The Role of Phytochemicals and Plant-Based Diets in Gestational Diabetes: Evidence from Clinical Trials
Abstract
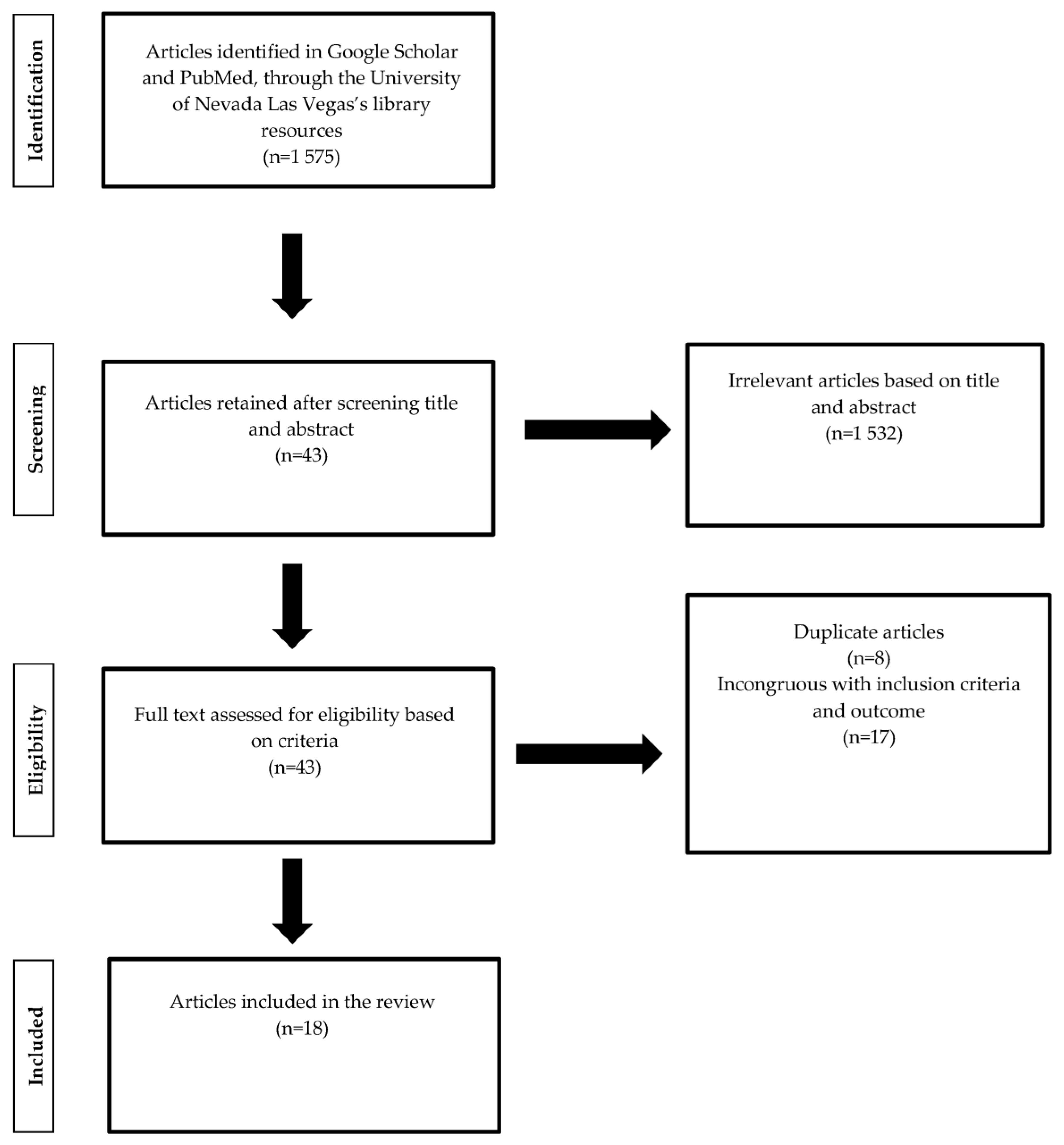
:1. Introduction
2. Methods
3. Results
3.1. Plant-Based Food Groups, Diet and GDM
3.1.1. Blood Glucose Markers
3.1.2. HOMA-IR
3.1.3. Blood Lipids
3.2. Plant-Based Supplements and GDM
3.2.1. Blood Glucose Markers
3.2.2. HOMA-IR
3.2.3. Blood Lipids
4. Discussion
4.1. Plant-Based Diets and Food Groups
4.2. Plant-Based Supplements
4.3. Strengths and Limitations
4.4. Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Casagrande, S.S.; Linder, B.; Cowie, C.C. Prevalence of gestational diabetes and subsequent Type 2 diabetes among U.S. women. Diabetes Res. Clin. Pract. 2018, 141, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.C.; Shah, N.S.; Petito, L.C.; Gunderson, E.P.; Grobman, W.A.; O’Brien, M.J.; Khan, S.S. Gestational Diabetes and Overweight/Obesity: Analysis of Nulliparous Women in the U.S., 2011–2019. Am. J. Prev. Med. 2021, 61, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Egan, A.M.; Vellinga, A.; Harreiter, J.; Simmons, D.; Desoye, G.; Corcoy, R.; Adelantado, J.M.; Devlieger, R.; Van Assche, A.; Galjaard, S.; et al. Epidemiology of gestational diabetes mellitus according to IADPSG/WHO 2013 criteria among obese pregnant women in Europe. Diabetologia 2017, 60, 1913–1921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, Y.; Li, W.; Liu, H.; Wang, L.; Zhang, S.; Li, W.; Liu, H.; Leng, J.; Shen, Y.; Tuomilehto, J.; et al. Effects of obesity and a history of gestational diabetes on the risk of postpartum diabetes and hyperglycemia in Chinese women: Obesity, GDM and diabetes risk. Diabetes Res. Clin. Pract. 2019, 156, 107828. [Google Scholar] [CrossRef]
- Harrison, C.L.; Lombard, C.B.; Strauss, B.J.; Teede, H.J. Optimizing healthy gestational weight gain in women at high risk of gestational diabetes: A randomized controlled trial. Obesity 2013, 21, 904–909. [Google Scholar] [CrossRef]
- Pantham, P.; Aye, I.L.; Powell, T.L. Inflammation in maternal obesity and gestational diabetes mellitus. Placenta 2015, 36, 709–715. [Google Scholar] [CrossRef] [Green Version]
- Davis, D.W.; Tallent, R.; Navalta, J.W.; Salazar, A.; Lyons, T.J.; Basu, A. Effects of Acute Cocoa Supplementation on Postprandial Apolipoproteins, Lipoprotein Subclasses, and Inflammatory Biomarkers in Adults with Type 2 Diabetes after a High-Fat Meal. Nutrients 2020, 12, 1902. [Google Scholar] [CrossRef]
- Xiao, J.; Bai, W. Bioactive phytochemicals. Crit. Rev. Food Sci. Nutr. 2019, 59, 827–829. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Superfruits: Phytochemicals, antioxidant efficacies, and health effects—A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1580–1604. [Google Scholar] [CrossRef]
