Immunohistochemistry and Mutation Analysis of SDHx Genes in Carotid Paragangliomas
Abstract
1. Introduction
2. Results
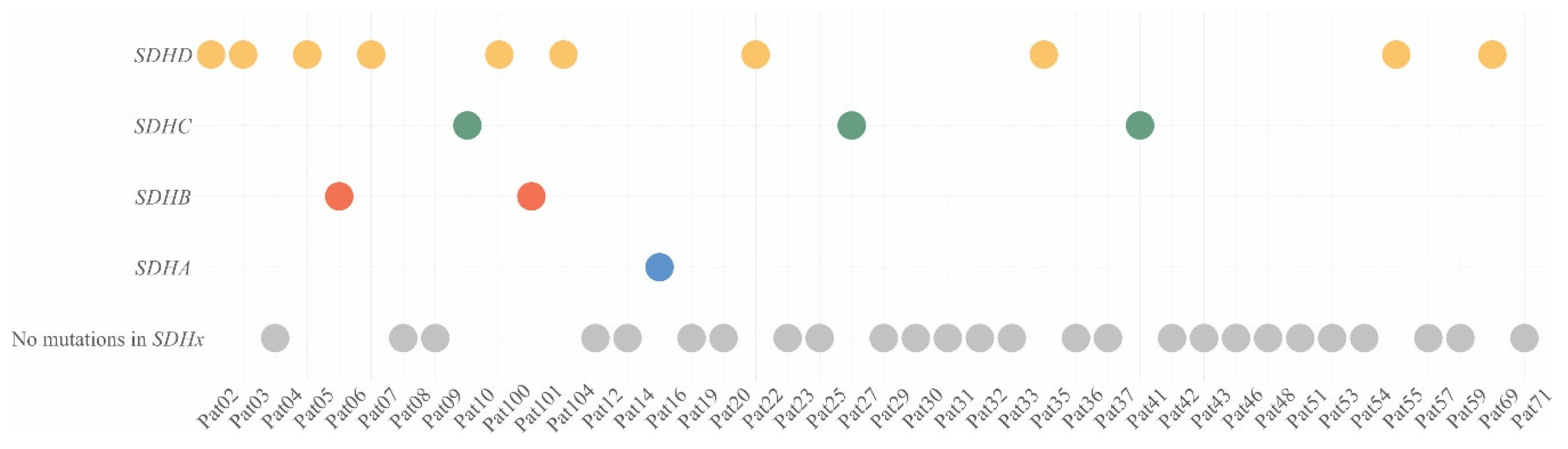
2.1. Pathogenic/Likely Pathogenic Variants of Susceptibility Genes in CPGLs
2.2. Correlation of SDHx Mutation Status with Their Immunostaining
2.3. Calculation of Diagnostic Accuracy
3. Discussion
4. Materials and Methods
4.1. Tumor Samples and Patients
4.2. DNA Extraction
4.3. Exome Sequencing
4.4. Immunohistochemistry
4.5. Correlation Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CPGL | carotid paraganglioma |
| SDHx | genes encoding for succinate dehydrogenase subunits |
| SDH | succinate dehydrogenase |
| IHC | immunohistochemistry |
| SFR | succinate to fumarate ratio |
References
- Prasad, S.C.; Paties, C.T.; Pantalone, M.R.; Mariani-Costantini, R.; Sanna, M. Carotid Body and Vagal Paragangliomas: Epidemiology, Genetics, Clinicopathological Features, Imaging, and Surgical Management. In Paraganglioma: A Multidisciplinary Approach; Codon Publications: Singapore, 2019; pp. 81–98. [Google Scholar]
- El-Naggar, A.K.; Chan, J.K.C.; Grandis, J.R.; Takata, T.; Slootweg, P.J. WHO Classification of Head and Neck Tumours; International Agency for Research on Cancer: Lyon, France, 2017. [Google Scholar]
- Myssiorek, D.; Persky, M.S.; Information, P.E.K.F.C. Treatment of carotid paraganglioma. Oper. Tech. Otolaryngol. Neck Surg. 2016, 27, 30–35. [Google Scholar] [CrossRef]
- Patel, S.R.; Winchester, D.J.; Benjamin, R.S. A 15-year experience with chemotherapy of patients with paraganglioma. Cancer 1995, 76, 1476–1480. [Google Scholar] [CrossRef]
- Lalya, I.; Mechchat, A.; Kebdani, T.; Hassouni, K.; Kanouni, L.; Andaloussi, K.; Elmarjani, M.; Hadadi, K.; Sifat, H.; Mansouri, H.; et al. Efficacité de la radiothérapie en première intention d’un paragangliome carotidien non résécable. J. des Mal. Vasc. 2011, 36, 185–188. [Google Scholar] [CrossRef]
- Kiziltan, H.S.; Ozucer, B.; Eris, A.H.; Veyseller, B. Bilateral Carotid Paraganglioma: Surgery and Radiotherapy. Clin. Med. Insights Case Rep. 2014, 7, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Boedeker, C.C. Paragangliomas and paraganglioma syndromes. GMS Curr. Top Otorhinolaryngol. Head Neck Surg. 2012, 10. [Google Scholar] [CrossRef]
- Udager, A.M.; Magers, M.J.; Goerke, D.M.; Vinco, M.L.; Siddiqui, J.; Cao, X.; Lucas, D.R.; Myers, J.L.; Chinnaiyan, A.M.; McHugh, J.B.; et al. The utility of SDHB and FH immunohistochemistry in patients evaluated for hereditary paraganglioma-pheochromocytoma syndromes. Hum. Pathol. 2018, 71, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.-X.; Khalimonchuk, O.; Schraders, M.; Dephoure, N.; Bayley, J.-P.; Kunst, H.; Devilee, P.; Cremers, C.W.R.J.; Schiffman, J.D.; Bentz, B.G.; et al. SDH5, a Gene Required for Flavination of Succinate Dehydrogenase, Is Mutated in Paraganglioma. Science 2009, 325, 1139–1142. [Google Scholar] [CrossRef] [PubMed]
