Spent Brewer’s Yeast as a Source of Insoluble β-Glucans
Abstract
:1. Introduction
2. Yeast Cell
2.1. Mechanical Strength of Yeast
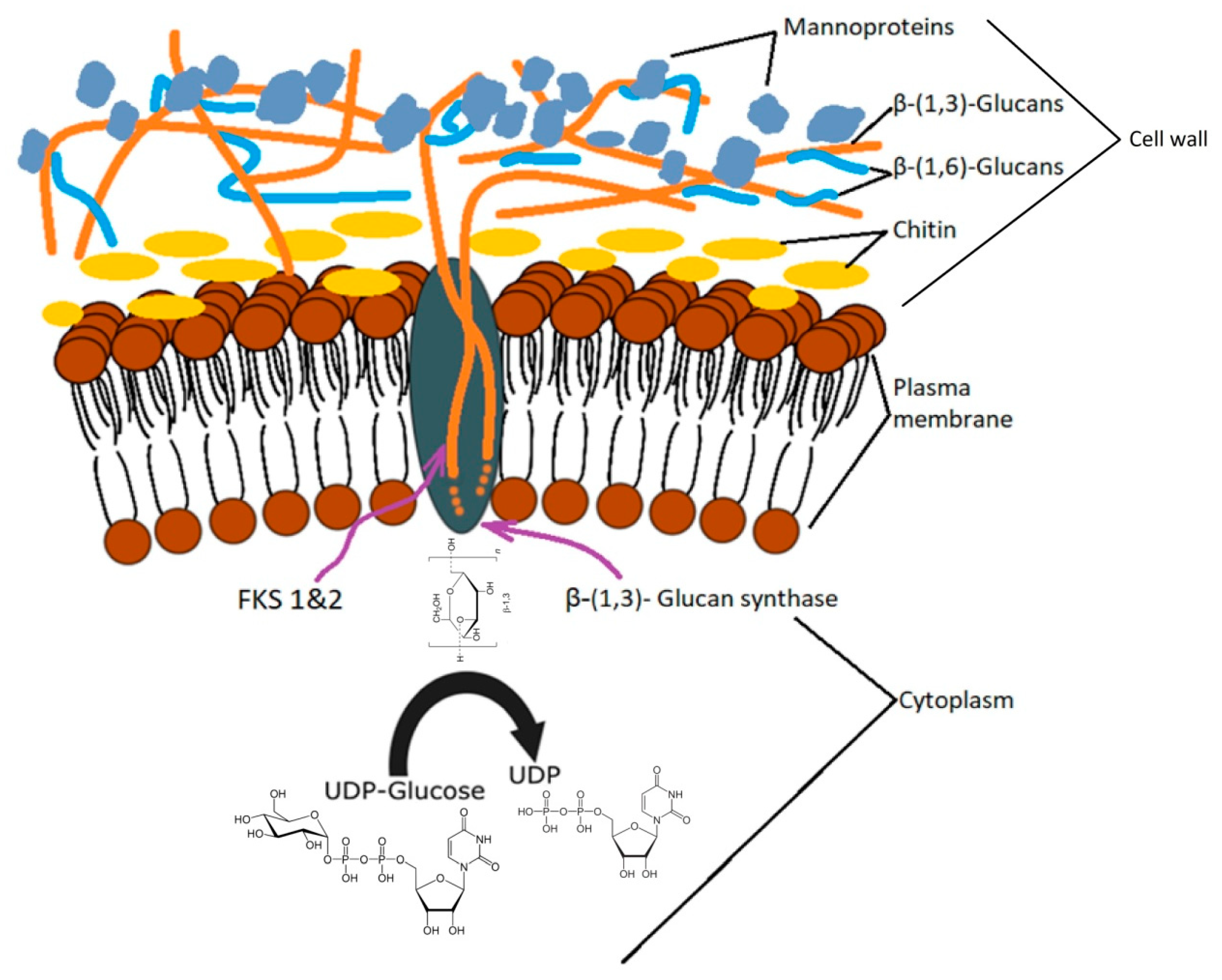
2.2. Cell Wall
2.3. Serial Repitching and the Thickness of the Cell Wall
3. Spent Brewer’s Yeast
3.1. Characterization
3.2. Bitter Compounds and the Possibility of Their Removal
3.3. Glucan Content
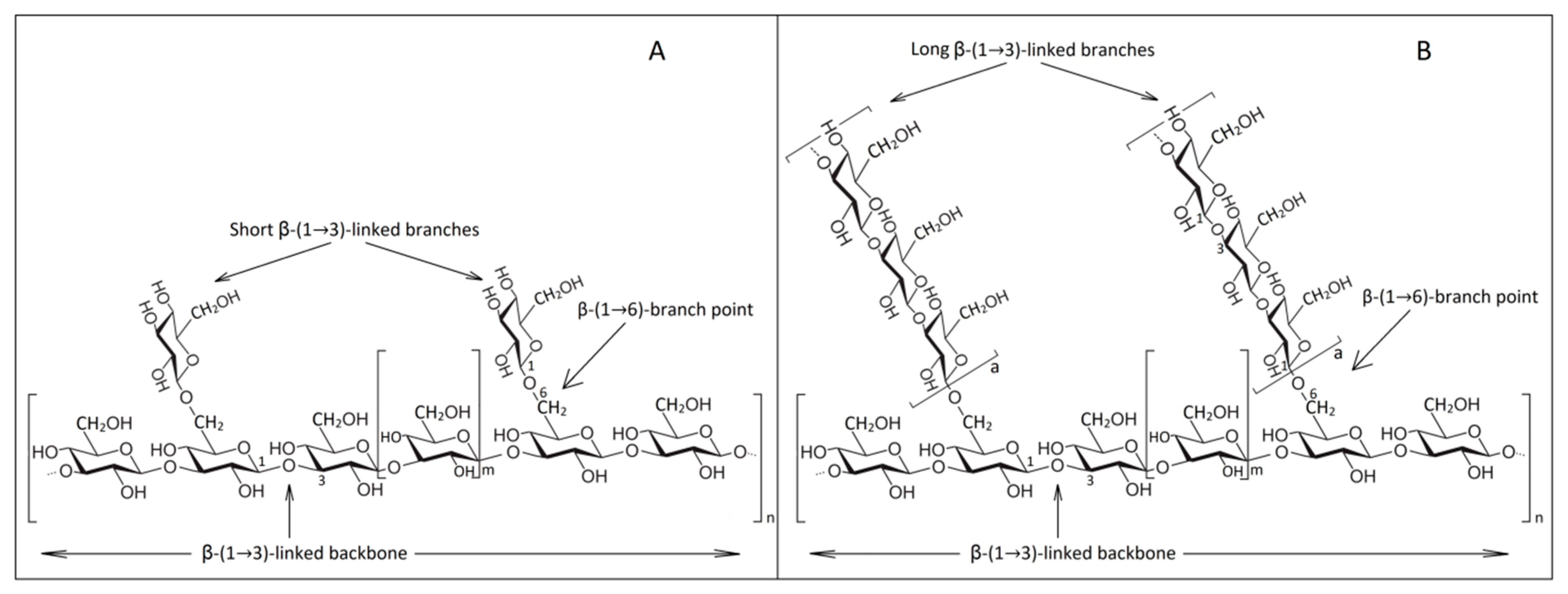
4. Beta-Glucans
4.1. Chemical Composition and Food Safety
4.2. Solubility
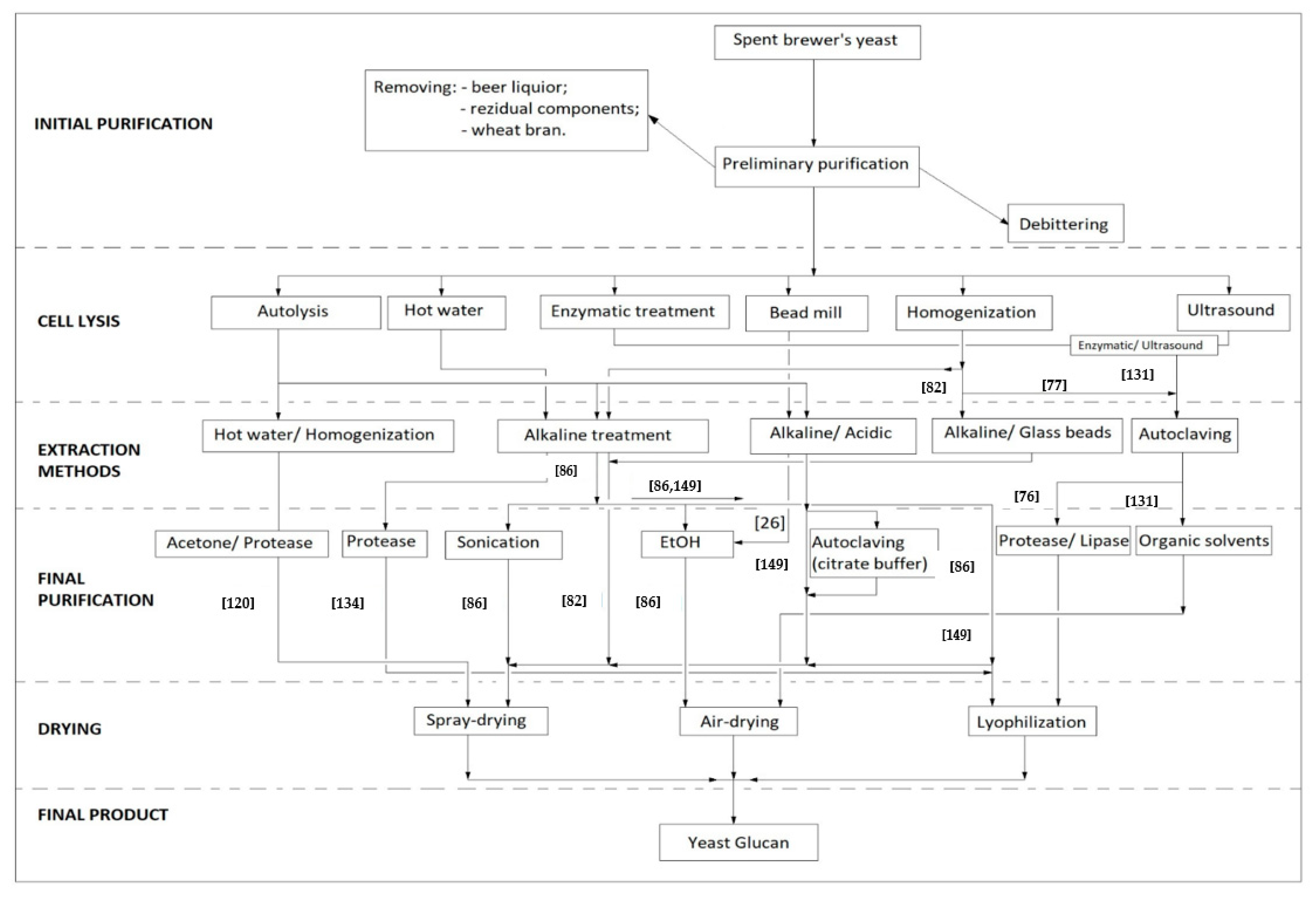
5. Extraction Methods
5.1. Initial Purification
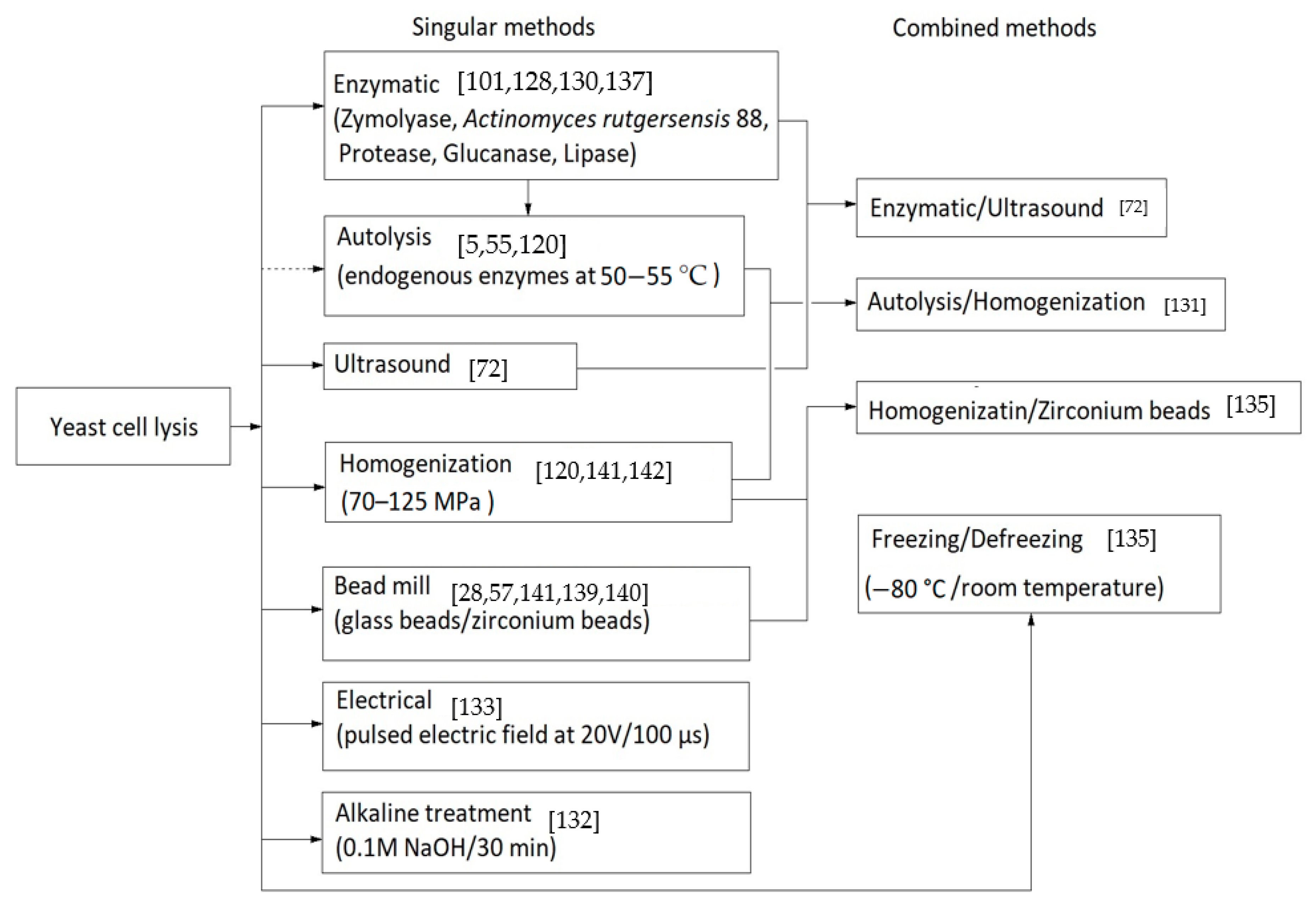
5.2. Cell Lysis and the Breakage of the Cell Wall
5.2.1. Autolysis
5.2.2. Enzymatic Treatment
5.2.3. Cell Disruption with Beads
5.2.4. High-Pressure Homogenization
5.3. The Proper Extraction
5.3.1. Alkaline/Alkaline-Acid Extraction
5.3.2. High-Pressure Homogenization
5.3.3. Enzymatic Extraction
5.3.4. Other Extraction Types
5.4. Final Purification of Extracted β-Glucans
5.5. Drying
6. Food Applications
6.1. Yogurt
6.2. Mayonnaise
6.3. Bread
6.4. Films
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Happy International Beer Day!—Product—Eurostat. Available online: https://ec.europa.eu/eurostat/web/products-eurostat-news/-/EDN-20190802-1 (accessed on 20 August 2020).
