A Novel Rare Missense Variation of the NOD2 Gene: Evidences of Implication in Crohn’s Disease
Abstract
1. Introduction
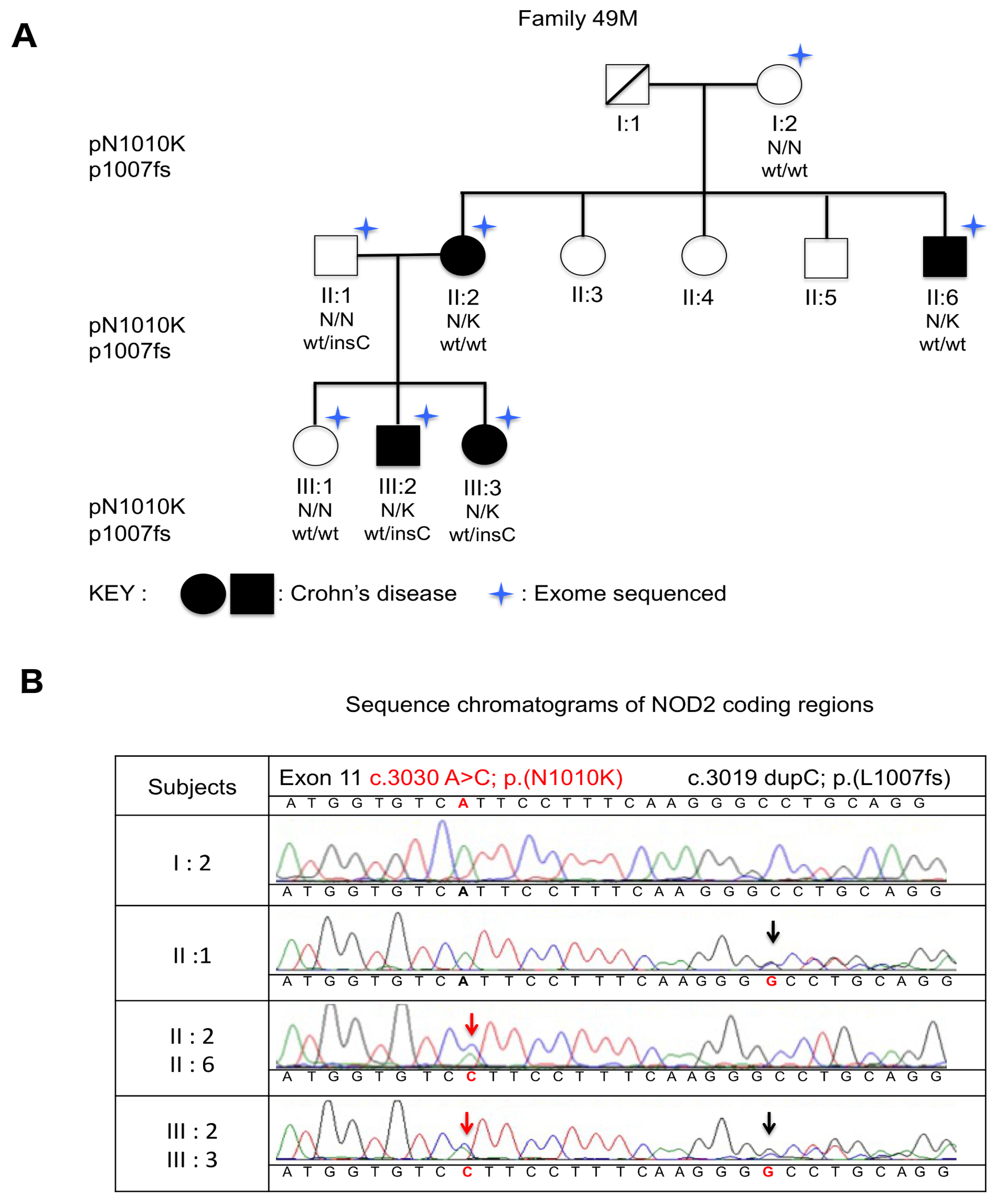
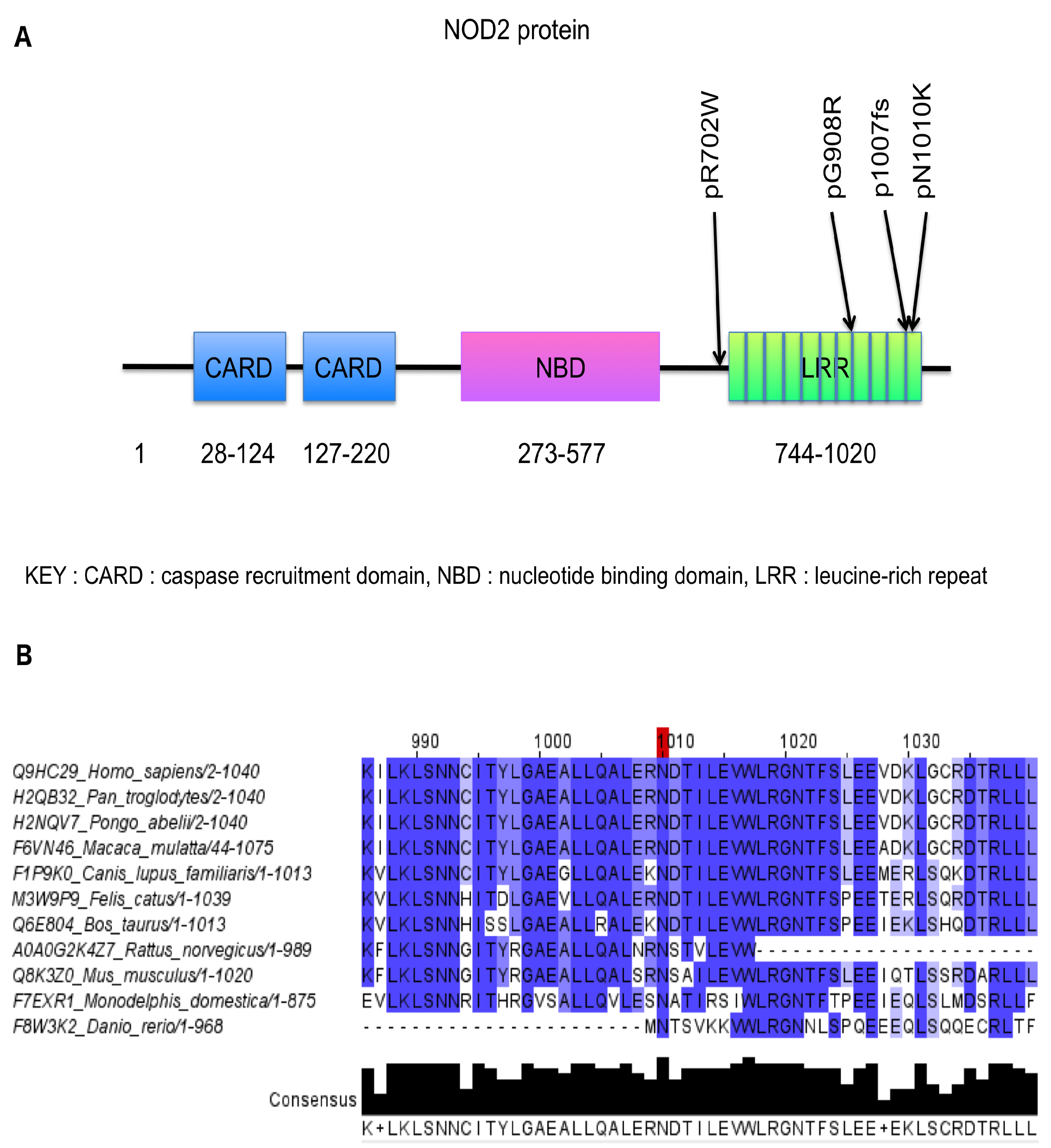
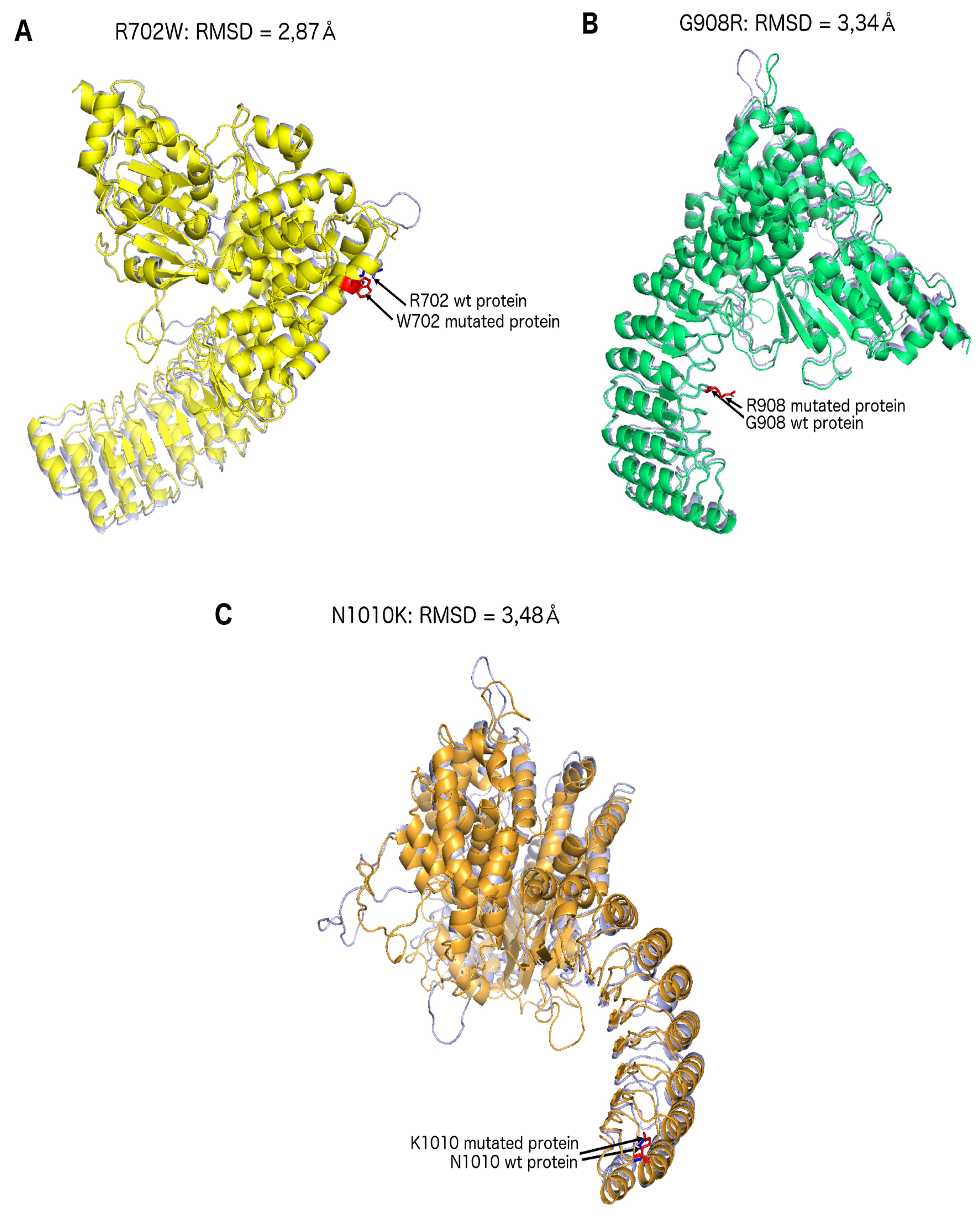
2. Results
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Exome Sequencing Analysis and Computer Analyses
4.3. Sanger Sequencing Confirmation of NOD2 Variants
4.4. Structural Predictions: NOD2
4.5. In Silico Predictions and Annotations
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CD | Crohn’s disease |
| DOAJ | Directory of open access journals |
| GWAS | Genome wide association study |
| IBD | Inflammatory Bowel Disease |
| LD | Linear Dichroism |
| LRR | Leucine-Rich Repeat |
| MAF | Minor Allele Frequency |
| MDPI | Multidisciplinary Digital Publishing Institute |
| NLR | NOD-like receptor |
| PCR | Polymerase Chain Reaction |
| RMSD | Root-Mean-Square-Deviation |
| TLA | Three letter acronym |
| WES | Whole Exome Sequencing |
References
- Qin, X. Etiology of inflammatory bowel disease: A unified hypothesis. World J. Gastroenterol. 2012, 18, 1708–1722. [Google Scholar] [CrossRef] [PubMed]
- Hugot, J.P.; Chamaillard, M.; Zouali, H.; Lesage, S.; Cézard, J.P.; Belaiche, J.; Almer, S.; Tysk, C.; O’Morain, C.A.; Gassull, M.; et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 2001, 411, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Lesage, S.; Zouali, H.; Cézard, J.-P.; Colombel, J.-F.; Belaiche, J.; Almer, S.; Tysk, C.; O’Morain, C.; Gassull, M.; Binder, V.; et al. CARD15/NOD2 Mutational Analysis and Genotype-Phenotype Correlation in 612 Patients with Inflammatory Bowel Disease. Am. J. Hum. Genet. 2002, 70, 845–857. [Google Scholar] [CrossRef] [PubMed]
