The Significance of Calcium in Photosynthesis
Abstract
1. Introduction
2. Mechanisms of Ca2+ Involved in Stomatal Movements
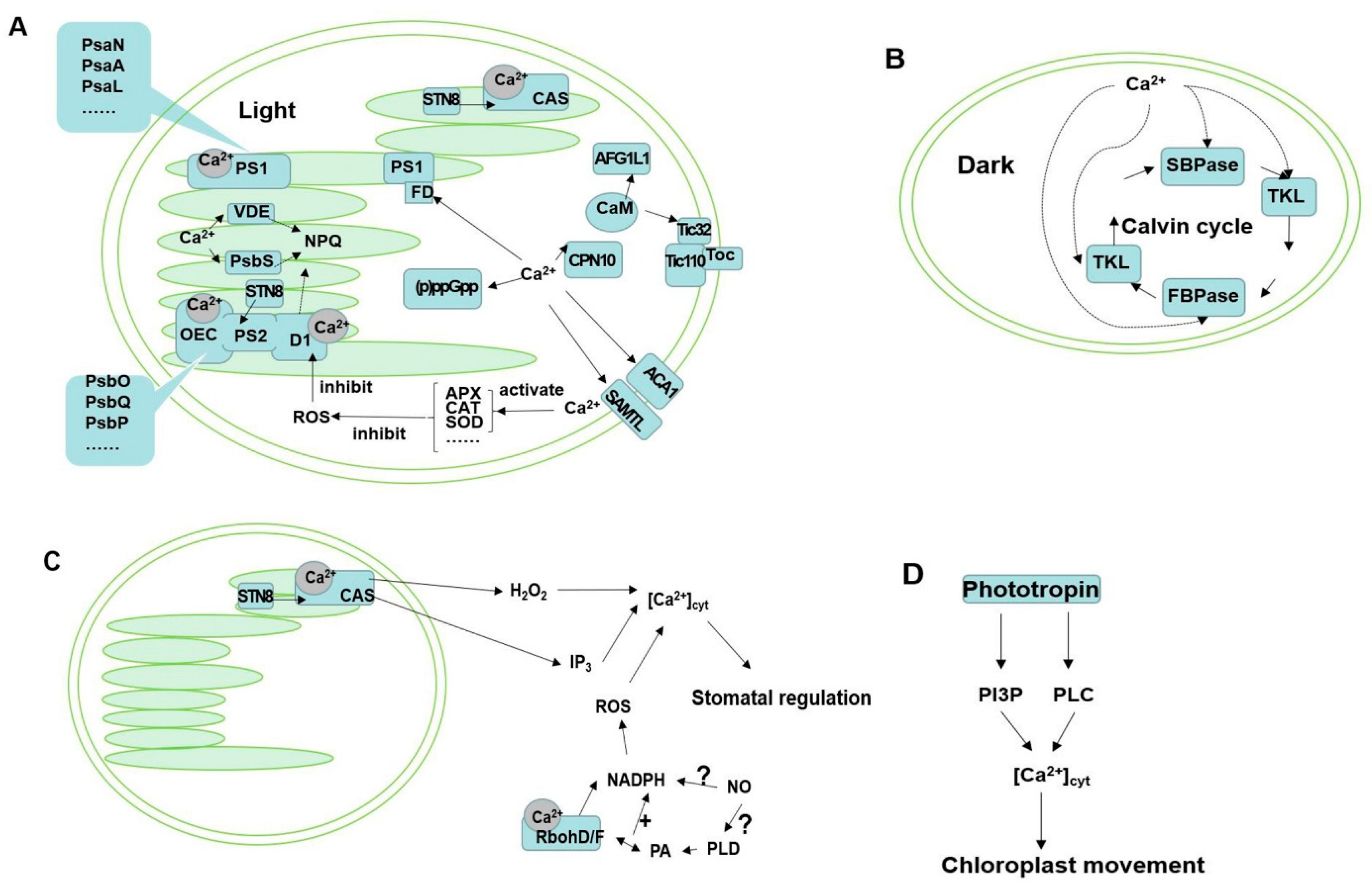
3. Ca2+ Is Involved in the Processes of Photosynthetic Reaction
4. Ca2+ Involved in Regulating Photosynthetic Enzyme Activity of Carbon Assimilation
5. Ca2+ Is Involved in the Mechanisms of Regulating Photoprotection
6. Ca2+ Is Involved in Chloroplast Movement
7. Other Ca2+-Related Chloroplast Proteins
8. Conclusions
Acknowledgements
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| ACA1 | Arabidopsis thaliana Ca2+-ATPase |
| AFG1L1 | ATPase family gene 1-like protein 1 |
| APX | Ascorbate peroxidase |
| CaM | Calmodulin |
| CAS | Ca2+ sensor |
| CAT | Catalase |
| CCM | CO2 concentration mechanism |
| CEF | Cyclic electron flow |
| Ch-CPN10 | 10 kDa chloroplast co-chaperonin |
| CP12 | 12 kDa chloroplast protein |
| Cytbf | Cytochrome b6f complex |
| D1 | The subunits of PS2 |
| E4P | Erythrose4-phosphate |
| FBPase | Fructose-l,6-bisphosphatase |
| FD | Ferredoxin |
| FNR | Ferredoxin NADP+ oxidoreductase |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| HLA3 | High-light activated 3 protein |
| H2O2 | Hydrogen peroxide |
| IP3 | The inositol 1,4,5-trisphosphate |
| LCIA | Low-CO2 (LC)-inducible protein A |
| LEF | Linear electron flow |
| NDH | NADPH dehydrogenase |
| NO | Nitric oxide |
| OEC | Oxygen-evolving complex |
| PA | Phosphatidic acid |
| Pl3P | Phosphatidylinositol 3-phosphate |
| PGR5 | Proton gradient regulation 5 |
| PGRL1 | PGR5-like photosynthetic phenotype1 |
| PLD | Phospholipase D |
| PLC | Phospholipase C |
| (p)ppGpp | Guanosine 5’-triphosphate (or 5’-diphosphate) 3’-diphosphate |
| PQ | Plastoquinone |
| PRK | Phosphoribulokinase |
| PS1 | Photosystem 1 |
| PS1-LHCI | PS1-light-harvesting |
| PS2 | Photosystem 2 |
| PsaA,-C,-D,-E,-H,-L,-N | The subunits of PS1 |
| PsbO,-P,-Q,-S,-H | The subunits of PS2 |
| RbohD/F | Respiratory burst oxidase homologues |
| ROS | Reactive oxygen species |
| SAMTL | S-adenosylmethionine transporter like? |
| SBPase | Sedoheptulose-1,7-bisphosphatase |
| SOD | Superoxide dismutase |
| STN8 | Thylakoid-associated kinases |
| Tic110; Tic32 | The subunit of chloroplast inner envelope protein complex |
| TKL | Transketolase |
| Toc | Chloroplast outer envelope protein complexes |
| TRX | Thioredoxin |
| VDE | Violaxanthin de-epoxidase |
| X5P | Xylulose5-phosphate |
References
- Liang, W.J.; Wang, M.L.; Ai, X.Z. The role of calcium in regulating photosynthesis and related physiological indexes of cucumber seedlings under low light intensity and suboptimal temperature stress. Sci. Hortic. 2009, 123, 34–38. [Google Scholar] [CrossRef]
- Knight, H.; Trewavas, A.J.; Knight, M.R. Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J. Cell Mol. Bio. 2010, 12, 1067–1078. [Google Scholar] [CrossRef]
- Shi, P.; Zeng, F.; Song, W.; Zhang, M.; Deng, R. Effects of calcium and lanthanum on ABA biosynthesis in Cucumber Leaves. Russ. J. Plant Physiol. 2002, 49, 696–699. [Google Scholar] [CrossRef]
- Vadassery, J.; Oelmüller, R. Calcium signaling in pathogenic and beneficial plant microbe interactions: what can we learn from the interaction between Piriformospora indica and Arabidopsis thaliana. Plant Signal. Behav. 2009, 4, 1024–1027. [Google Scholar] [CrossRef]
- Brand, J.J.; Becker, D.W. Evidence for direct roles of calcium in photosynthesis. J. Bioenerg. Biomembr. 1984, 16, 239–249. [Google Scholar] [CrossRef]
- Göhre, V.; Jones, A.M.; Sklenář, J.; Robatzek, S.; Weber, A.P. Molecular crosstalk between pamp-triggered immunity and photosynthesis. Mol. Plant-Microbe Interact. 2012, 25, 1083. [Google Scholar] [CrossRef]
- Hochmal, A.K.; Schulze, S.; Trompelt, K.; Hippler, M. Calcium-dependent regulation of photosynthesis. Biochim. Biophys. Acta. 2015, 1847, 993–1003. [Google Scholar] [CrossRef]
