Involvement of Capsaicin-Sensitive Lung Vagal Neurons and TRPA1 Receptors in Airway Hypersensitivity Induced by 1,3-β-D-Glucan in Anesthetized Rats
Abstract
:1. Introduction
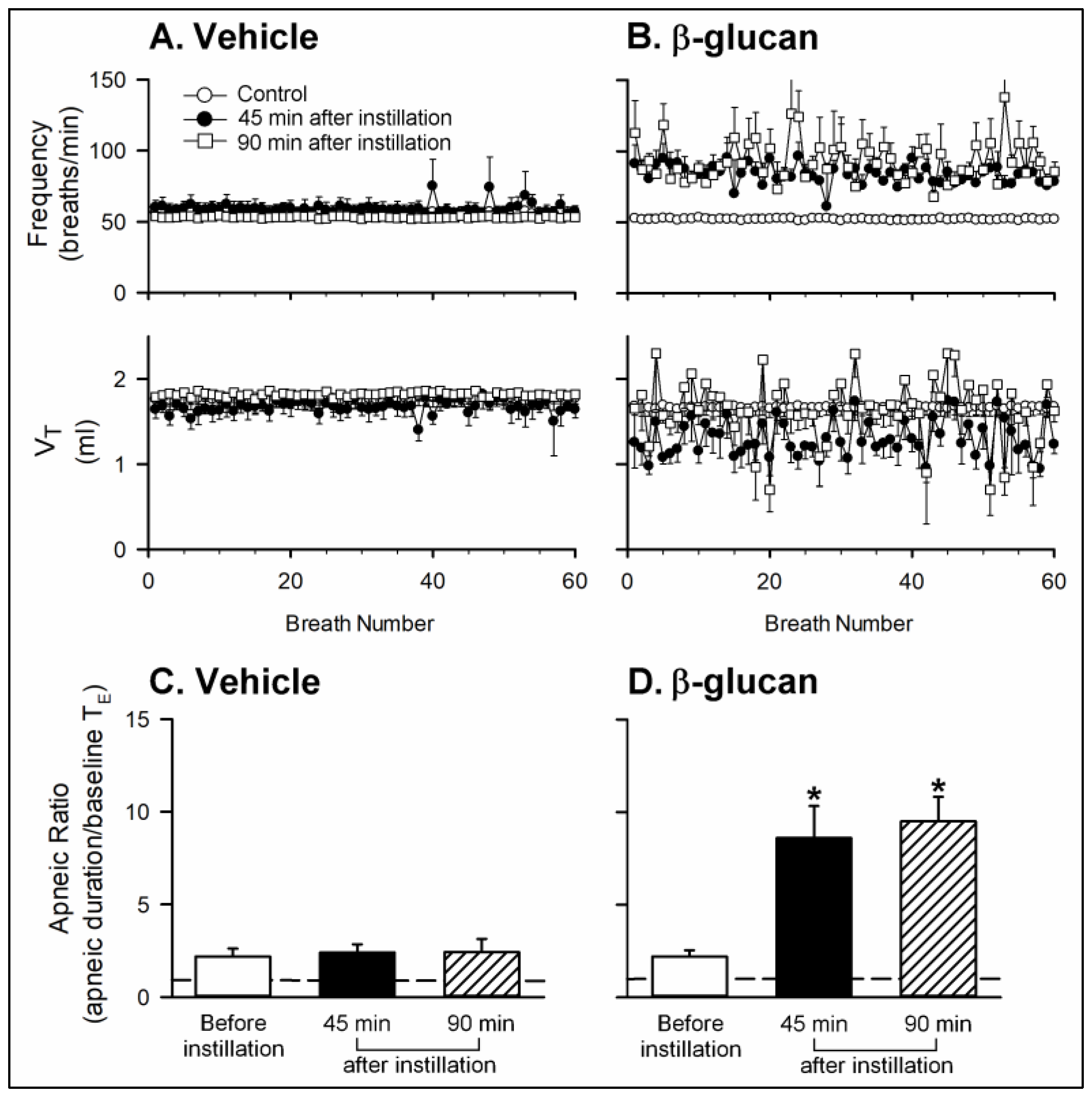
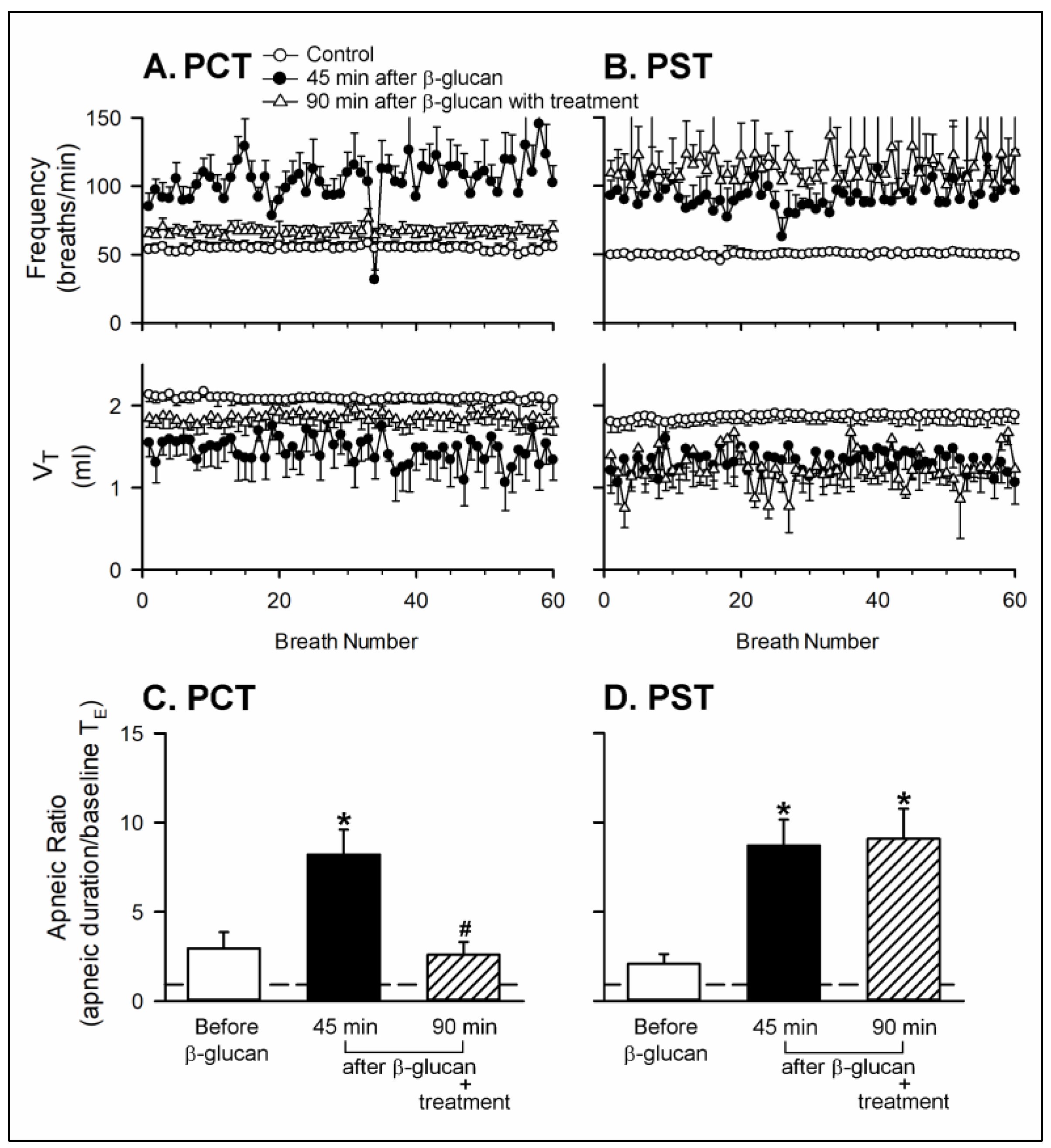
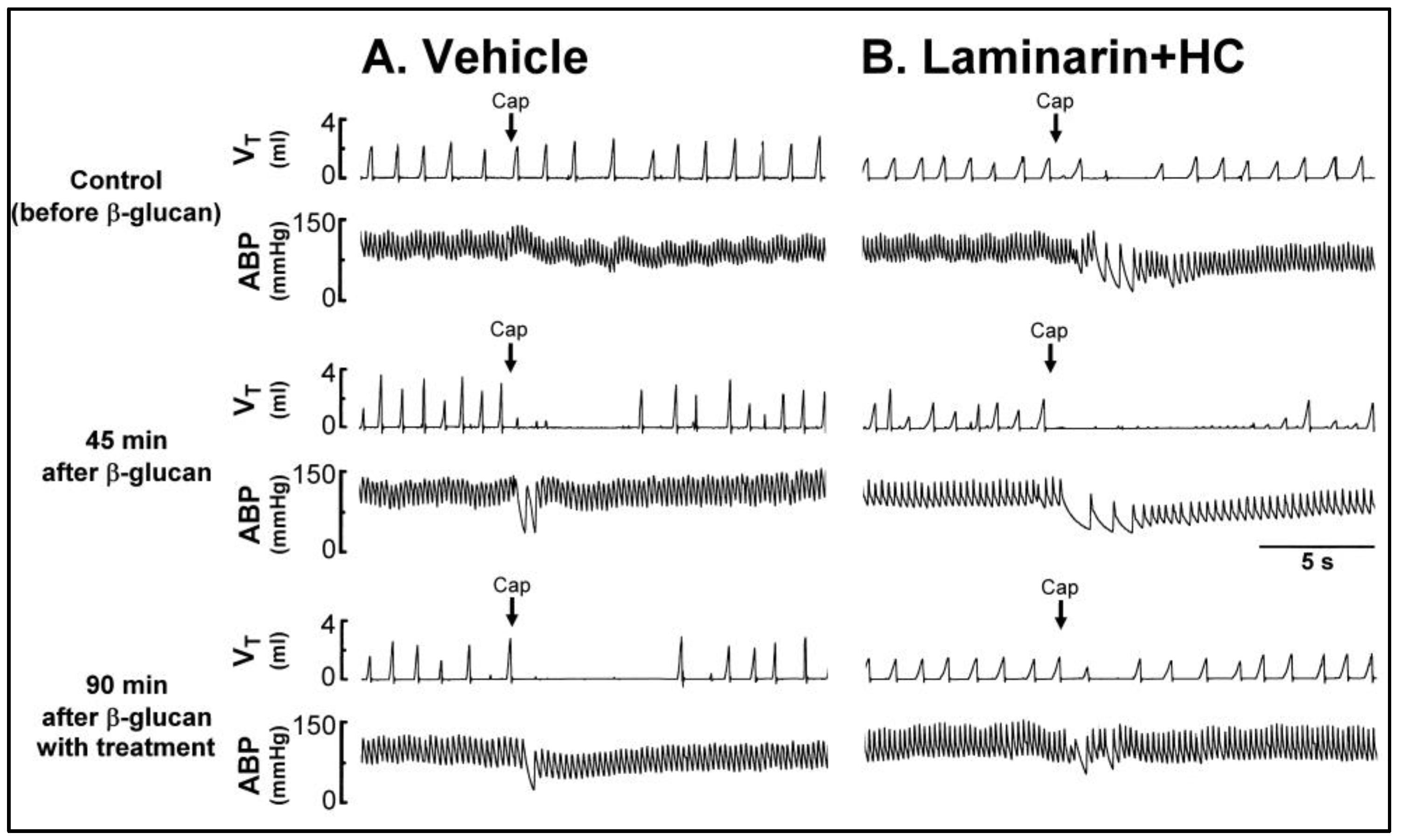
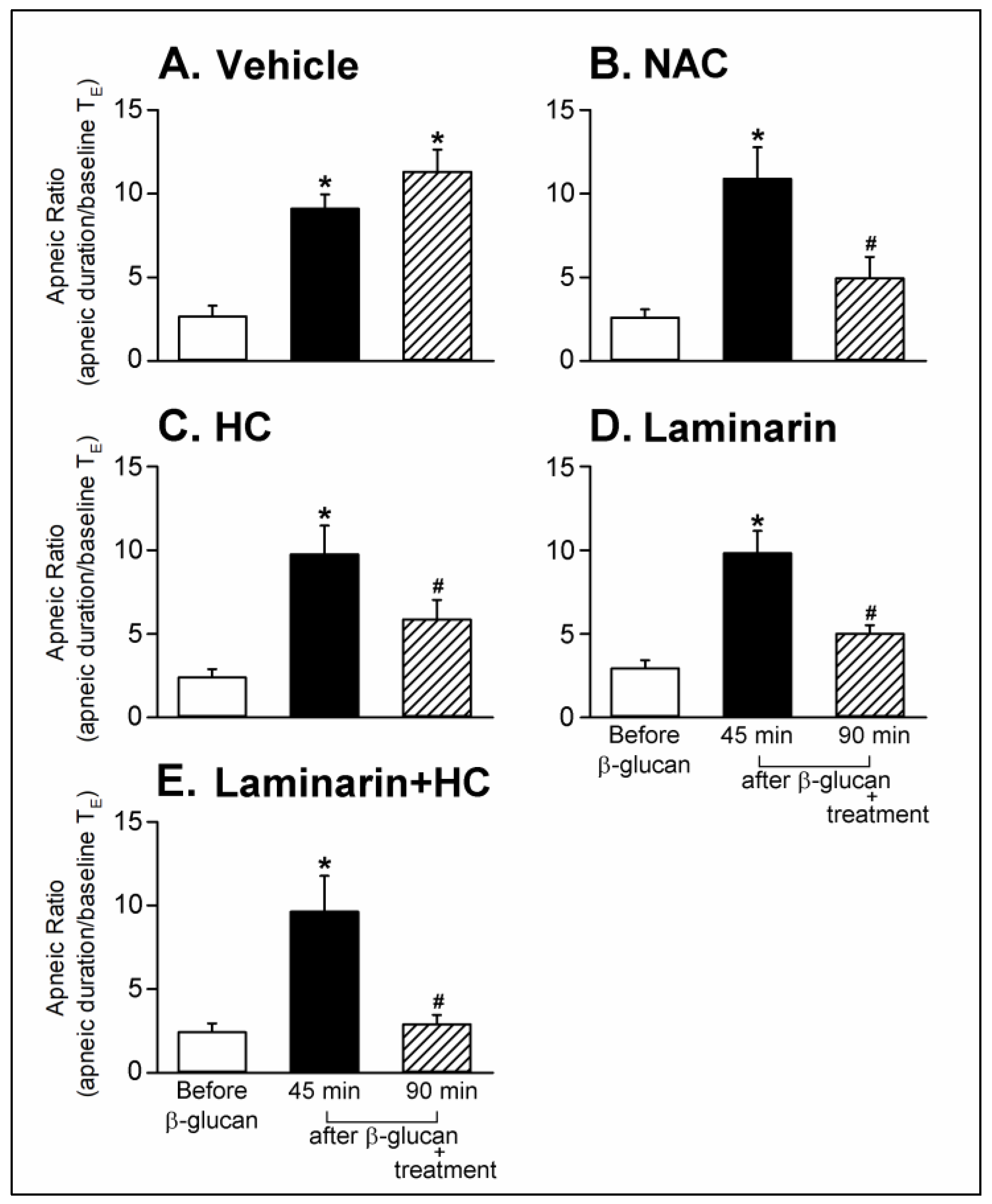
2. Results
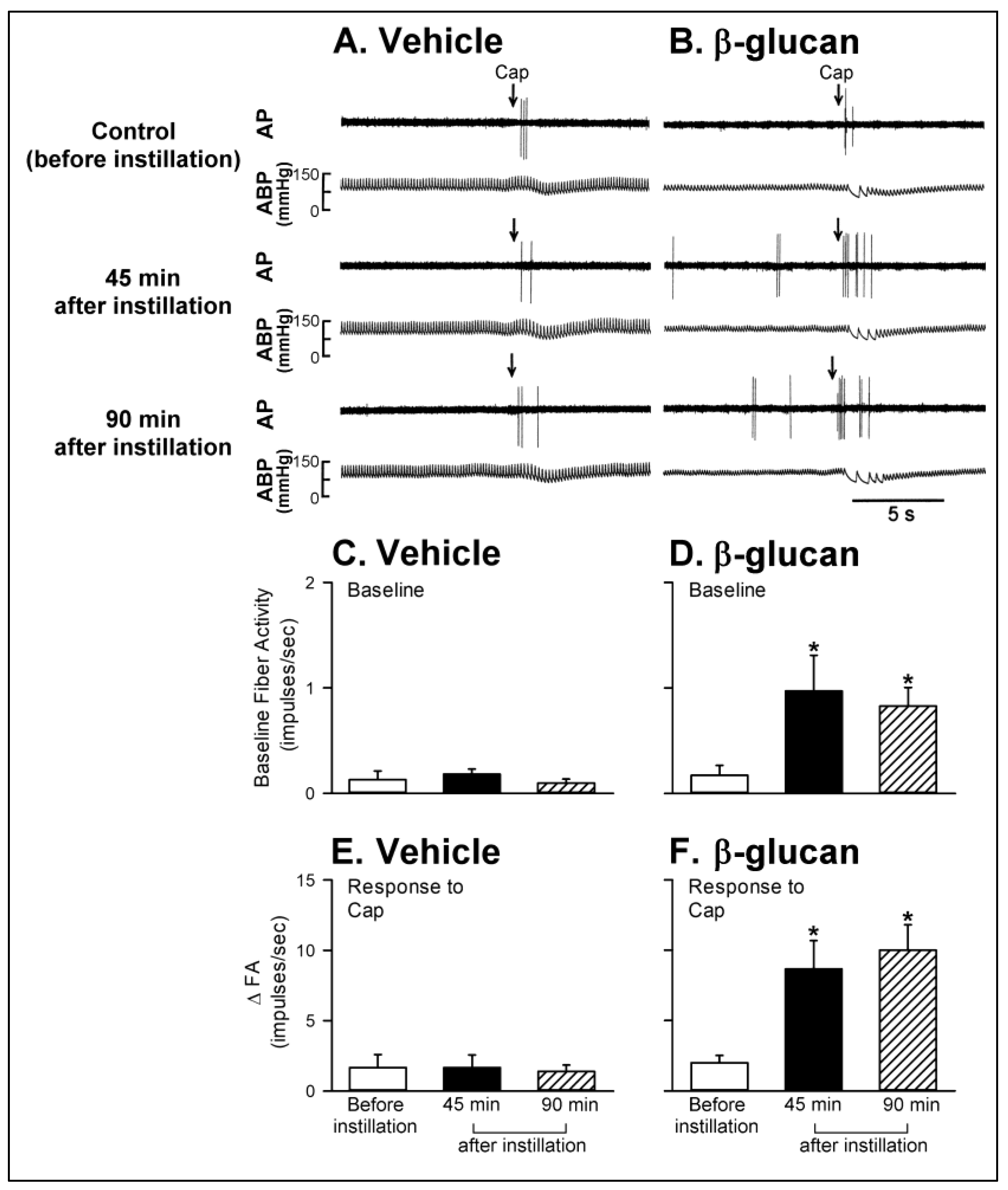
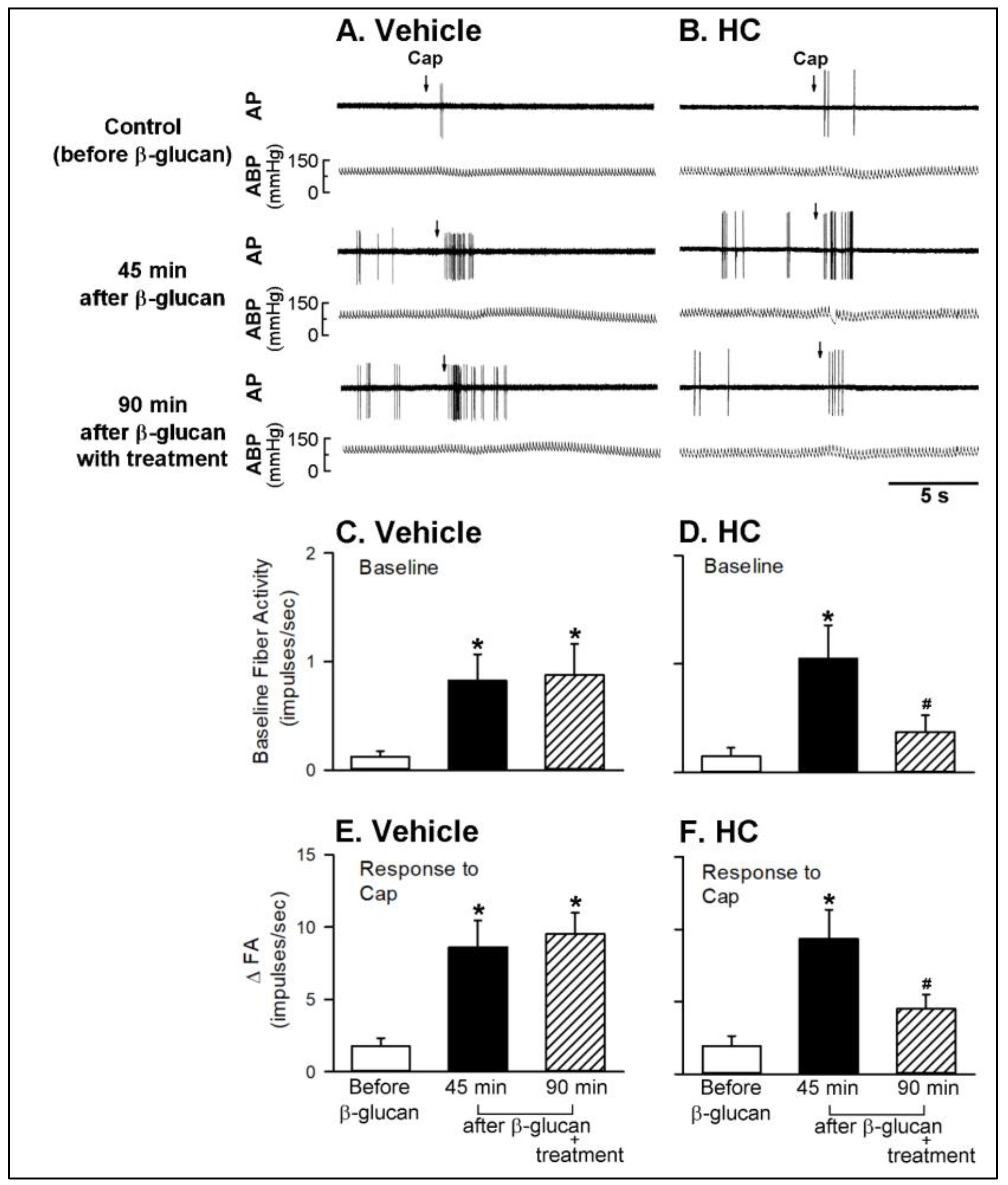
2.1. In Vivo Study
2.2. In Vitro Study
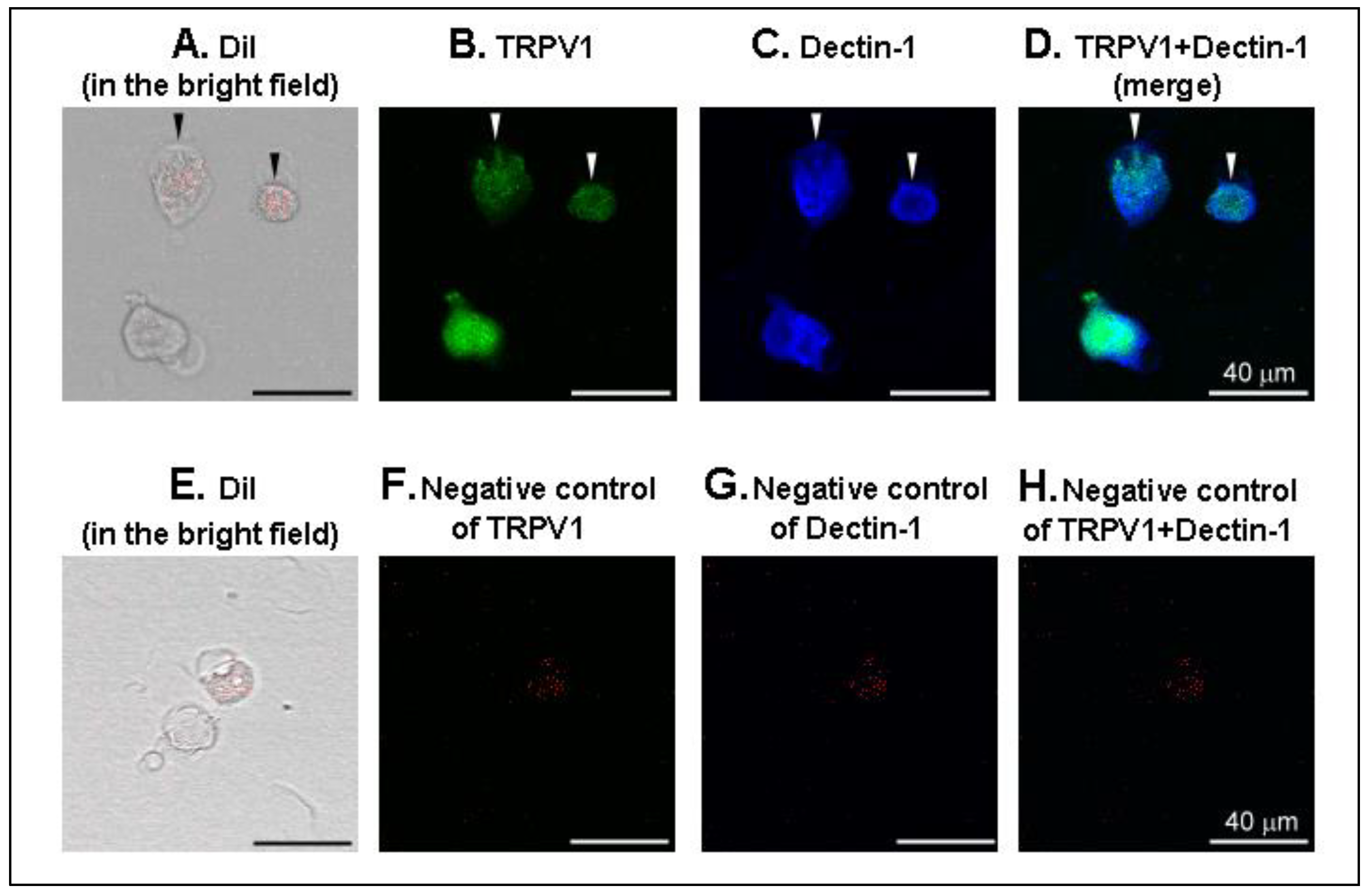
2.3. Immunofluorescence
3. Discussion
4. Materials and Methods
4.1. In Vivo Study
4.1.1. General Preparation
4.1.2. Airway Exposure to β-glucan
4.1.3. Measurement of Ventilatory Responses
4.1.4. Perineural Capsaicin Treatment (PCT) of Cervical Vagi
4.1.5. Measurement of Excitability of CSLV Afferents
4.1.6. Experimental Protocols
4.2. In Vitro Study
4.2.1. Identification of CSLV Neurons
4.2.2. Isolation and Culture of Nodose and Jugular Ganglion Neurons
4.2.3. Measurement of Ca2+ Transients on Isolated CSLV Neurons
4.2.4. Experimental Protocols
4.3. Immunofluorescence
4.4. Pharmacological Agents
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CSLV | capsaicin-sensitive lung vagal |
| ROS | reactive oxygen species |
| TRPA1 | transient receptor potential ankyrin 1 |
| iv | intravenous |
| TRPV1 | transient receptor potential vanilloid 1 |
| ip | intraperitonal |
| DRG | dorsal root ganglion |
| CGRP | calcitonin gene-related peptide |
References
- Rylander, R. Airborne (1→3)-β-D-glucan and airway disease in a day-care center before and after renovation. Arch. Environ. Health Int. J. 1997, 52, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Rylander, R.; Norrhall, M.; Engdahl, U.; Tunsater, A.; Holt, P.G. Airways inflammation, atopy, and (1→3)-β-D-glucan exposures in two schools. Am. J. Respir. Crit. Care Med. 1998, 158, 1685–1687. [Google Scholar] [CrossRef] [PubMed]
- Rylander, R.; Lin, R.-H. (1→3)-β-d-glucan—Relationship to indoor air-related symptoms, allergy and asthma. Toxicology 2000, 152, 47–52. [Google Scholar] [CrossRef]
- Wan, G.-H.; Li, C.-S.; Guo, S.-P.; Rylander, R.; Lin, R.-H. An airbone mold-derived product, β-1, 3-d-glucan, potentiates airway allergic responses. Eur. J. Immunol. 1999, 29, 2491–2497. [Google Scholar] [CrossRef]
