An Insight into the Stages of Ion Leakage during Red Blood Cell Storage
Abstract
1. Introduction
2. Results and Discussion
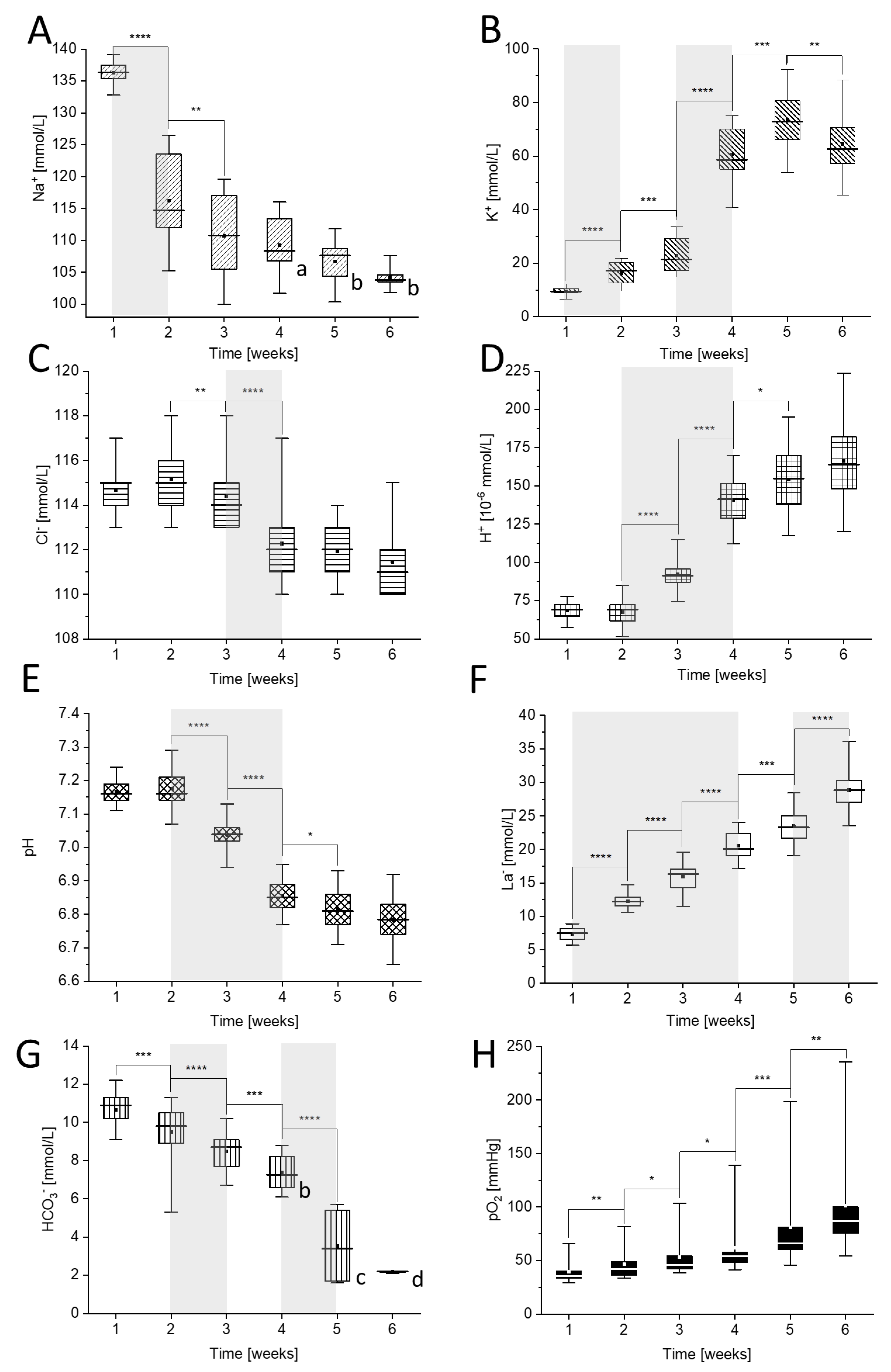
2.1. Analysis of the Ion Leakage Stages during pRBCs Storage
2.2. The Iimpact of Blood Group Type on the Ion Concentration
2.3. Relationship between Ion Exchange and RBCs Morphology
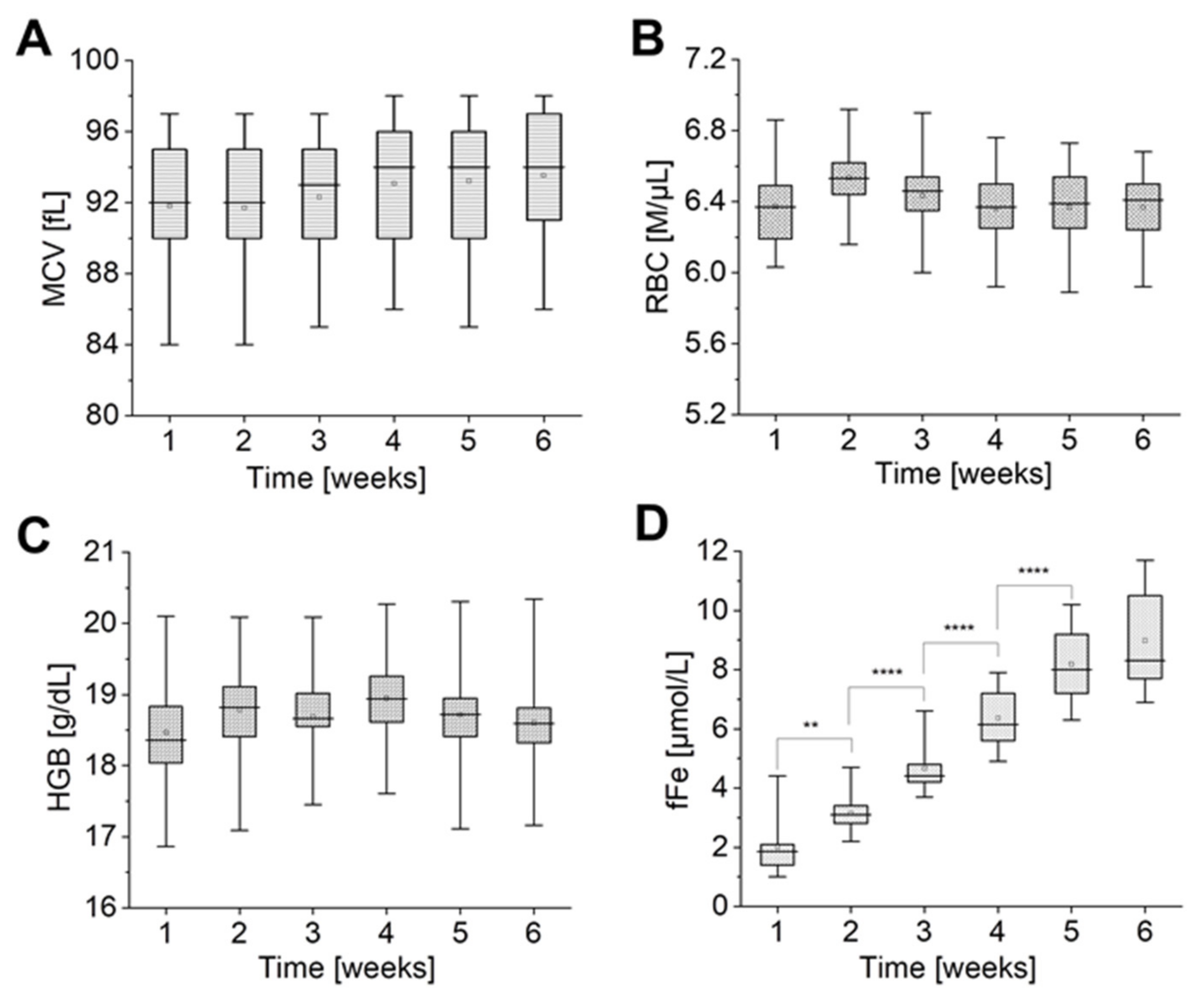
2.4. Relationship between Ion Exchange and Red Cell Quality Indices
3. Materials and Methods
3.1. Sample Preparation
3.2. Ions and Blood Gases Measurements
3.3. AFM Imaging
3.4. Haematologic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations:
| ATP | adenosine triphosphate |
| AFM | atomic force microscopy |
| 2,3-BPG | 2,3-bisphosphoglycerate |
| Ca2+ | calcium ion |
| Cl− | chloride ion |
| deoxyHb | deoxyhaemoglobin |
| CO2 | carbon dioxide |
| fFe | free iron |
| GLUT | glucose transporter |
| H+ | hydrogen ion |
| Hb | haemoglobin |
| HCO3− | bicarbonate ion |
| HCT | haematocrit |
| HGB | haemoglobin concentration |
| K+ | potassium ion |
| La− | lactate ion |
| Li+ | lithium ion |
| MCV | mean corpuscular volume |
| Mg2+ | magnesium ion |
| Na+ | sodium ion |
| O2 | dioxygen |
| oxyHb | oxyhaemoglobin |
| pO2 | partial pressure of O2 |
| RET | reticulocyte |
| RBC | red blood cell |
| CPD-SAGM | additive solution of citrate, phosphate, dextrose-saline, adenine, glucose, mannitol |
References
- Gevorkian, S.; Allahverdyan, A.; Gevorgyan, D.; Ma, W.-J.; Hu, C.-K. Can morphological changes of erythrocytes be driven by hemoglobin? Phys. A Stat. Mech. Appl. 2018, 508, 608–612. [Google Scholar] [CrossRef]
- Lux, S.E. Anatomy of the red cell membrane skeleton: Unanswered questions. Blood 2016, 127, 187–199. [Google Scholar] [CrossRef]
- De Rosa, M.C.; Alinovi, C.C.; Galtieri, A.; Scatena, R.; Giardina, B. The plasma membrane of erythrocytes plays a fundamental role in the transport of oxygen, carbon dioxide and nitric oxide and in the maintenance of the reduced state of the heme iron. Gene 2007, 398, 162–171. [Google Scholar] [CrossRef]
- Mazzulla, S.; Schella, A.; Gabriele, D.; Baldino, N.; Sesti, S.; Perrotta, E.; Costabile, A.; De Cindio, B. Oxidation of human red blood cells by a free radical initiator: Effects on rheological properties. Clin. Hemorheol. Microcirc. 2015, 60, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, V.; Diederich, L.; Keller, T.S.; Kramer, C.M.; Lückstädt, W.; Panknin, C.; Suvorava, T.; Isakson, B.E.; Kelm, M.; Cortese-Krott, M.M. Red Blood Cell Function and Dysfunction: Redox Regulation, Nitric Oxide Metabolism, Anemia. Antioxid. Redox Signal. 2017, 26, 718–742. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liu, L.; Hu, H.; Zhao, Q.; Xie, F.; Chen, K.; Liu, S.; Chen, Y.; Shi, W.; Yin, D. Age-related carbonyl stress and erythrocyte membrane protein carbonylation. Clin. Hemorheol. Microcirc. 2010, 46, 305–311. [Google Scholar] [CrossRef]
