Validation of a Novel Predictive Algorithm for Kidney Failure in Patients Suffering from Chronic Kidney Disease: The Prognostic Reasoning System for Chronic Kidney Disease (PROGRES-CKD)
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of Naïve Bayes Classifiers
2.2. PROGRES-CKD Training
2.3. Measures
2.3.1. Endpoint Definition
2.3.2. Input Variables
2.3.3. Definition of CKD Stages
2.4. Design and Setting of PROGRES-CKD Validation Studies
2.4.1. Study A
2.4.2. Study B
2.4.3. Study C
2.5. Statistical Analysis
3. Results
3.1. Cohort Characteristics
3.2. Model Discrimination in the Training and Validation Dataset from the FMC NephroCare Cohort
3.3. Model Discrimination in the GCKD Cohort
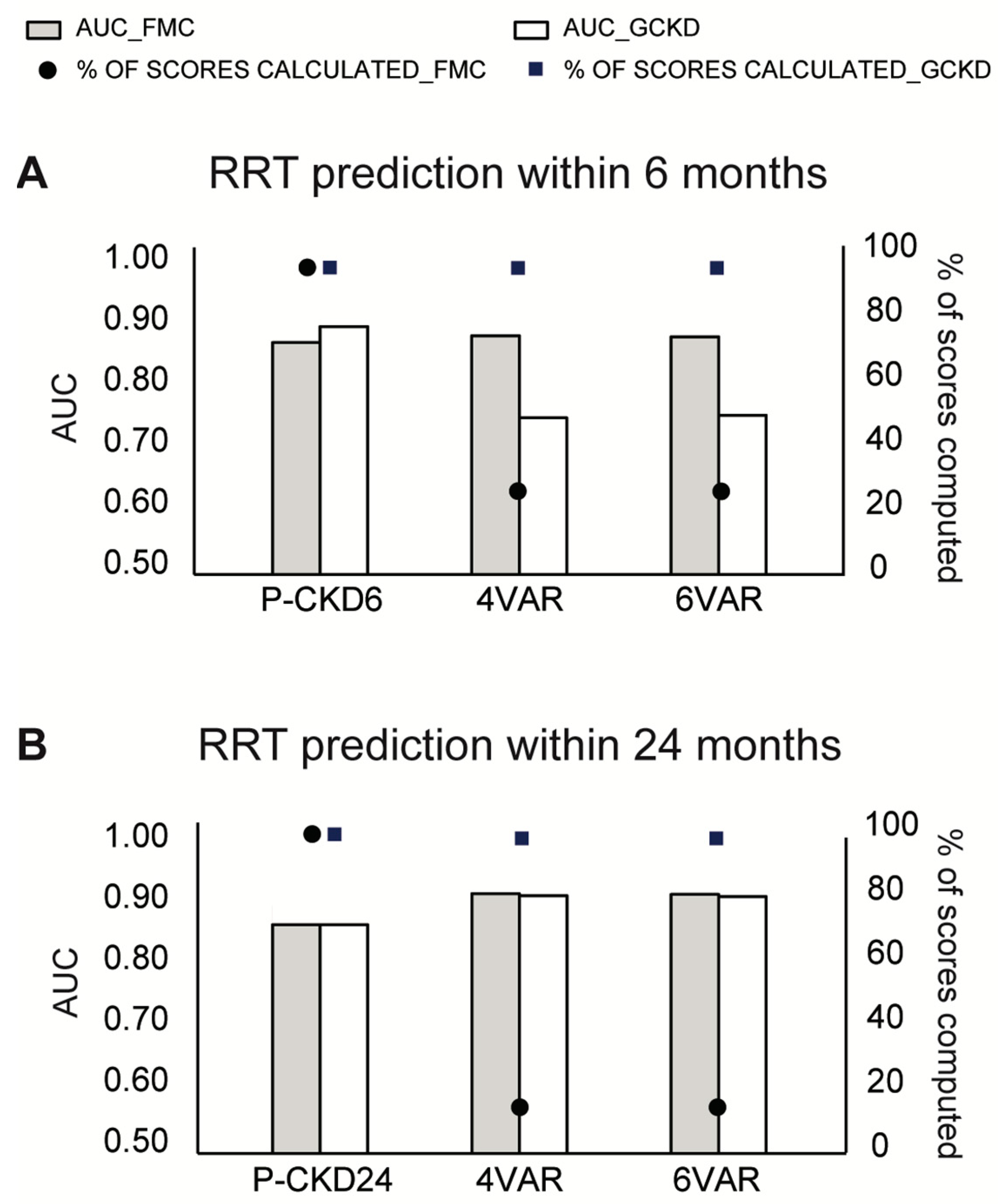
3.4. Comparison with KFRE Performance
3.5. Potential Impact Simulation
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peng, S.; He, J.; Huang, J.; Lun, L.; Zeng, J.; Zeng, S.; Zhang, L.; Liu, X.; Wu, Y. Self-management interventions for chronic kidney disease: A systematic review and meta-analysis. BMC Nephrol. 2019, 20, 142. [Google Scholar] [CrossRef] [PubMed]
- Navaneethan, S.D.; Shao, J.; Buysse, J.; Bushinsky, D.A. Effects of treatment of metabolic acidosis in CKD: A systematic review and meta-analysis. Clin. J. Am. Soc. Nephrol. 2019, 14, 1011–1120. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Greene, T.; Tighiouart, H.; Gansevoort, R.T.; Coresh, J.; Simon, A.L.; Chan, T.M.; Hou, F.F.; Lewis, J.B.; Locatelli, F.; et al. Change in albuminuria as a surrogate endpoint for progression of kidney disease: A meta-analysis of treatment effects in randomised clinical trials. Lancet Diabetes Endocrinol. 2019, 7, 128–139. [Google Scholar] [CrossRef]
- Inker, L.A.; Heerspink, H.J.L.; Tighiouart, H.; Levey, A.S.; Coresh, J.; Gansevoort, R.T.; Simon, A.L.; Ying, J.; Beck, G.J.; Wanner, C.; et al. GFR slope as a surrogate end point for kidney disease progression in clinical trials: A meta-analysis of treatment effects of randomized controlled trials. J. Am. Soc. Nephrol. 2019, 30, 1735–1745. [Google Scholar] [CrossRef] [PubMed]
- Campbell, G.A.; Bolton, W.K. Referral and comanagement of the patient with CKD. Adv. Chronic Kidney Dis. 2011, 18, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Levin, A. Consequences of late referral on patient outcomes. Nephrol. Dial. Transplant. 2000, 15, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Bartmańska, M.; Więcek, A. Chronic kidney disease and the aging population. G. Ital. Nefrol. 2014, 36, 1–5. [Google Scholar] [CrossRef]
