Beneficial Effects of Phenolic Compounds on Gut Microbiota and Metabolic Syndrome
Abstract
:1. Introduction
2. Metabolic Syndrome
- Elevated waist circumference;
- Elevated triglycerides (≥150 mg/dL; also during pharmacotherapy);
- Reduced high-density lipoprotein (HDL) cholesterol (<40 mg/dL; drug treatment for reduced HDL cholesterol is an alternate indicator);
- Elevated blood pressure (systolic ≥ 130 and/or diastolic ≥ 85 mm Hg; also during antihypertensive pharmacotherapy);
3. Healthy Microbiota Composition and Functions
4. Dysbiosis and Metabolic Syndrome
5. Classes of Phenolic Compounds and Their Biological Role for Human Health
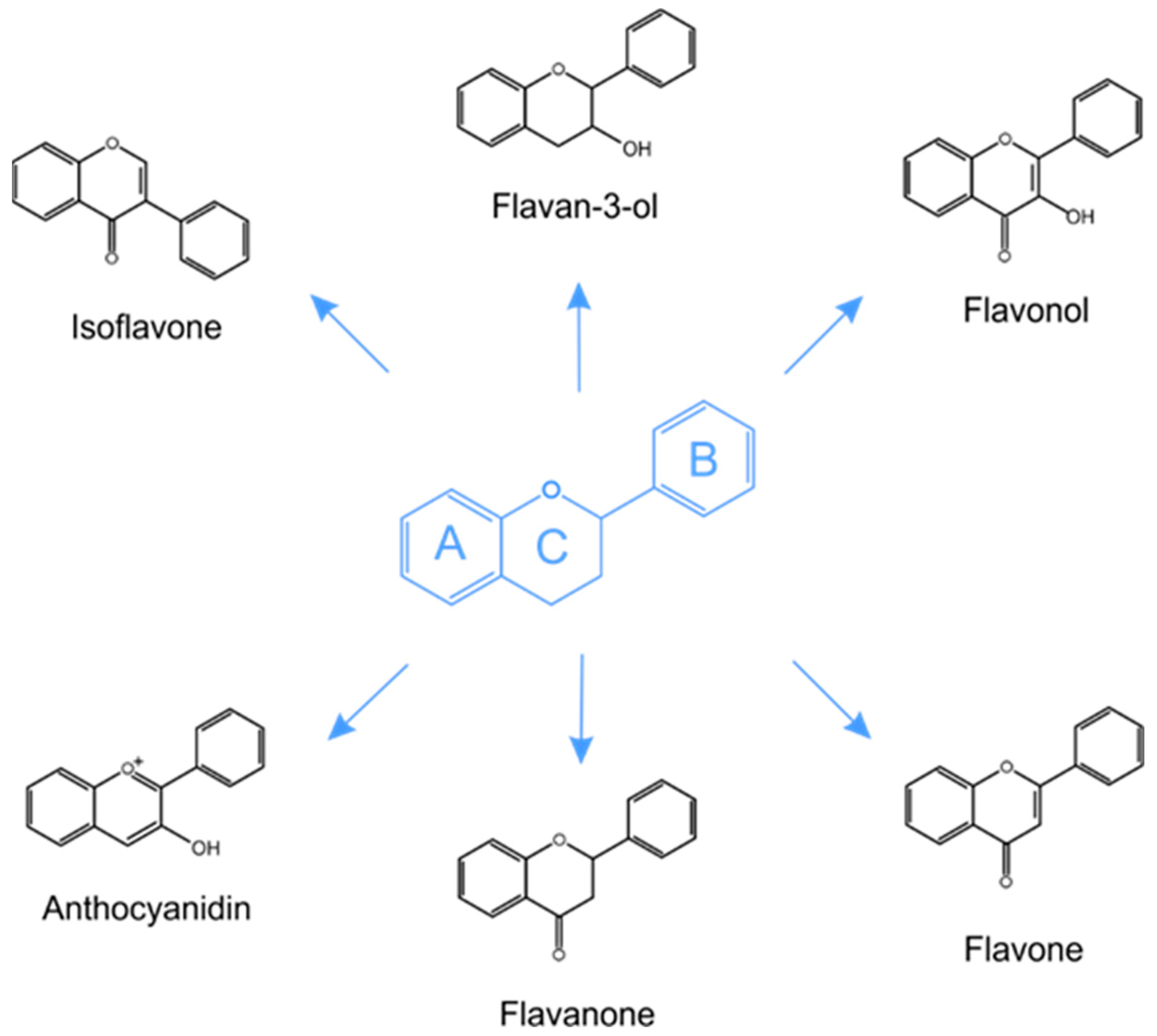
5.1. Structure and Classification of Phenolic Compounds in Plants
5.2. Polyphenolic Compounds as an Important Plant Component of the Diet
5.3. Health Benefit Properties of Plant Polyphenolic Compounds
6. Metabolism of Polyphenols by Gut Microbiota
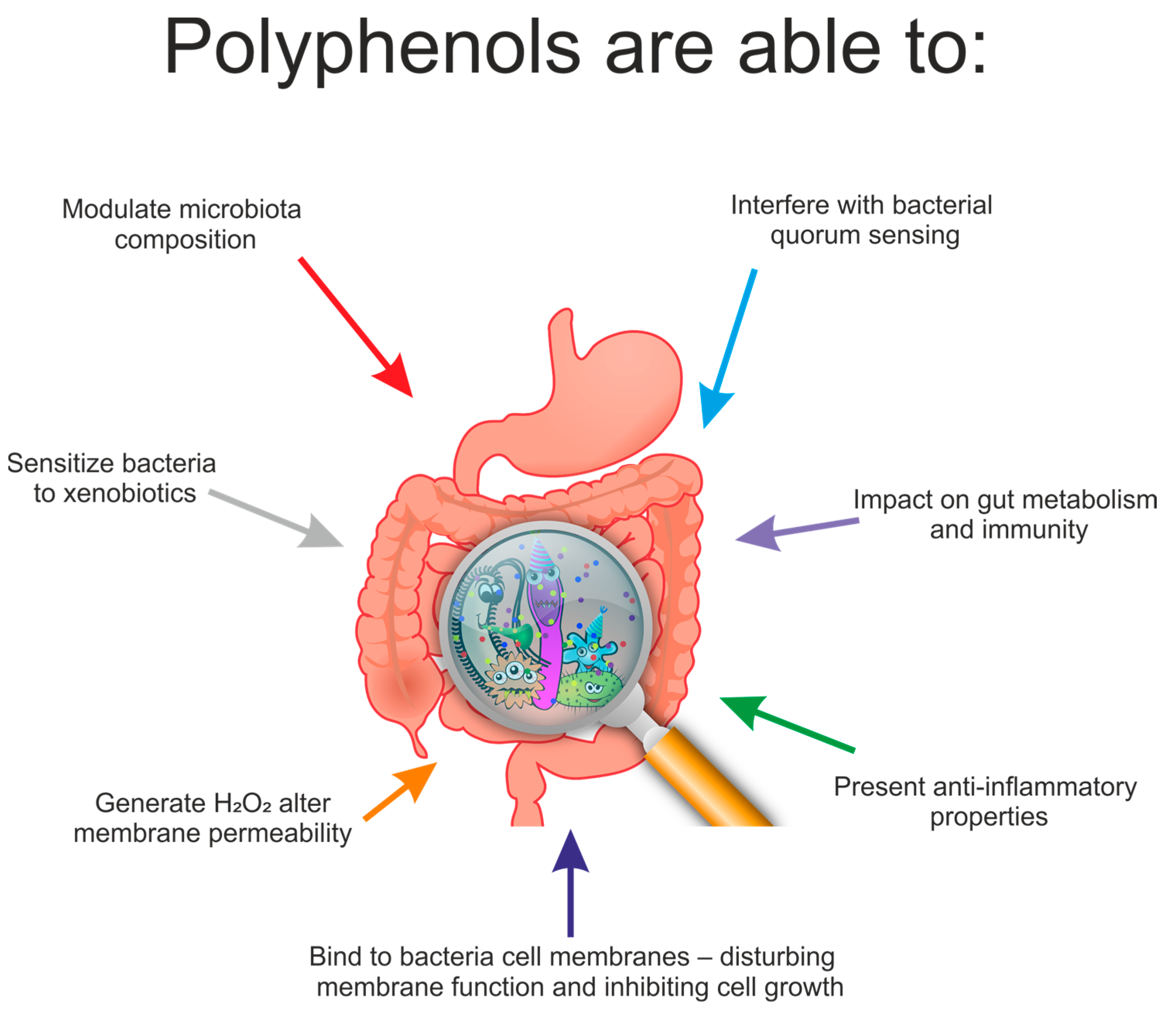
7. Effect of Polyphenols on the Composition of Gut Microbiota
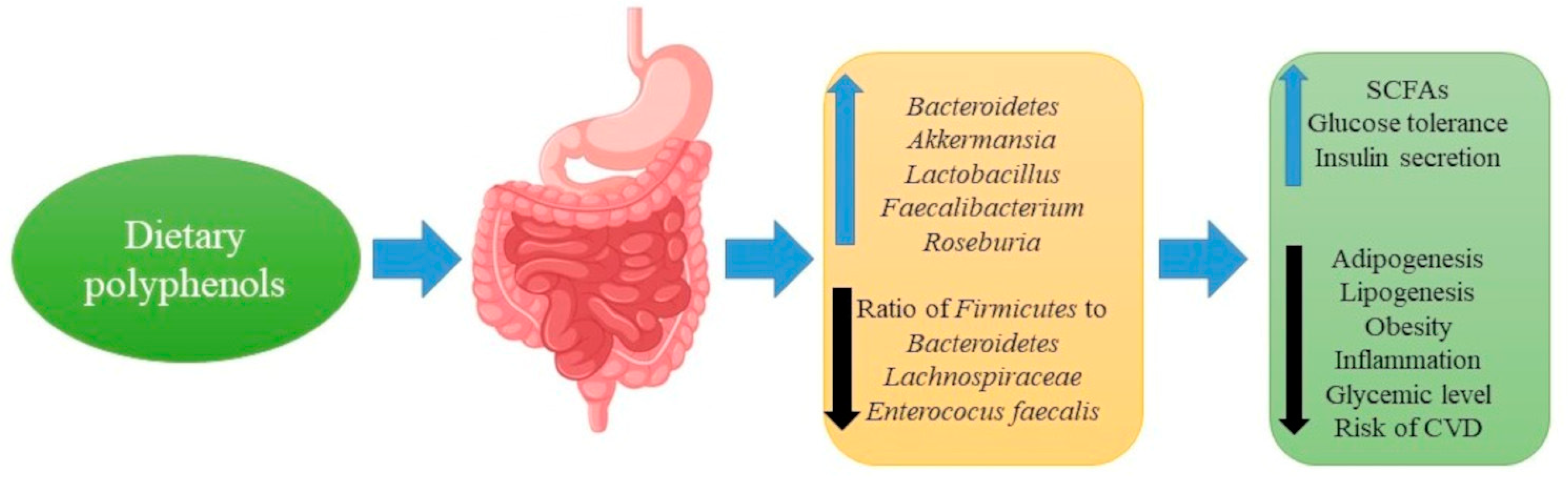
8. Polyphenols, Gut Microbiota and Metabolic Diseases
9. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peterson, J.; Garges, S.; Giovanni, M.; McInnes, P.; Wang, L.; Schloss, J.A.; Bonazzi, V.; McEwen, J.E.; Wetterstrand, K.A.; Deal, C.; et al. The NIH Human Microbiome Project. Genome Res. 2009, 19, 2317–2323. [Google Scholar] [CrossRef] [Green Version]
- Sender, R.; Fuchs, S.; Milo, R. Revised Estimates for the Number of Human and Bacteria Cells in the Body. PLoS Biol. 2016, 14, e1002533. [Google Scholar] [CrossRef] [Green Version]
- Goodacre, R. Metabolomics of a Superorganism. J. Nutr. 2007, 137 (Suppl. 1), 259S–266S. [Google Scholar] [CrossRef] [Green Version]
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A.; Tarko, T.; Satora, P.; Sroka, P. Interaction of Dietary Compounds, Especially Polyphenols, with the Intestinal Microbiota: A Review. Eur. J. Nutr. 2015, 54, 325–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, S.R.; Pop, M.; DeBoy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic Analysis of the Human Distal Gut Microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef] [Green Version]
- Thursby, E.; Juge, N. Introduction to the Human Gut Microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the Human Intestinal Microbial Flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grundy, S.M. Metabolic Syndrome: A Multiplex Cardiovascular Risk Factor. J. Clin. Endocrinol. Metab. 2007, 92, 399–404. [Google Scholar] [CrossRef]
- Grundy, S.M. Metabolic Syndrome Update. Trends Cardiovasc. Med. 2016, 26, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Mameli, C.; Zuccotti, G.V.; Carnovale, C.; Galli, E.; Nannini, P.; Cervia, D.; Perrotta, C. An Update on the Assessment and Management of Metabolic Syndrome, a Growing Medical Emergency in Paediatric Populations. Pharmacol. Res. 2017, 119, 99–117. [Google Scholar] [CrossRef]
- Cornier, M.-A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The Metabolic Syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef] [PubMed]
- Hess, P.L.; Al-Khalidi, H.R.; Friedman, D.J.; Mulder, H.; Kucharska-Newton, A.; Rosamond, W.R.; Lopes, R.D.; Gersh, B.J.; Mark, D.B.; Curtis, L.H.; et al. The Metabolic Syndrome and Risk of Sudden Cardiac Death: The Atherosclerosis Risk in Communities Study. J. Am. Heart Assoc. 2017, 6, e006103. [Google Scholar] [CrossRef] [PubMed]
- Uzunlulu, M.; Telci Caklili, O.; Oguz, A. Association between Metabolic Syndrome and Cancer. Ann. Nutr. Metab. 2016, 68, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Zimmet, P.Z. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus Provisional Report of a WHO Consultation. Diabet. Med. J. Br. Diabet. Assoc. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.-C.; James, W.P.T.; Loria, C.M.; Smith, S.C. Harmonizing the Metabolic Syndrome: A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasun, P. Mitochondrial Dysfunction in Metabolic Syndrome. Biochim. Biophys. Acta BBA Mol. Basis Dis. 2020, 1866, 165838. [Google Scholar] [CrossRef] [PubMed]
- Reyes, S.J.; Pak, T.; Moon, T.S. Metabolic Syndrome—Evidence-Based Strategies for Patient Optimization. Best Pract. Res. Clin. Anaesthesiol. 2020, 34, 131–140. [Google Scholar] [CrossRef]
- Cameron, A.J.; Shaw, J.E.; Zimmet, P.Z. The Metabolic Syndrome: Prevalence in Worldwide Populations. Endocrinol. Metab. Clin. N. Am. 2004, 33, 351–375. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bener, A.; Zirie, M.; Musallam, M.; Khader, Y.; Al-Hamaq, A. Prevalence of Metabolic Syndrome According to Adult Treatment Panel III and International Diabetes Federation Criteria: A Population-Based Study. Metab. Syndr. Relat. Disord. 2009, 7, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Giles, W.H.; Mokdad, A.H. Increasing Prevalence of the Metabolic Syndrome Among U.S. Adults. Diabetes Care 2004, 27, 2444–2449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oniszczuk, A.; Oniszczuk, T.; Gancarz, M.; Szymańska, J. Role of Gut Microbiota, Probiotics and Prebiotics in the Cardiovascular Diseases. Molecules 2021, 26, 1172. [Google Scholar] [CrossRef]