- Badior, K.E.; Casey, J.R. Molecular mechanism for the red blood cell senescence clock. IUBMB Life 2018, 70, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Pasini, E.M.; Lutz, H.U.; Mann, M.; Thomas, A.W. Red blood cell (RBC) membrane proteomics—Part I: Proteomics and RBC physiology. J. Proteom. 2010, 73, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Bagriantsev, S.N.; Gracheva, E.O.; Gallagher, P.G. Piezo Proteins: Regulators of Mechanosensation and Other Cellular Processes. J. Biol. Chem. 2014, 289, 31673–31681. [Google Scholar] [CrossRef]
- Kuchel, P.W.; Shishmarev, D. Accelerating metabolism and transmembrane cation flux by distorting red blood cells. Sci. Adv. 2017, 3, eaao1016. [Google Scholar] [CrossRef] [PubMed]
- Gnanasambandam, R.; Bae, C.; Gottlieb, P.A.; Sachs, F. Ionic Selectivity and Permeation Properties of Human PIEZO1 Channels. PLoS ONE 2015, 10, e0125503. [Google Scholar] [CrossRef]
- Rapetti-Mauss, R.; Picard, V.; Guitton, C.; Ghazal, K.; Proulle, V.; Badens, C.; Soriani, O.; Garçon, L.; Guizouarn, H. Red blood cell Gardos channel (KCNN4): The essential determinant of erythrocyte dehydration in hereditary xerocytosis. Haematologica 2017, 102, e415–e418. [Google Scholar] [CrossRef] [PubMed]
- Cahalan, S.M.; Lukacs, V.; Ranade, S.S.; Chien, S.; Bandell, M.; Patapoutian, A. Piezo1 links mechanical forces to red blood cell volume. eLife 2015, 4, e07370. [Google Scholar] [CrossRef]
- Danielczok, J.G.; Terriac, E.; Hertz, L.; Petkova-Kirova, P.; Lautenschläger, F.; Laschke, M.W.; Kaestner, L. Red Blood Cell Passage of Small Capillaries Is Associated with Transient Ca2+-mediated Adaptations. Front. Physiol. 2017, 8, 979. [Google Scholar] [CrossRef] [PubMed]
- Petkova-Kirova, P.; Hertz, L.; Makhro, A.; Danielczok, J.; Huisjes, R.; Llaudet-Planas, E.; Mañú-Pereira, M.D.M.; Corrons, J.-L.V.; Van Wijk, R.; Bogdanova, A.; et al. A Previously Unrecognized Ca2+-inhibited Nonselective Cation Channel in Red Blood Cells. HemaSphere 2018, 2, e146. [Google Scholar] [CrossRef]
- Kaestner, L.; Christophersen, P.; Bernhardt, I.; Bennekou, P. The non-selective voltage-activated cation channel in the human red blood cell membrane: Reconciliation between two conflicting reports and further characterisation. Bioelectrochemistry 2000, 52, 117–125. [Google Scholar] [CrossRef]
