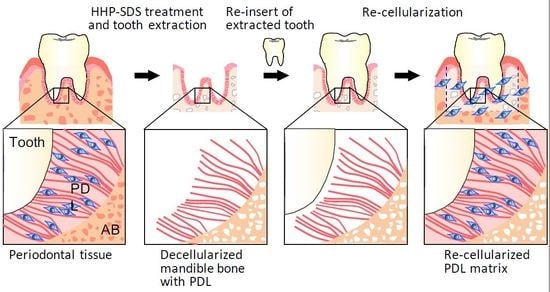
Extracellular Matrix Induces Periodontal Ligament Reconstruction In Vivo
Abstract
:1. Introduction
2. Results
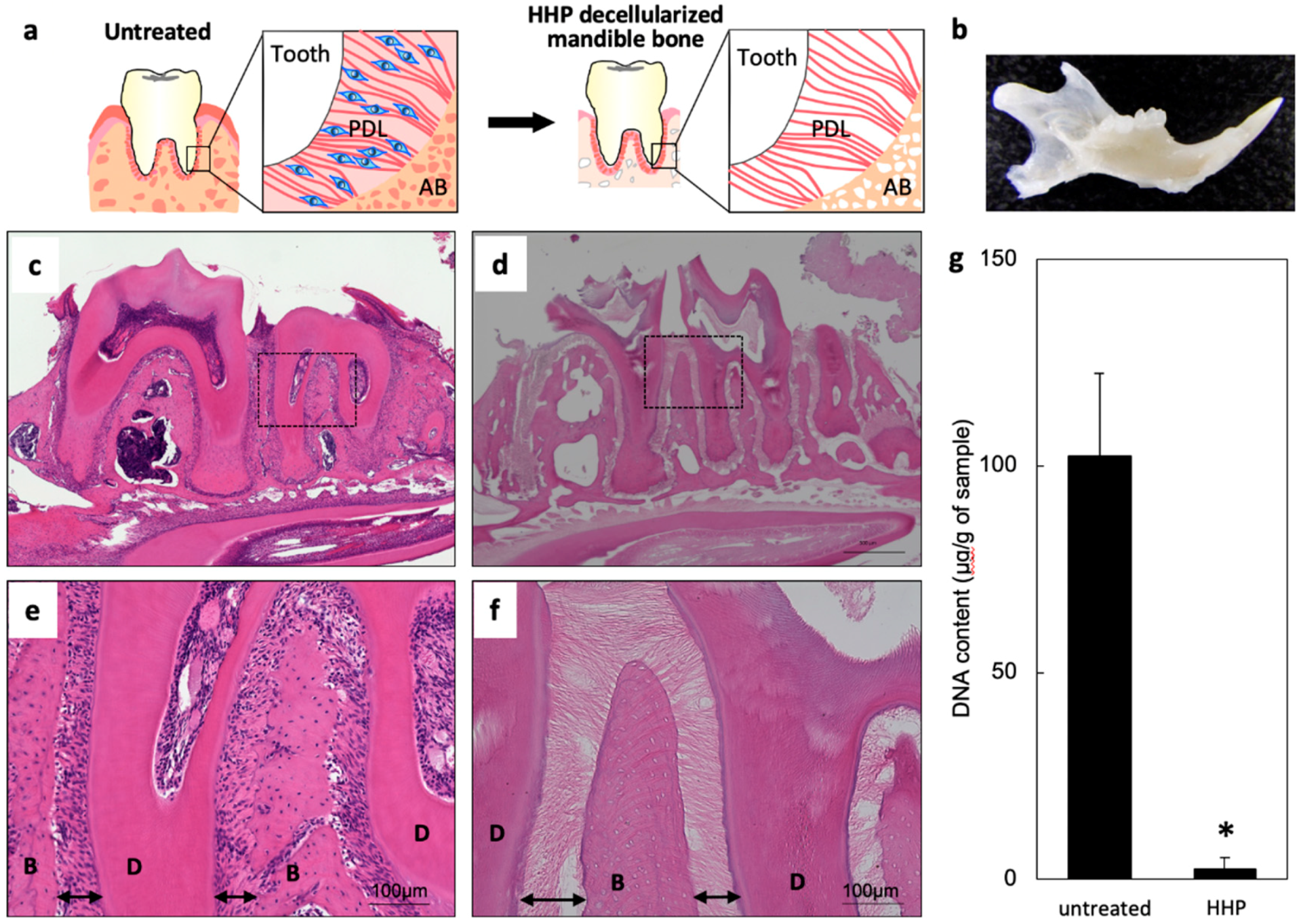
2.1. Decellularization of Mandible Bone including Tooth
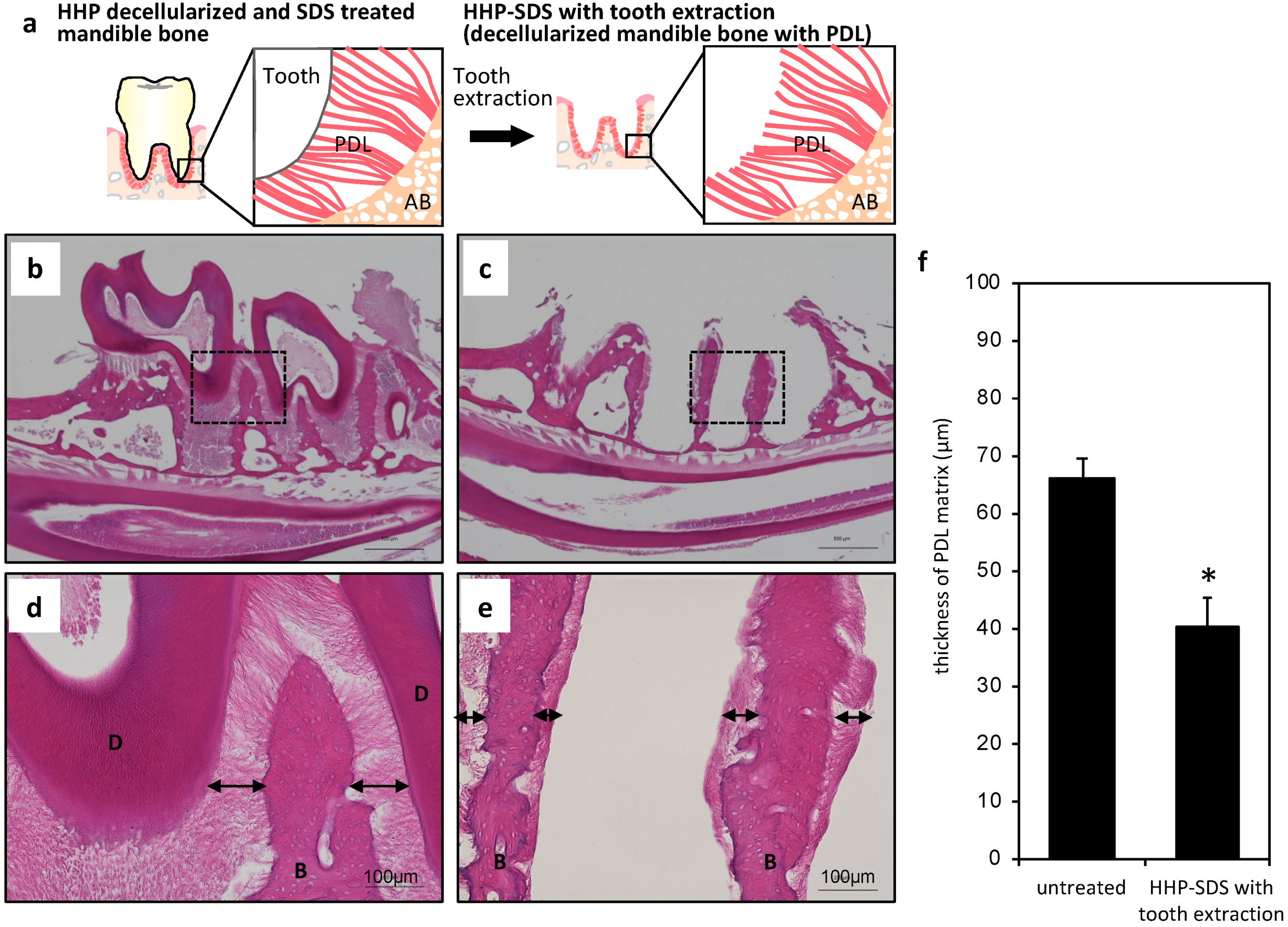
2.2. Preparation of Mandible Bone with PDL Matrix
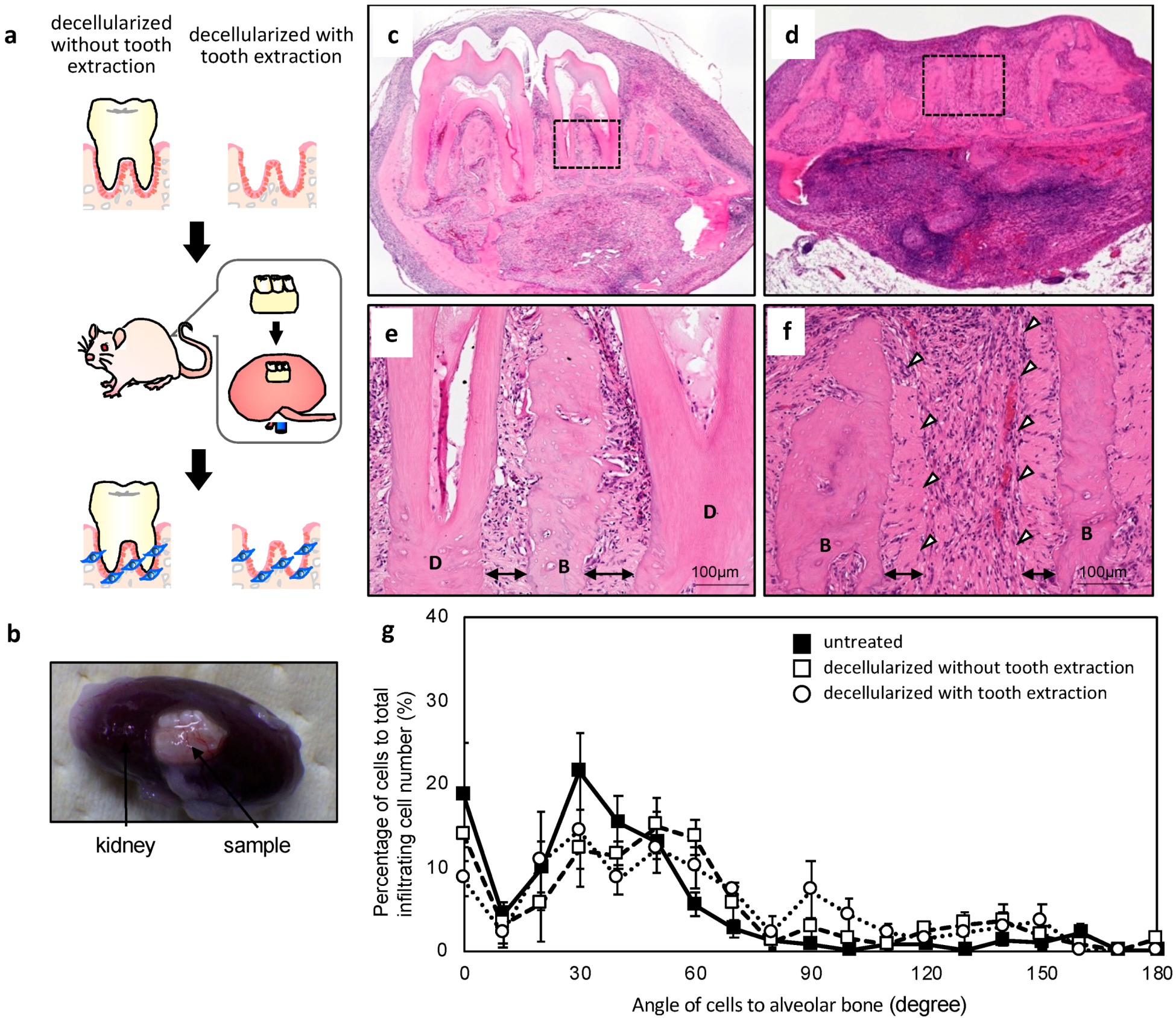
2.3. In Vivo Re-cellularization of HHP-decellularized Mandible Bone
2.4. Preparation of an Artificial Tooth Model
2.5. In Vivo Re-cellularization of Decellularized Mandible Bone with Re-inserted Artificial Tooth
3. Discussion
4. Materials and Methods
4.1. Preparation of Decellularized Mandible Bone
4.2. Preparation of PDL Matrix on Decellularized Mandible Bone
4.3. Histology
4.4. Quantification of Residual DNA
4.5. Subrenal Capsule Assay
4.6. Surface Modification of Extracted Teeth
4.7. SEM and Energy Dispersive X-ray Spectrometry
4.8. μCT
4.9. Quantitative Analysis of Cell Orientation
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iwata, T.; Yamato, M.; Tsuchioka, H.; Takagi, R.; Mukobata, S.; Washio, K.; Okano, T.; Ishikawa, I. Periodontal regeneration with multi-layered periodontal ligament-derived cell sheets in a canine model. Biomaterials 2009, 30, 2716–2723. [Google Scholar] [CrossRef] [PubMed]
