Mitochondrial Dynamics in Placenta-Derived Mesenchymal Stem Cells Regulate the Invasion Activity of Trophoblast
Abstract
:1. Introduction
2. Results
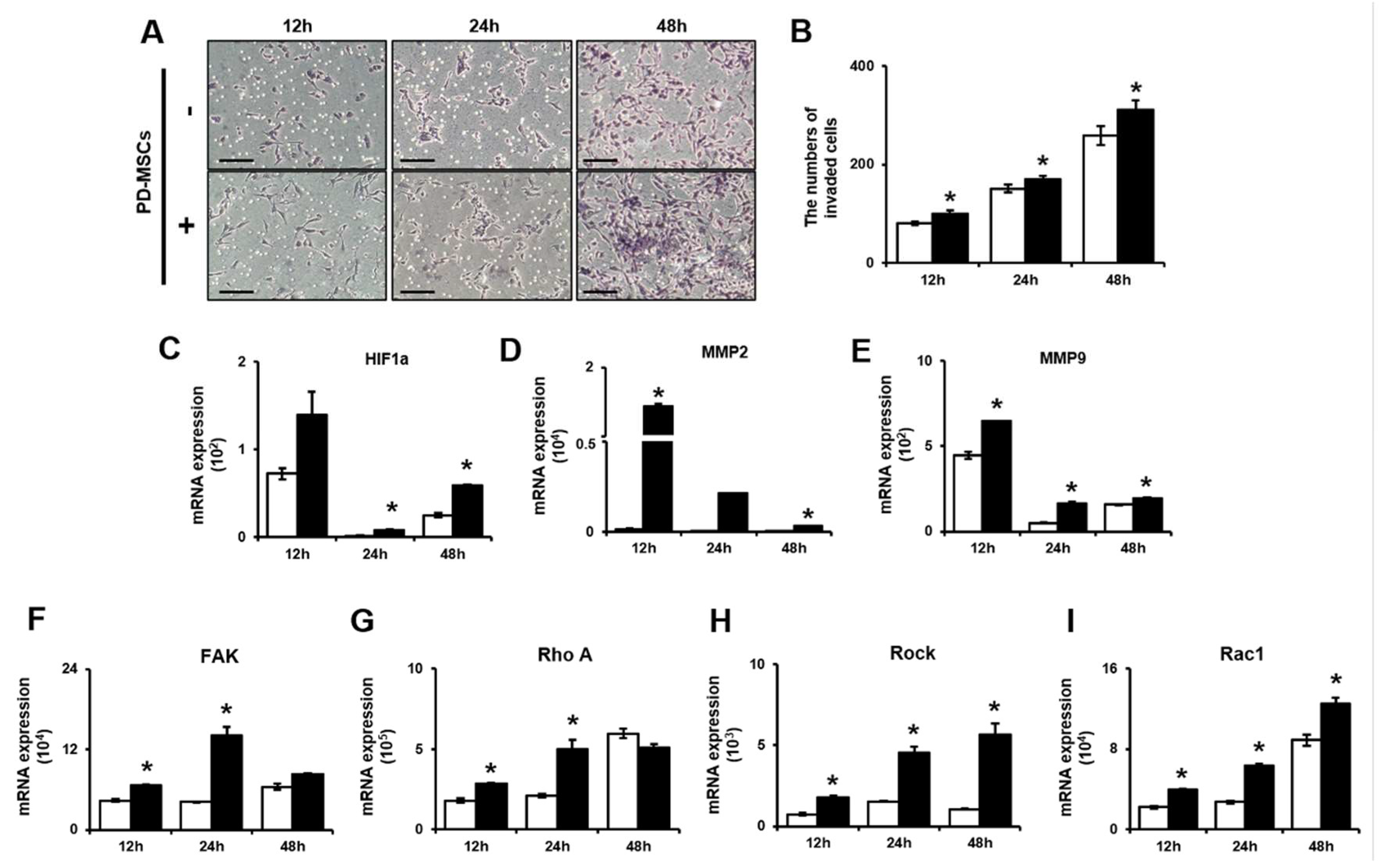
2.1. PD-MSCs Enhance the Invasion Ability of Trophoblasts via MMP-2/9 Activity and the Rho Family Pathway
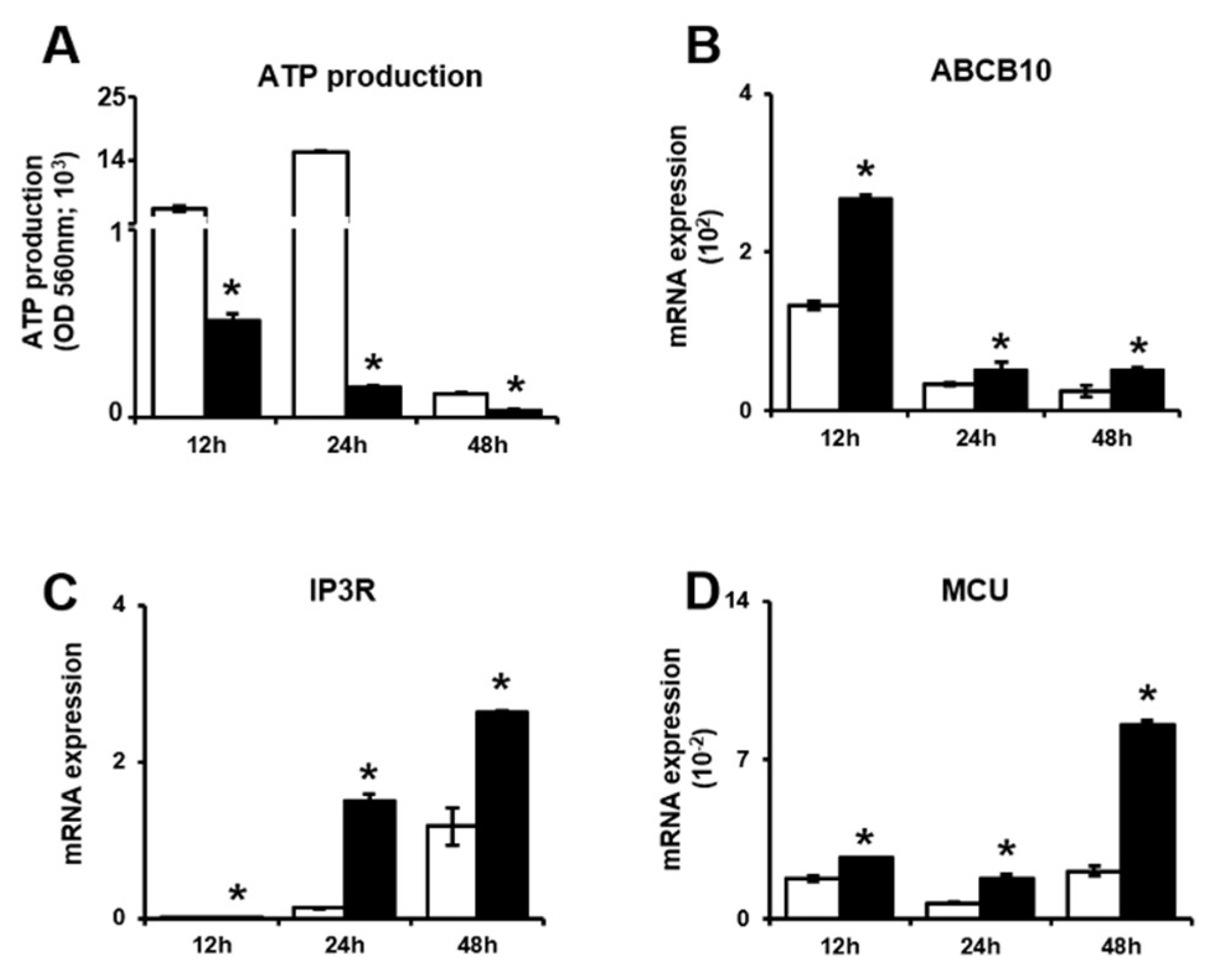
2.2. PD-MSCs Induce Changes in the Mitochondrial Function of Invasive Trophoblasts
2.3. PD-MSCs Regulated Glycolysis in Invasive Trophoblasts
2.4. PD-MSCs Changed the Mitochondrial Oxygen Consumption of Invasive Trophoblasts
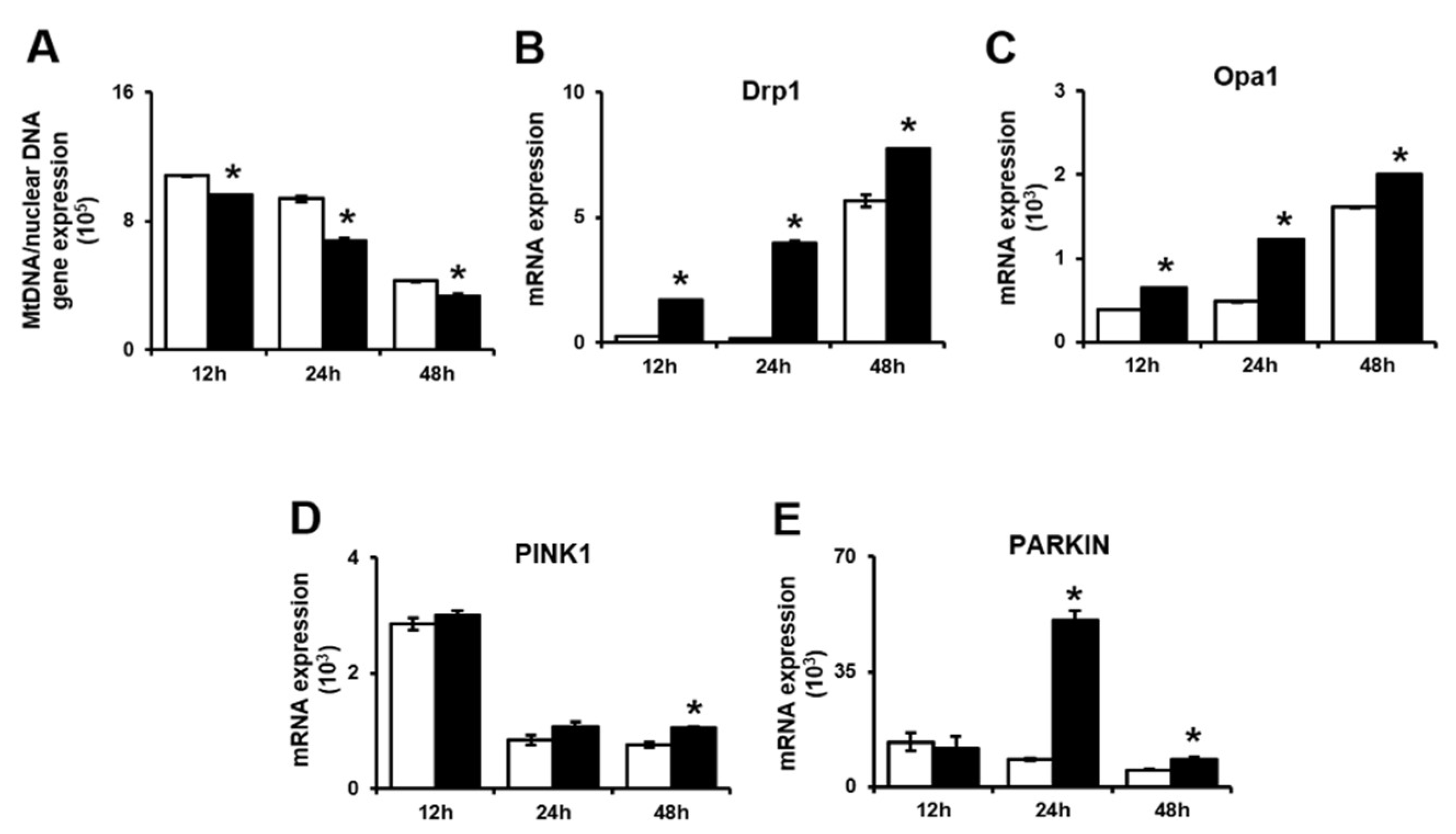
2.5. PD-MSCs Trigger Mitochondrial Autophagy in Invasive Trophoblasts
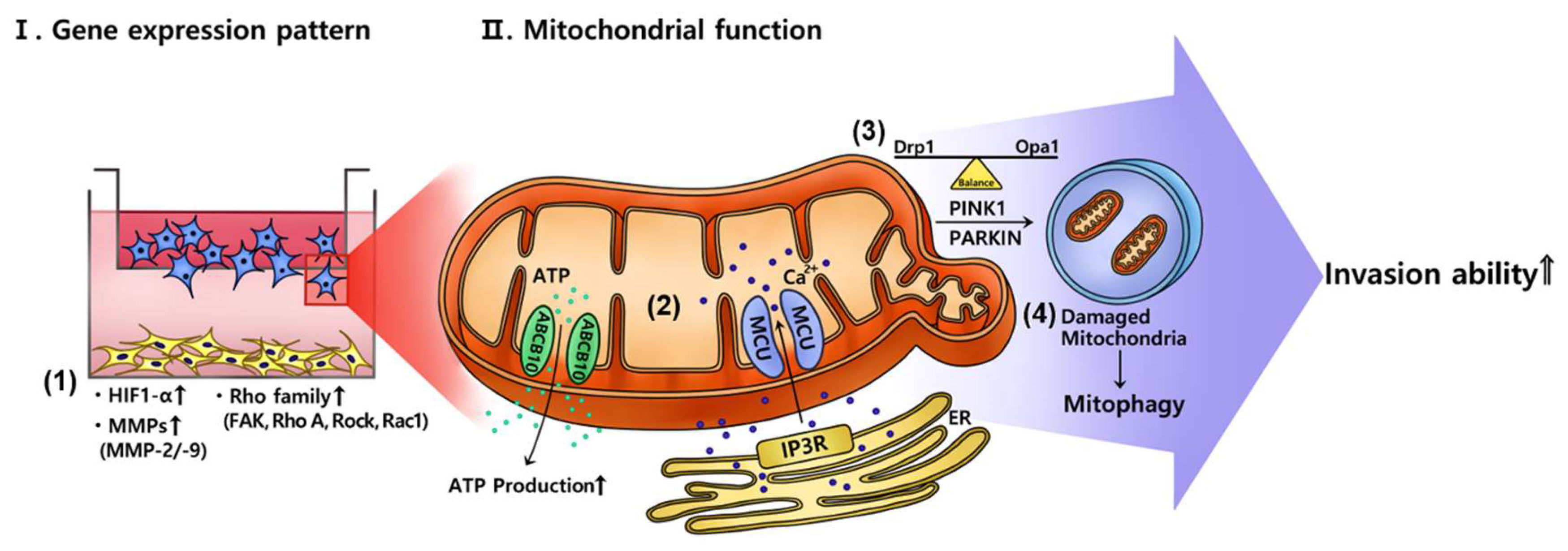
3. Discussion
4. Materials and Methods
4.1. HTR-8/SVneocells and Human Placenta-Derived Mesenchymal Stem Cells Culture
4.2. Quantitative Real-Time Polymerase Chain Reaction
4.3. gDNA Extraction and Mitochondrial DNA Copy Number Analysis
4.4. ELISA to Determine ATP Production
4.5. Invasion Assay
4.6. Seahorse XF24 Metabolic Flux Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABCB10 | ATP binding cassette sub-family 10 |
| BSA | Bovine serum albumin |
| ECAR | Extracellular acidification rate |
| ECM | Extracellular matrix |
| FAK | Focal adhesion kinase |
| hCG | Human chorionic gonadotropin |
| hFGF-4 | Human fibroblast growth factor 4 |
| HO | Heme oxygenase |
| HLA-G | Human leukocyte antigen G |
| HRP | Horseradish peroxidase |
| IP3R | Inositol triphosphate receptor |
| IUGR | Intrauterine growth restriction |
| MCU | Mitochondrial calcium uniporter |
| Mitophagy | Mitochondrial autophagy |
| MMPs | Matrix metallopeptidase |
| OCR | Oxygen consumption rate |
| PD-MSCs | Placenta-derived mesenchymal stem cells |
| PINK1 | PTEN-induced kinase 1 |
| PARKIN | 465-residue E3 ubiquitin ligase |
| PCR | Polymerase chain reaction |
| Rock | Rho-associated coiled-coil kinases |
References