- Stael, S.; Rocha, A.G.; Robinson, A.J.; Kmiecik, P.; Vothknecht, U.C.; Teige, M. Arabidopsis calcium-binding mitochondrial carrier proteins as potential facilitators of mitochondrial ATP-import and plastid SAM-import. FEBS Lett. 2011, 585, 3935. [Google Scholar] [CrossRef]
- Aronsson, H.; Jarvis, P. The chloroplast protein import apparatus, its components, and their roles. Plant Cell Monogr. 2009, 13, 1–35. [Google Scholar] [CrossRef]
- Yang, S.; Wang, F.; Guo, F.; Meng, J.J.; Li, X.G.; Dong, S.T.; Wan, S.B. Exogenous calcium alleviates photoinhibition of PSII by improving the xanthophyll cycle in peanut (Arachis Hypogaea) leaves during heat stress under high irradiance. PLoS ONE 2013, 8, e71214. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Han, S.; Tang, R.; Anderson, L.K.; Woerner, T.E.; Pei, Z.M. A cell surface receptor mediates extracellular Ca2+ sensing in guard cells. Nature 2003, 425, 196. [Google Scholar] [CrossRef] [PubMed]
- Nomura, H.; Komori, T.; Kobori, M.; Nakahira, Y.; Shiina, T. Evidence for chloroplast control of external Ca2+-induced cytosolic Ca2+ transients and stomatal closure. Plant J. 2008, 53, 988–998. [Google Scholar] [CrossRef]
- Petroutsos, D.; Busch, A.; Janssen, I.; Trompelt, K.; Bergner, S.V.; Weinl, S.; Holtkamp, M.; Karst, U.; Kudla, J.; Hippler, M. The chloroplast calcium sensor CAS is required for photoacclimation in Chlamydomonas reinhardtii. Plant Cell 2011, 23, 2950. [Google Scholar] [CrossRef] [PubMed]
- Vainonen, J.P.; Sakuragi, Y.; Stael, S.; Tikkanen, M.; Allahverdiyeva, Y.; Paakkarinen, V.; Aro, E.; Suorsa, M.; Scheller, H.V.; Vener, A.V.; et al. Light regulation of CAS, a novel phosphoprotein in the thylakoid membrane of Arabidopsis thaliana. FEBS J. 2008, 275, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.A.; Dangl, J.L.; Jones, J.D. Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc. Natl. Acad. Sci. USA 2002, 99, 517. [Google Scholar] [CrossRef]
- Kwak, J.M.; Mori, I.C.; Pei, Z.M.; Leonhardt, N.; Torres, M.A.; Dangl, J.L.; Bloom, R.E.; Bodde, S.; Jones, J.D.; Schroeder, J.I. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003, 22, 2623–2633. [Google Scholar] [CrossRef]
- Kimura, S.; Kaya, H.; Kawarazaki, T.; Hiraoka, G.; Senzaki, E.; Michikawa, M.; Kuchitsu, K. Protein phosphorylation is a prerequisite for the Ca2+ -dependent activation of Arabidopsis, NADPH oxidases and may function as a trigger for the positive feedback regulation of Ca2+, and reactive oxygen species. Biochim. Biophys. Acta. 2012, 1823, 398–405. [Google Scholar] [CrossRef]
- Pagliano, C.; Rocca, N.L.; Andreucci, F.; Deák, Z.; Vass, I.; Rascio, N.; Barbato, R. The extreme halophyte Salicornia veneta is depleted of the extrinsic PsbQ and PsbP proteins of the oxygen-evolving complex without loss of functional activity. Ann. Bot. 2009, 103, 505–515. [Google Scholar] [CrossRef]
- Heredia, P.; De, L.R.J. Calcium-dependent conformational change and thermal stability of the isolated PsbO protein detected by FTIR spectroscopy. Biochemistry 2003, 42, 11831–11838. [Google Scholar] [CrossRef]
- Popelkova, H.; Boswell, N.; Yocum, C. Probing the topography of the photosystem II oxygen evolving complex: PsbO is required for efficient calcium protection of the manganese cluster against dark-inhibition by an artificial reductant. Photosynth. Res. 2011, 110, 111–121. [Google Scholar] [CrossRef]
- Schmidt, S.B.; Powikrowska, M.; Krogholm, K.S.; Naumann-Busch, B.; Schjoerring, J.K.; Husted, S.; Jensen, P.E.; Pedas, P.R. Photosystem II Functionality in Barley Responds Dynamically to Changes in Leaf Manganese Status. Front. Plant Sci. 2016, 7, 1772. [Google Scholar] [CrossRef]