- Kaestner, L.; Bollensdorff, C.; Bernhardt, I. Non-selective voltage-activated cation channel in the human red blood cell membrane. Biochim. Biophys. Acta (BBA)-Biomembr. 1999, 1417, 9–15. [Google Scholar] [CrossRef]
- Hsu, K. Exploring the Potential Roles of Band 3 and Aquaporin-1 in Blood CO2 Transport–Inspired by Comparative Studies of Glycophorin B-A-B Hybrid Protein GP.Mur. Front. Physiol. 2018, 9, 733. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, A.D.; Engelman, D.M. Protein area occupancy at the center of the red blood cell membrane. Proc. Natl. Acad. Sci. USA 2008, 105, 2848–2852. [Google Scholar] [CrossRef]
- Hamasaki, N. The role of band 3 protein in oxygen delivery by red blood cells. Indian J. Clin. Biochem. 1999, 14, 49–58. [Google Scholar] [CrossRef]
- De Bruijne, A.; Vreeburg, H.; Van Steveninck, J. Kinetic analysis of l-lactate transport in human erythrocytes via the monocarboxylate-specific carrier system. Biochim. Biophys. Acta (BBA)-Biomembr. 1983, 732, 562–568. [Google Scholar] [CrossRef]
- Poole, R.C.; Halestrap, A.P. Transport of lactate and other monocarboxylates across mammalian plasma membranes. Am. J. Physiol. Physiol. 1993, 264, C761–C782. [Google Scholar] [CrossRef] [PubMed]
- Halestrap, A.P.; Price, N.T. The proton-linked monocarboxylate transporter (MCT) family: Structure, function and regulation. Biochem. J. 1999, 343, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Flatt, J.F.; Bawazir, W.M.; Bruce, L.J. The involvement of cation leaks in the storage lesion of red blood cells. Front. Physiol. 2014, 5, 214. [Google Scholar] [CrossRef]
- Gatto, C.; Milanick, M. Red blood cell Na pump: Insights from species differences. Blood Cells Mol. Dis. 2009, 42, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Quarmyne, M.-O.; Risinger, M.; Linkugel, A.; Frazier, A.; Joiner, C. Volume regulation and KCl cotransport in reticulocyte populations of sickle and normal red blood cells. Blood Cells Mol. Dis. 2011, 47, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Ovchynnikova, E.; Aglialoro, F.; von Lindern, M.; van den Akker, E. The shape shifting story of reticulocyte maturation. Front. Physiol. 2018, 9, 829. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.S.; Puchulu-Campanella, M.E.; Barber, L.A.; Palascak, M.B.; Joiner, C.H.; Low, P.S.; Cohen, R.M. Changes in the properties of normal human red blood cells during in vivo aging. Am. J. Hematol. 2012, 88, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Bize, I.; Taher, S.; Brugnara, C. Regulation of K-Cl cotransport during reticulocyte maturation and erythrocyte aging in normal and sickle erythrocytes. Am. J. Physiol. Physiol. 2003, 285, C31–C38. [Google Scholar] [CrossRef]