- Bausch, B.; Schiavi, F.; Ni, Y.; Welander, J.; Patocs, A.; Ngeow, J.; Wellner, U.; Malinoc, A.; Taschin, E.; Barbon, G.; et al. Clinical Characterization of the Pheochromocytoma and Paraganglioma Susceptibility Genes SDHA, TMEM127, MAX, and SDHAF2 for Gene-Informed Prevention. JAMA Oncol. 2017, 3, 1204–1212. [Google Scholar] [CrossRef]
- Burnichon, N.; Cascón, A.; Schiavi, F.; Morales, N.P.; Comino-Méndez, I.; Abermil, N.; Inglada-Pérez, L.; De Cubas, A.A.; Amar, L.; Barontini, M.; et al. MAX Mutations Cause Hereditary and Sporadic Pheochromocytoma and Paraganglioma. Clin. Cancer Res. 2012, 18, 2828–2837. [Google Scholar] [CrossRef]
- Korpershoek, E.; Favier, J.; Gaal, J.; Burnichon, N.; Van Gessel, B.; Oudijk, L.; Badoual, C.; Gadessaud, N.; Venisse, A.; Bayley, J.-P.; et al. SDHA Immunohistochemistry Detects Germline SDHA Gene Mutations in Apparently Sporadic Paragangliomas and Pheochromocytomas. J. Clin. Endocrinol. Metab. 2011, 96, 1472–1476. [Google Scholar] [CrossRef] [PubMed]
- Schiavi, F.; Savvoukidis, T.; Trabalzini, F.; Grego, F.; Piazza, M.; Amistà, P.; Demattè, S.; Piano, A.D.; Cecchini, M.E.; Erlic, Z.; et al. Paraganglioma Syndrome: SDHB, SDHC, and SDHD Mutations in Head and Neck Paragangliomas. Ann. N. Y. Acad. Sci. 2006, 1073, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Hermsen, M.; Sevilla, M.A.; Llorente, J.L.; Weiss, M.M.; Grimbergen, A.; Allonca, E.; Garcia-Inclán, C.; Balbín, M.; Suárez, C. Relevance of Germline Mutation Screening in Both Familial and Sporadic Head and Neck Paraganglioma for Early Diagnosis and Clinical Management. Cell. Oncol. 2010, 32, 275–283. [Google Scholar] [PubMed]
- Ding, Y.; Feng, Y.; Wells, M.; Huang, Z.; Chen, X. SDHx gene detection and clinical Phenotypic analysis of multiple paraganglioma in the head and neck. Laryngoscope 2018, 129, E67–E71. [Google Scholar] [CrossRef] [PubMed]
- Gaal, J.; Burnichon, N.; Korpershoek, E.; Roncelin, I.; Bertherat, J.; Plouin, P.-F.; De Krijger, R.R.; Gimenez-Roqueplo, A.-P.; Dinjens, W.N.M. Isocitrate Dehydrogenase Mutations Are Rare in Pheochromocytomas and Paragangliomas. J. Clin. Endocrinol. Metab. 2010, 95, 1274–1278. [Google Scholar] [CrossRef]
- Ercolino, T.; Becherini, L.; Valeri, A.; Maiello, M.; Gaglianò, M.S.; Parenti, G.; Ramazzotti, M.; Piscitelli, E.; Simi, L.; Pinzani, P.; et al. Uncommon clinical presentations of pheochromocytoma and paraganglioma in two different patients affected by two distinct novel VHL germline mutations. Clin. Endocrinol. 2008, 68, 762–768. [Google Scholar] [CrossRef]
- Richter, S.; Gieldon, L.; Pang, Y.; Peitzsch, M.; Huynh, T.; Leton, R.; Viana, B.; Ercolino, T.; Mangelis, A.; Rapizzi, E.; et al. Metabolome-guided genomics to identify pathogenic variants in isocitrate dehydrogenase, fumarate hydratase, and succinate dehydrogenase genes in pheochromocytoma and paraganglioma. Genet. Med. 2018, 21, 705–717. [Google Scholar] [CrossRef]