- Kohan-Ghadr, H.-R.; Kadam, L.; Jain, C.; Armant, D.R.; Drewlo, S. Potential role of epigenetic mechanisms in regulation of trophoblast differentiation, migration, and invasion in the human placenta. Cell Adhes. Migr. 2016, 10, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Le, H.T.; Atif, J.; Mara, D.L.; Castellana, B.; Treissman, J.; Baltayeva, J.; Beristain, A.G. ADAM8 localizes to extravillous trophoblasts within the maternal-fetal interface and potentiates trophoblast cell line migration through a beta1 integrin-mediated mechanism. Mol. Hum. Reprod. 2018, 24, 495–509. [Google Scholar] [CrossRef]
- Renaud, S.J.; Kubota, K.; Rumi, M.A.K.; Soares, M.J. The FOS Transcription Factor Family Differentially Controls Trophoblast Migration and Invasion. J. Biol. Chem. 2013, 289, 5025–5039. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Wang, W.; Ao, L.; Wang, Z.; Hao, X.; Zhang, H. Benzo[a]pyrene-7,8-diol-9,10-epoxide suppresses the migration and invasion of human extravillous trophoblast HTR-8/SVneo cells by down-regulating MMP2 through inhibition of FAK/SRC/PI3K/AKT pathway. Toxicology 2017, 386, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, P.; Black, S.; Huppertz, B. Endovascular Trophoblast Invasion: Implications for the Pathogenesis of Intrauterine Growth Retardation and Preeclampsia. Biol. Reprod. 2003, 69, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Wu, Y.; Chang, X.; Li, Y.; Wang, K.; Duan, T. Effects of Human Umbilical Cord Mesenchymal Stem Cells on Human Trophoblast Cell Functions In Vitro. Stem Cells Int. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.H.; Jung, J.; Na, K.-H.; Cho, K.J.; Yoon, T.K.; Kim, G.J. Effect of Mesenchymal Stem Cells and Extracts Derived from the Placenta on Trophoblast Invasion and Immune Responses. Stem Cells Dev. 2014, 23, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Motawi, T.M.; Sabry, D.; Maurice, N.W.; Rizk, S.M. Role of mesenchymal stem cells exosomes derived microRNAs; miR-136, miR-494 and miR-495 in pre-eclampsia diagnosis and evaluation. Arch. Biochem. Biophys. 2018, 659, 13–21. [Google Scholar] [CrossRef]
- Yang, C.; Lim, W.; Park, J.; Park, S.; You, S.; Song, G. Anti-inflammatory effects of mesenchymal stem cell-derived exosomal microRNA-146a-5p and microRNA-548e-5p on human trophoblast cells. Mol. Hum. Reprod. 2019, 25, 755–771. [Google Scholar] [CrossRef]