- Ifuku, K.; Nakatsu, T.; Kato, H.; Sato, F. Crystal structure of the PsbP protein of photosystem II from Nicotiana tabacum. EMBO Rep. 2004, 5, 362. [Google Scholar] [CrossRef]
- Reddy, V.S.; Ali, G.S.; Reddy, A.S. Genes encoding calmodulin-binding proteins in the Arabidopsis genome. J. Biol. Chem. 2002, 277, 9840–9852. [Google Scholar] [CrossRef]
- Fromme, P.; Jordan, P.; Krauss, N. Structure of photosystem I. Biochim. Biophys. Acta Bioenerg. 2001, 1507, 5–31. [Google Scholar] [CrossRef]
- Fischer, N.; Hippler, M.; Sétif, P.; Jacquot, J.; Rochaix, J. The PsaC subunit of photosystem I provides an essential lysine residue for fast electron transfer to ferredoxin. EMBO J. 1998, 17, 849–858. [Google Scholar] [CrossRef]
- Surek, B.; Kreimer, G.; Melkonian, M.; Latzko, E. Spinach ferredoxin is a calcium-binding protein. Planta 1987, 171, 565–568. [Google Scholar] [CrossRef]
- Raines, C.A. The Calvin cycle revisited. Photosynth. Res. 2003, 75, 1–10. [Google Scholar] [CrossRef]
- Koßmann, J.; Sonnewald, U.; & Willmitzer, L. Reduction of the chloroplastic fructose-1,6-bisphosphatase in transgenic potato plants impairs photosynthesis and plant growth. Plant J. 1994, 6, 637–650. [Google Scholar] [CrossRef]
- Kreimer, G.; Melkonian, M.; Holtum, J.A.M.; Latzko, E. Stromal free calcium concentration and light-mediated activation of chloroplast fructose-1,6-bisphosphatase. Plant Physiol. 1988, 86, 423–428. [Google Scholar] [CrossRef]
- Rocha, A.G.; Mehlmer, N.; Stael, S.; Mair, A.; Parvin, N.; Chigri, F.; Teige, M.; Vothknecht, U.C. Phosphorylation of Arabidopsis transketolase at Ser428 provides a potential paradigm for the metabolic control of chloroplast carbon metabolism. Biochem. J. 2014, 458, 313. [Google Scholar] [CrossRef]
- Tamoi, M.; Miyazaki, T.; Fukamizo, T.; Shigeoka, S. The Calvin cycle in cyanobacteria is regulated by CP12 via the NAD(H)/NADP(H) ratio under light/dark conditions. Plant J. 2005, 42, 504–513. [Google Scholar] [CrossRef]
- López-Calcagno, P.E.; Howard, T.P.; Raines, C.A. The CP12 protein family: a thioredoxin-mediated metabolic switch? Front. Plant Sci. 2014, 5, 9. [Google Scholar] [CrossRef]
- Rocha, A.; Vothknecht, U. Identification of CP12 as a novel calcium-binding protein in chloroplasts. Plants 2013, 2, 530–540. [Google Scholar] [CrossRef]
- Campbell, D.; Bruce, D.; Carpenter, C.; Gustafsson, P.; Oquist, G. Two forms of the photosystem II D1 protein alter energy dissipation and state transitions in the cyanobacterium Synechococcus sp. PCC 7942. Photosynth. Res. 1996, 47, 131–144. [Google Scholar] [CrossRef]
- Li, Z.L.; Burnap, R.L. Mutations of Arginine 64 within the putative Ca2+-binding lumenal interhelical a-b loop of the photosystem II D1 protein disrupt binding of the manganese stabilizing protein and cytochrome c550 in Synechocystis sp. PCC6803. Biochemistry 2001, 40, 10350. [Google Scholar] [CrossRef]
- Ware, M.A.; Belgio, E.; Ruban, A.V. Comparison of the protective effectiveness of NPQ in Arabidopsis plants deficient in PsbS protein and zeaxanthin. J. Exp. Bot. 2015, 66, 1259–1270. [Google Scholar] [CrossRef]
- Dominici, P.; Caffarri, S.; Armenante, F.; Ceoldo, S.; Crimi, M.; Bassi, R. Biochemical properties of the PsbS subunit of photosystem II either purified from chloroplast or recombinant. J. Biol. Chem. 2002, 277, 22750–22758. [Google Scholar] [CrossRef]
- Vainonen, J.P.; Hansson, M.; Vener, A.V. STN8 Protein Kinase in Arabidopsis thaliana Is Specific in Phosphorylation of Photosystem II Core Proteins. J. Biol. Chem. 2005, 280, 33679–33686. [Google Scholar] [CrossRef]
- Chigri, F.; Hörmann, A.; Stamp, A.; Stammers, D.K.; Bölter, B.; Soll, J.; Vothknecht, U.C. Calcium regulation of chloroplast protein translocation is mediated by calmodulin binding to Tic32. Proc. Natl. Acad. Sci. USA 2006, 103, 16051–16056. [Google Scholar] [CrossRef]