- Rivas, M.A.; Beaudoin, M.; Gardet, A.; Stevens, C.; Sharma, Y.; Zhang, C.K.; Boucher, G.; Ripke, S.; Ellinghaus, D.; Burtt, N.; et al. Deep resequencing of GWAS loci identifies independent rare variants associated with inflammatory bowel disease. Nat. Genet. 2011, 43, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Franke, A.; McGovern, D.P.B.; Barrett, J.C.; Wang, K.; Radford-Smith, G.L.; Ahmad, T.; Lees, C.W.; Balschun, T.; Lee, J.; Roberts, R.; et al. Genome-wide meta-analysis increases to 71 the number of confirmed Crohn’s disease susceptibility loci. Nat. Genet. 2010, 43, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Gower-Rousseau, C.; Salomez, J.L.; Dupas, J.L.; Marti, R.; Nuttens, M.C.; Votte, A.; Lemahieu, M.; Lemaire, B.; Colombel, J.F.; Cortot, A. Incidence of inflammatory bowel disease in northern France (1988–1990). Gut 1994, 35, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Gower-Rousseau, C.; Vasseur, F.; Fumery, M.; Savoye, G.; Salleron, J.; Dauchet, L.; Turck, D.; Cortot, A.; Peyrin-Biroulet, L.; Colombel, J.-F. Epidemiology of inflammatory bowel diseases: New insights from a French population-based registry (EPIMAD). Dig. Liver Dis. 2013, 45, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Vasseur, F.; Sendid, B.; Jouault, T.; Standaert-Vitse, A.; Dubuquoy, L.; Francois, N.; Gower-Rousseau, C.; Desreumaux, P.; Broly, F.; Vermeire, S.; et al. Variants of NOD1 and NOD2 genes display opposite associations with familial risk of Crohn’s disease and anti-saccharomyces cerevisiae antibody levels. Inflamm. Bowel Dis. 2012, 18, 430–438. [Google Scholar] [CrossRef]
- Ogura, Y.; Bonen, D.K.; Inohara, N.; Nicolae, D.L.; Chen, F.F.; Ramos, R.; Britton, H.; Moran, T.; Karaliuskas, R.; Duerr, R.H.; et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 2001, 411, 603–606. [Google Scholar] [CrossRef]