- Yen, I.W.; Lee, C.N.; Lin, M.W.; Fan, K.C.; Wei, J.N.; Chen, K.Y.; Chen, S.C.; Tai, Y.Y.; Kuo, C.H.; Lin, C.H.; et al. Overweight and obesity are associated with clustering of metabolic risk factors in early pregnancy and the risk of GDM. PLoS ONE 2019, 14, e0225978. [Google Scholar] [CrossRef]
- Hashim, S.A.; Mohd Yusof, B.N.; Abu Saad, H.; Ismail, S.; Hamdy, O.; Mansour, A.A. Effectiveness of simplified diabetes nutrition education on glycemic control and other diabetes-related outcomes in patients with type 2 diabetes mellitus. Clin. Nutr. ESPEN 2021, 45, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Barati, Z.; Iravani, M.; Karandish, M.; Haghighizadeh, M.H.; Masihi, S. The effect of oat bran consumption on gestational diabetes: A randomized controlled clinical trial. BMC Endocr. Disord. 2021, 21, 67. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Crew, J.; Ebersole, J.L.; Kinney, J.W.; Salazar, A.M.; Planinic, P.; Alexander, J.M. Dietary Blueberry and Soluble Fiber Improve Serum Antioxidant and Adipokine Biomarkers and Lipid Peroxidation in Pregnant Women with Obesity and at Risk for Gestational Diabetes. Antioxidants 2021, 10, 1318. [Google Scholar] [CrossRef] [PubMed]
- Basu, A.; Feng, D.; Planinic, P.; Ebersole, J.L.; Lyons, T.J.; Alexander, J.M. Dietary Blueberry and Soluble Fiber Supplementation Reduces Risk of Gestational Diabetes in Women with Obesity in a Randomized Controlled Trial. J. Nutr. 2021, 151, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Liu, H.; Li, Z.; Carughi, A.; Ge, S. Acute Effect of Pistachio Intake on Postprandial Glycemic and Gut Hormone Responses in Women With Gestational Diabetes or Gestational Impaired Glucose Tolerance: A Randomized, Controlled, Crossover Study. Front. Nutr. 2019, 6, 186. [Google Scholar] [CrossRef] [Green Version]
- Assaf-Balut, C.; Garcia de la Torre, N.; Duran, A.; Fuentes, M.; Bordiu, E.; Del Valle, L.; Familiar, C.; Ortola, A.; Jimenez, I.; Herraiz, M.A.; et al. A Mediterranean diet with additional extra virgin olive oil and pistachios reduces the incidence of gestational diabetes mellitus (GDM): A randomized controlled trial: The St. Carlos GDM prevention study. PLoS ONE 2017, 12, e0185873. [Google Scholar] [CrossRef]
- Sahariah, S.A.; Potdar, R.D.; Gandhi, M.; Kehoe, S.H.; Brown, N.; Sane, H.; Coakley, P.J.; Marley-Zagar, E.; Chopra, H.; Shivshankaran, D.; et al. A Daily Snack Containing Leafy Green Vegetables, Fruit, and Milk before and during Pregnancy Prevents Gestational Diabetes in a Randomized, Controlled Trial in Mumbai, India. J. Nutr. 2016, 146, 1453S–1460S. [Google Scholar] [CrossRef] [Green Version]
- Jamilian, M.; Asemi, Z. The Effect of Soy Intake on Metabolic Profiles of Women With Gestational Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2015, 100, 4654–4661. [Google Scholar] [CrossRef]