- Balsera, M.; Goetze, T.A.; Kovacs-Bogdan, E.; Schürmann, P.; Wagner, R.; Buchanan, B.B.; Soll, J.; Bölter, B. Characterization of Tic110, a Channel-forming Protein at the Inner Envelope Membrane of Chloroplasts, Unveils a Response to Ca2+ and a Stromal Regulatory Disulfide Bridge. J. Biol. Chem. 2009, 284, 2603–2616. [Google Scholar] [CrossRef]
- Tozawa, Y.; Nozawa, A.; Kanno, T.; Narisawa, T.; Masuda, S.; Kasai, K.; Nanamiya, H. Calcium-activated (p)ppGpp Synthetase in Chloroplasts of Land Plants *. J. Biol. Chem. 2007, 282, 35536–35545. [Google Scholar] [CrossRef]
- Yang, T.; Poovaiah, B.W. Arabidopsis Chloroplast Chaperonin 10 Is a Calmodulin-Binding Protein. Biochem. Biophys. Res. Commun. 2000, 275. [Google Scholar] [CrossRef]
- Malmström, S.; Askerlund, P.; Palmgren, M.G. A calmodulin-stimulated Ca2+-ATPase from plant vacuolar membranes with a putative regulatory domain at its N-terminus. FEBS Lett. 1997, 400, 324. [Google Scholar] [CrossRef]
- Bussemer, J.; Chigri, F.; Vothknecht, U.C. Arabidopsis ATPase family gene 1-like protein 1 is a calmodulin-binding AAA+-ATPase with a dual localization in chloroplasts and mitochondria. FEBS J. 2010, 276, 3870–3880. [Google Scholar] [CrossRef]
- Wang, P.; Song, C. Guard-cell signaling for hydrogen peroxide and abscisic acid. New Phytol. 2008, 178, 703–718. [Google Scholar] [CrossRef]
- Wang, S.W.; Li, Y.; Zhang, X.L.; Yang, H.Q.; Han, X.F.; Liu, Z.H.; Shang, Z.L.; Asano, T.; Yoshioka, Y.; Zhang, C.G.; et al. Lacking chloroplasts in guard cells of crumpled leaf attenuates stomatal opening: both guard cell chloroplasts and mesophyll contribute to guard cell ATP levels. Plant Cell Environ. 2014, 37, 2201–2210. [Google Scholar] [CrossRef]
- Leshem, Y.; Levine, A. Zooming into sub-organellar localization of reactive oxygen species in guard cell chloroplasts during abscisic acid and methyl jasmonate treatments. Plant Signal. Behav. 2013, 8. [Google Scholar] [CrossRef]
- Allaway, W.G.; Setterfield, G. Ultrastructural observations on guard cells of Vicia faba and Allium porrum. Can. J. Bot. 1972, 50, 1405–1413. [Google Scholar] [CrossRef]
- Lawson, T. Guard cell photosynthesis and stomatal function. New Phytol. 2009, 181, 13–34. [Google Scholar] [CrossRef]
- Song, Y.; Miao, Y.; Song, C.P. Behind the scenes: the roles of reactive oxygen species in guard cells. New Phytol. 2014, 201, 1121–1140. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, L.; Galbraith, D.W.; Song, C.P. Hydrogen peroxide is involved in abscisic acid-induced stomatal closure in Vicia faba. Plant Physiol. 2001, 126, 1438–1448. [Google Scholar] [CrossRef]
- Sparla, F.; Costa, A.; Schiavo, F.L.; Pupillo, P.; Trost, P. Redox regulation of a novel plastid-targeted β-amylase of Arabidopsis. Plant Physiol. 2006, 141, 840. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Yi, X.Q.; Han, A.D.; Liu, T.W.; Chen, J.; Wu, F.H.; Dong, X.J.; He, J.X.; Pei, Z.M.; Zheng, H.L. Calcium-sensing receptor regulates stomatal closure through hydrogen peroxide and nitric oxide in response to extracellular calcium in Arabidopsis. J. Exp. Bot. 2012, 63, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Weinl, S.; Held, K.; Schlücking, K.; Steinhorst, L.; Kuhlgert, S.; Hippler, M.; Kudla, J. A plastid protein crucial for Ca2+-regulated stomatal responses. New Phytol. 2008, 179, 675. [Google Scholar] [CrossRef]
- Wang, W.H.; Zheng, H.L. Mechanisms for calcium sensing receptor-regulated stomatal closure in response to the extracellular calcium signal. Plant Signal. Behav. 2012, 7, 289. [Google Scholar] [CrossRef]
- Allen, G.J.; Chu, S.P.; Schumacher, K.; Shimazaki, C.T.; Vafeados, D.; Kemper, A.; Hawke, S.D.; Tallman, G.; Tsien, R.Y.; Harper, J.F.; et al. Alteration of stimulus-specific guard cell calcium oscillations and stomatal closing in Arabidopsis det3 mutant. Science 2000, 289, 2338–2342. [Google Scholar] [CrossRef]