- Vokurkova, M.; Rauchova, H.; Dobesova, Z.; Loukotova, J.; Novakova, O.; Kunes, J.; Zicha, J. The Influence of Erythrocyte Maturity on Ion Transport and Membrane Lipid Composition in the Rat. Physiol. Res. 2016, 65, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Brugnara, C.; Tosteson, D.C. Cell volume, K transport, and cell density in human erythrocytes. Am. J. Physiol. Physiol. 1987, 252, C269–C276. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mohandas, N.; An, X. Membrane assembly during erythropoiesis. Curr. Opin. Hematol. 2011, 18, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Nikinmaa, M. Membrane transport and control of hemoglobin-oxygen affinity in nucleated erythrocytes. Physiol. Rev. 1992, 72, 301–321. [Google Scholar] [CrossRef] [PubMed]
- Forest, S.; Rapido, F.; Hod, E.A. Storage Lesion: Evolving Concepts and Controversies. In Diffuse Cystic Lung Diseases; Springer International Publishing: Berlin/Heidelberg, Germany, 2016; pp. 175–191. [Google Scholar]
- Zapala, B.; Zdzienicka, A.; Gawlik, K.; Maziarz, B. Reference values of analytes in laboratory diagnostics. In Laboratory diagnostics with Elements of Clinical Biochemistry; Edra Urban & Partner: Wrocław, Poland, 2017; pp. 874–886. [Google Scholar]
- Casey, J.R.; Grinstein, S.; Orlowski, J. Sensors and regulators of intracellular pH. Nat. Rev. Mol. Cell Biol. 2009, 11, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Rhoades, R.; Bell, D.R. Medical Physiology: Principles for Clinical Medicine, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2009; ISBN 9780781768528. [Google Scholar]
- Kaczmarska, M.; Grosicki, M.; Bulat, K.; Mardyla, M.; Szczesny-Malysiak, E.; Blat, A.; Dybas, J.; Sacha, T.; Marzec, K.M. Temporal sequence of the human RBCs’ vesiculation observed in nano-scale with application of AFM and complementary techniques. Nanomed. Nanotechnol. Biol. Med. 2020, 28, 102221. [Google Scholar] [CrossRef]
- Qi, Z.; Roback, J.D.; Voit, E.O. Effects of Storage Time on Glycolysis in Donated Human Blood Units. Metabolites 2017, 7, 12. [Google Scholar] [CrossRef]
- D’Alessandro, A.; D’Amici, G.M.; Vaglio, S.; Zolla, L. Time-course investigation of SAGM-stored leukocyte-filtered red bood cell concentrates: From metabolism to proteomics. Haematologica 2011, 97, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Gadsby, D.C.; Bezanilla, F.; Rakowski, R.F.; De Weer, P.; Holmgren, M. The dynamic relationships between the three events that release individual Na+ ions from the Na+/K+-ATPase. Nat. Commun. 2012, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lew, V.L.; Daw, N.; Etzion, Z.; Tiffert, T.; Muoma, A.; Vanagas, L.; Bookchin, R.M. Effects of age-dependent membrane transport changes on the homeostasis of senescent human red blood cells. Blood 2007, 110, 1334–1342. [Google Scholar] [CrossRef] [PubMed]