- Asemi, Z.; Samimi, M.; Tabassi, Z.; Esmaillzadeh, A. The effect of DASH diet on pregnancy outcomes in gestational diabetes: A randomized controlled clinical trial. Eur. J. Clin. Nutr. 2014, 68, 490–495. [Google Scholar] [CrossRef] [Green Version]
- Asemi, Z.; Tabassi, Z.; Samimi, M.; Fahiminejad, T.; Esmaillzadeh, A. Favourable effects of the Dietary Approaches to Stop Hypertension diet on glucose tolerance and lipid profiles in gestational diabetes: A randomised clinical trial. Br. J. Nutr. 2013, 109, 2024–2030. [Google Scholar] [CrossRef]
- Asemi, Z.; Samimi, M.; Tabassi, Z.; Sabihi, S.S.; Esmaillzadeh, A. A randomized controlled clinical trial investigating the effect of DASH diet on insulin resistance, inflammation, and oxidative stress in gestational diabetes. Nutrition 2013, 29, 619–624. [Google Scholar] [CrossRef]
- Hajimoosayi, F.; Jahanian Sadatmahalleh, S.; Kazemnejad, A.; Pirjani, R. Effect of ginger on the blood glucose level of women with gestational diabetes mellitus (GDM) with impaired glucose tolerance test (GTT): A randomized double-blind placebo-controlled trial. BMC Complement. Med. Ther. 2020, 20, 116. [Google Scholar] [CrossRef]
- Faroughi, F.; Charandabi, S.M.; Javadzadeh, Y.; Mirghafourvand, M. Effects of Garlic Pill on Blood Glucose Level in Borderline Gestational Diabetes Mellitus: A Randomized Controlled Trial. Iran. Red Crescent Med. J. 2018, 20, e60675. [Google Scholar] [CrossRef]
- Zhang, H.; Su, S.; Yu, X.; Li, Y. Dietary epigallocatechin 3-gallate supplement improves maternal and neonatal treatment outcome of gestational diabetes mellitus: A double-blind randomised controlled trial. J. Hum. Nutr. Diet. 2017, 30, 753–758. [Google Scholar] [CrossRef]
- Gao, F.; Wang, G.; Wang, L.; Guo, N. Phytosterol nutritional supplement improves pregnancy and neonatal complications of gestational diabetes mellitus in a double-blind and placebo-controlled clinical study. Food Funct. 2017, 8, 424–428. [Google Scholar] [CrossRef]
- Maged, A.M.; Torky, H.; Fouad, M.A.; GadAllah, S.H.; Waked, N.M.; Gayed, A.S.; Salem, A.K. Role of antioxidants in gestational diabetes mellitus and relation to fetal outcome: A randomized controlled trial. J. Matern. Fetal Neonatal Med. 2016, 29, 4049–4054. [Google Scholar] [CrossRef]
- Li, Q.; Xing, B. A Phytosterol-Enriched Spread Improves Lipid Profile and Insulin Resistance of Women with Gestational Diabetes Mellitus: A Randomized, Placebo-Controlled Double-Blind Clinical Trial. Diabetes Technol. Ther. 2016, 18, 499–504. [Google Scholar] [CrossRef]
- Yuan, L.J.; Qin, Y.; Wang, L.; Zeng, Y.; Chang, H.; Wang, J.; Wang, B.; Wan, J.; Chen, S.H.; Zhang, Q.Y.; et al. Capsaicin-containing chili improved postprandial hyperglycemia, hyperinsulinemia, and fasting lipid disorders in women with gestational diabetes mellitus and lowered the incidence of large-for-gestational-age newborns. Clin. Nutr. 2016, 35, 388–393. [Google Scholar] [CrossRef]