- Young, S.-H.; Robinson, V.A.; Barger, M.; Porter, D.W.; Frazer, D.G.; Castranova, V. Acute inflammation and recovery in rats after intratracheal instillation of a 1? 3-ß-glucan (zymosan a). J. Toxicol. Environ. Health A 2001, 64, 311–325. [Google Scholar] [CrossRef]
- Lee, L.-Y.; Yu, J. Sensory nerves in lung and airways. Compr. Physiol. 2011, 4, 287–324. [Google Scholar]
- Spina, D. Airway sensory nerves: A burning issue in asthma? Thorax 1996, 51, 335–337. [Google Scholar] [CrossRef] [Green Version]
- Canning, B.J.; Mori, N.; Mazzone, S.B. Vagal afferent nerves regulating the cough reflex. Respir. Physiol. Neurobiol. 2006, 152, 223–242. [Google Scholar] [CrossRef]
- Netea, M.G.; Brown, G.D.; Kullberg, B.J.; Gow, N.A. An integrated model of the recognition of Candida albicans by the innate immune system. Nat. Rev. Microbiol. 2008, 6, 67–78. [Google Scholar] [CrossRef]
- Brown, G.D.; Herre, J.; Williams, D.L.; Willment, J.A.; Marshall, A.S.; Gordon, S. Dectin-1 mediates the biological effects of β-glucans. J. Exp. Med. 2003, 197, 1119–1124. [Google Scholar] [CrossRef] [Green Version]
- Steele, C.; Marrero, L.; Swain, S.; Harmsen, A.G.; Zheng, M.; Brown, G.D.; Gordon, S.; Shellito, J.E.; Kolls, J.K. Alveolar macrophage–mediated killing of Pneumocystis carinii f. sp. muris involves molecular recognition by the dectin-1 β-glucan receptor. J. Exp. Med. 2003, 198, 1677–1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, G.D.; Gordon, S. A new receptor for β-glucans. Nature 2001, 413, 36–37. [Google Scholar] [CrossRef] [PubMed]
- Young, S.-H.; Robinson, V.; Barger, M.; Whitmer, M.; Porter, D.; Frazer, D.; Castranova, V. Exposure to particulate 1→3-β-glucans induces greater pulmonary toxicity than soluble 1→3-β-glucans in rats. J. Toxicol. Environ. Health A 2003, 66, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Young, S.-H.; Cox-Ganser, J.M.; Shogren, E.S.; Wolfarth, M.G.; Li, S.; Antonini, J.M.; Castranova, V.; Park, J.-H. Pulmonary inflammation induced by office dust and the relation to 1→3-β-glucan using different extraction techniques. Toxicol. Environ. Chem. 2011, 93, 806–823. [Google Scholar] [CrossRef]