- Wang, C.R.; Yang, A.F.; Yue, G.D.; Gao, Q.; Yin, H.Y.; Zhang, J.R. Enhanced expression of phospholipase C 1 (ZmPLC1) improves drought tolerance in transgenic maize. Planta 2008, 227, 1127. [Google Scholar] [CrossRef]
- Viswanathan, C.; Zhu, J.K. Molecular genetic analysis of cold-regulated gene transcription. Philos. Trans. Royal Soc. Lond. 2002, 357, 877. [Google Scholar] [CrossRef]
- Tang, R.H.; Han, S.; Zheng, H.; Cook, C.W.; Choi, C.S.; Woerner, T.E.; Jackson, R.B.; Pei, Z.M. Coupling diurnal cytosolic Ca2+ oscillations to the CAS-IP3 pathway in Arabidopsis. Science 2007, 315, 1423–1426. [Google Scholar] [CrossRef]
- Yoshioka, H.; Numata, N.; Nakajima, K.; Katou, S.; Kawakita, K.; Rowland, O.; Jones, J.D.; Doke, N. Nicotiana benthamiana gp91phox homologs NbrbohA and NbrbohB participate in H2O2 accumulation and resistance to phytophthora infestans. Plant Cell 2003, 15, 706–718. [Google Scholar] [CrossRef]
- Sang, Y.; Cui, D.; Wang, X. Phospholipase d and phosphatidic acid-mediated generation of superoxide in Arabidopsis. Plant Physiol. 2001, 126, 1449–1458. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, H.; Zhang, Q.; Li, M.; Yan, M.; Wang, R.; Wang, L.; Welti, R.; Zhang, W.; Wang, X. Phospholipase Dα1 and phosphatidic acid regulate NADPH oxidase activity and production of reactive oxygen species in ABA-mediated stomatal closure in Arabidopsis. Plant Cell 2009, 21, 2357–2377. [Google Scholar] [CrossRef]
- Distéfano, A.M.; García-Mata, C.; Lamattina, L.; Laxalt, A.M. Nitric oxide-induced phosphatidic acid accumulation: a role for phospholipases C and D in stomatal closure. Plant Cell Environ. 2008, 31, 187–194. [Google Scholar] [CrossRef]
- Singh, R.; Parihar, P.; Singh, S.; Mishra, R.K.; Singh, V.P.; Prasad, S.M. Reactive oxygen species signaling and stomatal movement: Current updates and future perspectives. Redox Biol. 2017, 11, 213–218. [Google Scholar] [CrossRef]
- Tyryshkin, A.M.; Watt, R.K.; Baranov, S.V.; Dasgupta, J.; Hendrich, M.P.; Dismukes, G.C. Spectroscopic evidence for Ca2+ involvement in the assembly of the Mn4Ca cluster in the photosynthetic water-oxidizing complex. Biochemistry 2006, 45, 12876–12889. [Google Scholar] [CrossRef]
- Dau, H.; Haumann, M. Eight steps preceding O-O bond formation in oxygenic photosynthesis--a basic reaction cycle of the photosystem ii manganese complex. Biochim. Biophys. Acta. 2007, 1767, 472. [Google Scholar] [CrossRef]
- Nagao, R.; Suzuki, T.; Okumura, A.; Niikura, A.; Iwai, M.; Dohmae, N.; Tomo, T.; Shen, J.R.; Ikeuchi, M.; Enami, I. Topological analysis of the extrinsic PsbO, PsbP and PsbQ proteins in a green algal PSⅡ complex by cross-linking with a water-soluble carbodiimide. Plant Cell Physiol. 2010, 51, 718–727. [Google Scholar] [CrossRef]
- Kentaro, I.; Takumi, N. Structural Coupling of Extrinsic Proteins with the Oxygen-Evolving Center in Photosystem II. Front. Plant Sci. 2016, 7, 84. [Google Scholar] [CrossRef]
- Miqyass, M.; Marosvölgyi, M.A.; Nagel, Z.; Yocum, C.F.; Gorkom, H.J.V. S-state dependence of the calcium requirement and binding characteristics in the oxygen-evolving complex of photosystem II. Biochemistry 2008, 47, 7915. [Google Scholar] [CrossRef]
- Ferreira, K.N.; Iverson, T.M.; Maghlaoui, K.; Barber, J.; Iwata, S. Architecture of the photosynthetic oxygen-evolving center. Science 2004, 303, 1831–1838. [Google Scholar] [CrossRef]
- Perez-Navarro, M.; Neese, F.; Lubitz, W.; Pantazis, D.A.; Cox, N. Recent developments in biological water oxidation. Curr. Opin. Chem. Biol. 2016, 31, 113–119. [Google Scholar] [CrossRef]
- Najafpour, M.M.; Renger, G.; Hołyńska, M.; Moghaddam, A.N.; Aro, E.M.; Carpentier, R.; Nishihara, H.; Eaton-Rye, J.J.; Shen, J.R.; Allakhverdiev, S.I. Manganese Compounds as Water-Oxidizing Catalysts: From the Natural Water-Oxidizing Complex to Nano-sized Manganese Oxide Structures. Chem. Rev. 2016, 116, 2886–2936. [Google Scholar] [CrossRef]
