Disparities in the Evolution of the COVID-19 Pandemic between Spanish Provinces
Abstract
1. Introduction
2. Data and Methods
2.1. Database
2.2. Disaggregated Data from Spanish Provinces and Self-Governing Cities
2.3. Variables
2.4. Convergence and Phillips–Sul Methodology
- Order the N provinces according to their final values;
- Starting from the highest-order province, add adjacent provinces from our ordered list and estimate model (3). Then, select the core group by maximizing the value of the convergence t-statistic, subject to the restriction that it is greater than −1.65;
- Continue adding one province at a time of the remaining provinces to the core group, and reestimate model (3) for each formation. Use the sign criterion (t-statistic >0) to decide whether a state should join the core group;
- For the remaining provinces, repeat steps (ii)–(iii) iteratively and stop when clubs can no longer be formed. If the last group does not have a convergence pattern, conclude that its members diverge.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Definition | Unit | Source | Club 1 | Club 2 | Club 3 | Club 4 |
|---|---|---|---|---|---|---|
| Number of travelers to the province (average value of the period July–November 2020) | Travelers | INE [94] | 58,902 | 71,343 | 52,818 | 77,932 |
| Population density, 2020 | Inhabitants per km2 | INE [94] | 721 | 111 | 135 | 115 |
| Population in cities lower than 50,000, 2020 | % | INE [94] | 4.0 | 14.6 | 15.2 | 12.6 |
| Employed people in the agricultural sector, 2020, Q4 | % | INE [94] | 9.4 | 6.9 | 6.0 | 4.9 |
| Employed people in the industrial sector, 2020, Q4 | % | INE [94] | 11.3 | 17.2 | 15.6 | 11.8 |
| Employed people in the construction sector, 2020, Q4 | % | INE [94] | 5.5 | 7.1 | 7.2 | 7.5 |
| Employed people in the service sector, 2020, Q4 | % | INE [94] | 73.8 | 68.8 | 71.3 | 75.8 |
| Per capita GDP, 2018 | € | INE [94] | 19,843 | 24,416 | 24,989 | 22,458 |
| Human Development Index (HDI), 2014 | - | [95] | 0.8 | 0.8 | 0.8 | 0.8 |
| Maghreb population over total, 2020 | % | INE [94] | 1.8 | 2.5 | 1.7 | 1.9 |
| African population over total, 2020 | % | INE [94] | 2.1 | 3.1 | 2.2 | 2.4 |
| Central and South American population over total, 2020 | % | INE [94] | 1.0 | 2.3 | 2.6 | 3.2 |
| Population greater than 65, 2019 | % | INE [94] | 18.4 | 21.4 | 22.8 | 18.1 |
| Population between 16 and 30, 2019 | % | INE [94] | 16.3 | 14.7 | 14.3 | 16.1 |
| Average life of the population, 2019 | Years of age | INE [94] | 42.8 | 44.8 | 45.7 | 43.1 |
| Life expectancy at birth (LEB), estimation of the average age that population born in 2019 will be when they die | Years of age | INE [94] | 82.1 | 83.4 | 83.8 | 83.7 |
| Infant mortality rate (IMR), probability of deaths of resident children under one year of age per 1000 live births, 2019 | 10−3 | INE [94] | 3.6 | 2.5 | 2.5 | 3.5 |
| Number of physicians per 100,000, 2019 | Physicians per 100,000 inhabitants | INE [94] | 499 | 510 | 584 | 504 |
| Number of nurses per 100,000, 2019 | Nurses per 100,000 inhabitants | INE [94] | 33 | 25 | 41 | 54 |
| IgG seroprevalence at the second half of November 2020 (2nd COVID-19 wave) | % | ENE-COVID [74] | –5.5 | 6.2 | 8.3 | 7.4 |
| IgG global seroprevalence until November 2020 | % | ENE-COVID [74] | 7.3 | 8.7 | 11.1 | 11.4 |
| Positivity test rate (PTR), 24–30 November 2020 | % | [89] | 10.8 | 10.9 | 8.1 | 7.5 |
References
- Coronaviridae Study Group of the International Committee on Taxonomy of Viruses. The species Severe acute respiratory syndrome-related coronavirus: Classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020, 5, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, P.; Williams, S.; Harvala, H. Understanding the outcomes of COVID-19—Does the current model of an acute respiratory infection really fit? J. Gen. Virol. 2020, jgv001545. [Google Scholar] [CrossRef]
- Villani, L.; McKee, M.; Cascini, F.; Ricciardi, W.; Boccia, S. Comparison of Deaths Rates for COVID-19 across Europe During the First Wave of the COVID-19 Pandemic. Front. Public Health 2020, 8, 620416. [Google Scholar] [CrossRef]
- Tang, S.; Mao, Y.; Jones, R.M.; Tan, Q.; Ji, J.S.; Li, N.; Shen, J.; Lv, Y.; Pan, L.; Ding, P.; et al. Aerosol transmission of SARS-CoV-2? Evidence, prevention and control. Environ. Int. 2020, 144, 106039. [Google Scholar] [CrossRef]
- Harbourt, D.E.; Haddow, A.D.; Piper, A.E.; Bloomfield, H.; Kearney, B.J.; Fetterer, D.; Gibson, K.; Minogue, T. Modeling the stability of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) on skin, currency, and clothing. PLoS Negl. Trop. Dis. 2020, 14, e0008831. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Wang, W.; Gao, L.; Wang, Y.; Luo, K.; Ren, L.; Zhan, Z.; Chen, X.; Zhao, S.; Huang, Y.; et al. Transmission heterogeneities, kinetics, and controllability of SARS-CoV-2. Science 2021, 371, eabe2424. [Google Scholar] [CrossRef]
- Bulfone, T.C.; Malekinejad, M.; Rutherford, G.W.; Razani, N. Outdoor Transmission of SARS-CoV-2 and Other Respiratory Viruses: A Systematic Review. J. Infect. Dis. 2021, 223, 550–561. [Google Scholar] [CrossRef]
- Pifarré, I.; Arolas, H.; Acosta, E.; López-Casasnovas, G.; Lo, A.; Nicodemo, C.; Riffe, T.; Myrskylä, M. Years of life lost to COVID-19 in 81 countries. Sci. Rep. 2021, 11, 3504. [Google Scholar] [CrossRef]
- Spanish Royal Decree 463/2020, of 14 March 2020, Declaring the State of Alarm for the Management of the Health Crisis Situation Caused by COVID-19. Official State Gazette, 14 March 2020, no. 67, pp. 25390–25400. Available online: https://boe.es/boe/dias/2020/03/14/pdfs/BOE-A-2020-3692.pdf (accessed on 27 January 2021).