- Young, R.S.; Jones, A.M.; Nicholls, P.J. Something in the air: Endotoxins and glucans as environmental troublemakers. J. Pharm. Pharmacol. 1998, 50, 11. [Google Scholar] [CrossRef] [PubMed]
- Beijer, L.; Thorn, J.; Rylander, R. Mould exposure at home relates to inflammatory markers in blood. Eur. Respir. J. 2003, 21, 317–322. [Google Scholar] [CrossRef] [Green Version]
- Tada, H.; Nemoto, E.; Shimauchi, H.; Watanabe, T.; Mikami, T.; Matsumoto, T.; Ohno, N.; Tamura, H.; Shibata, K.; Akashi, S. Saccharomyces cerevisiae-and Candida albicans-derived mannan induced production of tumor necrosis factor alpha by human monocytes in a CD14-and Toll-like receptor 4-dependent manner. Microbiol. Immunol. 2002, 46, 503–512. [Google Scholar] [CrossRef]
- Gantner, B.N.; Simmons, R.M.; Canavera, S.J.; Akira, S.; Underhill, D.M. Collaborative induction of inflammatory responses by dectin-1 and Toll-like receptor 2. J. Exp. Med. 2003, 197, 1107–1117. [Google Scholar] [CrossRef] [Green Version]
- Sigsgaard, T.; Bonefeld-Jorgensen, E.C.; Kjaergaard, S.K.; Mamas, S.; Pedersen, O.F. Cytokine release from the nasal mucosa and whole blood after experimental exposures to organic dusts. Eur. Respir. J. 2000, 16, 140–145. [Google Scholar] [CrossRef] [Green Version]
- Lai, C.J.; Ho, C.-Y.; Kou, Y.R. Activation of lung vagal sensory receptors by circulatory endotoxin in rats. Life Sci. 2002, 70, 2125–2138. [Google Scholar] [CrossRef]
- Vesely, K.R.; Hyde, D.M.; Stovall, M.Y.; Harkema, J.R.; Green, J.F.; Schelegle, E.S. Capsaicin-sensitive C-fiber-mediated protective responses in ozone inhalation in rats. J. Appl. Physiol. 1999, 86, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Schelegle, E.S.; Alfaro, M.F.; Putney, L.; Stovall, M.; Tyler, N.; Hyde, D.M. Effect of C-fiber-mediated, ozone-induced rapid shallow breathing on airway epithelial injury in rats. J. Appl. Physiol. 2001, 91, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.Y.; Kou, Y.R.; Frazier, D.T.; Beck, E.R.; Pisarri, T.E.; Coleridge, H.M.; Coleridge, J.C. Stimulation of vagal pulmonary C-fibers by a single breath of cigarette smoke in dogs. J. Appl. Physiol. 1989, 66, 2032–2038. [Google Scholar] [CrossRef] [PubMed]