- Fedewa, S.A.; McClellan, W.M.; Judd, S.; Gutiérrez, O.M.; Crews, D.C. The association between race and income on risk of mortality in patients with moderate chronic kidney disease. BMC Nephrol. 2014, 15, 136. [Google Scholar] [CrossRef]
- Kadatz, M.J.; Lee, E.S.; Levin, A. Predicting Progression in CKD: Perspectives and Precautions. Am. J. Kidney Dis. 2016, 67, 779–786. [Google Scholar] [CrossRef]
- Tangri, N.; Kitsios, G.D.; Inker, L.A.; Griffith, J.; Naimark, D.M.; Walker, S.; Rigatto, C.; Uhlig, K.; Kent, D.M.; Levey, A.S. Risk prediction models for patients with chronic kidney disease a systematic review. Ann. Intern. Med. 2013, 158, 596–603. [Google Scholar] [CrossRef]
- Ramspek, C.L.; de Jong, Y.; Dekker, F.W.; van Diepen, M. Towards the best kidney failure prediction tool: A systematic review and selection aid. Nephrol. Dial. Transplant. 2020, 35, 1527–1538. [Google Scholar] [CrossRef]
- Dekker, F.W.; Ramspek, C.L.; Van Diepen, M. Con: Most clinical risk scores are useless. Nephrol. Dial. Transplant. 2017, 32, 752–755. [Google Scholar] [CrossRef]
- Echouffo-Tcheugui, J.B.; Kengne, A.P. Risk Models to Predict Chronic Kidney Disease and Its Progression: A Systematic Review. PLoS Med. 2012, 9, e1001344. [Google Scholar] [CrossRef]
- Collins, G.S.; Omar, O.; Shanyinde, M.; Yu, L.M. A systematic review finds prediction models for chronic kidney disease were poorly reported and often developed using inappropriate methods. J. Clin. Epidemiol. 2013, 66, 268–277. [Google Scholar] [CrossRef]
- Tangri, N.; Stevens, L.A.; Griffith, J.; Tighiouart, H.; Djurdjev, O.; Naimark, D.; Levin, A.; Levey, A.S. A Predictive Model for Progression of Chronic Kidney Disease to Kidney Failure. JAMA 2011, 305, 1553–1559. [Google Scholar] [CrossRef]
- Tangri, N.; Grams, M.E.; Levey, A.S.; Coresh, J.; Appel, L.J.; Astor, B.C.; Chodick, G.; Collins, A.J.; Djurdjev, O.; Raina Elley, C.; et al. Multinational Assessment of Accuracy of Equations for Predicting Risk of Kidney Failure ameta-analysis. JAMA 2016, 315, 164–174. [Google Scholar] [CrossRef]
- Van Rijn, M.H.C.; van de Luijtgaarden, M.; van Zuilen, A.D.; Blankestijn, P.J.; Wetzels, J.F.M.; Debray, T.P.; Brand, J.A.J.G.V.D. Prognostic models for chronic kidney disease: A systematic review and external validation. Nephrol. Dial. Transplant. 2021, 36, 1837–1850. [Google Scholar] [CrossRef]