- Marson, G.V.; de Castro, R.J.S.; Belleville, M.P.; Hubinger, M.D. Spent brewer’s yeast as a source of high added value molecules: A systematic review on its characteristics, processing and potential applications. World J. Microbiol. Biotechnol. 2020, 36, 1–22. [Google Scholar] [CrossRef]
- San Martin, D.; Orive, M.; Iñarra, B.; Castelo, J.; Estévez, A.; Nazzaro, J.; Zufía, J. Brewers’ Spent Yeast and Grain Protein Hydrolysates as Second-Generation Feedstuff for Aquaculture Feed. Waste Biomass Valorization 2020, 11, 5307–5320. [Google Scholar] [CrossRef]
- Ferreira, I.M.P.L.V.O.; Pinho, O.; Vieira, E.; Tavarela, J.G. Brewer’s Saccharomyces yeast biomass: Characteristics and potential applications. Trends Food Sci. Technol. 2010, 21, 77–84. [Google Scholar] [CrossRef]
- Tanguler, H.; Erten, H. Utilisation of spent brewer’s yeast for yeast extract production by autolysis: The effect of temperature. Food Bioprod. Process. 2008, 86, 317–321. [Google Scholar] [CrossRef]
- Apostu, P.M.; Mihociu, T.E.; Nicolau, A.I. Technological and sensorial role of yeast β-glucan in meat batter reformulations. J. Food Sci. Technol. 2017, 54, 2653–2660. [Google Scholar] [CrossRef] [PubMed]
- Ritala, A.; Häkkinen, S.T.; Toivari, M.; Wiebe, M.G. Single cell protein-state-of-the-art, industrial landscape and patents 2001–2016. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef]
- Jacob, F.F.; Striegel, L.; Rychlik, M.; Hutzler, M.; Methner, F.J. Spent yeast from brewing processes: A biodiverse starting material for yeast extract production. Fermentation 2019, 5, 51. [Google Scholar] [CrossRef] [Green Version]
- Liepins, J.; Kovačova, E.; Shvirksts, K.; Grube, M.; Rapoport, A.; Kogan, G. Drying enhances immunoactivity of spent brewer’s yeast cell wall β-d-glucans. J. Biotechnol. 2015, 206, 12–16. [Google Scholar] [CrossRef]
- Worrasinchai, S.; Suphantharika, M.; Pinjai, S.; Jamnong, P. β-Glucan prepared from spent brewer’s yeast as a fat replacer in mayonnaise. Food Hydrocoll. 2006, 20, 68–78. [Google Scholar] [CrossRef]
- Synytsya, A.; Novak, M. Structural analysis of glucans. Ann. Transl. Med. 2014, 2, 17. [Google Scholar] [CrossRef]
- Ruiz-Herrera, J.; Ortiz-Castellanos, L. Cell wall glucans of fungi. A review. Cell Surf. 2019, 5, 100022. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Gordon, S. A new receptor for β-glucans. Nature 2001, 413, 36–37. [Google Scholar] [CrossRef] [PubMed]
- Ursachi, V.; Gutt, G.; Engineering, F. Feedstocks used for production of 2nd and 3rd generation bioethanol-review. Food Environ. Saf. J. 2020, 19. [Google Scholar]
- Synytsya, A.; Novák, M. Structural diversity of fungal glucans. Carbohydr. Polym. 2013, 92, 792–809. [Google Scholar] [CrossRef]
- Kaur, R.; Sharma, M.; Ji, D.; Xu, M.; Agyei, D. Structural Features, Modification, and Functional-ities of Beta-Glucan. Fibers 2020, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- Lipke, P.N.; Ovalle, R. Cell wall architecture in yeast: New structure and new challenges. J. Bacteriol. 1998, 180, 3735–3740. [Google Scholar] [CrossRef] [Green Version]
- Novák, M.; Synytsya, A.; Gedeon, O.; Slepička, P.; Procházka, V.; Synytsya, A.; Blahovec, J.; Hejlová, A.; Čopíková, J. Yeast β(1-3),(1-6)-d-glucan films: Preparation and characterization of some structural and physical properties. Carbohydr. Polym. 2012, 87, 2496–2504. [Google Scholar] [CrossRef]
- Falch, B.H.; Espevik, T.; Ryan, L.; Stokke, B.T. The cytokine stimulating activity of (1→3)-β-d-glucans is dependent on the triple helix conformation. Carbohydr. Res. 2000, 329, 587–596. [Google Scholar] [CrossRef]
- Lodolo, E.J.; Kock, J.L.F.; Axcell, B.C.; Brooks, M. The yeast Saccharomyces cerevisiae—The main character in beer brewing. FEMS Yeast Res. 2008, 8, 1018–1036. [Google Scholar] [CrossRef] [Green Version]
- Nikulin, J.; Vidgren , V.; Krogerus , K.; Magalhães, F.; Valkeemäki, S.; Kangas-Heiska, T.; Gibson, B. Brewing potential of the wild yeast species Saccharomyces paradoxus. Eur. Food Res. Technol. 2020. [Google Scholar] [CrossRef]
- Sicard, D.; Legras, J.-L. Bread, beer and wine: Yeast domestication in the Saccharomyces sensu stricto complex. C. R. Biol. 2011, 334, 229–236. [Google Scholar] [CrossRef]
- Hartwell, L.H. Saccharomyces cerevisiae cell cycle. Bacteriol. Rev. 1974, 38, 164–198. [Google Scholar] [CrossRef]
- Smits, G.J.; van den Ende, H.; Klis, F.M. Differential regulation of cell wall biogenesis during growth and development in yeast. Microbiology 2001, 147, 781–794. [Google Scholar] [CrossRef] [Green Version]
- Burgess, S.M.; Powers, T.; Mell, J.C. Budding Yeast Saccharomyces cerevisiae as a Model Genetic Organism. Encycl. Life Sci. 2017. [Google Scholar] [CrossRef]
- Northcote, D.H.; Horne, R.W. The chemical composition and structure of the yeast cell wall. Biochem. J. 1952, 51, 232–236. [Google Scholar] [CrossRef] [Green Version]
- Kollár, R.; Reinhold, B.B.; Petráková, E.; Yeh, H.J.; Ashwell, G.; Drgonová, J.; Cabib, E. Architecture of the Yeast Cell Wall. J. Biol. Chem. 1997, 272, 17762–17775. [Google Scholar] [CrossRef] [Green Version]
- Aimanianda, V.; Clavaud, C.; Simenel, C.; Fontaine, T.; Delepierre, M.; Latgé, J.P. Cell wall β-(1,6)-glucan of Saccharomyces cerevisiae: Structural characterization and in situ synthesis. J. Biol. Chem. 2009, 284, 13401–13412. [Google Scholar] [CrossRef] [Green Version]
- Pelling, A.E.; Sehati, S.; Gralla, E.B.; Valentine, J.S.; Gimzewski, J.K. Local nanomechanical motion of the cell wall of Saccharomyces cerevisiae. Science 2004, 305, 1147–1150. [Google Scholar] [CrossRef]
- Gaboriaud, F.; Parcha, B.S.; Gee, M.L.; Holden, J.A.; Strugnell, R.A. Spatially resolved force spectroscopy of bacterial surfaces using force-volume imaging. Colloids Surf. B Biointerfaces 2008, 62, 206–213. [Google Scholar] [CrossRef]
- Powell, C.D.; Diacetis, A.N. Long term serial repitching and the genetic and phenotypic stability of brewer’s yeast. J. Inst. Brew. 2007, 113, 67–74. [Google Scholar] [CrossRef]
- Bühligen, F.; Lindner, P.; Fetzer, I.; Stahl, F.; Scheper, T.; Harms, H.; Müller, S. Analysis of aging in lager brewing yeast during serial repitching. J. Biotechnol. 2014, 187, 60–70. [Google Scholar] [CrossRef]
- Wang, J.; Ding, H.; Zheng, F.; Li, Y.; Liu, C.; Niu, C.; Li, Q. Physiological Changes of Beer Brewer’s Yeast During Serial Beer Fermentation. J. Am. Soc. Brew. Chem. 2019, 77, 10–20. [Google Scholar] [CrossRef]
- Chaudhari, R.D.; Stenson, J.D.; Overton, T.W.; Thomas, C.R. Effect of bud scars on the mechanical properties of Saccharomyces cerevisiae cell walls. Chem. Eng. Sci. 2012, 84, 188–196. [Google Scholar] [CrossRef] [Green Version]
- Klis, F.M.; Mol, P.; Hellingwerf, K.; Brul, S. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2002, 26, 239–256. [Google Scholar] [CrossRef]
- Barazani, B.; Warnat, S.; MacIntosh, A.J.; Hubbard, T. MEMS measurements of single cell stiffness decay due to cyclic mechanical loading. Biomed. Microdevices 2017, 19, 77. [Google Scholar] [CrossRef]
- Zarandi, M.M.; Bonakdar, A.; Stiharu, I. Investigations on Natural Frequencies of Individual Spherical and Ellipsoidal Bakery Yeast Cells. COMSOL Conf. 2010, 27, 5–6. [Google Scholar]
- de Nobel, J.G.; Klis, F.M.; Priem, J.; Munnik, T.; van den Ende, H. The glucanase-soluble mannoproteins limit cell wall porosity in Saccharomyces cerevisiae. Yeast 1990, 6, 491–499. [Google Scholar] [CrossRef]
