Wang, L.; Ruan, Y.-L. Understanding and manipulating sucrose phloem loading, unloading, metabolism, and signalling to enhance crop yield and food security. J. Exp. Bot. 2014, 65, 1713–1735. [Google Scholar] [CrossRef] [Green Version]
- Milne, R.J.; Perroux, J.M.; Rae, A.L.; Reinders, A.; Ward, J.M.; Offler, C.E.; Patrick, J.W.; Grof, C.P. Sucrose transporter localization and function in phloem unloading in developing stems. Plant Physiol. 2017, 173, 1330–1341. [Google Scholar] [CrossRef] [PubMed]
- Turgeon, R. The role of phloem loading reconsidered. Plant Physiol. 2010, 152, 1817–1823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayre, B.G.; Turgeon, R. Export of Photosynthates from the Leaf. In The Leaf: A Platform for Performing Photosynthesis; Springer: Berlin/Heidelberg, Germany, 2018; pp. 55–79. [Google Scholar]
- Milne, R.J.; Grof, C.P.; Patrick, J.W. Mechanisms of phloem unloading: Shaped by cellular pathways, their conductances and sink function. Curr. Opin. Plant Biol. 2018, 43, 8–15. [Google Scholar] [CrossRef]
- Eom, J.-S.; Cho, J.-I.; Reinders, A.; Lee, S.-W.; Yoo, Y.; Tuan, P.Q.; Choi, S.-B.; Bang, G.; Park, Y.-I.; Cho, M.-H. Impaired function of the tonoplast-localized sucrose transporter in rice, OsSUT2, limits the transport of vacuolar reserve sucrose and affects plant growth. Plant Physiol. 2011, 157, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.-Q.; Qu, X.-Q.; Hou, B.-H.; Sosso, D.; Osorio, S.; Fernie, A.R.; Frommer, W.B. Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science 2012, 335, 207–211. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, D.; Miao, Q.; Yang, J.; Xuan, Y.; Hu, Y. Essential role of sugar transporter OsSWEET11 during the early stage of rice grain filling. Plant Cell Physiol. 2017, 58, 863–873. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, C.M.; Müdsam, C.; Keller, I.; Zierer, W.; Czarnecki, O.; Corral, J.M.; Reinhardt, F.; Nieberl, P.; Fiedler-Wiechers, K.; Sommer, F. Vernalization alters sink and source identities and reverses phloem translocation from taproots to shoots in sugar beet. Plant Cell 2020, 32, 3206–3223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Bian, Y.; Hou, S.; Li, X. Sugar transport played a more important role than sugar biosynthesis in fruit sugar accumulation during Chinese jujube domestication. Planta 2018, 248, 1187–1199. [Google Scholar] [CrossRef] [PubMed]
- Godt, D.; Roitsch, T. The developmental and organ specific expression of sucrose cleaving enzymes in sugar beet suggests a transition between apoplasmic and symplasmic phloem unloading in the tap roots. Plant Physiol. Biochem. 2006, 44, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-Y.; Peng, Y.-B.; Pelleschi-Travier, S.; Fan, Y.; Lu, Y.-F.; Lu, Y.-M.; Gao, X.-P.; Shen, Y.-Y.; Delrot, S.; Zhang, D.-P. Evidence for apoplasmic phloem unloading in developing apple fruit. Plant Physiol. 2004, 135, 574–586. [Google Scholar] [CrossRef] [Green Version]
- Abelenda, J.A.; Bergonzi, S.; Oortwijn, M.; Sonnewald, S.; Du, M.; Visser, R.G.; Sonnewald, U.; Bachem, C.W. Source-sink regulation is mediated by interaction of an FT Homolog with a SWEET protein in potato. Curr. Biol. 2019, 29, 1178–1186.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, S.; Li, Y.; Li, X.; Sui, X.; Zhang, Z. Phloem unloading strategies and mechanisms in crop fruits. J. Plant Growth Regul. 2019, 38, 494–500. [Google Scholar] [CrossRef]
- Ru, L.; Osorio, S.; Wang, L.; Fernie, A.R.; Patrick, J.W.; Ruan, Y.-L. Transcriptomic and metabolomics responses to elevated cell wall invertase activity during tomato fruit set. J. Exp. Bot. 2017, 68, 4263–4279. [Google Scholar] [CrossRef] [Green Version]
- Tang, F.; Zhu, J.; Wang, T.; Shao, D. Water deficit effects on carbon metabolism in cotton fibers during fiber elongation phase. Acta Physiol. Plant. 2017, 39, 69. [Google Scholar] [CrossRef]
- Weber, H.; Buchner, P.; Borisjuk, L.; Wobus, U. Sucrose metabolism during cotyledon development of Vicia faba L. is controlled by the concerted action of both sucrose-phosphate synthase and sucrose synthase: Expression patterns, metabolic regulation and implications for seed development. Plant J. 1996, 9, 841–850. [Google Scholar] [CrossRef]
- Makino, A.; Mae, T. Photosynthesis and plant growth at elevated levels of CO2. Plant Cell Physiol. 1999, 40, 999–1006. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, F.; Hao, L.; Yu, J.; Guo, L.; Zhou, H.; Ma, C.; Zhang, X.; Xu, M. Elevated CO2 concentration induces photosynthetic down-regulation with changes in leaf structure, non-structural carbohydrates and nitrogen content of soybean. BMC Plant Biol. 2019, 19, 255. [Google Scholar] [CrossRef]