- Kang, M.W.; Tangri, N.; Kim, Y.C.; An, J.N.; Lee, J.; Li, L.; Oh, Y.K.; Kim, D.K.; Joo, K.W.; Kim, Y.S.; et al. An independent validation of the kidney failure risk equation in an Asian population. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Steil, H.; Amato, C.; Carioni, C.; Kirchgessner, J.; Marcelli, D.; Mitteregger, A.; Moscardo, V.; Orlandini, G.; Gatti, E. EuCliD®—A Medical Registry. Methods Inf. Med. 2004, 43, 83–88. [Google Scholar]
- Merello Godino, J.I.; Rentero, R.; Orlandini, G.; Marcelli, D.; Ronco, C. Results from EuCliD® (European Clinical Dialysis Database): Impact of shifting treatment modality. Int. J. Artif. Organs 2002, 25, 1049–1060. [Google Scholar] [CrossRef]
- Eckardt, K.U.; Bärthlein, B.; Seema, B.A.; Beck, A.; Busch, M.; Eitner, F.; Ekici, A.B.; Floege, J.; Gefeller, O.; Haller, H.; et al. The German Chronic Kidney Disease (GCKD) study: Design and methods. Nephrol. Dial. Transplant. 2011, 27, 1454–1460. [Google Scholar] [CrossRef] [PubMed]
- Collins, G.S.; Reitsma, J.B.; Altman, D.G.; Moons, K.G.M. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. BMJ 2014, 350, g7594. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Phung, D.; Tran, T.; Gupta, S.; Rana, S.; Karmakar, C.; Shilton, A.; Yearwood, J.; Dimitrova, N.; Ho, T.B.; et al. Guidelines for developing and reporting machine learning predictive models in biomedical research: A multidisciplinary view. J. Med. Internet Res. 2016, 18, e323. [Google Scholar] [CrossRef] [PubMed]
- McNally, R.J.; Heeren, A.; Robinaugh, D.J. A Bayesian network analysis of posttraumatic stress disorder symptoms in adults reporting childhood sexual abuse. Eur. J. Psychotraumatol. 2017, 8, 1341276. [Google Scholar] [CrossRef]
- Khan, S.; Ghalib, M.R. A naive-bayes approach for disease diagnosis with analysis of disease type and symptoms. Int. J. Appl. Eng. Res. 2015, 10, 29005–29014. [Google Scholar]
- Constantinou, A.C.; Yet, B.; Fenton, N.; Neil, M.; Marsh, W. Value of information analysis for interventional and counterfactual Bayesian networks in forensic medical sciences. Artif. Intell. Med. 2016, 66, 41–52. [Google Scholar] [CrossRef]