- Ruan, T.; Lin, Y.-J.; Hsu, T.-H.; Lu, S.-H.; Jow, G.-M.; Kou, Y.R. Sensitization by pulmonary reactive oxygen species of rat vagal lung C-fibers: The roles of the TRPV1, TRPA1, and P2X receptors. PLoS ONE 2014, 9, e91763. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Ho, C.-Y.; Tang, G.-J.; Kou, Y.R. Alleviation of wood smoke-induced lung injury by tachykinin receptor antagonist and hydroxyl radical scavenger in guinea pigs. Eur. J. Pharmacol. 2001, 425, 141–148. [Google Scholar] [CrossRef]
- Chung, K.F.; McGarvey, L.; Mazzone, S.B. Chronic cough as a neuropathic disorder. Lancet Respir. Med. 2013, 1, 414–422. [Google Scholar] [CrossRef]
- Lee, L.-Y.; Pisarri, T.E. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir. Physiol. 2001, 125, 47–65. [Google Scholar] [CrossRef]
- Maruyama, K.; Takayama, Y.; Sugisawa, E.; Yamanoi, Y.; Yokawa, T.; Kondo, T.; Ishibashi, K.; Sahoo, B.R.; Takemura, N.; Mori, Y. The ATP transporter VNUT mediates induction of dectin-1-triggered Candida nociception. Iscience 2018, 6, 306–318. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.S.; Hsu, C.-C.; Bien, M.-Y.; Hsu, H.-C.; Weng, H.-T.; Kou, Y.R. Activations of TRPA1 and P2X receptors are important in ROS-mediated stimulation of capsaicin-sensitive lung vagal afferents by cigarette smoke in rats. J. Appl. Physiol. 2010, 108, 1293–1303. [Google Scholar] [CrossRef]
- Jancso, G.; Such, G. Effects of capsaicin applied perineurally to the vagus nerve on cardiovascular and respiratory functions in the cat. J. Physiol. 1983, 341, 359–370. [Google Scholar] [CrossRef]
- Bhatia, M.; Zhi, L.; Zhang, H.; Ng, S.-W.; Moore, P.K. Role of substance P in hydrogen sulfide-induced pulmonary inflammation in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 2006, 291, L896–L904. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.D.; Gordon, S. Fungal β-glucans and mammalian immunity. Immunity 2003, 19, 311–315. [Google Scholar] [CrossRef] [Green Version]