- Pollán, M.; Pérez-Gómez, B.; Pastor-Barriuso, R.; Oteo, J.; Hernán, M.A.; Pérez-Olmeda, M.; Sanmartín, J.L.; Fernández-García, A.; Cruz, I.; Fernández de Larrea, N.; et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): A nationwide, population-based seroepidemiological study. Lancet 2020, 396, 535–544. [Google Scholar] [CrossRef]
- Fontana, L.M.; Villamagna, A.H.; Sikka, M.K.; McGregor, J.C. Understanding viral shedding of severe acute respiratory coronavirus virus 2 (SARS-CoV-2): Review of current literature. Infect. Control. Hosp. Epidemiol. 2020, 1–10. [Google Scholar] [CrossRef]
- Yu, C.Y.; Chan, K.G.; Yean, C.Y.; Ang, G.Y. Nucleic Acid-Based Diagnostic Tests for the Detection SARS-CoV-2: An Update. Diagnostics 2021, 11, 53. [Google Scholar] [CrossRef]
- Interterritorial Board of the Spanish National Health System Public Health Commission. The Public Health Commission Approves the Inclusion of Antigenic Tests as a Rapid Diagnostic and Screening Tool for COVID-19 (Press Note). 22 September 2020. Available online: https://www.mscbs.gob.es/gabinete/notasPrensa.do?id=5057 (accessed on 27 January 2021).
- Emery, J.C.; Russell, T.W.; Liu, Y.; Hellewell, J.; Pearson, C.A.; Knight, G.M.; Eggo, R.M.; Kucharski, A.J.; Funk, S.; CMMID COVID-19 Working Group; et al. The contribution of asymptomatic SARS-CoV-2 infections to transmission on the Diamond Princess cruise ship. eLife 2020, 9, e58699. [Google Scholar] [CrossRef]
- Buitrago-Garcia, D.; Egli-Gany, D.; Counotte, M.J.; Hossmann, S.; Imeri, H.; Ipekci, A.M.; Salanti, G.; Low, N. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: A living systematic review and meta-analysis. PLoS Med. 2020, 17, e1003346. [Google Scholar] [CrossRef]
- Wang, R.; Chen, J.; Hozumi, Y.; Yin, C.; Wei, G.W. Decoding Asymptomatic COVID-19 Infection and Transmission. J. Phys. Chem. Lett. 2020, 11, 10007–10015. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Guidelines for Non-Pharmaceutical Interventions to Reduce the Impact of COVID-19 in the EU/EEA and the UK. 24 September 2020. Stockholm. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/covid-19-guidelines-non-pharmaceutical-interventions-september-2020.pdf (accessed on 27 January 2021).
- Azuma, K.; Yanagi, U.; Kagi, N.; Kim, H.; Ogata, M.; Hayashi, M. Environmental factors involved in SARS-CoV-2 transmission: Effect and role of indoor environmental quality in the strategy for COVID-19 infection control. Environ. Health Prev. Med. 2020, 25, 66. [Google Scholar] [CrossRef]
- Nørgaard, S.K.; Vestergaard, L.S.; Nielsen, J.; Richter, L.; Schmid, D.; Bustos, N.; Braye, T.; Athanasiadou, M.; Lytras, T.; Denissov, G.; et al. Real-time monitoring shows substantial excess all-cause mortality during second wave of COVID-19 in Europe, October to December 2020. Eurosurveillance 2021, 26, 2002023. [Google Scholar] [CrossRef] [PubMed]
- Aleta, A.; Martín-Corral, D.; Pastore, Y.; Piontti, A.; Ajelli, M.; Litvinova, M.; Chinazzi, M.; Dean, N.E.; Halloran, M.E.; Longini, I.M., Jr.; et al. Modelling the impact of testing, contact tracing and household quarantine on second waves of COVID-19. Nat. Hum. Behav. 2020, 4, 964–971. [Google Scholar] [CrossRef]
- Hodcroft, E.B.; Zuber, M.; Nadeau, S.; Crawford, K.; Bloom, J.D.; Veesler, D.; Vaughan, T.G.; Comas, I.; Candelas, F.G.; Stadler, T.; et al. Emergence and spread of a SARS-CoV-2 variant through Europe in the summer of 2020. medRxiv 2020. [Google Scholar] [CrossRef]
- Gómez-Carballa, A.; Bello, X.; Pardo-Seco, J.; Pérez del Molino, M.L.; Martinón-Torres, F.; Salas, A. Phylogeography of SARS-CoV-2 pandemic in Spain: A story of multiple introductions, micro-geographic stratification, founder effects, and super-spreaders. Zool. Res. 2020, 41, 605–620. [Google Scholar] [CrossRef] [PubMed]
- Aleta, A.; Moreno, Y. Age differential analysis of COVID-19 second wave in Europe reveals highest incidence among young adults. medRxiv 2020. [Google Scholar] [CrossRef]
- WHO Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/region/euro/country/es (accessed on 27 January 2021).
- Lu, G.; Razum, O.; Jahn, A.; Zhang, Y.; Sutton, B.; Sridhar, D.; Ariyoshi, K.; von Seidlein, L.; Müller, O. COVID-19 in Germany and China: Mitigation versus elimination strategy. Glob. Health Act. 2021, 14, 1875601. [Google Scholar] [CrossRef]
- Randolph, H.E.; Barreiro, L.B. Herd Immunity: Understanding COVID-19. Immunity 2020, 52, 737–741. [Google Scholar] [CrossRef]
- Turner-Musa, J.; Ajayi, O.; Kemp, L. Examining Social Determinants of Health, Stigma, and COVID-19 Disparities. Healthcare 2020, 8, 168. [Google Scholar] [CrossRef]
- Andersen, L.M.; Harden, S.R.; Sugg, M.M.; Runkle, J.D.; Lundquist, T.E. Analyzing the spatial determinants of local Covid-19 transmission in the United States. Sci. Total Environ. 2021, 754, 142396. [Google Scholar] [CrossRef]
- Mollalo, A.; Vahedi, B.; Rivera, K.M. GIS-based spatial modeling of COVID-19 incidence rate in the continental United States. Sci. Total Environ. 2020, 728, 138884. [Google Scholar] [CrossRef] [PubMed]
- Baum, C.F.; Henry, M. Socioeconomic Factors influencing the Spatial Spread of COVID-19 in the United States. Boston College Working Papers in Economics. Boston College Department of Economics: 2020. Available online: https://ideas.repec.org/p/boc/bocoec/1009.html (accessed on 27 January 2021).
- Chin, T.; Kahn, R.; Li, R.; Chen, J.T.; Krieger, N.; Buckee, C.O.; Balsari, S.; Kiang, M.V. U.S. county-level characteristics to inform equitable COVID-19 response. medRxiv 2020. [Google Scholar] [CrossRef]
- Snyder, B.F.; Parks, V. Spatial variation in socio-ecological vulnerability to Covid-19 in the contiguous United States. Health Place 2020, 66, 102471. [Google Scholar] [CrossRef]
- Maiti, A.; Zhang, Q.; Sannigrahi, S.; Pramanik, S.; Chakraborti, S.; Cerda, A.; Pilla, F. Exploring spatiotemporal effects of the driving factors on COVID-19 incidences in the contiguous United States. Sustain. Cities Soc. 2021, 68, 102784. [Google Scholar] [CrossRef] [PubMed]
- Congosto, M.; Arias, M.H. montera34/escovid19data, Collection of COVID-19 Data by Provinces in Spain. GitHub 2020. Available online: https://github.com/montera34/escovid19data (accessed on 9 December 2020).