- Kumar Singh, A.; Cabral, C.; Kumar, R.; Ganguly, R.; Kumar Rana, H.; Gupta, A.; Rosaria Lauro, M.; Carbone, C.; Reis, F.; Pandey, A.K. Beneficial Effects of Dietary Polyphenols on Gut Microbiota and Strategies to Improve Delivery Efficiency. Nutrients 2019, 11, 2216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut Microbiota in Health and Disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, M.; Nakayama, J. Development of the Gut Microbiota in Infancy and Its Impact on Health in Later Life. Allergol. Int. Off. J. Jpn. Soc. Allergol. 2017, 66, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Odamaki, T.; Kato, K.; Sugahara, H.; Hashikura, N.; Takahashi, S.; Xiao, J.; Abe, F.; Osawa, R. Age-Related Changes in Gut Microbiota Composition from Newborn to Centenarian: A Cross-Sectional Study. BMC Microbiol. 2016, 16, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Z.; Huang, S.; Zou, D.; Dong, D.; He, X.; Liu, N.; Liu, W.; Huang, L. Metabolic Shifts and Structural Changes in the Gut Microbiota upon Branched-Chain Amino Acid Supplementation in Middle-Aged Mice. Amino Acids 2016, 48, 2731–2745. [Google Scholar] [CrossRef] [PubMed]
- Man, A.W.C.; Zhou, Y.; Xia, N.; Li, H. Involvement of Gut Microbiota, Microbial Metabolites and Interaction with Polyphenol in Host Immunometabolism. Nutrients 2020, 12, 3054. [Google Scholar] [CrossRef] [PubMed]
- Belizário, J.E.; Faintuch, J.; Garay-Malpartida, M. Gut Microbiome Dysbiosis and Immunometabolism: New Frontiers for Treatment of Metabolic Diseases. Mediators Inflamm. 2018, 2018, 2037838. [Google Scholar] [CrossRef]
- Koppel, N.; Rekdal, V.M.; Balskus, E.P. Chemical Transformation of Xenobiotics by the Human Gut Microbiota. Science 2017, 356. [Google Scholar] [CrossRef] [PubMed]
- Aron-Wisnewsky, J.; Doré, J.; Clement, K. The Importance of the Gut Microbiota after Bariatric Surgery. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Alden, N.; Lee, K. Pathways and Functions of Gut Microbiota Metabolism Impacting Host Physiology. Curr. Opin. Biotechnol. 2015, 36, 137–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerdes, V.; Gueimonde, M.; Pajunen, L.; Nieuwdorp, M.; Laitinen, K. How Strong Is the Evidence That Gut Microbiota Composition Can Be Influenced by Lifestyle Interventions in a Cardio-Protective Way? Atherosclerosis 2020, 311, 124–142. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Zeng, M.Y.; Núñez, G. The Interplay between Host Immune Cells and Gut Microbiota in Chronic Inflammatory Diseases. Exp. Mol. Med. 2017, 49, e339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Chen, B.; Zhao, L.; Li, H. The Gut Microbiota: Emerging Evidence in Autoimmune Diseases. Trends Mol. Med. 2020, 26, 862–873. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.-F.; Li, X.; Li, H.-X.; Zhang, Q.; Zhou, H.-W.; He, Y.; Li, P.; Fu, C.; Zhang, X.-H.; et al. Disordered Intestinal Microbes Are Associated with the Activity of Systemic Lupus Erythematosus. Clin. Sci. Lond. Engl. 2019, 133, 821–838. [Google Scholar] [CrossRef] [PubMed]
- Mandl, T.; Ekberg, O. Dysphagia in Systemic Disease. Med. Radiol. 2017, 237–245. [Google Scholar] [CrossRef]
- Vieira, S.M.; Hiltensperger, M.; Kumar, V.; Zegarra-Ruiz, D.; Dehner, C.; Khan, N.; Costa, F.R.C.; Tiniakou, E.; Greiling, T.; Ruff, W.; et al. Translocation of a Gut Pathobiont Drives Autoimmunity in Mice and Humans. Science 2018, 359, 1156–1161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suganya, K.; Son, T.; Kim, K.-W.; Koo, B.-S. Impact of Gut Microbiota: How It Could Play Roles beyond the Digestive System on Development of Cardiovascular and Renal Diseases. Microb. Pathog. 2020, 104583. [Google Scholar] [CrossRef] [PubMed]
- Randrianarisoa, E.; Lehn-Stefan, A.; Wang, X.; Hoene, M.; Peter, A.; Heinzmann, S.S.; Zhao, X.; Königsrainer, I.; Königsrainer, A.; Balletshofer, B.; et al. Relationship of Serum Trimethylamine N-Oxide (TMAO) Levels with Early Atherosclerosis in Humans. Sci. Rep. 2016, 6, 26745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; van Esch, B.C.A.M.; Wagenaar, G.T.M.; Garssen, J.; Folkerts, G.; Henricks, P.A.J. Pro- and Anti-Inflammatory Effects of Short Chain Fatty Acids on Immune and Endothelial Cells. Eur. J. Pharmacol. 2018, 831, 52–59. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The Gut-Brain Axis: Interactions between Enteric Microbiota, Central and Enteric Nervous Systems. Ann. Gastroenterol. Q. Publ. Hell. Soc. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Asano, Y.; Hiramoto, T.; Nishino, R.; Aiba, Y.; Kimura, T.; Yoshihara, K.; Koga, Y.; Sudo, N. Critical Role of Gut Microbiota in the Production of Biologically Active, Free Catecholamines in the Gut Lumen of Mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G1288–G1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Kiely, B.; Cryan, J.F.; Dinan, T.G. Effects of the Probiotic Bifidobacterium Infantis in the Maternal Separation Model of Depression. Neuroscience 2010, 170, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Castro-Nallar, E.; Bendall, M.L.; Pérez-Losada, M.; Sabuncyan, S.; Severance, E.G.; Dickerson, F.B.; Schroeder, J.R.; Yolken, R.H.; Crandall, K.A. Composition, Taxonomy and Functional Diversity of the Oropharynx Microbiome in Individuals with Schizophrenia and Controls. PeerJ 2015, 3, e1140. [Google Scholar] [CrossRef]