- Taylor, P.R.; Brown, G.D.; Reid, D.M.; Willment, J.A.; Martinez-Pomares, L.; Gordon, S.; Wong, S.Y. The β-glucan receptor, dectin-1, is predominantly expressed on the surface of cells of the monocyte/macrophage and neutrophil lineages. J. Immunol. 2002, 169, 3876–3882. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.D.; Topley, N.; Alobaidi, H.M.; Harber, M.J. Activation of human polymorphonuclear leucocytes by particulate zymosan is related to both its major carbohydrate components: Glucan and mannan. Immunology 1986, 58, 117. [Google Scholar]
- Astarie-Dequeker, C.; N’Diaye, E.-N.; Le Cabec, V.; Rittig, M.G.; Prandi, J.; Maridonneau-Parini, I. The mannose receptor mediates uptake of pathogenic and nonpathogenic mycobacteria and bypasses bactericidal responses in human macrophages. Infect. Immun. 1999, 67, 469–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czop, J.K. The role of β-glucan receptors on blood and tissue leukocytes in phagocytosis and metabolic activation. Pathol. Immunopathol. Res. 1986, 5, 286–296. [Google Scholar] [CrossRef]
- Maruyama, K.; Takayama, Y.; Kondo, T.; Ishibashi, K.; Sahoo, B.R.; Kanemaru, H.; Kumagai, Y.; Martino, M.M.; Tanaka, H.; Ohno, N. Nociceptors boost the resolution of fungal osteoinflammation via the TRP channel-CGRP-Jdp2 axis. Cell Rep. 2017, 19, 2730–2742. [Google Scholar] [CrossRef] [Green Version]
- Rahman, I.; Adcock, I.M. Oxidative stress and redox regulation of lung inflammation in COPD. Eur. Respir. J. 2006, 28, 219–242. [Google Scholar] [CrossRef]
- Nassenstein, C.; Kwong, K.; Taylor-Clark, T.; Kollarik, M.; MacGlashan, D.M.; Braun, A.; Undem, B.J. Expression and function of the ion channel TRPA1 in vagal afferent nerves innervating mouse lungs. J. Physiol. 2008, 586, 1595–1604. [Google Scholar] [CrossRef]
- Taylor-Clark, T.E.; Undem, B.J. Sensing pulmonary oxidative stress by lung vagal afferents. Respir. Physiol. Neurobiol. 2011, 178, 406–413. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, E.S.; Fernandes, M.A.; Keeble, J.E. The functions of TRPA1 and TRPV1: Moving away from sensory nerves. Br. J. Pharmacol. 