- Kjærulff, U.B.; Madsen, A.L. Bayesian Networks and Influence Diagrams: A Guide to Construction and Analysis; Springer: New York, NY, USA, 2013. [Google Scholar]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding Algorithms for Defining Comorbidities in ICD-9-CM and ICD-10 Administrative Data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., III; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Oellgaard, J.; Gæde, P.; Rossing, P.; Persson, F.; Parving, H.-H.; Pedersen, O. Intensified multifactorial intervention in type 2 diabetics with microalbuminuria leads to long-term renal benefits. Kidney Int. 2017, 91, 982–988. [Google Scholar] [CrossRef]
- Fogelfeld, L.; Hart, P.; Miernik, J.; Ko, J.; Calvin, D.; Tahsin, B.; Adhami, A.; Mehrotra, R.; Fogg, L. Combined diabetes-renal multifactorial intervention in patients with advanced diabetic nephropathy: Proof-of-concept. J. Diabetes Its Complicat. 2017, 31, 624–630. [Google Scholar] [CrossRef]
- Peeters, M.J.; Van Zuilen, A.D.; Brand, J.A.J.G.V.D.; Bots, M.L.; Van Buren, M.; Dam, M.A.G.J.T.; Kaasjager, K.A.H.; Ligtenberg, G.; Sijpkens, Y.W.J.; Sluiter, H.E.; et al. Nurse Practitioner Care Improves Renal Outcome in Patients with CKD. J. Am. Soc. Nephrol. 2013, 25, 390–398. [Google Scholar] [CrossRef]
- Linden, A. Measuring diagnostic and predictive accuracy in disease management: An introduction to receiver operating characteristic (ROC) analysis. J. Eval. Clin. Pract. 2006, 12, 132–139. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Vickers, A.J.; Cook, N.R.; Gerds, T.; Gonen, M.; Obuchowski, N.; Pencina, M.J.; Kattan, M.W. Assessing the performance of prediction models: A framework for traditional and novel measures. Epidemiology 2010, 21, 128–138. [Google Scholar] [CrossRef]
- Delong, E.R.; Delong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Nielsen, T.D.; Jensen, F.V. Bayesian Networks and Decision Graphs; Springer: New York, NY, USA, 2009. [Google Scholar]
- Sun, L.; Zou, L.-X.; Chen, M.-J. Make Precision Medicine Work for Chronic Kidney Disease. Med. Princ. Pract. 2016, 26, 101–107. [Google Scholar] [CrossRef]
- Lerner, B.; Desrochers, S.; Tangri, N. Risk Prediction Models in CKD. Semin. Nephrol. 2017, 37, 144–150. [Google Scholar] [CrossRef]
- Bargman, J.M. Timing of Initiation of RRT and Modality Selection. Clin. J. Am. Soc. Nephrol. 2015, 10, 1072–1077. [Google Scholar] [CrossRef][Green Version]
- Allon, M. Vascular access for hemodialysis patients: New data should guide decision making. Clin. J. Am. Soc. Nephrol. 2019, 14, 954–961. [Google Scholar] [CrossRef]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Levey, A.S.; Coresh, J. Chronic kidney disease. Lancet 2012, 379, 165–180. [Google Scholar] [CrossRef]