- Conlon, M.A.; Bird, A.R. The Impact of Diet and Lifestyle on Gut Microbiota and Human Health. Nutrients 2014, 7, 17–44. [Google Scholar] [CrossRef] [PubMed]
- Tremaroli, V.; Bäckhed, F. Functional Interactions between the Gut Microbiota and Host Metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Blandino, G.; Inturri, R.; Lazzara, F.; Di Rosa, M.; Malaguarnera, L. Impact of Gut Microbiota on Diabetes Mellitus. Diabetes Metab. 2016, 42, 303–315. [Google Scholar] [CrossRef]
- Fernandes, R.; Viana, S.D.; Nunes, S.; Reis, F. Diabetic Gut Microbiota Dysbiosis as an Inflammaging and Immunosenescence Condition That Fosters Progression of Retinopathy and Nephropathy. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1876–1897. [Google Scholar] [CrossRef] [PubMed]
- Olszowy, M. What Is Responsible for Antioxidant Properties of Polyphenolic Compounds from Plants? Plant Physiol. Biochem. 2019, 144, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.C. Microbial Ecology of the Gastrointestinal Tract. Annu. Rev. Microbiol. 1977, 31, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A Concise Overview on the Chemistry, Occurrence, and Human Health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [Green Version]
- Roche, A.; Ross, E.; Walsh, N.; O’Donnell, K.; Williams, A.; Klapp, M.; Fullard, N.; Edelstein, S. Representative Literature on the Phytonutrients Category: Phenolic Acids. Crit. Rev. Food Sci. Nutr. 2017, 57, 1089–1096. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Waksmundzka-Hajnos, M.; Sherma, J.; Kowalska, T. Thin Layer Chromatography in Phytochemistry; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and Bioefficacy of Polyphenols in Humans. I. Review of 97 Bioavailability Studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Jiménez, J.; Neveu, V.; Vos, F.; Scalbert, A. Systematic Analysis of the Content of 502 Polyphenols in 452 Foods and Beverages: An Application of the Phenol-Explorer Database. J. Agric. Food Chem. 2010, 58, 4959–4969. [Google Scholar] [CrossRef]
- Murkovic, M. Phenolic Compounds: Occurrence, Classes, and Analysis. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 346–351. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef] [PubMed]
- Kroon, P.A.; Faulds, C.B.; Ryden, P.; Robertson, J.A.; Williamson, G. Release of Covalently Bound Ferulic Acid from Fiber in the Human Colon. J. Agric. Food Chem. 1997, 45, 661–667. [Google Scholar] [CrossRef]
- Williamson, G.; Holst, B. Dietary Reference Intake (DRI) Value for Dietary Polyphenols: Are We Heading in the Right Direction? Br. J. Nutr. 2008, 99 (Suppl. 3), S55–S58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karam, J.; Bibiloni, M.d.M.; Tur, J.A. Polyphenol Estimated Intake and Dietary Sources among Older Adults from Mallorca Island. PLoS ONE 2018, 13, e0191573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scalbert, A.; Williamson, G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Tsao, R. Dietary Polyphenols, Oxidative Stress and Antioxidant and Anti-Inflammatory Effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Hurrell, R.; Egli, I. Iron Bioavailability and Dietary Reference Values. Am. J. Clin. Nutr. 2010, 91, 1461S–1467S. [Google Scholar] [CrossRef] [PubMed]
- Santos-Buelga, C.; Scalbert, A. Proanthocyanidins and Tannin-like Compounds—Nature, Occurrence, Dietary Intake and Effects on Nutrition and Health. J. Sci. Food Agric. 2000, 80, 1094–1117. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.; Huh, K.; Kim, Y.; Joung, H.; Park, M. High Serum Isoflavone Concentrations Are Associated with the Risk of Precocious Puberty in Korean Girls. Clin. Endocrinol. 2011, 75, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, A.; Hirose, M.; Takahashi, S.; Ogawa, K.; Shirai, T.; Ito, N. Forestomach and Kidney Carcinogenicity of Caffeic Acid in F344 Rats and C57BL/6N x C3H/HeN F1 Mice. Cancer Res. 1991, 51, 5655–5660. [Google Scholar]
- Mennen, L.I.; Walker, R.; Bennetau-Pelissero, C.; Scalbert, A. Risks and Safety of Polyphenol Consumption. Am. J. Clin. Nutr. 2005, 81, 326S–329S. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Yu, D.; Sun, J.; Liu, X.; Jiang, L.; Guo, H.; Ren, F. Interaction of Plant Phenols with Food Macronutrients: Characterisation and Nutritional–Physiological Consequences. Nutr. Res. Rev. 2014, 27, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagliazucchi, D.; Helal, A.; Verzelloni, E.; Conte, A. The Type and Concentration of Milk Increase the in Vitro Bioaccessibility of Coffee Chlorogenic Acids. J. Agric. Food Chem. 2012, 60, 11056–11064. [Google Scholar] [CrossRef]