- Kanemoto, K.; Yamashita, Y.; Ozawa, T.; Imanishi, N.; Nguyen, N.T.; Suwa, R.; Mohapatra, P.K.; Kanai, S.; Moghaieb, R.E.; Ito, J. Photosynthetic acclimation to elevated CO2 is dependent on N partitioning and transpiration in soybean. Plant Sci. 2009, 177, 398–403. [Google Scholar] [CrossRef]
- Eichelmann, H.; Talts, E.; Oja, V.; Padu, E.; Laisk, A. Rubisco in planta k cat is regulated in balance with photosynthetic electron transport. J. Exp. Bot. 2009, 60, 4077–4088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkin, O. New Phytologist and the ‘fate’of carbon in terrestrial ecosystems. New Phytol. 2015, 205, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Stitt, M. Rising CO2 levels and their potential significance for carbon flow in photosynthetic cells. Plant Cell Environ. 1991, 14, 741–762. [Google Scholar] [CrossRef]
- Wang, N.; Nobel, P.S. Doubling the CO2 concentration enhanced the activity of carbohydrate-metabolism enzymes, source carbohydrate production, photoassimilate transport, and sink strength for Opuntia ficus-indica. Plant Physiol. 1996, 110, 893–902. [Google Scholar] [CrossRef] [Green Version]
- Cock, J.H.; Yoshida, S. Changing sink and source relations in rice (Oryza sativa L.) using carbon dioxide enrichment in the field. Soil Sci. Plant Nutr. 1973, 19, 229–234. [Google Scholar] [CrossRef]
- Nakano, H.; Yoshinaga, S.; Takai, T.; Arai-Sanoh, Y.; Kondo, K.; Yamamoto, T.; Sakai, H.; Tokida, T.; Usui, Y.; Nakamura, H. Quantitative trait loci for large sink capacity enhance rice grain yield under free-air CO2 enrichment conditions. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Hasegawa, T.; Sakai, H.; Tokida, T.; Nakamura, H.; Zhu, C.; Usui, Y.; Yoshimoto, M.; Fukuoka, M.; Wakatsuki, H.; Katayanagi, N. Rice cultivar responses to elevated CO2 at two free-air CO2 enrichment (FACE) sites in Japan. Funct. Plant Biol. 2013, 40, 148–159. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, T.; Sakai, H.; Tokida, T.; Usui, Y.; Yoshimoto, M.; Fukuoka, M.; Nakamura, H.; Shimono, H.; Okada, M. Rice free-air carbon dioxide enrichment studies to improve assessment of climate change effects on rice agriculture. Improv. Model. Tools Assess Clim. Chang. Eff. Crop Response 2016, 7, 45–68. [Google Scholar]
- Nakano, H.; Makino, A.; Mae, T. Effects of panicle removal on the photosynthetic characteristics of the flag leaf of rice plants during the ripening stage. Plant Cell Physiol. 1995, 36, 653–659. [Google Scholar]
- Shinano, T.; Osawa, M.; Soejima, H.; Osaki, M. Effect of panicle removal on cytokinin level in the xylem and nitrogen uptake activity of rice. Soil Sci. Plant Nutr. 2006, 52, 331–340. [Google Scholar] [CrossRef] [Green Version]
- Shimono, H.; Suzuki, K.; Aoki, K.; Hasegawa, T.; Okada, M. Effect of panicle removal on photosynthetic acclimation under elevated CO2 in rice. Photosynthetica 2010, 48, 530–536. [Google Scholar] [CrossRef]
- Jing, L.-Q.; Wu, Y.-Z.; Zhuang, S.-T.; Wang, Y.-X.; Zhu, J.-G.; Wang, Y.-L.; Yang, L.-X. Effects of CO2 enrichment and spikelet removal on rice quality under open-air field conditions. J. Integr. Agric. 2016, 15, 2012–2022. [Google Scholar] [CrossRef] [Green Version]
- Fabre, D.; Yin, X.; Dingkuhn, M.; Clement-Vidal, A.; Roques, S.; Rouan, L.; Soutiras, A.; Luquet, D. Is triose phosphate utilization involved in the feedback inhibition of photosynthesis in rice under conditions of sink limitation? J. Exp. Bot. 2019, 70, 5773–5785. [Google Scholar] [CrossRef] [PubMed]
- Page, E.R.; Liu, W.; Cerrudo, D.; Lee, E.A.; Swanton, C.J. Shade avoidance influences stress tolerance in maize. Weed Sci. 2011, 59, 326–334. [Google Scholar] [CrossRef]
- Chen, X.; Yao, Q.; Gao, X.; Jiang, C.; Harberd, N.P.; Fu, X. Shoot-to-root mobile transcription factor HY5 coordinates plant carbon and nitrogen acquisition. Curr. Biol. 2016, 26, 640–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, P.M.R.; Rodrigues, M.A.; Goncalves, A.Z.; Kerbauy, G.B. Exposure of Catasetum fimbriatum aerial roots to light coordinates carbon partitioning between source and sink organs in an auxin dependent manner. Plant Physiol. Biochem. 2019, 135, 341–347. [Google Scholar] [CrossRef]