- Jacoby, L.L.; McElree, B.; Trainham, T.N. Automatic influences as accessibility bias in memory and Stroop tasks: Toward a formal model. In Attention and Performance XVII: Cognitive Regulation of Performance: Interaction of Theory and Application; Elsevier: Amsterdam, The Netherlands, 1999; pp. 461–486. [Google Scholar]
- Qin, S.; Hermans, E.J.; van Marle, H.J.F.; Luo, J.; Fernández, G. Acute Psychological Stress Reduces Working Memory-Related Activity in the Dorsolateral Prefrontal Cortex. Biol. Psychiatry 2009, 66, 25–32. [Google Scholar] [CrossRef]
- Eva, K.W.; Norman, G.R. Heuristics and biases—A biased perspective on clinical reasoning. Med. Educ. 2005, 39, 870–872. [Google Scholar] [CrossRef]
- Mumford, A.D.; Banning, A.P. Minimising delays to thrombolysis in patients developing acute myocardial infarction in hospital. Postgrad. Med. J. 1997, 73, 491–495. [Google Scholar] [CrossRef][Green Version]
- Croskerry, P.; Norman, G. Overconfidence in Clinical Decision Making. Am. J. Med. 2008, 121, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Croskerry, P. A Universal Model of Diagnostic Reasoning. Acad. Med. 2009, 84, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Moons, K.G.M.; Kengne, A.P.; Grobbee, D.E.; Royston, P.; Vergouwe, Y.; Altman, D.G.; Woodward, M. Risk prediction models: II. External validation, model updating, and impact assessment. Heart 2012, 98, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Moosa, M.R.; Maree, J.D.; Chirehwa, M.T.; Benatar, S.R. Use of the “accountability for reasonableness” approach to improve fairness in accessing dialysis in a middle-income country. PLoS ONE 2016, 11, e0164201. [Google Scholar]
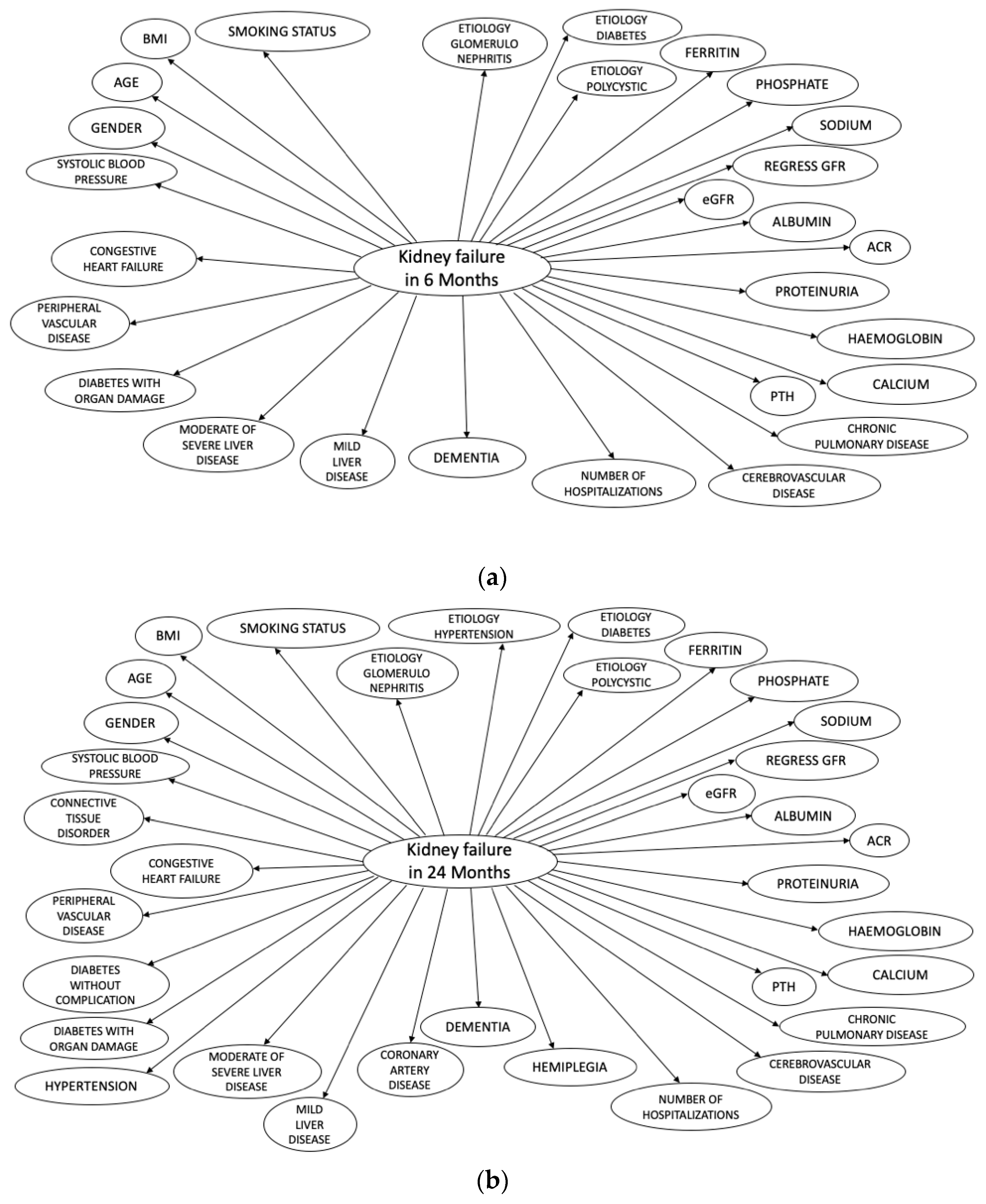
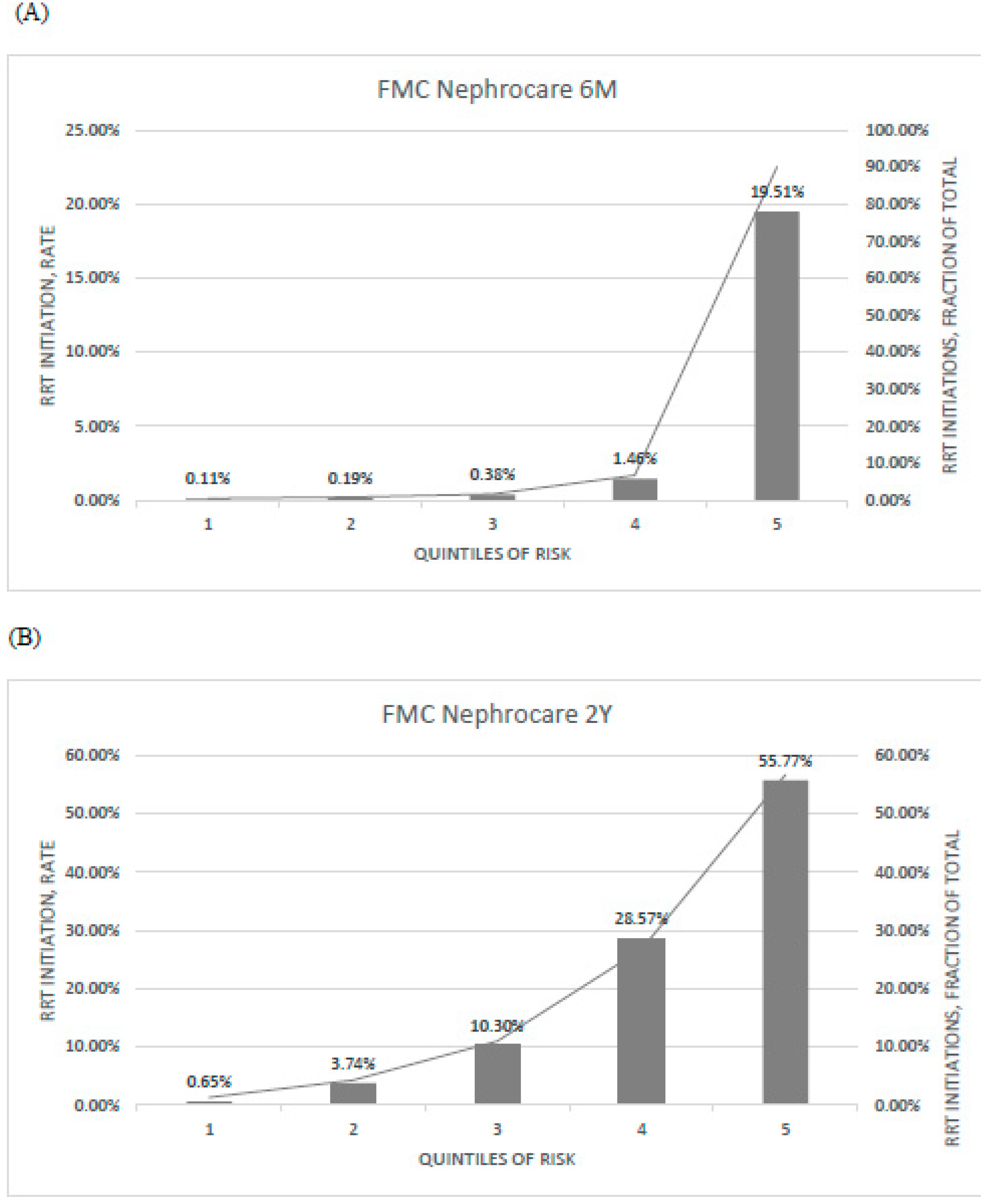


| PROGRES-CKD-6 | PROGRES-CKD-24 | ||
|---|---|---|---|
| Group | Variable | n = 28 | n = 34 |
| Demographics and anthropometrics | |||
| Age | X | X | |
| Gender | X | X | |
| BMI, Kg/m2 | X | X | |
| Smoking status | X | X | |
| Kidney function | |||
| Albumin, g/dL | X | X | |
| Albumin Creatinine Ratio (ACR), mg/mmol ** | X | X | |
| Calcium, mg/dL | X | X | |
| eGFR, (ml/min/173 m2) | X | X | |
| regressGFR * | X | X | |
| Hemoglobin, g/dL | X | X | |
| Phosphate, mg/dL | X | X | |