- Interterritorial Board of the Spanish National Health System. Agreement Providing for Public Health Measures against COVID-19 for the Celebration of the Christmas Holidays. 2 December 2020. Available online: https://www.mscbs.gob.es/gabinetePrensa/notaPrensa/pdf/02.12031220103636499.pdf (accessed on 27 January 2021).
- Spanish Act 16/2003, of 28 May 2003, on Cohesion and Quality of the National Health System. Official State Gazette, 29 May 2003, no. 128, pp. 20567–20588. Law with Amendments until 10 June 2020. Available online: https://www.boe.es/buscar/pdf/2003/BOE-A-2003-10715-consolidado.pdf (accessed on 27 January 2021).
- Spanish Royal Decree 926/2020, of 25 October 2020, which Declares the State of Alarm to Contain the Spread of Infections caused by SARSCoV-2. Official State Gazette, 25 October 2020, no. 282, pp. 91912–91919. Available online: https://www.boe.es/boe/dias/2020/10/25/pdfs/BOE-A-2020-12898.pdf (accessed on 27 January 2021).
- Spanish Royal Decree 956/2020, of 3 November 2020, which Extends the State of Alarm Declared by Royal Decree 926/2020, of 25 October, by which the State of Alarm is Declared to Contain the Spread of Infections caused by SARS-CoV-2. Official State Gazette, 4 November 2020, no. 291, pp. 95841-95845. Available online: https://www.boe.es/boe/dias/2020/11/04/pdfs/BOE-A-2020-13494.pdf (accessed on 27 January 2021).
- European Centre for Disease Prevention and Control. COVID-19 Testing Strategies and Objectives, 15 September 2020. ECDC: Stockholm, 2020. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/TestingStrategy_Objective-Sept-2020.pdf (accessed on 27 January 2021).
- European Commission. Commission Recommendation of 28.10.2020 on COVID-19 Testing Strategies, Including the Use of Rapid Antigen Tests. Brussels, 2020. Available online: https://ec.europa.eu/health/sites/health/files/preparedness_response/docs/covid19_testingstrategies_recommendation_en.pdf (accessed on 27 January 2021).
- European Commission. Commission Recommendation of 18.11.2020 on the Use of Rapid Antigen Tests for the Diagnosis of SARS-CoV-2 Infection. Brussels. 2020. Available online: https://ec.europa.eu/health/sites/health/files/preparedness_response/docs/sarscov2_rapidantigentests_recommendation_en.pdf (accessed on 27 January 2021).
- European Centre for Disease Prevention and Control. Options for the Use of Rapid Antigen Tests for COVID-19 in the EU/EEA and the UK, ECDC Technical Guidance. ECDC: Stockholm, 19 November 2020. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/Options-use-of-rapid-antigen-tests-for-COVID-19.pdf (accessed on 27 January 2021).
- Interterritorial Board of the Spanish National Health System. Resolution of 30 September 2020 Publishing Agreement of the Interterritorial Board of the Spanish National Health System on the Declaration of Coordinated Actionsin Public Health to Respond to Situations of Special Risk due to Uncontrolled Transmission of SARS-CoV-2 Infections. Official State Gazette, 1 October 2020, no. 260, pp. 83224-83232. Available online: https://www.boe.es/boe/dias/2020/10/01/pdfs/BOE-A-2020-11590.pdf (accessed on 27 January 2021).
- Interterritorial Board of the Spanish National Health System. Coordinated Response Actions to Control the Transmission of COVID-19. 22 October 2020. Available online: https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Actuaciones_respuesta_COVID-19_ENG.pdf (accessed on 27 January 2021).
- Council of the European Union. Draft Council Recommendation on a Coordinated Approach to the Restriction of Free Movement in Response to the COVID-19 Pandemic. Adopted by EU Member States on 13 October. 12 October 2020. Available online: https://data.consilium.europa.eu/doc/document/ST-11689-2020-REV-1/en/pdf (accessed on 27 January 2021).
- Barro, R.J.; Sala-i-Martin, X. Convergence. J. Politic. Econ. 1992, 100, 223–251. [Google Scholar] [CrossRef]
- Phillips, P.C.; Sul, D. Transition modeling and econometric convergence tests. Econometrica 2007, 75, 1771–1855. [Google Scholar] [CrossRef]
- Phillips, P.C.; Sul, D. Economic transition and growth. J. Appl. Econ. 2009, 24, 1153–1185. [Google Scholar] [CrossRef]
- Panopoulou, E.; Pantelidis, T. Convergence in per capita health expenditures and health outcomes in the OECD countries. Appl. Econ. 2012, 44, 3909–3920. [Google Scholar] [CrossRef]
- Panopoulou, E.; Pantelidis, T. Cross-state disparities in us health care expenditures. Health Econ. 2013, 22, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Clemente, J.; Lázaro-Alquézar, A.; Montañés, A. Does the Great Recession contribute to the convergence of health care expenditures in the US States? Int. J. Environ. Res. Public Health 2020, 17, 554. [Google Scholar] [CrossRef]
- Duncan, R.; Toledo, P. Inequality in body mass indices across countries: Evidence from convergence tests. Econ. Hum. Biol. 2019, 33, 40–57. [Google Scholar] [CrossRef]
- Kasman, S.; Kasman, A. Convergence in obesity and overweight rates across OECD countries: Evidence from the stochastic and club convergence tests. Empirical Econ. 2020, 1–34. [Google Scholar] [CrossRef]
- Christopoulos, K.; Eleftheriou, K. Premature mortality in the US: A convergence study. Soc. Sci. Med. 2020, 258, 113141. [Google Scholar] [CrossRef]
- González-Álvarez, M.A.; Lázaro-Alquézar, A.; Simón-Fernández, M.B. Global Trends in Child Obesity: Are Figures Converging? Int. J. Environ. Res. Public Health 2020, 17, 9252. [Google Scholar] [CrossRef]
- Hodrick, R.J.; Prescott, E.C. Postwar US business cycles: An empirical investigation. J. Money Credit Bank. 1997, 29, 1–16. [Google Scholar] [CrossRef]
- Ravn, M.O.; Uhlig, H. On adjusting the Hodrick-Prescott filter for the frequency of observations. Rev. Econ. Stat. 2002, 84, 371–376. [Google Scholar] [CrossRef]
- Brant, R. Assessing proportionality in the proportional odds model for ordinal logistic regression. Biometrics 1990, 46, 1171–1178. [Google Scholar] [CrossRef]
- Gibbs, H.; Nightingale, E.; Liu, Y.; Cheshire, J.; Danon, L.; Smeeth, L.; Pearson, C.A.B.; Grundy, C.; Kucharski, A.J.; Eggo, R.M.; et al. Human movement can inform the spatial scale of interventions against COVID-19 transmission. medRxiv 2020. [Google Scholar] [CrossRef]
- Gösgens, M.; Hendriks, T.; Boon, M.; Steenbakkers, W.; Heesterbeek, H.; van der Hofstad, R.; Litvak, N. Trade-offs between mobility restrictions and transmission of SARS-CoV-2. J. Royal Soc. Interf. 2021, 18, 20200936. [Google Scholar] [CrossRef]