- Zeeman, S.C.; Kossmann, J.; Smith, A.M. Starch: Its metabolism, evolution, and biotechnological modification in plants. Annu. Rev. Plant Biol. 2010, 61, 209–234. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.M.; Stitt, M. Coordination of carbon supply and plant growth. Plant Cell Environ. 2007, 30, 1126–1149. [Google Scholar] [CrossRef]
- Pyl, E.-T.; Piques, M.; Ivakov, A.; Schulze, W.; Ishihara, H.; Stitt, M.; Sulpice, R. Metabolism and growth in Arabidopsis depend on the daytime temperature but are temperature-compensated against cool nights. Plant Cell 2012, 24, 2443–2469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poire, R.; Wiese-Klinkenberg, A.; Parent, B.; Mielewczik, M.; Schurr, U.; Tardieu, F.; Walter, A. Diel time-courses of leaf growth in monocot and dicot species: Endogenous rhythms and temperature effects. J. Exp. Bot. 2010, 61, 1751–1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazdanbakhsh, N.; Sulpice, R.; Graf, A.; Stitt, M.; Fisahn, J. Circadian control of root elongation and C partitioning in Arabidopsis thaliana. Plant Cell Environ. 2011, 34, 877–894. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Encke, B.; Krohn, N.; Hoehne, M.; Stitt, M.; Pyl, E.T. Relationship between starch degradation and carbon demand for maintenance and growth in Arabidopsis thaliana in different irradiance and temperature regimes. Plant Cell Environ. 2015, 38, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Amiard, V.; Demmig-Adams, B.; Mueh, K.E.; Turgeon, R.; Combs, A.F.; Adams, W.W. Role of light and jasmonic acid signaling in regulating foliar phloem cell wall ingrowth development. New Phytol. 2007, 173, 722–731. [Google Scholar] [CrossRef]
- Lambers, H.; Oliveira, R.S. Photosynthesis, Respiration, and Long-Distance Transport: Long Distance Transport of Assimilates. In Plant Physiological Ecology; Springer: Berlin/Heidelberg, Germany, 2019; pp. 173–186. [Google Scholar]
- Amiard, V.; Mueh, K.E.; Demmig-Adams, B.; Ebbert, V.; Turgeon, R.; Adams, W.W. Anatomical and photosynthetic acclimation to the light environment in species with differing mechanisms of phloem loading. Proc. Natl. Acad. Sci. USA 2005, 102, 12968–12973. [Google Scholar] [CrossRef] [Green Version]
- Amist, N.; Singh, N. The role of sugars in the regulation of environmental stress. In Plant Life under Changing Environment; Elsevier: Amsterdam, The Netherlands, 2020; pp. 497–512. [Google Scholar]
- Yang, L.Y.; Yang, S.L.; Li, J.Y.; Ma, J.H.; Pang, T.; Zou, C.M.; He, B.; Gong, M. Effects of different growth temperatures on growth, development, and plastid pigments metabolism of tobacco (Nicotiana tabacum L.) plants. Bot. Stud. 2018, 59, 5. [Google Scholar] [CrossRef] [Green Version]
- Choquette, N.E.; Ainsworth, E.A.; Bezodis, W.; Cavanagh, A.P. Ozone tolerant maize hybrids maintain Rubisco content and activity during long-term exposure in the field. Plant Cell Environ. 2020, 43, 3033–3047. [Google Scholar] [CrossRef]
- Hebbar, K.; Apshara, E.; Chandran, K.; Prasad, P.V. Effect of elevated CO2, high temperature, and water deficit on growth, photosynthesis, and whole plant water use efficiency of cocoa (Theobroma cacao L.). Int. J. Biometeorol. 2020, 64, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Aiqing, S.; Somayanda, I.; Sebastian, S.V.; Singh, K.; Gill, K.; Prasad, P.; Jagadish, S.K. Heat stress during flowering affects time of day of flowering, seed set, and grain quality in spring wheat. Crop Sci. 2018, 58, 380–392. [Google Scholar] [CrossRef]
- Kumar, R.R.; Goswami, S.; Shamim, M.; Mishra, U.; Jain, M.; Singh, K.; Singh, J.P.; Dubey, K.; Singh, S.; Rai, G.K. Biochemical defense response: Characterizing the plasticity of source and sink in spring wheat under terminal heat stress. Front. Plant Sci. 2017, 8, 1603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso, M.P.; Abbate, P.E.; Mirabella, N.E.; Merlos, F.A.; Panelo, J.S.; Pontaroli, A.C. Analysis of sink/source relations in bread wheat recombinant inbred lines and commercial cultivars under a high yield potential environment. Eur. J. Agron. 2018, 93, 82–87. [Google Scholar] [CrossRef]
- Fan, Y.; Ma, C.; Huang, Z.; Abid, M.; Jiang, S.; Dai, T.; Zhang, W.; Ma, S.; Jiang, D.; Han, X. Heat priming during early reproductive stages enhances thermo-tolerance to post-anthesis heat stress via improving photosynthesis and plant productivity in winter wheat (Triticum aestivum L.). Front. Plant Sci. 2018, 9, 805. [Google Scholar] [CrossRef]