- Saura-Calixto, F.; Díaz-Rubio, M.E. Polyphenols Associated with Dietary Fibre in Wine: A Wine Polyphenols Gap? Food Res. Int. 2007, 40, 613–619. [Google Scholar] [CrossRef] [Green Version]
- Jucá, M.M.; Filho, F.M.S.C.; de Almeida, J.C.; da Silva Mesquita, D.; de Moraes Barriga, J.R.; Dias, K.C.F.; Barbosa, T.M.; Vasconcelos, L.C.; Leal, L.K.A.M.; Ribeiro, J.E.; et al. Flavonoids: Biological Activities and Therapeutic Potential. Nat. Prod. Res. 2020, 34, 692–705. [Google Scholar] [CrossRef] [PubMed]
- Paszkiewicz, M.; Budzyńska, A.; Różalska, B.; Sadowska, B. Immunomodulacyjna Rola Polifenoli Roślinnych. Postepy Hig. Med. Dosw. 2012, 66, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Vazhappilly, C.G.; Ansari, S.A.; Al-Jaleeli, R.; Al-Azawi, A.M.; Ramadan, W.S.; Menon, V.; Hodeify, R.; Siddiqui, S.S.; Merheb, M.; Matar, R.; et al. Role of Flavonoids in Thrombotic, Cardiovascular, and Inflammatory Diseases. Inflammopharmacology 2019, 27, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Han, S.; Gu, Z.; Wu, J. Advances and Impact of Antioxidant Hydrogel in Chronic Wound Healing. Adv. Healthc. Mater. 2020, 9, 1901502. [Google Scholar] [CrossRef] [PubMed]
- De Moura, F.B.R.; Justino, A.B.; Ferreira, B.A.; Espindola, F.S.; de Araújo, F.A.; Tomiosso, T.C. Pro-Fibrogenic and Anti-Inflammatory Potential of a Polyphenol-Enriched Fraction from Annona Crassiflora in Skin Repair. Planta Med. 2019, 85, 570–577. [Google Scholar] [CrossRef]
- Khan, H.; Sureda, A.; Belwal, T.; Çetinkaya, S.; Süntar, İ.; Tejada, S.; Devkota, H.P.; Ullah, H.; Aschner, M. Polyphenols in the Treatment of Autoimmune Diseases. Autoimmun. Rev. 2019, 18, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.; Kwon, D.; Um, E.; Kim, B. Could Polyphenols Help in the Control of Rheumatoid Arthritis? Molecules 2019, 24, 1589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassanzadeh, P.; Arbabi, E.; Atyabi, F.; Dinarvand, R. Ferulic Acid Exhibits Antiepileptogenic Effect and Prevents Oxidative Stress and Cognitive Impairment in the Kindling Model of Epilepsy. Life Sci. 2017, 179, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Morzelle, M.C.; Salgado, J.M.; Massarioli, A.P.; Bachiega, P.; de Rios, A.O.; Alencar, S.M.; Schwember, A.R.; de Camargo, A.C. Potential Benefits of Phenolics from Pomegranate Pulp and Peel in Alzheimer’s Disease: Antioxidant Activity and Inhibition of Acetylcholinesterase. J. Food Bioact. 2019, 5, 136–141. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Hong, G.; Li, X.; Zhang, Y.; Xu, Z.; Mao, L.; Feng, X.; Liu, T. Synthesis and Activity towards Alzheimer’s Disease in Vitro: Tacrine, Phenolic Acid and Ligustrazine Hybrids. Eur. J. Med. Chem. 2018, 148, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.Y.; Jeon, M.-T.; Jung, U.J.; Kim, D.W.; Moon, G.J.; Kim, S.R. Perspective: Therapeutic Potential of Flavonoids as Alternative Medicines in Epilepsy. Adv. Nutr. 2019, 10, 778–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Yu, Z.; Xia, J.; Zhang, X.; Liu, K.; Sik, A.; Jin, M. Anti-Parkinson’s Disease Activity of Phenolic Acids from Eucommia Ulmoides Oliver Leaf Extracts and Their Autophagy Activation Mechanism. Food Funct. 2020, 11, 1425–1440. [Google Scholar] [CrossRef] [PubMed]
- Giuliano, C.; Cerri, S.; Blandini, F. Potential Therapeutic Effects of Polyphenols in Parkinson’s Disease: In Vivo and in Vitro Pre-Clinical Studies. Neural Regen. Res. 2021, 16, 234. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural Polyphenols: An Overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef] [Green Version]
- Pejcic, T.; Tosti, T.; Džamić, Z.; Gašić, U.; Vuksanović, A.; Dolićanin, Z.; Tesic, Z. The Polyphenols as Potential Agents in Prevention and Therapy of Prostate Diseases. Molecules 2019, 24, 3982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajasekar, J.; Perumal, M.K.; Vallikannan, B. A Critical Review on Anti-Angiogenic Property of Phytochemicals. J. Nutr. Biochem. 2019, 71, 1–15. [Google Scholar] [CrossRef]
- Tung, W.-C.; Rizzo, B.; Dabbagh, Y.; Saraswat, S.; Romanczyk, M.; Codorniu-Hernández, E.; Rebollido-Rios, R.; Needs, P.W.; Kroon, P.A.; Rakotomanomana, N.; et al. Polyphenols Bind to Low Density Lipoprotein at Biologically Relevant Concentrations That Are Protective for Heart Disease. Arch. Biochem. Biophys. 2020, 694, 108589. [Google Scholar] [CrossRef]
- Bahadoran, Z.; Mirmiran, P.; Azizi, F. Dietary Polyphenols as Potential Nutraceuticals in Management of Diabetes: A Review. J. Diabetes Metab. Disord. 2013, 12, 43. [Google Scholar] [CrossRef] [Green Version]