- Liu, Y.; Morgenstern, C.; Kelly, J.; Lowe, R.; Jit, M.; CMMID COVID-19 Working Group. The impact of non-pharmaceutical interventions on SARS-CoV-2 transmission across 130 countries and territories. BMC Med. 2021, 19, 40. [Google Scholar] [CrossRef]
- Fan, C.; Lee, S.; Yang, Y.; Oztekin, B.; Li, Q.; Mostafavi, A. Effects of population co-location reduction on cross-county transmission risk of COVID-19 in the United States. Appl. Netw. Sci. 2021, 6, 14. [Google Scholar] [CrossRef]
- Chang, S.; Pierson, E.; Koh, P.W.; Gerardin, J.; Redbird, B.; Grusky, D.; Leskovec, J. Mobility network models of COVID-19 explain inequities and inform reopening. Nature 2021, 589, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Eggo, R.M.; Kucharski, A.J. Secondary attack rate and superspreading events for SARS-CoV-2. Lancet 2020, 395, e47. [Google Scholar] [CrossRef]
- Loo, B.; Tsoi, K.H.; Wong, P.; Lai, P.C. Identification of superspreading environment under COVID-19 through human mobility data. Sci. Rep. 2021, 11, 4699. [Google Scholar] [CrossRef]
- Robina-Ramírez, R.; Medina-Merodio, J.A.; Moreno-Luna, L.; Jiménez-Naranjo, H.V.; Sánchez-Oro, M. Safety and Health Measures for COVID-19 Transition Period in the Hotel Industry in Spain. Int. J. Environ. Res. Public Health 2021, 18, 718. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pose, A.; von Berlepsch, V. Does Population Diversity Matter for Economic Development in the Very Long Term? Historic Migration, Diversity and County Wealth in the US. Eur. J. Population. Rev. Eur. Demograph. 2018, 35, 873–911. [Google Scholar] [CrossRef] [PubMed]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef]
- Nixon, J.; Ulmann, P. The relationship between health care expenditure and health outcomes. Eur. J. Health Econ. 2006, 7, 7–18. [Google Scholar] [CrossRef]
- Tapia Granados, J.A.; Ionides, E.L. Population health and the economy: Mortality and the Great Recession in Europe. Health Econ. 2017, 26, e219–e235. [Google Scholar] [CrossRef]
- Trias-Llimós, S.; Riffe, T.; Bilal, U. Monitoring life expectancy levels during the COVID-19 pandemic: Example of the unequal impact of the first wave on Spanish regions. PLoS ONE 2020, 15, e0241952. [Google Scholar] [CrossRef]
- Vignesh, R.; Shankar, E.M.; Velu, V.; Thyagarajan, S.P. Is Herd Immunity Against SARS-CoV-2 a Silver Lining? Front. Immunol. 2020, 11, 586781. [Google Scholar] [CrossRef]
- Ke, R.; Romero-Severson, E.; Sanche, S.; Hengartner, N. Estimating the reproductive number R0 of SARS-CoV-2 in the United States and eight European countries and implications for vaccination. J. Theoretical Biol. 2021, 110621. [Google Scholar] [CrossRef] [PubMed]
- Carlos III Health Institute. National Seroepidemiological Study of SARS-CoV-2 Infection in Spain (ENE-COVID study), Fourth Round. 15 December 2020. Available online: https://www.mscbs.gob.es/gabinetePrensa/notaPrensa/pdf/15.12151220163348113.pdf (accessed on 27 January 2021).
- Fiocchi, A.; Jensen-Jarolim, E. SARS-COV-2, can you be over it? Arguments for the Immune passport. World Allergy Organ. J. 2021, 100514. [Google Scholar] [CrossRef] [PubMed]
- Sherina, N.; Piralla, A.; Du, L.; Wan, H.; Kumagai-Braesh, M.; Andréll, J.; Braesch-Andersen, S.; Cassaniti, I.; Percivalle, E.; Sarasini, A.; et al. Persistence of SARS-CoV-2 specific B- and T-cell responses in convalescent COVID-19 patients 6-8 months after the infection. Med 2021, 2, 281–295. [Google Scholar] [CrossRef]
- Dan, J.M.; Mateus, J.; Kato, Y.; Hastie, K.M.; Yu, E.D.; Faliti, C.E.; Grifoni, A.; Ramirez, S.I.; Haupt, S.; Frazier, A.; et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021, 371, eabf4063. [Google Scholar] [CrossRef]
- Gaebler, C.; Wang, Z.; Lorenzi, J.; Muecksch, F.; Finkin, S.; Tokuyama, M.; Cho, A.; Jankovic, M.; Schaefer-Babajew, D.; Oliveira, T.Y.; et al. Evolution of antibody immunity to SARS-CoV-2. Nature 2021, 591, 639–644. [Google Scholar] [CrossRef] [PubMed]
- Wajnberg, A.; Amanat, F.; Firpo, A.; Altman, D.R.; Bailey, M.J.; Mansour, M.; McMahon, M.; Meade, P.; Mendu, D.R.; Muellers, K.; et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 2020, 370, 1227–1230. [Google Scholar] [CrossRef]
- Long, Q.X.; Tang, X.J.; Shi, Q.L.; Li, Q.; Deng, H.J.; Yuan, J.; Hu, J.L.; Xu, W.; Zhang, Y.; Lv, F.J.; et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef]
- Cañete, P.F.; Vinuesa, C.G. COVID-19 Makes B Cells Forget, but T Cells Remember. Cell 2020, 183, 13–15. [Google Scholar] [CrossRef]
- Seow, J.; Graham, C.; Merrick, B.; Acors, S.; Pickering, S.; Steel, K.; Hemmings, O.; O’Byrne, A.; Kouphou, N.; Galao, R.P.; et al. Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat. Microbiol. 2020, 5, 1598–1607. [Google Scholar] [CrossRef]
- Chen, Y.; Zuiani, A.; Fischinger, S.; Mullur, J.; Atyeo, C.; Travers, M.; Lelis, F.; Pullen, K.M.; Martin, H.; Tong, P.; et al. Quick COVID-19 Healers Sustain Anti-SARS-CoV-2 Antibody Production. Cell 2020, 183, 1496–1507.e16. [Google Scholar] [CrossRef]
- Miyasaka, M. COVID-19 and immunity: Quo vadis? Int. Immunol. 2021, dxab008. [Google Scholar] [CrossRef] [PubMed]
- Lavine, J.S.; Bjornstad, O.N.; Antia, R. Immunological characteristics govern the transition of COVID-19 to endemicity. Science 2021, 741–745. [Google Scholar] [CrossRef]
- Wyllie, D.; Mulchandani, R.; Jones, H.E.; Taylor-Phillips, S.; Brooks, T.; Charlett, A.; Ades, A.E.; Makin, A.; Oliver, I.; Moore, P.; et al. SARS-CoV-2 responsive T cell numbers are associated with protection from COVID-19: A prospective cohort study in keyworkers. medRxiv 2020. [Google Scholar] [CrossRef]
- Harvey, R.A.; Rassen, J.A.; Kabelac, C.A.; Turenne, W.; Leonard, S.; Klesh, R.; Meyer, W.A., 3rd; Kaufman, H.W.; Anderson, S.; Cohen, O.; et al. Association of SARS-CoV-2 Seropositive Antibody Test with Risk of Future Infection. JAMA Int. Med. 2021, 181, 672–679. [Google Scholar] [CrossRef]
- Edridge, A.; Kaczorowska, J.; Hoste, A.; Bakker, M.; Klein, M.; Loens, K.; Jebbink, M.F.; Matser, A.; Kinsella, C.M.; Rueda, P.; et al. Seasonal coronavirus protective immunity is short-lasting. Nat. Med. 2020, 26, 1691–1693. [Google Scholar] [CrossRef] [PubMed]
- Spanish Directorate-General of Public Health, Spanish Department of Health. Main Monitoring Indicators for COVID-19 for 17-30 November 2020. 3 December 2020. Available online: https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/informe_covid_es_publico_2020-11-30.pdf (accessed on 27 January 2021).