| Urine protein, g/24 h | X | X | |
| Parathyroid hormone, ng/L | X | X | |
| Sodium, mmol/L | X | X | |
| Ferritin, microg/L | X | X | |
| Etiology of kidney disease | |||
| Diabetes | X | X | |
| Hypertension | X | ||
| Glomerulonephritis | X | X | |
| Polycystic | X | X | |
| Comorbidities | |||
| Cerebrovascular disease | X | X | |
| Chronic Pulmonary Disease | X | X | |
| Congestive heart failure | X | X | |
| Connective tissue disorder | X | ||
| Coronary artery disease | X | ||
| Dementia | X | X | |
| Diabetes with organ damage | X | X | |
| Diabetes without complications | X | ||
| Hemiplegia | X | ||
| Hypertension | X | ||
| Mild liver disease | X | X | |
| Moderate or severe liver disease | X | X | |
| Peripheral vascular disease | X | X | |
| Other | |||
| Number of hospitalizations | X | X | |
| Systolic blood pressure | X | X | |
| FMC Cohort | GCKD Cohort | |||
|---|---|---|---|---|
| Variable | n | Mean ± SD or Median (IQR) or n (%) | n | Mean ± SD or Median (IQR) or n (%) |
| Stage 3 | 11,965 | 11,965 (53.1%) | 3593 | 3593 (88.54%) |
| Stage 4 | 8026 | 8026 (35.62%) | 460 | 460 (11.34%) |
| Stage 5 | 2544 | 2544 (11.29%) | 5 | 5 (0.12%) |
| Age (year) | 22,535 | 72.15 ± 11.7 | 4058 | 62.12 ± 10.50 |
| BMI (kg/cm2) | 21,655 | 30.63 ± 10.92 | 4015 | 30.03 ± 5.91 |
| eGFR ((mL/min/1.73 m2) | 22,535 | 31.93 ± 13.4 | 4058 | 41.92 ± 9.76 |
| Albumin (g/dL) | 19,004 | 4.19 ± 0.4 | 4055 | 3.85 ± 0.42 |
| Ferritin (µg/L) | 7303 | 222.18 ± 260.98 | 1044 | 200.48 ± 196.11 |
| Hemoglobin (g/dL) | 21,916 | 12.65 ± 1.83 | 3978 | 13.49 ± 1.69 |
| Phosphate (mg/dL) | 20,362 | 3.65 ± 0.74 | 4058 | 3.45 ± 0.64 |
| Calcium (mg/dL) | 20,686 | 9.36 ± 0.73 | 4058 | 9.07 ± 0.63 |
| Sodium (mmol/L) | 20,612 | 140.17 ± 3.16 | 4057 | 139.70 ± 3.14 |
| PTH (ng/L) | 9466 | 131.84 ± 150.12 | 0 | - |
| ACR (mg/mmol) | 90 | 138.67 ± 568.28 | 3999 | 393.63 ± 888.48 |
| Proteinuria (g/24 h) | 8780 | 3.58 ± 150.29 | 0 | - |
| Systolic (mmHg) | 17,963 | 137.33 ± 18.41 | 4030 | 140.27 ± 20.53 |
| CRP (mg/L) | 13,468 | 4.23 (7.63) | 4056 | 2.41 (4.27) |
| Glucose (mg/dL) | 19,499 | 126.45 ± 48.59 | 0 | - |
| HDL Cholesterol (mg/dL) | 7074 | 48.3 ± 16.74 | 4051 | 50.72 ± 17.35 |
| LDL Cholesterol (mg/dL) | 7084 | 107.59 ± 219.29 | 4051 | 116.33 ± 42.93 |
| Triglyceride (mg/dL) | 15,191 | 142.77 (95.72) | 4050 | 173.38 (126.45) |
| hsTNT (ng/L) | 0 | - | 3976 | 13 (11) |