- Kukuczka, B.; Magneschi, L.; Petroutsos, D.; Steinbeck, J.; Bald, T.; Powikrowska, M.; Fufezan, C.; Finazzi, G.; Hippler, M. Proton Gradient Regulation5-Like1-Mediated Cyclic Electron Flow Is Crucial for Acclimation to Anoxia and Complementary to Nonphotochemical Quenching in Stress Adaptation. Plant Physiol. 2014, 165, 1604–1617. [Google Scholar] [CrossRef]
- Golbeck, J.H. Structure and function of photosystem I. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 3204–3210. [Google Scholar] [CrossRef]
- Fischer, N.; Setif, P.; Rochaix, J.D. Targeted mutations in the psaC gene of chlamydomonas reinhardtii: preferential reduction of FD at low temperature is not accompanied by altered electron flow from photosystem I to ferredoxin. Biochemistry 1997, 36, 93–102. [Google Scholar] [CrossRef]
- Munekage, Y.; Hojo, M.; Meurer, J.; Endo, T.; Tasaka, M.; Shikanai, T. PGR5 Is Involved in Cyclic Electron Flow around Photosystem I and Is Essential for Photoprotection in Arabidopsis. Cell 2002, 110, 0–371. [Google Scholar] [CrossRef]
- Iwai, M.; Takizawa, K.; Tokutsu, R.; Okamuro, A.; Takahashi, Y.; Minagawa, J. Isolation of the elusive supercomplex that drives cyclic electron flow in photosynthesis. Nature 2010, 464, 1210–1213. [Google Scholar] [CrossRef]
- Terashima, M.; Petroutsos, D.; Hudig, M.; Tolstygina, I.; Trompelt, K.; Gäbelein, P.; Fufezan, C.; Kudla, J.; Weinl, S.; Finazzi, G.; et al. Calcium-dependent regulation of cyclic photosynthetic electron transfer by a CAS, ANR1, and PGRL1 complex. Proc. Natl. Acad. Sci. USA. 2012, 109, 17717–17722. [Google Scholar] [CrossRef]
- Ishida, S.; Takabayashi, A.; Ishikawa, N.; Hano, Y.; Endo, T.; Sato, F. A Novel Nuclear-Encoded Protein, NDH-Dependent Cyclic Electron Flow 5, is Essential for the Accumulation of Chloroplast NAD(P)H Dehydrogenase Complexes. Plant Cell Physiol. 2009, 50, 383. [Google Scholar] [CrossRef]
- Peltier, G.; Aro, E.M.; Shikanai, T. NDH-1 and NDH-2 Plastoquinone Reductases in Oxygenic Photosynthesis. Ann. Rev. Plant Biol. 2015, 67, 55–80. [Google Scholar] [CrossRef]
- Lascano, H.R.; Casano, L.M.; Martín, M.; Sabater, B. The Activity of the Chloroplastic Ndh Complex Is Regulated by Phosphorylation of the NDH-F Subunit1. Plant Physiol. 2003, 132, 256–262. [Google Scholar] [CrossRef]
- Rojas-González, J.A.; Soto-Súarez, M.; García-Díaz, Á.; Romero-Puertas, M.C.; Sandalio, L.M.; Mérida, Á.; Thormählen, I.; Geigenberger, P.; Serrato, A.J.; Sahrawy, M. Disruption of both chloroplastic and cytosolic FBPase genes results in a dwarf phenotype and important starch and metabolite changes in Arabidopsis thaliana. J. Exp. Bot. 2015, 66, 2673–2689. [Google Scholar] [CrossRef]
- Portis, A.R.; Heldt, H.W. Light-dependent changes of the Mg2+ concentration in the stroma in relation to the Mg2+ dependency of CO2 fixation in intact chloroplasts. Biochim. Biophys. Acta. 1976, 449, 434–446. [Google Scholar] [CrossRef]
- Villafranca, J.J.; Axelrod, B. Heptulose synthesis from nonphosphorylated aldoses and ketoses by spinach transketolase. J. Biol. Chem. 1971, 246, 3126–3131. [Google Scholar] [CrossRef]
- Delobel, A.; Graciet, E.; Andreescu, S.; Gontero, B.; Halgand, F.; Laprévote, O. Mass spectrometric analysis of the interactions between CP12, a chloroplast protein, and metal ions: a possible regulatory role within a PRK/GAPDH/CP12 complex. Rapid Commun. Mass Spectrom. 2010, 19, 3379–3388. [Google Scholar] [CrossRef]
- Choudhury, N.K.; Behera, R.K. Photoinhibition of photosynthesis: role of carotenoids in photoprotection of chloroplast constituents. Photosynthetica 2001, 39, 481–488. [Google Scholar] [CrossRef]