- Guppy, M.; Attwood, P.V.; Hansen, I.A.; Sabaratnam, R.; Frisina, J.; Whisson, M.E. pH, Temperature and Lactate Production in Human Red Blood Cells: Implications for Blood Storage and Glycolytic Control. Vox Sang. 1992, 62, 70–75. [Google Scholar] [CrossRef]
- Gallagher, P.G. Disorders of erythrocyte hydration. Blood 2017, 130, 2699–2708. [Google Scholar] [CrossRef] [PubMed]
- Lew, V.L.; Tiffert, T. On the Mechanism of Human Red Blood Cell Longevity: Roles of Calcium, the Sodium Pump, PIEZO1, and Gardos Channels. Front. Physiol. 2017, 8, 977. [Google Scholar] [CrossRef] [PubMed]
- Kaestner, L.; Bogdanova, A.; Egee, S. Calcium Channels and Calcium-Regulated Channels in Human Red Blood Cells. In Retinal Degenerative Diseases; Springer: Berlin/Heidelberg, Germany, 2019; Volume 1131, pp. 625–648. [Google Scholar]
- Lagerberg, J.W.; Korsten, H.; Van Der Meer, P.F.; De Korte, D. Prevention of red cell storage lesion: A comparison of five different additive solutions. Blood Transfus. 2017, 15, 456–462. [Google Scholar] [PubMed]
- Bryk, A.H.; Wiśniewski, J.R. Quantitative Analysis of Human Red Blood Cell Proteome. J. Proteome Res. 2017, 16, 2752–2761. [Google Scholar] [CrossRef] [PubMed]
- Ferru, E.; Giger, K.; Pantaleo, A.; Campanella, E.; Grey, J.; Ritchie, K.; Vono, R.; Turrini, F.; Low, P.S. Regulation of membrane-cytoskeletal interactions by tyrosine phosphorylation of erythrocyte band 3. Blood 2011, 117, 5998–6006. [Google Scholar] [CrossRef]
- Kudelka, M.R.; Ju, T.; Heimburg-Molinaro, J.; Cummings, R.D. Simple Sugars to Complex Disease—Mucin-Type O-Glycans in Cancer. In Advances in Cancer Research; Elsevier BV: Amsterdam, The Netherlands, 2015; Volume 126, pp. 53–135. [Google Scholar]
- Stamler, J.S.; Telen, M.J. Functions of blood group antigens. In Molecular Hematology 4e; Wiley: Hoboken, NJ, USA, 2019; pp. 285–296. [Google Scholar]
- Wiley, J.S. Co-ordinated Increase of Sodium Leak and Sodium Pump in Hereditary Spherocytosis. Br. J. Haematol. 1972, 22, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Yazdanbakhsh, K.; Lomas-Francis, C.; Reid, M.E. Blood groups and diseases associated with inherited abnormalities of the red blood cell membrane. Transfus. Med. Rev. 2000, 14, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Pillai, A.D.; Addo, R.; Sharma, P.; Nguitragool, W.; Srinivasan, P.; Desai, S.A. Malaria parasites tolerate a broad range of ionic environments and do not require host cation remodelling. Mol. Microbiol. 2013, 88, 20–34. [Google Scholar] [CrossRef] [PubMed]