| Uric Acid (mg/dL) | 20,273 | 6.68 ± 1.61 | 4058 | 7.40 ± 1.92 |
| Gender (M) | 22,535 | 11,349 (50.36%) | 4058 | 2510 (61.85%) |
| Etiology Diabetes | 22,535 | 3614 (16.04%) | 4058 | 666 (16.41%) |
| Etiology Polycystic | 22,535 | 477 (2.12%) | 4058 | 157 (3.87%) |
| Etiology Hypertension | 22,535 | 5281 (23.43%) | 4058 | 1011 (24.91%) |
| Etiology Glomerulonephrite | 22,535 | 987 (4.38%) | 4058 | 623 (15.35%) |
| Smoking status: ex-smoker | 3502 | 3502 (15.54%) | 1819 | 1819 (44.96%) |
| Smoking status: no smoker | 10,066 | 10,066 (44.67%) | 1649 | 1649 (40.76%) |
| Smoking status: smoker | 2274 | 2274 (10.09%) | 578 | 578 (14.29%) |
| Alcohol: abuse | 8636 | 8636 (38.32%) | 771 | 771 (19.10%) |
| Alcohol: moderate | 0 | 0 (0%) | 3265 | 3265 (80.90%) |
| Alcohol: abstinence | 6984 | 6984 (30.99%) | 0 | 0 (%) |
| Peripheral Vascular Disease | 22,535 | 1875 (8.32%) | 4058 | 424 (10.45%) |
| Coronary Artery Disease | 22,535 | 4336 (19.24%) | 4058 | 908 (22.38%) |
| Congestive Heart Failure | 22,535 | 1887 (8.37%) | 4058 | 776 (19.12%) |
| Cerebrovascular Disease | 22,535 | 1876 (8.32%) | 4058 | 472 (10.52%) |
| Connective Tissue Disorder | 22,535 | 399 (1.77%) | 0 | - |
| Cancer | 22,535 | 2469 (10.96%) | 4058 | 532 (13.11%) |
| Diabetes | 22,535 | 9021 (40.03%) | 4058 | 1545 (38.07%) |
| Anemia | 22,535 | 9800 (43.49%) | 4058 | 1057 (26.05%) |
| Hypertension | 22,535 | 17,871 (79.3%) | 4058 | 3951 (97.36%) |
| Atrial Fibrillation | 22,535 | 2337 (10.37%) | 4058 | 876 (21.59%) |
| Diabetes Without Complications (CCI) | 22,535 | 3013 (13.37%) | 4058 | 1545 (38.07%) |
| Chronic Pulmonary Disease (CCI) | 22,535 | 1618 (7.18%) | 4058 | 285 (7.02%) |
| Psychiatric Disease | 22,535 | 177 (0.79%) | 0 | - |
| Liver Disease | 22,535 | 987 (4.38%) | 0 | - |
| RRT in 24 months | 9407 | 1817 (19.32%) | 3684 | 80 (2.17%) |
| RRT in 6 months | 18,504 | 801 (4.33%) | 3888 | 11 (0.28%) |
| Model | Validation Cohort | Comparator Model | AUC PROGRES-CKD | Delta AUC * | p-Value | Effective Sample Size |
|---|---|---|---|---|---|---|
| PROGRES-CKD-6 | ||||||
| FMC NephroCare | ||||||
| 4VAR | 0.90 | −0.012 | 0.3255 | 927 | ||
| 6VAR | 0.90 | −0.016 | 0.2220 | 927 | ||
| GCKD | ||||||
| 4VAR | 0.91 | −0.146 | 0.0016 | 459 | ||
| 6VAR | 0.91 | −0.149 | 0.0013 | 459 | ||
| PROGRES-CKD-24 | ||||||
| FMC NephroCare | ||||||
| 4VAR | 0.87 | 0.020 | 0.0483 | 1081 | ||
| 6VAR | 0.87 | 0.018 | 0.0888 | 1081 | ||