- Kasprzak, K.; Wojtunik-Kulesza, K.; Oniszczuk, T.; Kuboń, M.; Oniszczuk, A. Secondary Metabolites, Dietary Fiber and Conjugated Fatty Acids as Functional Food Ingredients against Overweight and Obesity. Nat. Prod. Commun. 2018, 13, 1073–1082. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Goel, N. Phenolic Acids: Natural Versatile Molecules with Promising Therapeutic Applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Appeldoorn, M.M.; Vincken, J.-P.; Gruppen, H.; Hollman, P.C.H. Procyanidin Dimers A1, A2, and B2 Are Absorbed without Conjugation or Methylation from the Small Intestine of Rats. J. Nutr. 2009, 139, 1469–1473. [Google Scholar] [CrossRef] [Green Version]
- Bode, L.M.; Bunzel, D.; Huch, M.; Cho, G.-S.; Ruhland, D.; Bunzel, M.; Bub, A.; Franz, C.M.A.P.; Kulling, S.E. In Vivo and in Vitro Metabolism of Trans-Resveratrol by Human Gut Microbiota. Am. J. Clin. Nutr. 2013, 97, 295–309. [Google Scholar] [CrossRef]
- Pasinetti, G.M.; Singh, R.; Westfall, S.; Herman, F.; Faith, J.; Ho, L. The Role of the Gut Microbiota in the Metabolism of Polyphenols as Characterized by Gnotobiotic Mice. J. Alzheimers Dis. JAD 2018, 63, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Gowd, V.; Karim, N.; Shishir, M.R.I.; Xie, L.; Chen, W. Dietary Polyphenols to Combat the Metabolic Diseases via Altering Gut Microbiota. Trends Food Sci. Technol. 2019, 93, 81–93. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of Polyphenols on Gut Microbiota and Implications in Human Health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, J.-H.; Duan, J.-A.; Jiang, S.; Qian, Y.-Y.; Qian, D.-W. Biotransformation and Metabolic Profile of Buddleoside with Human Intestinal Microflora by Ultrahigh-Performance Liquid Chromatography Coupled to Hybrid Linear Ion Trap/Orbitrap Mass Spectrometer. J. Chromatogr. B Anal. Technol. Biomed. Life. Sci. 2016, 1025, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.; Walle, T. Methylated Flavonoids Have Greatly Improved Intestinal Absorption and Metabolic Stability. Drug Metab. Dispos. Biol. Fate Chem. 2006, 34, 1786–1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Li, Q.; Zhao, T.; Zhang, Z.; Mao, G.; Feng, W.; Wu, X.; Yang, L. Biotransformation and Metabolism of Three Mulberry Anthocyanin Monomers by Rat Gut Microflora. Food Chem. 2017, 237, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Mayta-Apaza, A.C.; Pottgen, E.; De Bodt, J.; Papp, N.; Marasini, D.; Howard, L.; Abranko, L.; Van de Wiele, T.; Lee, S.-O.; Carbonero, F. Impact of Tart Cherries Polyphenols on the Human Gut Microbiota and Phenolic Metabolites in Vitro and in Vivo. J. Nutr. Biochem. 2018, 59, 160–172. [Google Scholar] [CrossRef]
- Beltrán, D.; Romo-Vaquero, M.; Espín, J.C.; Tomás-Barberán, F.A.; Selma, M.V. Ellagibacter isourolithinifaciens gen. nov., sp. nov., a new member of the family Eggerthellaceae, isolated from human gut. Int. J. Syst. Evol. Microbiol. 2018, 68, 1707–1712. [Google Scholar] [CrossRef]
- Theilmann, M.C.; Goh, Y.J.; Nielsen, K.F.; Klaenhammer, T.R.; Barrangou, R.; Abou Hachem, M. Lactobacillus Acidophilus Metabolizes Dietary Plant Glucosides and Externalizes Their Bioactive Phytochemicals. mBio 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barroso, E.; Van de Wiele, T.; Jiménez-Girón, A.; Muñoz-González, I.; Martín-Alvarez, P.J.; Moreno-Arribas, M.V.; Bartolomé, B.; Peláez, C.; Martínez-Cuesta, M.C.; Requena, T. Lactobacillus Plantarum IFPL935 Impacts Colonic Metabolism in a Simulator of the Human Gut Microbiota during Feeding with Red Wine Polyphenols. Appl. Microbiol. Biotechnol. 2014, 98, 6805–6815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, J.-P.; Wang, J.-H.; Liu, X. Metabolism of Dietary Soy Isoflavones to Equol by Human Intestinal Microflora—Implications for Health. Mol. Nutr. Food Res. 2007, 51, 765–781. [Google Scholar] [CrossRef] [PubMed]
- Setchell, K.D.R.; Brown, N.M.; Lydeking-Olsen, E. The Clinical Importance of the Metabolite Equol-a Clue to the Effectiveness of Soy and Its Isoflavones. J. Nutr. 2002, 132, 3577–3584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frankenfeld, C.L. O-Desmethylangolensin: The Importance of Equol’s Lesser Known Cousin to Human Health. Adv. Nutr. 2011, 2, 317–324. [Google Scholar] [CrossRef] [Green Version]
- Hur, H.G.; Lay, J.O.; Beger, R.D.; Freeman, J.P.; Rafii, F. Isolation of Human Intestinal Bacteria Metabolizing the Natural Isoflavone Glycosides Daidzin and Genistin. Arch. Microbiol. 2000, 174, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Niwa, T.; Osawa, T.; Suzuki, T. Characterization of an O-Desmethylangolensin-Producing Bacterium Isolated from Human Feces. Arch. Microbiol. 2010, 192, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, K.; Sugiyama, Y.; Sakano, T.; Ohigashi, H. Flavonols Enhanced Production of Anti-Inflammatory Substance(s) by Bifidobacterium Adolescentis: Prebiotic Actions of Galangin, Quercetin, and Fisetin. Biofactors Oxf. Engl. 2013, 39, 422–429. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, U.; Rubab, M.; Daliri, E.B.-M.; Chelliah, R.; Javed, A.; Oh, D.-H. Curcumin, Quercetin, Catechins and Metabolic Diseases: The Role of Gut Microbiota. Nutrients 2021, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Espín, J.C.; González-Sarrías, A.; Tomás-Barberán, F.A. The Gut Microbiota: A Key Factor in the Therapeutic Effects of (Poly)Phenols. Biochem. Pharmacol. 2017, 139, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front. Nutr. 2019, 6. [Google Scholar] [CrossRef] [Green Version]