- Health Alerts and Emergencies Coordination Center, Spanish Directorate-General of Public Health, Spanish Department of Health. Epidemiological Update on COVID-19 no. 212. 22 September 2020. Available online: https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Actualizacion_212_COVID-19.pdf (accessed on 27 January 2021).
- Haug, N.; Geyrhofer, L.; Londei, A.; Dervic, E.; Desvars-Larrive, A.; Loreto, V.; Pinior, B.; Thurner, S.; Klimek, P. Ranking the effectiveness of worldwide COVID-19 government interventions. Nat. Hum. Behav. 2020, 4, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Spanish Royal Decree 900/2020, of 9 October 2020, declaring the State of Alarm to Respond to Situations of Special Risk due to Uncontrolled Transmission of SARS-CoV-2 Infections. Official State Gazette, 9 October 2020, no. 268, pp. 86909–86915. Available online: https://www.boe.es/boe/dias/2020/10/09/pdfs/BOE-A-2020-12109.pdf (accessed on 27 January 2021).
- Gokmen, Y.; Baskici, C.; Ercil, Y. The impact of national culture on the increase of COVID-19: A cross-country analysis of European countries. Int. J. Intercult. Relations 2021, 81, 1–8. [Google Scholar] [CrossRef]
- Spanish National Statistics Institute (INE). Available online: https://www.ine.es/ (accessed on 27 January 2021).
- Montañés, A.; Olmos, L.; Reyes, M. Has the Great Recession affected the convergence process? The case of Spanish provinces. Econ. Model. 2018, 68, 360–371. [Google Scholar] [CrossRef]
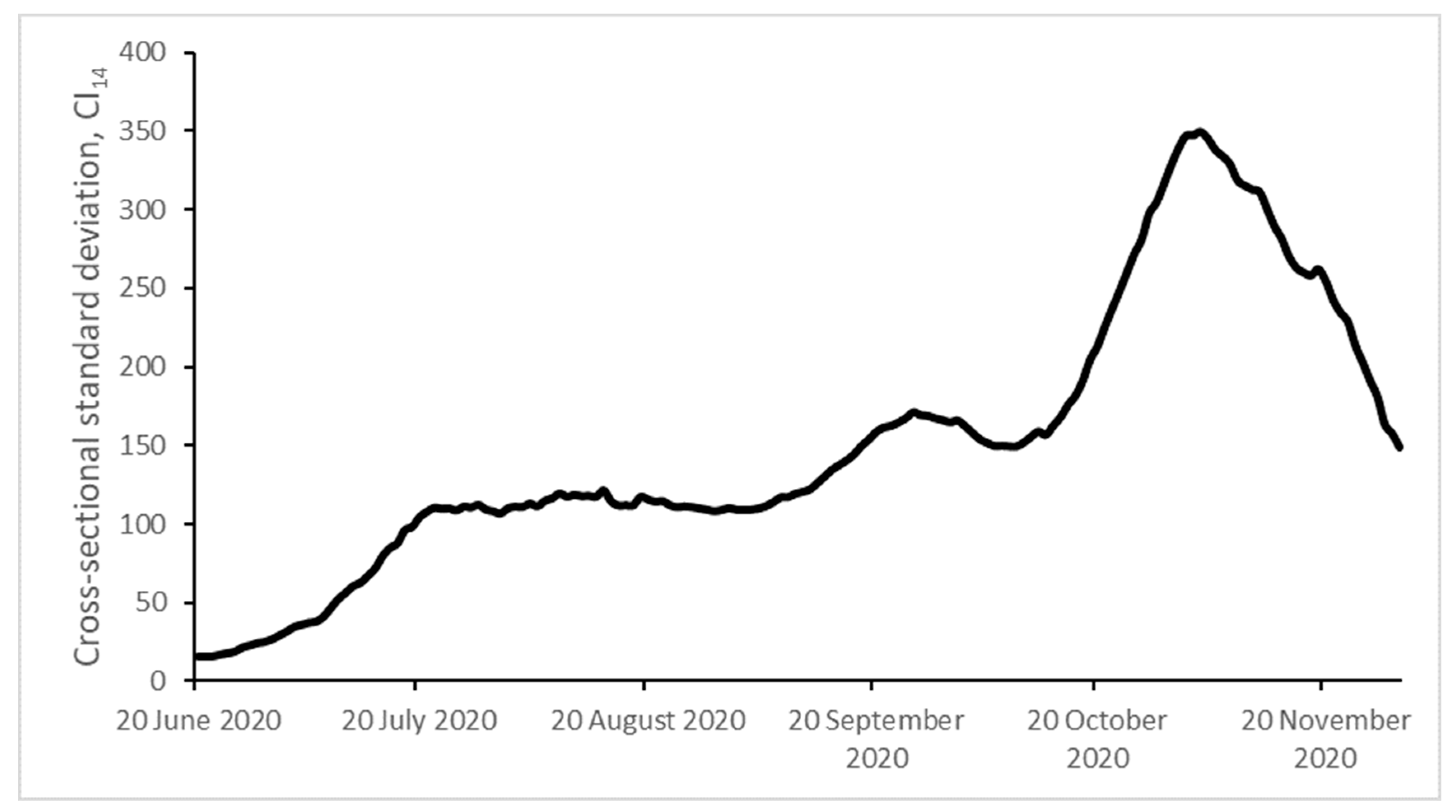
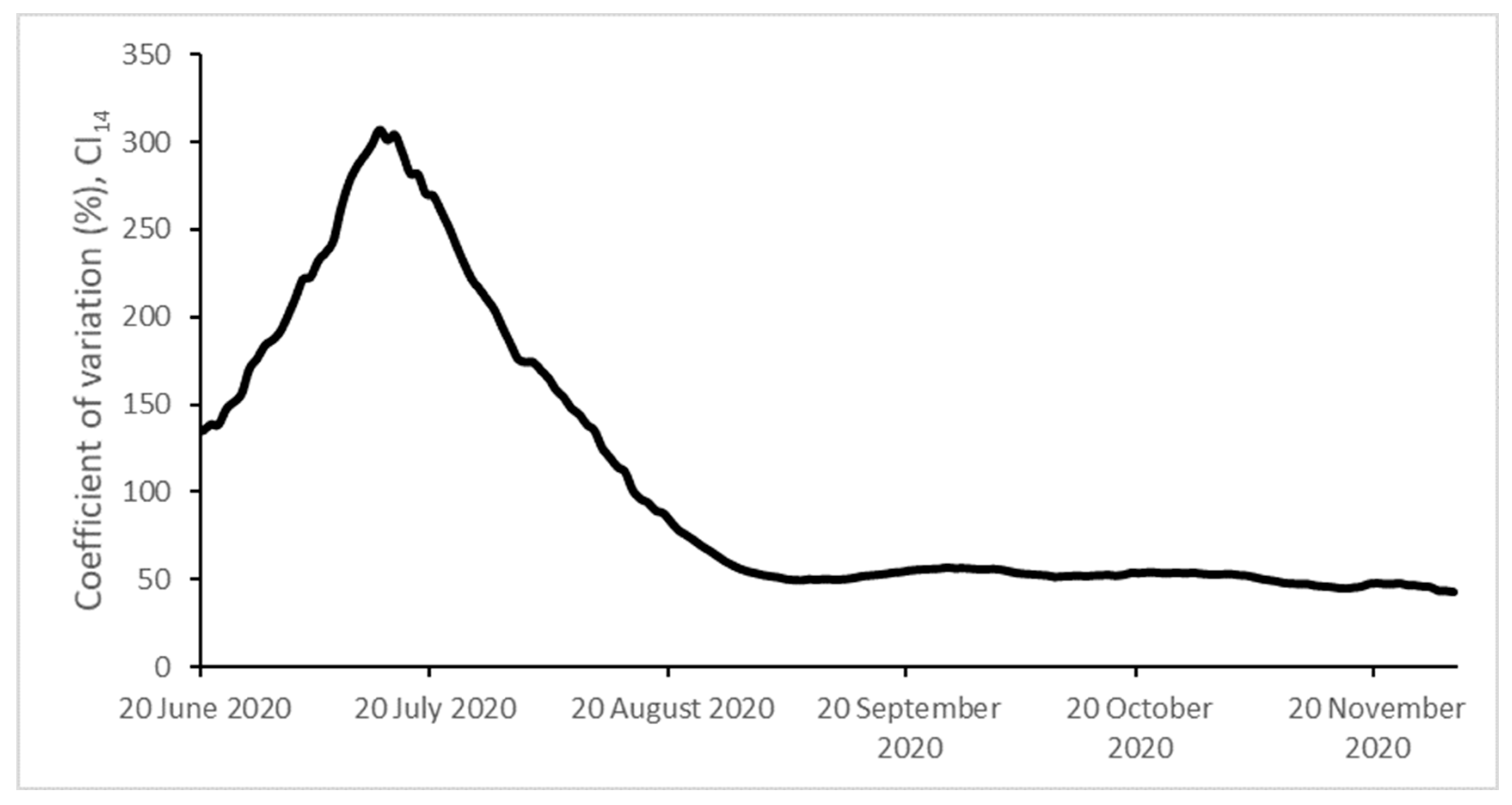
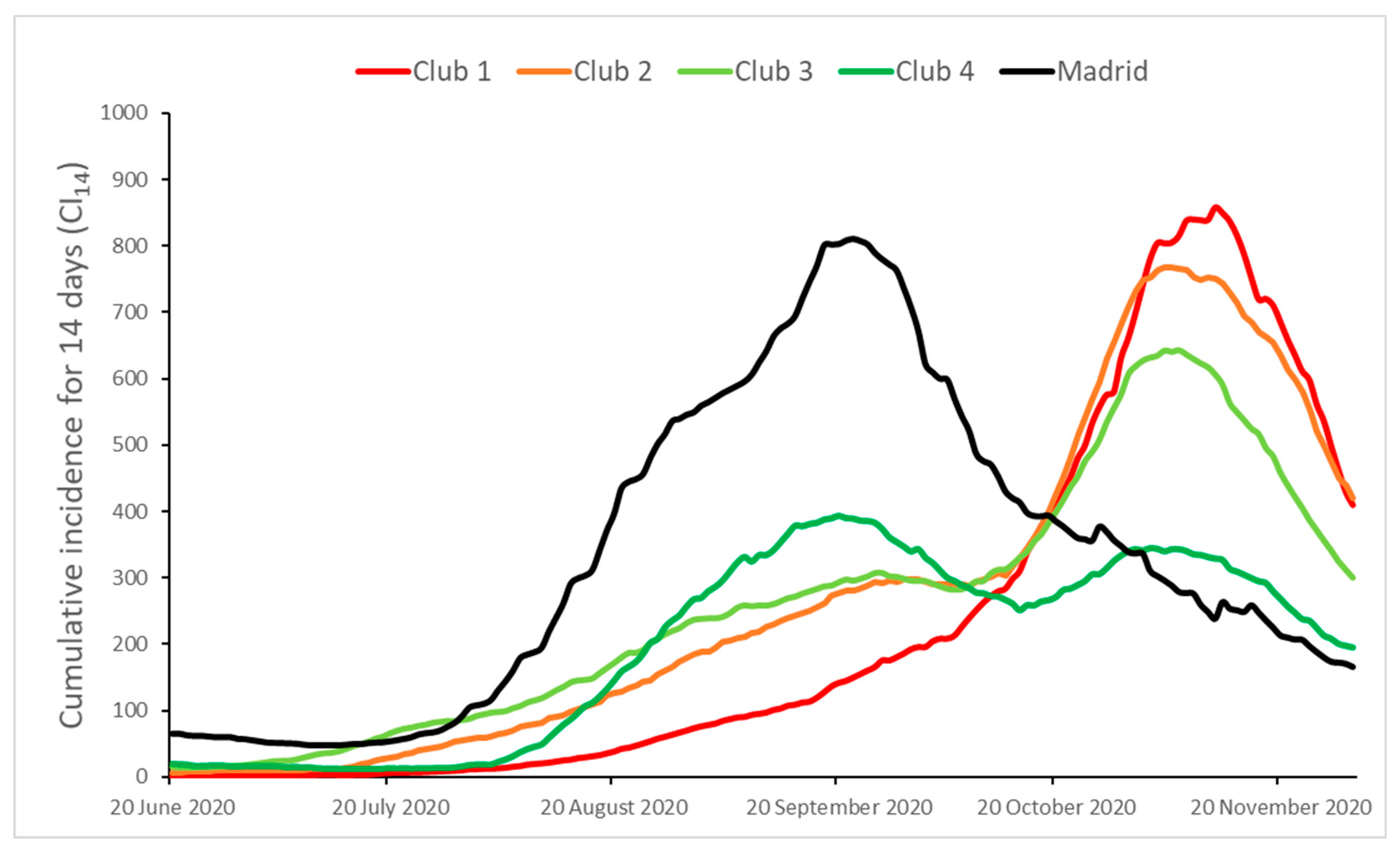
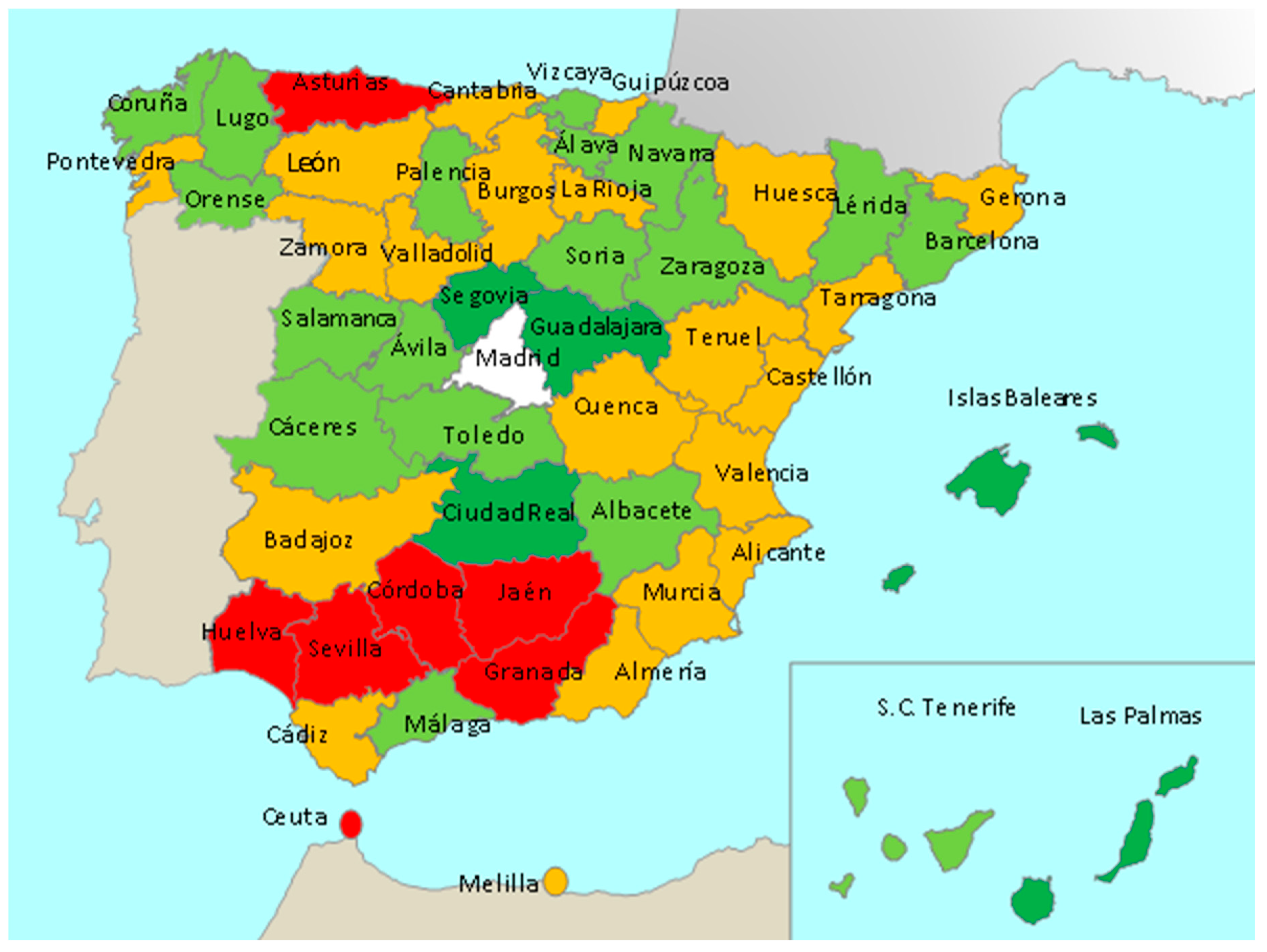
| Initial | Final | Max | Min | CV (%) | |
|---|---|---|---|---|---|
| Alacant/Alicante | 1.1 | 358.8 | 394.2 | 1.0 | 91.3 |
| Albacete | 8.8 | 330.3 | 510.1 | 7.0 | 77.2 |
| Almería | 0.8 | 379.2 | 482.8 | 0.7 | 72.4 |
| Araba-Álava | 9.2 | 370.4 | 545.9 | 8.7 | 66.7 |
| Asturias | 0.8 | 398.0 | 649.7 | 0.1 | 123.5 |
| Ávila | 19.0 | 241.7 | 783.4 | 1.9 | 88.6 |