- Mendanha, T.; Rosenqvist, E.; Hyldgaard, B.; Ottosen, C.-O. Heat priming effects on anthesis heat stress in wheat cultivars (Triticum aestivum L.) with contrasting tolerance to heat stress. Plant Physiol. Biochem. 2018, 132, 213–221. [Google Scholar] [CrossRef]
- Zhang, X.; Högy, P.; Wu, X.; Schmid, I.; Wang, X.; Schulze, W.X.; Jiang, D.; Fangmeier, A. Physiological and proteomic evidence for the interactive effects of post-anthesis heat stress and elevated CO2 on wheat. Proteomics 2018, 18, 1800262. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, M.; Burritt, D.J.; Gupta, A.; Tsujimoto, H.; Tran, L.P. Heat stress effects on source-sink relationships and metabolome dynamics in wheat. J. Exp. Bot. 2020, 71, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Impa, S.M.; Sunoj, V.S.J.; Krassovskaya, I.; Bheemanahalli, R.; Obata, T.; Jagadish, S.V.K. Carbon balance and source-sink metabolic changes in winter wheat exposed to high night-time temperature. Plant Cell Environ. 2019, 42, 1233–1246. [Google Scholar] [CrossRef]
- Wang, X.; Hou, L.; Lu, Y.; Wu, B.; Gong, X.; Liu, M.; Wang, J.; Sun, Q.; Vierling, E.; Xu, S. Metabolic adaptation of wheat grain contributes to a stable filling rate under heat stress. J. Exp. Bot. 2018, 69, 5531–5545. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann-Thoma, G.; van Bel, A.J.; Ehlers, K. Ultrastructure of minor-vein phloem and assimilate export in summer and winter leaves of the symplasmically loading evergreens Ajuga reptans L., Aucuba japonica Thunb., and Hedera helix L. Planta 2001, 212, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Schrier, A.A.; Hoffmann-Thoma, G.; van Bel, A.J. Temperature effects on symplasmic and apoplasmic phloem loading and loading-associated carbohydrate processing. Funct. Plant Biol. 2000, 27, 769–778. [Google Scholar] [CrossRef]
- Badri, M.A.; Minchin, P.E.; Lapointe, L. Effects of temperature on the growth of spring ephemerals: Crocus vernus. Physiol. Plant. 2007, 130, 67–76. [Google Scholar] [CrossRef]
- Lapointe, L. How phenology influences physiology in deciduous forest spring ephemerals. Physiol. Plant. 2001, 113, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Gandin, A.; Gutjahr, S.; Dizengremel, P.; Lapointe, L. Source-sink imbalance increases with growth temperature in the spring geophyte Erythronium americanum. J. Exp. Bot. 2011, 62, 3467–3479. [Google Scholar] [CrossRef] [Green Version]
- Ohashi, Y.; Nakayama, N.; Saneoka, H.; Fujita, K. Effects of drought stress on photosynthetic gas exchange, chlorophyll fluorescence and stem diameter of soybean plants. Biol. Plant. 2006, 50, 138–141. [Google Scholar] [CrossRef]
- Valliyodan, B.; Nguyen, H.T. Understanding regulatory networks and engineering for enhanced drought tolerance in plants. Curr. Opin. Plant Biol. 2006, 9, 189–195. [Google Scholar] [CrossRef]
- Porcel, R.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal influence on leaf water potential, solute accumulation, and oxidative stress in soybean plants subjected to drought stress. J. Exp. Bot. 2004, 55, 1743–1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Cui, K.; Xu, A.; Nie, L.; Huang, J.; Peng, S. Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol. Plant. 2015, 37, 9. [Google Scholar] [CrossRef]
- Cui, K.; Huang, J.; Xing, Y.; Yu, S.; Xu, C.; Peng, S. Mapping QTLs for seedling characteristics under different water supply conditions in rice (Oryza sativa). Physiol. Plant. 2008, 132, 53–68. [Google Scholar] [CrossRef]
- Hummel, I.; Pantin, F.; Sulpice, R.; Piques, M.; Rolland, G.; Dauzat, M.; Christophe, A.; Pervent, M.; Bouteillé, M.; Stitt, M. Arabidopsis plants acclimate to water deficit at low cost through changes of carbon usage: An integrated perspective using growth, metabolite, enzyme, and gene expression analysis. Plant Physiol. 2010, 154, 357–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.-S.; Li, F.-M.; Xu, H. Deficiency of water can enhance root respiration rate of drought-sensitive but not drought-tolerant spring wheat. Agric. Water Manag. 2004, 64, 41–48. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, W.; Zhang, B.; Xie, F. Effect of drought stress on sugar metabolism in leaves and roots of soybean seedlings. Plant Physiol. Biochem. 2020, 146, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Erice, G.; Louahlia, S.; Irigoyen, J.J.; Sanchez-Diaz, M.; Avice, J.-C. Biomass partitioning, morphology and water status of four alfalfa genotypes submitted to progressive drought and subsequent recovery. J. Plant Physiol. 2010, 167, 114–120. [Google Scholar] [CrossRef]