- Piekarska-Radzik, L.; Klewicka, E. Mutual Influence of Polyphenols and Lactobacillus Spp. Bacteria in Food: A Review. Eur. Food Res. Technol. 2021, 247, 9–24. [Google Scholar] [CrossRef]
- Stead, D. The Effect of Chlorogenic, Gallic and Quinic Acids on the Growth of Spoilage Strains of Lactobacillus Collinoides and Lactobacillus Brevis. Lett. Appl. Microbiol. 1994, 18, 112–114. [Google Scholar] [CrossRef]
- Parkar, S.G.; Stevenson, D.E.; Skinner, M.A. The Potential Influence of Fruit Polyphenols on Colonic Microflora and Human Gut Health. Int. J. Food Microbiol. 2008, 124, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Duda-Chodak, A. The Inhibitory Effect of Polyphenols on Human Gut Microbiota. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2012, 63, 497–503. [Google Scholar]
- Hervert-Hernández, D.; Pintado, C.; Rotger, R.; Goñi, I. Stimulatory Role of Grape Pomace Polyphenols on Lactobacillus Acidophilus Growth. Int. J. Food Microbiol. 2009, 136, 119–122. [Google Scholar] [CrossRef]
- Tzounis, X.; Vulevic, J.; Kuhnle, G.G.C.; George, T.; Leonczak, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P.E. Flavanol Monomer-Induced Changes to the Human Faecal Microflora. Br. J. Nutr. 2008, 99, 782–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, L.; Liu, L.; Ji, H.-F. Regulative Effects of Curcumin Spice Administration on Gut Microbiota and Its Pharmacological Implications. Food Nutr. Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tung, Y.-C.; Lin, Y.-H.; Chen, H.-J.; Chou, S.-C.; Cheng, A.-C.; Kalyanam, N.; Ho, C.-T.; Pan, M.-H. Piceatannol Exerts Anti-Obesity Effects in C57BL/6 Mice through Modulating Adipogenic Proteins and Gut Microbiota. Molecules 2016, 21, 1419. [Google Scholar] [CrossRef] [Green Version]
- Remely, M.; Ferk, F.; Sterneder, S.; Setayesh, T.; Roth, S.; Kepcija, T.; Noorizadeh, R.; Rebhan, I.; Greunz, M.; Beckmann, J.; et al. EGCG Prevents High Fat Diet-Induced Changes in Gut Microbiota, Decreases of DNA Strand Breaks, and Changes in Expression and DNA Methylation of Dnmt1 and MLH1 in C57BL/6J Male Mice. Oxid. Med. Cell. Longev. 2017, 2017, 3079148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masumoto, S.; Terao, A.; Yamamoto, Y.; Mukai, T.; Miura, T.; Shoji, T. Non-Absorbable Apple Procyanidins Prevent Obesity Associated with Gut Microbial and Metabolomic Changes. Sci. Rep. 2016, 6, 31208. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Xu, J.; Guo, T.L. Isoflavone Daidzein Regulates Immune Responses in the B6C3F1 and Non-Obese Diabetic (NOD) Mice. Int. Immunopharmacol. 2019, 71, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Hijona, E.; Aguirre, L.; Pérez-Matute, P.; Villanueva-Millán, M.J.; Mosqueda-Solis, A.; Hasnaoui, M.; Nepveu, F.; Senard, J.M.; Bujanda, L.; Aldámiz-Echevarría, L.; et al. Limited Beneficial Effects of Piceatannol Supplementation on Obesity Complications in the Obese Zucker Rat: Gut Microbiota, Metabolic, Endocrine, and Cardiac Aspects. J. Physiol. Biochem. 2016, 72, 567–582. [Google Scholar] [CrossRef] [PubMed]
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.T.; Portillo, M.P.; Martínez, J.A.; Milagro, F.I. Reshaping Faecal Gut Microbiota Composition by the Intake of Trans-Resveratrol and Quercetin in High-Fat Sucrose Diet-Fed Rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef]
- Hara, Y. Influence of Tea Catechins on the Digestive Tract. J. Cell. Biochem. 1997, 67, 52–58. [Google Scholar] [CrossRef]
- Clavel, T.; Fallani, M.; Lepage, P.; Levenez, F.; Mathey, J.; Rochet, V.; Sérézat, M.; Sutren, M.; Henderson, G.; Bennetau-Pelissero, C.; et al. Isoflavones and Functional Foods Alter the Dominant Intestinal Microbiota in Postmenopausal Women. J. Nutr. 2005, 135, 2786–2792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ankolekar, C.; Johnson, D.; Pinto, M.d.S.; Johnson, K.; Labbe, R.; Shetty, K. Inhibitory Potential of Tea Polyphenolics and Influence of Extraction Time Against Helicobacter Pylori and Lack of Inhibition of Beneficial Lactic Acid Bacteria. J. Med. Food 2011, 14, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Kohda, C.; Yanagawa, Y.; Shimamura, T. Epigallocatechin Gallate Inhibits Intracellular Survival of Listeria Monocytogenes in Macrophages. Biochem. Biophys. Res. Commun. 2008, 365, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-L.; Tsai, H.-L.; Peng, C.-W. EGCG Debilitates the Persistence of EBV Latency by Reducing the DNA Binding Potency of Nuclear Antigen 1. Biochem. Biophys. Res. Commun. 2012, 417, 1093–1099. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Li, X.-Y.; Shen, L. Modulation Effect of Tea Consumption on Gut Microbiota. Appl. Microbiol. Biotechnol. 