- Blajszczak, C.; Bonini, M.G. Mitochondria targeting by environmental stressors: Implications for redox cellular signaling. Toxicology 2017, 391, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.; Kim, J.; Bae, J.-S. ROS homeostasis and metabolism: A critical liaison for cancer therapy. Exp. Mol. Med. 2016, 48, e269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zsengellér, Z.K.; Rajakumar, A.; Hunter, J.T.; Salahuddin, S.; Rana, S.; Stillman, I.E.; Karumanchi, S.A. Trophoblast mitochondrial function is impaired in preeclampsia and correlates negatively with the expression of soluble fms-like tyrosine kinase 1. Pregnancy Hypertens. 2016, 6, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Deng, Q.; Luo, X.; Chen, Y.; Shan, N.; Qi, H. Oxidative stress-induced Gadd45alpha inhibits trophoblast invasion and increases sFlt1/sEng secretions via p38 MAPK involving in the pathology of pre-eclampsia. J. Matern. Fetal Neonatal. Med. 2016, 29, 3776–3785. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.P.; Chen, C.Y.; Wu, Y.H.; Chen, C.Y. Oxidative stress reduces trophoblast FOXO1 and integrin beta3 expression that inhibits cell motility. Free Radic. Biol. Med. 2018, 124, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Pickles, S.; Vigié, P.; Youle, R.J. Mitophagy and Quality Control Mechanisms in Mitochondrial Maintenance. Curr. Biol. 2018, 28, R170–R185. [Google Scholar] [CrossRef]
- Wu, N.N.; Zhang, Y.; Ren, J. Mitophagy, Mitochondrial Dynamics, and Homeostasis in Cardiovascular Aging. Oxidative Med. Cell. Longev. 2019, 2019, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.J.; McIntyre, R.L.; Janssens, G.E.; Houtkooper, R.H. Mitochondrial fission and fusion: A dynamic role in aging and potential target for age-related disease. Mech. Ageing Dev. 2020, 186, 111212. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, B.; Li, T.; Zhu, Y.; Luo, G.; Jiang, Y.; Tang, F.; Jian, Z.; Xiao, Y. AMPK activation serves a critical role in mitochondria quality control via modulating mitophagy in the heart under chronic hypoxia. Int. J. Mol. Med. 2017, 41, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Chen, Q. Hypoxia Activation of Mitophagy and Its Role in Disease Pathogenesis. Antioxidants Redox Signal. 