2012, 166, 510–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nassini, R.; Pedretti, P.; Moretto, N.; Fusi, C.; Carnini, C.; Facchinetti, F.; Viscomi, A.R.; Pisano, A.R.; Stokesberry, S.; Brunmark, C. Transient receptor potential ankyrin 1 channel localized to non-neuronal airway cells promotes non-neurogenic inflammation. PLoS ONE 2012, 7, e42454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, M.J.; Balasuriya, D.; Jeggle, P.; Goetze, T.A.; McNaughton, P.A.; Reeh, P.W.; Edwardson, J.M. Direct evidence for functional TRPV1/TRPA1 heteromers. Pflüg. Arch. Eur. J. Physiol. 2014, 466, 2229–2241. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, D.L.; Klein, B.S. Lung epithelium: Barrier immunity to inhaled fungi and driver of fungal-associated allergic asthma. Curr. Opin. Microbiol. 2017, 40, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Spann, K.; Snape, N.; Baturcam, E.; Fantino, E. The impact of early-life exposure to air-borne environmental insults on the function of the airway epithelium in asthma. Ann. Glob. Health 2016, 82, 28–40. [Google Scholar] [CrossRef] [Green Version]
- Gon, Y.; Hashimoto, S. Role of airway epithelial barrier dysfunction in pathogenesis of asthma. Allergol. Int. 2018, 67, 12–17. [Google Scholar] [CrossRef]
- Heijink, I.H.; Brandenburg, S.M.; Postma, D.S.; van Oosterhout, A.J. Cigarette smoke impairs airway epithelial barrier function and cell–cell contact recovery. Eur. Respir. J. 2012, 39, 419–428. [Google Scholar] [CrossRef] [Green Version]
- Crameri, R.; Blaser, K. Allergy and immunity to fungal infections and colonization. Eur. Respir. J. 2002, 19, 151–157. [Google Scholar] [CrossRef]
- Fukutomi, Y.; Taniguchi, M. Sensitization to fungal allergens: Resolved and unresolved issues. Allergol. Int. 2015, 64, 321–331. [Google Scholar] [CrossRef] [Green Version]
- Rajagopal, T.V.; Kant, S.; Verma, S.K.; Kushwaha, R.A.S.; Kumar, S.; Garg, R.; Srivastava, A.; Bajaj, D.K. Aspergillus sensitization in bronchial asthma: A separate phenotype. Allergy Asthma Proc. 2020, 41, e26–e32. [Google Scholar] [CrossRef]