- Philpott, D.J.; Sorbara, M.T.; Robertson, S.J.; Croitoru, K.; Girardin, S.E. NOD proteins: Regulators of inflammation in health and disease. Nat. Rev. Immunol. 2014, 14, 9–23. [Google Scholar] [CrossRef]
- Inohara; Chamaillard; McDonald, C.; Nuñez, G. NOD-LRR proteins: Role in host-microbial interactions and inflammatory disease. Annu. Rev. Biochem. 2005, 74, 355–383. [Google Scholar] [CrossRef] [PubMed]
- Inohara, N.; Ogura, Y.; Fontalba, A.; Gutierrez, O.; Pons, F.; Crespo, J.; Fukase, K.; Inamura, S.; Kusumoto, S.; Hashimoto, M.; et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J. Biol. Chem. 2003, 278, 5509–5512. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H. The genetics and immunopathogenesis of inflammatory bowel disease. Nat. Rev. Immunol. 2008, 8, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Gavel, Y.; von Heijne, G. Sequence differences between glycosylated and non-glycosylated Asn-X-Thr/Ser acceptor sites: Implications for protein engineering. Protein Eng. 1990, 3, 433–442. [Google Scholar] [CrossRef]
- Maekawa, S.; Ohto, U.; Shibata, T.; Miyake, K.; Shimizu, T. Crystal structure of NOD2 and its implications in human disease. Nat. Commun. 2016, 7, 11813. [Google Scholar] [CrossRef] [PubMed]
- Barnich, N.; Hisamatsu, T.; Aguirre, J.E.; Xavier, R.; Reinecker, H.-C.; Podolsky, D.K. GRIM-19 interacts with nucleotide oligomerization domain 2 and serves as downstream effector of anti-bacterial function in intestinal epithelial cells. J. Biol. Chem. 2005, 280, 19021–19026. [Google Scholar] [CrossRef] [PubMed]