- Iwata, T.; Yamato, M.; Washio, K.; Yoshida, T.; Tsumanuma, Y.; Yamada, A.; Onizuka, S.; Izumi, Y.; Ando, T.; Okano, T.; et al. Periodontal regeneration with autologous periodontal ligament-derived cell sheets—A safety and efficacy study in ten patients. Regen. Ther. 2018, 9, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Panduwawala, C.P.; Zhan, X.; Dissanayaka, W.L.; Samaranayake, L.P.; Jin, L.; Zhang, C. In vivo periodontal tissue regeneration by periodontal ligament stem cells and endothelial cells in three-dimensional cell sheet constructs. J. Periodontal Res. 2017, 52, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F. The extracellular matrix as a biologic scaffold material. Biomaterials 2007, 28, 3587–3593. [Google Scholar] [CrossRef] [PubMed]
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Crapo, P.M.; Gilbert, T.W.; Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 2011, 32, 3233–3243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, B.N.; Badylak, S.F. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl. Res. 2014, 163, 268–285. [Google Scholar] [CrossRef] [PubMed]
- Rana, D.; Zreiqat, H.; Benkirane-Jessel, N.; Ramakrishna, S.; Ramalingam, M. Development of decellularized scaffolds for stem cell- driven tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 942–965. [Google Scholar] [CrossRef]
- Nakamura, N.; Kimura, T.; Kishida, A. Overview of the Development, Applications, and Future Perspectives of Decellularized Tissues and Organs. ACS Biomater. Sci. Eng. 2017, 3, 1236–1244. [Google Scholar] [CrossRef]