- Hesse, B.D.; Goisser, M.; Hartmann, H.; Grams, T.E. Repeated summer drought delays sugar export from the leaf and impairs phloem transport in mature beech. Tree Physiol. 2019, 39, 192–200. [Google Scholar] [CrossRef]
- Aliche, E.B.; Theeuwen, T.; Oortwijn, M.; Visser, R.G.F.; van der Linden, C.G. Carbon partitioning mechanisms in POTATO under drought stress. Plant Physiol. Biochem. 2020, 146, 211–219. [Google Scholar] [CrossRef]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, H.; Wu, J.; Xie, F. Effect of drought stress during soybean R2–R6 growth stages on sucrose metabolism in leaf and seed. Int. J. Mol. Sci. 2020, 21, 618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, A.; Cao, S.; Li, Y.; Wang, H.; Wei, Y.; Shao, X.; Xu, F. Sucrose treatment suppresses programmed cell death in broccoli florets by improving mitochondrial physiological properties. Postharvest Biol. Technol. 2019, 156, 110932. [Google Scholar] [CrossRef]
- Cowan, A.K.; Freeman, M.; Björkman, P.-O.; Nicander, B.; Sitbon, F.; Tillberg, E. Effects of senescence-induced alteration in cytokinin metabolism on source-sink relationships and ontogenic and stress-induced transitions in tobacco. Planta 2005, 221, 801–814. [Google Scholar] [CrossRef]
- Rizhsky, L.; Liang, H.; Shuman, J.; Shulaev, V.; Davletova, S.; Mittler, R. When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. Plant Physiol. 2004, 134, 1683–1696. [Google Scholar] [CrossRef] [Green Version]
- Dai, N.; Schaffer, A.; Petreikov, M.; Shahak, Y.; Giller, Y.; Ratner, K.; Levine, A.; Granot, D. Overexpression of Arabidopsis hexokinase in tomato plants inhibits growth, reduces photosynthesis, and induces rapid senescence. Plant Cell 1999, 11, 1253–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Zhang, J.; Wang, Z.; Zhu, Q.; Liu, L. Abscisic acid and cytokinins in the root exudates and leaves and their relationship to senescence and remobilization of carbon reserves in rice subjected to water stress during grain filling. Planta 2002, 215, 645–652. [Google Scholar] [CrossRef]
- Westgate, M.E.; Grant, D.T. Effect of Water Deficits on Seed Development in Soybean: I. Tissue Water Status. Plant Physiol. 1989, 91, 975–979. [Google Scholar] [CrossRef] [Green Version]
- Krishnan, P.; Singh, R.; Verma, A.; Joshi, D.; Singh, S. Changes in seed water status as characterized by NMR in developing soybean seed grown under moisture stress conditions. Biochem. Biophys. Res. Commun. 2014, 444, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Muller, B.; Pantin, F.; Génard, M.; Turc, O.; Freixes, S.; Piques, M.; Gibon, Y. Water deficits uncouple growth from photosynthesis, increase C content, and modify the relationships between C and growth in sink organs. J. Exp. Bot. 2011, 62, 1715–1729. [Google Scholar] [CrossRef] [Green Version]
- Agasse, A.; Vignault, C.; Kappel, C.; Conde, C.; Gerós, H.; Delrot, S. Sugar transport & sugar sensing in grape. In Grapevine Molecular Physiology & Biotechnology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 105–139. [Google Scholar]
- Peuke, A.D. Correlations in concentrations, xylem and phloem flows, and partitioning of elements and ions in intact plants. A summary and statistical re-evaluation of modelling experiments in Ricinus communis. J. Exp. Bot. 2010, 61, 635–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, W.; Loka, D.A.; Fitzsimons, T.R.; Zhou, Z.; Oosterhuis, D.M. Potassium deficiency limits reproductive success by altering carbohydrate and protein balances in cotton (Gossypium hirsutum L.). Environ. Exp. Bot. 2018, 145, 87–94. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.A.; Kamiya, T.; Burritt, D.; Tran, L.-S.P.; Fujiwara, T. Plant Macronutrient Use Efficiency: Molecular and Genomic Perspectives in Crop Plants; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Zhao, H.; Sun, S.; Zhang, L.; Yang, J.; Wang, Z.; Ma, F.; Li, M. Carbohydrate metabolism and transport in apple roots under nitrogen deficiency. Plant Physiol. Biochem. 2020, 155, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Ning, P.; Yang, L.; Li, C.; Fritschi, F.B. Post-silking carbon partitioning under nitrogen deficiency revealed sink limitation of grain yield in maize. J. Exp. Bot. 2018, 69, 1707–1719. [Google Scholar] [CrossRef] [Green Version]
- Esteban, R.; Ariz, I.; Cruz, C.; Moran, J.F. Mechanisms of ammonium toxicity and the quest for tolerance. Plant Sci. 2016, 248, 92–101. [Google Scholar] [CrossRef] [Green Version]