- Girardelli, M.; Vuch, J.; Tommasini, A.; Crovella, S.; Bianco, A.M. Novel missense mutation in the NOD2 gene in a patient with early onset ulcerative colitis: Causal or chance association? Int. J. Mol. Sci. 2014, 15, 3834–3841. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Salem, M.; Boyd, M.; Bornholdt, J.; Li, Y.; Coskun, M.; Seidelin, J.B.; Sandelin, A.; Nielsen, O.H. Relation between NOD2 genotype and changes in innate signaling in Crohn’s disease on mRNA and miRNA levels. NPJ Genom. Med. 2017, 2, 3. [Google Scholar] [CrossRef] [PubMed]
- Molinié, F.; Gower-Rousseau, C.; Yzet, T.; Merle, V.; Grandbastien, B.; Marti, R.; Lerebours, E.; Dupas, J.L.; Colombel, J.F.; Salomez, J.L.; et al. Opposite evolution in incidence of Crohn’s disease and ulcerative colitis in Northern France (1988–1999). Gut 2004, 53, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Maiti, R.; Van Domselaar, G.H.; Zhang, H.; Wishart, D.S. SuperPose: A simple server for sophisticated structural superposition. Nucleic Acids Res. 2004, 117, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Kircher, M.; Witten, D.M.; Jain, P.; O’Roak, B.J.; Cooper, G.M.; Shendure, J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 2014, 46, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Ng, P.C.; Henikoff, S. Predicting deleterious amino acid substitutions. Genome Res. 2001, 11, 863–874. [Google Scholar] [CrossRef] [PubMed]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A method and server for predicting damaging missense mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef] [PubMed]
| R702W | G908R | L1007fs | N1010K | |
|---|---|---|---|---|
| ExAC MAF in Non-Finnish CEU | 0.04307 | 0.01187 | 0.02319 | 0 |
| GnomAD MAF | 0.02355 | 0.007589 | 0.01520 | 0 |
| Kaviar MAF | 0.2409 | 0.009595 | 0.01279 | 0 |
| CADD Phred | 24.6 | 29.8 | 35.0 | 22.6 |
| SIFT | 0.01 | 0.01 | N/A | 0 |
| PolyPhen2 | 0.72 | 0.986 | N/A | 0.996 |
| Grantham Score | 101 | 125 | N/A | 94 |
| Modelisation gap | 2.87 Å | 3.34 Å | N/A | 3.48 Å |