| Badajoz | 1.5 | 205.9 | 700.5 | 1.5 | 99.2 |
| Balears, Illes/Baleares, Islas | 5.4 | 185.6 | 335.8 | 4.6 | 72.0 |
| Barcelona | 11.5 | 234.9 | 809.0 | 9.6 | 83.3 |
| Bizkaia/Vizcaya | 23.5 | 367.2 | 745.5 | 3.1 | 73.7 |
| Burgos | 9.0 | 759.5 | 1387.8 | 3.4 | 97.8 |
| Cáceres | 5.1 | 260.8 | 508.7 | 2.3 | 87.0 |
| Cádiz | 2.2 | 524.0 | 579.9 | 1.2 | 112.2 |
| Cantabria | 3.6 | 338.0 | 547.4 | 1.9 | 91.9 |
| Castelló/Castellón | 3.3 | 309.3 | 485.0 | 2.2 | 103.4 |
| Ceuta | 0.1 | 218.2 | 838.7 | 0.1 | 116.0 |
| Ciudad Real | 44.6 | 297.1 | 510.9 | 19.6 | 76.0 |
| Córdoba | 0.3 | 296.9 | 776.5 | 0.1 | 104.3 |
| Coruña, A/Coruña, La | 4.0 | 224.0 | 349.8 | 2.9 | 82.1 |
| Cuenca | 25.0 | 520.6 | 1000.4 | 2.0 | 103.1 |
| Girona/Gerona | 11.9 | 299.7 | 964.5 | 8.0 | 102.7 |
| Gipuzkoa/Guipúzcoa | 3.5 | 569.9 | 1103.5 | 3.5 | 96.6 |
| Granada | 2.1 | 555.3 | 1485.3 | 2.1 | 128.7 |
| Guadalajara | 29.9 | 233.5 | 659.5 | 11.6 | 76.2 |
| Huelva | 0.2 | 380.7 | 576.4 | 0.2 | 137.6 |
| Huesca | 21.8 | 368.8 | 1429.7 | 21.8 | 80.6 |
| Jaén | 1.7 | 480.3 | 1012.5 | 0.9 | 119.8 |
| León | 9.6 | 465.9 | 897.0 | 2.2 | 104.0 |
| Lleida/Lérida | 59.3 | 360.3 | 731.6 | 59.3 | 53.4 |
| Lugo | 2.7 | 240.3 | 310.1 | 1.2 | 77.7 |
| Madrid | 65.9 | 166.4 | 810.7 | 48.4 | 71.2 |
| Málaga | 2.5 | 252.6 | 368.1 | 2.3 | 77.6 |
| Melilla | 2.3 | 420.9 | 1469.6 | 2.3 | 110.7 |
| Murcia | 1.2 | 279.7 | 730.6 | 1.2 | 84.6 |
| Navarra | 8.1 | 286.3 | 1270.4 | 8.1 | 84.7 |
| Ourense/Orense | 2.3 | 120.9 | 477.2 | 0.3 | 99.3 |
| Palencia | 24.8 | 607.5 | 1033.0 | 5.0 | 96.7 |
| Palmas, Las | 2.2 | 38.5 | 313.5 | 1.7 | 109.6 |
| Pontevedra | 4.9 | 274.2 | 359.3 | 0.6 | 115.0 |
| Rioja, La | 3.5 | 435.2 | 798.3 | 1.6 | 82.5 |
| Salamanca | 18.8 | 296.0 | 1046.6 | 2.4 | 91.4 |
| Santa Cruz de Tenerife | 1.2 | 123.8 | 125.1 | 0.2 | 79.4 |
| Segovia | 19.6 | 221.4 | 510.7 | 10.4 | 73.6 |
| Sevilla | 0.7 | 349.2 | 798.6 | 0.2 | 115.8 |
| Soria | 57.5 | 496.4 | 854.1 | 27.1 | 77.2 |
| Tarragona | 2.2 | 189.0 | 868.8 | 0.4 | 105.4 |