- Merlo, A.; De Quiros, S.B.; De Santa-María, I.S.; Pitiot, A.S.; Balbin, M.; Astudillo, A.; Scola, B.; Arístegui, M.; Quer, M.; Suárez, C.; et al. Identification of Somatic VHL Gene Mutations in Sporadic Head and Neck Paragangliomas in Association With Activation of the HIF-1?/miR-210 Signaling Pathway. J. Clin. Endocrinol. Metab. 2013, 98, 1661–1666. [Google Scholar] [CrossRef]
- Castro-Vega, L.J.; Buffet, A.; De Cubas, A.A.; Cascón, A.; Menara, M.; Khalifa, E.; Amar, L.; Azriel, S.; Bourdeau, I.; Chabre, O.; et al. Germline mutations in FH confer predisposition to malignant pheochromocytomas and paragangliomas. Hum. Mol. Genet. 2013, 23, 2440–2446. [Google Scholar] [CrossRef]
- Buffet, A.; Morin, A.; Castro-Vega, L.-J.; Habarou, F.; Lussey-Lepoutre, C.; Letouzé, E.; Lefebvre, H.; Guilhem, I.; Haissaguerre, M.; Raingeard, I.; et al. Germline Mutations in the Mitochondrial 2-Oxoglutarate/Malate Carrier SLC25A11 Gene Confer a Predisposition to Metastatic Paragangliomas. Cancer Res. 2018, 78, 1914–1922. [Google Scholar] [CrossRef]
- Van Nederveen, F.H.; Gaal, J.; Favier, J.; Korpershoek, E.; Oldenburg, R.A.; A De Bruyn, E.M.C.; Sleddens, H.F.B.M.; Derkx, P.; Rivière, J.; Dannenberg, H.; et al. An immunohistochemical procedure to detect patients with paraganglioma and phaeochromocytoma with germline SDHB, SDHC, or SDHD gene mutations: A retrospective and prospective analysis. Lancet Oncol. 2009, 10, 764–771. [Google Scholar] [CrossRef]
- Gill, A.J. Succinate dehydrogenase (SDH)-deficient neoplasia. Histopathology 2017, 72, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Papathomas, T.G.; Oudijk, L.; Persu, A.; Gill, A.J.; Van Nederveen, F.; Tischler, A.S.; Tissier, F.; Volante, M.; Matias-Guiu, X.; Smid, M.; et al. SDHB/SDHA immunohistochemistry in pheochromocytomas and paragangliomas: A multicenter interobserver variation analysis using virtual microscopy: A Multinational Study of the European Network for the Study of Adrenal Tumors (ENS@T). Mod. Pathol. 2015, 28, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Wallace, P.W.; Conrad, C.; Brückmann, S.; Pang, Y.; Caleiras, E.; Murakami, M.; Korpershoek, E.; Zhuang, Z.; Rapizzi, E.; Kroiss, M.; et al. Metabolomics, machine learning and immunohistochemistry to predict succinate dehydrogenase mutational status in phaeochromocytomas and paragangliomas. J. Pathol. 2020, 251, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Landrum, M.; Lee, J.M.; Benson, M.; Brown, G.R.; Chao, C.; Chitipiralla, S.; Gu, B.; Hart, J.; Hoffman, U.; Jang, W.; et al. ClinVar: Improving access to variant interpretations and supporting evidence. Nucleic Acids Res. 2017, 46, D1062–D1067. [Google Scholar] [CrossRef]
- Sherry, S.T.; Ward, M.-H.; Kholodov, M.; Baker, J.; Phan, L.; Smigielski, E.M.; Sirotkin, K. dbSNP: The NCBI database of genetic variation. Nucleic Acids Res. 2001, 29, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Tate, J.G.; Bamford, S.; Jubb, H.C.; Sondka, Z.; Beare, D.M.; Bindal, N.; Boutselakis, H.; Cole, C.G.; Creatore, C.; Dawson, E.; et al. COSMIC: The Catalogue Of Somatic Mutations In Cancer. Nucleic Acids Res. 2018, 47, D941–D947. [Google Scholar] [CrossRef]