- Ewald, D.R.; Sumner, S.C. Blood type biochemistry and human disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2016, 8, 517–535. [Google Scholar] [CrossRef] [PubMed]
- Deuticke, B. Transformation and restoration of biconcave shape of human erythrocytes induced by amphiphilic agents and changes of ionic environment. Biochim. Biophys. Acta (BBA)-Biomembr. 1968, 163, 494–500. [Google Scholar] [CrossRef]
- Karon, B.S.; Hoyer, J.D.; Stubbs, J.R.; Thomas, D.D. Changes in Band 3 oligomeric state precede cell membrane phospholipid loss during blood bank storage of red blood cells. Transfusion 2009, 49, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Pompeo, G.; Girasole, M.; Cricenti, A.; Boumis, G.; Bellelli, A.; Amiconi, S. Erythrocyte death in vitro induced by starvation in the absence of Ca2+. Biochim. Biophys. Acta (BBA)-Biomembr. 2010, 1798, 1047–1055. [Google Scholar] [CrossRef]
- Carelli-Alinovi, C.; Dinarelli, S.; Sampaolese, B.; Misiti, F.; Girasole, M. Morphological changes induced in erythrocyte by amyloid beta peptide and glucose depletion: A combined atomic force microscopy and biochemical study. Biochim. Biophys. Acta (BBA)-Biomembr. 2019, 1861, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Bailey, D.N.; Bove, J.R. Chemical and Hematological Changes in Stored CPD Blood. Transfusion 2003, 15, 244–249. [Google Scholar] [CrossRef]
- Moore-Igwe, B.; Jeremiah, Z.A.; Moore, T.C.A.B.; Adias, T.C. Storage Related Haematological and Biochemical Changes of CPDA-1 Whole Blood in a Resource Limited Setting. J. Blood Disord. Transfus. 2012, 3, 124. [Google Scholar] [CrossRef]
- Nogueira, D.; Rocha, S.; Abreu, E.; Costa, E.; Santos-Silva, A. Biochemical and cellular changes in leukocyte-depleted red blood cells stored for transfusion. Transfus. Med. Hemother. 2014, 42, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Ghezelbash, B.; Azarkeivan, A.; Pourfathollah, A.A.; Deyhim, M.; Hajati, E.; Goodarzi, A. Comparative Evaluation of Biochemical and Hematological Parameters of Pre-Storage Leukoreduction during RBC Storage. Int. J. Hematol. Stem Cell Res. 2018, 12, 35–42. [Google Scholar]
- Bosman, G.J.; Werre, J.M.; Willekens, F.L.A.; Novotný, V.M.J. Erythrocyte ageing in vivo and in vitro: Structural aspects and implications for transfusion. Transfus. Med. 2008, 18, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Barshtein, G.; Gural, A.; Manny, N.; Zelig, O.; Yedgar, S.; Arbell, D. Storage-Induced Damage to Red Blood Cell Mechanical Properties Can Be Only Partially Reversed by Rejuvenation. Transfus. Med. Hemother. 2014, 41, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Blasi, B.; D’Alessandro, A.; Ramundo, N.; Zolla, L. Red blood cell storage and cell morphology. Transfus. Med. 2012, 22, 90–96. [Google Scholar] [CrossRef]