| Teruel | 6.7 | 416.7 | 1153.3 | 6.0 | 78.6 |
| Toledo | 12.5 | 351.7 | 840.5 | 4.0 | 81.4 |
| València/Valencia | 4.8 | 379.5 | 454.3 | 3.5 | 85.5 |
| Valladolid | 17.3 | 743.1 | 1200.1 | 4.8 | 92.9 |
| Zamora | 6.4 | 587.7 | 1050.8 | 0.6 | 104.0 |
| Zaragoza | 7.3 | 317.3 | 1050.9 | 6.8 | 63.5 |
| Panel I. Testing for Convergence | ||||||
|---|---|---|---|---|---|---|
| Provinces | PS | |||||
| Full sample | −0.809 (−218.01) | |||||
| Panel II. Convergence Clubs | ||||||
| Panel A. Initial Estimation | Panel B. Adjacent Analysis | Panel C. Final Estimation | ||||
| Initial Clubs | Provinces | PS | Merging | PS | Final Clubs | Provinces |
| C1 | Asturias, Córdoba, Granada, Huelva, Jaén, Sevilla, Ceuta | 0.211 (10.56) | C1 + C2 | −0.46 (−412.57) | Club 1 | Asturias, Córdoba, Granada, Huelva, Jaén, Sevilla, Ceuta |
| C2 | Alicante, Almería, Badajoz, Burgos, Cádiz, Cantabria, Castellón, Cuenca, Gerona, Guipúzcoa, Huesca, León, Murcia, Pontevedra, La Rioja, Tarragona, Teruel, Valencia, Valladolid, Zamora, Melilla | 0.019 (1.30) | C2 + C3 | −0.1173 (−8.99) | Club 2 | Alicante, Almería, Badajoz, Burgos, Cádiz, Cantabria, Castellón, Cuenca, Gerona, Guipúzcoa, Huesca, León, Murcia, Pontevedra, La Rioja, Tarragona, Teruel, Valencia, Valladolid, Zamora, Melilla |
| C3 | Barcelona, Cáceres, La Coruña, Lérida, Lugo, Málaga, Navarra, Orense, Palencia, Santa Cruz de Tenerife, Zaragoza | 0.173 (11.86) | C3 + C4 | 0.023 (1.63) | Club 3 | Álava, Albacete, Ávila, Barcelona, Cáceres, La Coruña, Lérida, Lugo, Málaga, Navarra, Orense, Palencia, Salamanca, Santa Cruz de Tenerife, Soria, Toledo, Vizcaya, Zaragoza |
| C4 | Álava, Albacete, Ávila, Salamanca, Soria, Toledo, Vizcaya | 0.261 (17.40) | C4 + C5 | 0.145 (2.58) | Club 4 | Islas Baleares, Ciudad Real, Guadalajara, Las Palmas, Segovia |
| C5 | Islas Baleares, Las Palmas, Segovia | 0.613 (2.77) | C5 + C6 | 0.445 (14.15) | Divergent | Madrid |
| C6 | Ciudad Real, Guadalajara | 0.327 (23.75) | C6 + Divergent | −1.819 (−40.13) | ||