- Fei, B.B.; Ling, L.; Hua, C.; Ren, S.Y. Effects of soybean oligosaccharides on antioxidant enzyme activities and insulin resistance in pregnant women with gestational diabetes mellitus. Food Chem. 2014, 158, 429–432. [Google Scholar] [CrossRef]
- Zamani, B.; Milajerdi, A.; Tehrani, H.; Bellissimo, N.; Brett, N.R.; Azadbakht, L. Association of a plant-based dietary pattern in relation to gestational diabetes mellitus. Nutr. Diet. 2019, 76, 589–596. [Google Scholar] [CrossRef]
- Chen, Z.; Qian, F.; Liu, G.; Li, M.; Voortman, T.; Tobias, D.K.; Ley, S.H.; Bhupathiraju, S.N.; Li, L.J.; Chavarro, J.E.; et al. Prepregnancy plant-based diets and the risk of gestational diabetes mellitus: A prospective cohort study of 14,926 women. Am. J. Clin. Nutr. 2021, 114, 1997–2005. [Google Scholar] [CrossRef]
- Lee, Y.M.; Kim, S.A.; Lee, I.K.; Kim, J.G.; Park, K.G.; Jeong, J.Y.; Jeon, J.H.; Shin, J.Y.; Lee, D.H. Effect of a Brown Rice Based Vegan Diet and Conventional Diabetic Diet on Glycemic Control of Patients with Type 2 Diabetes: A 12-Week Randomized Clinical Trial. PLoS ONE 2016, 11, e0155918. [Google Scholar] [CrossRef] [Green Version]
- Reynolds, A.N.; Akerman, A.P.; Mann, J. Dietary fibre and whole grains in diabetes management: Systematic review and meta-analyses. PLoS Med. 2020, 17, e1003053. [Google Scholar] [CrossRef]
- Kahleova, H.; Petersen, K.F.; Shulman, G.I.; Alwarith, J.; Rembert, E.; Tura, A.; Hill, M.; Holubkov, R.; Barnard, N.D. Effect of a Low-Fat Vegan Diet on Body Weight, Insulin Sensitivity, Postprandial Metabolism, and Intramyocellular and Hepatocellular Lipid Levels in Overweight Adults: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e2025454. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Gonzalez-Lucan, M.; Fernandez-Fernandez, C.; Carneiro-Freire, N.; Seco-Filgueira, M.; Pedre-Pineiro, A.M. Effect of diet composition on insulin sensitivity in humans. Clin. Nutr. ESPEN 2019, 33, 29–38. [Google Scholar] [CrossRef]
- Wright, N.; Wilson, L.; Smith, M.; Duncan, B.; McHugh, P. The BROAD study: A randomised controlled trial using a whole food plant-based diet in the community for obesity, ischaemic heart disease or diabetes. Nutr. Diabetes 2017, 7, e256. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Yu, C.; Zhou, H.; Wei, X.; Wang, Y. Comparative evaluation for phytochemical composition and regulation of blood glucose, hepatic oxidative stress and insulin resistance in mice and HepG2 models of four typical Chinese dark teas. J. Sci. Food Agric. 2021, 101, 6563–6577. [Google Scholar] [CrossRef]