- Levin, D.E. Cell Wall Integrity Signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2005, 69, 262–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Ding, L.; Sun, J.; Boussetta, N.; Vorobiev, E. Yeast cell disruption strategies for recovery of intracellular bio-active compounds—A review. Innov. Food Sci. Emerg. Technol. 2016, 36, 181–192. [Google Scholar] [CrossRef]
- Backhaus, K.; Heilmann, C.J.; Sorgo, A.G.; Purschke, G.; de Koster, C.G.; Klis, F.M.; Heinisch, J. A systematic study of the cell wall composition of Kluyveromyces lactis. Yeast 2010, 27, 647–660. [Google Scholar] [CrossRef] [Green Version]
- Klis, F.M.; Boorsma, A.; de Groot, P.W.J. Cell wall construction in Saccharomyces cerevisiae. Yeast 2006, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Onofre, S.B.; Bertoldo, I.C.; Abatti, D.; Refosco, D. Chemical Composition of the Biomass of Saccharomyces cerevisiae—(Meyen ex E. C. Hansen, 1883) Yeast obtained from the Beer Manufacturing Process. Int. J. Environ. Agric. Biotechnol. 2017, 2, 558–562. [Google Scholar] [CrossRef]
- Peltzer, M.A.; Salvay, A.G.; Delgado, J.F.; de la Osa, O.; Wagner, J.R. Use of Residual Yeast Cell Wall for New Biobased Materials Production: Effect of Plasticization on Film Properties. Food Bioprocess. Technol. 2018, 11, 1995–2007. [Google Scholar] [CrossRef]
- Kondo, M.A. Yeast cell-surface display—Applications of molecular display. Appl. Microbiol. Biotechnol. 2004, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, S.; Kwiatkowski, S.E. Yeast (Saccharomyces cerevisiae) glucan polysaccharides-occurrence, separation and application in food, feed and health industries. Complex. World Polysacch. 2012, 47–70. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.H.; Fleet, G.H.; Rogers, P.L. Composition of the cell walls of several yeast species. Appl. Microbiol. Biotechnol. 1998, 50, 206–212. [Google Scholar] [CrossRef]
- Sun, C.; Lin, M.; Fu, D.; Yang, J.; Huang, Y.; Zheng, X.; Yu, T. Yeast cell wall induces disease resistance against Penicillium expansum in pear fruit and the possible mechanisms involved. Food Chem. 2018, 241, 301–307. [Google Scholar] [CrossRef]
- Orlean, P. Architecture and biosynthesis of the Saccharomyces cerevisiae cell wall. Genetics 2012, 192, 775–818. [Google Scholar] [CrossRef] [Green Version]
- Aimanianda, V. Transglycosidases and Fungal Cell Wall β-(1,3)- Glucan Branching. Mol. Biol. 2017, 6, 194. [Google Scholar] [CrossRef]
- Aimanianda, V.; Simenel, C.; Garnaud, C.; Clavaud, C. The Dual Activity Responsible for the Glucan in the Fungal Cell Wall. mBio 2017. [Google Scholar] [CrossRef] [Green Version]
- Kasahara, S.; Yamada, H.; Mio, T.; Shiratori, Y.; Miyamoto, C.; Yabe, T.; Nakajima, T.; Ichishima, E.; Furuichi, Y. Cloning of the Saccharomyces cerevisiae gene whose overexpression overcomes the effects of HM-1 killer toxin, which inhibits β-glucan synthesis. J. Bacteriol. 1994, 176, 1488–1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tartar, A.; Shapiro, A.M.; Scharf, D.W.; Boucias, D.G. Differential expression of chitin synthase (CHS) and glucan synthase (FKS) genes correlates with the formation of a modified, thinner cell wall in in vivo-produced Beauveria bassiana cells. Mycopathologia 2005, 160, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Sanz, A.B.; García, R.; Rodríguez-Peña, J.M.; Arroyo, J. The CWI pathway: Regulation of the transcriptional adaptive response to cell wall stress in yeast. J. Fungi 2018, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Li, M.; Zheng, F.; Niu, C.; Liu, C.; Li, Q.; Sun, J. Cell wall polysaccharides: Before and after autolysis of brewer’s yeast. World J. Microbiol. Biotechnol. 2018, 34, 137. [Google Scholar] [CrossRef]
- Ha, C.H.; Yun, C.W.; Paik, H.D.; Kim, S.W.; Kang, C.W.; Hwang, H.J.; Chang, H.I. Preparation and analysis of yeast cell wall mannoproteins, immune enchancing materials, from cell wall mutant Saccharomyces cerevisiae. J. Microbiol. Biotechnol. 2006, 16, 247–255. [Google Scholar]
- Wheatcroft, R.; Kulandai, J.; Gilbert, R.W.; Sime, K.J.; Smith, C.G.; Langeris, W.H. Production of β-Glucan-Mannan Preparations by Autolysis of Cells under Certain pH, Temperature and Time Conditions. U.S. Patent No. 6,444,448, 3 September 2002. [Google Scholar]
- Sedmak, J.J. Production of Beta-Glucans and Mannans. U.S. Patent No. 8,753,668, 17 June 2014. [Google Scholar]
- Cabib, E.; Roh, D.H.; Schmidt, M.; Crotti, L.B.; Varma, A. The Yeast Cell Wall and Septum as Paradigms of Cell Growth and Morphogenesis. J. Biol. Chem. 2001, 276, 19679–19682. [Google Scholar] [CrossRef] [Green Version]
- Vieira, E.F.; Carvalho, J.; Pinto, E.; Cunha, S.; Almeida, A.A.; Ferreira, I.M.P.L.V.O. Nutritive value, antioxidant activity and phenolic compounds profile of brewer’s spent yeast extract. J. Food Compos. Anal. 2016, 52, 44–51. [Google Scholar] [CrossRef]
- Francois, J.M.; Formosa, C.; Schiavone, M.; Pillet, F.; Martin-Yken, H.; Dague, E. Use of atomic force microscopy (AFM) to explore cell wall properties and response to stress in the yeast Saccharomyces cerevisiae. Curr. Genet. 2013, 59, 187–196. [Google Scholar] [CrossRef]
- Marson, G.V.; Machado, M.T.d.; de Castro, R.J.S.; Hubinger, M.D. Sequential hydrolysis of spent brewer’s yeast improved its physico-chemical characteristics and antioxidant properties: A strategy to transform waste into added-value biomolecules. Process. Biochem. 2019, 84, 91–102. [Google Scholar] [CrossRef]
- Wang, J.j.; Xu, W.n.; Li, X.; Li, J.; Li, Q. Absence of fks1p in lager brewing yeast results in aberrant cell wall composition and improved beer flavor stability. World J. Microbiol. Biotechnol. 2014, 30, 1901–1908. [Google Scholar] [CrossRef]
- Hernawan, T.; Fleet, G. Chemical and cytological changes during the autolysis of yeasts. J. Ind. Microbiol. 1995, 14, 440–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalayu, G. Serial re-pitching: Its effect on yeast physiology, fermentation performance, and product quality. Ann. Microbiol. 2019, 69, 787–796. [Google Scholar] [CrossRef]
- Li, X.E.; Wang, J.J.; Phornsanthia, S.; Yin, X.; Li, Q. Strengthening of cell wall structure enhances stress resistance and fermentation performance in lager yeast. J. Am. Soc. Brew. Chem. 2014, 72, 88–94. [Google Scholar] [CrossRef]
- Pinto, M.; Coelho, E.; Nunes, A.; Brandão, T.; Coimbra, M.A. Valuation of brewers spent yeast polysaccharides: A structural characterization approach. Carbohydr. Polym. 2015, 116, 215–222. [Google Scholar] [CrossRef]
- Thiago, R.d.S.M.; Pedro, P.M.d.; Eliana, F.C.S. Solid wastes in brewing process: A review. J. Brew. Distill. 2014, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Hayen, G.D.; Pollmann, D.S. Animal Feeds Comprising Yeast Glucan. U.S. Patent No. 6,214,337, 10 April 2001. [Google Scholar]
- Podpora, B.; Świderski, F.; Sadowska, A.; Piotrowska, A.; Rakowska, R. Spent Brewer’s Yeast Autolysates as a New and Valuable Component of Functional Food and Dietary Supplements. J. Food Process. Technol. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Rachwał, K.; Waśko, A.; Gustaw, K.; Polak-Berecka, M. Utilization of brewery wastes in food industry. PeerJ 2020, 8, e9427. [Google Scholar] [CrossRef]
- Thammakiti, S.; Suphantharika, M.; Phaesuwan, T.; Verduyn, C. Preparation of spent brewer’s yeast β-glucans for potential applications in the food industry. Int. J. Food Sci. Technol. 2004, 39, 21–29. [Google Scholar] [CrossRef]
- Desai, K.M.; Vaidya, B.K.; Singhal, R.S.; Bhagwat, S.S. Use of an artificial neural network in modeling yeast biomass and yield of b -glucan. Process. Biochem. 2005, 40, 1617–1626. [Google Scholar] [CrossRef]