2015, 22, 1032–1046. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, X.; Zhang, F.; Wang, M.; Xu, Y.; Tang, D.; Wang, J.; Qin, Y.; Liu, Y.; Tang, C.; et al. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol. 2017, 11, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-C.; Liao, T.-L.; Wei, Y.-H.; Tzeng, C.-R.; Kao, S.-H. Endocrine disruptor, dioxin (TCDD)-induced mitochondrial dysfunction and apoptosis in human trophoblast-like JAR cells. Mol. Hum. Reprod. 2010, 16, 361–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Wang, R.; Zhang, Q.; Mor, G.; Zhang, H. Benzo(a)pyren-7,8-dihydrodiol-9,10-epoxide induces human trophoblast Swan 71 cell dysfunctions due to cell apoptosis through disorder of mitochondrial fission/fusion. Environ. Pollut. 2018, 233, 820–832. [Google Scholar] [CrossRef] [PubMed]
- Drapalo, K.; Jozwiak, J. Parkin, PINK1 and DJ1 as possible modulators of mTOR pathway in ganglioglioma. Int. J. Neurosci. 2017, 128, 167–174. [Google Scholar] [CrossRef]
- Vives-Bauza, C.; Zhou, C.; Huang, Y.; Cui, M.; De Vries, R.L.; Kim, J.; May, J.; Tocilescu, M.A.; Liu, W.; Ko, H.S.; et al. PINK1-dependent recruitment of Parkin to mitochondria in mitophagy. Proc. Natl. Acad. Sci. USA 2010, 107, 378–383. [Google Scholar] [CrossRef] [Green Version]
- Narendra, D.P.; Jin, S.M.; Tanaka, A.; Suen, D.-F.; Gautier, C.A.; Shen, J.; Cookson, M.R.; Youle, R.J. PINK1 Is Selectively Stabilized on Impaired Mitochondria to Activate Parkin. PLoS Biol. 2010, 8, e1000298. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Dorn, G.W. PINK1-Phosphorylated Mitofusin 2 Is a Parkin Receptor for Culling Damaged Mitochondria. Science 2013, 340, 471–475. [Google Scholar] [CrossRef] [Green Version]
- Nagao, A.; Kobayashi, M.; Koyasu, S.; Chow, C.C.T.; Harada, H. HIF-1-Dependent Reprogramming of Glucose Metabolic Pathway of Cancer Cells and Its Therapeutic Significance. Int. J. Mol. Sci. 2019, 20, 238. [Google Scholar] [CrossRef] [Green Version]