- An, R.; Zong, A.; Chen, S.; Xu, R.; Zhang, R.; Jiang, W.; Liu, L.; Du, F.; Zhang, H.; Xu, T. Effects of oligosaccharides on the markers of glycemic control: A systematic review and meta-analysis of randomized controlled trials. Food Funct. 2022, 13, 8766–8782. [Google Scholar] [CrossRef]
- Tabatabaei-Malazy, O.; Nikfar, S.; Larijani, B.; Abdollahi, M. Influence of ascorbic acid supplementation on type 2 diabetes mellitus in observational and randomized controlled trials; a systematic review with meta-analysis. J. Pharm. Pharm. Sci. 2014, 17, 554–582. [Google Scholar] [CrossRef]
- Salehi-Sahlabadi, A.; Varkaneh, H.K.; Shahdadian, F.; Ghaedi, E.; Nouri, M.; Singh, A.; Farhadnejad, H.; Gaman, M.A.; Hekmatdoost, A.; Mirmiran, P. Effects of Phytosterols supplementation on blood glucose, glycosylated hemoglobin (HbA1c) and insulin levels in humans: A systematic review and meta-analysis of randomized controlled trials. J. Diabetes Metab. Disord. 2020, 19, 625–632. [Google Scholar] [CrossRef]
- Maharlouei, N.; Tabrizi, R.; Lankarani, K.B.; Rezaianzadeh, A.; Akbari, M.; Kolahdooz, F.; Rahimi, M.; Keneshlou, F.; Asemi, Z. The effects of ginger intake on weight loss and metabolic profiles among overweight and obese subjects: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 1753–1766. [Google Scholar] [CrossRef]
- Li, J.; Wang, R.; Xiao, C. Association between chilli food habits with iron status and insulin resistance in a Chinese population. J. Med. Food 2014, 17, 472–478. [Google Scholar] [CrossRef] [Green Version]
- Panchal, S.K.; Bliss, E.; Brown, L. Capsaicin in Metabolic Syndrome. Nutrients 2018, 10, 630. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.M.; Haastert, B.; Scherbaum, W.; Hauner, H. A phytosterol-enriched spread improves the lipid profile of subjects with type 2 diabetes mellitus--a randomized controlled trial under free-living conditions. Eur. J. Nutr. 2003, 42, 111–117. [Google Scholar] [CrossRef]
- Wang, J.F.; Zhang, H.M.; Li, Y.Y.; Xia, S.; Wei, Y.; Yang, L.; Wang, D.; Ye, J.J.; Li, H.X.; Yuan, J.; et al. A combination of omega-3 and plant sterols regulate glucose and lipid metabolism in individuals with impaired glucose regulation: A randomized and controlled clinical trial. Lipids Health Dis. 2019, 18, 106. [Google Scholar] [CrossRef] [Green Version]
| Authors, Year (Country) | Trial Design and Duration | Participants | Dietary Intervention | Control Group Diet | Blood Glucose | Blood Lipid Levels | Body Composition/ Body Weight |
|---|---|---|---|---|---|---|---|
| Barati, et al., 2021 (Iran) [12] | RCT; 4 weeks | Women with GDM (n = 104) | Standard GDM diet with 30 g oat bran with lunch and dinner | Standard GDM diet without oat bran | ↓ FBS | NR | Not statistically significant from control group |