- Stier, H.; Ebbeskotte, V.; Gruenwald, J. Immune-modulatory effects of dietary Yeast Beta-1,3/1,6-D-glucan. Nutr. J. 2014, 13, 38. [Google Scholar] [CrossRef] [Green Version]
- Opinion, S. Scientific Opinion on the substantiation of a health claim related to Yestimun® and immune responses pursuant to Article 13(5) of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1607. [Google Scholar] [CrossRef]
- Jaehrig, S.C.; Rohn, S.; Kroh, L.W.; Wildenauer, F.X.; Lisdat, F.; Fleischer, L.G.; Kurz, T. Antioxidative activity of (1→3), (1→6)-β-d-glucan from Saccharomyces cerevisiae grown on different media. LWT Food Sci. Technol. 2008, 41, 868–877. [Google Scholar] [CrossRef]
- Dwivedi, B.K.; Gibson, D.L. Processing of Spent Brewers’ Yeast for Food Use. Can. Inst. Food Technol. J. 1970, 3, 110–112. [Google Scholar] [CrossRef]
- Shotipruk, A.; Kittianong, P.; Suphantharika, M.; Muangnapoh, C. Application of rotary microfiltration in debittering process of spent brewer’s yeast. Bioresour. Technol. 2005, 96, 1851–1859. [Google Scholar] [CrossRef]
- Rakowska, R.; Sadowska, A.; Dybkowska, E.; Świderski, F. Spent yeast as natural source of functional food additives. Rocz. Panstw. Zakl. Hig. 2017, 68, 115–121. [Google Scholar]
- Aguilar-Uscanga, B.; François, J.M. A study of the yeast cell wall composition and structure in response to growth conditions and mode of cultivation. Lett. Appl. Microbiol. 2003, 37, 268–274. [Google Scholar] [CrossRef]
- Cabiscol, E.; Piulats, E.; Echave, P.; Herrero, E.; Ros, J. Oxidative stress promotes specific protein damage in Saccharomyces cerevisiae. J. Biol. Chem. 2000, 275, 27393–27398. [Google Scholar] [CrossRef]
- Tian, X.; Yang, P.; Jiang, W. Effect of Alkali Treatment Combined with High Pressure on Extraction Efficiency of β-d-Glucan from Spent Brewer’s Yeast. Waste Biomass Valorization 2019, 10, 1131–1140. [Google Scholar] [CrossRef]
- Simpson, W.J. Synergism Between Hop Resins and Phosphoric Acid and Its Relevance To the Acid Washing of Yeast. J. Inst. Brew. 1987, 93, 405–406. [Google Scholar] [CrossRef]
- Dixon, I.J.; Leach, A.A. The adsorption of hop substances on the yeast cell wall. J. Inst. Brew. 1968, 74, 63–67. [Google Scholar] [CrossRef]
- Marinescu, G.; Stoicescu, A.; Bolocan, A. Comparative study of spent brewer’s yeast β-glucans determination methods. Sci. Bull. 2010, 14, 23. [Google Scholar]
- Zechner-Krpan, V.; Petravić-Tominac, V.; Galović, P.; Galović, V.; Filipović-Grčić, J.; Srečec, S. Application of different drying methods on β-glucan isolated from spent brewer’s yeast using alkaline procedure. Agric. Conspec. Sci. 2010, 75, 45–50. [Google Scholar]
- Naser, H.M.A.; Zohri, A. Biotechnological β-glucan Production from Returned Baker’s Yeast and Yeast Remaining after Ethanol Fermentation. Egypt. Sugar J. 2019, 13, 29–43. [Google Scholar]
- Simard, R.E.; Bouksaim, M. Process for Brewer’s Yeast Debittering. U.S. Patent No. 5,716,653, 10 February 1998. [Google Scholar]
- Kim, J.-S. Washing for debittering of brewers yeast slurry. Korean J. Food Sci. Technol. 2001, 33, 205–208. [Google Scholar]
- Bzducha-Wróbel, A.; Kieliszek, M.; Błażejak, S. Chemical composition of the cell wall of probiotic and brewer’s yeast in response to cultivation medium with glycerol as a carbon source. Eur. Food Res. Technol. 2013, 237, 489–499. [Google Scholar] [CrossRef] [Green Version]
- Geller, A.; Shrestha, R.; Yan, J. Yeast-derived β-glucan in cancer: Novel uses of a traditional therapeutic. Int. J. Mol. Sci. 2019, 20, 3618. [Google Scholar] [CrossRef] [Green Version]
- Vetvicka, V. β-glucan as a new tool in vaccine development. Scand. J. Immunol. 2019, e12833. [Google Scholar] [CrossRef]
- Araújo, D.; Alves, V.D.; Lima, A.C.; Reis, S.; Freitas, F.; Reis, M.A.M. International Journal of Biological Macromolecules Novel hydrogels based on yeast chitin-glucan complex: Characterization and safety assessment. Int. J. Biol. Macromol. 2020, 156, 1104–1111. [Google Scholar] [CrossRef]
- Bacic, A.; Fincher, G.B.; Stone, B.A. Chemistry, Biochemistry, and Biology of 1-3 Beta Glucans and Related Polysaccharides; Academic Press: Cambridge, MA, USA, 2009; Available online: https://books.google.ro/books?hl=en&lr=&id=i3Jc8iZ6GHMC&oi=fnd&pg=PP1&dq=A.+Bacic,+G.+B.+Fincher,+and+B.+A.+Stone,+Chemistry,+biochemistry,+and+biology+of+1-3+beta+glucans+and+related+polysaccharides.+Academic+Press,+2009&ots=nDhJUcxCWj&sig=2XCRgpcaId7rjstUnmL2bmBDgcs&redir_esc=y#v=onepage&q&f=false (accessed on 20 November 2020).
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure—function relationships of immunostimulatory polysaccharides: A review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef]
- Brennan, C.S.; Cleary, L.J. The potential use of cereal (1→3,1→4)-β-d-glucans as functional food ingredients. J. Cereal Sci. 2005, 42, 1–13. [Google Scholar] [CrossRef]
- Kogan, G.; Alföldi, J.; Masler, L. 13C-nmr spectroscopic investigation of two yeast cell wall β-D-glucans. Biopolym. Orig. Res. Biomol. 1988, 27, 1055–1063. [Google Scholar] [CrossRef]
- Lesage, G.; Bussey, H. Cell Wall Assembly in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2006, 70, 317–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arroyo, J.; Farkaš, V.; Sanz, A.B.; Cabib, E. Strengthening the fungal cell wall through chitin–glucan cross-links: Effects on morphogenesis and cell integrity. Cell. Microbiol. 2016, 18, 1239–1250. [Google Scholar] [CrossRef]
- Kim, Y.-T.; Kim, E.-H.; Cheong, C.; Williams, D.L.; Kim, C.-W.; Lim, S.-T. Structural characterization of β-d-(1→3, 1→6)-linked glucans using NMR spectroscopy. Carbohydr. Res. 2000, 328, 331–341. [Google Scholar] [CrossRef]
- Varelas, V.; Tataridis, P.; Liouni, M.; Nerantzis, E.T. Application of different methods for the extraction of yeast β -glucan. E-Jst.Teiath.Gr. 2014, 1, 75–89. Available online: http://e-jst.teiath.gr/issues/issue_44/Varelas_44.pdf (accessed on 20 November 2020).
- Fesel, P.H.; Zuccaro, A. β-glucan: Crucial component of the fungal cell wall and elusive MAMP in plants. Fungal Genet. Biol. 2016, 90, 53–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waszkiewicz-Robak, B. Spent Brewer’s Yeast and Beta-Glucans Isolated from Them as Diet Components Modifying Blood Lipid Metabolism Disturbed by an Atherogenic Diet. Lipid Metab. 2013. [Google Scholar] [CrossRef] [Green Version]
- Chan, A.S.; Jonas, A.B.; Qiu, X.; Ottoson, N.R.; Walsh, R.M.; Gorden, K.B.; Harrison, B.; Maimonis, P.J.; Leonardo, S.M.; Ertelt, K.E. Imprime PGG-mediated anti-cancer immune activation requires immune complex formation. PLoS ONE 2016, 11, e0165909. [Google Scholar] [CrossRef]
- Shalaby, M.; Abo-Rya, M.; Motawei, A.-Z. Effect of Baking Process on Β-Glucan Content in Whole Barley Balady Bread. J. Food Dairy Sci. 2014, 5, 481–490. [Google Scholar] [CrossRef]
- EFSAPanelonDieteticProductsNutritionandAllergies(NDA), Scientific Opinion on the safety of ‘yeast beta-glucans’ as a Novel Food. EFSA J. 2011, 9, 2137. [CrossRef]
- Punere, D.D.E.; Aplicare, Î.N.; Comisiei, U.E.A. DECIZIA DE PUNERE ÎN APLICARE (UE) 2017/2078 A COMISIEI din 10 noiembrie 2017. 2017, Volume 2017, pp. 12–15. Available online: https://eur-lex.europa.eu/legal-content/RO/TXT/?uri=CELEX%3A32017D2078 (accessed on 20 November 2020).
- Oficial, J.; Europene, U. [notificat ă cu num ă rul C(2017) 7391]. 2017–2020. 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017D2078&from=EN (accessed on 20 November 2020).