2020, 104, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Jaquet, M.; Rochat, I.; Moulin, J.; Cavin, C.; Bibiloni, R. Impact of Coffee Consumption on the Gut Microbiota: A Human Volunteer Study. Int. J. Food Microbiol. 2009, 130, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Massot-Cladera, M.; Pérez-Berezo, T.; Franch, A.; Castell, M.; Pérez-Cano, F.J. Cocoa Modulatory Effect on Rat Faecal Microbiota and Colonic Crosstalk. Arch. Biochem. Biophys. 2012, 527, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P.E. Prebiotic Evaluation of Cocoa-Derived Flavanols in Healthy Humans by Using a Randomized, Controlled, Double-Blind, Crossover Intervention Study. Am. J. Clin. Nutr. 2011, 93, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Bialonska, D.; Kasimsetty, S.G.; Schrader, K.K.; Ferreira, D. The Effect of Pomegranate (Punica Granatum L.) Byproducts and Ellagitannins on the Growth of Human Gut Bacteria. J. Agric. Food Chem. 2009, 57, 8344–8349. [Google Scholar] [CrossRef]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A Purified Membrane Protein from Akkermansia Muciniphila or the Pasteurized Bacterium Improves Metabolism in Obese and Diabetic Mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chacar, S.; Itani, T.; Hajal, J.; Saliba, Y.; Louka, N.; Faivre, J.-F.; Maroun, R.; Fares, N. The Impact of Long-Term Intake of Phenolic Compounds-Rich Grape Pomace on Rat Gut Microbiota. J. Food Sci. 2018, 83, 246–251. [Google Scholar] [CrossRef]
- Queipo-Ortuño, M.I.; Boto-Ordóñez, M.; Murri, M.; Gomez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Cardona Diaz, F.; Andrés-Lacueva, C.; Tinahones, F.J. Influence of Red Wine Polyphenols and Ethanol on the Gut Microbiota Ecology and Biochemical Biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef]
- Etxeberria, U.; Fernández-Quintela, A.; Milagro, F.I.; Aguirre, L.; Martínez, J.A.; Portillo, M.P. Impact of Polyphenols and Polyphenol-Rich Dietary Sources on Gut Microbiota Composition. J. Agric. Food Chem. 2013, 61, 9517–9533. [Google Scholar] [CrossRef]
- Anhê, F.F.; Roy, D.; Pilon, G.; Dudonné, S.; Matamoros, S.; Varin, T.V.; Garofalo, C.; Moine, Q.; Desjardins, Y.; Levy, E.; et al. A Polyphenol-Rich Cranberry Extract Protects from Diet-Induced Obesity, Insulin Resistance and Intestinal Inflammation in Association with Increased Akkermansia Spp. Population in the Gut Microbiota of Mice. Gut 2015, 64, 872–883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, A.C.D.; Cecatti, C.; Fidélix, M.P.; Adorno, M.A.T.; Sakamoto, I.K.; Cesar, T.B.; Sivieri, K. Effect of Daily Consumption of Orange Juice on the Levels of Blood Glucose, Lipids, and Gut Microbiota Metabolites: Controlled Clinical Trials. J. Med. Food 2019, 22, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Wang, Y.; Lin, Y.; Lang, Y.; Li, E.; Zhang, X.; Zhang, Q.; Feng, Y.; Meng, X.; Li, B. Blueberry Polyphenols Extract as a Potential Prebiotic with Anti-Obesity Effects on C57BL/6 J Mice by Modulating the Gut Microbiota. J. Nutr. Biochem. 2019, 64, 88–100. [Google Scholar] [CrossRef]
- Kim, H.; Venancio, V.P.; Fang, C.; Dupont, A.W.; Talcott, S.T.; Mertens-Talcott, S.U. Mango (Mangifera Indica L.) Polyphenols Reduce IL-8, GRO, and GM-SCF Plasma Levels and Increase Lactobacillus Species in a Pilot Study in Patients with Inflammatory Bowel Disease. Nutr. Res. 2020, 75, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.A.; Han, M.J.; Kim, D.H. In Vitro Anti-Helicobacter Pylori Activity of Some Flavonoids and Their Metabolites. Planta Med. 1999, 65, 442–443. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ridaura, V.K.; Faith, J.J.; Rey, F.E.; Knight, R.; Gordon, J.I. The Effect of Diet on the Human Gut Microbiome: A Metagenomic Analysis in Humanized Gnotobiotic Mice. Sci. Transl. Med. 2009, 1, 6ra14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial Ecology: Human Gut Microbes Associated with Obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef] [PubMed]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia Muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, S.; Huang, K.; Zhao, C.; Xu, W.-T.; Sheng, Y.; Luo, Y.; Xiaoyun, H. Procyanidin Attenuates Weight Gain and Modifies the Gut Microbiota in High Fat Diet Induced Obese Mice. J. Funct. Foods 2018, 49, 362–368. [Google Scholar] [CrossRef]
- Kameyama, K.; Itoh, K. Intestinal Colonization by a Lachnospiraceae Bacterium Contributes to the Development of Diabetes in Obese Mice. Microbes Environ. 2014, 29, 427–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rong, S.; Hu, X.; Zhao, S.; Zhao, Y.; Xiao, X.; Bao, W.; Liu, L. Procyanidins Extracted from the Litchi Pericarp Ameliorate Atherosclerosis in ApoE Knockout Mice: Their Effects on Nitric Oxide Bioavailability and Oxidative Stress. Food Funct. 2017, 8, 4210–4216. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Arribas, M.V.; Bartolomé, B.; Peñalvo, J.L.; Pérez-Matute, P.; Motilva, M.J. Relationship between Wine Consumption, Diet and Microbiome Modulation in Alzheimer’s Disease. Nutrients 2020, 12, 3082. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, M.; Ho, C.-T.; Guo, X.; Wu, Z.; Weng, P.; Yan, M.; Cao, J. Metagenomics Analysis of Gut Microbiota Modulatory Effect of Green Tea Polyphenols by High Fat Diet-Induced Obesity Mice Model. J. Funct. Foods 2018, 46, 268–277. [Google Scholar] [CrossRef]