| Divergent | Madrid | |||||
| Marginal Effects | |||||
|---|---|---|---|---|---|
| Variable | Estimations | Club 1 | Club 2 | Club 3 | Club 4 |
| Travelers | −2.54 × 10−5 (−5.27) | 8.94 × 10−7 | 5.10 × 10−6 | −5.49 × 10−6 | −5.08 × 10−7 |
| Employed people in agricultural sector | −0.12 (−6.06) | 0.004 | 0.024 | −0.026 | −0.002 |
| Employed people in industrial sector | −0.288 (−11.96) | 0.010 | 0.058 | −0.062 | −0.006 |
| Central and South American immigrants | 1.84 (4.87) | −0.065 | −0.370 | 0.397 | 0.037 |
| Life expectancy at birth | 1.48 (4.10) | −0.052 | −0.298 | 0.321 | 0.030 |
| Capital of the region | −0.848 (−2.55) | 0.030 | 0.170 | −0.183 | −0.017 |
| Cut-points | |||||
| Cut-point 1 | 117.45 | ||||
| Cut-point 2 | 121.20 | ||||
| Cut-point 3 | 124.59 | ||||
| N | 51 | ||||
| Pseudo R2 | 0.34 | ||||
| Correctly classified cases | 69% | ||||
| Brant statistic | 7.09 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Mendoza, H.; Montañés, A.; Moliner-Lahoz, F.J. Disparities in the Evolution of the COVID-19 Pandemic between Spanish Provinces. Int. J. Environ. Res. Public Health 2021, 18, 5085. https://doi.org/10.3390/ijerph18105085
López-Mendoza H, Montañés A, Moliner-Lahoz FJ. Disparities in the Evolution of the COVID-19 Pandemic between Spanish Provinces. International Journal of Environmental Research and Public Health. 2021; 18(10):5085. https://doi.org/10.3390/ijerph18105085
Chicago/Turabian StyleLópez-Mendoza, Héctor, Antonio Montañés, and F. Javier Moliner-Lahoz. 2021. "Disparities in the Evolution of the COVID-19 Pandemic between Spanish Provinces" International Journal of Environmental Research and Public Health 18, no. 10: 5085. https://doi.org/10.3390/ijerph18105085
APA StyleLópez-Mendoza, H., Montañés, A., & Moliner-Lahoz, F. J. (2021). Disparities in the Evolution of the COVID-19 Pandemic between Spanish Provinces. International Journal of Environmental Research and Public Health, 18(10), 5085. https://doi.org/10.3390/ijerph18105085