- Dybas, J.; Bulat, K.; Blat, A.; Mohaissen, T.; Wajda, A.; Mardyla, M.; Kaczmarska, M.; Franczyk-Zarow, M.; Malek, K.; Chlopicki, S.; et al. Age–related and atherosclerosis–related erythropathy in ApoE/LDLR−/− mice. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165972. [Google Scholar] [CrossRef] [PubMed]
- Bulat, K.; Dybas, J.; Kaczmarska, M.; Rygula, A.; Jasztal, A.; Szczesny-Malysiak, E.; Baranska, M.; Wood, B.R.; Marzec, K.M. Multimodal detection and analysis of a new type of advanced Heinz body-like aggregate (AHBA) and cytoskeleton deformation in human RBCs. Analyst 2020, 145, 1749–1758. [Google Scholar] [CrossRef] [PubMed]
- Kozlova, Е.; Chernysh, А.; Moroz, V.; Sergunova, V.; Gudkova, О.; Kuzovlev, А. Nanodefects of membranes cause destruction of packed red blood cells during long-term storage. Exp. Cell Res. 2015, 337, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, I.; Al Marwani, A.; Nasr, K.M.; Kano, N.A.; Hadwan, T. Time Dependent Assessment of Morphological Changes: Leukodepleted Packed Red Blood Cells Stored in SAGM. BioMed Res. Int. 2016, 2016, 1–7. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimna, A.; Kaczmarska, M.; Szczesny-Malysiak, E.; Wajda, A.; Bulat, K.; Alcicek, F.C.; Zygmunt, M.; Sacha, T.; Marzec, K.M. An Insight into the Stages of Ion Leakage during Red Blood Cell Storage. Int. J. Mol. Sci. 2021, 22, 2885. https://doi.org/10.3390/ijms22062885
Zimna A, Kaczmarska M, Szczesny-Malysiak E, Wajda A, Bulat K, Alcicek FC, Zygmunt M, Sacha T, Marzec KM. An Insight into the Stages of Ion Leakage during Red Blood Cell Storage. International Journal of Molecular Sciences. 2021; 22(6):2885. https://doi.org/10.3390/ijms22062885
Chicago/Turabian StyleZimna, Anna, Magdalena Kaczmarska, Ewa Szczesny-Malysiak, Aleksandra Wajda, Katarzyna Bulat, Fatih Celal Alcicek, Malgorzata Zygmunt, Tomasz Sacha, and Katarzyna Maria Marzec. 2021. "An Insight into the Stages of Ion Leakage during Red Blood Cell Storage" International Journal of Molecular Sciences 22, no. 6: 2885. https://doi.org/10.3390/ijms22062885
APA StyleZimna, A., Kaczmarska, M., Szczesny-Malysiak, E., Wajda, A., Bulat, K., Alcicek, F. C., Zygmunt, M., Sacha, T., & Marzec, K. M. (2021). An Insight into the Stages of Ion Leakage during Red Blood Cell Storage. International Journal of Molecular Sciences, 22(6), 2885. https://doi.org/10.3390/ijms22062885