- Negishi, J.; Funamoto, S.; Kimura, T.; Nam, K.; Higami, T.; Kishida, A. Effect of treatment temperature on collagen structures of the decellularized carotid artery using high hydrostatic pressure. J. Artif. Organs. 2011, 14, 223–231. [Google Scholar] [CrossRef]
- Wu, P.; Nakamura, N.; Kimura, T.; Nam, K.; Fujisato, T.; Funamoto, S.; Higami, T.; Kishida, A. Decellularized porcine aortic intima-media as a potential cardiovascular biomaterial. Interact. Cardiovasc. Thorac. Surg. 2015, 21, 189–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, P.; Kimura, T.; Tadokoro, H.; Nam, K.; Fujisato, T.; Kishida, A. Relation between the tissue structure and protein permeability of decellularized porcine aorta. Mater. Sci. Eng. C 2014, 43, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Nakamura, N.; Morita, H.; Nam, K.; Fujisato, T.; Kimura, T.; Kishida, A. A hybrid small-diameter blood vessel fabricated from decellularized aortic intima-media and reinforced with electrospun fibers. J. Biomed. Mater. Res. Part A 2019, 107, 1064–1070. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Funamoto, S.; Sasaki, S.; Honda, T.; Hattori, S.; Nam, K.; Kimura, T.; Mochizuki, M.; Fujisato, T.; Kobayashi, H.; et al. Preparation and characterization of decellularized cornea using high-hydrostatic pressurization for corneal tissue engineering. Biomaterials 2010, 31, 3941–3948. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Funamoto, S.; Sasaki, S.; Negishi, J.; Honda, T.; Hattori, S.; Nam, K.; Kimura, T.; Mochizuki, M.; Kobayashi, H.; et al. Corneal Regeneration by Deep Anterior Lamellar Keratoplasty (DALK) Using Decellularized Corneal Matrix. PLoS ONE 2015, 10, e0131989. [Google Scholar] [CrossRef]
- Nakamura, N.; Sugano, K.; Nam, K.; Kimura, T.; Fujisato, T.; Kishida, A. A basic study of osteogenesis between decellularized cortical bone pieces for bone graft construction. Adv. Biomed. Eng. 2013, 2, 95–100. [Google Scholar] [CrossRef]
- Nakamura, N.; Kimura, T.; Nam, K.; Fujisato, T.; Iwata, H.; Tsuji, T.; Akio, K. Induction of in Vivo Ectopic Hematopoiesis by a Three-Dimensional Structured Extracellular Matrix Derived from Decellularized Cancellous Bone. ACS Biomater. Sci. Eng. 2019. [Google Scholar] [CrossRef]
- Oshima, M.; Inoue, K.; Nakajima, K.; Tachikawa, T.; Yamazaki, H.; Isobe, T.; Sugawara, A.; Ogawa, M.; Tanaka, C.; Saito, M.; et al. Functional tooth restoration by next-generation bio-hybrid implant as a bio-hybrid artificial organ replacement therapy. Sci. Rep. 2014, 4, 6044. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, N.; Oshima, M.; Tanaka, C.; Ogawa, M.; Nakajima, K.; Ishida, K.; Moriyama, K.; Tsuji, T. Functional tooth restoration utilising split germs through re-regionalisation of the tooth-forming field. Sci. Rep. 2015, 5, 18393. [Google Scholar] [CrossRef] [Green Version]