- de Avila Silva, L.; Condori-Apfata, J.A.; Marcelino, M.M.; Tavares, A.C.A.; Raimundi, S.C.J.; Martino, P.B.; Araujo, W.L.; Zsogon, A.; Sulpice, R.; Nunes-Nesi, A. Nitrogen differentially modulates photosynthesis, carbon allocation and yield related traits in two contrasting Capsicum chinense cultivars. Plant Sci. 2019, 283, 224–237. [Google Scholar] [CrossRef]
- Takei, K.; Takahashi, T.; Sugiyama, T.; Yamaya, T.; Sakakibara, H. Multiple routes communicating nitrogen availability from roots to shoots: A signal transduction pathway mediated by cytokinin. J. Exp. Bot. 2002, 53, 971–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, J.; Jia, Y.; Chen, H.; Zhang, L.; Yang, J.; Zhang, J.; Hu, X.; Ye, X.; Li, Y.; Zhou, Y. Growth, photosynthesis, and nutrient uptake in wheat are affected by differences in nitrogen levels and forms and potassium supply. Sci. Rep. 2019, 9, 1248. [Google Scholar] [CrossRef] [Green Version]
- Walch-Liu, P.; Neumann, G.; Bangerth, F.; Engels, C. Rapid effects of nitrogen form on leaf morphogenesis in tobacco. J. Exp. Bot. 2000, 51, 227–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Wang, M.; Sun, Y.; Gu, Z.; Wang, R.; Saydin, A.; Shen, Q.; Guo, S. Nitrate increased cucumber tolerance to Fusarium wilt by regulating fungal toxin production and distribution. Toxins 2017, 9, 100. [Google Scholar] [CrossRef] [Green Version]
- Hermans, C.; Hammond, J.P.; White, P.J.; Verbruggen, N. How do plants respond to nutrient shortage by biomass allocation? Trends Plant Sci. 2006, 11, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Samac, D.A.; Bucciarelli, B.; Allan, D.L.; Vance, C.P. Signaling of phosphorus deficiency-induced gene expression in white lupin requires sugar and phloem transport. Plant J. 2005, 41, 257–268. [Google Scholar] [CrossRef] [PubMed]
- de Groot, C.C.; Marcelis, L.F.; van den Boogaard, R.; Kaiser, W.M.; Lambers, H. Interaction of nitrogen and phosphorus nutrition in determining growth. Plant Soil 2003, 248, 257–268. [Google Scholar] [CrossRef]
- Cakmak, I.; Hengeler, C.; Marschner, H. Partitioning of shoot and root dry matter and carbohydrates in bean plants suffering from phosphorus, potassium and magnesium deficiency. J. Exp. Bot. 1994, 45, 1245–1250. [Google Scholar] [CrossRef]
- Morales, F.; Pavlovič, A.; Abadía, A.; Abadía, J. Photosynthesis in poor nutrient soils, in compacted soils, and under drought. In The Leaf: A Platform for Performing Photosynthesis; Springer: Berlin/Heidelberg, Germany, 2018; pp. 371–399. [Google Scholar]
- Pilot, G.; Gaymard, F.; Mouline, K.; Chérel, I.; Sentenac, H. Regulated expression of Arabidopsis Shaker K+ channel genes involved in K+ uptake and distribution in the plant. Plant Mol. Biol. 2003, 51, 773–787. [Google Scholar] [CrossRef]
- Deeken, R.; Geiger, D.; Fromm, J.; Koroleva, O.; Ache, P.; Langenfeld-Heyser, R.; Sauer, N.; May, S.T.; Hedrich, R. Loss of the AKT2/3 potassium channel affects sugar loading into the phloem of Arabidopsis. Planta 2002, 216, 334–344. [Google Scholar] [CrossRef]
- Tränkner, M.; Tavakol, E.; Jákli, B. Functioning of potassium and magnesium in photosynthesis, photosynthate translocation and photoprotection. Physiol. Plant. 2018, 163, 414–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cakmak, I.; Yazici, A.M. Magnesium: A forgotten element in crop production. Better Crop. 2010, 94, 23–25. [Google Scholar]
- Koch, M.; Busse, M.; Naumann, M.; Jákli, B.; Smit, I.; Cakmak, I.; Hermans, C.; Pawelzik, E. Differential effects of varied potassium and magnesium nutrition on production and partitioning of photoassimilates in potato plants. Physiol. Plant. 2019, 166, 921–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, Y.; Xu, G. Low magnesium with high potassium supply changes sugar partitioning and root growth pattern prior to visible magnesium deficiency in leaves of Rice (Oryza sativa L.). Am. J. Plant Sci. 2011, 2, 601. [Google Scholar] [CrossRef] [Green Version]
- Cakmak, I.; Hengeler, C.; Marschner, H. Changes in phloem export of sucrose in leaves in response to phosphorus, potassium and magnesium deficiency in bean plants. J. Exp. Bot. 1994, 45, 1251–1257. [Google Scholar] [CrossRef]
- Fischer, E.; Lohaus, G.; Heineke, D.; Heldt, H. Magnesium deficiency results in accumulation of carbohydrates and amino acids in source and sink leaves of spinach. Physiol. Plant. 1998, 102, 16–20. [Google Scholar] [CrossRef]