- Krause, G.H.; Weis, E. Chlorophyll fluorescence and photosynthesis: the basics. Annu. Rev. Plant Physiol. 1991, 42, 313–349. [Google Scholar] [CrossRef]
- Białasek, M.; Górecka, M.; Mittler, R.; Karpiński, S. Evidence for the involvement of electrical, calcium and ros signaling in the systemic regulation of non-photochemical quenching and photosynthesis. Plant Cell Physiol. 2017, 58, 207–215. [Google Scholar] [CrossRef]
- Yang, S.; Wang, F.; Guo, F.; Meng, J.J.; Li, X.G.; Wan, S.B. Calcium contributes to photoprotection and repair of photosystem II in peanut leaves during heat and high irradiance. J. Integr. Plant Biol. 2015, 57, 486–495. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Yamamoto, H.; Allakhverdiev, S.I.; Inaba, M.; Yokota, A.; Murata, N. Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery. EMBO J. 2014, 20, 5587–5594. [Google Scholar] [CrossRef]
- Harada, A.; Sakai, T.; Okada, K. Phot1 and Phot2 Mediate Blue Light-Induced Transient Increases in Cytosolic Ca2+, Differently in Arabidopsis Leaves. Proc. Natl. Acad. Sci. USA. 2003, 100, 8583–8588. [Google Scholar] [CrossRef]
- Aggarwal, C.; Łabuz, J.; Gabryś, H. Phosphoinositides Play Differential Roles in Regulating Phototropin1- and Phototropin2-Mediated Chloroplast Movements in Arabidopsis. PLoS ONE 2013, 8, e55393. [Google Scholar] [CrossRef]
- Yokota, E.; Tominaga, M.; Mabuchi, I.; Tsuji, Y.; Staiger, C.J.; Oiwa, K.; Shimmen, T. Plant villin, lily P-135-ABP, possesses G-actin binding activity and accelerates the polymerization and depolymerization of actin in a Ca2+-sensitive manner. Plant Cell Physiol. 2005, 46, 1690–1703. [Google Scholar] [CrossRef]
- Sato, Y.; Wada, M.; Kadota, A. Choice of tracks, microtubules and/or actin filaments for chloroplast photo-movement is differentially controlled by phytochrome and a blue light receptor. J. Cell Sci. 2001, 114, 269–279. [Google Scholar] [CrossRef]
- Ekkehard, S.; Meyer-Wegener, J.; Elfriede, S. No evidence for Ca2+ influx as an essential link in the signal transduction chains of either light-oriented chloroplast movements or Pfr-mediated chloroplast anchorage in Mougeotia. J. Photochem. Photobiol. B. 1990, 5, 331–341. [Google Scholar] [CrossRef]
- Takamatsu, H.; Takagi, S. Actin-Dependent Chloroplast Anchoring is Regulated by Ca2+-Calmodulin in Spinach Mesophyll Cells. Plant Cell Physiol. 2011, 52, 1973–1982. [Google Scholar] [CrossRef]
- Wang, L.; Yamano, T.; Takane, S.; Niikawa, Y.; Toyokawa, C.; Ozawa, S.I.; Tokutsu, R.; Takahashi, Y.; Minagawa, J.; Kanesaki, Y.; et al. Chloroplast-mediated regulation of CO2-concentrating mechanism by Ca2+-binding protein CAS in the green alga Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA. 2016, 113, 12586. [Google Scholar] [CrossRef]
- Yamano, T.; Toyokawa, C.; Fukuzawa, H. High-resolution suborganellar localization of Ca2+-binding protein CAS, a novel regulator of CO2-concentrating mechanism. Protoplasma 2018, 255, 1015–1022. [Google Scholar] [CrossRef]
- Reiland, S.; Finazzi, G.; Endler, A.; Willig, A.; Baerenfaller, K.; Grossmann, J.; Gerrits, B.; Rutishauser, D.; Gruissem, W.; Rochaix, J.D.; et al. Comparative phosphoproteome profiling reveals a function of the STN8 kinase in fine-tuning of cyclic electron flow (CEF). Proc. Natl. Acad. Sci. U.S.A. 2011, 108, 108,12955–12960. [Google Scholar] [CrossRef] [PubMed]
- Tikkanen, M.; Nurmi, M.; Kangasjärvi, S.; Aro, E.M. Core protein phosphorylation facilitates the repair of photodamaged photosystem II at high light. Biochim. Biophys. Acta Bioenerg. 2008, 1777, 1432–1437. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, R.S.; Nath, K.; Zulfugarov, I.S.; Lee, C.H. Production of superoxide from photosystem II-light harvesting complex II supercomplex in STN8 kinase knock-out rice mutants under photoinhibitory illumination. J. Photochem. Photobiol. B. 2016, 162, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Kovács-Bogdán, E.; Soll, J.; Bölter, B. Protein import into chloroplasts: The Tic complex and its regulation. Biochim. Biophys. Acta. 2010, 1803, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Hood, R.D.; Higgins, S.A.; Flamholz, A.; Nichols, R.J.; Savage, D.F. The stringent response regulates adaptation to darkness in the cyanobacterium Synechococcus elongatus. Proc. Natl. Acad. Sci. USA. 2016, 113, e4867–e4876. [Google Scholar] [CrossRef] [PubMed]