| Basu, et al., 2021 (US) [13] | Randomized parallel arm study; 18 weeks | Women at high risk of GDM (n = 34) | Supplemented with 2 cups whole blueberries and 12 g soluble fiber, dietary intakes recorded on 24 h food recall | Standard prenatal care based on USDA Dietary Guidelines for Americans for pregnant women | ↓ Postprandial blood glucose | NR | Body weight gain ↓ in Intervention group than control group |
| Basu, et al., 2021 (US) [14] | RCT; 18 weeks | Women at high risk of GDM and BMI ≥ 30 (n = 34) | Biweekly supply of 280 g frozen blueberries for snack and 12 g soluble fiber daily | Standard prenatal care based on USDA Dietary Guidelines for Americans for pregnant women | ↓ 1 h postprandial blood glucose ↑ Serum glucose ↑ Serum Insulin ↑ HOMA-IR ↓ Serum HbA1c | ↑ Total Cholesterol ↑ LDL Cholesterol ↑ Serum Triglycerides | Body weight gain ↓ in Intervention group than control group |
| Feng, et al., 2019 (China) [15] | Randomized, controlled, crossover study; same-day testing | Women with GIGT or GDM (n = 55) | Provided 42 g Pistachios to consume within 15 min | Provided 100 g Whole Wheat Bread to consume within 15 min | ↓ Blood glucose ↓ Blood Insulin | NR | NR |
| Assaf-Balut, et al., 2017 (Spain) [16] | Unicentric, clinic-based, prospective, randomized controlled trial with two parallel groups; 18 weeks | Women with FBG < 92 mg/dL (n = 874) | Same as control diet plus lifestyle provided with EVOO and pistachios, consumption of at least 40 mg EVOO and handful (25–30 g) pistachios | Basic MedDiet, ≥2 servings/day vegetables, ≥3 servings/day fruit (avoid fruit juice), 3 servings/day skimmed dairy products, whole grain cereals, 2–3 servings legumes/week, moderate–high fish consumption, low red and processed meat consumption, avoid refined grains, baked goods, soft drinks, fast foods, pre-cooked meals | ↓ FBG ↓ 2 h post glucose load ↓ HbA1c ↓ HOMA-IR | NR | ↓ GWG compared to control group |
| Sahariah, et al., 2015 (India) [17] | RCT; 32 weeks | Pregnant women at risk for GDM (n = 1008) | Snack containing leafy green vegetables in fresh (~30 g) or dried (~7.5 g) form, 12–16 g full-fat milk powder, and 4–60 g dried fruit, started pre-conception | Maintained regular diet | Fasting glucose, 120 min glucose, and fasting insulin are not statistically significant between groups | NR | Not statistically significant between groups |
| Jamilian, et al., 2015 (Iran) [18] | Randomized, parallel clinical trial; 6 weeks | Women with GDM (n = 68) | 0.8 g/kg protein (35% animal, 35% soy, 30% other plants) | 0.8 g/kg protein (70% animal, 30% plant) | ↓ FPG ↓ HOMA-IR | ↓ Total Cholesterol ↓ Triglycerides ↓LDL ↓ T/HDL-C ratio | Not statistically different from control |