- Lim, F.L.; Hashim, Z.; Than, L.T.L.; Md Said, S.; Hashim, J.H.; Norbäck, D. Respiratory health among office workers in Malaysia and endotoxin and (1, 3)-β-glucan in office dust. Int. J. Tuberc. Lung Dis. 2019, 23, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Falcon-Rodriguez, C.I.; De Vizcaya-Ruiz, A.; Rosas-Pérez, I.A.; Osornio-Vargas, Á.R.; Segura-Medina, P. Inhalation of concentrated PM2. 5 from Mexico City acts as an adjuvant in a guinea pig model of allergic asthma. Environ. Pollut. 2017, 228, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Kimberg, M.; Brown, G.D. Dectin-1 and its role in antifungal immunity. Med. Mycol. 2008, 46, 631–636. [Google Scholar] [CrossRef] [Green Version]
- Iliev, I.D. Dectin-1 exerts dual control in the gut. Cell Host Microbe 2015, 18, 139–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schelegle, E.S.; Chen, A.T.; Loh, C.Y. Effects of Vagal Perineural Capsaicin Treatment on Vagal Efferent and Airway Neurogenic Responses in Anesthetized Rats. J. Basic Clin. Physiol. Pharmacol. 2000, 11, 1–16. [Google Scholar] [CrossRef]
- Mansoor, J.K.; Hyde, D.M.; Schelegie, E.S. Pulmonary vagal reflexes and breathing pattern are not altered in elastase-induced emphysema in rats. Exp. Lung Res. 1997, 23, 441–457. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Ruan, T.; Lee, L.-Y.; Lin, Y.S. Stimulatory Effect of 5-Hydroxytryptamine (5-HT) on Rat Capsaicin-Sensitive Lung Vagal Sensory Neurons via Activation of 5-HT3 Receptors. Front. Physiol. 2019, 10. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Lee, L.-Y. Role of calcium ions in the positive interaction between TRPA1 and TRPV1 channels in bronchopulmonary sensory neurons. J. Appl. Physiol. 2015, 118, 1533–1543. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.-C.; Lin, Y.S.; Lin, R.-L.; Lee, L.-Y. Immediate and delayed potentiating effects of tumor necrosis factor-α on TRPV1 sensitivity of rat vagal pulmonary sensory neurons. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2017, 313, L293–L304. [Google Scholar] [CrossRef] [Green Version]
- Chung, C.-L.; Lin, Y.S.; Chan, N.-J.; Chen, Y.-Y.; Hsu, C.-C. Hypersensitivity of Airway Reflexes Induced by Hydrogen Sulfide: Role of TRPA1 Receptors. Int. J. Mol. Sci. 2020, 21, 3929. [Google Scholar] [CrossRef]


| Study | Response | Series | Group | Instillation (In Vivo)/Perfusion (In Vitro) | Stimulus | Treatment |
|---|---|---|---|---|---|---|
| In vivo | Pulmonary chemoreflex | 1 | 1 | β-glucan | Capsaicin | - |