- Field, B. Green magic: regulation of the chloroplast stress response by (p)ppGpp in plants and algae. J. Exp. Bot. 2018, 69, 2797–2807. [Google Scholar] [CrossRef] [PubMed]
- Sugliani, M.; Abdelkefi, H.; Ke, H.; Bouveret, E.; Robaglia, C.; Caffarri, S.; Field, B. An ancient bacterial signaling pathway controls chloroplast function to regulate growth and development in Arabidopsis. Plant Cell 2016, 28, 661. [Google Scholar] [CrossRef] [PubMed]
- Koumoto, Y.; Shimada, T.; Kondo, M.; Hara-Nishimura, I.; Nishimura, M. Chloroplasts Have a Novel Cpn10 in Addition to Cpn20 as Co-chaperonins in Arabidopsis thaliana. J. Biol. Chem. 2001, 276, 29688–29694. [Google Scholar] [CrossRef] [PubMed]
| Protein | Function Related to Photosynthesis | References | Function Related to Calcium | References |
|---|---|---|---|---|
| CAS | Stomatal regulation; photosynthetic electron flow; Regulate CCM | [12,13] | Ca2+-binding | [12,13,14,15] |
| RbohD/F | Stomatal regulation | [16,17] | Ca2+-binding | [18] |
| PsbO | The OEC subunit protein; | [19] | The Mn4CaO5 cluster as co-factor | [20,21] |
| PsbQ/PsbP | The OEC subunit protein; | [19,22] | The Cl- and Ca2+ as essential co-factors | [19,23] |
| PsaN | Regulate photosynthetic electron flow | [24] | Regulated by Ca2+/CaM | [24] |
| PsaA/PsaL | The PS1 subunit proteins; | [25] | Possibly a Ca2+ coordinate the two proteins | [25] |
| FD | Electron transport of PS 1 | [26] | High affinity with Ca2+ | [27] |
| FBPase/SBPase | The Calvin cycle key enzymes | [28,29] | Regulated by Ca2+ | [30] |
| TKL | The Calvin cycle key enzymes | [31] | Ca2+-dependent phosphorylation | [31] |
| CP12 | Regulate the Calvin cycle | [32,33] | Ca2+-binding | [34] |
| D1 protein | Regulate NPQ | [35] | Ca2+-binding | [36] |
| PsbS | Regulate NPQ | [37] | Regulated by Ca2+ | [38] |
| VDE | Regulate xanthophyll cycle | [10] | Regulated by Ca2+ and CaM | [10] |
| STN8 | Phosphorylate thylakoid membrane proteins | [39] | Interaction with CAS | [7] |
| Tic110 | Chloroplast inner envelope protein | [40] | Regulated by Ca2+ | [41] |
| Tic32 | Chloroplast inner envelope protein | [40] | Regulated by Ca2+ | [41] |
| (p)ppGpp | The regulator in chloroplast function | [42] | Ca2+-binding | [42] |
| ch-CPN10 | Assist chloroplast protein folding | [43] | CaM-binding | [43] |
| SAMTL | Chloroplast inner envelope protein | [8] | Regulated by Ca2+ | [8] |
| ACA1 | Chloroplast inner envelope protein | [44] | Ca2+ ATPase | [44] |
| AFG1L1 | Chloroplast protein | [45] | CaM-binding | [45] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Yang, S.; Wan, S.; Li, X. The Significance of Calcium in Photosynthesis. Int. J. Mol. Sci. 2019, 20, 1353. https://doi.org/10.3390/ijms20061353
Wang Q, Yang S, Wan S, Li X. The Significance of Calcium in Photosynthesis. International Journal of Molecular Sciences. 2019; 20(6):1353. https://doi.org/10.3390/ijms20061353
Chicago/Turabian StyleWang, Quan, Sha Yang, Shubo Wan, and Xinguo Li. 2019. "The Significance of Calcium in Photosynthesis" International Journal of Molecular Sciences 20, no. 6: 1353. https://doi.org/10.3390/ijms20061353
APA StyleWang, Q., Yang, S., Wan, S., & Li, X. (2019). The Significance of Calcium in Photosynthesis. International Journal of Molecular Sciences, 20(6), 1353. https://doi.org/10.3390/ijms20061353