- Vaser, R.; Adusumalli, S.; Leng, S.N.; Sikic, M.; Ng, P.C. SIFT missense predictions for genomes. Nat. Protoc. 2015, 11, 1–9. [Google Scholar] [CrossRef]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A method and server for predicting damaging missense mutations. Nat Methods 2010, 7, 248–249. [Google Scholar] [CrossRef]
- Schwarz, J.M.; Cooper, D.N.; Schuelke, M.; Seelow, D. MutationTaster2: Mutation prediction for the deep-sequencing age. Nat. Methods 2014, 11, 361–362. [Google Scholar] [CrossRef]
- Chun, S.; Fay, J.C. Identification of deleterious mutations within three human genomes. Genome Res. 2009, 19, 1553–1561. [Google Scholar] [CrossRef]
- Siepel, A.; Bejerano, G.; Pedersen, J.S.; Hinrichs, A.S.; Hou, M.; Rosenbloom, K.; Clawson, H.; Spieth, J.; Hillier, L.W.; Richards, S.; et al. Evolutionarily conserved elements in vertebrate, insect, worm, and yeast genomes. Genome Res. 2005, 15, 1034–1050. [Google Scholar] [CrossRef] [PubMed]
- Pollard, K.S.; Hubisz, M.J.; Rosenbloom, K.R.; Siepel, A. Detection of nonneutral substitution rates on mammalian phylogenies. Genome Res. 2009, 20, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; on behalf of the ACMG Laboratory Quality Assurance Committee; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–423. [Google Scholar] [CrossRef] [PubMed]
- Heiden, M.G.V.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Sci. 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Krasnov, G.S.; Dmitriev, A.A.; Snezhkina, A.V.; Kudryavtseva, A.V. Deregulation of glycolysis in cancer: Glyceraldehyde-3-phosphate dehydrogenase as a therapeutic target. Expert Opin. Ther. Targets 2013, 17, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtseva, A.V.; Krasnov, G.S.; Dmitriev, A.A.; Alekseev, B.Y.; Kardymon, O.; Sadritdinova, A.F.; Fedorova, M.S.; Pokrovsky, A.V.; Melnikova, N.V.; Kaprin, A.D.; et al. Mitochondrial dysfunction and oxidative stress in aging and cancer. Oncotarget 2016, 7, 44879–44905. [Google Scholar] [CrossRef]
- Senyilmaz, D.; Teleman, A.A. Chicken or the egg: Warburg effect and mitochondrial dysfunction. F1000Prime Rep. 2015, 7, 41. [Google Scholar] [CrossRef]
- Zhikrivetskaya, S.O.; Snezhkina, A.V.; Zaretsky, A.R.; Alekseev, B.Y.; Pokrovsky, A.V.; Golovyuk, A.L.; Melnikova, N.V.; Stepanov, O.A.; Kalinin, D.V.; Moskalev, A.; et al. Molecular markers of paragangliomas/pheochromocytomas. Oncotarget 2017, 8, 25756–25782. [Google Scholar] [CrossRef]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxidative Med. Cell. Longev. 2019, 2019, 6175804. [Google Scholar] [CrossRef]
- Aldera, A.P.; Govender, D. Gene of the month: SDH. J. Clin. Pathol. 2017, 71, 95–97. [Google Scholar] [CrossRef]