| Patient Identification | Age at Diagnosis (y) | Location at Diagnosis | Behaviour at Diagnosis |
|---|---|---|---|
| II:2 N1010K | 30/A2 | L3 | B2 |
| II:6 N1010K | 24/A2 | L1 | B3 |
| III:2 1007fs + N1010K | 8/A1 | L3 | B1 |
| III:3 1007fs + N1010K | 15/A1 | L3 | B1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frade-Proud’Hon-Clerc, S.; Smol, T.; Frenois, F.; Sand, O.; Vaillant, E.; Dhennin, V.; Bonnefond, A.; Froguel, P.; Fumery, M.; Guillon-Dellac, N.; et al. A Novel Rare Missense Variation of the NOD2 Gene: Evidences of Implication in Crohn’s Disease. Int. J. Mol. Sci. 2019, 20, 835. https://doi.org/10.3390/ijms20040835
Frade-Proud’Hon-Clerc S, Smol T, Frenois F, Sand O, Vaillant E, Dhennin V, Bonnefond A, Froguel P, Fumery M, Guillon-Dellac N, et al. A Novel Rare Missense Variation of the NOD2 Gene: Evidences of Implication in Crohn’s Disease. International Journal of Molecular Sciences. 2019; 20(4):835. https://doi.org/10.3390/ijms20040835
Chicago/Turabian StyleFrade-Proud’Hon-Clerc, Sara, Thomas Smol, Frédéric Frenois, Olivier Sand, Emmanuel Vaillant, Véronique Dhennin, Amélie Bonnefond, Philippe Froguel, Mathurin Fumery, Nathalie Guillon-Dellac, and et al. 2019. "A Novel Rare Missense Variation of the NOD2 Gene: Evidences of Implication in Crohn’s Disease" International Journal of Molecular Sciences 20, no. 4: 835. https://doi.org/10.3390/ijms20040835
APA StyleFrade-Proud’Hon-Clerc, S., Smol, T., Frenois, F., Sand, O., Vaillant, E., Dhennin, V., Bonnefond, A., Froguel, P., Fumery, M., Guillon-Dellac, N., Gower-Rousseau, C., & Vasseur, F. (2019). A Novel Rare Missense Variation of the NOD2 Gene: Evidences of Implication in Crohn’s Disease. International Journal of Molecular Sciences, 20(4), 835. https://doi.org/10.3390/ijms20040835