| 2 | Vehicle of β-glucan | Capsaicin | - | |||
| 2 | 3 | β-glucan | Capsaicin | PCT | ||
| 4 | β-glucan | Capsaicin | PST | |||
| 3 | 5 | β-glucan | Capsaicin | Vehicle | ||
| 6 | β-glucan | Capsaicin | NAC | |||
| 7 | β-glucan | Capsaicin | HC | |||
| 8 | β-glucan | Capsaicin | Laminarin | |||
| 9 | β-glucan | Capsaicin | Laminarin + HC | |||
| Fiber activity | 4 | 10 | β-glucan | Capsaicin | - | |
| 11 | Vehicle of β-glucan | Capsaicin | - | |||
| 5 | 12 | β-glucan | Capsaicin | Vehicle of HC | ||
| 13 | β-glucan | Capsaicin | HC | |||
| In vitro | Ca2+ transient | 1 | 1 | β-glucan | Capsaicin | - |
| 2 | Vehicle of β-glucan | Capsaicin | - | |||
| 2 | 3 | β-glucan | Capsaicin | Laminarin |
| Vehicle of β-Glucan (n = 6) | β-Glucan (n = 48) | |
|---|---|---|
| MABP, mmHg | ||
| Before instillation | 117 ± 5 | 114 ± 2 |
| 45 min after instillation | 113 ± 6 | 107 ± 2 |
| 90 min after instillation | 114 ± 4 | 106 ± 2 |
| HR, beats/min | ||
| Before instillation | 348 ± 23 | 365 ± 4 |
| 45 min after instillation | 354 ± 30 | 356 ± 6 |
| 90 min after instillation | 347 ± 33 | 353 ± 7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.S.; Huang, I.-H.; Lan, S.-H.; Chen, C.-L.; Chen, Y.-Y.; Chan, N.-J.; Hsu, C.-C. Involvement of Capsaicin-Sensitive Lung Vagal Neurons and TRPA1 Receptors in Airway Hypersensitivity Induced by 1,3-β-D-Glucan in Anesthetized Rats. Int. J. Mol. Sci. 2020, 21, 6845. https://doi.org/10.3390/ijms21186845
Lin YS, Huang I-H, Lan S-H, Chen C-L, Chen Y-Y, Chan N-J, Hsu C-C. Involvement of Capsaicin-Sensitive Lung Vagal Neurons and TRPA1 Receptors in Airway Hypersensitivity Induced by 1,3-β-D-Glucan in Anesthetized Rats. International Journal of Molecular Sciences. 2020; 21(18):6845. https://doi.org/10.3390/ijms21186845
Chicago/Turabian StyleLin, You Shuei, I-Hsuan Huang, Sheng-Hsuan Lan, Chia-Ling Chen, Yueh-Yin Chen, Nai-Ju Chan, and Chun-Chun Hsu. 2020. "Involvement of Capsaicin-Sensitive Lung Vagal Neurons and TRPA1 Receptors in Airway Hypersensitivity Induced by 1,3-β-D-Glucan in Anesthetized Rats" International Journal of Molecular Sciences 21, no. 18: 6845. https://doi.org/10.3390/ijms21186845