- Del Rey, M.J.; Valín, Á.; Usategui, A.; Blanco, F.J.; Sánchez-Aragó, M.; Cuezva, J.M.; Galindo, M.; Bravo, B.; Cañete, J.D.; Blanco, F.J.; et al. Hif-1α Knockdown Reduces Glycolytic Metabolism and Induces Cell Death of Human Synovial Fibroblasts Under Normoxic Conditions. Sci. Rep. 2017, 7, 3644. [Google Scholar] [CrossRef] [Green Version]
- Highet, A.R.; Khoda, S.M.; Buckberry, S.; Leemaqz, S.; Bianco-Miotto, T.; Harrington, E.; Ricciardelli, C.; Roberts, C.T. Hypoxia induced HIF-1/HIF-2 activity alters trophoblast transcriptional regulation and promotes invasion. Eur. J. Cell Biol. 2015, 94, 589–602. [Google Scholar] [CrossRef]
- Lee, H.-J.; Jung, J.; Cho, K.J.; Lee, C.K.; Hwang, S.-G.; Kim, G.J. Comparison of in vitro hepatogenic differentiation potential between various placenta-derived stem cells and other adult stem cells as an alternative source of functional hepatocytes. Differentiation 2012, 84, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.-F.; Zhong, Z.-N.; Fu, X.-F.; Peng, D.-X.; Lu, G.-H.; Li, W.-H.; Xu, H.-Y.; Hu, H.-B.; He, J.-M.; Su, W.-Y.; et al. Comparison of cell proliferation, apoptosis, cellular morphology and ultrastructure between human umbilical cord and placenta-derived mesenchymal stem cells. Neurosci. Lett. 2013, 541, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-P. Placental villous mesenchymal cells trigger trophoblast invasion. Cell Adhes. Migr. 2014, 8, 94–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunt, S.Y.; Heiden, M.G.V. Aerobic Glycolysis: Meeting the Metabolic Requirements of Cell Proliferation. Annu. Rev. Cell Dev. Biol. 2011, 27, 441–464. [Google Scholar] [CrossRef] [Green Version]
- Mookerjee, S.A.; Gerencser, A.A.; Nicholls, D.G.; Brand, M.D. Quantifying intracellular rates of glycolytic and oxidative ATP production and consumption using extracellular flux measurements. J. Biol. Chem. 2017, 292, 7189–7207. [Google Scholar] [CrossRef] [Green Version]
- Heiden, M.G.V.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [Green Version]