- Huang, H.; Ostroff, G.R.; Lee, C.K.; Agarwal, S.; Ram, S.; Rice, P.A.; Specht, C.A.; Levitz, S.M. Relative Contributions of Dectin-1 and Complement to Immune Responses to Particulate β-Glucans. J. Immunol. 2012, 189, 312–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manners, D.J.; Masson, A.J.; Patterson, J.C. Structure of a β-(1→3)-d-glucan from yeast cell walls. Biochem. J. 1973, 135, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Petravić-tominac, V.; Panjkota-krbavčić, I. Biological effects of yeast β-glucans. Agric. Conspec. Sci. 2010, 75, 149–158. [Google Scholar]
- Zeković, D.B.; Kwiatkowski, S.; Vrvić, M.M.; Jakovljević, D.; Moran, C.A. Natural and modified (1→3)-β-D-glucans in health promotion and disease alleviation. Crit. Rev. Biotechnol. 2005, 25, 205–230. [Google Scholar] [CrossRef]
- Rice, P.J.; Adams, E.L.; Ozment-Skelton, T.; Gonzalez, A.J.; Goldman, M.P.; Lockhart, B.E.; Barker, L.A.; Breuel, K.F.; DePonti, W.K.; Kalbfleisch, J.H.; et al. Oral delivery and gastrointestinal absorption of soluble glucans stimulate increased resistance to infectious challenge. J. Pharmacol. Exp. Ther. 2005, 314, 1079–1086. [Google Scholar] [CrossRef]
- Seljelid, R.; Bögwald, J.; Lundwall, Å. Glycan stimulation of macrophages in vitro. Exp. Cell Res. 1981, 131, 121–129. [Google Scholar] [CrossRef]
- Graubaum, H.-J.; Busch, R.; Stier, H.; Gruenwald, J. A Double-Blind, Randomized, Placebo-Controlled Nutritional Study Using an Insoluble Yeast Beta-Glucan to Improve the Immune Defense System. Food Nutr. Sci. 2012, 3, 738–746. [Google Scholar] [CrossRef] [Green Version]
- Rieder, A.; Ballance, S.; Böcker, U.; Knutsen, S. Quantification of 1,3-β-D-glucan from yeast added as a functional ingredient to bread. Carbohydr. Polym. 2018, 181, 34–42. [Google Scholar] [CrossRef]
- Soto, E.; Kim, Y.S.; Lee, J.; Kornfeld, H.; Ostroff, G. Glucan particle encapsulated rifampicin for targeted delivery to macrophages. Polymers 2010, 2, 681–689. [Google Scholar] [CrossRef]
- Plavcová, Z.; Šalamúnová, P.; Saloň, I.; Štěpánek, F.; Hanuš, J.; Hošek, J. Curcumin encapsulation in yeast glucan particles promotes its anti-inflammatory potential in vitro. Int. J. Pharm. 2019, 568. [Google Scholar] [CrossRef]
- Bzducha-Wróbel, A.; Blłazejak, S.; Kawarska, A.; Stasiak-Rózańska, L.; Gientka, I.; Majewska, E. Evaluation of the efficiency of different disruption methods on yeast cell wall preparation for β-glucan isolation. Molecules 2014, 19, 20941–20961. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Wang, Q.; Cui, S.W.; Liu, H.Z. A new isolation method of β-d-glucans from spent yeast Saccharomyces cerevisiae. Food Hydrocoll. 2008, 22, 239–247. [Google Scholar] [CrossRef]
- Magnani, M.; Calliari, C.M.; de Macedo, F.C.; Mori, M.P.; Cólus, I.M.d.; Castro-Gomez, R.J.H. Optimized methodology for extraction of (1 → 3)(1 → 6)-β-d-glucan from Saccharomyces cerevisiae and in vitro evaluation of the cytotoxicity and genotoxicity of the corresponding carboxymethyl derivative. Carbohydr. Polym. 2009, 78, 658–665. [Google Scholar] [CrossRef]
- Sauter, M.; Freimund, S.; Dutler, H.; Kappeli, O.; Al-Ghazawi, A.; Schwarz, E.; Thomas, L.; Schoberl, H. Isolation of Glucan Particles and Uses Thereof. Isolation of Glucan Particles and Uses Thereof. U.S. Patent Application 10/343,604, 18 March 2004. [Google Scholar]
- Xiao, M.; Jiang, M.; Wu, K.; Yang, H.; Ni, X.; Yan, W.; Jiang, F. Investigation on curdlan dissociation by heating in water. Food Hydrocoll. 2017, 70, 57–64. [Google Scholar] [CrossRef]
- Gawronski, M.; Park, J.T.; Magee, A.S.; Conrad, H. Microfibrillar structure of PGG-Glucan in aqueous solution as triple-helix aggregates by small angle x-ray scattering. Biopolym. Orig. Res. Biomol. 1999, 50, 569–578. [Google Scholar] [CrossRef]
- Yan, J.; Allendorf, D.J.; Brandley, B. Yeast whole glucan particle (WGP) β-glucan in conjunction with antitumour monoclonal antibodies to treat cancer. Expert Opin. Biol. Ther. 2005, 5, 691–702. [Google Scholar] [CrossRef]
- Galichet, A.; Sockalingum, G.D.; Belarbi, A.; Manfait, M. FTIR spectroscopic analysis of Saccharomyces cerevisiae cell walls: Study of an anomalous strain exhibiting a pink-colored cell phenotype. FEMS Microbiol. Lett. 2001, 197, 179–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vieira, E.; Brandão, T.; Ferreira, I.M. Evaluation of Brewer’s spent yeast to produce flavor enhancer nucleotides: Influence of serial repitching. J. Agric. Food Chem. 2013, 61, 8724–8729. [Google Scholar] [CrossRef]
- González, S.S.; Barrio, E.; Querol, A. Molecular characterization of new natural hybrids of Saccharomyces cerevisiae and S. kudriavzevii in brewing. Appl. Environ. Microbiol. 2008, 74, 2314–2320. [Google Scholar] [CrossRef] [Green Version]
- Javmen, A.; Grigiškis, S.; Gliebutė, R. β-glucan extraction from Saccharomyces cerevisiae yeast using Actinomyces rutgersensis 88 yeast lyzing enzymatic complex. Biologija 2012, 58, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Khoomrung, S.; Chumnanpuen, P.; Jansa-Ard, S.; Nookaew, I.; Nielsen, J. Fast and accurate preparation fatty acid methyl esters by microwave-assisted derivatization in the yeast Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2012, 94, 1637–1646. [Google Scholar] [CrossRef]
- Tâm, T.M.; Duy, N.Q.; Minh, N.P.; Dao, D.T.A. Optimization of Βeta-Glucan extraction from waste brewer’s yeast Saccharomyces cerevisiae using autolysis, enzyme, ultrasonic and combined enzyme-ultrasonic treatment. Am. J. Res. Commun. 2013, 1, 149–158. [Google Scholar]
- Hunter, K.W.; Gault, R.A.; Berner, M.D. Preparation of microparticulate β-glucan from Saccharomyces cerevisiae for use in immune potentiation. Lett. Appl. Microbiol. 2002, 35, 267–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-W.; Tai, Y.-C. A micro cell lysis device. Sens. Actuators A Phys. 1999, 73, 74–79. [Google Scholar] [CrossRef]
- Mendes-Costa, M.C.; Moraes, W.B.C. Comparação entre os teores de carboidratos totais solúveis presentes em distintas frações da levedura Saccharomyces cerevisiae Meyen. Rev. Bras. Biol. 1999, 59, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Kot, A.M.; Gientka, I.; Bzducha-Wróbel, A.; Błażejak, S.; Kurcz, A. Comparison of simple and rapid cell wall disruption methods for improving lipid extraction from yeast cells. J. Microbiol. Methods 2020, 176. [Google Scholar] [CrossRef] [PubMed]
- Jacob, F.F.; Hutzler, M.; Methner, F.J. Comparison of various industrially applicable disruption methods to produce yeast extract using spent yeast from top-fermenting beer production: Influence on amino acid and protein content. Eur. Food Res. Technol. 2019, 245, 95–109. [Google Scholar] [CrossRef]
- Powell, C.D.; van Zandycke, S.M.; Quain, D.E.; Smart, K.A. Replicative ageing and senescence in Saccharomyces cerevisiae and the impact on brewing fermentations. Microbiology 2000, 146, 1023–1034. [Google Scholar] [CrossRef] [Green Version]
- Chae, H.J.; Joo, H.; In, M.-J. Utilization of brewer’s yeast cells for the production of food-grade yeast extract. Part 1: Effects of different enzymatic treatments on solid and protein recovery and flavor characteristics. Bioresour. Technol. 2001, 76, 253–258. [Google Scholar] [CrossRef]