- Ott, H.C.; Matthiesen, T.S.; Goh, S.K.; Black, L.D.; Kren, S.M.; Netoff, T.I.; Taylor, D.A. Perfusion-decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nat. Med. 2008, 14, 213–221. [Google Scholar] [CrossRef]
- Ott, H.C.; Clippinger, B.; Conrad, C.; Schuetz, C.; Pomerantseva, I.; Ikonomou, L.; Kotton, D.; Vacanti, J.P. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010, 16, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, T.; Muraoka, Y.; Matsuyama, H.; Kishida, A.; Akashi, M. Apatite coating on hydrophilic polymer-grafted poly(ethylene) films using an alternate soaking process. Biomaterials 2001, 22, 53–58. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, N.; Ito, A.; Kimura, T.; Kishida, A. Extracellular Matrix Induces Periodontal Ligament Reconstruction In Vivo. Int. J. Mol. Sci. 2019, 20, 3277. https://doi.org/10.3390/ijms20133277
Nakamura N, Ito A, Kimura T, Kishida A. Extracellular Matrix Induces Periodontal Ligament Reconstruction In Vivo. International Journal of Molecular Sciences. 2019; 20(13):3277. https://doi.org/10.3390/ijms20133277
Chicago/Turabian StyleNakamura, Naoko, Ai Ito, Tsuyoshi Kimura, and Akio Kishida. 2019. "Extracellular Matrix Induces Periodontal Ligament Reconstruction In Vivo" International Journal of Molecular Sciences 20, no. 13: 3277. https://doi.org/10.3390/ijms20133277