- Marchi, S.; Bittremieux, M.; Missiroli, S.; Morganti, C.; Patergnani, S.; Sbano, L.; Rimessi, A.; Kerkhofs, M.; Parys, J.B.; Bultynck, G.; et al. Endoplasmic Reticulum-Mitochondria Communication Through Ca2+ Signaling: The Importance of Mitochondria-Associated Membranes (MAMs). Adv. Exp. Med. Biol. 2017, 997, 49–67. [Google Scholar]
- Patron, M.; Raffaello, A.; Granatiero, V.; Tosatto, A.; Merli, G.; De Stefani, D.; Wright, L.; Pallafacchina, G.; Terrin, A.; Mammucari, C.; et al. The Mitochondrial Calcium Uniporter (MCU): Molecular Identity and Physiological Roles. J. Biol. Chem. 2013, 288, 10750–10758. [Google Scholar] [CrossRef] [Green Version]
- Tarasov, A.I.; Semplici, F.; Li, D.; Rizzuto, R.; Ravier, M.A.; Gilon, P.; Rutter, G.A. Frequency-dependent mitochondrial Ca(2+) accumulation regulates ATP synthesis in pancreatic beta cells. Pflugers Arch. 2013, 465, 543–554. [Google Scholar] [CrossRef] [Green Version]
- LeBleu, V.S.; O’Connell, J.T.; Herrera, K.N.G.; Wikman-Kocher, H.; Pantel, K.; Haigis, M.C.; De Carvalho, F.M.; Damascena, A.; Chinen, L.T.D.; Rocha, R.M.; et al. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat. Cell Biol. 2014, 16, 992–1003. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Lee, J.; Choi, C. Mitochondrial Network Determines Intracellular ROS Dynamics and Sensitivity to Oxidative Stress through Switching Inter-Mitochondrial Messengers. PLoS ONE 2011, 6, e23211. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, V.; Hay, N. Molecular Pathways: Reactive Oxygen Species Homeostasis in Cancer Cells and Implications for Cancer Therapy. Clin. Cancer Res. 2013, 19, 4309–4314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmad, W.; Ijaz, B.; Shabbiri, K.; Ahmed, F.; Rehman, S. Oxidative toxicity in diabetes and Alzheimer’s disease: Mechanisms behind ROS/ RNS generation. J. Biomed. Sci. 2017, 24, 1–10. [Google Scholar] [CrossRef]
- Hurd, T.R.; DeGennaro, M.; Lehmann, R. Redox regulation of cell migration and adhesion. Trends Cell Biol. 2012, 22, 107–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, K.; Uchida, T.; Yoshie, T.; Mizote, Y.; Ishikawa, F.; Katsuyama, M.; Shibanuma, M. A mitochondrial ROS pathway controls matrix metalloproteinase 9 levels and invasive properties in RAS-activated cancer cells. FEBS J. 2018, 286, 459–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parzych, K.R.; Klionsky, D.J. An Overview of Autophagy: Morphology, Mechanism, and Regulation. Antioxid. Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Qu, Y.; Zhao, F.; Li, H.; Mu, D.; Li, X. Regulation of autophagy by the nuclear factor kappaB signaling pathway in the hippocampus of rats with sepsis. J. Neuroinflamm. 2015, 12, 116. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Wang, G. Autophagy in Mitochondrial Quality Control. Atherosclerosis 2019, 1206, 421–434. [Google Scholar] [CrossRef]
- Heeman, B.; Haute, C.V.D.; Aelvoet, S.-A.; Valsecchi, F.; Rodenburg, R.J.; Reumers, V.; Debyser, Z.; Callewaert, G.; Koopman, W.J.H.; Willems, P.H.G.M.; et al. Depletion of PINK1 affects mitochondrial metabolism, calcium homeostasis and energy maintenance. J. Cell Sci. 2011, 124, 1115–1125. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.-J.; Jung, J.; Na, K.-H.; Moon, J.S.; Lee, H.-J.; Kim, J.; Kim, G.I.; Kwon, S.-W.; Hwang, S.-G. Anti-fibrotic effect of chorionic plate-derived mesenchymal stem cells isolated from human placenta in a rat model of CCl4-injured liver: Potential application to the treatment of hepatic diseases. J. Cell. Biochem. 2010, 111, 1453–1463. [Google Scholar] [CrossRef]
- Poidatz, D.; Dos Santos, E.; Gronier, H.; Vialard, F.; Maury, B.; De Mazancourt, P.; Dieudonne, M.N. Trophoblast syncytialisation necessitates mitochondrial function through estrogen-related receptor-gamma activation. Mol. Hum. Reprod. 2015, 21, 206–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| HIF1a | 5’-GTT TAC TAA AGG ACA AGT CA-3’ | 5’-TTC TGT TTG TTG AAG GGA G-3’ |
| FAK | 5’-GGA GCA TTG GGT CGG GAA CTA-3’ | 5’-CTC-AAT GCA GTT TGG AGG TGC-3’ |
| Rho A | 5’-TGG AAA GCA GGT AGA GTT GG-3’ | 5’-GAC TTC TGG GGT CCA CTT TT-3’ |
| ROCK1 | 5’-GAT CTT GTA GCT CCC GCA TCT GT-3’ | 5’-GAA GAA AGA GAA GCT CGA GA-3’ |
| Rac1 | 5’-TGA TGC AGG CCA TCA AGT GT-3’ | 5’-AGA ACA CAT CTG TTT GCG GAT AG-3’ |
| MMP2 | 5’-CGG CCG CAG TGA CGG AAA-3’ | 5’-CAT CCT GGG ACA GAC GGA AG-3’ |
| MMP9 | 5’-GAC GCA GAC ATC GTC ATC CAG TTT-3’ | 5’-GCC GCG CCA TCT GCG TTT-3’ |