- He, H.; Jin, X.; Ma, H.; Deng, Y.; Huang, J.; Yin, L. Changes of plant biomass partitioning, tissue nutrients and carbohydrates status in magnesium-deficient banana seedlings and remedy potential by foliar application of magnesium. Sci. Hortic. 2020, 268, 109377. [Google Scholar] [CrossRef]
- Hermans, C.; Bourgis, F.; Faucher, M.; Strasser, R.J.; Delrot, S.; Verbruggen, N. Magnesium deficiency in sugar beets alters sugar partitioning and phloem loading in young mature leaves. Planta 2005, 220, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Gottwald, J.R.; Krysan, P.J.; Young, J.C.; Evert, R.F.; Sussman, M.R. Genetic evidence for the in planta role of phloem-specific plasma membrane sucrose transporters. Proc. Natl. Acad. Sci. USA 2000, 97, 13979–13984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xuan, Y.H.; Hu, Y.B.; Chen, L.-Q.; Sosso, D.; Ducat, D.C.; Hou, B.-H.; Frommer, W.B. Functional role of oligomerization for bacterial and plant SWEET sugar transporter family. Proc. Natl. Acad. Sci. USA 2013, 110, E3685–E3694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.-Q.; Hou, B.-H.; Lalonde, S.; Takanaga, H.; Hartung, M.L.; Qu, X.-Q.; Guo, W.-J.; Kim, J.-G.; Underwood, W.; Chaudhuri, B. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature 2010, 468, 527–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durand, M.; Porcheron, B.; Hennion, N.; Maurousset, L.; Lemoine, R.; Pourtau, N. Water deficit enhances C export to the roots in Arabidopsis thaliana plants with contribution of sucrose transporters in both shoot and roots. Plant Physiol. 2016, 170, 1460–1479. [Google Scholar] [CrossRef] [Green Version]
- Ibraheem, O.; Dealtry, G.; Roux, S.; Bradley, G. The Effect of Drought and Salinity on the Expressional Levels of Sucrose Transporters in Rice (‘Oryza sativa’ Nipponbare) Cultivar Plants. Plant Omics 2011, 4, 68. [Google Scholar]
- Gowda, V.R.; Henry, A.; Yamauchi, A.; Shashidhar, H.; Serraj, R. Root biology and genetic improvement for drought avoidance in rice. Field Crop. Res. 2011, 122, 1–13. [Google Scholar] [CrossRef]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. New Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Voothuluru, P.; Anderson, J.C.; Sharp, R.E.; Peck, S.C. Plasma membrane proteomics in the maize primary root growth zone: Novel insights into root growth adaptation to water stress. Plant Cell Environ. 2016, 39, 2043–2054. [Google Scholar] [CrossRef]
- Etxeberria, E.; Pozueta-Romero, J.; Gonzalez, P. In and out of the plant storage vacuole. Plant Sci. 2012, 190, 52–61. [Google Scholar] [CrossRef] [Green Version]
- Schulze, W.X.; Schneider, T.; Starck, S.; Martinoia, E.; Trentmann, O. Cold acclimation induces changes in Arabidopsis tonoplast protein abundance and activity and alters phosphorylation of tonoplast monosaccharide transporters. Plant J. 2012, 69, 529–541. [Google Scholar] [CrossRef]
- Wang, L.; Yao, L.; Hao, X.; Li, N.; Qian, W.; Yue, C.; Ding, C.; Zeng, J.; Yang, Y.; Wang, X. Tea plant SWEET transporters: Expression profiling, sugar transport, and the involvement of CsSWEET16 in modifying cold tolerance in Arabidopsis. Plant Mol. Biol. 2018, 96, 577–592. [Google Scholar] [CrossRef]
- Klemens, P.A.; Patzke, K.; Deitmer, J.; Spinner, L.; Le Hir, R.; Bellini, C.; Bedu, M.; Chardon, F.; Krapp, A.; Neuhaus, H.E. Overexpression of the vacuolar sugar carrier AtSWEET16 modifies germination, growth, and stress tolerance in Arabidopsis. Plant Physiol. 2013, 163, 1338–1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Li, D.; Xu, X.; Ziska, L.H.; Zhu, J.; Liu, G.; Zhu, C. The potential role of sucrose transport gene expression in the photosynthetic and yield response of rice cultivars to future CO2 concentration. Physiol. Plant 2020, 168, 218–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khush, G.S. Green revolution: The way forward. Nat. Rev. Genet. 2001, 2, 815–822. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.J.; Watson, A.; Griffiths, C.A. Linking fundamental science to crop improvement through understanding source and sink traits and their integration for yield enhancement. J. Exp. Bot. 2020, 71, 2270–2280. [Google Scholar] [CrossRef]
- Lander, E.S.; Botstein, D. Mapping mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics 1989, 121, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Fernie, A.R.; Gutierrez-Marcos, J. From genome to phenome: Genome-wide association studies and other approaches that bridge the genotype to phenotype gap. Plant J. 2019, 97, 5–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, F. Pesticides, environment, and food safety. Food Energy Secur. 2017, 6, 48–60. [Google Scholar] [CrossRef]