- Ashley, D.; Marasini, D.; Brownmiller, C.; Lee, J.A.; Carbonero, F.; Lee, S.-O. Impact of Grain Sorghum Polyphenols on Microbiota of Normal Weight and Overweight/Obese Subjects during In Vitro Fecal Fermentation. Nutrients 2019, 11, 217. [Google Scholar] [CrossRef] [Green Version]
- Su, H.; Li, Y.; Hu, D.; Xie, L.; Ke, H.; Zheng, X.; Chen, W. Procyanidin B2 Ameliorates Free Fatty Acids-Induced Hepatic Steatosis through Regulating TFEB-Mediated Lysosomal Pathway and Redox State. Free Radic. Biol. Med. 2018, 126, 269–286. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Su, Q.; Liu, Y. Sinapine Reduces Non-Alcoholic Fatty Liver Disease in Mice by Modulating the Composition of the Gut Microbiota. Food Funct. 2019, 10, 3637–3649. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Liu, A.B.; Sun, S.; Ajami, N.J.; Ross, M.C.; Wang, H.; Zhang, L.; Reuhl, K.; Kobayashi, K.; Onishi, J.C.; et al. Green Tea Polyphenols Modify the Gut Microbiome in Db/Db Mice as Co-Abundance Groups Correlating with the Blood Glucose Lowering Effect. Mol. Nutr. Food Res. 2019, 63, e1801064. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Barbosa, R.M.; Almeida, L.; Laranjinha, J. Nitric Oxide and DOPAC-Induced Cell Death: From GSH Depletion to Mitochondrial Energy Crisis. Mol. Cell. Neurosci. 2011, 48, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Galati, G.; O’Brien, P.J. Potential Toxicity of Flavonoids and Other Dietary Phenolics: Significance for Their Chemopreventive and Anticancer Properties. Free Radic. Biol. Med. 2004, 37, 287–303. [Google Scholar] [CrossRef] [PubMed]
| Polyphenol | Dose and Time of Use (for Ani-Mal and Human Study) | Type of Study and Name of the Species | Changes in Microbiota | Ref. |
|---|---|---|---|---|
| Quercetin | 4, 20, 50 µg/mL in medium | In vitro study | ↓ Ruminococcus gauvreauii, Bacteroides galacturonicus, Lactobacillus sp. | [120] |
| Kaempferol, quercetin, myricetin and fisetin | 25 μM in medium | In vitro study | Little or no antibacterial effect against Bifidobacterium adolescentis | [113] |
| Tannic acid | 100.5 mg/mL in medium | In vitro study | ↑ Lactobacillus acidophilus | [121] |
| (c)-epicatechin | 150 mg/L and 1000 mg/L | In vitro study | ↑ Clostridium coccoides–Eubacterium rectale group, Bifidobacterium spp. and Escherichia coli ↓ C. histolyticum | [122] |
| (+)-catechin | 150 mg/L and 1000 mg/L | In vitro study | ↑ C. coccoides–Eubacterium rectale group | [122] |
| Curcumin | 100 mg/kg/day for 15 days | Animal study (mouse) | ↑ Prevotellaceae, Bacteroidaceae ↓ Rikenellaceae | [123] |
| Picetannol | 0.25% in diet for 18 weeks | Animal study (mouse) | ↑ Firmicutes, Lactobacillus ↓ Bacteroidetes | [124] |
| (−)-Epigallocatechin-3-gallate | 25 mg/kg/day for 4 months | Animal study (mouse) | ↓ Firmicutes/Bacteroidetes ratio | [125] |
| Polymeric procyanidins | 0.5% in diet for 20 weeks | Animal study (mouse) | ↑Akkermansia ↓ Clostridium, Lachnospiraceae, Bifidobacterium ↓ Firmicutes/Bacteroidetes ratio | [126] |
| Daidzein | 20 mg/kg/day during adulthood | Animal study (mouse) | Not specified | [127] |
| Picetannol (resveratrol analogue) | 45 mg/kg/day for 6 weeks | Animal study (rat) | Nonsignificant changes in Bacteroides and Firmicutes | [128] |
| Quercetin | 30 mg/kg/day for 6 weeks | Animal study (rat) | ↓ Erysipelotrichaceae, Bacillus, Eubacterium cylindroides | [129] |
| Polyphenon G® powder (purified preparation of tea-derived catechins) | 0.2% Polyphenon G® (0.07% tea catechins) for 3 weeks | Human intervention | ↑ Lactobacilli ↓ Enterobacteriaceae | [130] |
| Isoflavones | 100 mg/day for 15 days | Human intervention (postmenopausal women) | ↑ stimulated dominant microorganisms of the Clostridium coccoides-Eubacterium rectale cluster, Lactobacillus-Enterococcus group, Faecalibacterium prausnitzii subgroup and Bifidobacterium genus | [131] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasprzak-Drozd, K.; Oniszczuk, T.; Stasiak, M.; Oniszczuk, A. Beneficial Effects of Phenolic Compounds on Gut Microbiota and Metabolic Syndrome. Int. J. Mol. Sci. 2021, 22, 3715. https://doi.org/10.3390/ijms22073715
Kasprzak-Drozd K, Oniszczuk T, Stasiak M, Oniszczuk A. Beneficial Effects of Phenolic Compounds on Gut Microbiota and Metabolic Syndrome. International Journal of Molecular Sciences. 2021; 22(7):3715. https://doi.org/10.3390/ijms22073715
Chicago/Turabian StyleKasprzak-Drozd, Kamila, Tomasz Oniszczuk, Mateusz Stasiak, and Anna Oniszczuk. 2021. "Beneficial Effects of Phenolic Compounds on Gut Microbiota and Metabolic Syndrome" International Journal of Molecular Sciences 22, no. 7: 3715. https://doi.org/10.3390/ijms22073715