- Melendres, A.V.; Honda, H.; Shiragami, N.; Unno, H. Enzyme release kinetics in a cell disruption chamber of a bead mill. J. Chem. Eng. Jpn. 1993, 26, 148–152. [Google Scholar] [CrossRef] [Green Version]
- Heim, A.; Kamionowska, U.; Solecki, M. The effect of microorganism concentration on yeast cell disruption in a bead mill. J. Food Eng. 2007, 83, 121–128. [Google Scholar] [CrossRef]
- Dimopoulos, G.; Tsantes, M.; Taoukis, P. Effect of high pressure homogenization on the production of yeast extract via autolysis and beta-glucan recovery. Innov. Food Sci. Emerg. Technol. 2020, 62, 102340. [Google Scholar] [CrossRef]
- Delgado, J.F.; Peltzer, M.A.; Salvay, A.G.; de la Osa, O.; Wagner, J.R. Characterization of thermal, mechanical and hydration properties of novel films based on Saccharomyces cerevisiae biomass. Innov. Food Sci. Emerg. Technol. 2018, 48, 240–247. [Google Scholar] [CrossRef]
- Bacon, J.S.; Farmer, V.C.; Jones, D.; Taylor, I.F. The glucan components of the cell wall of baker’s yeast (Saccharomyces cerevisiae) considered in relation to its ultrastructure. Biochem. J. 1969, 114, 557–567. [Google Scholar] [CrossRef] [Green Version]
- Mejri, W.; Bornaz, S.; Sahli, A. Formulation of non-fat yogurt with β-glucan from spen brewer’s yeast. J. Hyg. Eng. Des. 2014, 8, 163–173. [Google Scholar]
- Thanardkit, P.; Khunrae, P.; Suphantharika, M.; Verduyn, C. Glucan from spent brewer’s yeast: Preparation, analysis and use as a potential immunostimulant in shrimp feed. World J. Microbiol. Biotechnol. 2002, 18, 527–539. [Google Scholar] [CrossRef]
- Pengkumsri, N.; Sivamaruthi, B.S.; Sirilun, S.; Peerajan, S.; Kesika, P.; Chaiyasut, K.; Chaiyasut, C. Extraction of β-glucan from Saccharomyces cerevisiae: Comparison of different extraction methods and in vivo assessment of immunomodulatory effect in mice. Food Sci. Technol. 2016, 37, 124–130. [Google Scholar] [CrossRef] [Green Version]
- Ruphuy, G.; Saloň, I.; Tomas, J.; Šalamúnová, P.; Hanuš, J.; Štěpánek, F. Encapsulation of poorly soluble drugs in yeast glucan particles by spray drying improves dispersion and dissolution properties. Int. J. Pharm. 2020, 576. [Google Scholar] [CrossRef]
- Zechner-Krpan, V.; Petravić-Tominac, V.; Gospodarić, I.; Sajli, L.; Daković, S.; Filipović-Grčić, J. Characterization of β-glucans isolated from brewer’s yeast and dried by different methods. Food Technol. Biotechnol. 2010, 48, 189–197. [Google Scholar]
- Petravić-Tominac, V.; Zechner-Krpan, V.; Berković, K.; Galović, P.; Herceg, Z.; Srečec, S.; Špoljarić, I. Rheological properties, water-holding and oil-binding capacities of particulate β-glucans isolated from spent Brewer’s yeast by three different procedures. Food Technol. Biotechnol. 2011, 49, 56–64. [Google Scholar]
- Bernaerts, T.M.M.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; van Loey, A.M. Evaluating microalgal cell disruption upon ultra high pressure homogenization. Algal Res. 2019, 42, 101616. [Google Scholar] [CrossRef]
- Kleinig, A.R.; Middelberg, A.P.J. On the mechanism of microbial cell disruption in high-pressure homogenisation. Chem. Eng. Sci. 1998, 53, 891–898. [Google Scholar] [CrossRef]
- Freimund, S.; Sauter, M.; Käppeli, O.; Dutler, H. A new non-degrading isolation process for 1, 3-β-D-glucan of high purity from baker’s yeast Saccharomyces cerevisiae. Carbohydr. Polym. 2003, 54, 159–171. [Google Scholar] [CrossRef]
- Borchani, C.; Fonteyn, F.; Jamin, G.; Paquot, M.; Blecker, C.; Thonart, P. Enzymatic process for the fractionation of baker’s yeast cell wall (Saccharomyces cerevisiae). Food Chem. 2014, 163, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Pham, K.N.; Nhut, N.D.; Cuong, N.M. New method for preparing purity β-D-glucans (beta-Glucan) from baker’s yeast (Saccharomyces cerevisiae). Sci. Technol. Dev. J. 2020, 23, 673–678. [Google Scholar] [CrossRef]
- Liu, H.; Bai, W.; He, L.; Li, X.; Shah, F.; Wang, Q. Degradation mechanism of Saccharomyces cerevisiae β-D-glucan by ionic liquid and dynamic high pressure microfluidization. Carbohydr. Polym. 2020, 241, 116123. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, Q.; Luo, X.; Xiao, Y.; Cai, W.; Ma, H. Effects and mechanisms of ultrasound- and alkali-assisted enzymolysis on production of water-soluble yeast Β-glucan. Bioresour. Technol. 2019, 273, 394–403. [Google Scholar] [CrossRef]
- da Silva Araújo, V.B.; de Melo, A.N.F.; Costa, A.G.; Castro-Gomez, R.H.; Madruga, M.S.; de Souza, E.L.; Magnani, M. Followed extraction of β-glucan and mannoprotein from spent brewer’s yeast (Saccharomyces uvarum) and application of the obtained mannoprotein as a stabilizer in mayonnaise. Innov. Food Sci. Emerg. Technol. 2014, 23, 164–170. [Google Scholar] [CrossRef]
- Machová, E.; Kogan, G.; Alföldi, J.; Šotés, L.; Šandula, J. Enzymatic and ultrasonic depolymerization of carboxymethylated β-1, 3-D-glucans derived from saccharomyces cerevisiae. J. Appl. Polym. Sci. 1995, 55, 699–704. [Google Scholar] [CrossRef]
- Teo, C.C.; Tan, S.N.; Yong, J.W.H.; Hew, C.S.; Ong, E.S. Pressurized hot water extraction (PHWE). J. Chromatogr. A 2010, 1217, 2484–2494. [Google Scholar] [CrossRef]
- Benito, O.; Alonso, E.; Lucas, S. Optimization of the extraction process of beta-glucans from barley. AIChE Annu. Meet. Conf. Proc. 2008, 2, 829–835. [Google Scholar]
- The European Commission. COMMISSION IMPLEMENTING DECISION (EU) 2017/2078—of 10 November 2017—Authorising an extension of use of yeast beta-glucans as a novel food ingredient under Regulation (EC) No 258/97 of the European Parliament and of the Council. Off. J. Eur. Union 2017, 2009, 12–15. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32017D2078&from=EN (accessed on 20 November 2020).
- Suphantharika, M.; Khunrae, P.; Thanardkit, P.; Verduyn, C. Preparation of spent brewer’s yeast β-glucans with a potential application as an immunostimulant for black tiger shrimp, Penaeus monodon. Bioresour. Technol. 2003, 88, 55–60. [Google Scholar] [CrossRef]
- Manners, D.J.; Masson, A.J.; Patterson, J.C.; Björndal, H.; Lindberg, B. The structure of a β-(1→ 6)-D-glucan from yeast cell walls. Biochem. J. 1973, 135, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Hromádková, Z.; Ebringerová, A.; Sasinková, V.; Šandula, J.; Hříbalová, V.; Omelková, J. Influence of the drying method on the physical properties and immunomodulatory activity of the particulate (1→ 3)-β-D-glucan from Saccharomyces cerevisiae. Carbohydr. Polym. 2003, 51, 9–15. [Google Scholar] [CrossRef]
- Ahmad, A.; Anjum, F.M.; Zahoor, T.; Nawaz, H.; Dilshad, S.M.R. Beta glucan: A valuable functional ingredient in foods. Crit. Rev. Food Sci. Nutr. 2012, 52, 201–212. [Google Scholar] [CrossRef]
- Yoo, M.S.; Lee, Y.T. Pasting properties of crude ß-glucan from spent brewer’s yeast on wheat flour and starch. Food Sci. Biotechnol. 2007, 16, 485–488. [Google Scholar]
- Marinescu, G.; Stoicescu, A.; Patrascu, L. The preparation of mayonnaise containing spent brewer’s yeast β-glucan as a fat replacer. Rom. Biotechnol. Lett. 2011, 16, 6017–6025. [Google Scholar]