- Paul, M.J.; Oszvald, M.; Jesus, C.; Rajulu, C.; Griffiths, C.A. Increasing crop yield and resilience with trehalose 6-phosphate: Targeting a feast—famine mechanism in cereals for better source—Sink optimization. J. Exp. Bot. 2017, 68, 4455–4462. [Google Scholar] [CrossRef] [Green Version]
- Wu, A.; Hammer, G.L.; Doherty, A.; von Caemmerer, S.; Farquhar, G.D. Quantifying impacts of enhancing photosynthesis on crop yield. Nat. Plants 2019, 5, 380–388. [Google Scholar] [CrossRef]
- Salesse-Smith, C.E.; Sharwood, R.E.; Busch, F.A.; Kromdijk, J.; Bardal, V.; Stern, D.B. Overexpression of Rubisco subunits with RAF1 increases Rubisco content in maize. Nat. Plants 2018, 4, 802–810. [Google Scholar] [CrossRef]
- Pendleton, J.; Smith, G.; Winter, S.; Johnston, T. Field investigations of the relationships of leaf angle in corn (zea mays l.) to grain yield and apparent photosynthesis 1. Agron. J. 1968, 60, 422–424. [Google Scholar] [CrossRef]
- Richards, R.A.; Cavanagh, C.R.; Riffkin, P. Selection for erect canopy architecture can increase yield and biomass of spring wheat. Field Crop. Res. 2019, 244, 107649. [Google Scholar] [CrossRef]
- Srivastava, A.C.; Ganesan, S.; Ismail, I.O.; Ayre, B.G. Effective carbon partitioning driven by exotic phloem-specific regulatory elements fused to the Arabidopsis thaliana AtSUC2 sucrose-proton symporter gene. BMC Plant Biol. 2009, 9, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wingler, A.; Purdy, S.; MacLean, J.A.; Pourtau, N. The role of sugars in integrating environmental signals during the regulation of leaf senescence. J. Exp. Bot. 2006, 57, 391–399. [Google Scholar] [CrossRef] [Green Version]
- Rosche, E.; Blackmore, D.; Tegeder, M.; Richardson, T.; Schroeder, H.; Higgins, T.J.; Frommer, W.B.; Offler, C.E.; Patrick, J.W. Seed-specific overexpression of a potato sucrose transporter increases sucrose uptake and growth rates of developing pea cotyledons. Plant J. 2002, 30, 165–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weichert, N.; Saalbach, I.; Weichert, H.; Kohl, S.; Erban, A.; Kopka, J.; Hause, B.; Varshney, A.; Sreenivasulu, N.; Strickert, M. Increasing sucrose uptake capacity of wheat grains stimulates storage protein synthesis. Plant Physiol. 2010, 152, 698–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaachra, A.; Vats, S.K.; Kumar, S. Heterologous expression of key C and N metabolic enzymes improves re-assimilation of photorespired CO2 and NH3, and growth. Plant Physiol. 2018, 177, 1396–1409. [Google Scholar] [CrossRef] [Green Version]
- Naqvi, S.; Zhu, C.; Farre, G.; Ramessar, K.; Bassie, L.; Breitenbach, J.; Conesa, D.P.; Ros, G.; Sandmann, G.; Capell, T. Transgenic multivitamin corn through biofortification of endosperm with three vitamins representing three distinct metabolic pathways. Proc. Natl. Acad. Sci. USA 2009, 106, 7762–7767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bachem, C.W.; van Eck, H.J.; de Vries, M.E. Understanding genetic load in potato for hybrid diploid breeding. Mol. Plant 2019, 12, 896–898. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, P.; Tang, D.; Yang, Z.; Lu, F.; Qi, J.; Tawari, N.R.; Shang, Y.; Li, C.; Huang, S. The genetic basis of inbreeding depression in potato. Nat. Genet. 2019, 51, 374–378. [Google Scholar] [CrossRef]
- Ghosh, S.; Watson, A.; Gonzalez-Navarro, O.E.; Ramirez-Gonzalez, R.H.; Yanes, L.; Mendoza-Suárez, M.; Simmonds, J.; Wells, R.; Rayner, T.; Green, P. Speed breeding in growth chambers and glasshouses for crop breeding and model plant research. Nat. Protoc. 2018, 13, 2944–2963. [Google Scholar] [CrossRef] [Green Version]
- Fernie, A.R.; Yan, J. De novo domestication: An alternative route toward new crops for the future. Mol. Plant 2019, 12, 615–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aluko, O.O.; Li, C.; Wang, Q.; Liu, H. Sucrose Utilization for Improved Crop Yields: A Review Article. Int. J. Mol. Sci. 2021, 22, 4704. https://doi.org/10.3390/ijms22094704
Aluko OO, Li C, Wang Q, Liu H. Sucrose Utilization for Improved Crop Yields: A Review Article. International Journal of Molecular Sciences. 2021; 22(9):4704. https://doi.org/10.3390/ijms22094704
Chicago/Turabian StyleAluko, Oluwaseun Olayemi, Chuanzong Li, Qian Wang, and Haobao Liu. 2021. "Sucrose Utilization for Improved Crop Yields: A Review Article" International Journal of Molecular Sciences 22, no. 9: 4704. https://doi.org/10.3390/ijms22094704