| Asemi, et al., 2014 (Iran) [19] | Two-arm parallel RCT; 4 weeks | Women with GDM (n = 52) | Calorie and protein composition are similar to control. Rich in fruits, vegetables, whole grains, and low-fat dairy and low in saturated fats, cholesterol, refined grains and sweets | 45–55% CHO, 15–20% protein, 25–30% total fat | ↓ Insulin Therapy | NR | Not statistically different from control |
| Asemi, et al. 2013 (Iran) [20] | Two-arm parallel RCT; 4 weeks | Women with GDM (n = 34) | Similar to control and rich in fruits, vegetables, whole grains, low-fat dairy and low in saturated fats, cholesterol, refined grains and sweets. Sodium was 2400 mg/d | 45–55% CHO, 15–20% protein, 25–30% total fat, 7 d menu cycle based on usual practice in GDM | ↓ 1 h GTT ↓ 2 h GTT ↓ 3 h GTT ↓ HbA1c | ↓ Total Cholesterol ↓ TAG ↑ HDL ↓ LDL ↓ T/HDL-C ratio | Not statistically significant from control |
| Asemi, et al., 2013 (Iran) [21] | Randomized, two-arm, parallel clinical trial; 4 weeks | Women with GDM (n = 32) | DASH diet is rich in fruits, vegetables, whole grains, low-fat dairy products and low in saturated fats, cholesterol, refined grains, and sweets. Sodium reduced to ˂2000 mg/d, 7 d menu cycle | Based on recommendations for acceptable dietary intake for GDM: 40–50% CHO, 10–20% Protein, 25–30% total fats | ↓ FPG ↓ Serum Insulin ↓ HOMA-IR | ↓ Total Cholesterol | Not statistically significant from placebo |
| Authors, Year (Country) | Trial Design and Duration | Participants | Dietary Intervention | Control Group Diet | Blood Glucose | Blood Lipid Levels | Body Composition/ Body Weight |
|---|---|---|---|---|---|---|---|
| Hajimoosayi, et al., 2020 (Iran) [22] | Double-blind, placebo-controlled RCT, 6 weeks | Women with GDM (n = 70), 24–28 weeks gestation | 1500 mg ginger, split into 3 tablets | Placebo pill, split into 3 tablets | ↓ FBS ↓ Serum insulin ↓ HOMA-IR | NR | Not significantly different from control group |
| Faroughi, et al., 2018 (Iran) [23] | Triple-blind, placebo-controlled RCT, 8 weeks | Women with prediabetes and borderline GDM (n = 44), 24–28 weeks gestation | 1 pill with 400 mg dry garlic powder (1200–1800 mg allicin and 2 g fresh garlic) | 1 placebo pill, made of starch | ↓ FBS ↓ GDM incidence after intervention | NR | Not significantly different from control group |
| Zhang, et al., 2017 (China) [24] | Double-blind, placebo-controlled RCT, maintained until delivery | Women with GDM (n = 326), diagnosed at onset of 3rd trimester (29 wks) | 1 capsule containing 500 mg EGCG | 1 placebo capsule containing 500 mg starch powder | ↓ FPG ↓ Serum insulin ↓ HOMA-IR ↓ HOMA-B ↑ QUICKI | NR | Not significantly different from control group |
| Gao, et al., 2017 (China) [25] | Double-blind, placebo-controlled RCT, maintained until delivery | Women with GDM (n = 276), diagnosed GDM at onset of 3rd trimester (29 wks) | Phytosterol-enriched margarine spread, 10 g serving of spread with 2 g of phytosterols, 2x daily | Regular margarine spread, 10 g serving, 2x daily | ↓ FPG ↓ HbA1c ↓ Serum insulin ↓ HOMA-IR ↓ HOMA-B ↑ QUICKI | ↓ Total Cholesterol ↓ LDL ↑ HDL ↓ TC/HDL ratio | Not significantly different from control group |