- Santipanichwong, R.; Suphantharika, M. Carotenoids as colorants in reduced-fat mayonnaise containing spent brewer’s yeast β-glucan as a fat replacer. Food Hydrocoll. 2007, 21, 565–574. [Google Scholar] [CrossRef]
- Satrapai, S.; Suphantharika, M. Influence of spent brewer’s yeast β-glucan on gelatinization and retrogradation of rice starch. Carbohydr. Polym. 2007, 67, 500–510. [Google Scholar] [CrossRef]
- Banchathanakij, R.; Suphantharika, M. Effect of different β-glucans on the gelatinisation and retrogradation of rice starch. Food Chem. 2009, 114, 5–14. [Google Scholar] [CrossRef]
- Martins, Z.E.; Pinho, O.; Ferreira, I.M.P.L.V.O. Impact of new ingredients obtained from brewer’s spent yeast on bread characteristics. J. Food Sci. Technol. 2018, 55, 1966–1971. [Google Scholar] [CrossRef] [PubMed]
- Suwannarong, S.; Wongsagonsup, R.; Suphantharika, M. Effect of spent brewer’s yeast β-D-glucan on properties of wheat flour dough and bread during chilled storage. Int. J. Biol. Macromol. 2020, 156, 381–393. [Google Scholar] [CrossRef] [PubMed]
- da Silva Guedes, J.; Pimentel, T.C.; Diniz-Silva, H.T.; da Cruz Almeida, E.T.; Tavares, J.F.; de Souza, E.L.; Magnani, M. Protective effects of β-glucan extracted from spent brewer yeast during freeze-drying, storage and exposure to simulated gastrointestinal conditions of probiotic lactobacilli. LWT 2019, 116, 108496. [Google Scholar] [CrossRef]
- Piotrowska, A.; Waszkiewicz-Robak, B.; Éwiderski, F. Possibility of beta-gtucan from spent brewer’s yeast addition to yoghurts. Pol. J. Food Nutr. Sci. 2009, 59, 299–302. [Google Scholar]
- Raikos, V.; Grant, S.B.; Hayes, H.; Ranawana, V. Use of β-glucan from spent brewer’s yeast as a thickener in skimmed yogurt: Physicochemical, textural, and structural properties related to sensory perception. J. Dairy Sci. 2018, 101, 5821–5831. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, J.S.; de França, V.R.; Venâncio, R.L.; Hasegawa, P.H.; de Oliveira, A.G.; Costa, G.A.N. β-Glucan from saccharomyces cereviseae in skim yogurt production. Biosci. J. 2019, 35, 620–628. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Pu, Q.; He, L.; Na, Y.; Wu, F.; Jin, Z. Rheological and sem studies on the interaction between spent brewer’s yeast β-glucans and κ-carrageenan. J. Texture Stud. 2009, 40, 482–496. [Google Scholar] [CrossRef]
- Mohammadian, T.; Nasirpour, M.; Tabandeh, M.R.; Mesbah, M. Synbiotic effects of β-glucan, mannan oligosaccharide and Lactobacillus casei on growth performance, intestine enzymes activities, immune-hematological parameters and immune-related gene expression in common carp, Cyprinus carpio: An experimental infection with Aeromonas hydrophila. Aquaculture 2019, 511, 634197. [Google Scholar] [CrossRef]
- Kiliç, G.B.; Akpinar, D. The effects of different levels of β-glucan on yoghurt manufactured with Lactobacillus plantarum strains as adjunct culture. J. Food Agric. Environ. 2013, 11, 281–287. [Google Scholar]
- Tessari, P.; Lante, A. A multifunctional bread rich in beta glucans and low in starch improves metabolic control in type 2 diabetes: A controlled trial. Nutrients 2017, 9, 297. [Google Scholar] [CrossRef] [PubMed]
- Bozbulut, R.; Şanlıer, N.; Döğer, E.; Bideci, A.; Çamurdan, O.; Cinaz, P. The effect of beta-glucan supplementation on glycemic control and variability in adolescents with type 1 diabetes mellitus. Diabetes Res. Clin. Pr. 2020, 169. [Google Scholar] [CrossRef] [PubMed]
- Puscaselu, R.; Severin, T.L.; Amariei, S. Use of Biopolymers in Designing Edible Packaging Materials for Food Industry Development of statistical models. Mater. Plast. 2019, 56, 199–204. [Google Scholar] [CrossRef]
- Lavorgna, M.; Piscitelli, F.; Mangiacapra, P.; Buonocore, G.G. Study of the combined effect of both clay and glycerol plasticizer on the properties of chitosan films. Carbohydr. Polym. 2010, 82, 291–298. [Google Scholar] [CrossRef]
- Sun, C.; Jin, L.; Cai, Y.; Zheng, X.; Yu, T. (1→3)-β-D-glucan from yeast cell wall: Characteristic and potential application in controlling postharvest disease of pear. Postharvest Biol. Technol. 2019, 154, 105–114. [Google Scholar] [CrossRef]
- Peltzer, M.; Delgado, J.F.; Salvay, A.G.; Wagner, J.R. β-Glucan, a Promising Polysaccharide for Bio-based Films Developments for Food Contact Materials and Medical Applications. Curr. Org. Chem. 2018, 22, 1249–1254. [Google Scholar] [CrossRef]
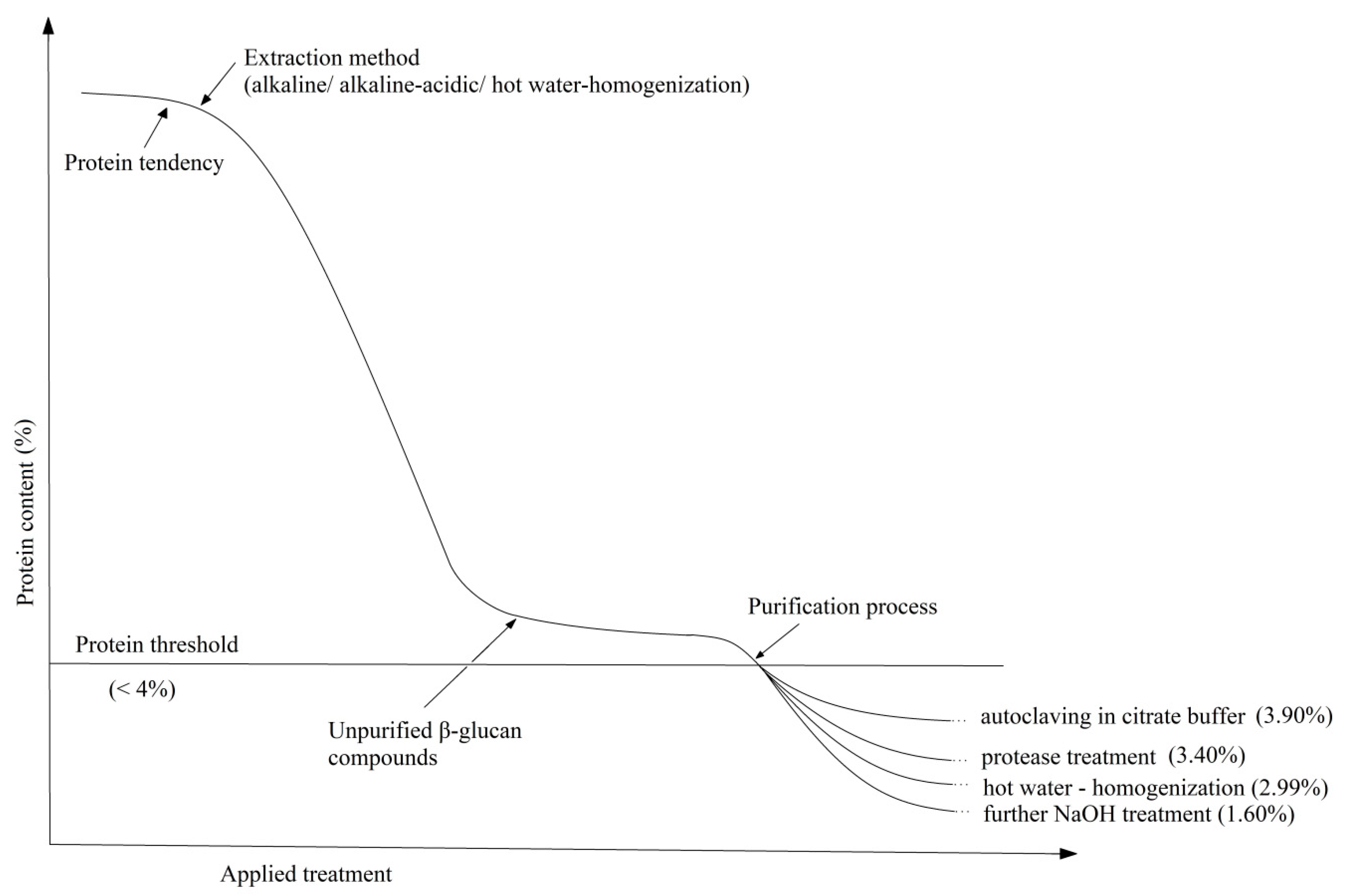
| Extraction Method | Purification Process | Total Carbohydrates (%) | β-Glucans (%) | Protein (%) | Mannans (%) | Lipids (%) | Ash (%) | Reference (%) |
|---|---|---|---|---|---|---|---|---|
| Alkaline-acid | no | 65.23 (59.61) * | 55.21 (23.59) * | 6.54 (43.47) * | nd | 6.54 (43.47) * | 0.55 (10.31) * | [72] |
| Alkaline-acid | Removal of mannoproteins by autoclaving in buffer (citrate) | 93.04 | 95.25 | 3.90 | nd | nd | nd | [149] |
| Alkaline | no | 91.73 | 92.00 | 5.17 | nd | nd | nd | [86] |
| Alkaline | Optimization of alkaline process for solubilizing the protein content | 91.8 (55.20) * | 50.50 (17.20) * | 1.6 (41.20) * | nd | nd | nd | [162] |
| High-pressure homogenization and alkaline | High-pressure homogenization | nd | 78.11 (16.16) * | 2.29 (51.89) * | 0.62 (9.75) * | 0.33 (3.21) * | nd | [82] |
| Hot water | Protease and lipase treatment (Savinase and Lipolase) | nd | 76.00 (30.00) * | 3.40 (32.2) * | nd | 0.9 (5.7) * | nd | [76] |
| Hot water | High-pressure homogenization, acetone and protease (Protamex) | 95.84 (35.09) * | 93.12 (20.58) * | 2.99 (59.67) * | 0.29 (12.72) * | tr (4.90) * | 3.87 (7.07) * | [120] |
| Properties | Extraction Method | Reference |
|---|---|---|
| Pasting properties on wheat flour and starch | Alkaline | [166] |
| Fat replacer in mayonnaise | Alkaline-acid | [10,167,168] |
| Thickening agent, water/oil binding capacity or as emulsifiers | Alkaline; Alkaline-acid; Alkaline-acid with mannoprotein removal | [72,149] |
| Gelling capacity and preventing rice starch retrogradation | Alkaline-acid | [169,170] |
| Improving the nutritional properties of bread and increasing the fiber content | Alkaline-acid | [171] |
| Improving the physical qualities of wheat flour dough and bread during refrigeration | - | [172] |
| Prebiotic activity and antioxidant action | Used as provided | [79] |
| Cryoprotective effect on lactic cultures of Lactobacillus spp. | Autoclavating at 121 °C | [173] |
| Yogurt formulation with up to 0.3% β-glucan | Used as provided | [174] |
| Thickening agent in low-fat yogurt | Used as provided; Alkaline | [144,175,176] |
| Fortifying role by strengthening the k-carrageenan network | Alkaline-acid | [177] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avramia, I.; Amariei, S. Spent Brewer’s Yeast as a Source of Insoluble β-Glucans. Int. J. Mol. Sci. 2021, 22, 825. https://doi.org/10.3390/ijms22020825
Avramia I, Amariei S. Spent Brewer’s Yeast as a Source of Insoluble β-Glucans. International Journal of Molecular Sciences. 2021; 22(2):825. https://doi.org/10.3390/ijms22020825
Chicago/Turabian StyleAvramia, Ionut, and Sonia Amariei. 2021. "Spent Brewer’s Yeast as a Source of Insoluble β-Glucans" International Journal of Molecular Sciences 22, no. 2: 825. https://doi.org/10.3390/ijms22020825