- Rana, H.; Rainville, I.R.; Vaidya, A. Genetic testing in the clinical care of patients with pheochromocytoma and paraganglioma. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Tischler, A.S. Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Paragangliomas. Head Neck Pathol. 2017, 11, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Pasini, B.; Stratakis, C.A.; Pasini, B. SDH mutations in tumorigenesis and inherited endocrine tumours: Lesson from the phaeochromocytoma-paraganglioma syndromes. J. Intern. Med. 2009, 266, 19–42. [Google Scholar] [CrossRef] [PubMed]
- Schiavi, F.; Boedeker, C.C.; Bausch, B.; Peczkowska, M.; Gomez, C.F.; Strassburg, T.; Pawlu, C.; Buchta, M.; Salzmann, M.; Hoffmann, M.M.; et al. Predictors and Prevalence of Paraganglioma Syndrome Associated With Mutations of the SDHC Gene. JAMA 2005, 294, 2057. [Google Scholar] [CrossRef]
- Gottlieb, E.; Tomlinson, I.P.M. Mitochondrial tumour suppressors: A genetic and biochemical update. Nat. Rev. Cancer 2005, 5, 857–866. [Google Scholar] [CrossRef]
- Gimenez-Roqueplo, A.-P.; Favier, J.; Rustin, P.; Mourad, J.-J.; Plouin, P.-F.; Corvol, P.; Rötig, A.; Jeunemaître, X. The R22X Mutation of the SDHD Gene in Hereditary Paraganglioma Abolishes the Enzymatic Activity of Complex II in the Mitochondrial Respiratory Chain and Activates the Hypoxia Pathway. Am. J. Hum. Genet. 2001, 69, 1186–1197. [Google Scholar] [CrossRef]
- Dekker, P.D.; Hogendoorn, P.C.; Kuipers-Dijkshoorn, N.; Prins, F.; Van Duinen, S.; Taschner, P.E.; Van Der Mey, A.; Cornelisse, C.J. SDHD mutations in head and neck paragangliomas result in destabilization of complex II in the mitochondrial respiratory chain with loss of enzymatic activity and abnormal mitochondrial morphology. J. Pathol. 2003, 201, 480–486. [Google Scholar] [CrossRef]
- Santi, R.; Rapizzi, E.; Canu, L.; Ercolino, T.; Baroni, G.; Fucci, R.; Costa, G.; Mannelli, M.; Nesi, G. Potential Pitfalls of SDH Immunohistochemical Detection in Paragangliomas and Phaeochromocytomas Harbouring Germline SDHx Gene Mutation. Anticancer Res. 2017, 37, 805–812. [Google Scholar] [CrossRef]
- Castelblanco, E.; Santacana, M.; Valls, J.; De Cubas, A.; Cascón, A.; Robledo, M.; Matias-Guiu, X. Usefulness of Negative and Weak–Diffuse Pattern of SDHB Immunostaining in Assessment of SDH Mutations in Paragangliomas and Pheochromocytomas. Endocr. Pathol. 2013, 24, 199–205. [Google Scholar] [CrossRef]
- Gill, A.J.; Benn, D.E.; Chou, A.; Clarkson, A.; Muljono, A.; Meyer-Rochow, G.Y.; Richardson, A.L.; Sidhu, S.B.; Robinson, B.G.; Clifton-Bligh, R. Immunohistochemistry for SDHB triages genetic testing of SDHB, SDHC, and SDHD in paraganglioma-pheochromocytoma syndromes. Hum. Pathol. 2010, 41, 805–814. [Google Scholar] [CrossRef]
- Pavlov, V.S.; Kalinin, D.V.; Lukyanova, E.N.; Golovyuk, A.L.; Fedorova, M.S.; Pudova, E.A.; Savvateeva, M.V.; Lipatova, A.V.; Guvatova, Z.G.; Kaprin, A.D.; et al. Multiple paragangliomas: A case report. BMC Med Genom. 2020, 13, 125. [Google Scholar] [CrossRef] [PubMed]