| HO-1 | 5’-TGG TGA TGG CCT CCC TGT ACC ACA TCT-3’ | 5’-AGA GCT GGA TGT TGA GCA GGA ACG CAG TCT-3’ |
| HO-2 | 5’-ATG TCA GCG GAA GTG GAA-3’ | 5’-GGG AGT TTC AGT GCT CGC |
| SOD1 | 5’-ATG GCG ACG AAG GCC GTG TGC GTG CTG AAG-3’ | 5’-TGC CTC TCT TCA TCC TTT GGC-3’ |
| ABCB10 | 5’-ATG GGC GAT ATC TAC GGA AAC TGA-3’ | 5’-GGC GAG CTG GAT AGG CAA AAT-3’ |
| IP3R | 5’-CGG AGC AGG GTA TTG GAA GGC-3’ | 5’-GTC CAC TGA GGG CTG AAA CT-3’ |
| MCU | 5’-CGT TTC CAG TTG AGA GAT GGC-3’ | 5’-GAT CCT CTG GTG TAC CGT CC-3’ |
| Drp1 | 5’-CTG ACG CTT GTG GAT TTA CC-3’ | 5’-CCC TTC CCA TCA ATA CAT CG-3’ |
| Opa1 | 5’-GCA GGA TTC AGC AGA TAA-3’ | 5’-CTC TTC TTC ATA TTC TCT TAT TAG C-3’ |
| PINK1 | 5’-TGG AGG ATT TAA CCC AGG AG-3’ | 5’-TTA CCA ATG GAC TGC CCT ATC A-3’ |
| PARKIN | 5’-TGG AGG ATT TAA CCC AGG AG-3’ | 5’-ACA GGG CTT GGT GGT TTT CT-3’ |
| GAPDH | 5’-CTC CTC TTC GGC AGC ACA-3’ | 5’-AAC GCT TCA CCT AAT TTG CGT-3’ |
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| Mitochondrial DNA | 5’-CCA CTG TAA AGC TAA CTT AGC ATT AAC-3’ | 5’-GTG ATG AGG AAT AGT GTA AGG AGT ATG G-3’ |
| Nuclear DNA | 5’-CCA GAA AAT AAA TCA GAT GGT ATG TAA CA-3’ | 5’-TGG TTT AGG GTT GCT TCC-3’ |
| Mitochondrial DNA probe | 5’-JOE-CCA ACA CCT CTT TAC AGT GAA ATG CCC CA-BHQ1-3’ | |
| Nuclear DNA probe | 5’-JOE-CAG CAC TTC TTT TGA GCA CAC GGT CG-BHQ1-3’ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seok, J.; Jun, S.; Lee, J.O.; Kim, G.J. Mitochondrial Dynamics in Placenta-Derived Mesenchymal Stem Cells Regulate the Invasion Activity of Trophoblast. Int. J. Mol. Sci. 2020, 21, 8599. https://doi.org/10.3390/ijms21228599
Seok J, Jun S, Lee JO, Kim GJ. Mitochondrial Dynamics in Placenta-Derived Mesenchymal Stem Cells Regulate the Invasion Activity of Trophoblast. International Journal of Molecular Sciences. 2020; 21(22):8599. https://doi.org/10.3390/ijms21228599
Chicago/Turabian StyleSeok, Jin, Sujin Jun, Jung Ok Lee, and Gi Jin Kim. 2020. "Mitochondrial Dynamics in Placenta-Derived Mesenchymal Stem Cells Regulate the Invasion Activity of Trophoblast" International Journal of Molecular Sciences 21, no. 22: 8599. https://doi.org/10.3390/ijms21228599