| GCKD | ||||||
| 4VAR | 0.85 | 0.030 | 0.0105 | 3999 | ||
| 6VAR | 0.85 | 0.027 | 0.0246 | 3999 | ||
| Experts | |||||
|---|---|---|---|---|---|
| PROGRES-CKD-24 | Expert 1 | Expert 2 | Expert 3 | Expert 4 | |
| AUC | 0.96 | 0.84 | 0.72 | 0.86 | 0.76 |
| Sensitivity | 0.76 | 0.80 | 0.50 | 0.75 | 0.60 |
| Specificity | 0.96 | 0.84 | 0.89 | 1.00 | 0.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellocchio, F.; Lonati, C.; Ion Titapiccolo, J.; Nadal, J.; Meiselbach, H.; Schmid, M.; Baerthlein, B.; Tschulena, U.; Schneider, M.; Schultheiss, U.T.; et al. Validation of a Novel Predictive Algorithm for Kidney Failure in Patients Suffering from Chronic Kidney Disease: The Prognostic Reasoning System for Chronic Kidney Disease (PROGRES-CKD). Int. J. Environ. Res. Public Health 2021, 18, 12649. https://doi.org/10.3390/ijerph182312649
Bellocchio F, Lonati C, Ion Titapiccolo J, Nadal J, Meiselbach H, Schmid M, Baerthlein B, Tschulena U, Schneider M, Schultheiss UT, et al. Validation of a Novel Predictive Algorithm for Kidney Failure in Patients Suffering from Chronic Kidney Disease: The Prognostic Reasoning System for Chronic Kidney Disease (PROGRES-CKD). International Journal of Environmental Research and Public Health. 2021; 18(23):12649. https://doi.org/10.3390/ijerph182312649
Chicago/Turabian StyleBellocchio, Francesco, Caterina Lonati, Jasmine Ion Titapiccolo, Jennifer Nadal, Heike Meiselbach, Matthias Schmid, Barbara Baerthlein, Ulrich Tschulena, Markus Schneider, Ulla T. Schultheiss, and et al. 2021. "Validation of a Novel Predictive Algorithm for Kidney Failure in Patients Suffering from Chronic Kidney Disease: The Prognostic Reasoning System for Chronic Kidney Disease (PROGRES-CKD)" International Journal of Environmental Research and Public Health 18, no. 23: 12649. https://doi.org/10.3390/ijerph182312649
APA StyleBellocchio, F., Lonati, C., Ion Titapiccolo, J., Nadal, J., Meiselbach, H., Schmid, M., Baerthlein, B., Tschulena, U., Schneider, M., Schultheiss, U. T., Barbieri, C., Moore, C., Steppan, S., Eckardt, K.-U., Stuard, S., & Neri, L. (2021). Validation of a Novel Predictive Algorithm for Kidney Failure in Patients Suffering from Chronic Kidney Disease: The Prognostic Reasoning System for Chronic Kidney Disease (PROGRES-CKD). International Journal of Environmental Research and Public Health, 18(23), 12649. https://doi.org/10.3390/ijerph182312649