- Burnichon, N.; Brière, J.-J.; Libé, R.; Vescovo, L.; Rivière, J.; Tissier, F.; Jouanno, E.; Jeunemaitre, X.; Bénit, P.; Tzagoloff, A.; et al. SDHA is a tumor suppressor gene causing paraganglioma. Hum. Mol. Genet. 2010, 19, 3011–3020. [Google Scholar] [CrossRef] [PubMed]
- Dwight, T.; Mann, K.; Benn, D.E.; Robinson, B.G.; McKelvie, P.; Gill, A.J.; Winship, I.; Clifton-Bligh, R. FamilialSDHAMutation Associated With Pituitary Adenoma and Pheochromocytoma/Paraganglioma. J. Clin. Endocrinol. Metab. 2013, 98, E1103–E1108. [Google Scholar] [CrossRef] [PubMed]
- Snezhkina, A.V.; Lukyanova, E.; Kalinin, D.V.; Pokrovsky, A.V.; Dmitriev, A.A.; Koroban, N.V.; Pudova, E.A.; Fedorova, M.S.; Volchenko, N.N.; Stepanov, O.A.; et al. Exome analysis of carotid body tumor. BMC Med Genom. 2018, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtseva, A.V.; Lukyanova, E.; Kalinin, D.V.; Zaretsky, A.R.; Pokrovsky, A.V.; Golovyuk, A.L.; Fedorova, M.S.; Pudova, E.A.; Kharitonov, S.L.; Pavlov, V.S.; et al. Mutational load in carotid body tumor. BMC Med Genom. 2019, 12, 39. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate long-read alignment with Burrows–Wheeler transform. Bioinformatics 2010, 26, 589–595. [Google Scholar] [CrossRef]
- Li, H.; Handsaker, B.; Wysoker, A.; Fennell, T.; Ruan, J.; Homer, N.; Marth, G.; Abecasis, G.; Durbin, R. The Sequence Alignment/Map format and SAMtools. Bioinformatics 2009, 25, 2078–2079. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010, 38, e164. [Google Scholar] [CrossRef]
- The 1000 Genomes Project Consortium A global reference for human genetic variation. Nature 2015, 526, 68–74. [CrossRef]
- Karczewski, K.; Weisburd, B.; Thomas, B.; Solomonson, M.; Ruderfer, D.M.; Kavanagh, D.; Hamamsy, T.; Lek, M.; Samocha, K.E.; Cummings, B.B.; et al. The ExAC browser: Displaying reference data information from over 60,000 exomes. Nucleic Acids Res. 2016, 45, D840–D845. [Google Scholar] [CrossRef] [PubMed]
- Karczewski, K.; Francioli, L.C.; Tiao, G.; Cummings, B.B.; Alföldi, J.; Wang, Q.; Collins, R.L.; Laricchia, K.M.; Ganna, A.; Birnbaum, D.P.; et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 2020, 581, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Glusman, G.; Caballero, J.; Mauldin, D.E.; Hood, L.; Roach, J.C. Kaviar: An accessible system for testing SNV novelty. Bioinformatics 2011, 27, 3216–3217. [Google Scholar] [CrossRef]
- Mitchell, A.; Attwood, T.K.; Babbitt, P.C.; Blum, M.; Bork, P.; Bridge, A.; Brown, S.D.; Chang, H.Y.; El-Gebali, S.; Fraser, M.I.; et al. InterPro in 2019: Improving coverage, classification and access to protein sequence annotations. Nucleic Acids Res. 2018, 47, D351–D360. [Google Scholar] [CrossRef]

| Pat | Gene | dbSNP ID | GeneBank | Pos | NC Change | AA Change | ClinSig | |
|---|---|---|---|---|---|---|---|---|
| Pat16 | SDHA | rs1061517;239661 | NM_004168 | chr5: 218471 | c.1A > G | p.Met1? | P/LP | |
| Pat06 | SDHB | rs727503415;165180 | NM_003000 | chr1: 17359564 | c.277T > C | p.Cys93Arg | P | |
| Pat101 | rs74315370;142763 | chr1: 17371320 | c.136C > T | p.Arg46* | P | |||