| Maged, et al., 2016 (Egypt) [26] | Placebo-controlled RCT, maintained until delivery | Women with GDM (n = 200) | 1 g L-ascorbic acid effervescent tablet | 1 placebo effervescent tablet | ↓ Insulin dosage | NR | NR |
| Li, et al., 2016 (China) [27] | Double-blind, placebo-controlled RCT, 16 weeks | Women with GDM (n = 222), diagnosed GDM at onset of 2nd trimester (13 wks) | Phytosterol-enriched margarine spread, 10 g serving of spread with 2 g of phytosterols, 2x daily | Regular margarine spread, 10 g serving, 2x daily | ↓ FPG ↓ Serum insulin ↓ HOMA-IR ↓ HOMA-B ↑ QUICKI | ↓ Triacylglycerol ↓ Total Cholesterol ↓ LDL ↑ HDL ↓ TC/HDL ratio | Not significantly different from control group |
| Yuan, et al., 2016 (China) [28] | Double-blind, placebo-controlled RCT, 4 weeks | Women with GDM (n = 44), between 22–33 weeks gestation | 0.625 g chili powder with 2.5 mg of capsaicin, 2x daily | 0.625 g chili powder with 0 mg of capsaicin, 2x daily | ↓ 2 h PG ↓ 2 h insulin ↓ 2 h HOMA-IR ↑ CGRP | ↓ Total cholesterol ↓ Triglycerides ↑ Serum apolipoprotein B | Not significantly different from control group |
| Fei, et al., 2014 (China) [29] | Placebo-controlled RCT, 8 weeks | Women with GDM (n = 97), currently on insulin treatment | Soybean oligosaccharides (combination of raffinose, stachyose, and sucrose) 10 g in 200–300 mL water orally, along with 3x injection of NovoRapid Insulin (short-term) before meals with a 1x injection of Novolin N insulin (intermediate-term) before sleep | 3x injection of NovoRapid Insulin (short-term) before meals with a 1x injection of Novolin N insulin (intermediate-term) before sleep | ↓ Fasting insulin ↓ HOMA-IR ↑ Adiponectin ↓ Total insulin dosage | NR | NR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaworsky, K.; DeVillez, P.; Basu, A. The Role of Phytochemicals and Plant-Based Diets in Gestational Diabetes: Evidence from Clinical Trials. Int. J. Environ. Res. Public Health 2023, 20, 4188. https://doi.org/10.3390/ijerph20054188
Jaworsky K, DeVillez P, Basu A. The Role of Phytochemicals and Plant-Based Diets in Gestational Diabetes: Evidence from Clinical Trials. International Journal of Environmental Research and Public Health. 2023; 20(5):4188. https://doi.org/10.3390/ijerph20054188
Chicago/Turabian StyleJaworsky, Kataryna, Pamela DeVillez, and Arpita Basu. 2023. "The Role of Phytochemicals and Plant-Based Diets in Gestational Diabetes: Evidence from Clinical Trials" International Journal of Environmental Research and Public Health 20, no. 5: 4188. https://doi.org/10.3390/ijerph20054188