| Pat10 | SDHC | - | NM_003001 | chr1: 161310387 | c.183G > A | p.Trp61* | LP* | |
| Pat27 | - | chr1: 161332121 | c.409delT | p.Trp137fs | LP* | |||
| Pat41 | rs786205147;189841 | chr1: 161310428 | c.224G > A | p.Gly75Asp | LP | |||
| Pat05 Pat22 Pat100 Pat104 | SDHD | rs104894302 | NM_003002 | chr11: 111959726 | c.305A > G | p.His102Arg | LP | |
| Pat02 | rs80338843;6893 | chr11: 111958640 | c.112C > T | p.Arg38* | P | |||
| Pat03 | - | chr11: 111957643 | c.13dupT | p.Trp5fs | LP* | |||
| Pat07 | rs104894307;6911 | chr11: 111957632 | c.1A > G | p.Met1? | P | |||
| Pat35 | - | chr11: 111959626 | c.205G > T | p.Glu69* | P* | |||
| Pat55 | - | chr11: 111965547 | c.335_338del | p.Thr112fs | LP* | |||
| Pat69 | - | chr11: 111959637 | c.217dupA | p.Ser33fs | LP* | |||
| Pat31 | IDH1 | rs121913499 | NM_005896 | chr2: 209113113 | c.394C > T | p.Arg132 Cys | LP | |
| Pat16 Pat35 | RET | rs77724903;13936 | NM_020975 | chr10: 43613908 | c.2372A > T | p.Tyr791Phe | P | |
| Pat27 | rs17158558 | chr10: 43620335 | c.2944C > T | p.Arg982Cys | P |
| Characteristic | Number of patients, n |
|---|---|
| Total number | 42 |
| Age, yr | |
| <40 | 15 |
| ≥40 | 27 |
| Sex | |
| Female | 28 |
| Male | 14 |
| Multifocal tumor | 2 |
| Bilateral CPGL | 1 |
| Recurrent patients | 1 |
| Familial history | |
| Positive | 1 |
| Negative | 0 |
| N/A | 41 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snezhkina, A.V.; Kalinin, D.V.; Pavlov, V.S.; Lukyanova, E.N.; Golovyuk, A.L.; Fedorova, M.S.; Pudova, E.A.; Savvateeva, M.V.; Stepanov, O.A.; Poloznikov, A.A.; et al. Immunohistochemistry and Mutation Analysis of SDHx Genes in Carotid Paragangliomas. Int. J. Mol. Sci. 2020, 21, 6950. https://doi.org/10.3390/ijms21186950
Snezhkina AV, Kalinin DV, Pavlov VS, Lukyanova EN, Golovyuk AL, Fedorova MS, Pudova EA, Savvateeva MV, Stepanov OA, Poloznikov AA, et al. Immunohistochemistry and Mutation Analysis of SDHx Genes in Carotid Paragangliomas. International Journal of Molecular Sciences. 2020; 21(18):6950. https://doi.org/10.3390/ijms21186950
Chicago/Turabian StyleSnezhkina, Anastasiya V., Dmitry V. Kalinin, Vladislav S. Pavlov, Elena N. Lukyanova, Alexander L. Golovyuk, Maria S. Fedorova, Elena A. Pudova, Maria V. Savvateeva, Oleg A. Stepanov, Andrey A. Poloznikov, and et al. 2020. "Immunohistochemistry and Mutation Analysis of SDHx Genes in Carotid Paragangliomas" International Journal of Molecular Sciences 21, no. 18: 6950. https://doi.org/10.3390/ijms21186950
APA StyleSnezhkina, A. V., Kalinin, D. V., Pavlov, V. S., Lukyanova, E. N., Golovyuk, A. L., Fedorova, M. S., Pudova, E. A., Savvateeva, M. V., Stepanov, O. A., Poloznikov, A. A., Demidova, T. B., Melnikova, N. V., Dmitriev, A. A., Krasnov, G. S., & Kudryavtseva, A. V. (2020). Immunohistochemistry and Mutation Analysis of SDHx Genes in Carotid Paragangliomas. International Journal of Molecular Sciences, 21(18), 6950. https://doi.org/10.3390/ijms21186950










